Note: Descriptions are shown in the official language in which they were submitted.
2134263
TARGET FOR USE IN THE PRODUCTION OF MOLYBDENUM-99
Field of the Invention
This invention is directed to the production of molybdenum-99 and, in
particular a target for production of molybdenum-99.
5 Backqround of the Invention
Molybdenum-99 (Mo-99) is the parent nucleus to technetium-99m (Tc-
99m). Tc-99m is used in nuclear medicine for liver, kidney, lung, blood pool, thyroid
and tumour scanning. Tc-99m decays to a stable isotope, technetium-99, emitting
a low energy gamma ray which can be detected outside the body and used to
10 recol1sl,.lct the image of an organ. Tc-99m is prefer,ed over many other radio
isotopes for nuclear medicine because of its short half-life of approximately 6 hours
which results in reduced radiation exposure of organs relative to the exposure given
by most other imaging radio isotopes.
Because of its short half-life Tc99m must be produced just prior to
15 administration. Tc-99m can be produced from its parent nucleus Mo-99 which has
a half-life of approximately 66 hours. Mo-99 is produced by nuclear fission of
uranium-235 (U-235). Production techniques for Mo-99 have been dcv010ped which
yield a suitable product for use in nuclear medicine. However, current production
techniques are complex and time consuming and result in considerable decay
20 losses. In addition, current production techniques create large quantities of high
level radioactive liquid waste, thus increasi"g production costs and reducing the
suitability of such processes for large scale commercial production of Mo-99. A
process for production of Mo-99 is required which reduces the amount of waste
produced.
213~263
- 2 -
A target for use in Mo-99 production having high heat transfer will allow
irradiation at high fluxes so that a high rate of fission is obtained. Targets having
high heat transfer have been proposed incorporating uranium embedded in an
aluminum matrix typically containing 79% by weight of aluminum and 21% by weight5 of uranium. However, the use of aluminum in the target presents serious
disadvantages in the production of Mo-99. The need to dissolve the aluminum
matrix in order to obtain the uranium requires a considerable period of time, adding
several hours to the production process. During this time, the radioactive materials
are decaying and ll,ererore final product is being lost. Moreover, the presence of
10 dissolvcd aluminum in the solution complicates the separation steps and renders it
diffficult to obtain pure products. Mercury is required as a catalyst in the process to
remove aluminum. Mercury is of course toxic, and thereby adds to process hazard.The relatively high volume of solution needed for dissolution of the large mass of
aluminum results in cor,esponding large volumes of radioactive waste solution. This
15 is diffficult and expensive to store, and cannot easily be disposed of in a safe way.
Other targ~l~ are known consisting of closed cylinders in which
uranium oxide or metal is electroplated about the inner surface. The cylinder ismade from stainless steel or zirconium alloy (zircaloy) and allows for a direct
exposure of the irradiated uranium for processing. However, such targets are useful
20 only in low power reactors since heat transfer is a problem at higher powers.
Summar,v of the Invention
A target has been invented for production of Mo-99 having effective
heat transfer without the use of aluminum and which is suitable for use in high
power reactors.
In accordance with a broad aspect of the present invention there is
provided a target for the production of Mo-99 comprising: a first outer wall member;
a second outer wall member; and, a layer of aluminum-free uranium or uranium
213~263
-- 3 -
oxide disposed therebetween, such that heat produced by fission of the uranium or
uranium oxide is transferred directly to the first and the second outer wall members.
In accordance with a further broad aspect of the present invention,
there is provided a process for producing a target for the production of Mo-99
5 comprising: loading aluminum-free uranium or uranium oxide between a pair of walls
such that the uranium or uranium oxide is in intimate contact with walls, and sealing
the uranium or uranium oxide within the walls.
