Note: Descriptions are shown in the official language in which they were submitted.
` 213Q86~
HOECHST A~TIENG~S~LSC~AFT HOE 93/F 349~ Dr.Fl/ws
Deecription
Substituted hetorocyclic carboxamidee, thoir preparation
and thoir use as pharmaceuticals
The invention relates to ~ubstituted hotorocyclic carbox~
amidee, to their pr-paration and to thoir u~o aB inh~-
bitors of prolyl-4-~ydroxyla~o, and to their use as
pharmaceuticals for treating fibrotic diseaee~.
Compounds which inhibit tho onzymo~ prolino hydroxylase
and lyeine hydroxyla~e bring about a vory soloctiva
inhibltion of collagon bio~ynthesis by thoir influenco on
the collagon-specific hydroxylation roaction~. In the
course of theso roactions, protein-bound prolino or
lysine is hydroxylatod by the enzymes proline hydroxylaso
or lyeino hydroxyla~o, resp~ctively. If thie reaction i~
prevented by inhibitors, there then arises a non-
functional, ~ubhydroxylated collagen ~olecule which can
only be eecreted by the cells into tho oxtrace~lular
space in small quantitios. Furthormor-, the eubhydroxy-
lated collagen cannot be incorporated into the collagenmatrix and i~ very readily degradod protoolytically.
The~- offect~ re~ult in a diminution of the overall quan-
tity of collagen which is doposited oxtracellularly.
Inhibitors of prolyl hydroxylase are thoreforo suitable
substances for use in~tho therapy of disoa~os in which
tho deposition of collagen~ makos a substantial contri-
bution to the clinical picture. Th-se d~soases includo,
inter alia, fibroses of the lung, liver and skin
(scloroderma and cLcatrizations following burns, in~urios
and surgical intor~ention) and also athorosclorosis.
It is known that the enzyme proline hydroxylase is
efficiently inhibited by pyridine-2,4-dicarboxylio acid
and pyridine-2,5-dicarboxylic ?cid (~. Ma~amaa et al.,
- :.,:..-
213486~
- 2 -
Eur. J. Biochem. 138 (1984) 239-245). ~owever, tho~e
compound~ are only active as inhibitor~ in cell culture
at vsry high concentrations (Tschan~, G. et al., Blocham. ~ ~ ~
J. 238 (1987) 625 to 633). ~;
Prodrugs of pyridino-2,4(5)-dicarboxylates are also
known. Those are describod in the relatively old German
Applicatlons P 42 33 124.2, P 42 38 506.7 and P 42 09
424Ø
N-Oxalylglyc~nes which are inhibitors of prolyl-4-
hydroxylase are dioclosod in J. Med. Chem. 1992, 35, 2652
to 2658 (Cunliffo et al.), and ~P-A-0 457 163 (Baader ot
al.).
Hydroxyisoquinolinecarboxylic acid glycyl~mldes and
hydroxycinnolinecarboxylic acid glycylamlde~ aro
disclosed in Biochem. Soc. Trans. 1991, 19, 812 to 815
(Franklin st al.). 3-~anzyloxypyrid$n--2-c~rboxylic ~cid
(L-threonyl)amide and 3-ben~yloxypyridine-2-carboxylic `~
acid ((Fmoc-Phg)-L-threonyl)amido hydrochlorid- are
disclosed in Liebigs Ann. Chem. 1986, 1 to 20, Ro~sler et
al.
It has now bqen found, ~urprisingly, that heterocyclic
carboxamides having an ether sub~tituent, a thioether ~;
substituont or an amino substituent in the ortho po~itlon
to the amide function have a strong inhibitory actlon on
prolyl-4-hydroxyla~e.
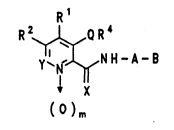
The compounds according to the invent~on conform to the
formula I ; '~
R 1 ~: : .`
R2~,N H-A- B ( I )
X , , .. :~ . ",,;
( )m
... .:, ~ ~.
' ' ' ,~: ~ ' .''
-`` 2134866
- 3
in which
Q i8 O, 8, NR' or a bond,
X i~ O or S,
Y 18 C-R3 or,
if Rl and R2 form a cyel-,
Y is N or CR3,
m i8 0 or 1,
A ~8 (Cl-C~)-alkylen~, whieh is optionally ~ub~titutod
by one or two ~ubstitu~t~ from the group halogen,
eyano, nitro, trifluoro~othyl, (Cl-C,)-alkyl, (Cl-
C~)-hydroxyalkyl, (Cl-C~)-alkoxy, -O-~CH,]~
C~H~ al~, proferably (C1-C,)-fluoroal~oxy, (C~
C~)-fluoroalkenyloxy, (Cl-C~)-fluoroalkynyloxy,
-OCF~C1 or -O-CF~-CHFC1, (C1-C6)-alkylmereapto, (C~
C~)-alkylsulflnyl, (Cl-C~)-alkyloulfonyl,
(Cl-C6)-allcylcarbonyl, (Cl-C6)-alkoxycarbonyl,
carbamoyl, N-(Cl-C~)-alkylcarbamoyl, N,N-di-(Cl-C~
alkylcarbamoyl, (Cl-C~)-alkylcarbonyloxy, (C3-C,)-
eycloalkyl, ph-nyl, b-nzyl, ph-noxy, benzyloxy,
anilino, N-methylanilino, phonylmereapto, ph nyl-
sulfonyl, phanyl~ulf~nyl, sulfamoyl, N-(Cl-C~
alkylsulfamoyl or N,N-di-(Cl-C~)-alkyl~ulfamoyl, or
by a ~ub~tituted (C~-Cll)-aryloxy, (C7-C1l)-aralkyl-
oxy, (Cc-Cll)-aryl or (C7-Cl,)-aralkyl radical whieh
earri-s in th- aryl i~ty 1, 2, 3, 4 or 5 id~ntieal
or difft~rent ~ub~tituent~ from the group halog-n,
eyano, nitro, trifluoromethyl, (Cl-C6)-alkyl, (Cl-
C~)-alkoxy, -O-[CH~ C~Ht~ al~, OCFsCl, -O-CF~
CHFCl, (Cl-C~)-alkylmeroapto, (Cl-C~)-alkylsulfinyl,
(Cl-C~)-alkylsulfonyl, (Cl-C,)-alkylearbonyl, (C1-C~
alkoxyearbonyl, earbamoyl, N-~Cl-C~)-alkylearbamoyl,
N,N-di-(C1-C~)-alkylearbamoyl, (C1-C~)-alkyl-
earbonyloxy, (C3-C~) -eycloalkyl, sulfamoyl, N-(C
C~)-alkyl~ul~amoyl or N,N-di-(C1-C~)-alkyl~ulfamoyl,
or ~` I:'~'`i!,'';'' `,'':
by the ~ub~tituent~ Rs of the a-carbon atom of an a-
amino acid, lt b-1ng po~lbl- to u~- th- natur~
- 2134866
- 4 -
L-a~ino acide and thoir D-lso~ores
B i~ an acid grouping from t~e group -CO~,
-CON~COR~', -CONHSOR'~, CONHSOlR~', -NHSO,CF"
tetrazolyl, imidazolyl or 3-hydroxyl~oxazolyl, whore
Rn' is aryl, h-teroaryl, (C3-C7)-cycloalkyl or
(C,-C~)-alkyl, optionally noeubstitutod by (C~-
C,l)-aryl, heteroaryl, OH, SH, (C,-C~)-alkyl,
(C1-C~)-alkoxy, (C~-C~)-thioalkyl, (C,-C~ ulfinyl, ;~
(C~-C~)-sulfo~yl, CF3, Cl, Br, F, I, NO" -COOH,
0 (C,-C5) -alkoxycarbonyl,NE~,mono-(C~-C~-alkyl)-amlno,
di-(Cl-C~-alkyl)-amino or (C~-C~)-perfluoroalkyl,
Rl, R~ and R3 aro identical or difforont and ar~ ~ydrogon,
hydroxyl, halogen, cyano, tri~luoromethyl, nitro,
carboxyl, (C,-C20)-alkyl, (C,-C,)-cycloalkyl, (C3-
C,)-cycloalkyl-(C~-C")-alkyl, (C3-C,)-cycloalkoxy,
(C3-C,)-cycloalkyl-(C,-Cl,)-alkoxy, (C3-C,)-cyclo- ~;~
alkyloxy-(C1-C~)-alkyl, (Cl-C~)-cycloalkyloxy-(C~
C")-alkoxy, (C3-C,) -cycloalkyl-(C,-C,)-alkyl-(C,-
C~)-alkoxy, (C3-C,) -cycloalkyl-(C,-C,)-alkoxy-(C,-
C6)-alkyl, (C3-C,) -cycloalkyloxy-(C,-C~)-alkoxy-(C~
C6)-alkyl, (C3-C,)-cyGloalkoxy-(C~-C~)-alkoxy-(C~ "'~
C,)-alkoxy, (C~-C,2)-aryl, (C7-C")-aralkyl, (C7-C") - .`; :~`f :`;
aralkenyl, (C7-C,c)-aralkynyl, (Cs-C,0)-alkonyl, (Cl- ~ t
Cl0)-alkynyl, (C,-C~0)-alkoxy, (C~-C~0)-alkenyloxy,
(Cl-Cl0)-alkynyloxy, retlnyloxy, (C1-Cl0)-alkoxy-(C,- .
C,l)-alkyl, (C,-C,l)-alkoxy-(C,-C,l)-alkoxy, (C,-C,l)-
alkoxy-(C~-C~)-alkoxy-(C~-C~)-alkyl, (C~-C")-aryloxy,
(C7-ClC) -aralkyloxy, (Cc-C")-aryloxy-(Cl-Cc)-alkoxy, . ~'!.. ',,~,~",'.'`.
(C~-C~)-aralkoxy-(C~-Cc)-alkoxy, (C~-C~)-hydroxy-' '; '~' ';'` ,`,.!, '~'', ','
alkyl, (C~-C~)-aryloxy-(C~-C~)-alkyl, (C7-Cl,)-
aralkoxy-(C,-C,)-alkyl, (C~-C")-aryloxy-(C,-C,)~
alkoxy-(CI-C~)-alkyl, (C7-C") -aralkyloxy-(C,-C,)-
alkoxy-(C,-C~)-alkyl, (C,-C,0)-alk~nyloxy-(C,-C~
alkyl, (C~-C,0)-alkynyloxy-(C~-C,)-alkyl, r-tinyloxy-
(C,-C~)-alkyl, -O-tC~ -C~H~ )F~, -OCF,Cl, -OCF,-
CHFCl, . .~
'~: ,: ." ' . . ~ .
(Cl-Cl0)-alkylcarbonyl, (C3-C,)-cycloalkylcarbonyl, .;~
.: ., .::
" , .:, ,.;, .
- 2134866
-- 5 --
(C,-Cl2)-arylcarbonyl, (C7-C~,)-arallcylcarbonyl,
s:~nnamoyl, (C2-C,0)-alk~slylcarbonyl, (C,-C20)-
alkynylcarbonyl,
(C,-C20)-alkoxycarbonyl, (c1-c12)-alkoxY-(c~-cl2)
alkoxycarbonyl, (C6-C12)-aryloxy¢arbonyl, (C7-C1~
aralkoxycarbonyl, (C3-C,) -cycloalkoxycarbonyl, (C2-
C20)-alkenyloxycarbonyl, rotinyloxycarbonyl, (C,-C,0)-
alkynyloxycarbonyl, (C,-C1~)-aryloxy-(C1-Cc)-alkoxy-
carbonyl, (C7-Cl~)-aralkoxy-(Cl-C~)-alkoxycarbollyl,
(C3-C,)-cycloallcyl-(C1-C~)-alkoxycarbonyl, (C3-C,,)-
cycloalkoxy-(C,-C~)-alkoxycarbonyl,
(C~-C~2)-alkylcarbonyloxy, (C3-C:~)-cycloalkylcarbon-
yloxy, (C6-C,2)-arylcarbonyloxy, (C7-C1,)-aralkyl-
carbonyloxy, cinnamoyloxy, (C2-C12)-alkonylcarbonyl-
oxy, (C2-C12)-alkynylcarbonyloxy,
~C,-C,2)-alkoxycarbonyloxy, (C,-C,2)-alkoxy-(C,-C,2)-
alkoxycarbonyloxy, (C,-C,2)-aryloxycarbonyloxy, (C7-
C,6)-aralkyloxycarbonyloxy, (C3-C,)-cycloalkoxycar-
bs~nyloxy, (C2-C~2)-alkcnyloxycarbonyloxy, (C,-Cls)-
alkynyloxycarbonyloxy,
.':. ~'.';''"'~:
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-(C1-
Cl2)-alkylcarbamoyl, N-(C3-C~)-cycloalkylcarbamoyl,
N,N-dicyclo- (C3 - C,) -alkylcarbamoyl, N-(C1-C10)-alkyl-
N- (C3-C,) -cycloalkylcarbamoyl, N-((C3-C~) -cycloalkyl-
(C~-C6)-alkyl)carbamoyl, N-(cl-c~)-alkyl-N-((c3-c~
cycloalkyl (C~-C6?,-alkyl)carbamoyl, N-(l)-dehydro-
abietyl¢arbamoyl, N-(C1-C,)-alkyl-N-(+)-dchydro-
abietylcarbamoyl, N-(C,-C12)-arylcarbamoyl, N- (C7-
C16)-aralkylcarbamoyl, N-(C1-Clo)-alkyl-N-(cc-c~
arylcarbamoyl, N-(C,-C~0)-alkyl-N-(C,-Cl~)-aralkyl-
carbamoyl, N-((C1-C1,)-alkoxy-(C1-C,0)-alkyl)car!~a-
moyl, N-((C,-C,6)-aryloxy-(C1-C10)-alkyl)carbamoyl,
N-((C7-Cl6)-aralkyloxy-(C1-C10)-alkyl)carbamoyl, N-
(C1-C10) -alkyl-N- ( (C1-C10) -alkoxy- (C,-C,0) -
alkyl) carbamoyl, N-(C1-C1o) -alkyl-N-((C,-C,2)-
.. ........
213~866
- 6 -
aryloxy(Cl-Cl0)-alkyl)carbamoyl, N-(Cl-C~O)-al~cyl-N-
((C7-Cl6)-aralkyloxy-(C,-C10)-allcyl)carbamoyl, or ~ ~ :
CON(C}I,)h, in which a C}12 group can be r~plac~d by O,
S, N-(Cl-C~)-allcylimino, N- (C3-C,) -cycloallcylimino,
N- (C3-C,) -cycloalkyl-(Cl-C~)-alkyl~ino, N-(C,-Cl,)-
arylimino, N- (C7-Cl~) -arallcyli~no or N-(C1-C~)-
alkoxy-(Cl-C,)-alkylimino, and h ic from 3 to 7,
a aarbamoyl radical of tho fonhula II
- 1 , " ~
R' H
--CO~ I NR ~~~--T ( I I ), ~ ~;
L 5
in which
R~ ie the subetituent of an a-amis~o acid to wh~oh
the L- and D-amino acidc belong,
e ie 1, 2, 3, 4 or 5, and
T ie OH, OR or NR-R--, whore
R-, R^- and R--- are ~dentical or diffsront and are
hydrogen, (C6-Cl2)-aryl, (C7-Cll)-arallcyl, (Cl-C,)-
alkyl, (C3-C,)-cycloalkyl, (~)-dehydroabiotyl,
(Cl-C~ lkoxy-(Cl-C~)-alkyl,(C7-Cl,)-aralkoxy-(Cl-
C,)-alkyl, (C~-Cl~)-aryloxy-(Cl-C~)-alkyl, (Cl-Cl0)-
alkanoyl, optionally eubetituted (C7-Cl~)-aralk-
anoyl or optionally eubetituted (C~-Cl,)-aroyl, or
R~ and R-- togothor are -lCE~,]b, in which a C~, group
oan be roplac~d by O, S, SO, SO" N-acylamlno, N-
(Cl-C~0)~allcoxy~arbonyl~n~no, N-(Cl~C~)-alkylimino,
N- (C3-C,) -cycloallcylimino, N-(C~-C~)-cycloallcyl-
(Cl-C~)-alkylimino, N-(C~-Cl~)-arylimino, N-(C7Cl~
aralkylimino or N-(Cl-C~)-alkoxy-(Cl-C~)-alkyl-
imino, and h ie from 3 to 7,
carbamoyloxy, N-(C,-C")-alkylcarbamoyloxy, N,N-dl-
(Cl-Cl2)-alkylcarbamoyloxy, N- (C3-C,) -cycloalkylcarb-
amoyloxy, N- (C~-C,;,)-arylcarbamoyloxy,
' ' .'' '' .: "" '
",.,
`` 213~866 : ~ ~
_
N-(C7-C~-aralkylcarbamoyloxy, N-(Cl-C10)-al ~l-N-
(C6-Cl2)-arylci~rbamoyloxy,N-(C~-ClO)-alkyl-N-(C7-C~
aralkylcarbamoyloxy, N-((Cl-C~0)-al ~l)carbamoyloxy,
N-((C,-C,2)-aryloxy-(C,-C10)-al~yl)car~ ~ ylo ~, N-
((C7-C") -aralkyloxy-(C~-C,0)-al ~l)carbamoyloxy, N-
(cl-clo)-al~yl-N-((cl-clo)-alkoxy-(cl-clo)-al~yl)-
carbAmnyloxy, N-(C,-C10)-al~l-N-(~C~-C~2)-arylo~-(C,-
C10)-alkyl)carbamoylo~, N-(C~-C,0)-alkyl-N-((C7-C~
aralkyloxy-(C,-C,0)-alkyl)carbamoyloxy,
amino, (Cl-C,2)-alkylamino, di-(C,-Clj)-al ~1 ~ no,
(C,-C,)-cycloal~ylamino, (C3-C~2)-alk~nyl ~ no, (C3-
Cl2)-alkynylamino, N-(C,-C,2)-aryl~no, N-(C7-C1~
aralkylamino, N-alkyl-aralkylamino, N-al ~l-aryl-
amino, (C,-Cl2)-alkoxyA~no, (C,-C12)-alkoxy-N-(C,-
C~0)-alkyl ~ino,
(C,-C,3) -alk~oylamino, (C3-C~)-cycloalkanoylamino,
(C~-C~2)-aroyl ~ino, (C7-Cl6)-aral~anoyl~no, (C~
C,2)-alkanoyl-N-(C,-C,0)-alkylamino, (C3-C,)-cyclo-
alkanoyl-N-(C~-C10)-alkylamino, (C~-C~2)-aroyl-N-
(C,-C,0)-alkyl ~ no, (c7-cll)-aralkanoyl-N-(cl-clo)
alkylamino,
~C,-C,2)-alkanoylamino-(C,-C,)-alkyl, (C3-C,)-cyclo-
alkanoylamino-(C,-C~)-alkyl, (C~-C12)-aroylamlno-(C,-
C,)-alkyl, (C7-C,6)-aralkanoyl ~ no-(C,-C~)-alkyl,
2i amlno-(C,-C,0)-alkyl, N-(Cl-C,0)-alkylamlno-(C,-i~O)~
alkyl, N,N-dl(C,-C,o)-alkylamino-(C~-C~0)-alkyl, (C~
C,?-cycloalkylamino-(C,-C10)-alkyl, (C~-C20)-al~yl-
mercapto, (C1-C20)-alkyloulfinyl, (C1-C20)-alkyloul-
fonyl, (C~-C~2)-arylm-rcapto, (C~-C~2)-arylculfinyl,
(C~-C~2)-aryloulfonyl, (C7-C~)-aralkylmorcapto,
(C7-C~6)-aralkylculfinyl, (C7-C,6)-aralkyl~ulfonyl,
(Cl-C12)-alkylmercapto-(C~-C~)-alkyl, (C,-C,j)-alkyl~
culf$nyl-(C~-C~)-alkyl, (C~-C~2)-alkylsulfonyl-(C~-C~)-
alkyl, ~C~-Cl2)-arylmerc~pto-(C,-C~)-alkyl, (C~-C,2)-
arylsulfinyl-(Cl-C,)-alkyl,(C~-Cl2)-aryloulfonyl-(Cl-
C6)-alkyl, (C7-C,~)-aral ~lmorcapto-(C,-C~)-alkyl,
::~:: ::. .:
2134866
-- 8 --
(C7-C16) -aralkylsulfinyl-(Cl-C~)-alkyl, (C7-c~
aralkylsulforyl-(C1-C~)-alkyl, : ~ ;
~ulfamoyl, N-(Cl-Cl0)-alkyl~ulfamoyl, N,N-di-(Cl-Clo)- ~ :
alkylsulfamoyl, (C,-C,)-cyeloalkyl~ulfamoyl, : ~
N-(C,-C,2)-aryleulfamoyl, - .~-
N-(C7-Cl~)-aralkylsulfamoyl, : , .;
N-(C~-C,O)-alkyl-N-(C~-C~l)-arylsulfamoyl, ~ .
N-(C~-C,O)-alkyl-N- (C7-C") -aralkylsulfamoyl,
(C1-C,0)-alkyl~ulfona~ido,
N-((C,-C,0)-alkyl)-(C~-C,0)-alkylsulfonam~do,~C7-C
aralkyl~ùlfona~ldo or N-((C~-C~O)-alkyl- (C7-C~
aralkylsulfonamido,
where the radlcals whieh conta~n an aryl radleal
can, for the~r part, bs ~ubstltuted on the aryl by
from 1 to 5 idontical or dlfforant radlcal~ from the
hydroxyl, halogon, cyano, trifluoromethyl, nltro,
carboxyl, (C,-C~)-alkyl, (C3-C,)-cyclo-lkyl, (C3-C~ ".,~
cycloalkyl-(Cl-Cl,)-alkyl, (C3-C,) -cycloalkoxy,
(C 3-C~) -C y C 1 0 a 1 k y 1 - (Cl- Cl,) - a 1 k o x y, :~
(C3-C,) -cycloalkyloxy-(Cl-Cl,)-alkyl, (C,-C,)-cyclo-
alkyloxy-(Cl-Cl,)-alkoxy, (C3-C,) -cycloalkyl-(Cl-C~
alkyl-(Cl-C,)-alkoxy, (C3-C,) -cycloalkyl-(Cl-C,)-
alkoxy-(Cl-C,)-al~yl, (C3-C,) -cycloalkyloxy-(Cl-C~
alkoxy-(Cl-C~)-alkyl, (C3-C,) -cycloalkoxy-(Cl-C,)- .
alkoxy-(Cl-C,)-alkoxy, (C,-Cl,)-aryl, (C7-C,~) -aralkyl,
(C~-Cl~)-allcenyl, (C,-Cl,)-alkynyl, (Cl-Cl~)-alkoxy, ... ;''.~', ,',:`:.
(Cl-Cl,)-alkanyloxy, (Cl-Cl,)-alkoxy-(Cl-Cl,)-alkyl, ~` `' .; .;~
(Cl-Cl~)-alkoxy- (Cl-C12) -alkoxy, (Cl-Cl,)-alkoxy-(Cl- .. .
C,)-alkoxy-(Cl-C~)-alkyl, (C~-Cl,)-aryloxy, (C7-C
aralkyloxy, (C~-Cl2)-aryloxy-(Cl-C~)-alkoxy, (C7-C
aralkoxy-(Cl-C,)-alkoxy, (Cl-C,)-hydroxyalkyl, (C~
Cl~)-aryloxy-(Cl-C,)-alkyl, (C7-Cl~)-aralkoxy-(Cl-C~)- :~-: : ;
alkyl, (C~-Cl,)-aryloxy-(Cl-C,)-alkoxy-(Cl-C,)-alkyl, `.. ~.
( C7-Cl~) - aralkyloxy-(Cl-C,)-alkoxy-(Cl-C,)-alkyl, ~ ~ :
-O-tCH,.]~-C~H(,~ )F~, -OCF,Cl, OCF2-CHFCl,
(Cl-C12)-alkylcarbonyl, (C3-C,)-cyeloalkylcarbonyl,
:: . : ~'
2131866
.
g
(C6-Cl2)-arylcarbonyl, (C,-Cl,~-arallcylcarbonyl,
(C,-Cl2)-alkoxycarbonyl, (el-e12)-alkoxY-(cl-cl2)
alkoxycarbonyl, (C,-Cl2)-aryloxycarbonyl, (C,-Cl,)-
aralkoxycarbonyl, (C,-C,)-cycloalkoxycarbonyl, (C2-
Cl2)-allcenyloxycarbonyl, (C2-Cl2)-allcynyloxyearbonyl,
(C~-C~2)-aryloxy-(Cl-C~)-alkoxycarbonyl, (C7-cl~
aralkoxy-(Cl-C,)-alkoxycarbonyl, (C,-C,)-cycloalkyl-
(C1-C~)-allcoxycarbonyl, (C,-C")-eyeloalkoxy-(Cl-C~
al;coxyc:arbonyl,
(Cl-Cl2)-allcylearbonyloxy, (C,-C,)-eyeloallcylearbo-
nyloxy, (Cc-C12)-arylearbonyloxy, (C7-Cl~)-arallcyl-
carbonyloxy, cinna~oyloxy, (C2-Cl2)-alkenyl¢arbonyl-
oxy, (C2-Cl2)-alkynylcarbonyloxy,
(Cl-Cl2)-alkoxycarbonyloxy, (C1-C12)-alkoxy-(Cl-Cl2)-
alkoxycarbonyloxy, (C,-Cl2)-aryloxycarbonyloxy, (C7-
Cl6)-aralkyloxycarbonyloxy, (C~-C~)-cycloalkoxycar-
bonyloxy, (C2-Cl2)-alkenyloxycarbonyloxy, (C2-Cl2)-
alkynyloxycarbonyloxy,
carbamoyl, N-(Cl-Cl2)-alkylcarbamoyl, N,N-dl-(Cl-C12)-
alkylcarbamoyl, N- (C3-C,) -cyeloalkylcarbamoyl, N,N-
d~cyelo- (C3-C,) -alkylcarbamoyl, N-(C1-C10)-alkyl-N-
(C3-C,) -cy¢loalkyl¢arbamoyl, N-( (C3-C,) -eycloallcyl-
(Cl-C~)-alkyl)carbamoyl, N-(Cl-C~)-alkyl-N-( (C3-C,) -
cycloalkyl- (Cl-C6) -alkyl)earbamoyli N-(l)-dehydro-
abietylcarb~oyl, N-(C1-C~)-alkyl-N-(I)-dohydro-
abiotyl¢a*am!oyl~ N-(C~-Cl~)-aryl¢arb~oyl, N- (C7-
Cl~)-aralkyl¢arbamoyl, N-(Cl-c30)-alkyl-N-(cc-clc)-
aryl¢arbamoyl, N-(C1-C10~-alkyl-N-(C7-Cl~)-arallcyl-
carbamoyl, N- ((Cl-Cl,) -alkoxy- (Cl-C10) -alkyl) -
carbamoyl, N- ( (C,-Cl6) -aryloxy- (C1-C10) -
alkyl)carbu~oyl, N-((c7-cl~)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl,
N-(Cl-C"0)-alkyl-N-((Cl-C1O)-alkoxy-(Cl-C1O)-alkyl)-
¢arbamoyl, N-(Cl-C10)-alkyl-N-((Cc-Cll)-aryloxy-(Cl-
C10)-alkyl)¢arb~noyl, N-(cl-c~o)-alkyl-N-((c7-cl6)-
-,.
" ,
,, ~ .
213~866
.
- 10 - ,,
aralkyloxy-(Cl-C10)-allcyl)carbamoyl, CON(C~)h, $n
which a C}Iil group can b~ roplaced by O, S, N-(Cl-C,)~
alkyl~m~no, N- (C3-C,) -cycloalkyl~m~no, N-(C,-C~
cycloalkyl-(Cl-C~)-alkyl~m~no, N-(C~-Cl2)-aryl~m~no,
N-(C7-Cl6)-aralkyl~m~no or N-(Cl-C")-alkoxy-(Cl-Cs)-
alkylimino, and h ic from 3 to 7,
carbamoyloxy, N-(C1-C~j,)-alkylcarbamoyloxy, N,N-d~
(Cl-Cl~)-alkylcarbamoyloxy, N-(C~-C~)-cycloallcyl~
carbamoyloxy, N-(C~-Cl~)-arylcarbamoyloxy, N-(C,- `: -~ : j
Cl~)-aralkylcarbamoyloxy, N-(Cl-C10)-allcyl-N-(C~
Cll)-arylcarbamoyloxy, N-(cl-clo)-allcyl-N-(c7-cl~
arallcylcarbamoyloxy, N-((Cl-C10)-alkyl)carl:~amoyloxy,
N-~(C6-Clj~)-aryloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
((C7-Cl6)-aralkyloxy-(Cl-C10)-allcyl)carbamoyloxy, N-
(Cl-C10) -alkyl-N- ( (Cl-C10) -alkoxy- (Cl-C~O) -
alkyl)carbamoyloxy, N-(cl-clo)-allcyl-N-((
aryloxy-(C,-C10)-allcyl)carbamoyloxy,N-(C1-C10)-allcyl~
N-((C7-Cl6)-arallcyloxy-(C1-C10)-alkyl)carb~oyloxy,
: , ; ~ .
amino, (Cl-Cl2) -alkylamino, di-(C1-C1,)-alkylamino,
(C3-C,) -cycloalkylamino, (C~-Cl~)-allcenyl~m~no, (C3- ~:~' ,,.. :, .
