Note: Descriptions are shown in the official language in which they were submitted.
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
```` - 213~018
Thioimidates, their preparation and their use
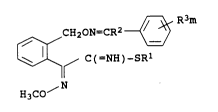
The present invention relates to thioimidates of the formula I
~-~ R3m
~ CH20N=CR2 ~ )
~ ~ C(=NH)-SR1 I
NOCH3
where the substituents and the index have the following meanings:
15 R1 is Cl-C6-alkyl;
R2 is Cl-C3-alkyl or cyclopropyl;
m is 0, 1, 2 or 3, it being possible for the radicals R3 t~
be different if m is 2 or 3;
R3 is cyano, nitro, hydroxyl, mercapto, halogen, amino,
Cl-C6-alkyl, cyano-Cl-C6-alkyl, nitro-Cl-C6-alkyl,
Cl-C6-alkoxy-Cl-C6-alkyl, Cl-C6-haloalkyl,
C3-C6-cycloalkyl, Cl-C6-alkoxy, Cl-C6-haloalkoxy,
C3-C6-cycloalkoxy, Cl-C6-alkylthio, Cl-C6-alkylamino,
di-Cl-C6-alkylamino,
C2-C6-alkenyl, C2-C6-alkenyloxy, C2-C6-alkynyl,
C2-C6-alkynyloxy,
phenyl, phenoxy,
C(=O)Ra, C(=O)ORa, C(=O)NRaRb, C(=S)NRaRb,
OC(=O)Ra, NRaC(=O)Rb, NRaC(=O)ORb,
S(=O)Ra, S(=O)ORa,
CRa=NRb, C(Ra)=NORb, C(XRa)=NORb, N=CRaRb,
or two adjacent R3 together are a CH=CH-CH=CH group;
X is oxygen, sulfur or amino (NRC);
Ra,Rb and
RC independently of one another are hydrogen or C1-C6-alkyl,
and their salts.
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
`` ` 2137018 _ 2
The invention additionally relates to processes for preparing
these compounds, in particular using compounds of the formula IX
~ CH2-Z
Y IX
N ~
OCH3
where the substituents have the following meanings:
Y is CN, C(=S)NH2, CO2H or CONH2, and
15 Z is hydrogen, halogen or a
-ON=CR ~ R3m
group
R2, R3 and m having the meaning given above, as intermediates. The
invention additionally includes compositions against An;r-l pests
and harmful fungi, which contain the compounds I, the use of the
compounds for preparing such compositions and their use for con-
25 trolling An;r-l pests or harmful fungi.
Thioimidates are known for pest control from the literature
(EP-A 528 681).
30 It is an object of the present invention to provide novel thio-
imidates having an improved and widened spectrum of action.
We have found that this object is achieved by the thioimidates I
defined at the outset. We have additionally found processes and
35 intermediates for preparing the compounds I, compositions con-
tA;n;ng them and their use for controlling An;r~l pests or harm-
ful fungi. The compounds I according to the invention are acces-
sible in various ways.
40 They are particularly advantageously obtained in the way drawn up
in the following reaction scheme, starting from a toluene deriva-
tive of the formula II.
BASF Aktiengesellschaft 930627 0.Z. 0050/44487
` 2137018
~ C;3 Stage 1 ~ CH2Ha1
NOCH3 NOCH3
II III
~Stage 2
~ Co2H stage 3a ~ ~ CN R3m
NOCH3 NOCH3
VII V
~Stage 3.b~Stage 3
20 CH2ON=CR2 ~ R3m CH2ON=CR2 ~ R3m
CONH2Stage 3c ~ ~ CSNH2
NOCH3 NOCH3
VIII VI
~Stage 4
CH2ON=CR2 ~ R3m
C(=NH)-SR
NOCH3
The individual reactions are in this case customarily carried out
as follows:
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
`~ ~ 4 2137Q18
1. Preparation of the benzyl halide III (EP Appl.
No. 93 113 372.6)
~ CH3 ~ C~2HcaN1
NOCH3 NOCH3
II III
Hal in the formula III is a halogen atom, in particular chlorine
or bromine.
15 The halogenating agents used are customary organic or inorganic
compounds, eg. chlorine, bromine or n-bromosuccinimide. N-Bromo-
succinimide is preferably used.
This reaction is carried out at from 0 C to 150 C, preferably from
20 40-C to 100-C.