In accordance with a further broad aspect of the present invention,
there is provided a target for the production of Mo-99 comprising: a first tubular
10 member; a second tubular member arranged concentrically with the first memberand a layer of aluminum-free uranium or uranium oxide disposed therebetween,
such that heat produced by fission of the uranium or uranium oxide is transferred
directly to the first and second members.
Brief Description of the Drawings
A further, detailed, description of the invention, briefly described above,
will follow by reference to the following drawings of specific embodiments of the
invention, which depict only typical embodiments of the invention and are therefore
not to be considered limiting of its scope. In the drawings:
Figure 1 shows a perspective, cutaway view of a target according to the
present invention; and,
Figure 2 shows a perspective, cutaway view of another embodiment of a
target according to the present invention; and,
Figure 3 shows a flow diagram of a process for using the target of the
present invention.
213426~
_ -- 4 --
Detailed Descri, liGn of the Present Invention
The target of the present invention comprises a first wall member and
a second wall member which sandwich a layer of uranium or uranium oxide
5 therebetween. The layer can be in the form of uranium oxides such as, U02 or
U3O8, in powder form, uranium metal foil, uranium metal foil oxidized to U02 andelectrodeposited U02 or U3O8. In a preferred embodiment, the uranium or uranium
oxide is highly enriched. The outer wall members are in contact with the uraniumor uranium oxide layer such that the target has effective heat transfer during fission.
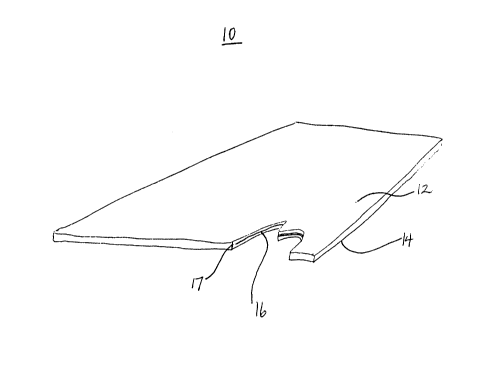
Referring to Figure 1 there is shown a view of a target 10 according to
the present invention, cutaway to reveal its inner contents. Target 10 comprises a
first wall member 12, a second wall member 14 and a layer of uranium 16
therebetween. Wall members 14 and 16 are rolled to be in intimate contact with
layer 16 to provide for effective heat transfer and to stabilize the uranium within the
target. Edges 17 of wall members 14 and 16 are then sealed such as by welding.
Referring to Figure 2 there is shown a view of another target 110
according to the present invention. Target 110 comprises an inner wall member
112, an outer wall member 114 and a layer of uranium oxide 116 therebetween.
End caps 118 are provided to seal a gap formed between the wall members 112,
114 during loading of the uranium oxide.
Wall members are produced from any suitable material for use in
nuclear reactor environments, such as, for example zirconium alloy. Stainless steel
can be used but is not preferred because of its high neutron absorption when
compared to zirconium alloy. To provide close contact between the uranium or
uranium oxide layer and the wall members and thereby effective heat transfer during
fission, the members are preferably compressed about the layer, such as by rolling
or swaging. In an alternate embodiment, the uranium or uranium oxide is in closecontact with at least one member and the target is helium filled to provide for heat
21~4263
- 5 --
l,al1sfer. However, it is to be noted that helium filling provides good heat transfer
across small gaps, such as less than about 1 mm. Heat transfer by means of
helium filling is diminished substantially as the space between the wall members of
the target is increased. The outer wall members are adapted to facilitate exposure
5 and dissolution of the layer after irradiation. For example, where uranium foil is
used, the zirconium alloy surfaces are anodized prior to application of the foil to
facilitate removal of the foil after irradiation.
The uranium or uranium oxide is loaded between the wall members in
a thin layer and in an amount to give the desired power level such as for example
about 100 mg/cm2 and, thereby, the desired Mo-99 production. In a preferred
embodiment, an annular target, generally as shown in Figure 2, is 470 mm in length
having an inner diameter of 13 mm and an outer diameter of 15 mm and has loaded
therein about 20 9 of uranium oxide.
