Note: Descriptions are shown in the official language in which they were submitted.
2 1 3 3 4 3 2 1 PILOT 8 ~
Temperature-Sensitive Color-Memorizing
Microcapsulated Pigment
The ~ubject matter of thi~ application i8 related to that
di~clo~ed in copending Application~ No. 2,138,897 and 2,138,900.
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a temperature-
sensitive color-memorizing microcapsulated pigment,
more specifically, to a temperature-sensitive color-
memorizing microcapsulated pigment which exhibits a
wide hysteresis range of coloring/decloring alternately
and reversibly caused by temperature changes and
remains in the colored/decolored state at ambient
temperatures without being continuously exposed to
high/low temperatures required for causing the
colored/decolored state.
Related Backqround Art
A temperature-sensitive color-memorizing
microcapsulated material of the above-mentioned kind is
disclosed in Japanese Patent Publication No. 4-17154.
A widely-used, typical reversible thermochromatic
material exhibiting thermochromatism at a certain
color-change-causing temperature can be, at ambient
~ temperatures, only in one of its two states, that is,
in the state maintained at ambient temperature. The
other state can not be maintained unless the material
is continuously exposed to a temperature beyond the
color-change-causing temperature in a case that the
2133932
-- 2
color-change-causing temperature is higher than ambient
temperature, or to a temperature below the color-
change-causing temperature in a case that the color-
change-causing temperature is lower than ambient
temperatures. The temperature-sensitive color-
memorizing material disclosed in said patent
publication, however, can selectively maintain, at
ambient temperatures, both of its two states obtained
at temperatures higher/lower than the color-change-
causing temperature. By heating and cooling thematerial the two states can be alternately selected to
be maintained at ambient temperatures. As a result,
the temperature-sensitive color-memorizing material has
been applied to various kinds of fields such as
temperature-sensitive recording materials, toys,
ornaments, printing materials, and so on.
As described in the Japanese Patent Publication
No. 4-17154, the above-mentioned color-memorizing
effect consists in thermochromism obtained only by
systems having, as a constituent, compound(s) selected
from a specific group of esters included in the group
of esters used to control color forming reactions. In
other words, the ester to be employed in the material
can not be freely chosen and the applications of the
temperature-sensitive color-memorizing material are
limited. Accordingly, new materials of this kind which
are more effective and more useful have been greatly
21339:32
demanded.
SUMMARY OF THE INVENTION
The object of the present invention is to provide
a temperature-sensitive color-memorizing
microcapsulated pigment capable of a wide range of
applications by finding compounds which function as
reaction media for exhibiting the above-mentioned
color-memorizing effect and which can be selected more
easily.
The present invention is based on the finding of
the inventors that a system employing, as the reaction
media, one or more specific aliphatic acid alcohol
esters obtained from a specific group of monohydric
aliphatic alcohols having odd numbers of carbon atoms
and a group of aliphatic carboxylic acids having even
numbers of carbon atoms exhibits thermochromism
characteristics having wide hysteresis ranges (~H) and
excellent color memorizing effects.
A temperature-sensitive color-memorizing
microcapsulated pigment according to the present
invention is the combination in a homogenous compatible
state of, as indispensable components, (A) an electron-
donating chromatic organic compound, (B) an electron-
accepting compound, and (C) one or more esters employed
to control color forming reactions. The one or more
esters are ones which realize the characteristics of
2133932
reversible color change of the temperature-sensitive
color-memorizing material having a wide hysteresis
range with respect to the relation between color
densities and temperatures. These three kinds of
indispensable components in the homogenous compatible
state are enclosed or occluded in microcapsules and
change colors with a hysteresis range (~H) from 8 C to
30 C.
The ester(s) are selected from the group
consisting of: (1) aliphatic acid alcohol esters
obtained from monohydric aliphatic alcohols having odd
numbers (not less than 9) of carbon atoms and aliphatic
carboxylic acids having even numbers of carbon atoms
and/or (2) aliphatic acid alcohol esters having 17 to
23 carbon atoms obtained from aliphatic carboxylic
acids having even numbers (10 to 16) of carbon atoms
and either n-pentyl alcohol or n-heptyl alcohol.
