Note: Descriptions are shown in the official language in which they were submitted.
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
HOLLOW FIBER MEMBRANE INCORPORATING A SURFACTANT AND PROCESS
FOR PREPARING SAME
SPECIFICATION
TO ALL WHOM IT MAC.' CONCERN:
Be it known that we, Louis C. Cosentino, a resident of
Plymouth, Hennepin county, Minnesota, Robert T. Hall II, a
resident of Welch, Dakota county, Minnesota, Robert G. Andrus, a
resident of New He>pe, Hennepin county, Minnesota, Paul D. Brinda,
a resident of Robbinsdale, Hennepin county, Minnesota and Randal
M. Wenthold, a ree>ident of Belle Plain, LeSuer county, Minnesota,
all citizens of the United States have invented new and useful
improvements in a
HOLLOW FIBER MEMBRANE INCORPORATING A SURFACTANT
AND PROCESS FOR PREPARING SAME
of which the following is a specification.
1
CA 02136006 1999-08-30
' ' H10 94/00222 PCT/US93/03211
Backcrround of t:he Invention
1. Field of the' Invention.
This invention relates generally to improved
asymmetrical microporous hollow fibers incorporating a low
molecular weight. surfactant and to the process for the
production of the hollow fibers. In particular, the invention
relates to asymrnetrical, microporous hollow fibers having
improved flux and rewetting characteristics. The process
involves passing a polymeric solution through an outer annulus
of die to create an annular stream and passing a precipitating
fluid through the inner orifice of the die creating a stream
within the annular stream resulting in hollow fiber formation.
The process further involves incorporating a low molecular
weight surfactant on or into the hollow fibers at any one of
several steps during the fiber manufacturing process.
2. Description of the Related Art.
Microporous, hollow fibers are polymeric capillary
tubes having an outside diameter equal to about 1 mm or less
and whose walls function as semipermeable membranes. The fibers
are useful in separation processes involving transport mainly
through sorption and diffusion. Such processes include
dialysis, including hemodialysis, ultrafiltration,
hemofiltration, blood separation, drug release in artificial
organs and water- filtration. These applications have various
requirements including pore size, strength, biocompatibility,
cost and speed of production and reproducibility.
Conventional art hollow fibers for these uses have
typically included regenerated cellulose materials and modified
polyacrylonitril.e material. However, it is difficult to control
the porosity anc~ pore size of these fibers, and for some
applications, composite membranes consisting of an ultra-thin
layer contiguou:~ with a more porous substrate are needed to
provide the necessary strength.
Conventional art hollow fiber membranes have also been
prepared from hydrophobic polymers such as polysulfones and
polyaromatic pol.yamide polymers. The hydrophobic nature of the
polymer present: difficulties in using these membranes in
2
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
aqueous systems,, and therefore, hydrophilic polymers have been
incorporated directly into the fibers.
For example, Klein, et al., U.S. Patent No. 4,051,300,
discloses a process for the preparation of hollow microporous
fibers capable of withstanding from 600 psi to 2000 psi applied
pressure without. collapse. However, the process disclosed by
Klein is slow and time consuming. Further, fibers so prepared
are designed on7_y to be used as the support structure of a final '
composite membrane. The actual selective membrane is applied as
an ultrathin coating to this support structure in an additional
step or steps.
Heilmann, U.S. Patent No. 4,906,375, discloses a
process comprising wet spinning a polymer solution made up of a
solvent, about 7_2 to 20 wt.~ of a first, hydrophobic polymer and
2-10 wt.% of a hydrophilic polyvinylpyrrolidone polymer and
simultaneously passing through a hollow internal core a
precipitant solution comprising an aprotic solvent in
conjunction with at least 25 wt.~ non-solvent. However, the
hollow fiber membranes so produced have limitations in
hydrophilicity, water flux, etc. and by their very nature are
limited in use t:o dialysis applications.
In a :separate line of development of fiber membranes,
surfactants have' been incorporated into membrane manufacturing
processes. For example, Wrasidlo et al., U.S. Patent No.
4,432,875, discloses film or fiber membranes comprising a
hydrophobic polymer and, baked onto the membrane a polymeric,
high molecular weight surfactant. The polymeric surfactant
apparently take:; the place of the hydrophilic polymer in the
Klein and Heilmann references. The fiber produced using the
Wrasidlo proces~~, however, is limited to sheet membranes and is
not able to be adapted to the manufacture of hollow fiber
membranes. Further, the "baking on" of the surfactant in
Wrasidlo resulta in a fiber that is costly to manufacture, thus
making the fiber's use economically impractical for smaller
companies.
Moreover, most conventional art fibers utilize
glycerol to impart the rewetting and flux characteristics of the
3
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
fiber. However,, the addition of glycerol to the fiber makes the
fiber costly to manufacture. Further, the glycerol must be
thoroughly rinsed prior to use or it will contaminate the piping
system. This makes glycerol-coated fibers inefficient, costly
and time consum_Lng for the end user. In addition, if the
glycerol were not used in the fiber, the fiber would have a much
lower flux rate.,
While the conventional art fibers discussed above are
useful in many applications, there is and always has been a
trade-off among properties including tensile strength,
elasticity, porosity, flux, and sieving characteristics
including molecular size cutoff, solute clearance, etc. Thus,
new membranes are constantly needed which can offer advantages
in particular applications with given property requirements.
The fiber membranes discussed above each have their own
particular advantages and disadvantages, e.g., Klein teaches a
fiber which can withstand high pressures present in reverse
osmosis systems, Heilmann discloses a fiber which is tailored to
dialysis systems, having lower flux and more stringent sieving
properties, and Wrasidlo discloses membranes for reverse osmosis
and filtration processes. However, not one of these references
teach a hollow, microporous membrane which is equally suitable
in processes such as hemofiltration, hemodialysis and water
purification.
A hollow fiber membrane that could be applied across a
large range of applications would provide a decided advantage
over conventional art fiber membranes. Additionally, a new and
useful process is needed that will ensure that a low cost hollow
fiber is available to both large and small companies.
Specifically, a new and useful process is needed that allows the
incorporation of: low molecular weight surfactants into hollow
fiber membranes that does not require the use of high
temperatures to ensure the incorporation of the surfactant into
and/or onto the fibers. Further, a new and useful hollow fiber
is needed that c:an be autoclaved repeatedly without the loss of
the rewetting characteristic and one which does not rely on
glycerol for rewettability.