Cl~)-alkynylamino, N-(C6-Cl~)-arylamino, N-(C7-Cl~
aralkylamino, N-alkyl-arallcylamlno, N-alkyl-aryl- j :; .r: ",'
amino, (C1-C1~)-alkoxyamino, (C1-C1~)-alkoxy-N-(C1- :~
C10)-alkylamino, ; ~ . A'.`'
(C1-C1,)-alkanoylamlno, (C3-C,) -cycloalkanoylamino, ;.
(C6-C1,)-nroylamlno, (C7-C16)-aralkanoylamino, (C1- ::
C~-alkanoyl-N-(Cl-C10)-alkylamino, (C3-C,) -cyclo- .
alkanoyl-N-(C1-C10)-alkylamino, (C~-C1,)-aroyl-N-(C1- ~
C~0)-alkylamino, (C7-Cll)-aralkanoyl-N-(C1-C10)-alkyl- `
amino, . ~
(Cl-Cl~) -alkanoylamino-(Cl-C~) -alkyl, (C3-C,) _ ` ~ `
cycloalkanoyl~n~no-(Cl-C~)-alkyl, (C6-Cl,)-aroylamino-
(C,-C~)-a;Lkyl, (C7-C16)-aralkanoylamino-(C1-C,)-allcyl,
amino-(Cl-C10)-allcyl, N-(Cl-C10)-alkylamino-(Cl-C,O)-
alkyl, N,N-di-(Cl-C10)-alkylamino-(Cl-ClO)-allcyl, ~. : ~ ~:.. ; .
:.~ ,:. ..
'" "' ' ': ~'~
213~86~
11 .
(C~-C~)-cycloal~ylamino-(Cl-Cl0)-alkyl,
(Cl-Cl2)-allcylmercapto, (Cl-Cl2)-allcyl~ulfinyl, (Cl-
C~2)-alkylsulfonyl, (C~-Clc)-arylmorcapto, (C~-Cl,)~
aryleulfinyl, (C6-Cl~)-arylsulfonyl, (C7 -Cl~
aralkylmercapto, (C~-Cl~)-aral~yl~ulfinyl or (C7-C16)-
aral~ylsulfonyl,
Rl and R2 or R2 and R3 form a chain tCH2]o ~n which ono or
two CH2 groups of ths chnin, which is ~aturat~d or
unuaturated by a C-C doublo bond, are optionally
replaced by 0, S, S0, S02 or NR', and o 1~ 3, 4 or
5, and
R' i~ hydrogen, (C6-Cl~)-aryl, (Cl-C~)-alkyl, (Cl-C,)-
alkoxy-(Cl-C~)-al~yl, (C7-C~ aral~oxy-(Cl-C,)-alkyl,
(c6-cl2)-aryloxy-(cl-c~)-al~yl~ (Cl-C10)-al~anoyl,
optionally sub~tituted (C7-C~ aral~anoyl or
optionally ~ub~titutod (C6-Cl2)-aroyl, whore
the radical~ Rl and R2 or R2 and R3, togother with
the pyrldine or pyrldazlne carrylng th~m, preferably
form a 5, 6, 7, 8-tetrahydrolsoqulnollne rlng, a
5, 6, 7, 8-tetrahydrogulnollne rlng or a 5, 6, 7, 8-
tetrahydrocinnoline rlng,
or
Rl and R2 or Rs and R3 form a carbocycllc or a
h-terocycllc, 5- or 6-membered aromatic rlng, where
the radlcals Rl and R2 or R2 and R3, together w~th
the pyrldlne or pyridazine carrylng them, preferabiy
form the followlng optlonally ~ub~tltuted
h-terocyclic ring sy~temss
Thlenopyrldlne~
Furanopyrldines,
Pyridopyrldln-s,
Pyrlmidinopyridlne~,
Imidazopyridinos,
.
i~ :' ~',' '"''
2 1 3 4 8 6 6
- 12
Thiazolopyridinoe,
Oxazolopyridinee,
Quinolino, ~oguinolino and ;~
Cinnolina,
whoro quinolino, ieoquinoline or cinnollno pre~
forably eatiefy the formulae la, lb and lc
R~2 ~ ~R~ ~ OR~
1~ 1 b ; : . :. .`
, ,~: ., :: ., :.........
R 2 o
~ Q R~
R22~q~ : ~ :
`N~
1 C , ; ~ .
and the eubstituent~ R~l to Rss, in oach caeo independontly
of each othor, have the meaning of Rl, R2 and R3, :
., : ~ I I ' , .,: . : :: . '
R~ ie, if Q iB a bond, halogon, nitrilo or trifluoro-
mothyl, or, if Q ie O, S or NR', a braDch-d or
unbranched (Cl-CsO)-alkyl radical, an uneub~titutod, :~
~aturatod fluoroalkyl radical of the formula lC~]~
C~H~s~ )F~, a (C~-C1c)-aryl radical, a (Cj-C1,)-aralkyl
radical, a heteroaryl radical or a hetoroaralkyl : . .;
radical, . .:
.;'.;,,'.'"'",.:~
,~ ,, ."~
.
213~866
- 13 -
whore the~ rad~cals are s~bstituted by one or more
radical~ from th~ group
hydroxyl, halogen, cyano, tr~fluoromothyl, n~tro,
carboxyl, (C1-C,2)-alkyl, (C3-C,) -cycloalkyl, (C3-C,)-
cycloalkyl-(C,-Cl2)-alkyl, (C3-C,) -cycloalkoxy, (C3-C,) -
cycloalkyl-(C~-C~)-alkoxy, (C3-c~) -cycloal~yloxy-(C~-C~2)-
alkyl, (C3-C,) -cycloalkyloxy- (C,-C,2) -al~oxy, (C3-C,) -
cycloalkyl-(C~-C~)-al~yl-(C~-C~)-alkoxy, (C3-C,) -GyC10-
alkyl-(C,-C,)-alkoxy-(C,-C,)-alkyl, (C,-C,)-cycloalkyloxy-
(C~-C~)-alkoxy-(C~-C~)-alkyl, (C3-C,) -cycloalkoxy-(C~-C~
alkoxy-(C,-C,)-alkoxy, (C~-C")-aryl, (C7-C") -aral~yl,
(C~-Cl2)-alkonyl, (C2-C~)-alkynyl, (Cl-C~2)-al~coxy, (C,-
C")-alkoxy-(C1-C~)-alkyl, (c~-c~2)-alkoxY-(c~-c~2)-
alkoxy, (C,-C,2)-alkoxy-(C,-C,)-alkoxy-(C,-C,)-alkyl, (C,-
Cl,)-aryloxy, (C7-C~c)-aralkyloxy, (C,-C")-aryloxy-(C~
C6)-alkoxy, ~C7-C~)-aralkoxy-(C~-C~)-alkoxy, (C,-C,)-
hydroxyalkyl, (Cc-C~)-aryloxy-(C,-C,)-alkyl, (C7-C")-
aralkoxy-(C1-C~)-alkyl, (C~-C")-aryloxy-(C~-C~)-alkoxy-
(C~-C~)-alkyl, (C7-C")-aralkyloxy-(C,-C~)-alkoxy-(C~-C~
alkyl, ~O~lC~,]~~C~ lg~F~, -OCF2Cl, -OCF,-CHFCl,
..: :: ,:
(C~-C")-alkylcarbonyl, (C3-C~) -cycloalkylcarbonyl, (C,-
C~2)-arylcarbonyl, (C7-C")-aralkylcarbonyl, c~nnamoyl,
(C2-C")-alkenylcarbonyl, (C,-C,2)-aklynylcarbonyl,
(C~-C~2)-alkoxycarbonyl, (C~-C")-alkoxy-(C~-C")-alkoxy-
carbonyl, (C~-C,2)-aryloxycarbonyl, (C7-C~)-aralkoxy-
carbonyl, (C3-C,)-cycloalkoxycarbonyl, (C~-C")-alko~yl-
oxycarbonyl, (C2-C~2)-~lkynyloxyoarbonyl, (C~-C")-aryl-
oxy-(C,-C~)-alkoxycarbonyl, (C7-C")-aralkoxy-(C,-C,)-
alkoxycarbonyl~(c3-C~) -cycloalkyl-(C~-C~)-alkoxycarbonyl,
(C3-C,) cycloalkoxy-(C~-C~)-alkoxycarbonyl,
(C~-C~2)-alkylcaxbonyloxy, (C3-C,) -cycloalkylcarbonyloxy,
(C~-C")-arylcarbonyloxy, (C7-C") -aralkylcarbonyloxy,
clnnamoyloxy, (C,-C")-alk~nylcarbonyloxy, (C,-C~
alkynylcarbonyloxy,
. - :;::: ..,, :.. ., :.,
(C~-C1,)-alkoxycarbonyloxy,(C~-C~2)-alkoxy-(C,-C,2)-alkoxy-
: ::.:; . . ~
213~866 ~ :
- 14 - ~-
carbonyloxy, (C,-Cl,)-aryloxycarbonyloxy, (C,-Cl,)-aralkyl-
oxycarbonyloxy, (C3-C,)-cycloalkoxycarbonyloxy, (C,-Cl,)-
alkenyloxycarbonyloxy, (C,-Cl,)-al~ynyloxycarbonyloxy,
carbamoyl, N-(C~-Cs,)-al~ylcarbamoyl, N,N-di-(Cl-C~
alkylcarbamoyl, N- (C3-C,) -cycloalkylcarba~oyl, N,N-dl-
cyclo- (C3-C~) -alkylcarbamoyl, N-(Cl-C10~-alkyl-N- (C3 C,)
cycloalkylcarbamoyl, N-((C3-C~)-cycloalkyl-(C~-C~
alkyl)carbamoyl, N-(cl-c~)-alkyl-N-((c3-c~) -cycloalkyl-
(Cl-C~)-alkyl)carbamoyl, N-(~)-dehydroabi-tylcarbamoyl, ; '~
N-(Cl-C,)-alkyl-N-(~)-dehydroab~etylcarbamoyl, N-(C,-Cl2)-
arylcarbamoyl, N- (C7-C") -aralkylcarbamo~l, N-(Cl-C1o)-
alkyl-N-(C~-Cl~)-arylcarbamoyl, N-(Cl-Cl0)-al~yl-N- (C7-
Cl6)-aralkylcarbamoyl, N((cl-clo)-alkoxy-(cl-clo)- -'
alkyl)carbamoyl, N-((C~-Cl~)-aryloxy-(Cl-Cl~)- ; -
alkyl)carbamoyl, N-((C7-Cl,)-aralkyloxy-(Cl-Cl0)-
alkyl)carbamoyl, N-(Cl-Cl0)-alkyl-N-((Cl-C10)-alkoxy-(Cl-
Cl0)-alkyl)carbamoyl, N-(Cl-ClD)-alkyl-N-((C~-Cl~)-aryloxy- ~;
(Cl-Cl0)-alkyl)carbamoyl, N(cl-clo)-alkyl-N-((c7-cl~
aralkyloxy-(C1-Cl0)-alkyl)carbamoyl, CON(CH~)h, in which
20 a CH, group can be replaced by O, S, N-(Cl-C,)-alkyllmino, ;~
N- (C3-C,) -cycloalkyllmlno, N-(C3-C,)-cycloalkyl-(Cl-C~
alkyllmino, N-(C,-Cl,)-aryllmlno, N-(C7-Cl~)-aralkyllmino
or N-(Cl-C~)-alkoxy-(Cl-C~)-alkyl~mlno, ~nd h ~e from 3 to
7, or by
a carbamoyl radlcal of the formula II
. :' ~ .~ ', ,~'~' ':' ":'
R X H ~ :
C O - - N R l ~ `r ( I I ) . ~ :
S ::.. '; ::;':.. -,
ln whlch ~ ;~
RY le the eubstituent of an a-amino acld to which the
L- and D-amlno acids belong,
e le 1, 2, 3, 4 or 5, and
T is OH, OR or NR-R--, where
. ,,. ~",,.
2134~66
- 15 ~
R-, R--, and R--- are ident~cal or dlfforont and aro
hydrogon, (C~-C,2)-aryl, (C7-Cll)-aral~yl, (Cl-C~
alkyl, (C3-C,)-cycloalkyl, (I)-do~ydroabietyl, (C
C,)-allcoxy-(Cl-C~)-alkyl, (C7-Cl~) -aralkoxy-(Cl-C~
alkyl, (C~-Cl~)-aryloxy-(Cl-C~)-alkyl, (Cl-C10)-
alkanoyl, optlonally cubctitutod (C~-C16) -aralkanoyl
or optionally ~ub~tituted (C,-Cl2)-aroyl, or
R and R togother aro -~ ]h~ in w~ch a CEI~ group can
be replaced by O, S, SO, SO~, N-aeyla3~no, N-(Cl-
C,0)-alkoxycarbonyl~m~no, N-(C1-C,)-~lkylimino, N-
(C~-Ca) -cycloallcylimino, N- (C3-C,) -cycloalkyl-
(C~-C~)-alkylimino, N- (C6-C~2) -arylimino, N- (C,-C,6)~
aralkylimino or N-(C~-C,)-alkoxy-(C~-C,)-alkyllmino,
and h i~ from 3 to 7, or by
carbamoyloxy, N- (Cl-C~2) -alkylcarbamoyloxy, N,N-di-(C~
Cl2)-alkylcarbamoyloxy, N-(C3-C~)-cycloalkylcarbamoyloxy,
N-(Cc-C,2)-arylcarbamoyloxy, N-(C,-Cl~)-aralkyl-
carbamoyloxy,N-(Cl-C10)-alkyl-N- (CC-C12) -arylcarbamoyloxy,
N-(Cl-C10)-alkyl-N-(C7-Clc)-aralkylcarbamoyloxy, N-((Cl-C~O)-
alkyl)carbamoyloxy, N-((c~-cl2)-aryloxy-(cl-clo)
-alkyl)carbamoyloxy, N-((C7-Clc)-aralkyloxy-(Cl-ClO)-
alkyl)carba~oyloxy, N-(Cl-C10)-alkyl-N-((Cl-C10)-alkoxy-
(C,-C1~)-alkyl)carbamoyloxy, N-(Cl-C10)-alkyl-N-((C~-Cl2)-
aryloxy-(Cl-C,0)-alkyl)carbamoyloxy, N-(Cl-C10)-alkyl-N-
((C7-Cl6)-aralkyloxy-(Cl-C~0)-alkyl)carbamoyloxy,
amlno, (Cl-cl2) -alkylamlno, di- (Cl-cl2) -alkylamlno, (C3-
C~)-cycloalkylamlno, ~C3-C12) -allcenylamino, (C3-C12)-
alkynylamlno, N-(C~-C,2)-arylamino, N-(C,-Cll)-aral~yl-
amino, N-alkyl-aralkylamlno, N-alkyl-arylamlno, (Cl-Cl2)-
alkoxyamino, (C~-C12) -alkoxy-N-(C,-C,0)-allcyl~ no,
(Cl-C,2)-alkanoylamlno, (C3-C,)-cycloalkanoylamlno, (C,-
C,2)-aroylamlno, (C,-C,c)-aralkanoylamlno, (Cl-Cl2)-alka-
noyl-N-(Cl-C10)-alkylamino, (C3-C,) -cycloallcanoyl-N-(Cl-
C10)-alkylamlno, (Cc-Cl2)-aroyl-N-(Cl-ClO)-alkylamlno, (C,-
C"~-aralkanoyl-N-(C,-C,0)-alkylamlno,
213~866
- 16 -
(C1-C")-alkanoylamino-(C1-C~)-alkyl, (C3-C,) -eyelo-
alkanoylamino-(Cl-C,)-alkyl, (C~-C12) -aroylamino-(Cl-C~)-
alkyl, (C7- C16) - aral~anoylamino-(C1-C~)-alkyl, amino-(Cl-
C10)-alkyl, N-(C1-C1O)-alkylamlno-(C1-C1O)-alkyl~ N,N-di~C1-
C1o)-alkYlamino-(C1-C1O)-alkyl, (C~-C,)-eyeloalkylamlno-
(C1-C10)-alkyl,
(C1-C12)-alkylmercapto, (C1-C12)-alkylsulfi~yl, (C1-Cl2)-
alkylsulfonyl, (C6-C12) -arylmereapto,(C,-C,)-aryl~ulfinyl,
(Cc-Cl2)-arylsulfonyl, (C7-Cl~)-aralkylmorcapto, (C7-Cl6)-
aralkylsulfinyl, (C7-C1~)-aralkyl~ulfonyl,
sulfamoyl, N-(Cl-C10)-alkyl~ulfamoyl, N,N-di-(Cl-C1o)-
alkylsulfamoyl, (C3-Ca)-eyeloalkylsulfamoyl,
N-(C~-C12)-aryleulfamoyl,
N-(C7-C1c)-aralkylsulfamoyl,
N-(C~-C10)-alkyl-N-(C~-Cl2)-arylsulfamoyl, N-(Cl-clo)
alkyl-N-(C7-C1~)-aralkylsulfamoyl,
(C1-C10)-alkylsulfonamldo,
N-((C1-C10)-alkyl)-(Cl-ClO)-alkylsulfonamido, (C7-C~
aralkyl~ulfonamido or N-((C~-C10)-alkyl-(C7-Cl,)-aralkyl-
~ulfonamido, where the radleals whieh ¢ontain an aryl
radieal ean, for their part, bo sub~tituted on tho aryl
by from 1 to 5 ldentieal or differont radioal~ from the
group:
hydroxyl, halogon, eyano, trifluoromethyl, nit~o,
carboxyl, (C1-C12)-alkyl, (C3-C,) -eyeloalkyl, (C3-C,)-
cycloalkyl-(C1-C~2)-alkyl, (C3-C,) -eyeloalkoxy, (C3-C,) -
eycloalkyl- (Cl-C12) -alkoxy, (C3-C,) -eycloalkoyloxy-(C1-C1~
alkyl, (C3-C,) ~-eyeloalkyloxy- (C~-C12) -alkoxy, (C,-c~? -
cycloalkyl-(Cl-C~)-alkyl-(C1-C~)-alkoxy, (C3-C,) -eyelo-
alkyl-(Cl-C,)-alkoxy-(Cl-C~)-alkyl, (C,-C,)-eyeloalkyloxy-
(C1-C,)-alkoxy-(C1-C,)-alkyl, (C3-C~) -eyeloalkoxy-(C1-C,)-
alkoxy-(Cl-C~)-alkoxy, (C~-C12)-aryl, (C7-C1c~-aralkyl,
(C2-C12) -alkenyl, (C2-cl2) -alkynyl, (Cl-cl2) -alkoxy, (C1-
Cl2)-alkoxy-(C1-Cl2)-alkyl, (el-C1~)-alkoXy-(cl-cl2)-
alkoxy, (Cl-C,2)-alkoxy-(C1-C,)-alkoxy-(C1-C,)-alkyl, (C,-
C12)-aryloxy, (C7-C1c)-aral~yloxy, (Cc-Cl2)-aryloxy-(Cl-
C,)-alkoxy, (C7-C1,)-aralkoxy-(C1-Cc)-alkoxy,
.: . .: : ~:,.' ~:
' .:: .. " :::':i:
. . ~ : . , . :~ ~
,; :
213~866
- 17 - -
(Cl-C,)-hydroxyalkyl, (C,-Cl~)-aryloxy-(Cl-C,)-al~yl, (C7-
Cl~)-aralkoxy-(C,-C,)-alkyl, (C~-Cl2~-aryloxy-(Cl-C~)-alkoxy-
(Cl-C6) -alkyl, (C,-Cl,)-aralkyloxy-(Cl-C~)-alkoxy-(~l-C~
alkyl, -O-[CH~]~-CrH~ )F~, -OCF,Cl~ -OCF,-CHFCl, -~
:: .
(Cl-Cl~)-alkylcarbonyl, (C3-C,)-cycloal~ylcarbonyl, (C,-
Cl,)-arylcarbonyl, (C7-Cl~)-arallcylcarbonyl, ;
(Cl-Cl~)-alkoxycarbonyl, (Cl-Cl,)-alkoxy-(Cl-Cl2)-alkoxy-
carbonyl, (C~-Cl~)-aryloxycarbonyl, (C7-C~ ralkoxycarb-
o~yl, (C3-C~)-cycloalkoxycarbonyl, (C,-C1,)-alkonyloxy-
10 carbonyl, (C,-C12) -alkynyloxycarbonyl, (C~-C12) -aryloxy-
(Cl-C~)-alkoxycarbonyl, (C7-Cl~)-aralkoxy-(Cl-C~)-alkoxy-
carbonyl, (C3-C,)-cycloalkyl-(Cl-C,)-alkoxycarbonyl,
(C3-C~)-cycloalkoxy-(Cl-C~)-alkoxycarbonyl,
(Cl-Cl,)-alkylcarbonyloxy, (C3-C,)-cycloal~ylcarbonyloxy, ~;~ s
15 (C~-Cl,)-arylcarbonyloxy, (C7-C1~)-aral~ylcarbonyloxy, :~
cinnamoyloxy, (C,-Cl,)-alkonylcarbonyloxy, (C~-Cl,)~
alkynylcarbonyloxy,
. ~ !
(Cl-Cl~)-alkoxycarbonyloxy, (Cl-Cl~)-alkOxy- (Cl~Cla) ~
alkoxycarbonylcxy, (C~-Cl~)-aryloxycarbonyloxy, (C7-C
20 aralkyloxycarbonyloxy, (C3-C~)-cycloalkoxycarbonyloxy, :~
(C,-Cl,)-alkonyloxycarbonyloxy, (C,-Cl2)-allcynyloxy~
carbonyloxy, :~
carbamoyl, N-(Cl-Cl~)-alkylcarbamoyl, N,N-dl-(Cl-C1,)- ... :. ~
alkylcarbamoyl, N-(C3-C,)-cycloalkylcarbamoyl, N,N-di- : ;;
cyclo-(C3-C,)-alkylcarbamoyl, N-(C1-C10)-alkyl-N-(C3-C,)-
cycloalkylcarbamoyl, N-((c3-c!)-cycloalkyl-(cl-c~
alkyl)carbamoyl, N-(Cl-C~)-alkyl-N-((C3-C,)-cycloalkyl-
(Cl-C~)-alkyl)carbamoyl, N-(l)-dehydroabietylcarbamoyl, : ,
N-(Cl-C~)-alkyl-N-(~)-dehydroabletylcarb~moyl, N-(C~-C1,~
30 arylcarbamoyl, N-(C7-C1~)-aralkylcarbamoyl, N-(Cl-Clo)- ; ;:~ ::
alkyl-N-(C~-Cl~)-arylcarbamoyl, N-(Cl-Cl0)-al~yl-N-(C7-
Cl~)-aralkylcarbamoyl, N-((cl-clo)-alkoxy-(cl-clo)
alkyl)carbamoyl, N-((c~-cl~)-aryloxy-(cl-clo)-
alkyl)carbamoyl, N-((C7-C1~)-aral~yloxy-(C1-C10)-
alkyl)oarbamoyl, N-(Cl-C~0)-alkyl-N-((C1-C10)-alkoxy~
': ~ '
:
- 213~866 ` ~:
- 18 -
(C1-C10)-alkyl)carbamoyl, N-(cl-clo)-al~yl-N-((cc-cl2) -
aryloxy-(C,-C10)-al~yl)carbamoyl, N-(C,-C10)-al~yl-N-((C,- .
Cl6)-aralkyloxy-(Cl-C10)-al~yl)carbamoyl, CON(CH2)~, ln . :
which a CH2 group can be replac~d by O, ~, N-(C1-C,)-
alkylimino, N-(C,-C,)-cycloalkylimino, N- (C3-C,) -cyclo-
alkyl-(C,-C~)-alkylimino, N-(C~-C12)-aryl~m~no, N-(C~-C1~)-
aralkylimino or N-(C1-C~)-alkoxy-(C1-C~)-al~ylimi~o, and h
i8 from 3 to 7,
carbamoyloxy, N-(C1-C")-alkylcarbamoyloxy, N,N-di-(C1-
C1~)-alkylcarbamoyloxy, N- (C3-C,) -cycloal~ylcarbamoyloxy,
N- (C6-cl6) -arylcarbamoyloxy, N-(C,-Cl~)-aralkyloarbamoyl-
oxy, N-(Cl-C10)-alkyl-N- (C6-C12) -aryl~arba~oyloxy, N-(C~
C~0)-alkyl-N- (C7-ClC) -aralkylcarbamoyloxy, N-((Cl-clo)
alkyl)carbamoyloxy, N-((C,-Cl~)-arYlXY-(cl-clo)- :; :~
alkyl)carbamoyloxy, N-((C,-Cl,)-aralkyloxy-(C1-C10)-
alkyl)carbamoyloxy, N-(Cl-C10)-alkyl-N-((Cl-ClO)-alkoxy-(Cl- ::~
C10)-alkyl)carbamoyloxy, N-(cl-clo)-alkyl-N-((cc-cL~) - : .
aryloxy-(Cl-C10)-alkyl)carbamoyloxy, N-(C1-C10)-alkyl-N-
((C7-C1~)-aralkyloxy-(C1-C10)-alkyl)carbamoyloxy, . - .
20 amlno, (Cl-C1,)-alkylamino, d~-(C1-C1~)-alkylamino, (C3-C,) -:~
cycloalkylamino, (C3-Cl,) -al~onylamino, (C3-C~) -alkynyl-
amino, N-(C,-C1,)-arylamino, N-(C7-C11)-aralkylamlno,
N-alkyl-aralkylamino, N-alkyl-arylamino, (Cl-C1,)-
alkoxyamlno, (C1-C1,)-alkoxy-N-(C1-C10)-alkyl~no,
(C1-C")-alkanoylamino, (C3-C,) -cycloalkanoylamino, (C~
Cl~)-aroylamino, (C,-Cl,)-arallcanoylamino, (Cl-Cl~)-alka- .,'. ::~
noyl-N-(C1-C10~-alkylamino, (C3-C,) -cycloalkanoyl-N-(cl-
C10)-alkylamino, (C,-C1,)-aroyl-N-(C1-C10)-alkylamino, (C,-
C11)-aralkanoyl-N-(Cl-C10)-alkylam~no, i; ~ ;
(C1-C1,)-alkanoylamino-(C1-C,)-alkyl, (C,-C~)-cyclo-
alkanoylamino-(C1-C~)-alkyl, (Cc-C1,)-aroylamino-(C1-C~)- : .- .::.:i::.. :
alkyl, (C,-C1c)-aralkanoylumino-(Cl-C,)-alkyl, amino-(C1-
C10)-alkyl, N-(C1-C10)-alkylamino-(Cl-ClO)-alkyl, N,N-di-
(cl-clo)-alkylamino-(cl-clo)-alkyl~ ( C3 - C,) - cycloalkyl-
35 ~mino-(C1-C10)-alkyl, ~ :
- ~', :~'' ~:., '
! ',, "' ., .. ' ', ~: '
213~866
- 19 -
(Cl-C12)-alkylmercapto, (Cl-C~ alkylsulfinyl, (C~-C~
al~ylsulfonyl, (C6-Clc)-arylmercapto, (C~-C1,)-aryl-
sulfi~yl, (C~-C")-arylsul~onyl, (C7-C")-aralkylmercapto,
(C7-C1~)-aralkyleulfinyl or (C7-C~)-aralkyl~ulfonyl, and
R~ i~ R", providod that Q ha~ the m~aning of NR', where
R~ and R" aro identical or difforent and ar~ hydro-
gon, (C,-Cl,)-aryl, (C7-Cl)-aral~yl, (C1-C~)-alkyl,
(~1~C~)-alkoxy-(Cl-c~)-alkyl~ (C7-Cl~)-aral~oxy-(C,- ~ 8
C~)-alkyl, (C~-C,2)-aryloxy-(C1-C~)-al~yl, (Cl-C~0)-
alkanoyl, optionally ~ubstitut-d (~7-C1,)-aralkanoyl
or optionally sub~titut-d (C~-C12) -aroyl, or
R' and R" togothor are -~CH,lh, in which a CH~ group
can be roplacsd by 0, S, N-acylimino or N-(C1-C~o)~
alkoxycarbonylimino, and ~`;
f i3 l to 8,
g is 0 or 1 to (2f~
x i~ 0 to 3,
h i8 3 to 7,
lncludlng tho physiologically act$ve ~alts, ~ ;
with 3-bonzyloxypyridine-2-carboxyllc acid (L-throonyl)-
amido and 3-benzyloxypyrldine-2-carboxylic aoid ((Fmoc-
Phg)L-threonyl)amlde hydrochlorlde being xcept-d.
In general, aryl is understood to mean carbocyclic and
hoterocycllc aromatlc ring ~ystems. In partlcular, it ~8 ;
under~tood to lnclude phenyl-substituted, blphonyl-
~ubstltuted, naphthyl-~ubstltut~d or un~ubstituted 5- and
6-membered heteroaromatlc rlngs havlng 1, 2 or 3 nltrog-n
and/or oxygen and/or ~ulfur atoms, 8uch as dQrlvatlv~ of
pyrldyl, pyrldazyl, pyrimldyl, pyrazyl, imldazolyl, ; ~ -
triazolyl, thlenyl, oxazolyl and thiazolyl, and their
benzo-fu~od derivative~
The inv~ntion also mbraces salts of the compounds of th~
formula I.
:: .