Further, the above-mentioned aliphatic acid
alcohol esters may be selected from aliphatic acid
alcohol esters having 17 to 37 carbon atoms obtained
from n-nonyl alcohol, n-undecyl alcohol, n-tridecyl
alcohol or n-pentadecyl alcohol.
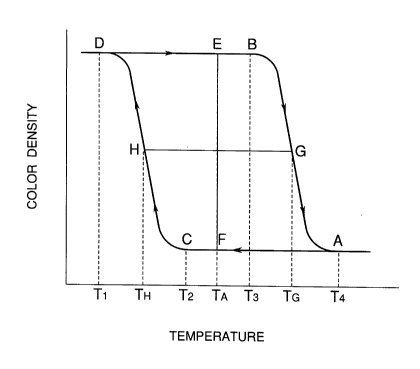
BRIEF DESCRIPTION OF THE DRAWING
Figure illustrates graphically a relationship
between color density and temperature indicating the
hysteresis characteristic of the temperature-sensitive
21~3932
-- 5
color-memorizing microcapsulated pigment according to
the present invention.
The relationship between color density and
temperature indicating the hysteresis characteristic of
the temperature-sensitive color-memorizing
microcapsulated pigment according to the present
invention will be described with respect to Figure.
In Figure with the axis of ordinates, color
densities, and the axis of abscissas, temperatures, the
color density changes as the temperature changes as is
indicated by the arrows. Point A indicates the color
density at a temperature T4, the lowest temperature
which can cause a completely decoloring state
(hereinafter referred as coloring/decoloring threshold
temperature). Point B indicates the color density at a
temperature T3, the highest temperature at which a
completely colored state can be maintained (hereinafter
referred as highest color-maintaining temperature).
Point C indicates the color density at a temperature T2,
the lowest temperature at which a completely decoloring
state can be maintained (hereinafter referred as lowest
decoloring maintaining temperature). And point D
indicates the color density at a temperature T1, the
highest temperature which can cause a completely
coloring state (hereinafter referred as coloring-
completing temperature). At a temperature TA~ both of
two phases, that is, a colored state indicated by point
2133932
-- 6 --
E and a decolored state indicated by point F can be
obtained. As is clearly understood, within a
temperature range, including the temperature TA' in
which the colored state and the decolored state are
compatible with each other, the obtained colored or
decolored state can be maintained. The length of the
segment EF corresponds to the contrast of
coloring/decoloring. And the length of the segment HG
intersecting the middle point of the segment EF
corresponds to the range of the hysteresis with respect
to temperatures (hereinafter referred as hysteresis
range ~H). The greater the hysteresis range ~H is, the
easier the obtained colored or decolored state can be
maintained. According to the experiments carried out
by the inventors, the obtained colored or decolored
state can be practically maintained if ~H appears
within a range from 8 C to 30 C. The difference ~t
between the coloring/decoloring threshold temperature T4
and the highest color-maintaining temperature T3
corresponds to sharpness of color change, wherein ~t
should be from 1 C to 10 C to be effective for
practical use.
In addition, the temperature range from T3 to T2,
including TA' within which both the colored state and
the decolored state can be substantially maintained
should be from 2 C to 30 C to be effective for
practical use.
,~133932
The ratio of said three kinds of components of the
present invention is determined according to desired
color densities, color-change-causing temperatures,
color change behavior and the types of respective
components employed. In general, however, 0.1 to 50
parts of the component (B), more preferably 0.5 to 20
parts thereof, is employed against 1 part of the
component (A) in order to obtain desirable properties.
Similarly, 1 to 800 parts of the component (C), more
preferably 5 to 200 parts thereof, is employed against
1 part of the component (A). Note that parts are
measured by weight ratio.