4
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
Summary of the Invention
It is an obj ect of the hollow fiber membrane
incorporating a surfactant and the process for preparing the
same provided in accordance with the present invention to solve
the problems outlined above that have heretofore inhibited the
successful production of a cost-efficient fiber having a broad
range of applications. The microporous hollow fiber membrane in
accordance with the present invention enables the use of a
unique hollow fiber that, as will be shown, has greatly improved
flux and rewetting characteristics than conventional art fibers.
"Flux," as used herein, is a measure of the volume of water
passed by the membrane under pressure for a given time and area.
"Rewetting" and similar words such as rewettable, etc., as used
herein, is a de:3cription of the ability of a membrane to
maintain a significant level of flux after cycles of wetting and
drying the membz-ane .
The ho7_low fiber includes about 75 to 99 dry wt.% of a
hydrophobic polysulfone polymer, about 0.1 to 20 dry wt.% of a
hydrophilic polyvinylpyrrolidone polymer and about 0.001 to 10
dry wt.% of a low molecular weight surfactant. The hollow fiber
membrane has a flux of at least about 5 X 10-5 mL/min/CM2/mmHg
and maintained ~~ignificant flux characteristics for up to at
least five use and drying cycles. In addition, as will be shown,
the fiber has superior rewetting characteristics, "rewetting"
being defined as the ability of the fiber's flux to continuously
return after at least five drying and wetting cycles.
In addition, the invention includes a method of
manufacturing the fibers. This process includes the steps of
(a) forming an annular liquid by passing a polymeric solution
comprising about: 5 to 25 wt.% of a hydrophobic polysulfone
polymer and about 1 to 25 wt.% of a hydrophilic polyvinyl-
pyrrolidone polymer dissolved in an aprotic solvent and having a
viscosity of about 100 to 10,000 cps through an outer annular
orifice of a tube-in-orifice spinneret, (b) passing a
precipitating solution comprising about 0.1 to 100 wt.% of an
organic solvent and about 0.1 to 160 wt.% of water into the
center of the annular liquid through the inner tube of the
5
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
spinneret, (c) passing the polymer precipitate through the
atmosphere or an augmented atmosphere, (d) quenching the polymer
precipitate in a bath to form a hollow fiber, (e) contacting the
polymer precipitate with a solution comprising about 0.01 to 10
wt.~ of a low molecular weight surfactant, and (f) taking up the
fiber at a rate of about 125 - 250 ft/min.
A second embodiment incorporates the low molecular
weight surfactant solution into the polymeric solution prior to
precipitation (:step (a) above) while a third embodiment contacts
the surfactant :solution with cut and formed bundles of hollow
fibers.
One of the advantages of the present invention is that
hollow fibers treated with surfactant retain, as will be shown,
their "rewettinc~" character after repeated washing and
autoclaving without the use of glycerol. Another advantage of
the present invention is that the surfactant may be incorporated
on and/or into t:he hollow fiber without the need to covalently
bond the fiber as in heat bonded surfactant to fiber. More
significantly, t:he present invention provides a ready-to-use,
rewettable hollow fiber membrane without the use of glycerol.
These and other objects and advantages of the present
invention will become apparent during the course of the
following detailed description and appended claims. The
invention may best be understood with reference to the
accompanying drawings, disclosure and examples wherein an
illustrative embodiment is shown.
Brief Description of the Drawings
Figure 1 is a side elevational diagram with parts cut
away depicting t:he process of the present invention;
Figure 2 is a side elevational detail view of the dry-
jet wet spinning spinneret used in the process of the present
invention;
Figure 3 is a fragmentary sectional detail view of the
orifices of the spinneret.
6
PCT/US93/03211
WO 94/00222 ~ 1 ~ 6 0 0 6
~Prailed Dew~r~~~'~~~ of the Preferred Embodiment
The invention is directed to a microporous, hollow
fiber that includes a hydrophobic polymer, a hydrophilic polymer
and a low molecular weight surfactant. The hydrophobic polymer
is preferably a polysulfone polymer, polyethersulfone,
poly(arylsulfone), poly(aryl ether sulfone) or a
poly(phenylsulfone). The polysulfone polymer is preferably a
poly(arylsulfone). More preferably, the polysulfone polymer is
a poly(oxy-1,4-phenylene sulfonyl-1,4-phenyleneoxy-1,4-
phenyleneisopropylidene-l,4phenylene) polymer having the formula
( -OC6H4C (CH3 ) ZC6H9SO2C6H4- ) n with the accompanying structure
(I) ~ 0 ~ CHI
O S O O O C ~- O
1
CHl
O n
available from Amoco Chemicals Corp. (Atlanta, Georgia)) under
the UDEL mark; or
a polyether sulfone having the formula (-O-C6H4SO2C6H4-) with the
accompanying structure:
(II)
O __
0 O S O
l o
n
available from ICI Americas, Inc. (Wilmington, Delaware) under
the VICTREX mark; or
or a poly(arylsulfone) available from Amoco Chemicals Corp.
(Atlanta, Georgia) under the RADEL mark, or a mixture thereof.
7
WO 94/00222 ~ 1 3 6 0 0 6 PCT/US93/03211
Most preferably, the polysulfone polymer is a polysulfone of''"''
formula (I).
The polysulfone polymers preferably have a molecular
weight of about 20,000 to 100,000. More preferably, the ,
molecular weight is about 55,000 to 65,000 and most preferably,
the molecular weight is about 60,000 to 65,000. If the
molecular weight of the polymer is greater than about 100,000,
the viscosity of the polymeric solution may become too great for
processing. On the other hand, if the molecular weight of the
polysulfone polymer is less than about 20,000, the viscosity of
the polymeric solution may become too low,to produce,a fiber and
any fiber formed may be too weak for processing.
The hydrophilic polymer not only supplies
hydrophilicity to the hollow fiber membrane but also markedly
improves its porosity as well. The hydrophilic polymer may be
water soluble cellulose, starch derivatives,
polyvinylpyrrolidone, polyethylene glycols. Preferably, the
hydrophilic polymer is polyvinylpyrrolidone ("PVP").