Suitable solvents are aliphatic hydrocarbons such as pentane,
hexane, cyclohexane and petroleum ether, aromatic hydrocarbons
such as toluene, o-, m- and p-xylene, and halogenated hydrocar-
25 bons such as methylene chloride, chloroform, chlorobenzene andtetrachloromethane, particularly preferably tetrachloromethane.
Mixtures of the solvents mentioned can also be used.
30 In general, the halogenating agents are used in an equimolar
amount, in an excess or if appropriate as a solvent.
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
` ~ 5 2137018
2. Preparation of the oxime ether V (cf. EP-A 463 488,
EP-A 460 575, EP-A 472 300)
CH2Hal
CN +HON=CR ~ R3m
NOCH3
III IV
CH2ON=CR2 ~ R3m
~ ~ CN
NOCH3
V
This reaction of III with IV is customarily carried out at from
0 C to 100 C, preferably from 15 C to 60 C, in an inert organic -
20 solvent in the presence of a base.
Suitable solvents are aliphatic hydrocarbons such as pentane,hexane, cyclohexane and petroleum ether, aromatic hydrocarbons
such as toluene, o-, m- and p-xylene, halogenated hydrocarbons
25 such as methylene chloride, chloroform and chlorobenzene, ethers
such as diethyl ether, diisopropyl ether, tert-butyl methyl
ether, dioxane, anisole and tetrahydrofuran, nitriles such as
acetonitrile and propionitrile, ketones such as acetone, methyl
ethyl ketone, diethyl ketone and tert-butyl methyl ketone,
30 alcohols such as methanol, ethanol, n-propanol, isopropanol,
n-butanol and tert-butanol, and dimethyl sulfoxide and dimethyl-
formamide, particularly preferably acetonitrile, dimethylforma-
mide and tetrahydrofuran.
35 Mixtures of the solvents mentioned can also be used.
Suitable bases are generally inorganic compounds such as alkali
metal and alkaline earth metal hydroxides such as lithium hydrox-
ide, sodium hydroxide, potassium hydroxide and calcium hydroxide,
40 alkali metal and alkaline earth metal oxides such as lithium
oxide, sodium oxide, calcium oxide and magnesium oxide, alkali
metal and alkaline earth metal hydrides such as lithium hydride,
sodium hydride, potassium hydride and calcium hydride, alkali
metal amides such as lithium amide, sodium amide and potassium
45 amide, alkali metal and alkaline earth metal carbonates such as
lithium carbonate and calcium carbonate, and alkali metal hydro-
gen carbonates such as sodium hydrogen carbonate, organometallic
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
- 2l37nls
compounds, in particular alkali metal alkyls such as methyl-
lithium, butyllithium and phenyllithium, alkylmagnesium halides
such as methylmagnesium chloride, and alkali metal and alkaline
earth metal alkoxides such as sodium methoxide, sodium ethoxide,
5 potassium ethoxide, potassium tert-butoxide and dimethoxymagne-
sium, additionally organic bases, eg. tertiary amines such as
trimethylamine, triethylamine, triisopropylethylamine and
N-methylpiperidine, pyridine, substituted pyridines such as col-
lidine, lutidine and 4-dimethylaminopyridine, and also bicyclic
10 amines.
Sodium methoxide, sodium ethoxide and potassium hydroxide are
particularly preferred.
15 In general, the bases are employed in an equimolar amount, but
they can also be used in an excess or if appropriate as a
solvent.
In general, the starting materials III and IV are reacted with -
20 one another in equimolar amounts. It can be advantageous for the
yield to employ IV in an excess or deficit based on III.
The compounds IV needed for the reaction are known from the prior
literature-(cf. Houben-Weyl, Vol. E14b, p. 287-367) or can be
25 prepared similarly to the processes described there.
3. Preparation of the thiocarboxamide VI (cf. Houben-Weyl,
Vol. E5, pp. 1253-1255)
R3m ~-~ R3m
CH20N=CR2 ~ CH20N=CR
~ ~ CN ~ ~ CSNHz
V NOCH3 NOCH3
This reaction with hydrogen sulfide is customarily carried out at
from 10 C to 180 C, preferably from 20 C to 80 C, in an inert or-
ganic solvent in the presence of a base.