In an embodiment, uranium oxide in the form of a finely divided powder
is vibration packed into an annular gap formed between the wall members. In an
another embodiment, a film of uranium oxide is electrodeposited onto the wall
members. In still another embodiment, uranium metal or oxidized uranium metal isdisposed between the wall members.
To produce a target having a packed powder layer of uranium oxide,
the wall members are positioned such that a uniform annular gap of between about0.10 and 0.20 mm is formed between the members. The edges of the wall
members are sealed to contain the powder, such as by insertion of end caps or
welding, and the powder is vibration packed into the gap such as, for example, by
use of a Syntron vibrator. The outer walls are then rolled or swaged to compressthe uranium oxide to the desired density of about 6.5 to 11 g/cm3 and to cause the
wall members to be in intimate contact with the uranium oxide.
A target is produced using electrodeposition by first washing one or
2139263
-- 6 --
both wall members in preparation for electrodeposition of the uranium oxide. Theuranium oxide is electrodeposited over the surface of the wall members such thatit will be disposed between the wall members in the assembled target and such that
a total amount of about 100 mg/cm2 is disposed between the walls. Such
5 electrodeposition is affected by any known method suitable for uranium loading. For
example, the uranium oxide can be electrodeposited by use of a bath containing
0.042 M uranyl nitrate and 0.125 M ammonium oxalate, the pH being adjusted to 7.2
with NH40H. Uranium is elect,odeposited to suitable thicknesses by use of current
of 0.9 amperes, 1.5 volts and a temperature of about 93C. The wall member
10 having the ele~,tlodeposited layer thereon is then heated to 500C.
After electrodeposition, the walls are dipped in nitric acid to remove a
portion of the uranium oxide such that a portion of the wall is exposed for sealing
the target. The walls are then positioned in close relation and sealed at the edges.
The walls are then pressed such as by rolling or swaging or, alternatively, the space
15 between the walls is helium filled, to provide for good heat transfer.
A target having uranium metal or oxidized uranium metal foil therein is
prepared by placing the foil between wall members which have, preferably, been
anodized. The members are then rolled or swaged to provide intimate contact
between the metal and the walls. The edges are sealed by any suitable means
20 such as by welding.
The target can be of any suitable shape which will allow heat transfer
through each wall member such as, for example, a plate assembly, as shown in
Figure 1, an annular assembly, as shown in Figure 2, or other suitable shapes that
provide for direct heat l,ansrer from the uranium or uranium oxide through the walls
25 to a heat sink or cooling fluid. As an example, some targets generally as described
in relation to Figure 3, have been successfully irradiated at target powers of 18.2
kW/g of U-235.
213~263
_ -- 7 -
Referring to Figure 3, a flow diagram of a preferred process for
production of Mo-99 and management of the waste produced therefrom is shown.
Steps 1 to 4 pertain to the irradiation of uranium oxide and recovery of Mo-99.
Steps 5 to 8 pertain to a process for management of a waste stream after Mo-99
5 recovery.
Mo-99 is produced by placement of a target containing uranium-235
into the irradiation zone of a nuclear reactor, particle generator or neutron particle
source. The target can be according to the present invention or, alternatively, any
suitable target containing uranium or uranium oxide which is substantially free of
10 aluminum. After a suitable period of irradiation, such as up to about 21 days, the
target is removed and cooled for a suitable period such as, for example, for 2 to 16
hours.
The Mo-99 is recovered by a process comprising opening the target to
expose the uranium and dissolving the uranium or uranium oxide in nitric acid
15 solution. Dissolution requires at least stoichiometric equivalents of nitric acid for
each gram of uranium-235 irradiated. However, this may be increased depending
on the form of uranium or uranium oxide used. For example, 5 to 40 ml of 2 to 16N nitric acid are required to dissolve each gram of U-235, depending on the form U-
235 with powder forms of uranium oxide requiring the least amount of nitric acid.