The PVP generally consists of recurring units of the
formula (-C(CQH6N0)HCH2-)n with the accompanying structure:
~~
~ 0
I
-~ cx - c~u ~
The PVP is useful to increase the solution viscosity
of a polymeric spinning solution or dope. Further, this polymer
is water soluble and the majority of the polymer may be
8
WO 94/00222 ~ ~ ~ ~ ~ ~ ~ PCT/US93/03211
dissolved from the formed fiber to increase its porosity. As
some of the PVP may remain in the fiber, the fiber's wettability
by an aqueous media is increased.
However, to further increase the wettability of the
fiber by an aqueous media, the fiber incorporates a low
molecular weight surfactant. This surfactant may be amphoteric,
zwitterionic, nonionic, anionic, cationic, or the surfactant may
include a mixture of surfactant types. A representative, non-
limiting list of useful amphoteric surfactants includes
lauroamphocarboxyglycinate, e.g., MIRANOL 2MHT MOD available
from Miranol, Inc. (Dayton, New Jersey) or synergistic
constituents thereof. A representative, non-limiting list of
useful zwitterionic surfactants includes B-N-alkylaminopropionic
acids; N-alkyl-B-iminodipropionic acids, fatty acid imidazoline
carboxylates, N-alkyl betaines, sulfobetaines, sultaines, and
amino acids (e.g., asparagine, L-glutamine, etc.). A
representative, nonlimiting list of useful nonionic surfactants
include alkoxylated alkylamines, ethanol, isopropanol, methanol
glycerine, alkylpyrrolidones, linear alcohol alkoxylates,
difunctional block copolymer surfactants with terminal secondary
hydroxyl groups, difunctional block copolymers with terminating
primary hydroxyl groups, fluorinated alkyl esters, N-
alkylpyrrolidones, alkoxylated amines, and
poly(methylvinylether/maleic anhydride) derivatives. Other
suitable surfactants would include oligomeric or non-monomeric
species containing a C12-18 aliphatic and/or aromatic
hydrophobic moiety and a hydrophilic functionality within the
same molecule. A representative, non-limiting list of anionic
9
PCT/US93/03211
WO 94/00222
surfactants include aromatic hydrophobic based acid esters and
anionic flourochemical surfactants. A representative, non-
limiting list of cationic surfactants includes methylbis-
hydrogenated tallow amido-ethyl, 2-hydroxy-ethyl ammonium methyl
sulfate, water soluble quaternized condensate polymers, and
cocoalkyl bis (2-hydroxyethyl) methyl, ethoxylated chlorides.
Preferably, the surfactant is an aromatic hydrophobic based acid
ester, an alkoyxlated fatty amine, an alkoxylated alkylamine or
a lauroampho-diacetate/sodium trideceth sulfate. More
preferably, the surfactant is an alkoxylated cocoamin~. Most
preferably, the surfactant is an ethoxylated
(2-15 EO) cocoamine.
The preferred embodiment utilizes a low molecular
weight surfactant. Thus, useful surfactants are generally non-
polymeric and/or oligomeric surfactants having molecular weights
of less than about 2,000. Preferably, the surfactant:has a
molecular weight of about 300 to 1,500, more preferably, about
700 to 1,200, and most preferably about 800 to 1,000 . If the
molecular weight of the water soluble surfactant is too high,
longer soaking and rinsing times would be required in most
cases, interfering with the efficiency of the process. On the
other hand, if the molecular weight of the water soluble
surfactant is too low the surfactant may wash off too quickly
resulting in an unwettable fiber. Naturally however, this is
contingent upon the particular surfactant's critical micelle
concentration (CMC) which allows one to draw comparisons between
theoretical monolayer coverage (i.e surfactant to surface area)
and perfor~ance and solvating characteristics such as dependence
PCT/US93/03211
WO 94/00222
on pH, dissolved solids which affect the efficiency of a given
surfactant toward fiber coverage.
For reasons of cost and effectiveness, it is preferred
that the final fiber prior to potting contains from
substantially about 0.001% to 10.0% by weight of the surfactant
(1.0 X 10-5g to 0.18 of surfactant/lg of fiber), more preferably
from substantially about 0.1% to 2.0% by weight of the
surfactant (0.0018 to 0.028 of surfactant/1g of fiber) and most
preferably from substantially about 0.1% to 0.5% by weight of
the surfactant (0.0018 to 0.058 of surfactant/1g of fiber) when
the surfactant is incorporated into the polymeric dope solution
in accordance with the present invention.
When the surfactant is contacted with the formed fiber
in the quenching bath series or when bundles of fibers are
soaked in the surfactant solution, it is preferred that the
final fiber prior to potting contains from substantially about
0.001% to 10.0% by weight of the surfactant (1.0 X 10-58 to O.lg
of surfactant/1g of fiber), more preferably from substantially
about 0.1% to 2.0% by weight of the surfactant (0.0018 to 0.028
of surfactant/1g of fiber) and most preferably from
substantially about 0.1% to 0.5% by weight of the surfactant
(0.0018 to 0.058 of surfactant/1g of fiber). It is also
preferred that the final fiber after potting contains amounts of
surfactant substantially equal to those designated above.
It should be noted that the actual concentration of
surfactant in the soaking solution is dictated by processing
restraints. At higher concentrations, flush time needed to
remove excess surfactant from the fiber is increased. At lower
11
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
concentrations, longer soaking times are required to obtain
effective membranes.
The surfactant interacts with the hollow fiber to
become associated with the fiber probably through an absorption
and/or adsorption phenomenon, i.e., the surfactant is co-
miscible with and/or absorbed on the fiber surface. The
inventors hypothesize that this is most likely accomplished by
hydrogen bonding, dipole-dipole attractions and Van der Walls
forces. There is no evidence to suggest that covalent bonding
is involved. In addition, the inventors hypothesize that the
surfactant utilized in accordance with the present invention may
act to change the conformational nature of the polymer thus
imparting the superior characteristics.
The hollow fiber membranes in accordance with the
present invention have improved flux characteristics as
discussed previously. The fiber surface may well be modified
with surfactant so as to reduce inter and/or intra molecular
surface tension and/or water wettability. This may enable the
opening of previously closed pore structure helping to account
for increased water flux across the membrane. When the treated
fibers were examined under high magnification scanning electron
microscopy (SEM), no apparent change in fiber structure was
noted. Further, it also appears that the effective molecular
size cutoff is numerically increased using the membranes of the
present invention. For example, using bovine serum albumin
(BSA) as a molecular marker (0.5 g/L), a BSA (in reverse osmosis
water) rejection test showed a significant increase in the
effective pore aize for the surfactant treated fiber. A non-
12
~1 X60 06
treated fiber, on the other hand, showed approximately 99%
BSA rejection as opposed to treated fibers which showed a 70%
rejection of BSA. The surfactant does not wash out of the
fiber completely, even with repeated use and drying cycles.