Suitable solvents are aliphatic hydrocarbons such as pentane,
hexane, cyclohexane and petroleum ether, aromatic hydrocarbons
such as toluene or o-, m- and p-xylene, halogenated hydrocarbons
such as methylene chloride, chloroform and chlorobenzene, ethers
45 such as diethyl ether, diisopropyl ether, tert-butyl methyl
ether, dioxane, anisole and tetrahydrofuran, ketones such as ace-
tone, methyl ethyl ketone, diethyl ketone and tert-butyl methyl
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
" - 2137018
ketone, alcohols such as methanol, ethanol, n-propanol, isopro-
panol, n-butanol and tert-butanol, and dimethyl sulfoxide and di-
methylformamide, particularly preferably dimethylformamide and
tetrahydrofuran.
Mixtures of the solvents mentioned can also be used.
Suitable bases are generally inorganic compounds such as alkali
metal and alkaline earth metal hydroxides such as lithium hydrox-
10 ide, sodium hydroxide, potassium hydroxide and calcium hydroxide,alkali metal and alkaline earth metal oxides such as lithium ox-
ide, sodium oxide, calcium oxide and magnesium oxide, alkali
metal and alkaline earth metal carbonates such as lithium carbon-
ate and calcium carbonate, and ~lk~l; metal hydrogen carbonates
15 such as sodium hydrogen carbonate, alkali metal and alkaline
earth metal alkoxides such as sodium methoxide, sodium ethoxide,
potassium ethoxide, potassium tert-butoxide and dimethoxymagne-
sium, additionally organic bases, eg. tertiary amines such as
trimethylamine, triethylamine, triisopropylethylamine and
20 N-methylpiperidine, pyridine, substituted pyridines such as col-
lidine, lutidine and 4-dimethylaminopyridine, and also bicyclic
amines.
Diethylamine, triethylamine, pyridine, potassium carbonate,
25 sodium carbonate, potassium hydroxide and sodium hydroxide are
particularly preferred.
In general, the bases are employed in catalytic amounts, but they
can also be used in an equimolar amount, in an excess or if ap-
30 propriate as a solvent.
In addition to the direct reaction of V to give VI describedabove, it is also possible first to convert V to the carboxylic
acid VII and then to convert VII to the corresponding thiocarbox-
35 amide VI via the carboxamide VIII.
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
821~7018
~ R3m ~ R3m
5~ ~ CN ~ ~ CO2H
NOCH3 NOCH3
V VII
CH2ON=CR2 ~ R3m
~ __~ VI
~ CONH2
NOCH3
15VIII
The hydrolysis of V to VII is customarily carried out by reacting
with aqueous acid or base, if appropriate with addition of an in-
ert solvent, at from 20 C to 100 C (cf. Organikum l9th Edition,
(1993) pp. 441-444.
To prepare VIII, the carboxylic acid VII is in general reacted
with ammonia in an inert solvent at from 0 C to 100 C, if ap-
propriate using an activating reagent (cf. Houben-Weyl, Vol. E5,
pp. 941-1044).
The further reaction of VIII to give VI is carried out in a man-
ner known per se using a sulfurizing agent in an inert solvent at
from 50 C to 150 C (cf. Houben-Weyl, Vol. E5, pp. 1242-1245).
30 4. Preparation of the thioimidate I
CH2ON=CR2 ~ R3m CH20N=CR2 ~ R3m
~ ~ CSNH2 --~ ~ ~ C(=NH)-SR
NOCH3 NOCH3
VI
The thioimidates I are customarily obtained from the thiocarboxa-
40 mides VI by reacting with an alkylating agent in an inert organic
solvent in the presence of a base. In general, the alkylating
agents used are alkyl halides, in particular chlorides, bromides
and iodides, or alkyl sulfates.
~ ~ASF Aktiengesell~chaft 930627 O.Z. 0050/44487
" ~ 9 2137018
Suitable solvents are, in particular, toluene, dichloromethane,
diethyl ether, dioxane, tetrahydrofuran, dimethylformamide and
dimethyl sulfoxide.
5 Preferred bases are potassium hydroxide, potassium carbonate,
sodium carbonate, sodium hydride, sodium methoxide, sodium ethox-
ide and potassium tert-butoxide.
In addition, suitable alkylating agents are also trialkyloxonium
10 salts, in particular trialkyloxonium tetrafluoroborate. Suitable
solvents in this case are, in particular, toluene, dichloro-
methane, diethyl ether, tetrahydrofuran and dioxane. A reaction
of this type is disclosed, for example, in EP-A 528 681.
15 In the processes for preparing the compounds I outlined above, in
general it is insignificant whether the -CN group is first con-
verted to the desired group (-C(=NH)-SR1) or whether the toluene
methyl group is first converted. Correspondingly, the conversions
can be performed in any desired sequence.