20 Where it is necess~ry to submerge the target, amounts greater than this may be
required. To reduce the amount of waste produced the volume of acid used should
be as little as conveniently possible to provide dissolution. Immersion in the acid is
maintained until the layer is dissolved. The time for dissolution is not critical and
should be optimized on a cost benefit analysis in terms of amount dissolved versus
25 time spent. Gases rele~sed during exposure of the uranium or uranium oxide layer
and dissolution thereof are collected for off-gas treatment. In an embodiment, the
target is punctured to release fission products such as Xe-133 and 1-131 prior to
target decladding and dissolution.
213~26~
After the uranium or uranium oxide has dissolved, the target is
removed from the acid solution and is managed as low level waste. Mo-99 is
recovered from the acid solution by contacting with an adsorbent. In an
embodiment, the acid solution is passed at least once through an alumina column.5 The alumina column useful in the preferred method is prepared by dissolving
aluminum oxide in 1N nitric acid to form a slurry. A column packed with 150 ml to
250 ml of wet aluminum oxide is sufficient to absorb 100 to 2000 six day Ci of Mo-
99. The alumina column containing adsorbed Mo-99 is passed to treatment for
removal of Mo-99.
After recovery of Mo-99, waste acid solution remains which contains
uranium nitrite. Such waste is p~ssed to a process wherein it is converted to solid
uranium oxide. The process includes de-watering, such as for example, by boiling,
and heating to about 500 C in the presence of oxygen to allow oxidation and
calcindlion. In a preferred embodiment, suitable time is provided prior to
15 evaporation for decay of isotopes having a short-half life.
In one embodiment, waste solution is passed to an evaporation cell,
wherein it is boiled to remove the water, and then to a calciner where it is further
heated to about 500C in the presence until solid uranium oxide and calx thereof is
20 formed. Alternately, the waste solution is passecl directly to a calciner where
evaporation and crllcin~liGn are combined.
Any suitable calciner can be used such as an in-pot calciner where
temperatures will be increased from 400C to 650C, or a rotary calciner where
calcination can be affected at temperatures of 400C to 500C. Waste in the form25 of stable, ceramic-like uranium oxide calx is obtained by the process and is suitable
for long term storage in sealed canisters.
Examples
2134263
Four targets, generally as shown in Figure 2, containing 18.5 9 of U-
235/target in the form of highly enriched uranium oxide powder were irradiated for
10 days at a target power of 60.7 kW. Similarly, sixteen targets containing 2.4 9 of
U-235/target in the form of aluminum-uranium alloy (79% Al, 21% U) were irradiated
5 for 10 days at 15.5 kW.
After irradiation, the targets were cooled and processed to recover Mo-
99. The targets containing uranium oxide were opened and l,ealed with 2 N nitricacid until completely dissolved. The targets containing aluminum-uranium alloy was
dissolved in 2 N nitric acid containing Hg(NO3)2 until completely dissolved. The10 resulting solutions were passed though a alumina column to recover the Mo-99.
Liquid waste remaining after the recovery was allowed decay time
followed by evaporation and calcination. Results are shown in Table 1.
Table I
Plocess Parameters Al-U UO2
Target power (kW/g U-235) 6.46 3.28
Mo-99 yield from irradiation (Ci/g U-235) 229 140
Plocess time (hours) 28.5 21.0
Mo-99 yield from processing (Ci/g U-235) 153 101
Volume liquid waste/g U-235 (ml) 200 18
Volume of calcined waste/g U-235 (ml) 13 1.3
The target of the offers faster process time over previous Al-containing
targets. Thus, less Mo-99 is lost due to decay during processing.
It will be apparent that many other change-s may be made to the
25 illustrative embodiments, while falling within the scope of the invention and it is
intended that all such changes be covered by the claims appended hereto.