213~866
- 20 -
The formation of ~alt~ w~th ba~io reagento can take place
once, twice or three times on th~ acidio groups of tho
compounds of the formula I (i - radicals B, Rl, R~, R3
and R~), in particular on tho radicals B, R' and/or R~
Examples of reagonts boing usod are nlcoholat~, hy-
droxides, carbo~ates, hydrog n carbonatos! hydrogon
phosphates, organometallic compounds of tho alkali and
alkaline earth element~, the elements of the third and
fourth main group~ of tho period~c systom, and tho
elements of the transition met~l~
amines, optionally substituted 1 to 3 times by (C,-C,)-
hydroxyalkyl, (C,-C~)-alkoxy-(C,-C,)-alkyl, phenyl, benzyl
or (C,-C,)-alkyl, which oan be substituted 1 to 3 t~mes
by hydroxyl or (Cl-C~)-alkoxy, for example tromethane
(Tri~ buffer), 2-aminoethanol, 3-aminopropanol,
hydroxylamine, dimothylhydroxylamin-, 2-methoxy~thyl-
amine, 3-ethoxypropylamine, and
ba~ic amino ac~d~ and amlno dorlvative~, ~uoh a~ amino
acid ester~, hl~tidlne, arginine and lysi~e, and their
derivatives, and also
"" '.: '"' ' ;'~'
pharmaceuticals which contain a baslc group, such as, for
xample, ~Amiloride, ~Verapamil and beta blocker~
~ - ,. ::: ;j ,- ..~:,
The lnventlon al~o r~lates to the compounds according to
formula I, plus 3-b-nzyloxypyrldlne-2-carboxyllc acld (L-
threonyl)amld~3 and 3-bsnzyloxypyridine-2-carboxylic aoid
((Fmoc-Phg)L-thr~olnyl)~mido hydrochlorldo for uco as
pharmaceutlcal~
..` ,~ :
Compound~ of the formula I are of great int-r-~t, ln
whlch
30 Q ~ 0, ~, NR' or a bond,
X 18 0, ~
Y 1~ CR3 or, ;
lf R~ and Rl form a cycle,
Y 1~ N or CR3,
,
:: .
213~866
- 21 -
m i8 0 or 1
Compounds of tho formula I are vory lmportant, in whieh
Q is 0, NR' or a bond,
X is 0
5 Compound~ of the formula I aro al~o very lmportant in
which Q is 8 and X i~ 0 ~ ~ ;
Compounds of tho formula I aro of particular importa~ee
in whieh
Q i~ 0, NR' or a bond, ;;, ~ `
1 0 X i 8 0,
Y is CR3 or, if R' and R' form a eyelo, N or CR3,
m is 0 or 1,
A i~ (Cl-C3) -allcyleno whieh is opt~ onally substitutod
once by halogon, eyano, trifluoromethyl, (Cl-C,)~
alkyl, (Cl-C6)-hydroxyallcyl, (Cl-C,)-alkoxy or -0-
[C}~2~"--Cf~It,f,l ~F~" or
A is -CHR5-, whero R5 18 onts of the ~ub~tituents of
the a-ear}~on atom of an a-amino aeld, in partieular ~ ;
of a natural L-amino acid and of its D-isom r,
B i8 C0~%,
:.
R2 is hydrogon, (Cl-C,0)-alkyl, (C,-C,0)-alkonyl, (C,-
C,0)-alkynyl, (Cl-C~O)-alkoxy, (C,-C,0)-allcenyloxy,
(C2-C,O) -alkynyloxy, r-tinyloxy, (Cl-C~0) -alkoxy- (Cl-
C3) -alkyl, (C,-C,0~ -allc-nyloxy- (C1-C~) -alkyl,
retinyloxy- (Cl-C~) -alkyl, (C,-C,0) -alkynyloxy- (Cl-
C~)-alkyl, halogen, eyano, trifluoromethyl, (Cl-C~
hydroxyalkyl, (Cl-C,0)-alkanoyl, (C7-Cl~)-aralkanoyl,
(C~-Cl~) -aroyl, (C~-Cl2) -aryl, (C7-Cl~) -arallcyl,
~O~ ~CH2~"-CrH~t.~l s"F", N}~'Rn, (Cl-C1O) -alkylm-rcapto,
(Cl-C10)-alkyl~ulfiDyl, (Cl-C10)-alkylsul~onyl, (C~
Cl,)-arylm~rcapto, (C~-Cl,)-arylsulflnyl~ (C,-Cl,)-
arylsulfonyl, (C7-Cl,)-arallcyl~oreapto,
'.,''' ~'''',''~'''.,''
:: :: :;: :.::, . . :,:
2l3~s6e
- 22 -
(C7-Cl~)-aralkylsulfinyl, (C7-C~2)-aralkylsulfonyl,
(Cc-cl2)-aryloxy~ (C7-CI6)-aralkyloxy, earboxyl, (Cl-
C20)-alkoxycarbonyl, (Cl-Cl2)-alkoxy- (Cl-Cla) -alkoxy-
carbonyl, (C,-Cl2)-aryloxycarbo~yl, (C7-Cl~ ral-
koxycarbonyl, (C,-C,)-eycloalkoxyearbonyl, (C,-
C20)-alkonyloxycarbonyl, r~tinyloxycarbonyl,
(C2-C20)-alkynyloxycarbonyl, (C3-C,) -cyeloalkyl-(Cl-
Cc)-alkoxycarbonyl, (C,-C~)-eyeloal~oxy-(Cl-C~
alkoxyearbonyl, (C~-C32)-aryloxy-(Cl-C~)-alkoxy~
carbonyl, (C,-C16) -aralkoxy-(C1-C~)-alkoxyearbonyl,
earbamoyl, N-(C1-Cl2)-alkylearbumoyl, N,N-di-(C,-Cl2)~
alkylearbamoyl, N- (C3-C,) -eyeloalkylearbam~yl, N,N-
dieyelo (C3-C,) -alkylearbamoyl,N-(Cl-Cl0)-al~yl-N- (C3-
C,)-eyeloal~ylearbamoyl, N- (C3-C,) -eyeloalkyl-(C -C~
alkyl)earbamoyl, N-(cl-c~)-alkyl-N-((c3-c~) -eyelo-
alkyl-(Cl-C~)-alkyl)earbamoyl, N-(l)-dehydroabietyl-
carbamoyl, N-(Cl-C~)-alkyl-N-(~)-dehydroab~otyl~
earbamoyl, N-(C~-C1,)-arylcarbamoyl, N-(C,-C")-
aralkylcarbamoyl, N-(Cl-Cl0)-alkyl-N-(C~-Cl~)-aryl-
carbamoyl, N-(Cl-Cl0)-alkyl-N-(C7-Cl~)-aralkylcar-
bamoyl, N-((Cl-Cl2)-alkoxy-(C -Cl0)-alkyl)earba_oyl,
N-((C~-C1~)-aryloxy-(Cl-Cl0)-alkyl)earbamoyl, N-~(C,-
Cl~)-aralkyloxy-(C1-Cl0)-alkyl)earbamoyl, N-(Cl-C1o)~
alkyl-N-((Cl-Cl0)-alkoxy-(Cl-C10)-alkyl)earbamoyl, N-
(Cl-Cl0)-alkyl-N-((C,-C1,)-aryloxy-(Cl-Cl0)-
alkyl)earbamoyl, N-(cl-clo)-alkyl-N-((c7-cl~)
aralkyloxy-(C1-C10)-al~yl)earbamoyl, CON(CE~)~, in
whieh a C~2 group ean be replaeed by O, S, N-(C1-C,)-
alkylimlno, j N! ~C3-C,) -eyeloalkylimino, N- (C3-C,) -
eyeloalkyl-(C1-C~)-alkylimino, N-(C~-C1~)-aryllmlno,
N- (C7-C~) -aralkyllmlno or N-(C1-C~)-alkoxy-(C1-C,)-
alkyllmino, and h i~ from 3 to 7,
.
, .., ~ .
where aryl i~ ~ub~tltut-d in tho manner a- defined for
and R',
5 R1 and R3 are idontleal or difforont and are hydrogen,
halogen, (C1-C1,)-alkyl, (C1-C~2)-alkoxy, -O-[C~ -C~
213~66
- 23 -
~, (c~-cl2)-alkoxy-(cl-cl2)-al~yl, (~-C~
alkoxy- (C~-C12) -alkoxy, (C,-C,2) -alkoxy-(C~-C~
alkoxy-(C2-C~)-alkyl, (C7-C~) -aralkyloxy, (C3-C,) -
cycloalkyl, (C3-C,) -cycloalkyl-(C,-C,)-alkyl, (C3-
C~)-cycloalkyloxy, (C3-C,) -cycloal~yl-(C~-C~)-alkoxy,
(C3-C,) -cycloalkyloxy-(C,-C,)-~l~yl, (C~-C,)-cyclo-
alkyloxy-(C,-C~)-alkoxy, (C,-C,)-cycloal~yl-(C~-C,)-
alkyl-(C,-Cc)-alkoxy, (C3-C,) -cycloalkyl-(C~-C~
alkoxy-(C,-C~)-alkyl, (C3-C,) -cycloalkoxy-(C~-C~
alkoxy-(C,-C,)-alkyl, NRYRZ, (C,-C,)-alkylm rcapto,
(C,-C,)-alkyl~ulf~nyl or (C~-C,)-alkyl~ulfonyl, (C,-
Cl~)-arylmercapto, (C,-C")-aryl~ulfinyl, (C~-C
arylsulfonyl, SCj-C")-aralkylmercapto, (C7-C
aralkyl~ulflnyl, (C7-C,l)-aralkyl~ulfonyl,
oubstituted (C~-Cl2)-aryloxy-(C,-C~)-alkyl, (C7-C
aralkoxy-(C1-C6)-alkyl, (C~-Cl~)-aryloxy-(C~-C~
alkoxy-(C,-Cc)-al~yl, (C7-Cl~)-aralkyloxy-(C,-Cs)-
alkoxy-(C,-C~)-alkyl, (C,-C1,)-aryloxy, (C7-C,1)~
aralkyloxy, (C6-C12)-aryloxy-(C1-C~)-alkoxy or (C7-
C~)-Aralkoxy-(C~-Cc)-alkoxy, wh-ro an aromatic
radical carrie~ by 1, 2, 3, 4 or 5 idontical or
difforont sub~tituont~ from tho group hydrogen,
halogon, cyano, nitro, trlfluoromothyl, (C,-C
alkyl, (C,-C,6) -alkonyl, (C,-C6) -hydroxyalkyl, (C
C16) -alkoxy, (C,-C,6) -alkonyloxy, -O-[CH~]~-C~H~ F~
-OCF~Cl, -O-CF~-CHFCl, (C,-C,)-alkylmorcapto, (C~-C6)-
alkylsulfinyl, (C~-C6) -alkyl~ulfonyl, (C,-C~)-al~yl-
carbonyl, (C,-C6)-alkoxycarbonyl, carbamoyl, N-
(C,-C~)-alkylcarbamoyl, N,N-di-(C~-C~)-alkyl-
carbamoyl, (Ç~-C~)-alkylcarbonyloxy, (C,-C~)-cyclo-
alkylcarbamoyl, ph-nyl, bonzyl, phenoxy, benzyloxy,
NRYRZ, phonylmorcapto, phonylculfonyl, ph~nyl-
~ulfinyl, ~ulfamoyl, N-(C,-C~)-alkyloulfamoyl or N,N-
di-(C,-C~)-alkylsulfamoyl, or optionally carrie~ up
to 3 of tho abovomontloned ldontlcal or diffor-nt
~ub~titu~nt~, and two ad~acont carbon atom~ of th~
aralkyloxy radical togethsr carry a chaln-tCH~
and/or -CH=C~-CH3C~-, whero a C~ group of tho chaln
1~ optionally roplaced by O, S, SO, SO, or NR',
213~86B
- 24 -
R~ and R2 or Rl and R3 form a chain [CH~o~ where o is 3,
4 or 5, or
form, together wlth the pyridine or pyridazine
carryi~g thQm, a einnollns ring, a quinollno ring or
an lsoquinoline ring,
R~ i~, if Q is a bond, fluorine, chlorine or bro~ino,
or, if Q is 0 or NR', a branehod or unbranehod (C~
C20) -alkyl radieal, wh~eh can eontain up to 3 C-C
multiple bonds, u~ uneubetitutod ~aturatod fluoro-
alkyl rndical of the formula tC~2]~-Ct~(2~ F~, a
(C6-Cl,)-aryl radieal or a (C7-Cl,)-aralkyl radieal,
which can contain up to 2 C-C multiple bonds $n tho
alkyl chain, or an h~toroaryl rad$cal or an het~ro-
aryl alkyl radical, whero these radieals are ~ubsti-
tutod by one or ro radicals from the group
~ydroxyl, fluorine, ehlorine, eyano, tri~luoro-
methyl, earboxyl, (Cl-Cl,)-alkyl, (C3-C,) -cyeloalkyl,
(C3-C,) -cyeloalkyl-(Cl-Cl~)-alkyl, (C3-C,) -eyelo-
alkoxy, (C3-C,) -cyeloalkyl-(Cl-Cl2)-alkoxy, (C3-C,) -
cycloalkyloxy-(Cl-Cl,)-alkyl, (C3-C,) -eyeloalkyloxy-
(C,-C12)-alkoxy, (C3-C,) -eyeloalkyl-(Cl-C,)-alkyl-(Cl-
C6)-alkoxy, (C~-C,)-eyaloalkoxy-(Cl-C,)-alkoxy-(Cl-
C,)-alkoxy, (C~-Cl2)-aryl, (C7-Cl~)-aralkyl, (C~-Cl,)-
alkenyl, (C2-Cl2)-alkynyl, (Cl-C")-alkoxy, (Cl-Cl2)-
alkoxy-(C,-Cl2)-alkoxy, (Cl-Cl,)-alkoxy-(Cl-C~
alkoxy-(Cl-C,)-alkyl, (C~-C12) -aryloxy, (C,-C
aralkyloxy, (C~-C12) -aryloxy-(Cl-C~)-alkoxy, lC7-
C16) -aralkoxy-(Cl-C~)-alkoxy, (Cl-C,)-hydroxyalkyl,
-0-lCH2.~-c~H~2~ )F~
(Cl-C12) -alkylearbonyl, (C,-C,)-eyeloalkylearbonyl,
(C6-C12) -aryloarbonyl, (C7-Clc)-aralkylearbonyl,
(Cl-C12) -alkoxyearbonyl, (Cl-cl2) -alkoxy- (Cl-c12) -
alkoxyearbonyl, (C6-C~2)-aryloxyearbonyl, (C7-Cl,)-
aralkoxycarbonyl, (C~-C~)-¢yeloalkoxyearbo~yl, (C2-
Cl2)-alkonyloxyearbonyl, (C2-Cl2)-alkynyloxyearbonyl,
213~86G
- 25 -
(C3-C,) -cycloalkyl-(Cl-C~)-alkoxycarbonyl,
(Cl-Cl,)-allcylcarbonyloxy, (C~-C,)-cycloalkyl-
carbonyloxy, (C~-C12) -arylcarbonyloxy, (C,-C~
aralkylcarbonyloxy, carbamoyl, N-(Cl-Cl2)-allcylcar- ~ -
bamoyl, N,N-d~-(Cl-Cl2)-alkylcarbamoyl, N- (C3-C,) -
cycloalkylcarbamoyl, N,N-dicyclo- (C3-C,) -alkyl-
carbamoyl, N-(Cl-Cl0)-alkyl-N-(C,-C,)-cyoloallcylcar-
b a m o y l, N - ( ( C 3 - C, ) - c y c l o a l k y l - ( Cl- C~
allcyl)carbamoyl, N-(Cl-C,)-alkyl-N-( (C3-C,) -cyelo-
alkyl-(C1-Cc)-alkyl)carbamoyl, N-(l)-dehydroabiotyl-
carbamoyl, N-(Cl-C~)-allcyl-N-(I)-dohydroabietyl-
carbamoyl, N-(C~-Cl,)-arylcarbamoyl, N- (C7-Cl~) -
aralkylcarb~moyl, N-(Cl-Cl0)-alkyl-N-(C~-Cl~)-aryl-
carbamoyl, N-(Cl-ClO)-alkyl-N-(C,-Cl,)-aralkylcarba-
moyl, N-((Cl-Cl0)-alkoxy-(Cl-Cl0)-alkyl)carbamoyl, N-
((C~-Cl~)-aryloxy-(Cl-Cl0)-alkyl)carbamoyl, N-((C,-
Cl~)-aralkyloxy-(Cl-C,0)-alkyl)carbamoyl, CON(CHl)~ ln
whlch a CH2 group can be replaced by O, N-(Cl-
C~)-alkyllmino, N-(C3-C8)-cycloalkylimino, N- (C3-C,) -
cycloalkyl-(Cl-C~)-alkylim~no, N-(C~-Cl,)-arylimino
or N- (C7-Cl~) -aralkylimino, and h 18 from 3 to 6,
where the radlcals which contain an aryl radlcal
aan, for th-lr part, be ~ub~ltutesd on tho aryl by
from 1 to 5 ldontlcal or dlfferent radlcal~ from tho ;~ r "~ ''''~'
groups
hydroxyl, fluorine, chlor~ne, cyano, trlfluoro-
methyl, carboxyl, (Cl-Cl~)-alkyl, (C3-C,) -cyeloalkyl,
(Cl-C~)-alkoxy, (C3-C~)-'cycloalkoxy, (C~-Cl2)-alkoxy- ,.
earbonyl, ~ ~
N-(Cl-C~)-alkylcarbamoyl, N,N-d~-(C1-C~)-alkyl- ~ ~ ~'',' ';''''~J'','
carbamoyl or N- (C3-C,) -cycloalkylcarbamoyl, and
R~ 1~ R, provided Q ha~ tho m-aning o~ NR', whoro R'
and R~ aro id-ntlcal or difforent and are hydrog-n, ;
(Cl-C,)-alkyl, or (C,-Cll)-arallcyl which i~ optlonally
213~866
- 26 -
substituted onco by fluor~ne, chlorine or (C,-C,)- . ;~ ~ ~
alkoxy, ~ ~ .
RY and R~ are identical or difforent and are hydrogon,
(C~-C~ aryl, (Cl-C~0)-alkyl, (C~-C10)-cycloal~yl,
(C,-C,)-alkoxy-(C,-C~)-alkyl, (C7-cl2) -aralkoxy-(Cl- ~;~
C,)-alkyl, (C,-Cl,)-aryloxy-(Cl-C,)-alkyl, (Cl-C10)-
alkanoyl, optionally substituted (C,-Cl,)-aral~anoyl
or optionally sub~tituted (C,-Cl,)-aroyl, or
RY and R~ together are -[CH,~, in which a CH, group can
be replaced by O, S, N-(Cl-C~)-alka~oylimlno or N-
(Cl-C~)-alkoxycarbonylimlno, and ~ -
f is l to 8, ~ ~
g is 0 or 1 to (2f ~ 1), -:; ;~:.::;.. ~-.. ~.
h is 3 to 6,
15 x is 0 to 3, and
n is 3 or 4.
Compound~ of the formula I are preferred in whlch
Q i~ O, NR' or a bond,
X is O,
20 Y is CR3 or, if Rl and R' form a cycle, N or CR',: ; -
m io 0, ~ :
A i~ (Cl-C3)-alkylene which i~ optionally ~ubstituted
once by halogen, cyano, trlfluoromethyl, (Cl-C6)-
alkyl, (C,-C6~-hydroxyalkyl, (Cl-C6)-alkoxy or
-O-lC~,]X-Ct~ F~ or : ::
: I ' ` ~ ! ' ~ .
A i~ -C~R5-, where R5 is one of the substituent~ of th-
a-carbon atom of an a-amino acid, ln partioular of
a natural ~-amino aoid and of it~ D-lsomor,
: :-
. ~, ":.. ,:
B is CO,H
:. . :,: ,. : ~, ~:
30 R' is hydrogen,.(Cl-C20)-alkyl, (Cl-C,0)-alkonyl, (C,- ::
C20)-alkenyloxy, (C2-C20)-nlkynyloxy, retinyloxy,
'''' "'''.'~"'"
213~866 ~ ~ ~
- 27 -
(Cl-Cl0)-alkoxy-(C,-C,)-alkyl, (C,-C,0)-alkoxy-(C,-
C3) -alkyl, (C~-C20)-al~enyloxy-(C~-C~)-alkyl,
retinyloxy-(C~-C~-alkyl, (C2-C~o) -alkynyloxy-(C~
C,)-alkyl, (C1-C,0)-alkoxy, haloge~, eyano,
trlfluoromethyl, (C~-C")-hydroxyalkyl, (C~-C~0)-
alkanoyl, (C7-C~2)-aralkanoyl, (C~-C~)-aroyl,
-O-tCH~-C~(s~ F~, Na~a~ (cl-clo) -al~ylmor¢apto,
(C~-C~0)-al~yleulf~nyl, (C~-C~)-al~ylsulfonyl, (C~
C~2)-arylmercapto, (Cc-C~)-arylsul~y}, (C~-C~2)-
aryl~ulfonyl, (C~-C~aralkylmoseapto, (C7-C")-
aralkyleulfinyl, (C7-C,2)-aralkyl~ulfonyl, (C,-C~
aryloxy, (C,-C~)-aral~yloxy, earboxyl, (C,-C,0)-
alkoxyearbonyl, (C,-C~2)-alkoxy-(C,-C~
alkoxyearbonyl, (C~-Cl,)-aryloxyearbo~yl, (C,-C")-
aralkoxycarbonyl, (C3-C,) -cycloalkoxycarbonyl, (C2-
C,0)-alkenyloxycarbonyl,retinyloxycarbonyl, (C~-C20)-
alkynyloxyearbonyl, (C3-C~) -cyeloalkyl-(C~-C~
alkoxycarbonyl, (C3-C,)-~ycloalkoxy-(C~-Cc)-
alkoxycarbonyl, (C,-C1,)-aryloxy-(C,-C~)-alkoxy-
carbonyl, (C7-C,c)-aralkoxy-(C,-C,)-alkoxycarbonyl,
carbamoyl, N-(Cl-C")-alkylearbamoyl, N,N-di-(C,-CL,)-
alkylcarbamoyl, N-(C3-C~)-cycloalkylearbamoyl, N,N-
dicyclo(C3-C~)-alkylearbamoyl,N-(C~-C~0)-alkyl-N-(C3-
C~)-cycloalkyl¢arbamoyl, N-(C3-C~)-cyeloalkyl-(C,-Cc)-
alkyl)carbamoyl, N-(C,-C,)-alkyl-N-((C3-C,)-cyclo-
alkyl-(C~-C~)-alkyl)carbamoyl, N-(~)-dohydroabiotyl-
carbamoyl, N-(C,-C,)-alkyl-N-(I)-dehydroabiotyl-
carbamoyl, N-(C~-C~ arylcarbamoyl, N-(C,-C~
aralkylcarbamoyl, N-(Cl-C,0)-alkyl-N-(C,-C~,)-aryl-
carbamoyl! N-lC1-C~0)-alkyl-N-(C,-C~2)-aralkylcarb-
amoyl, N-((C,-C")-alkoxy-(C,-C,0)-alkyi)carbamoyl, N-
((C~-C~,)-aryloxy-(C,-C~0)-alkyl)carbamoyl, N-((C7-
C~)-aralkyloxy-(C,-C,0)-alkyl)carbamoyl, N-(C1-C~o)~
alkyl-N-((C~-C,0)-alkoxy-(C~-C~O)-alkyl)earbamoyl, N-
(C,-C,0)-alkyl-N-((C,-C")--ryloxy-(C,-C~O)-
alkyl) carbamoyl, N- (C,-C,0) -alkyl-N- ( (C,-C~
- aralkyloxy- (C,-C,O) -alkyl) carbamoyl, or CON(Cl!I,)h, ln
whlch a CH~ group can be replaced by 0, S, N- (Cl-C,) -
alkylimino, N- (C3-C,) -cycloalkyllmlno,
213~66
- 28 -
N-(C3-C~)-cycloallcyl-(C1-C4)-allcyl~m~no, N- (C6-C~
aryl~mino, N- (C,-C16) -aralJcyl~m~no or N-(C1-C4)-
alkoxy-(Cl-Cc)-alkyl~m~no, and h i~ from 3 to 6,
wherQ aryl ls ~ubstituted Jn tho mannQr aEI dof~ned for
and R3,
Rl and R3 are ident~cal or diff~r-nt and are hydrogen,
halogen, (Cl-Cl,)-alkyl, (C1-C~)-alkoxy,
-O- lCEI~]J-C~ al~l~ (cl-cl~) -alkoxy-(C1-Cl,)-allcyl,
(Cl-C,)-alkoxy-(Cl-Cl,)-alkoxy, (Cl-Cl,)-alkoxy-(C,~
C~)-alkoxy-(C~-C6)-alkyl, (C7-C 1) -~ralkyloxy, (C3-
C~)-cycloalkyl, (C3-C~)-cycloalkyl-(C1-C~)-al~cyl,
(C3-C,) -cycloallcyloxy, (C3-C,)-cycloalkyl-(Cl-C,)-
alkoxy, (C3-C~)-cycloalkyloxy-(Cl-C~)-alkyl, (C3-C,)-
cycloalkyloxy-(Cl-C,)-allcoxy, (C3-C,)-cycloallcyl-(Cl-
C6) -alkyl-(Cl-C~)-alkoxy, (C3-C,)-cycloalkyl-(Cl-Cc)-
alkoxy- (C,,-C6) -allcyl, (C3-C,)-cycloallcoxy-(Cl-cc)-
alkoxy-(Cl-C~)-alkyl, NRYRZ, (Cl-C,)-alkylmercapto,
(C,-C,)-allcyl~ulfinyl or (Cl-C,)-alkylsulfonyl, (C,-
Cl,)-arylmercapto, (C,-Cl,)-aryl~ulfinyl, (C,-Cl2)-
aryl~ulfonyl, (C,-Cl,)-aralkylmercapto, (C,-Cl,)-
aralkylsulfinyl, (C,-Cll)-aralkylsulfonyl,
sub~t~tutod (C6-Cl,)-aryloxy-(Cl-C,)-alkyl, (C,-Cll)-
aralkoxy-(Cl-C~)-alkyl, (C~-C1~)-aryloxy-(Cl-C~
alkoxy-(C1-C~)-alkyl, (C7-C~l) -aralkyloxy-(C1-C~
alkoxy-(Cl-C,)-alkyl, (C,-Cl2)-aryloxy, (C7-C~
aralkyloxy, (C,-Cl,)-aryloxy-(Cl-C,)-alkoxy or
(C,-Cll)-aralkoxy- (Cl-C6) -alkoxy, where an aromatic
radical carri~ by 1, 2, 3, 4 or 5 identlcal or
different sub~tituQnt~ from the group hydrogen,
halogen, cyano, nitro, trifluoromothyl, (Cl-Cl~
alkyl, (Cl-Cl,)-alkonyl, (Cl-C,)-hydroxyalkyl,
(Cl-Cl,) -alkoxy, (Cl-Cl,) -allc-nyloxy,
--[CH~]x~CtH(~ F~ -OCF,Cl, -O-CF,-CHFCl, (C1-C,)-
alkylmercapto, (Cl-C6)-alkyl~ulfinyl, (Cl-C~)-alkyl-
~ulfonyl, (Cl-C,)-alkylcar}~onyl, (Cl-C,)-alkoxy-
carbonyl, carbamoyl, N-(Cl-C~)-alkylcarbamoyl, N,N-
di-(Cl-C~)-alkylcarba~oyl, (Cl-C,)-alkylcarbonyloxy,
., .,, .: .,
213~86G
- 29 -
(C~-C~)-cycloalkylcarbamioyl, phenyl, b~zyl, phonoxy,
benzyloxy, NRYR~i, phenylmercapto, phonylsulfonyl,
phenylsulfinyl, sulfamoyl, N- (C1-C,) -alkylsulfamoyl
or N,N-di- (Cl-C~) -all~ylsulfamoyl, or optlonally
carries up to 3 of tho abovementloned ide~lt~ cal or
difforent eubstituents, and two ad~acsnt carbon
atoms of the aralkyloxy radlcal togeth~r carry a
chain -tCH,-~ and/or -C~l.CEI-CE~.C~I-, whero a C~l~ group
of the chain ~8 optionally roplacod by 0, S, SO, SO, : ~ -
or NRY,
Rl and R' or R2 and R3 can form a chain tC~,] O~ whore o i8 :
3, 4 or 5, and :
",
R4 , provided Q is a bond, is chlorine os, if Q i~3 0 or
NR~, is a branched or u~branchsd (Cl-C10)-allcyl
radical, which can contain one or two C-C multlple ~ ~
bonds, or an unsubstituted fluoroalkyl radical of - :::
the formula - LcH2]~,-c~ 2~ y~F~ or (Cl-C,) -alkoxy- (C~
Cc) -alkyl, (Cl-C,) -alkoxy- (C1-C~) -alkoxy- (Cl-C~) -alkyl ~ ~;
or a radical of the f ormula Z, . .
~: : ::.