In the above reactions, the compounds IX defined at the outset
are particularly preferably used as intermediates. The corre-
sponding compounds, in which the -C=N- double bond has the E con-
figuration with respect to the -OCH3 group and the radical -Y, are
25 in some cases known from the literature (EP-A 477 631,
EP-A 463 488, EP Appl. No. 93 113 372.6) or can be obtained by
the processes described there.
The compounds I (Z configuration with respect to the -OCH3 group
30 and the -C(=NH)-SRl radical) obtA;nAhle from the intermediates IX
are additionally useful intermediates for the preparation of the
known active compounds of the formula X
~-~ R3m
~ CH20N=CR2 ~
CONHCH3 X
H3CO~
where the radicals R2 and R3 and the index m have the above
meanings.
These compounds X are obtained, for example, in the manner indi-
45 cated in the following reaction scheme.
8ASF Aktiengesellschaft 930627 O.Z. 0050/44487
21~7(~18
R3m ~ R3m
~ CH20N=CR2 ~ =~ CH20N=CR2 ~ =~)
5 ~ ~ C(=NH)-SRl ~ ~ COSR
N \ N ~
OCH3 OCH3
I.A XI
(Z configuration)
CH2ON=CR2 ~ R3m CH2ON=CR2 ~ R3m
15 ~ CONHCH3 ~ ~ CONHCH3
N ~ ~ N
OCH3 CH30
X.A X
(Z configuration)
The hydrolysis of the thioimidates I.A is carried out at from 0 C
to 100 C, preferably from 0 C to 50 C, in particular from 0 C to
30 C, in an inert solvent in the presence of an acid (cf. Liebigs.
25 Ann. Chem. 768 (1976); Can. J. Chem. 48, (1970) 3662).
Suitable solvents are toluene, diethyl ether, dioxane, tetra-
hydrofuran, methanol, ethanol and dimethylformamide.
30 The thioester XI thus obtained is then reacted with methylamine
at from 0 C to 60 C, preferably from 0 C to 30 C, in particular
from 0-C to 25-C, in an inert organic solvent, if appropriate in
the presence of water.
35 The desired active compounds having the E configuration are
obtained from the compounds X.A thus obtained having the Z con-
figuration by addition of acid or photochemically.
Because of the basic character of the NH group, the compound I is
40 able to form salts or adducts with inorganic or organic acids or
with metal ions.
Examples of inorganic acids are hydrohalic acids such as hydro-
fluoric acid, hydrochloric acid, hydrobromic acid and hydriodic
45 acid, sulfuric acid, phosphoric acid and nitric acid.
BASF AXtiengesell~chaft 930627 O.Z. 0050/44487
' ' ~ 1 1
Suitable organic acids are, for example, formic acid, carbonic
acid and alkanoic acids such as acetic acid, trifluoroacetic
acid, trichloroacetic acid and propionic acid, and glycolic acid,
thiocyanic acid, lactic acid, succinic acid, citric acid, benzoic
5 acid, cinnAm;c acid, oxalic acid, alkanesulfonic acids (sulfonic
acids cont~;n;ng straight-chain or branched alkyl radicals having
1 to 20 carbon atoms), arylsulfonic acids or -disulfonic acids
(aromatic radicals such as phenyl and naphthyl which carry one or
two sulfonic acid groups), alkylphosphonic acids (phosphonic
10 acids cont~;n;ng straight-chain or branched alkyl radicals having
1 to 20 carbon atoms), arylphosphonic acids or -diphosphonic
acids (aromatic radicals such as phenyl and naphthyl which carry
one or two phosphonic acid radicals), it being possible for the
alkyl or aryl radicals to carry further substituents, eg.
15 p-toluenesulfonic acid, salicylic acid, p-aminosalicylic acid,
2-phenoxybenzoic acid, 2-acetoxybenzoic acid etc.
Suitable metal ions are in particular the ions of the elements of
the second main group, in particular calcium and magnesium, of -
20 the third and fourth main group, in particular aluminum, tin andlead, and also of the first to eighth subgroup, in particular
chromium, manganese, iron, cobalt, nickel, copper, zinc and
others. The metal ions of the elements of the subgroups of the
fourth period are particularly preferred. The metals can be pres-
25 ent here in the various valencies befitting them.