The hollow fiber membranes may be formed using tube-in-
orifice spinning procedures. In particular, the hydrophobic
polymer and hydrophilic polymer are formed into a polymeric
solution comprising an aprotic solvent. An aprotic solvent
is a solvent which is not proton-releasing under processing
conditions, i.e., having non-acidic or non-ionizable hydrogen
atoms. Preferably, this solvent is also soluble in water. A
representative, non-limiting list of aprotic solvents useful
in the invention includes dimethylformamide (DMF), dimethyl-
sulfoxide (DMSO), dimethylaetamide (DMA), n-methylpyrrolidone
and mixtures thereof. Preferably, the solvent is DMA.
Depending on the desired property of the hollow fiber, a
small amount of another solvent may be added instead of using
a pure aprotic solvent. Preferably the additional solvent is
a lower alcohol. This may enhance the precipitation of the
polymer in the fiber formation.
Preferably, about 11-25 wt.%, more preferably, about 14-
16 wt.%, and most preferably, about 15 wt.s of the fiber
forming hydrophobic polymer are dissolved in the aprotic di-
methylacetamide solvent. When less than about il wt.% of the
13
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
hydrophobic polymer is used, the fibers formed are not strong
enough to withstand the stresses involved in the high speed
process of the present invention. On the other hand, when the
level of hydrophobic polymer exceeds about 25 wt.~, a fiber
having inferior hydraulic properties is produced.
The hydrophilic polymer is dissolved in the solvent at
a concentration c>f about 0.1-5 wt.~, more preferably, about 2-4
wt.~, and most preferably, about 3 wt.~. When the hydrophilic
polymer is included in the dope solution above about 5 wt.~, the
resulting fibers are stiff and difficult to manufacture. A
similar result is seen when the amount of hydrophilic polymer is
less than about 0.1 wt.~.
The polymeric solution has a viscosity of about 700-
2300 cps, preferably about 1400-1700 cps, and most preferably,
about 1500 cps at: 25°C, as measured on a Brookfield viscometer.
The solution is ~>referably filtered to remove any entrained
particles (contaminants or undissolved components) to prevent
apparatus blockade.
The polymeric solution is spun from the outer,
annular orifice c>f a tube-in-orifice spinneret. A precipitating
solution is deli~~ered to the tube of the spinneret. The
precipitating solution preferably includes a protic solvent, an
aprotic solvent and water and combinations thereof. To some
extent, the composition of the precipitating solution affects
the porosity, clearance, tensile strength, wall thickness, inner
and outer diameters and flux properties of the fiber. The
practitioner of ordinary skill in the art will recognize that
the precipitating solution compositions outlined in the
14
WO 94/00222 PCf/US93/03211
X1-360 06
following tables are helpful to direct the practitioner in
selecting a useful formulation for a desired fiber end use. The
selection of particular components and proportions is obviously
up to the practitioner. The composition of the precipitating
solution effective to produce a hollow fiber membrane for use in
hemodialysis is illustrated below in Table I.
TABLE I
More Most
Preferred preferred preferred
Lower alcohol 30-90wt.~ 65-90wt.~ 75-85wt.~
Water 10-35wt.~ 10-35wt.~ 10-35wt.o
Aprotic Solvent
Precipitating solutions effective to produce a hollow
fiber membrane for use in a hemofilter operation may comprise
the components in proportions as illustrated in Table II.
TABLE II
More Most
Preferred preferred Preferred
Lower alcohol 30-90wt.~ 50-85wt.~ 80-85wt.~
Water 10-35wt.$ 10-30wt.~ 15.25wt.o
Aprotic solvent 0-50wt.~ 5-35wt.~ 10-20wt.o
Precipitating solutions effective to produce a hollow
fiber membrane for use in a blood filter to separate red blood
cells from higher molecular weight materials may comprise the
components in proportions as illustrated in Table III.
15
WO 94/00222 ~ 1' ,~- ~ ~-. ~ ~.' PCT/US93/03211
., ~.r
TABLE III
More Most
Preferred Preferred preferred
Lower alcohol 30-90wt.% 30-60wt.% 35-45wt.%
Water 10-35wt.% 10-30wt.% 15-25wt.%
Aprotic solvent 0-50wt.% 20-50wt.% 35-45wt.%
Precipitating solutions effective to produce a hollow
fiber membrane for use in water filtration may comprise the
components in proportions as illustrated in Table IV.
TABLE IV
More Most
Preferred Preferred Preferred
Lower alcohol 30-98wt.% 30-60wt.% 35-45wt.%
Water 2-35wt.% 2-30wt.% 2-25wt.%
Aprotic Solvent 0-90wt.% 20-50wt.% 35-45wt.%
The above tables are merely offered to guide the
practitioner in formulating fiber precipitation solutions.
Indeed, the practitioner may decide that it is advantageous to
operate in a "Preferred" range for one component while operating
in a "Most Preferred" range for another.
Representative, non-limited examples of lower alcohols
include methanol, ethanol, n-propanol, is-propanol, n-butanol, t-
butyl alcohol, iso-propanol, n-butanol, t-butyl alcohol,
isobutyl alcohol or a mixture thereof. Preferably, the alcohol
comprises methanol, ethanol, n-propanol, isopropanol, n-butanol
or a mixture thereof. Various polyols, lower alcohols,
glycerine etc. and/or aqueous solutions of inorganic salts may
also be used. More preferably, the alcohol comprises
isopropanol.
16
;, .
WO 94/00222 ~ ~ PCT/US93/03211
~1~fi0 Ofi
The water which may be used in the precipitating
liquid may be tap water, deionized water or water which is a
product of reverse osmosis. Preferably the water is deionized
water which has first been treated by reverse osmosis.
The aprotic solvent used in the precipitating solution
may again be dimethylformamide (DMF), dimethylsulfoxide (DMSO),
dimethylacetamide (DMA), n-methylpyrrolidone and mixtures
thereof. Preferably, the aprotic solvent is the same as that
used in the polymeric fiber forming solution. Most preferably,
the aprotic solvent is DMA.