-[C H2lV-1O]w-[CHJ, E (Z) ~ : ~; s
whero E i~ a oubstitutod phenyl radlcal of the
f orsnula F
R~ R~
~ R ~ ( F )
R l C R ; .~. . .
or a (C3-C,)-cycloalkyl radical, whoro
v i0 O, 1, 2, 3, 40 5 or 6, w 18 O or 1, and t ~8 O, . ::
1, 2 or 3, with the re~trictlon that v is not egual :~;
to O if w is 1, and R', R7, R~, R9 and R10 are iden- -;
tical or d~fferent and are hydrogen, halogen, cyano,
nitro, trifluoromethyl, (C,-C~)-alkyl, ;~
,. ,.":
:' :,"' '.:;':'
'~';'''',,.,,,'', `.`'''''~'''. "'' ', j
2134866
- 30 -
(C~-C~)-cycloalkyl, (Cl-C~)-alkoxy, -O-~CH,]~-C~H~
F~, -O~F~Cl, -O-CF, CHFCl, (Cl-Cc)-al~yl~ercapto,
(Cl-C~)-hydroxyalkyl, (C1-C~)-alkoxy-(Cl-Cc)-alkoxy,
(Cl-C~)-alkoxy-(Cl-C~)-alkyl, (Cl-C~)-alkylsulfinyl,
(Cl-C~)-alkylsulfonyl, (C,-C,)-alkylcarbonyl, (Cl-C,)-
alkoxycarbonyl, carbamoyl, N-(C~-C,)-alkylcarbu~oyl,
N!N-di-(Cl-C,)-alkylcarba yl, (C7-Cll)-aralkylcarba- `
moyl which i~ optionally ~ubetituted by fluorine, ~-
chlorine, bromine, trifluorom~thyl or (C~-C~)-alkoxy,
N- (C3-C,) -cycloal~ylcarbamoyl, N-(C~-C,)-~ycloal~yl-
(Cl-C~)-alkylcarbamoyl, (C1-C,)-alkylcarbonyloxy,
phenyl, b~nzyl, phonoxy, b~nzyloxy, NRYR~, ~u¢h a~
amino, nnilino, N-m thylan~lino, ph-nylmercapto,
phenyleulfonyl, phonyl~ulflnyl, ~ulfamoyl, N-(Cl-C,)-
alkylsulfamoyl or N,N-dil-(Cl-C~)-alkylsulfamoyl, or
~wo ad~acent ~ubetltuents togeth-r ars a chain
-~C~ -C~.C~-C~.C~-, where a CH, group of th- cha~n
iB optionally r~placed by O, S, SO, SO, or NRY, and
where a heteroaryl radical can carry 1, 2 or 3
sub~tituents, and a cycloalkyl radical one ~ ;
sub~tituent, from the above group, and
R~ ie R~, providod Q hac the meaning of NR', where ~ ;
R' ~ hydrog~3n or methyl, and ;~ ;
R i~ benzyl, and
if Rl and/or R3 have the meaning of (C~-Cl~)-aryloxy, (C7-
Cll)-aralkyloxy, (C~-Cl,)-aryloxy-(Cl-C~)-alkoxy, (C7-Cll)-
aralkoxy-(Cl-C~)-alkoxy or a corre~ponding radical con-
taining terminal cycloalkyl group~, this radical lc ; ; ~ i;
preferably then a radical of tho formula D ~ ~
OZ (D), or ,~ ~
if ~1 and/or R3 havo the moaning of (C7-Cll)-aralkyl, ~ ,`
~C,-Cll)-aryloxy-(Cl-C~)-alkyl, (C7-Cll)-aralkoxy-(Cl-C,)-
alkyl or a corro~ponding radical containing torminal ; ```
cycloal~yl groups, thi~ radical i~ pr~forably then a ;~
radical of the formula Z,
:: . . : ..
2134865
- 31 -
RY and RZ aro ldent~ c81 or difforent and aro hydrogon, : ~:
(C6-Cl2)-aryl, (Cl-ClO)-allcyl, (C3-C10)-cycloallcyl, ~,
(Cl-C,)-alkoxy-(Cl-C,)-alkyl, (C7-Cl,)-aral~oxy-(C,-
C~)-alkyl, (C~-C,s)-aryloxy-(Cl-C~)-al~yl, (Cl-C10)-
alkanoyl, optionally cubstituted (C7-Cl6~-aralkanoyl
or optlonally ~ubstitutod (C~-Cl2)-~royl, or
RY and RZ aro togothor -tC~2~, in which a CE~ group ca~ ,t.''
be replaced by 0, S, N-(Cl-C~)-alka~oylimino or N- -~
(Cl-C~)-alkoxycarbonylimlno, and ;~
f ~ 1 to 8,
. .
g ie 0 or 1 to (2f
h i~ 3 to 6,
x is 0 to 3, and
n is 3 or 4. ; :-~
~ . ~ .. -.~-.... .
lS Co~pound~ o~ tho formula I ar~ particularly pr~ferred in ..
which ;~
X is 0, :; ~ ' ~'J '. ,1.~'~''~''`
Y i~ CR3 and, additionally, N lf Rl and R2 form a
cyclo,
m i8 0~ '''J''',"'",''~ ,''
A 1~ -CHR~-, whor- R' i~ the ~ubstituont of th- a-
carbon atom of an a-amino acid, in partlcular of a :~
natural L-amlno acid or lt~ D-lsomer,
B is C02H,
.. -
R' is hydrogen, bromino, chlorino, cyano, (Cl-C~
alkyl, (C~-C,)-alkoxy, (Cl-Cl,)-alkoxymothyl, (C~-C~
alkonyloxymothyl, (C~-C~)-alkynyloxymothyl, :.:~
carbamoyl, N-(Cl-C10)-al~ylcarbamoyl, N-((Cl-C~2)- .. -
alkoxy-(Cl-C~)-alkyl)carbamoyl, N,N-di-(Cl-C,)-
alkylcarbamoyl, N(C~-C,)-cycloalkylcarbamoyl, N-(C,- .
- Cl2)-phenylcarbamoyl, N-(C7-Cl2)-phonylalkylcarba- ; `
moyl, N-(Cl-Cc)-alkyl-N-(C,-Cl2)phenylcarbamoyl, N- j : -
(Cl-C6)-alkyl-N-(C7-Cl,)-phonylalkylcarbamoyl, :~
,., ,,. .~ ~
'' :' ~`
2 1 3 ~ 8 6 6
- 32 -
N-((C,-C~)-alkoxy-(Cl-C~)-alkyl)earbamoyl, oarboxyl,
(Cl-C~O)-allcoxycarbonyl, (C3-C20)-alkenyloxycarbo~yl,
ret~nyloxyearbonyl, (C3-C,)-eyoloalkoxyearbonyl,
(C3-C~)-eyeloalkyl-(Cl-C~)-al~oxyearbo~yl, (C3-C,)-
eyeloalkoxy-(Cl-C,)-alkoxyearbonyl, phenyl-(C~-C~)-
alkoxyearbonyl, phonoxy-(Cl-C,)-alkoxyearbonyl or
benzyloxy-(Cl-C,)-al~oxyearbonyl, wher- a ph~nyl
radleal 1~ su~etituted ln the mann-r ae defined $or
Rl and R3, and one of the radieale
Rl or R3 ~e hydrogen and the other a radieal from the
group hydrogen, fluorln~, ehlorln~, (Cl-C,)-alkyl,
(Cl-C1O)-alkoxy, (Cs-C~)-cycloalkyl, (Cs-C~)-cyclo-
alkyl-(Cl-C6)-alkyl, (C5-C,) -eyeloalkyloxy, (C5-C,)
cycloalkyl-(Cl-C~)-al~oxy, (Cs-C,)-cycloalkyloxy-(C
C~)-alkyl, (C5-C,) -cycloalkyloxy-(C1-C~)-al~oxy, (C5-
C,)-eyeloalkyl-(Cl-C~)-alkyl-(Cl-C~)-al~oxy, (Cs-C,)~
cycloalkyl-(Cl-C~)-alkoxy-(Cl-C~)-alkyl, (C5-c~
cycloalkoxy-(Cl-C~)-alkoxy-(Cl-C2)-alkyl,
-O-[CH2]~-C~K(2f~l~,F~,(Cl-C,)-alkoxy-(Cl-C~)-alkyl,(Cl-
C,)-alkoxy-(Cl-C,)-alkoxy, (Cl-C,)-alkoxy-(Cl-C~
alkoxy-(Cl-C2)-alkyl, eubet~tut~d (C,-Cl2)-ph-noxy,
(C7-Cll)-phonylalkyloxy, (C~-Cl2)-phenoxy-(Cl-C~
alkoxy or (C7-cll) -ph-nylalkoxy-(Cl-C~)-alkoxy,
phenoxy-(Cl-C~)-alkyl, (C7-Cll)-phenylalkyloxy-
(Cl-C~)-alkyl, phenoxy-(Cl-C~)-alkoxy-(Cl-C~-alkyl
or (C7-C~l)-phenylalkyloxy-(Cl-C~)-alkoxy-(Cl-C2)-
alkyl, whor- an aromatlc radleal le ~ubetltut-d by
1, 2 or 3 ldentlcal or dlfferent subetituents from
the group 1uor,1ne, chlorlne, eyano, trifluoro-
methyl, (Cl-Cl2)-alkyl, (C2-Cl2)-alkenyl, (C2-Cls)-
alkenyloxy or (Cl-Cl~)-alkoxy,
Rl and R2, wlth the pyrldlne earrylng them, form a 5, 6,
7, 8-tetrahydrolsoqulnoline rlng,
R~ le a branehed or unbraneh~d (Cl-C10)-alkyl rad1eal,
(Cl-C~)-alkoxy-(Cl-C~)-alkyl or a radleal of th~
formula Z,
', ~ ~' ;`'' ,''~'''. '
---`` 213~8~6
- 33 -
,~CH2lV-1Olw-~cH2J~-E (Z) '' '
whero E i8 a ~ub~t~ tutod phenyl radlcal of tho
f onnula F
R ~ R
_~_ RS ( ~
R 1 0 R9 ~ ~ -
or a (C3-C,)-eyeloallcyl radleal, wh-r~ v 1EI 0, l, 2 -;
or 3, w i8 O, and t ean b- 0 or 1, and ln wh~ eh R',
R7, R', R9 and Rl ~r~ ~dent~cal or differ-nt ~d are
hydrogen, fluorine, chlortno~ cyano, trlfluoro-
m o t hy l , ( C , - C ~ ) - a l ky l , ( C l - C , ) - a l k oxy ,
~O~ ~CH~;]~~C~H~ )Fg~ N- (Cl-C~) -alkylear~amoyl, N,N-
di- (C,-C6) -alkylcarbamoyl, N- (C,-C,) -cyeloalkylearba-
moyl, N-(~)-dehydroab~otylaminocarbonyl, or (C7-Cll)-
phenylalkylcarbamoyl, which ~ s optionally
tituted by fluorinn, ehlorine, trlfluoromethyl
or (C,-C~)-alkoxy, or whoro RC and R' or R7 and R', ~ a
togother w$th the phonyl ring carying th-m, form
naphthalono dorivatives ~;
: . . ~ , : , .,
If Rl or R' ha~ th- moaning of (C~-Cl2)-phenoxy, (C7-Cll)- ~ ~
~ ,....... .
ph-nylalkyloxy, (C~-Cl,) -phenoxy- (Cl-C~) -alkoxy, (C,-Cl,) -
phonylalkoxy- (C,-C,) -alkoxy, (C,-C,) -cycloalkyloxy- (C~-C,) - ~ ~ `
alkoxy, (C,-C~) -eycloalkoxy- (Cl-C,) -alkoxy or (C5-C,) - ~ .~ . ..... ... /
cyeloalkyl- (Cl-C~) -alkyl- (Cl-C~) -alkoxy, thi~ radical i8
th-n, o~pecially, a radical of tho formula D
OZ (D), or
- . .:: ~. ::; .
. :,, :. .. ,:,,
if Rl or R3 has tho mo~ing of phenyl, phonoxy-(C,-C,)-
25 alkyl, (C7-Cll) -ph-nylalkyl (C7-Cll) -phenylalkyloxy- (Cl-C") -
alkyl, (C~-C~) -cycloalkyl, (C~-C~) -cycloalkyl- (Cl-C~
allcyl, (C~-C~) -cycloalkoxy- (Cl-C~) -alkyl, (Cs-C~) -eyclo- ~ -
alkyl- (Cl-C~) -alkoxy- (Cl-C,) -alkyl or (C,-C~) -cycloalkoxy-
'~.',,,,..~
- 213~866
- 34 -
(C,-C~)-alkoxy-(Cl-C,)-alkyl, th~ radlcal i8 thon,
e~pocially, a radical of tho formula Z in which, ln both
ca~es,
v is 1, 2, 3 or 4, w is 0 and t is 0, or
v is 1, 2, 3 or 4, w i~ 1 and t is 0, or
v is 1, 2, 3 or 4, w ~8 1, t 1~ 1, and
f is 1 to 4, .
g i~ 0 or 1 to (2f~
x i~ 0 or 1. ::~
Compounds of the formula I ar~ very particularly pre-
f~rred in which -~
Q i8 O, . `
X i~ o,
Y i~ CR3, -
m is 0,
A i~ a -CH2- group which can b~ ~ub~tituted by a ~ - m
methyl group, ~ ,":,a
8 is -CO2H,
R2 is hydrogen, (Cl-C,)-alkoxy, (Cl-Cl~)-alkoxymothyl,
(Cl-Cl~)-alk~nyloxymethyl, retlnyloxymethyl, N-(C1- .
C~0)-alkylcarbamoyl, N-((Cl-Cl,)-alkoxy-(Cl-C3)-
alkyl)carbamoyl, N,N-di-(Cl-C~)-alkylcarbamoyl, N~
(C5-C~)-cycloalkylcarbamoyl, N-phenylcarbamoyl, N-
phenyl-(Cl-C~)-alkylcarbamoyl, carboxyl, (C~-Cl~ . P
alkoxycarbonyl, (C2-Cl~)-alk~nyloxycarbonyl, . . ~
ret~nyloxycarbonyl, (C~-C~)-cyclonlkoxycarbonyl, (C5- ~ ,.. ."~,.` ;,;
C,)-cycloalkyl-(Cl-C,)-alkoxycarbonyl or phenyl-(C
C~)-alkoxycarbonyl, where a phonyl radical i~
sub~t~tutod in the manner a~ defined for Rl and R3,
and one of th~ radicals
R1 or R3 1~ hydrogen and the other radical i~ a radical ;~
from the group hydrog-n, (C,-C~0)-alkoxy, (C,-C,)-
cycloalkylo.~y, (C,-C,)-cycloalkyl-(Cl-C,)-alkoxy,
~O~~CH~l~~CtH~s~ g~Fy~ (Cl-C~)-alkoxy-(Cl-C~)-alkoxy,
cubstituted (C6-Cl,)-ph~noxy, (C7-C11)-phenylalkyloxy,
(C~-C12) -phenoxy-(Cl-C~)-alkoxy or (C7-Cll)-phsnyl-
~;, ,
213~866
- 35 -
alkoxy-(C~-C,)-al~oxy, wh~r- an aromatic rad~c~l le
substitutcd by 1, 2 or 3 ldentical or dlffor-nt
eubst~tuente from the group fluorine, chlor~n~s,
cyano, trlfluoromothyl, (C1-C10)-alkyl, (Cl-C10)-
alkoxy or (Cl-C10)-alkenyloxy, and
: ~ " ., ' ~
R~ is a branchod or unbranchod (Cl-C~)-alkyl rad~cal or .. :.. .. `
a r~dlcal of tho formula Z,
-lCH~lv-lOlw-lCHalt-E (Z)
where B ia a eubstitutod ph~anyl radical o tho
lC formula F
R~ R7
~ R~ ( F ) , ~ ` ~
t o
R . .~
.-. :,.`:.
or a (C3-C,)-cycloalkyl radical, whero .
v 1~ O, 1, 2 or 3, w is 0, and t can b- 0 or 1, and ~. -;:: .;;
in which R6, R', R', R9 and Rl aro ~dent~cal or
different and aro hydrogen, fluorino, chlorlne, ~ . . .
cyano, trifluoromethyl, (Cl-C~)-alkyl, (C~-C6)- . .:
alkoxy, -O-lCH,~X-CtH~lt~l9)F9, N-(Cl-C,)-alkylcarba-
moyl, N,N-dl-(Cl-C~)-alkylcarb~moyl, N-(C,-C,)-
cycloalkylcarbamoyl, N-(I)-dohydroabietylamlnocar-
bonyl substituted benzyl radlcal, and f i8 1 to 4, ; . :
g l~ 0 or 1 to (2f~ and x 1~ 0 or 1.
Compound~ of the formula I aro partlcularly preferr-d ln ..
whlch . ~:
Q 18 O,
X 18 O, .; .
25 Y is CR3, ~:~
m 1~ 0,
B 18 -CO2H,
A 1~ a -CH~-group,
,. ., ~ .
213~g66
- 36 -
Rl $~ hydrogen, (Cl-C~)-alkoxy or ~O~tC~,]~~C~HO~.lg,Fg,
R2 is hydrogen, N-(C~-C,0)-alkylcarbamoyl, N-((Cl-C~
alkoxy-(C,-C,)-alkyl)carbamoyl,N,N-di-(C~-C,)-alkyl-
carbamoyl, N-(Cs-Cc)-cycloalkylcarb~moyl, N-phonyl-
carbamoyl,N-phenyl-(C~-C~)-alkylcarbamoyl,carboxyl,
(C~-C~)-alkoxycarbonyl, (C2-C~)-alkenyloxycarbonyl,
rotinyloxycarbonyl, (C~-C,)-cycloalkoxycarbonyl, (C5-
C6)-cycloalkyl-(C~-C~)-alkoxycarbonyl or phenyl-(C
C~)-alkoxycarbonyl, whero a phonyl radical 1~
sub~tituted by 1 or 2 ld-ntloal or difforont
sub~tituente from the group fluorine, chlorine,
cyano, trifluoromethyl, (Cl-C,0)-alkyl, (C,-C,0)-
alkenyloxy or (C,-C~0)-alkoxy, and
R3 i~ hydrogen, (C,-C5) -alkoxy or (C5-C,) -cycloalkyl-~C,-
C2)-alkoxy, wher~ one of the ~ubstltuent~ R1 and R3
i~ hydrogen, ~ 6 i
R~ i~ a branched or unbranched (C,-C,)-al~yl radical, or
a 2-phonylethyl radlcal, or a bonzyl radical
~ub~tituted by 1 or 2 radlcal~ from tho group flu-
orlne, chlorlne, cyano, trlfluoromethyl, (Cl-C,)-
alkyl, (C,-C,)-alkoxy, -O-~CX2]~-C~H~ )F~, N-(Cl-C,)-
alkylcarba~oyl, N,N-di-(C,-C,)-alkylcarbamoyl,N-(C,-
C,)-cycloalkylcarbamoyl or N-(~)-dehydroabletyl-
aminocarbonyl, and f 1~ 1 to 4, g 1~ 0 or 1 to
(2f~1) and x 1
: : :
Compound~ of the formula I are preferred to the hlghe~t
degree ln whlch
Q 1~ O,
X 1~ O,
Y i8 CR3,
m i~ 0,
A 1~ a -CH,-group,
B 18 -CO
R~ 18 hydrogen,
213~866
- 37
R~ i~ hydrogen, N-(C1-C10)-alkylcarbamoyl, N-((Cl-C~
al~oxy-(Cl-C,)-alkyl)carbamoyl, N-~yclohexylcar~
bamoyl, N-phenylcarbamoyl, N-(phenyl-(Cl-Cs)-al~yl)-
carbamoyl, whero, in the last two caco~, the phenyl
radlcal can carry a fluorins subotituent, (Cl-Clo3-
alkyl oub~tituent or (Cl-ClO)-alkoxy cub~tituent,
carboxyl, (Cl-Cl,)-alkoxycarbonyl, (Cs-Clc)-alkenyl~
oxycarbonyl, retinyloxycarbonyl, (C5-C~) -cyclo-
al~oxycarbonyl or bonzyloxycarbonyl,
R3 i8 hydrogan, (Cl-C~)-alkoxy or 2-(cyclohexyl)ethyl-
oxy, whore one of tho ~ubet~tuento Rs and R~ io
hydrogon, ; ~
R~ i~ a branchod or uDbranched (Cl-C~)-alkyl radical or ~ . .:;
a benzyl radical which ic ~ubotitutod once by flu-
orine, chlorino, trifluoromethyl, (Cl-C~)-al~yl or .
( Cl - C3 ) -alkoxy~
Compounds of the formula I are aloo preferred to the ;.
highe3t degree in which
Q i~ 8, ;. ;
20 X i8 O, ,.
Y i8 CR3,
m io 0,
A io a -CHs- group,
i l~ - COsH,
Rl io hydrogen,
Rl io hydrogen, N-(Cl-C10)-al~ylcarbamoyl, N-((Cl-Cl,)-
alkoxy- (Cl-C3) -al~yl)carbamoyl, N-cyclohexylcarha-
moyl, N-phenylcarbamoyl, N-(phenyl-(Cl-Cs)al~yl)-
carb~moyl, wherQ, in the laot two ca~e~, the phenyl
radical can carry a fluorin~ ~ubotituent, (Cl-C10)-:.; ::;: :~:
alkyl oubotituent or (Cl-C10)-alkoxy ~ubctituent, ::
carboxyl, (Cl-Cl,)-alkoxycarbonyl, (C~-Cl~)-alkenyl- :.
oxycarbonyl, retinyloxycarbo~yl, (C5-C,)-cycloalk-
oxy~arbonyl or benzyloxycarbonyl,
,, ~ ", ,,; "
~' ' ".:. ' `.:
2134866
- 38 -
R3 is hydrogen, (Cl-C,)-~lkoxy or 2-(cyclohexyl)-
ethyloxy, whore one of tho substituont~ R' and R' ~o
hydrogen, and
R~ 18 a branched or unbranch-d (~,-C~)-alkyl radical or
a benzyl radical which lc substltut-d onco by flu-
orine, ohlorln-, trifluoromothyl, (Cl-C~)-alkyl or
(Cl-C3)-alkoxy
The compound~ of the formula I aro al~o profqrr-d to the
highe~t degree ~n wh~ch
10 Q i~ S, ~-
Y i8 CR3
m is 0,
A is a -CH2-group, -
15 B i8 -CO~H, ' ~' ~,,, ~'~ ,'''~'"'',~`'~','~''!'~
Rl iB hydrogen, ~ >-
R2 is carboxyl or (Cl-Cl,)-alkoxycarbonyl,
R3 is hydrogen, and ;
R~ is a branch-d or unbranch~d (Cl-C~)-alkyl radlcal
Tho compound of tho formula I i~ al~o pr-forred to the
hlgh-~t dogreo in which
Q i~ 0,
X ia 0,
Y i~ CR3, whor~ R3 i~ hydrogon, ~ -
m i~ 0,
A 18 a -CH2-group,
B is -C02H-,
Rl and R2, togethor with th- pyridin- carrying them, form
an i~oqulnollno ring havlng an unsubstltut-d b~nzo
moloty, and
R~ 1~ methyl
" ;'~ . ' : ^~;: ,
,, ~, ,.., ~
Tho compound of tho formula I 1~ also pref-rr-d to tho
highe~t degr-- in which -~
Q ;8 0,
t . ''~
'':': ' " ',', "' .', ' " ' '~;
21~866
- 39 - ;
X is 0,
Y ie CR3, -~
m ie 0,
A ie a -C~-group,
5 B i~ -C0~
Rl is hydrogon,
R' and R3, togother with the pyridine carrying them, form
a quinolino ring having an unsubstituted benzo ~;
moioty, and
R4 is mothyl.
, .
Tho invention al80 ~mbracee prodrugs for tho compound~ o~ :
tho formula (I), which prodrugs bring about an i~hl~it~on -~
of collagon blosynthosie in vivo by liborating compounds ~ i~
of tho formula I or thoir ealte.
,~
"
Finally, the invontion also ~mbracoe prodruga which, by
liberating compounide of the formula I or their ealta,
bring about i~n lnhlbitory effect in vivo on prolyl-4-
hydroxylaee.
.
Prodrug groupinge aro chomical groupe whlch, ln vlvo, -
,
0 - aro convortod ~nto the carboxylato group of the
compounde of th~ formula I, and/or
- can bo cleav~d from the amldo N atom, and/or
- can bo converted into a pyrldlno ring. ~ ;~
., -: .~
-, . . ::.::
Thoee prodrug groupe which aro ouitable are known to tho
porson skllled in the~art.
:,. :::
~he followlng prodrug grouplngs receive partlcular
montlons ;
for the carboxylate group, ester groupe, amlde group~
hydroxymothyl groups and aldehydo group~, and tholr
derlvatlvess for the pyrldino N atom, N-oxide~ and N-
alky~ deriratlvoe; and fox the pyrldlne rlng, 1,4-
dihydropyridine derivatives or tetrahydropyridino
derivativee. ~ i
'.." ~:
,: ~.,,,i:
213~866
- 40 -
The invention relatos to tho u~e of c Q ounds of tho
formula I, and also the phy~iologically tolarated ~alt~
for inhibiting collagon biosynthesis.
The inventlon relatos to the use of compounds of the
formula I, and also tho physiologically tolerat~d ~alts,
for inhibiting prolyl-4-hydroxylase. ~ -~
The inve~tion also relate~ to the use o~ compou~ds of the ~ ;~
formula I, and al80 the physiologically toleratod salt~
for producing a pharmaceutical against fibrotic disease~
The invention also relates to the use of compound~ of the
formula I, and al~o the physiologically tolerated salts,
for producing a pharmaceutical against fibrotic diseases
of the liver, the lung and the skin.
::
Finally, the invention relates to the compounds of the ~ ;
formula I for use as pharmaceutical~
The invention relate~, in particular, to the compounds of -
the formula I for use as fibrosuppressive ag-nte.
The invention al~o relate~ to a procese for pr-paring
compounds of the formula I.
20 The oompound~ of the formula I, in whi¢h A i~ a ~
~ubstituted alkylene moiety, B i~ C02~, Y is CR' and m is ;;~ ;
0 or 1, are prepared by ;~
,, ~ j , , , , :
11.) Rea¢ting pyridine-2-carboxylic acids of th- formula
II (R~3 is H) with the amino esters of th- formula
III to form tho amid- e~ters of the formula IV, or i-
i2.) Reacting pyridins-2-carboxylic estere of the for~ula; ; -~
II (R~3 is lower al~yl), under the conditions of
aminolysis, to form the compounds of the formula IV;
and
2131866
,--
- 41 -
ii) liberating the compound~ of the formula I from th~ir
eeters of the formula IVs with, whero appropriate,
iii) the oompounde of the formula IV boing prepared by
alkylation of compounds of the for~ula V with R~
iand, where appropriate, : ; :
: .. . ~
i~) the compounds o~ th~ Formula IV being convert~d, :
pro~ided Q is 0 or NR', into thoir pyridine N-oxidee :-:
IV' (R'~ iB (C3-C3,)-alkyl or benzyl) and the l~Att~r :~:
being hydrolyzod to form the pyridino N-oxid~s of
tho formula I' (R2~ ie H). :
Scheme 1 . :
~C22~2~ -c~2l~2~
l V
~ - ~
~ X t ~ ~ ~
", , ... . .. ~.
R
J R2~NH-~-CO2R2~ ~
R~ . ;
O ' . ' ,' " ,.,
V .-: - .::`.
:, ' ' ', ' ~'',
O R ' ~ N - A - t O, 2
A - C O, ~ , "'~ : ; . .
o O : ~ . .','"'.''' :'''::'
v~ c,-e")-~ . ~n~
I ~ 12~ N . . ~ ~
' ~ .,; ',;: .
;, . ~ ' ' . ,:
' ,,:,~. ;:.',:",'
:''; ~',.'.'' i
R~3 ie H or (C,-C16)-alkyl, .
;: :.;. .: ,.
.,, : .:
'".;' .~ ' '" ' ''','', ' ' ~ '
6", ' ' ;; ~
213~866
- 42 -
R~ i B X, (Cl-Cl,)-alkyl or bonzyl,
X ie a leaving group, ln particular halogen, OSO~Me or
OSO~phenyl
The methods of carboxyl actlvation and the condensatlon
reactions known from peptide ch d ~try are sultable
procosse~ for the amide formation (reactlon il)
The eub~tance~ which are known to the per~on skllled ln
the art, such as thlonyl chloride, oxalyl chloride,
pivaloyl chloride, chloroformate derivativo~, or N,N'-
carbonyldiimidazolo, can b- u~ed a~ roag-nt~ for tho
carboxylic acid activatlon The aotlvated dorlvAtive~ of
the compounds of the formula II aro prepared ln situ and
then rsacted with tho amide derivativee of tho ~ormula
III
A~ exa~ple of a euitable conden~ing agent ie the
combination of N,N~-dicyclohexylcarbodllmldo/N-hydroxy-
lH-benzotriazolo and N-ethylmorphollne
Sultablo aolvents aro dichlorom thane, t-trachloro-
methane, butyl acotato, othyl acotnt-, tolu-n-, totra-
hydrofuran, dlmethoxy-tha~e, 1,4-dloxano, ac-tonltrlle,
N,N-dlmethylformamld-, N,N-dim-thylacotam$do, dimothyl
~ulfoxldo, nltromethano and/or pyrldlne
The compoundo of the formula I, ln whlch R~ and R3 aro
hydrogen and R~ 18 a carboxyl eubstltuent, a carbamoyl
~ub~3titu-nt or an ! est~r ~ub~tltuont, wero proparod a~
outllned ln schomes 1, 2 and 3
,, , . " . :,. .:
8cheme 2 lllu~trat-r3 th- preparatlon of tho compound~ of
the formula TI ln whlch Rl lo a c~rboxyllc acid
~ubstltuont, or lts derlvatlve, and Rl and R' aro hydro-
gen
:, '. . ,: '" ' ~'
Th- 3-~3ubstituted 5-carboxypyridlne-2-carboxyllc e~3ter~
of the formula XI and thelr isomer~ of the for~ula XII
'
2134866
- 43 -
are prepared from the pyrldlno-2,5-dicarboxyllc die~ti~rs
of tho formula VII.