Each component may be a mixture of plural kinds of
materials. Thus, so long as the characteristics of the
pigment is not hindered, anti-oxidants, ultraviolet
light absorbents, infrared absorbents, dissolving
assistants, and so on may be added.
In addition, ordinary non-thermochromic pigments
may be added so that the color change can occur from
one color [1] to another [2] instead of
coloring/decoloring.
The components (A), (B) and (C) will be described
in detail.
The component (A), that is, the electron-donating
chromatic organic compound(s) employed in the pigment
according to the present invention may be selected from
the group of conventionally known compounds consisting
2133932
- 8 -
of diphenyl methane phthalides, fluorans, diphenyl
methan azaphthalides, indolyl phthalides, phenyl
indolyl phthalides, phenyl indolyl azaphthalides,
styrinoquinolines, and so on.
The examples of this group are as follows:
3,3-bis(p-dimethylaminophenyl)-6-dimethyl-
aminophthalide, 3-(4-diethylaminophenyl)-3-(1-ethyl-2-
methylindol-3-yl)phthalide, 3-(4-diethylamino-2-
ethoxyphenyl)-3-(1-ethyl-2-methylindol-3-yl)-4-
azaphthalide, 1,3-dimethyl-6-diethylaminofluoran, 2-
chloro-3-methyl-6-dimethylaminofluoran, 3-dibutylamino-
6-methyl-7-anilinofluoran, 3-diethylamino-6-methyl-7-
anilinofluoran, 3-diethylamino-6-methyl-7-
xylidinofluoran, 2-(2-chloroanilino)-6-
dibutylaminofluoran, 3,6-dimethoxyfluoran, 3,6-di-n-
butoxyfluoran, 1,2-benz-6-diethylaminofluoran, 1-2-
benz-6-dibutylaminofluoran, 1,2-benz-6-
ethylisoamylaminofluoran, 2-methyl-6-(N-p-tolyl-N-
ethylamino)fluoran, 2-(N-phenyl-N-methylamino)-6-(N-p-
tolyl-N-etylamino)fluoran, 2-(3'-
trifluoromethylanilino)-6-diethylaminofluoran, 3-
chloro-6-cyclohexylaminofluoran, 2-methyl-6-
cyclohexylaminofluoran, 3-methoxy-4-
dodecoxystylinoquinoline, and so on.
The component (B), that is, the electron-accepting
compound may be selected from the group consisting of
compounds having active proton(s), pseudo-acidic
- o -
compounds (which are not real acids but act as acids in
the compositions to develop the color of the component
(A)), compounds having electron hole(s).
The compounds having active proton(s) include:
monophenol and/or polyphenol groups, as compounds
having phenolic hydroxyl group(s), that is, compounds
having monohydric and/or polyhydric phenolic hydroxyl
group(s) and optionally having substituent(s) selected
from alkyl groups, aryl groups, acyl groups, alkoxy
carbonyl groups, carboxy groups, esters thereof, amide
groups, halogen atoms, and so on; phenols having two or
three identical substituents, such as bis or tris
phenols; phenol-aldehyde condensation resins; metal
salts of the above compounds having phenolic hydroxyl
group(s); and so on.