The proportions of the alcohol, water and aprotic
solvent which make up the precipitating solution influence the
morphology, clearance, permeability, selectivity, etc. of the
hollow fiber membrane. It is generally preferred that the
proportion of water in the precipitating solution remain
relatively low, about 2 to 35 wt.%, to ensure that a fiber
having desirable characteristics is produced. If the
precipitating liquid contains less than about 2 wt.% water, the
resultant precipitation of the polymers may be too slow to form
a fiber. On the other hand, a precipitating liquid that has a
concentration of water greater than about 35 wt.'% may result in
a fiber having decrease flux with a small pore size.
As indicated above, the polymeric dope is pumped,
filtered and directed to the outer, ring orifice of a tube-in-
orifice spinneret. At the same time, the precipitating liquid
is pumped to the inner coaxial tube of the spinneret. These two
solutions are then delivered from the spinneret in a manner such
that the polymer dope forms an annular sheath surround a flow of
17
WO 94/00222 ~ ~ ~ ~ Q ~ PCT/US93/03211 .
precipitating liquid within the annulus. Preferably, the
spinneret head is maintained at a temperature of about 5-85~C,
more preferably, about 15-25°C, and most preferably, about
18°C. The polymeric dope is subjected to a pressure of about 0-
1400 kPa, more preferably, about 140-1000 kPa, and most
preferably, about 350-850 kPa. In a preferred embodiment, the
polymer dope is spun through a ring orifice having an outside
diameter of about 0.018 to 0.040 inches (about 460 to 1.016
microns) and an inside diameter of about 0.008 to 0.010 inches
(about 200 to 254 microns).
At the same time, precipitating liquid is pumped
through the tube of the spinnerette at a pressure of about 0-
1000 kPa, preferably about 0-100 kPa, and most preferably, about
1-20 kPa. In a preferred embodiment, the precipitating liquid
or diluent solution is delivered through a tube having an
outside diameter of substantially about 0.010 inches (about 254
microns) and an inside diameter of substantially about .005
inches (about 127 microns).
In a preferred embodiment, in order to produce a
hollow fiber having an approximately 380 micron outside diameter
and an approximately 280 micron inside diameter, the polymer
dope is delivered to the spinnerette at a rate of substantially
about 1.0-10 mL/min, more preferably, about 2-5 mL/min, most
preferably, about 3 mL/min, and the precipitating liquid is
delivered at a rate of at least about 1.0-10 mL/min, more
preferably, about 2-5 mL/min, and most preferably, about 2-3
mL/min. The spinnerette is oriented in a manner such that fiber
production is driven by fluid flow and by removal from the
18
WO 94/00222 ~ ~ ~ S ~ p 6 PCT/US93/03211
spinnerette by gravity effects. Preferably, the fiber emerges
from the spinnerette and is pulled by gravity and the take-up
speed in a nearly vertical direction downwards.
In order to provide satisfactory fibers in the
practice of the invention, laminar fluid flow should be
maintained both within the spinneret head and the spun fluids
which interact to precipitate the fiber. If turbulent flow is
present in the spinneret head, especially within the channels
which convey the polymeric dope, gas pockets may develop and
ultimately form large voids in the spun fiber. Turbulent flow
within the spun fluids may also result in voids within the
fiber.
It is helpful to visualize the spinnerette dimensions
by resort to ratios of the annular orifice for passage of the
polymeric dope and the coaxial tubular orifice for passage of
the diluent or precipitating solution. One helpful ratio is the
ratio of the cross-sectional area of the annular orifice to
tubular orifice. Preferably, the ratio is greater than about
1:1, more preferably, the ratio is about 2:1 to 5:1, and most
preferably, the ratio of the annular orifice to tubular orifice
cross-sectional area is about 3:1 to 4:1. Another helpful
dimensional ratio is the annular ring thickness to tube inside
diameter. Preferably, the ratio is greater than about 1:1, more
preferably, the ratio is about 2:1 to 7:1, and most preferably,
the ratio of the annular ring thickness to tube inside diameter
is about 3:1 to 6:1. A third helpful dimensional ratio is the
outside diameter of the annular orifice to tube inside diameter.
Preferably, this ratio is greater than about 2:1, more
19
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
preferably, the ratio is about 3:1 to 10:1, and most preferably,
the ratio of the annular outside diameter to tube inside
diameter is about 5:1 to 8:1.
As the fiber emerges from the spinneret, it drops in a
substantially downward vertical direction over a distance of
about 0.1-10 m, more preferably, about .5 to 2.0 m, and most
preferably, about 1.0 to 1.5 m. This allows the precipitating
liquid to substantially precipitate the polymer in the annular
dope solution forming the solid fiber capillary before it is
immersed in a quenching solution. Between the spinneret and the
quenching bath, the fiber drops through the atmosphere, air, air
with a particular relative humidity, an augmented atmosphere,
e.g., a mixture of air or air with a particular relative
humidity and a c~as, an inert gas, or a mixture thereof.
Preferably, for ease in processing and to produce a high quality
fiber, the fiber- drops through air maintained at a temperature
of O°C to 100°C, more preferably, the air is maintained at a
temperature of 5°C to 50°C and most preferably at 15°C to
25°C.
Preferably the air is also maintained at a relative humidity of
substantially about 10~ to 90~, more preferably from
substantially about 20~ to 80~ and most preferably from
substantially about 40~ to 65~. This gaseous atmosphere may be
relatively stagnant, or there can be fluid flow. Preferably,
the flow rate is sufficient to allow complete air change over in
the spinning environment once every 30 minutes. In one
preferred embodiment, the gas flow is about 10 L/min.
Next, t:he fiber is submerged in a tank comprising
water and 0-10 wt.~ other materials. Again, the water may be
WO 94/00222 ~ ~ ~ ~ ~ ~ 6 " PGT/US93/03211
tap, deionized water, or the product of a reverse osmosis
process. The temperature of the quenching bath is preferably
between about 0° to 100°C, more preferably, about 15°C to
45°C,
and most preferably, about 35°C. The water temperature directly
affects the performance of the fiber. Lower temperatures can
reduce the flux of the resulting fiber. Increasing the
quenching bath temperature can increase the flux of the fiber.
The fiber is preferably immersed in the quenching bath
for a period of about 0.1 to 10 seconds, preferably about 0.1 to
5 seconds, and most preferably, about 1 second. This residence
time permits the full precipitation of the hydrophobic polymer
to form the microporous hollow fiber. The quenching bath also
helps to remove the excess, unprecipitated polymers as well as
some of the hydrophilic polymer, the water soluble solvent and
precipitating liquid.