The oxidation of the pyridine-2,5-dicarboxylatos of tho
formula VII is d~scribed in J. Chem. Soc. Per~in Tra~.
2, 1978, 34-38 and in J. Org. Chem. 25 (1960) 565 to 568
(M.L. Peterson).
The halogenation (chlorination) of the pyrid~no N-ox~d,~,s
of the formula VIII with thionyl chloride and the reac-
tion of the 3-chloropyridine-2,5-dicarboxyllc diostor
(Formula IX) with alcoholat~s (Q i,s o or 8) can be
carried out in analogy with tho proco,~ do,scrlbed in the
patent specification 7~H 658 651 (LONZA), where M 18 a
singly charged or doubly chargod metal ion, preferably
from the first or second mai~ group of the periodic
syetem.
In analogy with the known literaturo (7-A: vol. 68, 1968,
68 840 h), the monoesters of tho formula XII are pre~
pared, under hydrolysis condltions, from the substituted
pyridine-2,5-dicarboxylic dio~ters of the formula Xb.
Seloctive hydrolysis using ~u(II) salts, J. Delarge in
Pharmaceutica Acta Holvetiao 44, 637-643, 1969, repre-
,s,-nt~ anothar procos~ for preparing the compounds of the
formula XII from tho diesters of the formula Xb. 1~ ;
,:-, . :,.: ,,,
. : ;
The compounds of the formula XII thus obtained are
reaoted with the,amino estor~ of the formula III to form
the compounds of tho formula IV (SchQme 2).
,:~
Ths pyridine-2-carboxylic ester-5-carboxylates of the
formula XI can be prepared, under eeiterification condi-
tlons, from substituted pyridino-2,5-dicarboxylic acids
of tho formula Xa (see CA: vol. 68, 1968, 68840 h).
~ultable conditions are, for examplo, eE,terification with
mothanol in the presence of sulfuric acid, it being
necessary to choose the reaction time 80 that complete
' " ::' "
"....
II:A . i; i ~ .
' ; . ~;
213~866
- . .
- 44 -
esterification to form tho d~e~ter product only ta~o~
placo to a ~ocondary oxtent, or ~o that tho diostor ~ -
products can bo ~oparatsd off a~ by-product~.
Tho compounds of tho formula XI aro convsrtod w~th amlnoo
or alcohole $nto ths 5-carboxylic acid doriv~tl~o of the
formula XIV (Scheme 3).
These are then hydrolysod to form tho ~iompounds of th~
formula II (R23 i~ ~), which compou~d~ aro sub~oqu-ntly
reacted in analogy with Scheme 1.
Scheme 2
RO2C~ 2 ~ ~`CO2R
V l I I
V I I
h~logen~tion
( R ~ H, lower ~Ikyl ) ~
:` ,' '~ ''''".: ' .",'
UOR~ e r
R O ~ C~O R U ( ~ ) O R ~ R O 2 C~¢~L
~C O 2 R C O 2 R
I X
X a : R H L C I, a ~
X b : R lower ~Ikyl 1~ metd ion, :
~ingly or doubly
\ ch rged
\ O O . 5 ,
: '~ '' :`"'"'
U 2 C~O R ~C O H
X I R Iower dkylX I I R Iow ~Ikyl
H, \l - A - C O 2 R ~
I V ' .,
213~866
,
- 45 -
Scheme 3
ROIl o r HNRR
~02C ~R~ X I I I ' R2 QR~
~C 2 low ~1 ~C 2 lower~l
X I X I V R2 . COOR, CO-NR R
The 2-hydroxymethylpyridlnes of the ~or~ula VI-, which
are dir~clo~ed in ~P-A-O 304 732, EP-A-O 321 385 a~d EP-A-
0 208 452, can bo used a~ ~ntermodiatee for preparing ~ -
5 derlvativo~ (Rl) which are eubstitut-d in th- 4 posltlon ~ -
. .
Scheme 4 ~ -
R2 ~ QR Id 1i ~2
R~ CH20H R C02~
V I I I ( R 1 ~ H )
v I o ~ ~ I c : o R 4 O ~ me~yl)
V l ~ ~ l l b : O R ~ O i~ n ( ~ n benzyl ) ; ~ -
',
Th- 3-0-benzyl derlvative~ of the formula VIb were al~o
obtained in an analogous manner, a~ de~cribod ln thoso
documents
.. : ,:
The compounds of the~ formulas VIa and VIb wor- react-d
wlth an oxldlzlng agent, preforably wlth RMnO~ ln agueous
alkalino msdlum, to form th- pyridino-2-carboxylic acid
dorivatlv-s of tho formula TI (of Schemo 4)
Th- proparation of sub~tltuted pyridine-2-carboxylic ~ ;
acids 18, for example, dlsclo~od ln DE-A-353 046, and for ~; ,;
3-(3-chlorophonoxy)pyridine-2-carboxylic acld and 3-(3- ~ `
methylphonoxy)pyridlno-2-carboxylic ncid in J Med Chem
1975, 18, pp 1-8, Vlllani ot al ; ~or
' ~
: , . .
213~8S6
-
- 46 -
3,5-diethoxypyridine-2-carboxylic acld ln J. Med. Chem.
1974, 17, pp. 172-181, French et al.; and for 3-mothyl-
thiopyridine-2-carboxylic acid and 3-benzylthlopyridine-
2-carboxylic acid in J. Med. Chem. 1974, 17, pp. 1065-
1071, Blank et al.; and for 3-methoxypyridine-2,5-d~car-
boxylic acid in CH-PS 658 651.
The compounds of the formula I are iDhlbltors of prolyl-
4-hydroxyla~e. Tho lnhibition of thls onzyme w~e
determined a~ described by ~aulo und G~nzler in Annal.
Biochem. 184, 219 to 297 (1990).
TAe novel compounda of the formula I pos~ece valuablo
pharmacological propertios and exh~bit, in particular,
antifibrotic acti~ity.
T~e antifibrotic effect can, for example, be determined
u~ing the model of carbon tetrachloride-induced hopatic
fibro~is. For this, rats aro troated twic- a waek with
CCl~ (1 ml/kg) - dls~olved in olivo oil. Tho oubstanco
under te~t is administered daily, whero appropriate oven
twice a day, por 08, or intrnperitonoally - dle~olvod in
a ~uitable toleratod solvont. Tho extent of tho hopatic
fibro~i~ is detormined by hi~tology, and the proportion
of collagon in tho llvor 1~ analyzod by means of deter-
mining hydroxyproline - a~ describod in Rivirikko t al.
(Anal. Biochem. 19, 249 f. (1967)). The fibrogenic ac-
tivity can be meaeur-d by the radioimmunologlcal detor-
mination of collagen fragments and procollagon peptidos
in the ~erum. Injthis model, the novol,compounds !aro
active at a concontration of from 1 to 100 mg/~g.
~ :..-. .
The fibrogenic activlty can be moasured by radiolmmuno-
logi¢al determination of the N-terminal propsptido of
¢ollagen type III or of the N-torminal or C-terminal
cro~slinking domain of collagen type IV (79 collage~ or
type IV collagen NCl) in the serum. - ~ ~;
~ ~ '
For this purposo, mea~urement~ wero made of the ~?~:
213~866
- 47 -
concentrations of hydroxyproline, procollagen III
peptide, 78 collagen and type IV collagen NC in the llver
of
a) untreated rat~ (control)
b) rats which wero adminlster-d carbon tetrachlorido
(CCl" control)
c) rat~ which were first administered CCl" and tho,n a
novel compound
(this te~t method 1D described by Rouillor, C., ~xpcri-
mental toxlc ln~ury of the llv~r; in Tho Llver, C.
Rouillor, vol. 2, 5. 335 to 476, New York, Acade~ic
Press, 1964).
The novel compounds cu~ al~o b- demon~trated to b~ active
ln the following ~ystem~
Inhibition of hepatic prolyl-4-biydroxylase ln vlvo~
Thi~ model i~ used to demonstrate the acute inhlbition of
prolyl-4-hydroxyla~e in vlvo. For thi~, r~ts of both
~exe~ (healthy or with induced hepat$c flbrosi~) are
administered (intraporitoneally, intravonously or per 08)
the substance under tast or th- corrosponding ~ohicle
and, after thio, aro giv-n 1~C-L-proline (250 ~Ci/kg of
bodyweight), which is administerod intraperiton-ally.
There then follows a socond lntraperltoneal admiini~
tration of l"C-L-prolino (250 ~C~/kg of bodywelght).
Finally, the animals are exsang~inat-d under pento-
barbital ane~thesla and the liver~ removed. The hepatic
collagen was purifiod ~by dlgo~tlon with popsln and
fractlonal ammonium eulfate precipitation in conformity
with published protocol~ (Ref. 1 and 2). The purifiod
liver collagen was hydrolyzed and the content of 1'~C-
hydroxyprolino and 1~C-proline wa~ determirod by mean~ of
amino acid analy~ls u~ing lon exchange chromatography.
Inhibitlon of prolyl-4-hydroxylaso is s,hown by a d-croase
in the quotient 1'~-hydroxyprollne/[1~C-hydroxy~
proline~1"C-proline]. 2,2'-Dipyridyl i~ u~ed as the
reference substanco. (1: Cho~kier, M. 1986, Hepatocyte
:
21348~6
- 48 -
collagen production $n vivo in normal rat~, J. Clin.
Invest. 78: 333-339 and 2: Oga~a I., qt al. 1991, M~nor
contribut$on of hopatocytos to collagen productlon in
normal and oarly f$brotic livors, ~opatology 14:
361-367).
Inhibition of prolyl-4-hydroxyla~e in cell cultur-s:
~: , .,; ,;:
The following cell types are us-d for t~sting inhibitor~
of prolyl-4-hydroxyla~o in coll cultur~c: `
Normal _uman skin fibroblasts, (N~DF), rat liver
opithelial cells, (ref. 1) and primary fat storing c-lls
from rat liver (ref. 2). For thi~, the coll~ ar- oult~
. , ,
vated in the pro~ence of inhibitors. At the ~amo time,
the collagen which iB nowly ~ynthesized during thi~
period ~8 metabolically labelled with 4-3H-L-proline and ;~
~C-prol$ne. The iafluonce of the test cubst~ncee on tho
: .~
degree of hydroxylation of the collagen is then deter-
mined in iaccordance with the method of Cho~kier et al
(ref. 3). 2,2~-Dipyridyl i8 ~mployod as tho r~feronce
sub~tance. (1.: Schrode, W., Mecke, D., G-bhard, R. 1990,
Induction of glutamine synthqtase in periportal hepato-
cyte~ by co-cultivation with a liver pithelial cell
line, Eur. ~. Coll. Biol. 53: 35-41; 2. Blomhoff, R.,
Borg T. 1990, I~olation and cultivation of rat liv-r
~tellate cell~, Mothods Enzymol. 190: 59-71s and 3.:
Cho~kier, M. Peterkofsky, B. Batema~, J. 1980, A new
m-thod for dot-rmlning the xtent of prolino hydroxy-
lation by m-a~uring changes in the ration of [4-'X]:t~C~
proline in collagenase,dig-sts, Anal. Biochom. 108: 385-
393).
Tho compound~ of the formula I may be used as medicament~
in the form of pharmaceutical pr-paration~, which contain
tho compound~, whoro appropriat- together with toleratod
pharmac-utical oxcipient~. The compounds can bo u~od a~
medicines, for example ln tho form of pharmaceutical ~`
preparation~ which contain the~e compound~ in a mixture
together with a pharmaceutical, organic or inorganic ; ;;
':'.: :.. ~ '~
. - .: ,::
::' , :" ' :,; : `~
213~866
- 49 -
excipient which i8 suitablo for ~nteral, percutaneoua or
parenteral adm~nietration, such aA, for sxampl~, water,
gum arabic, gelat~n, lactoeQ, starch, magneeium stearate,
talc, vogetable oilB, polyalkylene glycol~, vaeelino,
etc.
Por this purposs, they can be adminietor-d orally ~n
doses of from 0.1 to 25 mg/kg/day, proferAbly of fro~
to 5 mg/kg/day, or parenterally ln doeoe of from 0.01 to
5 mg/kg/day, proferably of from 0.01 to 2.5 mg/kg/day, ln
particular of from 0.5 to 1.0 mg/kg/d~y. Tho doeage can
aleo be increased ln sovere ca~e~. In many casee, how-
evor, emaller doeo~ aro al~o sufflcient. Thsee data rofor
to an adult of about 75 kg ln weight.
The novel conyounds of tho formula I are doeignatod
eubetltutod hoterocyclic carboxyllc acld glycylamidee,
prefsrably pyrldlne-2-carboxyllc acld glycylamide~, ln
the examplos doecrlbod bolow.
This mode o~ deelgnatlon le underetood to moan
subetltutod N-carboxymothylpyridine-2-carboxamldoe.
20 Anothor optlon ie to claseify th~m ae eubetitut-d N- ; ~
(pyridyl-2-carbonyl)glycinee. ,;
Examplo 1 ~ ~ `
3-Mothoxy-4-(2,2,2-trifluoroothyloxy)pyridlns-2-
carboxyllc acid glycylamide
a) 2-Mothyl-3-methoxy-4-chloropyridine N-oxide
11.2 g (80.5 mmol) of 3-mothoxy-2-mothyl-4(1~)-pyrldone -
were heatod under reflux for 10 houro ln 100 ml of -~
phoephorus oxychloride. Subeogu-ntly, th~ mixturo wae
conc-ntxated and 30 ml of toluen- were add~d to each 2 ml
volume5 concentration thon took placo onco agaln and the
r-eldue wa~ takon up ln 150 ml of water, wlth the pH of
the mixture then belng adjuetod to 11 wlth ~,CO3; thle
mixturo was thon extractod with dichloromethano and tho
organic phaee was waehod with wator, dr~ed and freed from
." '; ;~'~'~: ,';;
2134866
~ 50
solvent
8 g of ths product were obtained, under ~tandard con-
d~tlons, from the pale brown oil (9 g) using m-chloropor-
benzoic acid in d~chloromothano, m p 88 to 8gC (fro~
5 petroleum other) ; ;
b) 2-Methyl-3-methoxy-4-(2,2,2-trifluoroethoxy)pyrldino
N-oxide
6 7 g of potas~ium tert-butoxide were added ln portions,
at -20C, while otirring and undor a nitrogon atmosph-ro,
to 20 ml of trifluoroethanol Ater tho mixture had beon
warmed to 0C, 5 2 g (30 mmol) of 2-methyl-3-methoxy-4-
chloropyridlne N-oxide wero added in portlon~ The
mixturo was heated under reflux for 3 hours, ~nd then
coolod down to room temperatureS a further 3 45 g of
potassium tert-butoxide w~ro then add~d and the mixture
was heatod under roflux for 2 hours After it had coolod
down, 40 ml of water wore addod to the r~act~on m~xture, ~-
which was thon extractod with dichloromethane; the
extract was then dried over MgSO~ and freed from ~olvont
ln vacuo Tho rosulting oily product wa~ ~ub~-cted to
furthor roaction
c) 3-Methoxy-4-(2,2,2-trifluoroethoxy)-2-hydroxymethyl-
pyr~dlne
. ~ .
8 g (33 8 mmol) of tho a~ovo compound wer- dis~olv-d in ~ ~ ;
25 16 ml of glacial acotic acid, and 24 ml of acotic ~ ~;
anhydrid- wore addod, at 80C and while stlrring, to this
mixturo The reaction mixturo was hoated at 110C for 2
houra and thon coolod down to 80C; 40 ml of methanol ;-~
wer- th-n addod to it dropwlse Sub~equontly, th~ mixture
30 wa~ conc-ntrated ln vacuo, and tho oily residue addod to ;
75 ml of 2N m thanolic NaOH, with thi~ mixturo boing
etirred for 30 minutes Following treatment with active -~
charcoal, and filtration, the mixture was conc~ntrated in ~ .',,',.'.,'t
vacuo and 50 ml of water wero addod to tho rosidue, after -~`
~,' ~, "'' ',
213~866
. .
- 51 -
which extract~on took placo w~th dichloromethan~; the
extract was dried (NgS0~) and conc~ntratod, and the
residue was treatod with dii60propyl othar 3 9 g of the
product wero obtained ~n th- form of eolorloss erystal~
m p 107 to 108C
d) 3-Methoxy-4-(2,2,2-trifluoroothyloxy)pyridino-2-
earboxylic acid
0 8 g (3 3 mmol) of the abovo alcohol was dis~olv-d in a
solution composed of 0 3 g of potao~ium hydroxide and
25 ml of water, and 1 6 g of pota~ium p-rmanganate were
addod in portions at 100C and whil- otirring Aft-r
decolorlzation, the manganese dioxide which had formed
was filtered off with ~uction fro~ the hot m~xture and
waehed twice with hot waters ~he filtrat~ wae concen-
trated in va~uo to 1/3 of the volume, ad~u~ted to p~with conc agueous hydrochlorlc acid, und eoncentrat~d in
vacuo; the rosldue was treatod w~th ~ydrous thanol and
tho undis~olved matorial was filtored off 0 73 g of
product, m p 157, was obtainod frc~ t~o filtrate
~ . ,
o) 3-Mothoxy-4-(2,2,2-trifluoroothyloxy)pyridine-2-
carboxylic acid (glycyl thyl oster) umlde
0 58 g (2 3 mmol) of tho above carboxyllc acid wae
~u~pendod in 100 ml of anhydrou~ t-trahydrofuran, aft-r
whlch 322 mg (2 3 mmol) of glycin- thyl e~ter
hydrochlorlde, 0 64 ml (5 mmol) of N--thylmorpholino,
350 mg (2 6 mmol) of l-hydroxy-l~-b-nzotriazole and 53j7
mg (2 6 mmol) of N,N'-dlcyclohexylcarbodilmlde were add-d
at 20C and while etlrrlng, and tho mixture was th~n
stirred at 20C ~or 48 hours Undls~olvod matorial wa~
th-n f~ltsred off and th~ flltrat- wae eonc-ntrated ln
vacuot the residue wa~ tak-n up ln ~thyl acetate ancl
undl~eolved materlal wa~ filterod off; th- filtrate wae
~tirred together with 100 ml of a ~aturated, aqueous
~olution of Na bicarbonate, and th- organic phase waB
dried and concontratod in vacuo; the r-eidue wae
:, ~:,,;: ..
:, . ,: :,
`
213~866
.
- 52 -
cryetallized using diisopropyl othor 0 45 g of tho
colorlese cryetalline product wero obtained, m p 80 to
82C
f) 0 4 g (1 2 mmol) of tho above oet-r wa~ added to
50 ml of a 1 5 N mothanolic solution of sodium hydroxid~,
and this mixturo wa~ ~tirrod at 20C for 30 mi~utes It
wae then concantrated in vacuo and tho ros$duo was taken
up in 50 ml of wator; this mixtur~ wa~ ad~u~ted to pH 1
with comp aqu00us hydrochlortc acid and tho small amount
of undissolved material was flltor~d of~; th- filtrate
was concentrated in vacuo and the residue wae treated
wlth 50 ml of anhydroue othanol; thi~ mlxturo WaB
filtered and tho filtrate wa~ concentratod and brought to
crystallizatlon using diothyl eth-r 0 32 g of the title
15 compound wae obtained, m p 163 to 165C (with gae
evolution)
Example 2
4-Chloro-3-methoxypyridino-2-carboxylic acid glycylamide
a) 4-Chloro-2-hydroxymethyl-3-methoxypyridin-
20 30 g (173 mmol~ of 4-chloro-3-methoxy-2-methylpyridine N-
oxide (cf Example la) w-r- di~olv~d in 100 ml of
glaclal acetic acid, after which 150 ml o~ acetic
anhydride w-re added dropwis-, at 80C and while ~tir~
rlng, and th- mixtur~ was then stirred at 110C for 2
25 hours The mixture was thon cooled down to 80C ~nd 200
ml of methanol were added dropwi~e; the mixturo was then
heated to boiling for 15 minutes and, aft~r having beon
cooled down, concentrated in vacuo; the residue was taken
up in methanol and thi~ mixture was allowed to flow into
30 300 ml of a 1 5 N methanolic solution of ~odium
hydroxide, with this mixture then boing stlrred at 20C
for 30 minutes and concentrated in vacuo; th- residue wa~
takon up in wator, and this mixture extract~ad three timos
with dichloromethane, with the organic phase boing driod
and concentratod; tho residuo was crystallizod using
.' ',~ ~;
213~866
- 53 -
potroleum athor. 23 g of product were obtainod, m.p. 64
to 6SC.
: -:
b) 4-Chloro-3-methoxypyridino-2-carboxylic acid
8.65 g (50 mmol) of tho above alcohol were di~sol~ed ln
a mixturo comFosed of 0.8 g of potasslum hydroxide and
60 ml of wator, after which potas-ium pesm~ganat~ was
addod in portions, at 60C and while ~tirring, until no
moro discoloration could bo soen (12 g, 75 mmol). Aft-r
1 hous at 60C, the ma3ganeso dioxide was filtered off
wlth suction and then washed wlth hot waterS the flltrate
was concontrated ln vacuo to 200 ml and ad~ustod, while
cooling, to pH 1 with aguoou~ conc. ~Cl. After grindlng,
tho product csystallizss out in a~sociation with cooling.
Additional product can be obtalnod from tho mother liguor
by troatment with potroleum other. Total guantity, 4.2 g,
m.p. 116 to 117C (with ga~ evolution).
,
c) 4-Chloro-3-mothoxypyridino-2-carboxyl~cacid(glycyl
othyl ester) umide
4.7 g (25 mmol) of ~he above carboxyllc acld were
~uspended ln 200 ml of anhydrou~ dichloromethans, and
after that 3.5 g (25 mmol) of glyclne ethyl estor
hydrochlorldo, 6.4 ml (50 mmol) of N-ethylmorpholino,
3.8 g (28 mmol) of l-hydroxy-lH-bonzotriazolo and 5.15 g
(25 mmol) of N,N'-dicyclohoxylcarbodilmido were added
~eguentially, at 2qC a,nd while tirring, and the mixture
was th-n stirred at 20C for 20 hours. ~ndis~olved
mat-rial wa~ then ~$1t-rod off and the organic phase was
~haken with a saturated, aguoous solution of odium
carbonate, driod and ooncentrated in vacuoS th~ rosiduo
(6 g of oil) wa~ chromatographod on ~ilica g~l u-ing
~thyl acetato and 5.4 g of oily product wero obtained.
d) The t$tlo compound wa~ obtain-d by hydrolyz$ng tho
abovs othyl ester. For th$~, 0.7 g (2.6 ~mol) of th$~
213~866
- 54 -
est-r wa~ dlssolved in 50 ml of methisnol/water (3 1), and
after that 170 mg (7 ~mol) of lithium hydroxldo wero
added at 20C and while stirring Aft~r 30 minut~, the
mixture wa8 concentrated $n vacuo; tho resldue w
brought to p~ 1 with conc aquoous hydrochloric a¢ld and
thl~ mlxture was concentrated ln vacuo; the r~slduo was
troatod twlce with i~n~ydrous thanol and the ethi~nollc
phaso was concentrated; the re~ldue wa~ treated with hot
ethyl acetate and the amorphous re~ldu- was drlod on an
oil pump 0 31 g of the tltle compound was obtalned
Example 3
4-Butyloxy-3-mothoxypyrldine-2-carboxylic acld glycyl-
amide
M p 137 to 139C (with gas evolutlon, from tetrahydro-
furan)
..
-. : . :-.
: . .. ~ . . .,:
ExampleEi 4 to 16 were prepar~d in an analogou~ mannors
Example 4
3,4-Dlmethoxypyr$dine-2-carboxylic acld glycylam~d~
Example 5
3-Ethyloxy-4-(3-mothoxybenzyloxy)pyrldlne-2-carboxylic
acid glycylamlde
Exampl- 6
4-~exyloxy-3-mothoxypyrldine-2-carboxylic acld glycyl~
amlde - ~-
.., ~ . .. .
25 Examplo 7 ~ ,",;
3-Mothoxy-4-(3-methyl-1-butyloxy)pyrldlne-2-carboxylic ~ ~-
acld glycylamide
Example 8 ; ~`~
4-(4-Fluorobenzyloxy)-3-msthoxypyrldlno-2-carboxyllc acid
30 glycylamlde -
: :''::''' .":'.
. ~
",, ',,.~ ,: ';
, i ~ . ~ .:: .
:: .
.: : :. ,: .::
2~3~866
- 55 -
Examplo 9
3-Methoxy-4-(4-trifluoromothylbonzyloxy)pyrldlno-2-
carboxyllc acid glycyl ~ido
Bxamplo 10
3-Mothoxy-4-(2,2,3,3,3-pentafluoropropyloxy)pyridlne-2-
carboxylic ac$d glyeylamido :~
Examplo 11
4-(2,2,3,3,4,4,4-~optafluorobutyloxy)-3-methoxypyrldln~
2-carboxylic acid glycylamide
.
Example 12
4-(3-Mothoxybenzyloxy)-3-mothoxypyridlno-2-carboxylic
acid glycylamido ~;
:
Example 13
3-Ethyloxy-4-(2,2,2-trifluoroothyloxy)pyrldlne-2-carboxy- : .
lic acid glycylamide
~ '
Example 14
4-Butyloxy-3-ethyloxypyrldine-2-carboxyllc acld glycyl-
amlde
~. . -
::, :~: . ~ . .: . .
Example 15
3-Mothoxy-4-((phenoxyothyl)oxy)pyrldine-2-carboxyllc acid
glycylamide :`
: ,,:
Examplo 16 ;~ .; n-
3-Ethyloxy-4-bonzyaoxypyridino-2-carboxylie aeld glyeyl- ~ ~.
amide
25 Example 17 :~
3,6-Dimethoxypyridlno-2-carboxylic acid glyeylamide ~ jÇ;~`~
a1 3,6-Dlmethoxy-2-methylpyridino N-oxido .~ -
1.15 g (50 mmol) of ~odlum were dis~olvod ln 100 ml of
anhydrou~ methanol, and aftor that 7.4 g (40 mmol) of
, "".,",",,",,,,,,,~
: . ' ; , .
213~8~6
- 56 -
3-methoxy-2-methyl-6-nitropyridine N-oxide woro added at
20C and while ~tirring The mixture was th~n heatad to
reflux for 3 hours and, aft~r having boen cooled down,
concentrated in vacuo; tho re~iduo was ta~en up in wat-r
And this mixturo was oxtractod with d$chloromethane; the
organlc pha~e was dri-d and concentrated and the r~ldue
wa~ cry~tallized using diisopropyl ether 7 g of product
wore obtained, m p 63 to 65C
.~
b) 3,6-Dimethoxy-2-hydroxymethylpyridine
7 g (41 4 mmol) of the abov~ compound were react-d with
glacial acet$c acid/acetic anhydride in analogy with
Examplo lc) and the rosulting acetate was hydrolyzed
using 1 5 N methanolic sodium hydrox$de solution 5 6 g
were obtainod of oily product which wa~ sub~octed to
further reaction under c)
c) 3,6-Dimethoxypyridine-2-carboxylic acid
5 6 g (33 mmol) of the abovo compound and 2 4 g of
potas~ium hydroxide wer- di~solv-d in 150 ml of wat-r,
and after that 15 g (100 mmol) of potasslum p-rmanganate
w-re addod in portion~ at 60C and whilo ~tirring Th-
mangano~- dloxide which had formed wa~ thon filt-r-d off
with ~uction and wa~h-d twico with hot wator; the com-
bined wator pha~e was concentrated to 100 ml, ad~u~ted to
pH 1 with conc agu~ou~ hydrochloric acid while being
cool-d with ice, and concentrated in vacuo; the r-~idu~
was treat-d with othyl acetat- and othanol, and undi~
solv-d materlal wa~ filterod off from this mixturo, with
th- filtrate being concentrat-d in vacuo The r-sidu- wa~
cry~tallized u~ing di-thyl th-r 4 g of product were
obtained, m p 131-132C (with gas volution)
d) 3,6-Dimethoxypyridine-2-carboxylic acid (glycyl
ethyl oster) amid-
2 2 g (12 m~ol) of the abov~ carboxylic acid wero
~'' ' ''''" ''"'
~ ,::; ::':':
,
2~34866 ~:-
.