The examples of the electron-accepting component
(B) are as follows:
phenol, o-cresol, tert-butylcatechol, nonylphenol,
n-octylphenol, n-dodecylphenol, n-stearylphenol, p-
chlorophenol, p-bromophenol, o-phenylphenol, p-hydroxy-
n-butyl benzoate, p-hydroxy-n-octylbenzoate, resorcin,
dodecyl gallate, 2,2-bis(4'-hydroxyphenyl) propane,
4,4-dihydroxydiphenyl sulfone, 1,1-bis(4'-
hydroxyphenyl)ethane, 2,2-bis(4'-hydroxy-3-
methylphenyl)propane, bis(4-hydroxyphenyl)sulfide, 1-
phenyl-1,1-bis(4'-hydroxyphenyl)ethane, 1,1-bis(4'-
hydroxyphenyl)-3-methylbutane, 1,1-bis(4'-
2133932
-- 10 --
hydroxyphenyl)-2-methylpropane, 1,1-bis(4'-
hydroxyphenyl)n-hexane, 1,1-bis(4'-hydroxyphenyl)n-
heptane, 1,1-bis(4'-hydroxyphenyl)n-octane, 1,1-bis(4'-
hydroxyphenyl)n-nonane, 1,1-bis(4'-hydroxyphenyl)n-
decane, 1,1-bis(4'-hydroxyphenyl)n-dodecane, 2,2-
bis(4'-hydroxyphenyl)butane, 2,2-bis(4'-
hydroxyphenyl)ethyl propionate, 2,2-bis(4'-
hydroxyphenyl)-4-methylpentane, 2,2-bis(4'-
hydroxyphenyl)hexafluoropropane, 2,2-bis(4'-
hydroxyphenyl)n-heptane, 2,2-bis(4'-hydroxyphenyl)n-
nonane, and so on.
Though the above-mentioned compounds having
phenolic hydroxyl group(s) can exhibit the most
effective thermochromism characteristics, the component
(B) may be selected from aromatic carboxylic acids,
aliphatic carboxylic acids having 2 to 5 carbon atoms,
metal salts of carboxylic acids, acidic phosphates and
metal salts thereof, 1,2,3-triazol and derivatives
thereof.
The examples of the component (C), the esters, are
as follows:
n-pentadecyl acetate, n-tridecyl butylate, n-
pentadecyl butylate, n-undecyl caproate, n-tridecyl
caproate, n-pentadecyl caproate, n-nonyl caprylate, n-
undecyl caprylate, n-tridecyl caprylate, n-pentadecyl
caprylate, n-heptyl caprate, n-nonyl caprate, n-undecyl
caprate, n-tridecyl caprate, n-pentadecyl caprate, n-
21~3~
pentyl laurate, n-heptyl laurate, n-nonyl laurate, n-
undecyl laurate, n-tridecyl laurate, n-pentadecyl
laurate, n-pentyl myristate, n-heptyl myristate, n-
nonyl myristate, n-undecyl myristate, n-tridecyl
myristate, n-pentadecyl myristate, n-pentyl palmitate,
n-heptyl palmitate, n-nonyl palmitate, n-undecyl
palmitate, n-tridecyl palmitate, n-pentadecyl
palmitate, n-nonyl stearate, n-undecyl stearate, n-
tridecyl stearate, n-pentadecyl stearate, n-nonyl
eicosanoate, n-undecyl eicosanoate, n-tridecyl
eicosanoate, n-pentadecyl eicosanoate, n-nonyl
behenate, n-undecyl behenate, n-tridecyl behenate, n-
pentadecyl behenate.
As long as the hysteresis characteristics are not
greatly affected, the component (C) of the present
invention selected from the above-mentioned esters may,
if desirable, also contain other kinds of esters,
alcohols, carboxylic acids, ketones, amides, and so on.
In this case, preferably 20 parts of additive(s) or
less can be added against 100 parts of the component
(C) (by weight ratio) in order to obtain desired
excellent color-memorizing effect.
The three kinds of indispensable components (A),
(B) and (C) in the homogenious compatible state are
occluded or enclosed into microcapsules according to
known encapsulation techniques. By micro granulation
(0.5 to 50 ,um, more preferably 1 to 30 ,um) a wider
~13393~
- 12 -
hysteresis range AH can be obtained. As the components
are protected in the capsule membranes, their
properties are not degraded upon contact with
chemically active substances such as acidic substances,
alkaline substances, peroxides, and so on, or with
other kinds of solvent components. At the same time,
thermal resistance can be obtained.
The examples of available encapsulation techniques
are interfacial polymerization, in SltU polymerization,
in-liquid curing coating, phase separation from an
aqueous solution, phase separation from an organic
solvent, in-gas suspending coating, spray drying, and
so on, which are properly selected depending on the
intended use. The surfaces of microcapsules, if
preferable in practical use, may be coated with an
additional resin membrane to improve stability and/or
surface characteristics.