In the definitions of the compounds I and IX given at the outset,collective terms were used which are generally representative of
the following substituents:
H~logen: fluorine, chlorine, bromine and iodine;
Alkyl: straight-chain or branched alkyl groups having 1 to 3 or
6 carbon atoms, eg. C1-C6-alkyl such as methyl, ethyl, propyl,
35 1-methylethyl, butyl, 1-methylpropyl, 2-methylpropyl, 1,1-di-
methylethyl, pentyl, l-methylbutyl, 2-methylbutyl, 3-methylbutyl,
2,2-dimethylpropyl, l-ethylpropyl, hexyl, l,l-dimethylpropyl,
1,2-dimethylpropyl, l-methylpentyl, 2-methylpentyl, 3-methyl-
pentyl, 4-methylpentyl, l,l-dimethylbutyl, 1,2-dimethylbutyl,
40 1,3-dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-
dimethylbutyl, l-ethylbutyl, 2-ethylbutyl, 1,1,2-trimethylpropyl,
1,2,2-trimethylpropyl, l-ethyl-l-methylpropyl and 1-ethyl-2-
methylpropyl;
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
12 2137018
Alkyl~rino: an amino group which carries a straight-chain or
branched alkyl group having 1 to 6 carbon atoms as mentioned
above;
5 Dialkyl~oino: an amino group which carries two straight-chain or
branched alkyl groups which are independent of one another and
each have 1 to 6 carbon atoms as mentioned above;
Haloalkyl: straight-chain or branched alkyl groups having 1 to 6
10 carbon atoms, it being possible in these groups for the hydrogen
atoms to be partly or completely replaced by halogen atoms as
mentioned above, eg. C1-C2-halogenalkyl such as chloromethyl,
dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, chlorofluoromethyl, dichlorofluoromethyl,
15 chlorodifluoromethyl, 1-fluoroethyl, 2-fluoroethyl, 2,2-difluoro-
ethyl, 2,2,2-trifluoroethyl, 2-chloro-2-fluoroethyl, 2-chloro-
2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-trichloro-
ethyl and pentafluoroethyl;
20 Alkoxy: straight-chain or branched alkyl groups having 1 to 6
carbon atoms as mentioned above, which are bonded to the struc-
ture via an oxygen atom (-O-);
Alkoxyalkyl: straight-chain or branched alkyl groups having 1 to
25 6 carbon atoms as mentioned above, which in any desired position
carry a straight-chain or branched alkoxy group having 1 to 6
carbon atoms as mentioned above;
Halo~l~o-y: straight-chain or branched alkoxy groups having 1 to
30 6 carbon atoms as mentioned above, it being possible in these
groups for the hydrogen atoms to be partly or completely replaced
by halogen atoms as mentioned above, eg. C1-C2-haloalkoxy such as
chloromethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy,
difluoromethoxy, trifluoromethoxy, chlorofluoromethoxy, dichloro-
35 fluoromethoxy, chlorodifluoromethoxy, l-fluoroethoxy, 2-fluoro-
ethoxy, 2,2-difluoroethoxy, 2,2,2-trifluoroethoxy, 2-chloro-2-
fluoroethoxy, 2-chloro-2,2-difluoroethoxy, 2,2-dichloro-2-fluoro-
ethoxy, 2,2,2-trichloroethoxy and pentafluoroethoxy;
40 Alkylthio: straight-chain or branched alkyl groups having 1 to 6
carbon atoms as mentioned above, which are bonded to the struc-
ture via a sulfur atom (-S-), eg. C1-C6-alkylthio such as methyl-
thio, ethylthio, propylthio, l-methylethylthio, butylthio,
1-methylpropylthio, 2-methylpropylthio, l,l-dimethylethylthio,
45 pentylthio, l-methylbutylthio, 2-methylbutylthio, 3-methylbutyl-
thio, 2,2-di-methylpropylthio, l-ethylpropylthio, hexylthio,
l,l-dimethylpropylthio, 1,2-dimethylpropylthio,
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
, 13 21~70~8
1-methylpentylthio, 2-methylpentylthio, 3-methylpentylthio,
4-methylpentylthio, l,1-dimethylbutylthio, 1,2-dimethylbutylthio,
1,3-dimethylbutylthio, 2,2-dimethylbutylthio, 2,3-dimethylbutyl-
thio, 3,3-dimethylbutylthio, 1-ethylbutylthio, 2-ethylbutylthio,
5 1,1,2-trimethylpropylthio, 1,2,2-trimethylpropylthio, 1-ethyl-1-
methylpropylthio and 1-ethyl-2-methylpropylthio;
Alkenyl: straight-chain or branched hydrocarbon groups having 2
to 6 carbon atoms and a double bond in any desired position, eg.