After the quenching bath, the fiber may be further
rinsed to remove additional unprecipitated polymers and
solvents. This rinsing may be accomplished in a water bath
arrangement. Preferably, the additional rinse is achieved in a
water bath having a water temperature of about 0°C-100°C, more
preferably, about 15°C-45°C, and most preferably, about
35°C.
The fiber is then wound on a take-up reel. The take-up reel is
preferably rotating at a speed such that the fiber is being
wound at about 90-150$ of the rate at which it is being formed
at the spinneret. More preferably, the fiber is being wound at
a rate substantially equal to that at which it is being
produced. In other words, the fiber is taken up with enough
speed (i) to create a fiber of the desired size and (ii) to
21
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
apply sufficient tension to the fiber such that it will remain
taut in the take-up guide unaffected by ambient air currents,
i.e. there is no "draft."
The sup°factant may be incorporated into or onto the
hollow fiber mennbrane through a number of mechanisms. The
polymeric spinning solution itself may comprise about 0.01 to 10
wt.% of surfactant. In other useful embodiments, about 0.01 to
wt.% of a surfactant may be incorporated into the quenching
bath, rinse bath, take-up reel bath, a surfactant bath or any
10 other process step wherein the gelled tube or precipitated
hollow fiber is contacted with an aqueous or organic solution,
or both. In another embodiment, the fiber is cut and formed
into bundles that are then soaked in a surfactant solution.
Preferably, the hollow fiber membrane or gelled
polymeric solution has a contact time with a surfactant solution
of less than about 10 seconds. If the surfactant is
incorporated into the quenching bath, rinse bath or take-up reel
bath, the fiber's residence time in the solution is about 4 to
48 hours. In another embodiment, the fibers are cut and bundled
prior to soaking in the surfactant solution for less than 72
hours, more preferably for less than 30 hours and most
preferably for less than 24 hours.
The surfactant solution may be contacted with the
gelled polymer or polymeric precipitate at a temperature of
about O°C to 100°C. more preferably, the hollow fiber or
polymeric precipitate is contacted with a surfactant solution at
a temperature of: about 20°C to 50°C, and most preferably at
about 40°C to 50°C.
22
PCT/US93/03211
WO 94/00222 ~ ~ ~ ~ o ~ 6
The hollow fibers may then be dried by any method
appropriate to general manufacturing procedures including but
not limited to air, heat, vacuum, or any combination thereof.
The hollow fibers may be further processed to form useful
articles including hemodialyzer cartridges, hemofilters, blood
filters, water filters, etc., having improved performance
levels.
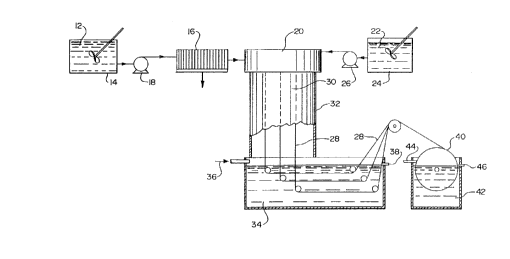
~Pra;lar~ nASr_r,'_g~ion of the Drawings
The process of the present invention may be generally
described by referring to the drawings. A polymeric dope
solution 12 including a polysulfone polymer and
polyvinylpyrrolidone polymer dissolved in an aprotic solvent is
prepared in a mixing vessel 14. The solution is then filtered
in a filter press 16 and delivered by means of a pump 18 to a
dry-jet wet spinning spinneret apparatus 20. This apparatus is
discussed in further detail below.
Simultaneously, a diluent or precipitating solution 22
is prepared in a second mixing vessel 24 from water and a lower
alcohol. The diluent solution 22 is also delivered to the
spinneret apparatus 20 by means of pump 26. The dope solution
23 and diluent solution 22 are spun from the spinneret apparatus
20 to form a hollow fiber 28. The hollow fiber 28 drops through
a volume of gaseous fluid 30 which is enclosed within a pipe 32
until the fiber reaches the surface of a quenching bath 34.
Water is circulated through the quenching bath 34 in an overflow
manner, i.e., a continuous flow of water 36 is supplied to the
quenching bath 34, and the excess fluid overflows and is
removed, e.g., at 38. The fiber 28 is then directed out of the
23
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
quenching bath 34 and is wound on a take-up reel 40 which is
immersed in a second, rinsing bath 42, and the excess fluid
overflows the bath and is removed, e.g., at 46.
The hol_Low fiber 28 thus produced may then be removed
from the take-up reel 40 and further processed. An example of
further processing includes cutting fibers 28 to a uniform
length, bundling them and drying them in any conventional
manner.
Referring to Figures 2 and 3, the details of a
spinneret head 102 which is part of the dry-yet-wet spinning
spinneret apparatus 20 is illustrated. The dope solution 12
enters through a dope port 104, is directed to an annular
channel 106, and flows out of an annular orifice 18 in a
generally downward direction. The diluent solution 22 enters
the spinneret head 102 through a diluent port 110, is directed
through an inner channel 112 and flows out through a tubular
orifice 114 which is in a generally concentric orientation with
respect to the annular orifice 108.
Again, in a preferred embodiment of the present
invention, the hollow fibers 28 formed may be cut into bundles
(not shown) of a constant length and soaked in an aqueous
surfactant solution (not shown) as discussed above. Water flux
is determined by a test developed in-house. Specifically, the
water flux is measured on test mat size (0.02 to 0.08 m2) bundles
which are potted in a polycarbonate cylindrical case. A
transmembrane pressure of 5 psi is maintained across the unit as
reverse osmosis water is pumped through one of two side ports
(one side port clamped off), exiting out one of two end ports
24
.
WO 94/00222 PCT/US93/03211
~1~~0 A6 .
(one end port clamped off). The water is collected via
. graduated cylinder on a timed basis to determine flux. Dzying
of the membrane may be accomplished by circulating dry air
through and around the hollow fiber membranes. The flux of a
fiber which has been cycled in this manner can be compared to
its original values to determine the membrane's rewettability.
The process is repeated in duplicate to insure reproducibility.
The following specific examples which c~,ntain the best
mode, can be used to further illustrate the invention. These
examples are merely illustrative of the invention and do not
limit its scope.