According to the present invention, one or more
specific aliphatic acid alcohol esters obtained by
esterification of monohydric aliphatic alcohols having
odd numbers of carbon atoms and aliphatic carboxylic
acid having even numbers of carbon atoms are employed
in the composition exhibiting thermochromism as
reaction media of color forming reactions caused by
donation/acceptance of electron(s). The resultant
composition exhibits thermochromism with a wide
hysteresis range ~H with respect to the relation
2133932
- 13 -
between color densities and temperatures. Such a wide
hysteresis range ~H can not be realized when one or
more aliphatic acid alcohol esters obtained from
aliphatic alcohols having even numbers of carbon atoms
are employed. In addition, as the above-mentioned
composition is enclosed or occluded in microcapsules
and used in the form of encapsulated micro granules,
effective color-memorizing characteristics having a
preferable hysteresis range ~H from 8 C to 30 C can be
obtained.
To manifestation of said excellent characteristics
has not been fully explained, but the same
characteristics and behavior of thermochromism, which
will be shown in the measurement data, were repeatedly
observed in the embodiments according to the present
invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The preferred embodiments according to the present
invention will be described below, wherein the present
invention is not limited thereto.
First, the preparation of each embodiment of the
microcapsuled pigment is described. And then the
measuring method employed to measure hysteresis
characteristics of said microcapsulated pigment with
respect to temperature changes is described.
Note that the ratios of components are described
- 14 -
2 ~ ~ 39 3 2
by weight ratio.
[Preparation of Microcapsulated Pigment]
(A) 1.5 parts of 1.2-benz-6-diethylamino-fluoran
serving as an electron-donating chromatic organic
compound, (B) 5.0 parts of 2.2-bis(4'-hydroxyphenyl)-4-
methylpentane as a phenol compound and (C) 50.0 parts
of ester(s) according to the present invention were
heated at lZ0 C to be in a homogenious compatible
state, and mixed to a mixture solution consisting of 10
parts of EPON828 (epoxy resin manufactured by Yuka
Shell Epoxy K.K.) and 10 parts of methyl ethyl ketone.
The resultant solution was added dropwise to 100 parts
of a 10~ aqueous solution of gelatin and agitation was
performed so that fine droplets were formed. ~urther,
an aqueous solution prepared by dissolving 5 parts of
Curing Agent U (adduct compound obtained by adding
amines to epoxy resin, manufactured by Yuka Shell Epoxy
K.K.) in 45 parts of water was gradually added to the
above mixture being agitated. The resultant mixture
was further agitated at 80 C for about 5 hours to
obtain a mixture containing microcapsulated pigment.
This mixture was subjected to centrifugal separation to
obtain microcapsulated pigment with water content of
40~ (weight percentage).
[Measuring Method of Hysteresis Characteristics]
An ink was prepared by dispersing 40 parts of the
microcapsulated pigment obtained as described above in
*Trade-mark
213393~
60 parts of emulsion of ethylene/vinyl acetate. The
ink was printed on a sheet of woodfree paper with a
screen printing machine. The printed sheet was heated
and cooled as described in the following, and the
behavior of color change was plotted.
The printed paper was set at the proper position
of a color-difference meter [color-difference meter TC-
3600 manufactured by K.K. Tokyo Denshoku]. The printed
sheet was heated and cooled, and heated again in a
range of 50 centigrade degrees, wherein the
heating/cooling was carried out at a rate of 10
centigrade degrees/min. For example, Embodiment 1 at
its initial temperature of -20 C was heated up to 30 C
at the rate of 10 centigrade degrees/min, and then
cooled to -20 C at the same rate. Luminosity at
various temperatures which was measured by the color-
difference meter was plotted to obtain the relation
between color densities (measured as luminosity) and
temperatures, as is shown in Fig. 1. Thus,
temperatures T1, T2, T3, T4~ TH ( determined as the
temperature at which 50~ of the highest color density
of the ink is observed during the coloring process), TG
(determined as the temperature at which 50~ of the
highest color density of the ink is observed during
decolonizing process), and ~H (the length of the
segment HG). Table 1 shows the temperature-sensitive
color change characteristics of the microcapsulated
2133932
- 16 -
pigments employing esters of respective embodiments
according to the present invention.