- 57 -
suspended in 300 ml of anhydrous dichloromothano, ~nd
after that 1.68 g (12 mmol) of glycine thyl ostor
hydrochloride, 3.25 ml (25 mmol) of N-~thylmorpholine,
1.62 g (12 mmol) of l-hydroxy-l~-benzotriazole and 5.2 g
(12 mmol) of N-cyclohexyl-N'-(2-morpholinoethyl)carbo-
diimide mothyl-p-toluen~ulfonato wero add~d, w~ilo
~tirring, and tho mixtur~ was then ~tirr~d at 20C for
20 hour~. Tho ~mall amount Oe undio~olved mat~rial wa~
then filtered off ~nd the filtr~te wa~ cha~-n once with
wator ~d ~hen with a ~aturatod, aquoou~ solution of Nn
bicarbonate; the organic pha~e was dr~od and concentrated
in vacuo and tho re~idue was crystallizod using diloo-
propyl ethor. 2 g of product wore obtainod, m.p. 93 to
95C.
e) The title compound wa~ obtalned by hydrolyzing 0.6 g
(2.24 mmol) of the above ethyl oster at 20C u~lng 120 mg
of lithium hydroxide in 60 ml of methucol/wator (3
After concentration had taken place in ~acuo, the re~idue
was acidified and extracted, at 20C, with tetrahydro-
furan; the filtrate wa~ concentrated in vacuo and the
yellow, re~inous re~idue wa~ cry~tallized u~ing diethyl ~ -
other. 0.14 g wero obtalned of the title compound, m.p.
130C (decomposition), which i~ ~trongly hygroccopic. The
re~idue from the in~pis~ated reaction mixturo wae then
extracted three tim-~ with 50 ml of hot aceton~ on each
occa~ion and the in~pis~ation re~idue wac cryctalllzcd
u~lng dlethyl ethor. A further 0.35 g of tho title
compound wa~ obtainod, m.p. 155C (decomposition).
Example 18
3,5-Diethoxypyridine-2-carboxylic ac$d ylycylamide
~Sxample 19
3-Methoxy-6-(3-methyl-1-butyloxy)pyridine-2-carboxylic ~ ;
acid glycylamide ~ ;
M.p.: 105 to 107C (from aqueou~ hydrochloric acid, p~ 3
to 4)
":~
213~86~
- 58 -
Exsmple 20
3-Benzyloxy-4-(3-ethyloxypropyloxy)pyridine-2-oarboxyllc
scid glycylamide
M.p. 118-120C (from acetone)
According to lH NMR, tho product contains ~pproximatsly
15 % of the 3-hydroxy dorivativo.
Example 21
3-Benzyloxy-4-hexyloxypyridi~o-2-csrboxylic acid glycyl-
M.p. i30 to 132C (from agueou~ hydrochloric acid)
Example 22 ~ ,j?~
6-(2-Butoxyethyloxy)-3-mothoxypyridlne-2-carboxyllc ac~d
glycylamide
: ,,., ~.... ~
Example 23
6-(2-Cyclohexyl)ethyl)oxy-3-methoxypyridine-2-carboxylic
acid glycylam~do ~-
M.p. from 70C (~intering from 50C, from aqueou~ hydro-
chloric ac~d, p~ 3)
Examplo 24
20 3-Ethyloxy-6-~ethylpyridino-2-carboxylic acid glycyl- ````~
amid~
: ', , .: ,', . . .
Example 25
6-Bonzyloxy-3-methoxypyr;d~ne-2-carboxylic acid glycyl-
amide
"' . ,. ~
25 Example 26 '! ' ;,.. ',.'
3-Benzyloxypyr$dine-2-carboxylic acid glycylam~do
M.p. 142-144C
Examplo 27.1
3-Methoxypyrldine-2-carboxyli¢ acid glycylamlde
amorphous ~ub~tanco, pr-pared by hydrolyzing tho 3-
methoxypyridine-2-carboXlic acid (glycyl othyl ost-r)
amlde, m.p. 141 to 142C (with ga~ ovolutlon, from
2~ 3~866
- 59 -
diothyl other).
This ethyl ester was obtalned by catalytic hydrogenation
of 4-chloro-3-methoxypyridine-2-carboxylic acid (glycyl
othyl estor~ ~mido (ooo ~x~mple 2c), whlch wa~ obtainod
from 4-chloro-3-mothoxypyrldine-2-carboxylic acid (m.p.
119 to 120C, from 4-chloro-3-mothoxy-2-mothylpyridine N-
oxide by reaction with acotic anhydride/glacial acotic
acid and subseguont oxidation of tho 2-hydroxy~ethyl-
pyridino dorivativo) (~oe Examplo 2a, b3 ~nd glyclno
othyl ootor hydrochlorido.
Example 27.2
3-Methoxypyridino-2-carboxylic acid glycylamlde
hydrochloride
a) 4-Chloro-3-methoxypyridine-2-carboxylicacid(glycyl
benzyl e~ter) ~do ~ ~-
The product was obtained, in analogy with Examplo 90a), ~ ~', ,'`''''',J~
from 4-chloro-3-methoxypyridino-2-carboxyllc acid (cf.
Example 2b), glycine benzyl ostor tosylate, N-ethylmor-
pholine, l-hydroxy-lH-benzotriazole and CMC, m.p. 57-
20 58C. ~ ~
..: , ,., , :'
b) The title compound wao obtained by hydrog-nating the
above product in mothanol/tetrahydrofuran (1:1) uoing Pd ;~
on charcoal (10 %) in a hydrogenatlon v-~el. After
removing the catalyot, and freeing from the oolv-nt, the
product was crystallized using acetone, m.p. 168C (with
foaming). ;~
' ~: ~ ' '.:,
Examplo 28
3~Ethoxypyridins-2-carboxylic acid glycylamide ~ - ~
: ' ' '
, :, .
Examplo 29 ;
3-Propyloxypyridine-2-carboxylic acid glycylamide
.
213~866
- 60 -
Example 30
3-Butyloxypyr~d~ne-2-carboxyl~c acid glycylam~de
a) 3-n-Butyloxypyr~dine-2-carboxyl~c ac~d
:"-,
6 g ~150 mmol) of N~H (60 %, in minoral oil) were added
in portions, at 20C and whll- stirrlng, to 9 8 g
(70 mmol) of 3-hydroxypyridino-2-carboxyli~ acld ln
150 ml of N,N-dimothylac-tamide Aftor 30 m~nut-s, 15 ml
(140 mmol) of butyl bromide wero added dropwiso and the
mixture was hoatod at between 95C and 125C for 2 5
hour~ After havlng boen cooled down, ~he mixture wa~
concentrated ~n vacuo, treatod w~th an aqu-ou~ solution
of Ni~ blcarbonato and oxtractod wlth dichloromethanes
aftor drying, tho residuo was puri~ied by chromatography
on sllica gel uolng ethyl acetate
The 13 g of oily product thus obtained were lntroducod
into 250 ml of a 1 5 N mothanollc ~olutlon of sodlum
hydroxide, and the m~xturo wa~ then ~tlrrod at 20C for
30 minutes and concentrated ln vacuo; the residue was
ta~en up in 200 ml of water and thi~ mixturo wae
extracted with dichloromethano and the ~quoouo phas- was
ad~u~t-d to pH 1 with conc aquoou~ hydrochlorlc acld~
conc-ntration took place ln vacuo and tho re~ldue wao
troatod wlth othyl acetato and thon wlth anhydrous
ethanol Tho r-~ultlng ~olutions wore conc~ntr~tod and
the reoidue wa~ crystallized using acetono 9 3 g were
obtained of product (m p 93-95C) which, accordlng to lH
NMR, otlll contalnod approxlmately 20 % of 3-hydroxy-
pyrldino-2-carboxylic acld
b) 2 8 g (20 mmol) of glyclne ethyl ster
hydrochlorld-, 5 2 ml (40 mmol) of N-ethylmorphollno,
2 7 g (20 mmol) of l-hydro~y-lH-benzotrlazole and 3 0 ml
(20 mmol) of N,N'-dllsopropylcarbodilmid- were added, at
20C and whlle stirrlng, to 4 g (20 mmol) of th- above
product in 200 ml of anhydrous tetrahydrofuran and 100 ml
of anhydrous acetonltrile, and the m~xture wa~ then
otlrred at 20C for 20 hours
, ,''': .
2134866
- 61 -
Aftor wor~ing up ~treatmont with Na bicarbonate oolution,
removal of precipitated diisopropyluroa), 3 5 g of oily -
product, which ~till contained N,N'-diisopropyluroa, w~ro ~ -
obtained following chromatography on oilica gol (ethyl
ac-tato/n-hoptane 1 1; then puro othyl acotato)
Thi~ mixture was introduced, at 20C and whllo ~tirring, ~ ;
into 150 ml of a l S N methanolic ~olution of sodlum
hydroxide and the total mixtur- wa~ stirred for 30 ~ ;s-
minutes
Thi~ latter mixtur- wa~ th n conoontrat-d in vacuo and
the re~idue wao ta~en up in waterS thi~ mixtur- wac ~n
turn oxtractod with 200 ml of dlchlorom than- and the
agueou~ pha~e wa~ brought to pH 1 u~ing conc aqueouc 8Cl
and concentrated in vacuo; th- residuo wao tr-at-d with
anhydrous ethanol and thon with N,N-dim thylfo-m~mide,
wlth ach extract boing f~lt-r-d to romove undis~olved
mat~rial and th-n con¢entrat-d and each of the r-oiduoo ~- m;-
being cry~talliz-d u~ing thyl acetat- 1 65 g of th~
title compound w re obtained from the ethanol phaoe ~
20 (~lightly contaminat-d according to '8 NMR, m p 170C ~ ~"X,
with gac evolution) and a furth-r 0 63 g from the
dimethylformamide phaoo (m p 182C, with ga~ ovolution)
~xa~plo 31 ~ -
3-~4-Chlorob-nzyloxy)pyridino-2-carboxylic acid glycyl-
amld-
a) 4-Chlorob-nzyl 3-(4-chlorob-nzyloxy)pyrldine-2-
carboxylate
8 4 g (60 mmol) of 3-hydroxypyridine-2-carboxyllc acid
w-r- alkylated (3 houro, 110C) with 5 2 g (approximatoly
130 Iol~ 60 ~) of ~odium hydride and 19 3 g (120 mmDl)
of 4-chlorobonzyl chlorld- ln N,N-dlm-thylacotamido in
analogy with ~xampl- 30a) Aft-r concontration in ~acuo
and xtraction wlth Na bicarbonate solution, th- r-cidu-
wao purifi-d on oillca gol u~lng h-ptano/ethyl aoetat-
(lsl), and 14 8 g of th- product were cry~tallizod from
appropriate fractiono u~ing diioopropyl eth-r, m p 92 to
213~866
.
- 62 -
94C.
b) 3-(4-Chlorobenzyloxy)pyridine-2-carboxylic acid
9.7 g (25 mmol) of the above e~ter were hydrolyzed w$th
200 ml of a 1.5 N mothanolic ~olution of eodlum hydroxide
(24 h, 20C). After working up (concontratlon, taking up
of the reslduo in wator, extraction w~th dlchlorometh~ne
and acidificatlon), 6.5 g of product were obtainod, ~.p.
144C (from water, decompo~ition).
c) 3-(4-Chlorobenzyloxy)pyrldine-2-car~oxyllc (glycyl
othyl ostor~ amide
3.2 g (12 mmol) of the above pyridine-2-carboxylic acid
were reactod, in analogy with example 17d), with 1.7 g
(12 mmol) of glycine ethyl ~ster hydrochlorido, 1.62 g
(12 mmol) of l-hydroxy-(lH)-benzotriazolo, 3.3 ml (25
mmol) of N-ethylmorpholine and 5.2 g (12 mmol) of N-
cyclohexyl-N'-(2-morpholinoethyl)carbodiimlde ~othyl-p-
toluene~ulfonat~. After working up, 3.0 g of tho product
were cry~tallized u~ing dii~opropyl ether, m.p. 106 to
108C.
.
d) Th~ title ¢ompound wa~ obtnined by hydrolyzi~g the
abov- ethyl e~t-r. 120 mg (5 mmol) of lithium hydroxide
w-re added to 0.9 g (2.5 ",ol) of the ethyl e~ter in
60 ml of methanol/wator (3:1), and the mlxtur~ wae
~tirrod at 20C for l hour. It wa~ then concentratod in
vacuo and the re~ulting agueou~ pha~- was ad~u~tod to
pH 3; the ro~ulting precipitate wa~ filtored off with
~uction, wa~hed with water and dried in vacuo. 0.52 g of
tho title compound wa~ obtained, m.p. 155 to 157C. ~-
Examplo 32
3-(3-Nethoxybenzyloxy)pyridine-2-carboxylic acid glycyl-
amlde
' ,: '', '~`;'
.,, "
~ ":,''' "
213 ~866
- 63 -
a) 3-Methoxybenzyl 3-(3-mothoxybenzyloxy)pyrldlno-2-
carboxylate
In analogy with Examplo 38a), 10 g of ths product woro
obtalned as a colorles~ oil, which was sub~octed to
further reactlon, from 8.4 g (60 mmol) of 3-hydroxy~
pyrid~na-2-carboxylic acid and 3-methoxybenzyl chlorlde
following chromatography on ~ilica gol.
i ~ . ~",
b) 3-(3-Methoxybenzyloxy)pyr$dlne-2-carboxyllc acid
10 g of the above e~tsr were hydrolyz-d in 300 ml of a ;~
1.5 N methanolic eolutlon of eodlum hydroxide. 7.5 g of
product were obtained, m.p. 147C (docomposltion, from
agueou~ hydrochlorlc acld)
c) 3-(3-Me~hoxybenzyloxy)pyrldlne-2-carboxyllc acid ;
(glycyl ethyI ester) amlde
3.2 g (12 mmol) of th~ above car~oxyllc ncid were reacted
in analogy wlth Example 31c). 3.6 g of olly crudo product
wero i~olated which, accordlng to the H NMR opectrum,
etill contalned N-ethylmorphollne. The pure cub~tanc-,
m.p. 135 to 137C (from dlleopropyl ether/ethyl acetate)
wa~ obtalned from thl~ crude product.
d) 2.1 g (6 mmol) of the above product wor~ hydrolyzed
u~lng O.4 g of NaO~ ln 60 ml of methanol. Followlng
acldlflcatlon to p~ 3, 1.6 g of th~ title compound wore
obtained aa a colorle~s cry~talline ~ub~tanco, m.p. 89 to
91C (from aguoou~ hydrochlorlc acid).
Ex~mplo 33
3-(2-Phonylethyloxy)pyridlne-2-carboxyllc acld glyayl-
amld- ~odlum salt
~,
a) 3-(2-Phenylethyloxy)pyrldine-2-carboxyllc acld
In analogy with Example 30a), 8.4 g (60 mmol) of
213~866
- 64 -
3-hydroxypyrid$ne-2-carboxylic acid w~re al~ylatad with
Na~/2-phenylethyl bromide in N,N-dim~thylacetamide. The
10 g of oily product obtained after purification by
column chromatography were hydrolyzod w~th methanoliG
eod~um hydroxide solution in analogy with Examplo 30a).
3 g of product were obtained (m.p. 145C (with fo~ming~
from acetono)) which, according to the 1~ NMR spectrum,
contained approximately 25 % of 3-hydroxypicolinic acid.
:-, :, :.- ,.
b) 3-((2-Phenylothyl)oxy)pyridin--2-carboxylic acld
(glycyl ethyl ester) ~do
, -: " ~
2.9 g of the abo~e compound wer~ roacted, ln ~nalogy w~th
Example 30b), w~th glycino ethyl estor hydrochloride, N-
ethylmorpholine, 1-hydroxy-lH-benzotriazole and N,N-
dicyclohexylcarbodiimide. After working up, the crude
product wa~ chromatographed on ailica g-1 using ethyl
acetate. 3-Hydroxypyridine-2-carboxylic acid (glyoyl
ethyl e~ter) amide was initlally eluted ao a by-product
and cry~tallized fro~ appropriate fraction~ using petro-
leum other; 1.1 g (m.p. 86 to 88C, strong fluore~conce
in W light). The product was then crystallized from
appropriate fractions using diisopropyl ethor, and 1.7 g
of the product wero obtained, m.p. 73-75C.
c) The titl- compound wa~ obtained by hydrolyzing
0.99 g (3 mmol) of the above ethyl e~ter using 100 ml of
a lN methanolic solution of sodium hydroxide. After the
mixture had been stirred at 20C for 1 hour, it wa~
conc-ntrated and the rosldue was dissolved ln a little
water~ this latter mixture was extracted wlth dichloro-
methano and the agueous pha~e was acidified, while being
cooled with i¢e, to p~ 1 using conc. agusou~ hydrochloric
a~id and then concontrated in vacuo; the residuo was
oxtracted twico with totrahydrofuran and the extracts
were concentrated~ the r-sidue was di~solvod in a little
water/tstrahydrofuran (lsl), and 252 mg (3 mmol) of
~odium bicarbonate wore added to thls ~olution. This
mlxture was concentrat-d to dryne~ and th- r-~idue was
,
213~866
- 65 -
crystallizsd using anhydrous ~thanol. 0.38 g of the title
compound was obtained as sodium ~alt, m.p. ~ 300C. ~ ~ -
Exismple 34 ~ -~
3-(4-Trifluoromethylbonzyloxy)pyridiae-2-carboxyllc acid
glycylamide
M.p. 161 to 163C (from aquoous hydrochloric acid, pH 3)
, ~ .; :.. :,-
Example 35
3-(4-(2-Propyl)benzyloxy)pyridine-2-carboxylic acid ` j ;
glycylamide ~odium ~alt
10 M.p.-108C (with decomp., from d~l~opsopyl other)
Example 36 ~;
3-(4-Fluorobenzyloxy)pyridino-2-carboxylic acid glycyl-
amide
M.p.: 135 to 138C (from agueous hydrochloric acid, pH 3
to 4)
Example 37
3-(4-(2-(4-Msthoxyphenyl)ethylamino)carbonyl)b nzyloxy)-
pyridine-2-carboxylic acid glycylamid-
M.p. 168-170C (from dichloromothan-) -
Tho following xample no~. 38-64 w-re preparod in an
analogous manner:
Exi~mple 38
3-(2,4-Dichlorobenzyloxy)pyridine-2-carboxylic acid
glycylamido , ;~
25 Examplo 39 ,
3-(3-Fluorobonzyloxy)pyridine-2-carboxylic acid glycyl- ~;~
amido
~:.... ,:; .
Exunplo 40
- 3-(3-Chlorobenzyloxy)pyridine-2-carboxyllc acid glycyl-
30 i3mide ;~
, ,, ., :: ::
, ~'' ' ~`', "' '''",
: ' ' ~ ,', ` ':
213~866
. ,
- 66 -
Example 41 .
3-(3,4-Dichlorobenzyloxy)pyridine-2-carboxylic acid
glycylamide -- .
Example 42
5 3-(3-Trifluoromotkylbenzyloxy)pyridine-2-carboxylic acid ; :::
glycylamido
Examplo 43 . ~
3-(4-Trifluoromethoxybonzyloxy)pyridine-2-carboxylic acld ::
glycylamide
Example 44
3-(3-Ethoxybenzyloxy)pyridino-2-carboxylic acid glycyl-
amido .
Example 45
3-(4-Cyanobenzyloxy)pyridino-2-carboxylic acid glycyl-
amide
Exa~plo ~6
3-((2-Pyridylmethyl)oxy)pyridine-2-carboxylic acid : :
glycylamido hydrochloride
:'.' . '..'.
Exampl- 47 ~ .
3-((3-Pyrldylmothyl)oxy)pyridine-2-carboxylic acid
glycylamido hydrochloride .. :
Examplo 48 .~:
3-((4-Pyrldylmethyl)oxy)pyridino-2-carboxylic acid ~ ~:
glycylamido hydrochlorido ~:
Examplo 49
3-((2-Thionylmethyl)oxy)pyridino-2-oarboxylic acid
glycylamldo
Exampl- 50 ~.;
3-(3,5-Dim~thoxybenzyloxy)pyridino-2-carboxylic acid
30 glycylamide -~
:. ~, ": :,
: ~ . ., :. .
... ,-
.. .
,,: , :
213~866
- 67 - ;~
,
Example 51
3-Cyclohoxyloxypyridine-2-carboxyllc acid glyeyla~ido
Example 52 ~ ~ :
3-(3-Phenylpropyloxy)pyridine-2-carboxylic acid glycyl- . -
amide
. :~
Examplo 53 .
3-(4-Phenylbutyloxy)pyridino-2-carboxylic aeid glycyl-
amide :
Examplo 54
3-(((4-Nethoxy-2-pyridyl)methyl)oxy)pyridino-2^earboxylic
acid glyeylamide :
Example 55
3-(((4-Ethoxy-2-pyridyl)mothyl)oxy)pyr~dino-2-carboxylic -
acid glyeylamide
-: -
Example 56
3-Methylthiopyridine-2-earboxylic acid glycylamide ~
~ .
Example 57
3-Ben~ylthiopyridino-2-earboxylic aeid glyeylamide : ~:;
Examplo 58 ~ .
3-(3-Chloroph-noxy)pyridine-2-earboxylie aeid glyeyl~
amide
Ex~mple 59 :~
3-~3-Methoxyphonoxy)pyridino-2-earboxylie aeld glyeyl-
amido
. ,.:.. :: .. : ::
~: . :..;.:: ~ ,:
25 Example 60 :~
3-Phenoxypyridine-2-earboxylie acid glyeylamide
:::, : .,
Example 61
3-~utyloxypyridine-2-earboxylie aeid ~-alanylamide
2134866
~ .. ..
..:..:.
- 68 -
Example 62
3-Butyloxypyridino-2-cnrboxylic acid D-alanylamido
'
Example 63
3-Benzyloxypyridine-2-carboxylic acid ~-alanyl~nide
Example 64
3-(3-Methylbutyloxy)pyridino-2-carboxyllc ac~d ~-leucyl-
amide
Example 65
4-Methoxyi~oquinoline-3-carboxylic acid glycylamide
a) Methyl 1,2-dihydro-4-hydroxy-1-oxoi~oqulnoline-3-
carboxylato, wa~ propared a~ de~cribed (~. Suzuki et ;~
al., Synthe~ie 1978, 461). -
b) Mothyl 1,2-dihydro-4-methoxy-1-oxoisoguinoline-3-
carboxylato, from a) u~i~g (trimethyl~ilyl)-
diazomethano in methanol/ac-tonitrile, m.p. 177 to ;
179C (ethyl acetate/hoptano).
c) Mothyl 1-chloro-4-mothoxyi~oquinolin--3-carboxylate,
from b) using pho~phoru~ oxychlorido, m.p. 108C
(othyl ac-tate).
~' .~ .. ,', ' ., .:
20 d) Mothyl 4-mothoxyisoguinolino-3-carboxylate, from c) ;
uoi~g hydrogon/Pd/C, m.p. 129C (from mothyl tert- ~ ~`
butyl other).
',":':,':
o) 4-Methoxyi~oguinolino-3-carboxylic acid, from d) by ~ -
hydroly~i~, m.p. 185-189C (from aguoouc hydro-
chloric acid). ``
f) 4-Mothoxyi~oquinolino-3-¢arboxylic ~cid (glycyl ~ ~;
mothyl o~tor) amido, from e) u~ing glycine mothyl
e~tor hydrochlorido, DCC, HOBT, THF and NEM, oily
~ubstance (crude product).
,:., ''.'"'",,;' ''''
213~866
- 69 -
g) The titlo compound wae obtained by hydrolyzing the
abovs ~ethyl eetor, m.p. 147C (fram agueoue hydro-
chloric acid)
Examplss 66 to 76 were obtained in an analogou~ mannsr
from ths correeponding ieoguinoline-3-carboxylia acid~ or
the 5,6,7,8-tetrahydro derivativos, reepecti~sly~
Exampls 66
4-Ethoxyisoquinoline-3-carboxylic acid glycylumlde
Examplo 67
10 4-Propyloxyieoguinoline-3-carboxylic acid glycylamido .
~xampls 68
4-(3-Nethylbutyloxy)ieoguinolin~-3-carboxylic acid
glycylamide
.,:. :,,
Example 69 - ~
4-Methoxy-5,6,7,8-tetrAhydroieoquinoline-3-carboxylic ~ :
acid glycylamide
. I ~, ~ .
Examplo 70
4-(3-Mothylbutyloxy)-5,6,7,8-tetrahydroieoguinoline-3-
carboxylic acid glycylamido
: :. . .,:. ~";.
20 Examplo 71 . ::~
4-Ethoxy-5,6,7,8-tstrahydroieoguinollno-3-carboxylic acid
01ycylamide ~,~ ;,:':,.'~.;~::'
, ~ ~, ,, . .:
Examplo 72
4-Bsnzyloxy-5,6,7,8-t~trahydroieoguinolino-3-carboxylic
25 acid glycylamide -
.
Examplo 73
4-Benzyloxyi~oguinoline-3-carboxylic acid glycylamids : ~: '~A .
''~ ~''~`"''''''.
213~866
- 70 -
Example 74
4-(3-Methoxybenzyloxy)-5,6,7,8-tetrahydroisoguinoline-3-
carboxylic acid glycylamide
Example 75
7-Bromo-4-methoxyisoguinollno-3-carboxylic acid glycyl-
amide
Example 76
7-Methoxy-4-methoxyisoguinolin~-3-carboxyllc acid
glycylamide
Example 77
3-Mothoxy-6-((3-methylbutyloxy)methyl)pyridlne-2-
carboxylic acid glycylamlde ~
.,~ ; : ~ :,,
Example 78
3-Methoxy-6-((cyclohexyloxy)methyl)pyrldln--2-carboxylic
15 acld glycylamide ;
Ex~mplo 79 ,
3-Methoxy-6-benzyloxymothylpyridine-2-carboxylic acld
glycylamide
Exampl~ 80 to 91 were propared ln accordanco wlth the
procesc-s de~crib~d in Schemes 1, 2 and 3
Example 80
5-Carboxy-3-methoxypyridlne-2-carboxyllc acld glycyl-
amlde
270 mg of tho tltle compound ~rom Example 81 wer-
hydrolyzod at 20C uslng 50 ml of lN methanollo NaO~
After 30 minute~, the mlxture was concentrated ln vacuo
and tho resldue wae dl~solved in 50 ml of water; thl~
~olutlon was oxtracted with dlethyl ethor and tho aguoous ;
phase wa~ ad~u~ted to p~ 1 wlth conc~ agueou~ hydro- ;~
chloric acid and thon concentrated ln vacuo; tho wator
was removed azeotropioally from the resldue u~lng ethyl
213~866
- 71 -
acetate and the ros~due wa~ then troated with othanol and
thi~ mixture wa~ concentrated; the r~iduo was cry~tal-
lized u~ing d~ethyl othor. 230 mg ware obtainod of the
titlo compound, m.p. 173C (with gas evolution, sintering
at 170C), whlch, according to the lH NMR sp-ctrum, ~till
contain~ approximatoly 20 ~ of an impurity.
.
The title compound wa~ al~o obtained by hydrolyzing
0.45 g of 5-((-1-butyloxy)carbonyl)-3-methoxypyridin--2-
carboxylic acid N-(((1-butyloxy)carbonyl)me~hyl)amido,
m.p. 80-81C (from petroloum ether~, using 50 ml of 1.5 N ~;~
methanolic NaO~. 0.23 g was obtainod of tho titlo com-
pound, m.p. 198-200~ (from an othanolic pha~o w~o~o
re~idue, following concontration, wa~ crystallizod ucing
diethyl ethor). According to tho '~ NMR ~poctrum and MS,
the aub~tance contain~ approximately 5-10 % of it~ othyl
e~tor.
The isomeric 2-carboxy-3-methoxypyridine-5-carboxylic
acid glycylamido wa~ obtalned in an nnalogou~ mann-r,
m.p. from 65C (~intering from 45C, with foaming, from
20 diethyl othor, ~ygroscopic). -
'' ; ': ~ :: :
Examplo 81 ~ ~ `
5-M-thoxycarbonyl-3-methoxypyridine-2-carboxylic acid ;~
glycylamid~
a) 5-Nethoxycarbonylpyridine-2-cnrboxylic acld l-ox~de
12 g (60 mmol) of dimothyl pyridine-2,5-dicarboxylato
woro ~uspsnded in 30 ml of glacial acotic acid, and aft-r -~ ;
that 13 ml of hydrogen peroxid- ~35 %) wer- added at 20C
and whil- ctirring. Tho mixture wa~ than hoat-d to 100C
(intornal temp-rature), whilo ~tirring, with a clear
solution boing for~od at 50C. Aftor the mixturo had b-en
~tirred at 100C for g0 minut-s, it wae allowed to cool
down to 20C and the cry~talline precipitate was filt~r-d
off w$th ~uction and wa~hed with wator; After drying,
7.5 g of product wero obtained, m.p. 160C (decomp.).
. .