A polymer solution was prepared by dissolving 15.1 by
weight of a polysulfone polymer having a molecular weight of
about 60,000 to 65,000 and 2.8$ by weight of PVP having a K-
value of about 80 to 87 in 81.6 dimethylacetomide with 0.5~ by
weight of an exthoxylated (15 EO) cocoamine surfactant. The
material was filtered and then pumped into a tube-in orifice
spinnerette at a rate of 3.5-3.7 ml/min at a temperature of
about 65-72°F.
A diluent solution containing 40~ by weight
isopropanol, 40~ by weight DMAC and 20~ by weight deionized,
reverse osmosis water was delivered to the spinnerette at a
temperature of 65-72°F and at a rate of 2.5-2.6 ml/min. The
polymeric dope solution was delivered through the outer, annular
orifice of the spinnerette having an outside dimension of about
.037 inches and an inside dimension of about .010 inches. The
WO 94/00222 ~ , PCT/US93/03211
diluent was delivered through a tube orifice within the annular
orifice having an inside diameter of about .005 inches. The
spinnerette head was maintained at about 70°F by means of a
water bath or run without a water bath and maintained at room
temperatures 68-74°F. The spinnerette discharged the column of
dope solution downward through air at a temperature of 68-80°F
and relative humidities of 20-60%. The fiber dropped through
this controlled environment 1.1 meters into a reverse osmosis
quenching water bath which was maintained constant at 90-100°F.
Reverse osmosis water was pumped into the quenching bath
resulting in overflow. The fiber was pulled at approximately 10
RPM into a second bath containing reverse osmosis water
maintained at a temperature of 90-100°F.
The fiber was removed from the take-up wheel, cut and
formed into bundles containing approximately 2,100 fibers of
about 30.5 cm. The fiber bundles were soaked in reverse osmosis
water with overflow maintained at 46-58°C. The bundles were
centrifuged and dried at 38-50°C in a convection oven.
A polymer solution was prepared by dissolving 16.2% by
weight of a polysulfone polymer having a molecular weight of
about 60,000 to 65,000 and 2.8% by weight of PVP having a K-
value of about 80 to 87 in 81.6% dimethylacetomide with 0.5% by
weight of an ethoxylated (15 EO) cocoamine surfactant. The
material was filtered and then pumped into a tube-in orifice
spinnerette as in Example 1.
A diluent solution containing 98% by weight
isopropanol, 0% by weight DMAC and 2% by weight deionized,
26
WO 94/00222 ~ PCT/US93/03211
~l~so os
reverse osmosis water was delivered to the spinnerette at a
temperature of 65-72°F and at a rate of 2.8-3.0 ml/min. The
polymeric dope solution was delivered through the outer, annular
orifice of the spinnerette having an outside dimension of about
.037 inches and an inside dimension of about .010 inches. The
diluent was delivered through a tube orifice within the annular
orifice having an inside diameter of about .005 inches. The
spinnerette head was maintained at about 70°F by means of a
water bath or run without a water bath and maintained at room
temperatures 68-74°F. The spinnerette discharged the column of
dope solution and diluent downward through air at a temperature
of 68-80°F and relative humidities of 20-60~. The fiber dropped
through this controlled environment 1.1 meters into a reverse
osmosis quenching water bath which was maintained constant at 90-
100°F. Reverse osmosis water was pumped into the quenching bath
resulting in overflow. The fiber was pulled at approximately 10
RPM into a second bath containing reverse osmosis water
maintained at a temperature of
90-100°F .
The fiber was removed from the take-up wheel, cut and
formed into bundles containing approximately 2,100 fibers of
about 30.5 cm. The fiber bundles were soaked in reverse osmosis
water with overflow maintained at 46-58°C. The bundles were
centrifuged and dried at 38-50°C in a convection oven.
Example 3
A polymer solution was prepared by dissolving 15.1 by
weight of a polysulfone polymer having a molecular weight of
about 60.000 to 65,000 and 2.8~ by weight of PVP having a K-
27
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
value of about 80 to 87 in 82.1% dimethylacetomide. The
material is filtered and then pumped into a tube-in orifice
spinnerette at a rate of 3.5-3.7 ml/min at a temperature of
about 65-72°F.
A diluent solution containing 80% by weight
isopropanol, 0% by weight DMAC and 20% by weight deionized,
reverse osmosis water was delivered to the spinnerette at a
temperature of E>5-72°F and at a rate of 2.5-2.6 ml/min. The
polymeric dope solution was delivered through the outer, annular
orifice of the :~pinnerette having an outside dimension of about
.020 inches and an inside dimension of about .010 inches. The
diluent was delivered through a tube orifice within the annular
orifice having an inside diameter of about .005 inches. The
spinnerette head was maintained at about 70°F by means of a
water bath or run without a water bath and maintained at room
temperatures 68-~74°F. The spinnerette discharged the column of
dope solution and diluent downward through air at a temperature
of 68-80°F and relative humidities of 20-60%. The fiber dropped
through this controlled environment 1.5 meters into a reverse
osmosis quenching water bath which was maintained constant at 90-
100°F. Reverse osmosis water was pumped into the quenching bath
resulting in overflow. The fiber was pulled at approximately 20
RPM into a second bath of reverse osmosis water maintained at a
temperature of 5~0-100°F.
The fiber was removed from the take-up wheel, cut and
formed into bundles containing approximately 6,000 fibers of
about 30.5 cm. The fibers were then placed for 24 hours in a
static soak tank: containing 1% by weight of an ethoxylated (15
28
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
EO) cocoamine surfactant and water maintained at 68°F to
100°F.
The bundles were centrifuged and dried at 38-50°C in a
convection oven.
Example 4
A polymer solution was prepared by dissolving 15.1% by
weight of a poly:~ulfone polymer having a molecular weight of
about 60,000 to E>5,000 and 2.8% by weight of PVP having a K-
value of about 80 to 87 in 82.1% dimethylacetomide. The
material is filtered and then pumped into a tube-in orifice
spinnerette at a rate of 3.5-3.7 ml/min at a temperature of
about 65-72°F.