Table 1
Temperature-Sensitive Color Change
Characteristics of the Embodiments
Embodi- ~ perature( C) T1 TH T2 T3 TG T4 ~H
ment No. Ester ~
1 n-undecyl caprylate -12 -8 -5 -2 2 7 10
2 n-nonyl caprate-14 -7 -5 8 12 15 l9
3 n-tridecyl caprate8 11 13 23 26 28 15
4 n-nonyl laurate-8 -2 1 11 14 17 16
n-undecyl laurate6 10 13 29 31 33 21
6 n-tridecyl laurate18 21 23 27 32 35 11
7 n-pentyl myristate-10 -4 -3 1 4 10 8
8 n-heptyl myristate-2 1 4 8 11 16 10
9 n-nonyl myristate5 7 9 19 21 24 14
n-undecyl myristate 16 18 19 30 32 34 14
11 n-pentyl palmitate0 5 8 13 15 20 10
12 n-heptyl palmitate6 9 11 21 23 27 14
13 n-nonyl palmitate13 16 18 28 30 33 14
14 n-undecyl palmitate 22 26 27 34 36 40 10
n-nonyl stearate22 25 26 35 37 40 12
16 n-undecyl behenate 42 44 45 51 53 58 9
_ l7 _
Microcapsulated pigments employing esters which
were obtained by esterification of monohydric aliphatic
alcohols having even numbers of carbon atoms and
aliphatic carboxylic acids were prepared in the same
manner as described above, and hysteresis ranges AH
thereof were measured as described. The results are:
n-butyl stearate ; 1 centigrade degree
n-octyl stearate ; 7 centigrade degrees
n-decyl myristate ; 7 centigrade degrees
n-decyl palmitate ; 5 centigrade degrees
n-decyl stearate ; 5 centigrade degrees
n-lauryl stearate ; 2 centigrade degrees and
myristyl myristate; 6 centigrade degrees.
[Application 1]
In the same manner as described above, a
microcapsulated pigment [T1=6 C and T4=33 C] exhibiting
reversible thermochromism of vermilion/colorless was
prepared, wherein 2-chloro-3-methyl-6-
diethylaminofluoran serving as the component (A), 2,2-
bis(4'-hydroxyphenyl) propane as the component (B) and
undecyl laurate as component (C) in a homogenious
compatible state were enclosed in the microcapsules.
40 parts of thus prepared microcapsulated pigment was
dispersed in 60 parts of emulsion of ethylene-vinyl
acetate to prepare a printing ink. English words, for
example, "DEVIL" and "ANGEL" were printed on a sheet of
woodfree paper in said ink, while the corresponding
213393~
- 18 -
Japanese translations "AKUMA" and "TENSHI" were printed
near their respective corresponding English words on
the same sheet in an ordinary ink (which is not
temperature sensitive nor change colors). Similarly,
other English words and corresponding Japanese
translations were printed on the sheet. Thus, an
English Word Excercize Sheet was made.
Both the English words and the Japanese
translations could be read on said English Word
Excercize Sheet. Then, the sheet was heated over 33 C,
when English words disappeared. This decolored state
of the English words was maintained when the sheet was
left at the room temperature of about 25 C. In this
case, only the Japanese words could be seen at the room
temperature. Next, the sheet was cooled below about
6 C, when the vermilion English words appeared. This
colored state of the English word was maintained when
the sheet was left at the room temperature. The
colored state and the decolored state of the English
words could be alternately and repeatedly obtained and
maintained at ambient temperatures, which is very
useful to the learners who want to memorize English
words.