, . ,::
213~866
- 72 -
b) Dimethyl 3-chloropyridin~-2,5-dicarboxylate
17 ml of thionyl chlorido, 35 ~1 of anhydrous chloroform
and 1 5 ml of N,N-dim-thylformamide wore hoated to 60C,
whilo stirring, and 7 5 g of tho above product w~ro then
added in portions at thi~ tomperatur~ The mixturo was
thon stirred at 60C for a further 60 minuto~, and, after
cooling, tho solvent and exeo88 rsagsnt were di~tllled
off in vacuo; dichloromethano wa~ added to the re~ldue,
and the N,N-dimethylform~m~de x ~Cl complex wns filtor-d
off with ~uetion and wa~hed w$th dichloromethuno
Approximately 15 ml of triethylamin- and 10 ml of
methanol wore addod, whil- ¢ooling, to the mothor liguor
and the mixturo was stirrod for 30 minute~ After eoneon-
trating by evaporation in vacuo, tho residuo was dia-
solvod ~n 50 ml of wator and this mixture wAs thenextracted 3 x with dichloromethane; th- organie phase was
dried and concentr~tod, and tho rssidue wa~ chr o to-
graphed on silica g-l u~ing n-heptano and n-heptane ethyl
acetate ~3 1) 5 3 g of product were crystallizsd, using
potroleum ether, from appropriato fractlon~, m~p 36 to
38C
c) 3-Mothoxypyridln--2,5-dlcarboxylic acid
53 g (0 231 mol) of tho abovo dloster wore di~solved in
500 ml of metha~ol, and after that 150 ml (0 81 1) of
eodlum methoxide solutlon (30 % ln mothanol) were added,
at 20C and whilo ~tirring, whereupon tho temporaturo
roeo to 30C The mixturo was heatod undor r-flux for
4 5 houre, 300 ml of wator wer~ add-d at 20C, and tho
mlxturo was thon ~tirrod at 35C for 30 minutes The
oxces~ mothanol w~s distillod off ln vacuo and the
aquoous phase was ad~u~ted to p~ 2, while eooling, with
half-concentrated aqueous hydrochloric ac~ds the color-
le88 crystalline product wa~ filtered off with euctlon
and dried 49 g were obtalned, m p 185C (gas vo-
lution); 2S5C (decomp )
~ .
',,.,,, , ., ,/" ,,",."~.. ... ..... .... ...
213~866
.
- 73 -
d~ Dimothyl 3-methoxypyr~dino-2,5-dicarboxylato, cf.
Exa~ple 90a)
o) S-Mothoxycarbonyl-3-msthoxypyrldin--2-carboxylic
~cid -.
The compound was obtain-d, as a mixture with tho ~someric
monomethyl sster (Gf. Examplo 90a)), fro~ 3.4 g (15 ol)
of the above dia~ter by hydroly~i~ with a d~lute olutio~
of methanolic sodium hydroxide (0.54 g of NaOH (13.5
mmol)). 1.8 g of monoo~ter m~xture, m.p. 152C, w~r-
obtatned in addltion to 0.8 g of unreaoted die~t-r.
::, .~, ;.:~..,
f) 5-Methoxycarbonyl-3-methoxypyridlne-2-carboxylic
acid (glycyl benzyl ester) amido ;~
1.8 g of tho above mixturo wore cond~nsod, in analogy
with Examplo 90b), wlth 2.9 g (8.6 mmol) of glyclne
benzyl eeter tosylat- in tho pre~once of N--thylmor-
pholine, l-hydroxy-l~-bonzotriazole and CMC. A~tor
working up, 2.3 g of oily mixture wore chromatogra~hed on
~ilica gel u~ing dichloromothane ~in the pres-nco of ~p
to 2 % methanol). 0.82 g of product wa~ obt~lnod, m.p.
108C. 0.6 g of th- olly l~omor was al~o lsolatod.
g) The tltle compound wa~ obtained by di~solvlng 65Q mg
of tho above benzyl ~ster ln 100 ml of totrahydrofuran/
methanol (1:1) and hydrogenatlng it u~ing Pd/C ~n a
hydrogenation vessel. Onco the cataly~t had b~en filtorod
off wlth ~uctlo~, the filtrato was concontrated and tho
reslduo was cry~tallizod u~lng diothyl othor. 380 mg woro
obtal~ed of a colorles~ crystalllne product, m.p. 158 to
160C. ;;
Ex~mple 82
5-(3-Pentyloxy)carbonyl-3-methoxypyrldine-2-carboxyllc
acld glycylamlde ;~;
.~ . ., ~.Z~,`.
, . . ,:.,,: ,
.. . .... .
2134 86G
- 74 -
Example 83
5-Cyclohexyloxycarbonyl-3-methoxypyridino-2-carboxyllc
acid glycyl~mide
Examplo 84
5-(n-Butylam$nocarbonyl)-3-methoxypyridino-2-carboxylic
acid glycylamide
Example 85 -
5-(2-Msthyl-2-butylaminocarbonyl)-3-methoxypyrid~ne-2-
carboxylic acid glycylamide
10 Example 86 - ;~
5-(Cyclohexylaminocarbonyl)-3-methoxypyridine-2-car- , -~
boxylic acid glycylamide
a) 5-(Cyclohexylaminocarbonyl)-3-methoxypyridine-2-car-
boxylic acid
Tho product was obtained, ln analogy with Ex mple 90b),
from 5-carboxy-3-m-thoxypyridin--2-carboxylic acid and
cyclohexylamine,
m.p. 155C (sintering at 80C, from aqu-ouc hydrochloric
acid).
:..~ . :: .. :..0 b) 5-~Cyclohexylaminocarbonyl)-3-methoxypyrldino-2-
carboxyllc acld ~glycyl ethyl e~ter) amlde
The product wa~ obtaln-d, ln analogy with Example 90c),
from th- above compound, m.p. 187 to 188C (from dl-thyl
eth~r)
c) The colorle~ crystalllne title compound wa~
obtalned by hydrolyzlng the abovo compound ln analogy
wlth Example 90c),
m.p. 110C (wlth foamlng, a desp-blac~ coloratlon at
240C).
Examplo 87
5-(Cyclohexylaminocarbonyl)-3-ethyloxypyridin--2-car-
boxylic acid glycylamido
- :,
.,~; ,
' . . ~: ~ ..,
213~866
- 75 -
Exampl~ 88
5-((2-Phonylothyl)aminocarbonyl)-3-mothoxypyridine-2~
carboxylic acid glycylamido --
Examplo 89
5~ )-Dehydroabietylaminoearbonyl)-3-methoxypyrldine-2-
carboxylic acid glycyl~mide
a) 5-(~)-Dohydroabi~tylamlnocarbonyl)-3-methoxy- ;- ~;
pyridine-2-carboxyllc acid
The resinou~ product wa~ obta~nod, in analogy with
Examplo 90n), fro~ 5-carboxy-3-mothoxypyridin--2-oar-
boxylic ~cid and ~I)-dehydroabiotyl~mine.
b) 5-((+)-Dehydroabi-tylaminocarbonyl)-3-mothoxy-
pyridino-2-carboxyllc acld (glycyl thyl e~ter)
amide
The product WaB obtained in analogy with Exa~ple 90c),
from the above compound, m.p. from 150C with foaming,
~intering at 120C, from di-thyl ethor).
c) Tho tltlo compound wa~ obtalnod by h~drolyzin~ th-
above compound ln analogy wlth ~xampl- 90 d),
m.p. 215C (sintoring at 150C, from aquoous hydrochloric
acld).
" ~"r~
Example 90
5-((2-(4-Fluoroph-nyl)othyl)aminocarbonyl)-3-methoxy- ; ~ ;~
pyrldine-2-carboxylic acid glycyl~mid-
a) Mothyl 5-carboxy-3-mothoxypyridin--2-carboxylate
10 g (50.7 ~mol) of the 3-mothoxypyridine-2,5-dicar-
boxyllc acid (Example 81c) were ~uspended ln 150 ml of ;
anhydrou~ m-thanol, and after that 2 ml of concentrat-d
~ulfurlc acid wer- add-d and the mlxture was heatod under
reflux for 3 hour~. Half of tho methanol was then
di~tilled off in vacuo and the residue was lntroduced ~;
, ~ , . .;: ,:
213486~
- 76 -
into 400 ml of ic8 water; the cry~talline re~idue wa~
filtered off with suction and wa~hed w~th water; the
residue wao dissolvod in 150 ml of a ~aturatod, aqueous
~olution of Na bicarbonate and this mixtur~ was extracted
twice with 80 ml of dichloromethane on each occa~ion; the
bicarbonate phaso was adju~ted to p~ 1, while cooling,
with half-concentrated aqueous hydrochloric acid and the
precipitat0d product was flltered o~f with ouction and
driod. 5 g of colorl-ss, crystalline ~ub~tance wore
obtained, m.p. 196 to 197C. 1.7 g of dimethyl e~ter,
m.p. 53 to 55C (from petrol-um sther), wero obtainod
from the dichloromethane phaoo.
b) 5-(((2-(4-Fluorophonyl)othyl)amino)carbonyl)-3-
methoxypyridine-2-carboxylic ~cid
3.2 g of methyl 5-carboxy-3-methoxypyridine-2-oarboxylate
were eu~pend~d in 300 ml of anhydrous dichloromethano,
and after that 2.0 ml (15 ~mol) of 2-(4-fluorophenyl)-
ethylamine, 1.95 ml (15 mmol) of N-ethylmorphollne, 2.2 g
(16.5 m~ol) of 1-hydroxy-1~-benzotriazole and 6.35 g
(15 mmol) of N-cyclohexyl-N'-(2-morpholinoethyl)carbodi-
imide methyl-p-tolueneoulfonato (CMC) were added sequen-
tially, at 20C and whilo otirring, and the mixture wao
otlrrod for 24 hours. Ths undis~olvod matorial wa~ then
filterod off and tho organic pha~e was extracted, in each
ca~e 3 x, with an aqueou~ solution of Na ~icarbonate,
with lN aqueous hydrochloric acid and w$th water, and the
organic pha~e was dried and concentrated. 3.7 g were
obtainod of methyl oster, m.p. 168 to 169C, which was
introduoed into 150 ml of 1.5N methanolic NaO~. After
30 minuteo, tho mlxture was concontratod and d~ssolved in
100 ml of wator, and this mlxture wa~ adjustod to p~ 1
with ~onc. aqueouo hydrochloric acid; the crystalline
precipitato was filterod off with suction, washed with
water and dried. 3.4 g of product wero obtained, m.p.
110C (with foaming, ~intering at 75~
213~86~
- 77 -
c) 5-(((2-(4-Fluorophenyl)othyl)amino)carbonyl)-3-
mothoxypyridine-2-carboxylic ncid (glycyl ~thyl
eeter) amide
In analogy with Exa~ple 90a), 3.2 g (10 mmol) of the
above compound wero reactod with 1.4 g (10 m~ol) of
glycine othyl eeter hydrochloride, N-othylmorpholine,
1-hydroxy-lH-benzotrlAzole and CMC. Following analogous
working up, 2.8 g of the colorless cryetallin~ product
were cryatallized using diisopropyl othsr, m.p. 170 to
171C.
d) The titlo compound wae obtained by hydrolyeing 1.0 g
of the above glycine othyl estor, at 20C, ln 1.5N
methanolic NaOH. 0.95 g of product, m.p. 206C (with
foaming) crystallizoe from aqueoue medium at p~ 3.
Example 91
5-((2-(4-Methoxyphenyl)othyl)aminocarbonyl)-3-ethyloxy-
pyridine-2-carboxylic acid glycylamide
.
Examples 92 to 105 were obtained from the corre~pondingly
eubst~tuted pyridine-2-carboxylic acid derivativee of the
formula II and glycine ethyl e~tor hydrochloride and with
~ubeequent hydrolyeis of the glycine othyl ester com-
pound-.
Example 92
5-Chloro-3-othyloxypyridine-2-carboxyl~c acid glycyl-
25 amldo ~;
.: .: . ;: ' ~`Example 93
5-Chloro-3-mothyloxypyridino-2-carboxylic acid glycyl-
amido
Example 94
30 5-Cyclohoxyloxymothyl-3-methoxypyridine-2-carboxylic acid -~
glycylamide
' ' ' '
213~86~
- 78 -
Example 95
5-(3-Methylbutyl)oxymothyl-3-m~thoxypyridlno-2-carboxyllc
acid glycylamido
Example 96
5-Benzyloxymethyl-3-ethyloxypyrtd$ne-2-carboxylic aoid
glycylamide
Example 97 .
3-((Cyclohoxyl)mothyloxy)pyrid~ne-2-carboxylic acid
glycylamido ~ :. ;.
:
Example 98
3-((2-Cyclohexyl)othyloxy)pyridine-2-carboxylic acid
glycylamide ~-~
Example 99
3-((3-Cyclohexyl)propyloxy)pyridino-2-cnrboxylic acid
glycylamide
Example 100
3-(3-Methylbutyloxy)pyridino-2-carboxylic ac~d glycyl-
amido
Example 101
3-~exyloxypyrldine-2-carboxylic acid glycylamide
Example 102
3-(4-Ethylbonzyloxy)pyridine-2-carboxylic acid glycyl- . :
amid~
,:
Example 103
3-(4-Propylbonzyloxy~pyridine-2-ca~boxylic acid glycyl-
amide
~, '' ',
Example 104
3-(4-~utylbenzyloxy)pyridine-2-carboxylic acid glycyl-
amide
; ..
213~866
- 79 -
Example 105
3-(4-tert-Butylbonzyloxy)pyridine-2-carboxyl~c ac~d
glycylamide
Examplee 106 to 188 wore prepared in ~alogy with
Examplee 80 to 91.
Example 106
5-Methoxycarbonyl-3-(2-methyl-1-propyloxy)pyridine-2-
carboxylic acid glycyl~mide
a) 3-(2-Methyl-1-propyloxy)pyridine-2,5-dicarboxylic
acid
In analogy with Example 81c), 3.5 g (146 mmol) of sodium
wero di~solved in 350 ml of 2-methyl-1-propanol (i80-
butyl alcohol), and a~ter that 13.7 g (55 mmol) of
3-chloropyridine-2-carboxylic acid ~thyl eet~r 5-carbox-
ylic acid methyl ostor (pr~pared in analogy with kxample81b)) wore addod at 20C and while stirsing. The mixturo
was thon etirred at 80C for 90 minute~ and, aft~r
cooling, concentrat~d in ~acuo; tho reeiduo wae takon up
in 200 ml of 1 N methanolic NaOH and this mixturo wae
stirred at 20C. Aftor 15 minutes, tho eolution bocamo
cloudy. Water wae added untll a clear solution wae
obtainod and thie wae etirrod for 1 hour and thon concen-
trated in vacuo; the aqueoue eolution wae acldifiod with
agueous hydrochloric acid and tho cry~tallino product was
$iltered off with euction, waahed and dri~d, and 10.6 g
,
of dicarboxylic acid were obtained, m.p. 192C (decomp.).
b) Dimethyl 3-(2-met~yl-1-propylo~y)pyrld~n~-2,5-dicar-
boxylate
The oily product wae obtained from the abovo dicarboxylic
acid undor eeterification conditions (methanol/sulfuric
acid) and after working up (washing w~th water and
extracting with ethyl acetate). ~; -
. , ,, :
~,""i,.. '~,fi ,~ "", ,,,,,"
213~66
- 80 -
c) S-Methoxycarbonyl-3-(2-methyl-1-propyloxy)pyridino-
2-carboxylic acid (glycyl banzyl oeter) anido
0.48 g (12 mmol) of NaO~, diseol~od in 50 ml of methanol,
wae added to 3.2 g (12 mmol) of the abovo dioeter ~n
25 ml of mothanol, and tho mixturo wae etirred at 65C
for 90 minutee. The mixture was thon acldifi-d, whilo
being coolod, with diluto agueoue hydrochloric acid and
froed from methanol in vacuo. 2.5 g (10 mmol) of tho
monoester mixture thus obtainod woro ~tirrod, ln analogy
with Examplo 90b), in 250 ~1 of dlchloro~e~hano, ~t 20C
for 24 hour~, together with 3.4 g (10 ~m~l) of glyc~ne
benzyl e~ter toeylate, 1.4 g (lO ,,ol) of l-hydroxy-(l~)-
benzotriazole, 2.6 ml ~20 mmol) of N-sthylmorpholine and
4.3 g (10 mmol) of CMC.
The undieeol~ed materlal was thon iltered off with
euction and tho filtrate was extracted with an agueous
solution of Na bicarbonate, with diluto hydroahloric acid
and with water; the organic phaee was drlod and concen-
tratsd and tho residue was chromatographod on eilica gal
uslng n-heptane/ethyl acetate (1:1). 0.8 g of aolorlese
product wae obtained from appropriate fra¢tiono, m.p. 103
to 105C. 1.1 g of the ieomeric roeinoue product were
al~o obtained.
d) Tho titlo compound wae obtained by dieeolving 0.7 g
of the above compound in 100 ml of totrahydrofuran/
mothanol (lsl) and hydrogenating it for 2 hour~ ueing Pd
on charcoal (10 %) in a hydrogenation ~oeeol. Tho cata-
lyet wae then filtor~d off with euction and the flltrate
: : . .
wae concentrated; tho reeidue wae cryetallized using
diieopropylether and 0.45 g wae obtainod of the titlo
compound, j~
m.p. approximatoly 70C (with foaming).
Tho isomeric compound wae obtainod in an analogoue ;~
manner, m.p. approximately 60C (with foaming, from
diieopropyl ether).
'., '':" -,,'' ~-
~ ''' `'`''
2131866
- 81 -
Example 107
5-Ethoxycarbonyl-3-(2-mothyl-1-propyloxy)pyrid~ 2-
carboxylic acid glycylamido
Examplo 108
5 5-Mothoxycarbonyl-3-(3-mothyl-1-butyloxy)pyridino-2-
carboxylic acid glycylamido
Examplo 109
5-Ethoxycarbonyl-3-ethoxypyridine-2-carboxyllc ~eid
glycylamide
10 Example 110
5-Ethoxycarbonyl-3-(1-propyloxy)pyridino-2-carboxyl~c
acid glyeylamido : ~:
Examplo 111 -
5-Ethoxycarbollyl-3-(2-propyloxy)pyridlne-2-carboxyl$c :~
15 acid glycylamido
Example 112 ~.:
3-Benzyloxy-5-othoxycarbonylpyridine-2-carboxylle acid ~ ~ :
glycylamido
Example 113
20 3- (4-Chlorob~nzyloxy) -5-ethoxycarbonylpyridine-2-
earboxylie aeid glycylamido
Examplo 114
5-E thoxyearbonyl-3- (4-fluorobenzyloxy) pyridine-2- -: :
earboxylie acid glycylamido .
25 Examplo 115
5-Ethoxyearbonyl-3- (4- (tr~ fluoromethyl) - :~
benzyloxy)pyridino-2-carboxylic aeid glycylamldo ;
Examplo 116 . .
5-Ethoxyearbonyl-3- (4- (trifluoromethoxy)be~zyloxy) - -~: :
pyridino-2-carboxylic acid glycyl~ide ~-
''''"
2131866
- 82 -
Example 117
5-Ethoxycarbonyl-3-(4-(2-propyl~benzyloxy)pyridino-2-
carboxylic acid glycylamide
Example 118
3-(4-Ethoxybenzyloxy)-5-ethoxycarbonylpyridine-2-car-
boxylic acid glycyla~ide
Example 119
5-~thoxycarbonyl-3-(3,4-dimethoxybenzyloxy)pyridi~-2-
carboxylic ac$d glycylamide
Exa~ple 120
5-Ethoxycarbonyl-3-(2-(4-fluorophenyl)ethyloxy)pyridine-
2-carboxylic acid glycyl~mide
Example 121 ~ ~;
5-Ethoxycarbonyl-3-(2,2,2-trifluoroothyloxy)pyridine-2-
carboxylic acid glycylamide
Example 122 -
3-Cyclohexyloxy-5-ethoxycarbonylpyridine-2-carboxylic
acid glycylamide ::
Example 123
5-Ethoxycarbonyl-3-(naphthyl-2-methyloxy)pyridine-2-
carboxylic ~cid glycylamido
Exampls 124 .;
5-Ethoxycarbonyl-3-(naphthyl-1-mothyloxy)pyridine-2-
carboxylic acid glycylamide
Example 125
5-Carboxy-3-(2-methyl-1-propyloxy)pyridine-2-carboxylic
acid glycylamide
The title compound wa~ obtained by hydrolyzing 0.3 g of -:
th~ title compound from Example 106, at 20~, in 50 ml of
a lN methanolic solution of ~odium hydroxide. After
1 hour, the ~ixture was concentrated in ~acuo and ~ -~
:
2131~66
"
- 83 -
extracted with diethyl other; the agueous pha~o wae
acidified, while bei~g coolod, with agueous hydroohlorlc
acid; the aqueou~ phass was concentrated and then fre~d
from water azeotropically using ethyl acetato and the
residuo was treatod with acetone; the solution W~8
concentrated and the residue was ery~talliz~d uoing
petroleum ethsr. 0.27 g of product was obtained, m.p.
80C (with foaming).
Exampla 126
5-Carboxy-3-(3-methyl-1-butyloxy)pyridine-2-earboxylic
acid glycylamide
Example 127
5-Carboxy-3-ethoxypyridine-2-carboxylic aeid glycylamide ~ -
Example 128 ;;~
5-Carboxy-3-propyloxypyridine-2-earboxylic acid glyeyl-
amide `~
Example 129 ; -
5-Carboxy-3-(2-propyloxy)pyridine-2-earboxylie acid
glycylamide
Examplo 130
3-Bonzyloxy-5-carboxypyridine-2-carboxylie aeid glyeyl-
amide
. :.~ '.
Example 131 ; -
5-Carboxy-3-(4-ehlorobenzyloxy)-2-carboxylicacidglycyl-
25 amide ;
Example 132
5-Car~oxy-3-(4-fluorobenzyloxy)pyridine-2-carboxylie ac~d
glyeylamide
Example 133
5-Carboxy-3-((4-trifluoromethyl)benzyloxy)pyridino-2-
carboxylie acid glyeylamide
213~8~6
:
' Bxample 134
:-~ 5^Carboxy-3-((4-trifluoromethoxy)banzyloxy)pyrldlne-2-
' carboxylic acid glycylamide
,~i
1 Examplq 135 -
1 5 5-Carbo~y-3-(4-(2-propyl)benzy~loxy)pyridi~-2-carboxylic
;~ acid glycylamide
~xample 136 :-
5-Car~oxy-3-(4-ethoxybenzyloxy)pyridine-2-carboxyllc acid `~
glycylamids :~
:: :
Example 137
5-Carboxy-3-(3,4-dimethoxybanzyloxy)pyridine-2-carboxylic E ;~
acid glycylamide
Example 138 ~`
~ . 5-Carboxy-3-(2-(4-fluorophe~yl)sthyloxy) pyr~dine-2- :~
: 15 carboxylic acid glycylamide ~. -
~ ' '. ~ ' '~,
~xample 139
5-Carboxy-3-(2,2,2-trifluoroethyloxy)pysidi~e-2-: ~'
carboxylic acid gylcylamlde
: ' '~
Example 140
20 5-Carboxy-3-cyclohexyloxypyridine-2-carboxylic a~ld ~` ;
glycylamide
~, . .
~x~mple 141
5-Carboxy-3-(naphthyll2-~ethyloxy)pyridine-2-carboxylic
acid glycylamide
Example 142
S-Carboxy-3-(naphthyl-~-methyloxy)pyridin2-2-carboxylic
acid glycyl~mide
Example 143
5-(3-Pentyloxy)carbonyl-3-(2-m~thyl-1-propyloxy)pyridine~
2-carboxylic acid glycylamid~
. :
;~ .
21~866
- 85 -
Example 144
5-(3-Pentyloxy)carbonyl-3-(3-me~hyl-1-butyloxy)pyridlne-
2-carboxylic aeid glycylamide
Example 145
3-Ethoxypyridine-5-(3-pentyloxy)earbonyl-2-e~rboxylic
acid glyeylamide --
Example 146
5-(3-Psntyloxy)earbonyl-3-propyloxypyridine-2-earboxylie
acid glyeylamide
Example 147
5-(3-Pentyloxy)carbonyl-3-(2-propyloxy)pyridine-2- :
carboxylie acid glycylamide
Example 148
3-~enzyloxy-5-(3-pentyloxy)earbonylpyridino-2-carboxylie
acid glyeylamide
Example 149
3-(4-Chlorobenzyloxy)-5-(3-pentyloxy)earbonylpyridine-2- -
carboxylie acid glycylamide
, ~
Example 150 :
3-(4-Fluorobenzyloxy)-5-(3-pentyloxy)carbonylpyridine-2-
earboxylie acid glycylamlde
Example 151
5-(3-Pentyloxy)earbonyl-3-((4-trifluorclmethyl)benzyloxy)-
pyridine-2-earboxylic acid glycylamide
Example 152
5-(3-Pentyloxy)carbonyl-3-((4-trifluoromethoxy)benzyl-
oxy)pyridine-2-earboxylie aeicl glyeylamid~
Example 153
5-(3-Pentyloxy)earbonyl-3-(4-(2-propyl)benzyloxy)-
pyridine-2-earboxylic aeid glycylamide
~; ~
213~866
- 86 -
Example 154
3-(4-Ethoxybsnzyloxy)-5-(3-pontyloxy)earbo~ylpyridino-2- ~ -
carboxylie acid glyeylamido
Examplo lS5
S 3-(3,4-Dimethoxybenzyloxy)-5-(3-pentyloxy)carbonyl-
pyridino-2-carboxylic acid glycyla~ido
Example 156
3-(2-(4-Fluorophenyl)-thyloxy)-5-(3-p~ntyloxy)carbo~yl-
pyrldine-2-carboxylic aeid glyeylamide
Exæmplo 157
5-(3-Pentyloxy)earbonyl-3-(2,2,2-trifluoroothyloxy)-
pyrldino-2-earboxylle aeld glyeylamido ~ -
Examplo 158
3-Cyelohoxyloxy-5-(3-pentyloxy)earbonylpyridine-2-
15 earboxylie aeid glyeylamide ;~
Exa~ple 159
3-(Naphthyl-2-m-thyloxy)-5-(3-pontyloxy)earbonylpyr~dino-
2-earboxylle aeid glyeylamido
Exampl- 160
3-(Naphthyl-1-m-thyloxy)-5-(3-pentyloxy)carbonylpyrldine-
2-earboxylie aeid glycylamido
Example 161
5-(4-~optyloxy)earbonyl-3-(2-methyl-1-propyloxy)pyridino-
,
2-earboxyllc aeld glycylfimldo
Examplo 162
5-(4-Hoptyloxy)carbonyl-3-othoxypyridine-2-earboxylic `~
acid glycyla~ide
Example 163
3-Benzyloxy-5-(4-heptyloxy)carbonylpyridino-2-carboxylie
30 aeid glyoylamido ;-
~, ..
.
2134~66
- 87 -
Example 164
3-(4-Chlorobenzyloxy)-5-(4-heptyloxy)carbonylpyridi~-2-
carboxylic ac~d glycylamide
Examplo 165
3-(4-Fluorobenzyloxy)-5-(4-heptyloxy)pyrid~ne-2-
carboxylic acid glycylamide
Example 166
5-(4-Heptyloxy)carbonyl-3-(4-(2-propyl)be~zyloxy)-
pyridine-2-carboxyliG acid glycylamido
Examplo 167
3-(2-Methyl-1-propyloxy)-5-(5-nonyloxy)carbonylpyridine- - -
2-carboxylic acid glycyl~m$de
. .
Example 168 ~ ,
3-Benzyloxy-5-(5-nonyloxy)carbonyl)pyridino-2-carboxylic
ac~d glycylamide
:,
Example 169
3-(4-Fluorobenzyloxy)-5-(5-nonyloxy)carbonylpyr~dine-2- . ~:
carboxylic acid glycylamide
Example 170
5-(5-Nonyloxy)carbonyl-3-(4-(2-propyl)benzyloxy)pyridi~e-
2-carboxylic acid glycylamide
Example 171
5-Geranyloxycarbonyl-3-othoxypyridine-2-aarboxylic acid
glycylamido
25 Ex~mplo 172 -
3-Benzyloxy-5-(geranyloxycarbonyl)pyridin~-2-carboxylic : ;~:
acid glycylamide ~: :
Examplo 173
3-(4-Chlorobenzyloxy)-5-(geranyloxycarbonyl)pyridi~e-2-
carboxylic acid glycylamide
. ' ~' .
,: '''
~`~ <:
21~866
- 88 -
Example 174
3-(4-Fluorobenzyloxy)-5-(goranyloxyc~rbonyl)pyrid$ne-2-
carboxylic acid glycylamide
Example 175
5-Geranyloxycarbonyl-3-(2-propyloxy)pyrid$ne-2-carboxylic
acid glycylamlde
Example 176
5-Farnosyloxycarbonyl-3-(2-propyloxy)pyr$din~-2-car-
boxylic acid glycylamide
10 Example 177 -~
3-Bonzyloxy-5-(farne~yloxycarbonyl)pyridine-2-carboxylic
acld glycylamlde ~ :~
Example 178 ::
5-Farnesyloxycarbonyl-3-(4-fluorobenzyloxy)pyr$dlno-2- ~;
carboxylic acid glycylamide
Example 179 . ~ :
5-Farn-~yloxycarbonyl-3-othoxypyridine-2-carboxylic acid
glycylamide
Examplo 180
3-Methoxy-5-(retinyloxycarbonyl)pyridine-2-carboxylic
acid glycylamide
~., .
Example 181
3-Ethoxy-5-(rotinyloxycarbonyl)pyridine-2-carboxylic acid
glycylamlde
Example 182
3-(2-Propyloxy)-5-(ret~nyloxycarbonyl)pyrldine-2-
carboxylic acid glycylamide
. . ~
213~86G
- 8~ -
Example 183
3-Benzyloxy-5-(retinyloxycarbonyl)pyridine-2-carboxylic
acid glycylamide
Example 184
3-(4-Fluorobenzyloxy)-5-(retinyloxycarbonyl)pyridine-2-
carboxylic acid glycylamide
Example 185
3-(3-Methoxybenzyloxy)-5-(ret~nyloxycarbonyl)pyridine-2-
carboxylic acid glycylamide
Example 186
5-Benzyloxycarbonyl-3-(4-(2-propyloxy)benzyloxy)pyridine-
2-carboxyllc acid glycylamide .