A diluent solution containing 90% by weight
isopropanol, 0% by weight DMAC and 10% by weight deionized,
reverse osmosis water was delivered to the spinnerette at a
temperature of 6~>-72°F and at a rate of 2.5-2.6 ml/min. The
polymeric dope solution was delivered through the outer, annular
orifice of the spinnerette having an outside dimension of about
.020 inches and an inside dimension of about .010 inches. The
diluent was delivered through a tube orifice within the annular
orifice having an inside diameter of about .005 inches. The
spinnerette head was maintained at about 70°F by means of a
water bath or run without a water bath and maintained at room
temperatures 68-74°F. The spinnerette discharged the column of
dope solution and diluent downward through air at a temperature
of 68-80°F and relative humidities of 20-60%. The fiber dropped
through this controlled environment 1.5 meters into a reverse
osmosis quenching water bath which was maintained constant at 90-
100°F. Reverse osmosis water was pumped into the quenching bath
29
CA 02136006 1999-08-30
' - WO 94/00222 PCT/US93/03211
resulting in overflow. The fiber was pulled at approximately
110% of the rate at which it is being formed into a second bath
containing 1% by weight of an ethoxylated (15 EO) cocoamine
surfactant and reverse osmosis water maintained at a temperature
of 90-100°F.
The fiber was removed from the take-up wheel, cut and
formed into bundles containing approximately 6,000 fibers of
about 30.5 cm. The bundles were centrifuged and dried at 38-
50°C in a convection oven.
Example 5
A polymer solution was prepared by dissolving 15.1% by
weight of a poly~~ulfone polymer having a molecular weight of
about 60,000 to E~5,000 and 2.8% by weight of PVP having a K-
value of about 80 to 87 in 82.1% dimethylacetomide. The
material is filtered and then pumped into a tube-in orifice
spinnerette at a rate of 3.5-3.7 ml/min at a temperature of
about 65-72°F.
A diluent solution containing 90% by weight
isopropanol, 0% by weight DMAC and 10% by weight deionized,
reverse osmosis water was delivered to the spinnerette at a
temperature of 65-72°F and at a rate of 2.5-2.6 ml/min. The
polymeric dope solution was delivered through the outer, annular
orifice of the spinnerette having an outside dimension of about
.020 inches and an inside dimension of about .010 inches. The
diluent was delivered through a tube orifice within the annular
orifice having an inside diameter of about .005 inches. The
spinnerette head was maintained at about 70°F by means of a
water bath or run without a water bath and maintained at room
PCT/US93/03211
WO 94/00222
temperatures 68-74°F. The spinnerette discharged the column of
dope solution and diluent downward through air at a temperature
of 68-80°F and relative humidities of 20-60%. The fiber dropped
through this controlled environment 1.5 meters into a reverse
osmosis quenching water bath which was maintained constant at 90-
100°F. Reverse osmosis water was pumped into the quenching bath
resulting in overflow. The fiber was pulled at approximately 20
RPM into a second bath containing 1% by weight of an ethoxylated
(2 EO) cocoamine surfactant and reverse osmosis water maintained
at a temperature of 90-100°F.
The fiber was removed from the take-up wheel, cut and
formed into bundles containing approximately 6,000 fibers of
about 30.5 cm. The bundles were centrifuged and dried at 38-
50°C in a convection oven.
Test Data
The membranes made from the examples above were
measured for flux, rewettability and diffusional flow rates.
' The water flux was measured on test mat size (0.02 to 0.08m2)
bundles which were potted in a polycarbonate cylindrical case.
A transmembrane pressure of 5 psi was maintained across the unit
as reverse osmosis water was pumped through one of two side
ports (one side port clamped off), exiting out one of two end
ports (one end port clamped off). The water was collected via
graduated cylinder on a timed basis to determine flux. Drying
of the membrane was accomplished by circulating dry air through
and around the hollow fiber membranes. The flux of fibers
cycled in this manner were compared to their original values to
determine the membrane's flux and rewettability characteristics.
31
CA 02136006 1999-08-30
WO 94/00222 PCT/US93/03211
The hollow fiber membranes were also tested for
diffusional air flow, a method of determining the integrity of a
membrane. When dry, air flow easily through the pores in the
membrane; when wet, air does not flow through an intact
membrane.
Membranes were wet with reverse osmosis water to fill the pores.
A transmembrane pressure equal to 30 psi was applied to the
upstream side of. the membrane. Air which diffuses through is
measured to determine the integrity of the membrane.
Test 1
A filter module comprising approximately 1.4 m2 of
membrane prepared in accordance with Example 1 was tested in
accordance with the previously disclosed flux test. The module
produced a water- flux of .0026 mL/min/mmHg/cm2. The module also
produced a diffusional air flow of 20 mL/min at 30 psi inlet air
pressure.
Test 2
A filter module comprising approximately 1.4 m2 of
membrane fabricated in accordance with Example 2 was tested for
its ability to re-wet upon successive dryings. Each wet dry
cycle trial con:~isted of the following steps:
1) flux test
2) diffusional flow test
3) air dried by blowing 20°C air through the lumen for 24
hours
32
WO 94/00222 ~ 1 3 6 ~ ~ ~ PCT/US93/03211
The results are tabulated below:
Flux Diff. Flow
Trial 1 .0020 21
Trial 2 .0030 27
Trial 3 .0034 29
'trial 4 .0036 39
best 3
A filter module comprising approximately 1.4 m2 of
membrane fabricated in accordance with Example 3 was tested for
its rewetting characteristics and its ability to remove bovine
serum albumin from blood. Each rewetting test trial consisted
of the following steps:
1) flux test
2) air dried by flowing 20°C air through the lumen for 24
hours
3) rewet
The results are tabulated below:
Trial 1 .000428
Trial 2 .000446
Trial 3 .000457
Trial 4 .000454
Trial 5 .000476
The bovine serum albumin rejection rate was 80~.
Test 4
A filter module comprising approximately 3.0 m2 of
membrane fabricated in accordance with Example 4 was tested for
33
WO 94/00222 ~ ~ A ~ PCT/US93/03211
its initial flux rate and diffusional flow characteristics. The
module produced a water flux of .00146 and a diffusional air
flow of 53 mL/min.
A filter module comprising approximately 3.Om2 of
membrane fabricated in accordance with Example 5 was tested for
its initial flux rate and diffusional flow characteristics. The
module produced a water flux of .00092 and a diffusional air
flow of 51 mL/min.
Although the description of the preferred embodiment
has been presented, it is contemplated that various changes,
including those mentioned above, could be made without deviating
from the spirit of the present invention. It is therefore
desired that the present embodiment be considered in all
respects as illustrative, not restrictive, and that reference be
made to the appended claims rather than to the foregoing
description to indicate the scope of the invention.
34