[Application 2]
(A) 1,3-dimethyl-6-diethylaminofluoran, (B) 2,2-
bis(4'-hydroxyphenyl)-4-methylpentane and (C) n-nonyl
stearate in a homogenious compatible state were
2~3~932
- 19 -
microcapsulated according to interfacial polymerization
of epoxy resin and amine to obtain microcapsulated
pigment having a mean diameter of 10 ~um. The resultant
microcapsulated pigment exhibited reversible
thermochromism of orange/colorless [T1=22 C and
T4=40 C].
5 parts of this microcapsulated pigment, 1 part of
non-color-changing yellow pigment and 94 parts of
polyethylene chips were fused and mixed at about 170 C
to prepare chips exhibiting thermochromism.
Then, the chips were molded at about 150 C with a
blow molding machine to obtain a plastic gold fish
exhibiting thermochromism. This gold fish was orange
in a temperature range from 25 C to 35 C, but turned
yellow when put into hot water over 40 C. This yellow
state was maintained when the gold fish was left in the
o
temperature range from 25 C to 35 C. When cooled below
22 C, the gold fish turned orange. This orange state
was maintained when the gold fish was left in the
temperature range from 25 C to 35 C. The orange state
and the yellow state were alternately and repeatedly
maintained in the temperature range from 25 C to 35 C.
[Application 3]
(A) 3-dibutylamino-6-methyl-7-anylinofluoran, (B)
1,1-bis(4'-hydroxyphenyl)-3-methylbutane and (C)
undecyl myristate in a homogenious compatible state
were microcapsulated according to interfacial
213393~
- 20 -
polymerization of epoxy resin and amine to obtain
microcapsulated pigment having a mean diameter of 10
,um. The resultant microcopsulated pigment exhibited
reversible thermochromism of black/colorless [Tl=16 C
and T4=34 C].
An ink was prepared by dispersing said
microcapsulated pigment in emulsion of etylene-vinyl
acetate. The ink was printed on woodfree white paper
with a screen printing machine using a 180-mesh screen
to prepare a recording sheet exhibiting thermochromism.
The resultant recording sheet was black at ambient
temperatures, but turned white when heated over 34 C.
This white state was maintained when the sheet was left
at the room temperature of about 25 C. Then, the sheet
was cooled below about 16 C, when it turned black.
This black state was maintained when the sheet was left
at the room temperature.
The black state and the white state of the
recording sheet could be alternately and repeatedly
obtained and maintained at ambient temperatures. On
the recording sheet in the black state at the room
temperature, white images could be drawn with a heating
type thermopen (generating heat of 45 C). On the other
hand, on the recording sheet in the white state, black
images could be drawn with a cooling type thermopen (of
3 C). In both cases, images could be maintained at the
room temperature.
2133932
- 21 -
The microcapulated pigment according to the
present invention effectively exhibits reversible
thermochromism of coloring/decoloring with a hysteresis
range (~H) from 8 C to 30 C with respect to color-
densities and temperatures. Both of the two states ofthermochromism obtained at temperatures higher/lower
than the color-change-causing temperature are
alternately memorized and maintained at ambient
temperatures. By heating or cooling the pigment, both
of the two state, one at a time, can be reversibly
obtained and maintained. In addition, as such
thermochromism is derived from employing, as reaction
media of color forming reactions, one or more aliphatic
esters obtained from esterification of monohydric
aliphatic alcohols having odd numbers of carbon atoms
and aliphatic carboxylic acids having even number of
carbon atoms, a wide hysteresis range ~H and excellent
color-memorizing effect can be realized. Accordingly
the microcapsulated pigment according to the present
invention is of great use in the practical fields of
applications.
The microcapsulated pigment according to the
present invention may be used for various kinds of
painting and printing as paint and printing ink, or
fused and mixed with thermally plastic resin and wax to
be used as on excipient in various form.