Example 187 -: ;
5-Benzyloxycarbonyl-3-(4-fluorobenzyloxy)pyridine-2-car-
boxylic acid glycylamide
Example 188
5-Butyloxycarbonyl-3-benzyloxypyridine-2-carboxyllc acid
glycylamide
Example 189
5-(((4-Ethoxyphenyl)amino)carbonyl)-3-methoxypyridine-2-
carboxylic acid glycylamid~, analogouely to Exa~ple 191
Example 190
5-(((4-Ethoxyphenyl)amino)carbonyl)-3-benzyloxypyridine-
2-carboxylic acid glycylamide
~xamplo 191
5-(((4-(1-Butyloxy)phonyl)amino)carbonyl)-3-methoxy-
pyridine-2-carboxylic acid glycylamide ~ :
a) Methyl 5-(((4-(1-butyloxy)phenyl)amino)carbonyl)-3- . .
methoxypyridine-2-carboxylat~
' : '
213486~
- so -
3.2 g (15 mmol) of methyl S-carboxy-3-methoxypyrid~ne-2-
carboxylate (cf. Example 90a)) were reacted, ln nnalogy
with Example 90b), with 2.5 g (15 mmol) of 4-n-butoxy-
anillne and the reagents do~cribed in that example. 3.9 g
of product were crystallized using diethyl ~th~r (m.p.
138 to 141C.
~ .
b) 5-(((4-(1-Butyloxy)phenyl)amino)car~o~yl)-3-methoxy-
pyridino-2-carboxylic ac$d ~-~
3.2 g of the above e~ter were hydrolyzed at 20C ucing
100 ml of 1.5 N mathanolic aodium hydroxide oolution.
2.7 g of product were obta~ned from agueous hydrochloric
acid, m.p. 128 to 130C, sintering from 120C.
c) 5-(((4-(1-Butyloxy)phenyl)amino)c rbonyl)-3-~othoxy-
pyridine-2-carboxylic acid N-~(ethoxyc~rbonyl)-
methyl) am~de
2.7 g (7.8 mmol) of the a~ovo pyridine-2-carboxylic acid
were stirred in 500 ml of anhydroue dichloromethano, at
20C for 24 h, togather with 1.1 g (7.8 mmol) of glycine
ethyl oster hydrochlorido, 3.0 ml (23.4 ~^1) of N-
ethylmorpholine, 1.2 g (8.6 m~ol) of 1-hydroxy-1~-bonzo-
trlazolQ and 3.3 g (7.8 mmol) of CMC.
Tho undissolved material was then filtored off and tho
organic pha~e was extracted ~equentially with 200 ml each
of water, aquoous Na bicarbonato ~olution, lN aqueous
hydrochloric acid and water, dried over Mg sulfate and
concentrated in vacuo; the residue wa~ cry~tallized ueing
diethyl ethor. 2.4 g of product were obtained, m.p. 193-
195C.
d) The titlo compound was obtained by hydrolyzing 1.0 g
of the above glycine oster, at 20C, in 100 ml of a 1.5 N
methanolic solution of sodium hydroxide. After 30 m~n,
the mixturo wa~ concontrate~ in vacuo ~nd the roEidue wa~
diesolved i~ water; this solution was oxtracted with
2~3~86~
:'``
- 91 -
diethyl ether and the aqueous solut~on ad~u~tod to p~ 3
u~ing agueous hydrochloric ~cid. When tke ~olution wa~
cooled with ice, 390 mg of the title compound crystal-
lized, m.p. 230C, sinterlng at 193C.
5 Example 192 .
5-(((4-(1-Butyloxy)phenyl)amino)carbonyl)-3-(4-fluoro-
benzyloxy)pyridine-2-c~rboxyllc acid glycylamide
Example 193
5-(((4-(1-Butyloxy)ph~nyl)amlno)carbonyl)-3-b~nzyloxy-
pyridine-3-carboxylic acid glycylamide
Example 194
3-(2-Methyl-1-propyloxy)pyridine-2,5-dicarboxylic acid
diglycylæmide
N.p. 103-105C (from 0thyl acetate)
Example 195
5-(Di-N,N-ethylaminocarbonyl)-3-~thoxypyridin~-2-car-
boxylic acid glycylamide, amorphou3 ~ub~tance, prepared-~ ~ :
in analogy with Ex~ple 223 u~ing N,N-di~thylamino.
Example 196
5-(N-Bonzyl-N-methylamlnocarbonyl)-3-methoxypyridine-2-
carboxylic acld glycylamlde
Ex~mple 197
5-Farne~yloxyc~rbo~yl-3-mothoxypyridine-2-carboxylic acid -:
glycylamide
Example 198
5-Geranyloxycarbonyl-3-methoxypyrldin~-2-carboxylic acid ::
glycylamide -~
.
Example 199
5-(Farnesyloxymethyl)-3-methoxypyridins-2-cArboxylic acid
glycylamide
213 ~866
- 92 -
Example 200
5-(Geranyloxymethyl)-3-methoxypyridille-2-carboxyl~c acid
glycylamide
Example 201
5 5-Retinyloxymethyl-3-methoxypyridine-2-carboxylic acid
glycylamide
Example 202
5-Retinyloxymethyl-3-ethyloxypyridi~e-2-carboxylic ac:id
glycylamide
10 Example 203
N-(Carboxymethyl)-4-methoxycinnoline-3-carboxamide
Examples 204 to 209 were prepared in ~nalogy with Example
191: ~ -
Example 204
15 5- (((4-(1-Hexyloxy)phenyl)amino)carbonyl)-3-methoxy-
pyridine-2-carboxylic acid N-(carboxy~ethyl)am$de
a) Methyl 5-(((4-(1-hexyloxy)phenyl)amino)car}~onyl)-3-
methoxy-2-carboxylate was E~repared from methyl 5-
carboxy-3-mqthoxypyridine-2-carboxylate and 4-hexyl-
oxyaniline, m.p. 118-119C (from diethyl ether). ;~-
b) 5-(((4-(1-Hexyloxy)phenyl)~mino)carbonyl)-3-methoxy- ;
pyridine-2-carboxylic acid, 160-162C, ~intering at
148C (from aqueouc hydrochloric
acid/tetrahydrofuran)
c) 5-(((4-(l Hexyloxy)phenyl)amino)carbonyl)-3-methoxy-
pyridin0-2-carboxylic acid N-((ethoxycarbonyl)-
me~thyl)amide was obtain~d, in analogy with Example
l91c), from 4.2 g of the above compound. 4.0 g of
product were crystallized u~ing ethyl acetate, m.p.
157-159C.
213 186~
:`.
_ 93 _
d) The title compound wa~ obtainsd by hydrolyzing 1.2 g
of tho above oster, at 20C, ueing 100 ml of a 1.5N
methanolic solution of codium ~ydroxide. Following
concentration ln vacuo, acidi~icatio~ to p~ 1 took
place in water/totrahydrofuran u~ing aquoou~ hydro-
chloric acid; tho mlxturo was concontrated in v~cuo
and the reeidue wa~ cry~tallized ufling acetone. 840
mg of product were obtained, m.p. 193-195C.
Example 205
5-(((4-(1-Decyloxy)phenyl)amino)carbonyl)-3-methoxy-
pyridine-2-carboxylic acid N-(carboxy~ethyl)amide
Example 206 -
5-(((4-(1-Decyl)phenyl)~;no)carbonyl)-3-methoxypyridine-
2-carboxylic acid N-(carboxymethyl)amide
-'- .
a) 5-(((4-(1-Decyl)phenyl)amino)carbonyl)-3-mothoxy-
pyridine-2-carboxylic acid N-((othoxycarbonyl)-
methyl)amide was prepared from 5-(((4-n-decyl-
phenyl)amino)carbonyl)-3-methoxypyridin~-2-
carboxylic acid (m.p. 160C (decomp.); from aqueou~
hydrochlorlc acid/THF) and glycin~ othyl ~ter
hydrochloride, m.p. 155-157C (fron dii~opropyl
othsr).
~ -: . .
b) The title compound wa~ obtained by hydrolyzing 1.5 g
of tho abovo eeter in 200 ml of a 1 N methanolic
~olution of ~odium hydroxido. 1.4 g of product, m.p.
195C ~decomp.), were isolated from aqu~ou~ hydro-
chloric acid/t~trahydrofura~.
Examplo 207
5-(((4-~eranyloxyphenyl)amino)carbonyl)-3-mothoxy-
pyrldine-2-carboxylic acid N-(carboxymethyl)amide
21318~6
- 94 -
Example 208
5-(((4-(1-Octyloxy)phenyl)am~no~carbonyl)-3-~ethoxy-
pyridine-2-carboxylic acid N-(carboxymethyl)a~lde
Example 209
5-(((3-(1-Octyloxy)propyl)amino)carbonyl)-3-methoxy-
pyridine-2-carboxylic acid N-~car~oxymethyl)umide
Example 210
5-((1-Butoxy)mothyl)-3-mothoxypyridi~e-2-carboxylic ac~d
N-(carboxymethyl)am~do
Example 211
5-((1-Hoxyloxy)methyl)-3-mothoxypyridine-2-Carboxylic
acid N-(carboxymethyl)amido
Example 212 ~ :
5-((1-Octyloxy)methyl)-3-mothoxypyridine-2-carboxylic
15 acid N-(carboxymethyl)amide ~ ~ :
Examplo 213 -
5-((1-~ex-3-onyloxy)methyl)-3-methoxypyridine-2-car-
boxylic acid N-(carboxymothyl)amide :p
Exa~ple 214
20 5-((1-Docyloxy)methyl)-3-m~thoxypyridine-2-carboxylic : . :
acid N-(carboxymethyl~amide
Examplo 215
5-((l-Dodecyloxy)methyl)-3-methoxypyrldine-2-carboxylic :~
acid N-(carboxymothyl)amide -~
25 Example 216 ~:
5-((1-~exadecyloxy)methyl)-3-methoxypyridlne-2-carboxyllc
acid N-(carboxymethyl)amide
Examplo 217
3-S4-(((+)-Dohydroabietylamino)carbonyl)benzyloxy)pyri-
30 di~e-2-carboxylic acid N-(carboxymethyl)amido : :
213~866
;
- 95 -
a) 4-(((~)-Dehydroabietylamino)oarbonyl)chloromethyl-
benzeno was obtained from 4-chloromethylbonzoic acid
and (~)-dehydroabiotyl~mine, ~.p. 170-172C (from
othyl acetate/hoptano (1:1)).
b) 3-(4-(((~)-Dehydroabiotylamino)carbonyl)benzyloxy)-
pyridino-2-carboxylic acid N-((~thyloxycarbonyl)-
mothyl)amido, m.p. approximat~ly 80C (a~orphou~
substance, from ethyl aootato).
c) Tho titlo compound was obtained by hydrolyzing the
above es10r, m.p. 125C (w~th ~oaming, ~rom dii~o-
propyl ether).
Example 218
3-Mothoxyguinoline-2-carboxylic acid N-(oarboxymothyl)-
amido
a) 2-Acetyl-3-hydroxyquinoline, ~nown from D.W. Bayne
et al., J. Chem. Soc. Chem. Comm. 1975, 782 (M.p.
106C from agueouR hydrochloric acid).
.: ',' .
b) 2-Acetyl-3-mothoxyquinolino, from a) ucing pota~sium
carbonate/methyl lodido in acotone, oily crude ;~
product.
c) 3-Methoxyguinoline-2-carboxylic acid, from b) u~ing
potaa~ium hypochlorite in water/dioxane, m.p. 123C
(from methyl tort-butyl ether).
.
d) 3-Methoxyguinoline-2-carboxylic acid N-((methoxy-
carbonyl)methyl)amido, from c) u~ing DCC~ ~OBT, THP,
NEM and glyc$ne mothyl o~tor hydrochlorido.
o) Tho title co~pound wa~ obtained by hydrolyzing the
above ester, m.p. 106C (from othyl ac~tate).
'' ~' ''
':
213~866
- 96 -
Example 219
5-(((4-(1-Butyloxy)phenyl)amino)carbonyl)-3-chloropyri-
dine-2-carboxylic acid N-(carboxyme~hyl)amid~
a) Methyl 5-carboxy-3-chloropyridine-2-csr~oxylate w~s
prepared in analogy with Example 90a), m.p. 182-
184C (from aqueou~ hydrochloric acid).
.
b) Methyl 5-(((1-butyloxy)phenyl)amino)carbonyl)-3-
chloropyridine-2-carboxylate wa~ obtalned from the
above ~ompound us~ng oxalyl chloride and 4-(1-butyl-
oxy)aniline, m.p. 121-123C (from di~thyl other).
c) 5-(((4-(1-Butyloxy)phenyl)smino)carbonyl)-3-~hloro-
pyrldine-2-carboxylic acid by hydrolyzing the
product from b), m.p. 163-164C (from agueou~ hydro-
chloric acid).
d) 5-(((4-(1-Butyloxy)phenyl)æmino~carbonyl~-3-~hloro-
pyridine-2-carboxyli~ acid N-((ethyloxycarbonyl)-
methyl)amide waa obtained, in ~nalogy with ~xample
90b), from tho abo~e ~u~tsnce by conden~ation (N-
ethylmorpholine, l-hydroxy-lH-benzotrlszole and CMC)
with glyclne ethyl ostor hydrochloride, m.p. 177-
179C (from othanol).
e) ~he titlo compound was obtainad by hydrolyzi~g the
above o~tor, M.p. 190C (with docompo~ition, from
aqueou~ hydrochlorlc acid).
Example 220
3-(N-Benzyl-N-methylamino)-5-(((4-(1-butyloxy)phe~yl)-
smlno)carbonyl)pyr~dine-2-carboxylic Acid N-(carboxy-
methyl)amide
0.5 g (1.23 mmol) of the title compound from Exampl~ 219
wa~ stirred ln 10 ml of N-be~zyl-N-methylamino, firstly
at 100-110C for 2 h and then at 130C for a further 2 h.
After the mixture had been cooled down, it was introduced
. ~. ...
2~34866
~nto 100 ml of lN hydrochloric acid ~nd th~ h~lf-
crystalline precipitate wa~ taken up in dichloromethane;
the undiseolved mat~rial was filtersd off and the residue
was crystallized; 0.2 g of the title compound, m.p. 155-
5 157 C .
~xample 221
3-(N-Benzylamino)-5-(((4-(1-butyloxy)phenyl)amino)car-
bonyl)pyridine-2-carboxylic acid N-(carboxy~ethyl)A~de -~
0.5 g (1.23 mmol) of the title compound from Example 219
wa~ ~tirred in 10 ml of bon~ylamine, fir~tly at 120C for
2 h and then at 135C for 1.5 h. After the mixture hnd
been cooled down, it was acidifiod and the precipitat~d
re~in wae dissolved in dichloromethane; thi~ ~olution wa~
dried and conaentrated and the re~idue wa8 chromato-
graphed on ~ilica gel us$ng ethyl ac~tate (with the
addition o~ up to 20 % methanol). 0.1 g of th~ titl~
compound wa~ cry~tallized from appropriate f~action~
u~ing dii~opropyl other, m.p. 185-190C.
Example 222
:
3-(4-Chlorobenzyloxy)pyridine-2-carboxyllc acid N-
(carboxymethyl)amide 1-oxido
a) 3-(4-Chlorobenzyloxy)pyridine-2-carboxylic acid N-
((ethyloxy)methyl)amide 1-oxide
0.7 g (2 mmol) of the compound from Example 31c) wa~
di~olved in dichloromethane and roacted with 1.41 ~
g of 3-chloroperbenzoic acid. After the mixtura had ~;
been ~tirrod at 20C for 1 h, ammonia wa~ pa~ed in ;
until thero wac no further pr~cipitation; tho
procipitats wa~ filtered off and the filtrate waa ~-
concontratod and the oily residuo cryctallized ucing
diethyl ether, m.p. 70-72C.
b) 0.18 g of the title compound, m.p. 206-208C
213~866
- 98 -
(sintering at 200C, fro~ aqueous hydrochloric acid)
was obtained by hydrolyzing 0.3 g of tke above
compound.
Example 223
5-(((3-(1-Butyloxy)propyl)P~no)carbonyl)-3-methoxypyri-
dine-2-c~rboxylic acid N-(carboxymethyl)amido
a) Methyl 5-(((3-(l-butyloxy)propyl) a~ no)carbonyl)-3-
methoxypyridine-2-carboxylic acid
1.7 ml of oxalyl chloride (20 mmol), and al80 2 drops of
N,N-dimethylformamide, dissolved in tctrahydrofuran, were
added dropwiEie, at 10C and whlle itirr~ng, to 2.1 g
(10 mmol) of methyl 5-carboxy-3-methoxypyridine-2-car-
boxylate in 100 ml of i~nhydrouEi totrahydrofurian and the
reaction mixture wa~i ~itirred at 10C for 30 min and at
20C for 1 h. It was then concentrated and the residue
was dis~iolved in dichloromethane; 6.8 ~l (50 mmol) of
triethyla~ine, and then 1.3 g (1.5 ml, 10 mmol) of 3-
butoxypropylamine, diseolved i~ dichloromsthanei, wore
added, at 0C, to thi~i siolution.
Aftor 30 min, the mlxture was allowed to warm to room
tomperature and waE extracted with water, a Eiolution of
Na bicarbonate and aqueouE lN ~Cl; the organic phas~ waEi
dried and concentrated and the residue wa~i crystallized
u~ing diethyleth~r/petroleium ether (3:1). 2.3 g of
product were obtained, m.p. 51-53C.
b) 5-(((3-(1-Butyloxy)propyl)amino)carbonyl)-3-methoxy-
pyridine-2-carboxylic acid N-((benzyloxycarbonyl)-
methyl)a~ide
~ . . .
The above subEitance was hydrolyz0d by Eitandard pro-
c0dures, and 1.5 g (5 mmol) of the amorphous 5-(((3-(1-
butyloxy)propyl)amino)carbonyl)-3-methoxypyridine-2-
carboxylic acid, which wa~i dried on an o~l pump, was
reacted with glycine benzyl oster toEiylate, ;
.
213~866
- 99
N-ethylmorpholine, l-hydroxy-l~-benzotriazole and ~MC (a8
de~cribed). 1.42 g of the product were cry~tallized using
acetone, m.p. 97-99C. -
c) 1.3 g of the above benzyl e~ter were hydrogenatod in
100 ml of tetrahydro~uran/methanol (1:1) u~$ng Pd/C
(10 %) in a hydrogenation ves~el. 0.8 g of the title
compound wae crystallized u~ing diethyl othor, m.p.
155-157C.
Example 224
5-(((3-(1-Lauryloxy)propyl)amino)carbonyl)-3-methoxy-
pyridine-2-carboxyllc acid N-(carboxym~thyl)amide
a) 5-(((3-Lauryloxypropyl)am$no)c~rbonyl)-3-methoxy- -
pyridine-2-carboxyl~c acid N-((benzyloxycarbonyl)-
methyl) mide was obtained, i~ analogy with Example 223,
u~ing 3-lauryloxypropylamine, m.p. from 109-111C (from
diisopropyl ether).
b) 1.3 g of the above benzyl e~ter were hydrogenated aa
dQscribed under 223c). 0.9 g of the titlo compound wero
obtained from petroloum ether, m.p. from 120C.
20 Example 225 -;
5-(((2-Methoxyethyl)amino)carbonyl)-3-methoxypyridine-2- ~ - -
carboxylic acid N-(carboxymethyl)amide ~ i
The title compound wa~ prepared in analogy with Example
223 u~ing 2-methoxyethylamine.
a) 5-(~(2-Methoxyethyl)amino)carbonyl)-3-~ethoxypyri-
dine-2-sarboxyllc acid, m.p. 160-161C (wlth ga~
evolution, from ethyl acetate)
b) 5-(((2-Methoxyethyl)amino)carbo~yl)-3-methoxypyri-
dine-2-carboxylic acid N-((benzyloxycarbonyl)-
methyl)amide wa~ ~ry~tallized u~ing dii~opropyl
ether, m.p. 129-131C.
:
213~366
- 100 -
c) The title compound was obtained, aa above, from the
benzyl ester, m.p. 186-188C (from diethyl ether).
Example 226
N-(3-Benzyloxypyridyl-2-carbonyl)alanine racemate,
M.p. 186-187C (from pentane/ethyl acetate)
Example 227
N-(3-Benzyloxypyr~dyl-2-carbonyl)-L-phenylalanine,
M.p. 100-101C (from pentane/ethyl acetate).
Example 228
5-((1-Butyloxy)carbonyl)-3-methoxypyridin~-2-carboxylic
acid N-(carboxymethyl)amide trifluoroacetate
a) Di-(1-butyl) 3-methoxypyr~dine-~,5-dicarboxylate
5.0 g of dimethyl 3-methoxypyridine-2,5-dicarboxylate
(cf. Example 90a)) were dissolvod in 100 ml of 1-butanol,
and after that 1.5 ml of conc. ~ulfuric were added and
the mixture wa~ hoated to boiling for 2 h, with a part of
the solvent bsing distilled off. After the mixture had
been cooled, it was concentrated in vacuo and the rosidue
was taken up ~n dichloro~ethane; this solution wa~
oxtr~ct-d with a oaturated, aqueou~ ~olution of Na
bicarbonato and the organic phase was dried and concen-
trated. 6 g of o$1y crude product.
., .
b) ~is[5-((1-butyloxy)carbonyl)-3-methoxypyridine-2-
carboxylic acid]-Cu(II) complex
.
6 g (20 mmol) of the above product were addod, disaolved
in 10 ml of mothanol, to a solution of 4.8 g (20 mmol) of
Cu(II) nitrate x 3 ~,0 in 100 ml of methanol, and t:he
mixture was heated to boiling for 4 h. It wa~ then cooled
down to 0-5C and the cry~talline precipitate was fil-
terod off with suction and washed with diethyl ether.
4.2 g of bluo-groen, cry~talline product were obtained,
.p. 267C (wlth decomposition).
'' ;' `' '' ~' '
.: ~
2i31866
- 101 -
c) 5-((1-Butyloxy)carbonyl)-3-mothoxypyridine-2-car-
boxylic acid
4 g of the above Cu complex were suisipended in 75 ~1 of
1,4-dioxane. ~2S gaisi w~,~i pai~ised in, while the mixture was
being stirred, for 30 min, ~nd the eedlment ~CuS) wh~ich
had precipitated out wai~i filtered off with i~iuction
through kie3elguhr and then washed with 1,4-dioxane
(continued introduct~on Or H,S did not l~ad to any
further precipitation); the filtrate w~i6i concentratod in
vacuo. The residue wae crystii~llized uciiny potroleum
ether, m.p. 96-9SC.
d3 5-((1-Butyloxy)carbo~yl)-3-methoxypyridino-2-
carboxylic acid N-((tert-butyloxyc~rbonyl)-
methyl)amide
0.76 g (3 mmol) of the a~ovo pyridinecarboxylic acid wa,~
condensed with 0.52 g (3 mmol) of glycine tert-butyl
ester hydrochloride, 1.2 ~1 (9 ol) of N-~thylmorpho-
line, 0.45 g (3.3 ~mol) of 1-hydroxy~ )-benzotriazole
and 1.3 g (3 mmol) of CMC. 0.8 g of product wa~c obtained,
m.p. 50-52C (from peitroleum ther).
ei) The title compound wac obtain~d by ~dding 2.7 ml of
trlfluoroacetic acid, at 20C, to 0.4 g of the above
tert-butyl ei~iteir in dichloromethane. Aftsr 20 h, the
mixture was concentrated in vacuo and 0.2 g W~8 obtained
of the colorleei~i, cryi~tall$ne, strongly hygroi~icop~c
product, which, on being filtered off with ,aiuction,
deliquesced on the suction filteir.
Example 22g
5-Ethyloxycarbonyl-3-methoxypyridine-2-carboxylic acid
N-(carboxymethyl)amide
Example 230
3-Methoxy-5-((1-propyloxy)carbonyl)pyridine-2-carboxyl~c
acid N-(carboxymethyl)amide
213~866
- 102 -
Example 231
5-(~ exyloxy)carbonyl)-3-mathoxypyridine-2-carboxylic
acid N-(carboxymethyl)amide
Exa~ple 232 ~-
3-Methoxy-5-((1-pentyloxy)carbonyl)pyr$dino-2-carboxylic
acid N-(carboxymethyl)amido
Example 233
5-((1-Heptyloxy)carbo~yl)-3-methoxypyridino-2-carboxyl~c
acid N-(carboxymethyl)amlde
Example 234
3-Methoxy-5-((1-octyloxy)carbonyl)pyridine-2-carboxylic
acid N-~carboxymethyl)amide
~xamples 235-238 were prepared in an analogous manner to
Example 228 proceeding from 3-(2-propyloxy)pyridine-2,5-
dicarboxylic acid or the corre~ponding dial~yl esters.
Examplo 235
5-Ethyloxycarbonyl-3-(2-propyloxy)pyridine-2-carboxylic
acid N-(carboxymethyl)amide ~`
~ ..
Examplo 236
5-((1-Butyloxy)carbonyl)-3-(2-propyloxy)pyridine-2-
carboxylic acid N-(carboxymethyl)amide
Example 237 . .
5-((1-Hexyloxy)carbonyl)-3-(2-propyloxy)pyridino-2- :
carboxylic acid N-(carboxymethyl)amide
",
25 Example 238 - --.;
5-((-1-Octyloxy)carbonyl)-3-(2-propylc-xy)pyridine 2-
carboxylic acid N-(c~rboxymethyl)amide
Example 239
5-Carboxy-3-(methylthio)pyridine-2-carboxylic ~cid
N-(carboxymethyl)amide di~odium salt
' ' ~
2134~66
- 103 -
a) 3-(Methylthio)pyridino-2,5-dicarboxyllc acid
4.6 g (12 mmol) of dibenzyl 3-c~loropyridine-2,5-dicar-
boxylate were dissolvod, at 20C ~nd while ~tirring, in
30 ml of dimethyl sulfoxido, and aftor that 5.0 g
(70 mmol) of sodium thiomethoxido wore ~ddod, in as~oci-
ation with which the temperaturo roce to 80C. The
reaction mixture was he~tod ~t 140C for 1 h and then
cooled down; water wa~ added ~nd tho oily layor w~
~eparatod off; conc. hydrochloric acid (p~ 1) was ~dded
to the aqueouo DMS0 pha~e and the preciplt~tod product
waQ filter0d off with suction. 2.8 g wsro obtained of a
yellow cry~talline product, m.p. 223C (with decom-
position).
b) Dimethyl 3-(methylthio)pyridine-2,5-dicarboxylate
50 ml of 1,4-dioxane, 40 ml of tetrahydrofuran and 0.5 ml
of conc. ~ulfuric aoid were added to 2.8 g of the above
compound in 150 ml of mothanol, ~nd tho mixture w2s
heated ~o reflux for 2 h, during which tim0 a eolution
wa~ formed. After tho ~olution had been cooled down, it
was concentrated in vacuo and 100 ml of an aqueou~
solution of Na bicarbonato were addod to the rosidue; the
latter mixture wae oxtracted with dichloro~ethane and the
organic phase was dried a~d concentrated. 1.4 g were
obtained of tho yellow, crystallino product, ~.p. 103-
105C.
c) 5-Methoxycarbonyl-3-(methylthio)pyridine-2
carboxylic acid-Cu(II) complex
1.3 g of the ~bova dimethyl 3-msthylthiopyridine-2,5-
dicarboxylate were reacted in analogy with Example 228~
1.3 g of grsenish/cry~talline product were obtained, m.p.
~ 330C.
d) 5-Methoxy-3-(methylthio)pyridine-2-carboxylic acid
- ::
213~866
- 104 -
1.3 g of the above compound were ro~cted in analogy with
Example 228c), 0.72 g of product, m.p. 183-185C.
e) 5-Methoxycarbonyl-3-(methylthio)pyr~din~-2-
carboxylicac$dN-((1-butyloxy)carbonyl)methyl)amldo
S ~he compound was obtained by condensing O.68 g (3 mmol)
of the above pyridinecarboxylic acid wit~ 0.91 g (3 ol)
of glycine 1-butyl e~ter tosylata (1-hydroxy-1~-benzotri-
azole, N-ethylmorpholine ~nd CMC). 0.55 g of pale yellow
product was obtained, m.p. 47-49C (frcm potroleum
10 ether) .
f) The title compound wa~ obtained by hydroly~ing
O.45 g (1.3 mmol) of the above e~ter u~ing 50 ml of 1 N
methanolic NaO~. The clear yollow ~olution hecamo cloudy
after 30 min. Aftor 2 h, the precipitate was filtered off
with ~uction, washed twice w~th ~ethanol and dried in
vacuo. 0.32 g of the title compound wa~ obtained, m.p.
345C (decomp.).
' ~'
: .
~:: - . .
.~ - ,,~ ,
- "~ ~
, ~, ,,,.,~-,,.
-., ~:
,'' ~',' ''' -,' ,'.''`' ~'
,: ,; .~ . . .
: : .