10 ethenyl, 1-propenyl, 2-propenyl, 1-methylethenyl, 1-butenyl,
2-butenyl, 3-butenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl,
1-methyl-2-propenyl, 2-methyl-2-propenyl, 1-pentenyl, 2-pentenyl,
3-pentenyl, 4-pentenyl, 1-methyl-1-butenyl, 2-methyl-1-butenyl,
3-methyl-1-butenyl, 1-methyl-2-butenyl, 2-methyl-2-butenyl,
15 3-methyl-2-butenyl, 1-methyl-3-butenyl, 2-methyl-3-butenyl,
3-methyl-3-butenyl, 1,1-dimethyl-2-propenyl, 1,2-dimethyl-1-
propenyl, 1,2-dimethyl-2-propenyl, 1-ethyl-1-propenyl,
1-ethyl-2-propenyl, 1-hexenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl,
5-hexenyl, 1-methyl-1-pentenyl, 2-methyl-1-pentenyl, 3-methyl- -
20 1-pentenyl, 4-methyl-1-pentenyl, 1-methyl-2-pentenyl, 2-methyl-
2-pentenyl, 3-methyl-2-pentenyl, 4-methyl-2-pentenyl, 1-methyl-
3-pentenyl, 2-methyl-3-pentenyl, 3-methyl-3-pentenyl, 4-methyl-
3-pentenyl, 1-methyl-4-pentenyl, 2-methyl-4-pentenyl, 3-methyl-
4-pentenyl, 4-methyl-4-pentenyl, 1,1-dimethyl-2-butenyl, 1,1-di-
25 methyl-3-butenyl, 1,2-dimethyl-1-butenyl, 1,2-dimethyl-2-butenyl,
1,2-dimethyl-3-butenyl, 1,3-dimethyl-1-butenyl, 1,3-dimethyl-2-
butenyl, 1,3-dimethyl-3-butenyl, 2,2-dimethyl-3-butenyl, 2,3-di-
methyl-1-butenyl, 2,3-dimethyl-2-butenyl, 2,3-dimethyl-3-butenyl,
3,3-dimethyl-1-butenyl, 3,3-dimethyl-2-butenyl, 1-ethyl-1-
30 butenyl, 1-ethyl-2-butenyl, 1-ethyl-3-butenyl, 2-ethyl-1-
butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl, 1,1,2-trimethyl-
2-propenyl, 1-ethyl-1-methyl-2-propenyl, 1-ethyl-2-methyl-1-
propenyl and 1-ethyl-2-methyl-2-propenyl;
35 ~ losy: straight-chain or branched alkenyl groups having 2 to
6 carbon atoms as mentioned above, which are bonded to the
structure via an oxygen atom (-o-);
Alkynyl: straight-chain or branched hydrocarbon groups having 2
40 to 6 carbon atoms and a triple bond in any desired position, eg.
ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl,
1-methyl-2-propynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pent-
ynyl, 1-methyl-2-butynyl, 1-methyl-3-butynyl, 2-methyl-3-butynyl,
3-methyl-1-butynyl, 1,1-dimethyl-2-propynyl, 1-ethyl-2-propynyl,
45 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-
-2-pentynyl, 1-methyl-3-pentynyl, 1-methyl-4-pentynyl, 2-methyl-
-3-pentynyl, 2-methyl-4-pentynyl, 3-methyl-1-pentynyl,
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
` - 21370~8
14
3-methyl-4-pentynyl, 4-methyl-1-pentynyl, 4-methyl-2-pentynyl,
1,1-dimethyl-2-butynyl, 1,1-dimethyl-3-butynyl, 1,2-dimethyl-
3-butynyl, 2,2-dimethyl-3-butynyl, 3,3-dimethyl-1-butynyl,
1-ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl and
5 1-ethyl-1-methyl-2-propynyl;
Alkynylosy: straight-chain or branched alkynyl groups having 2 to
6 carbon atoms as mentioned above, which are bonded to the
10 structure via an oxygen atom (-O-);
Cycloalkyl: monocyclic alkyl groups having 3 to 6 carbon ring
members, such as cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl;
Cyclc~l~o~y: monocyclic alkyl groups having 3 to 6 carbon ring
members as mentioned above, which are bonded to the structure via
an oxy group (-O-);
20 Compounds I are also particularly preferred in which Rl is
C1-C4-alkyl, in particular methyl or ethyl.
In addition, compounds I are preferred in which R2 is cyclopropyl.
25 Further particularly preferred compounds I are those in which R2
is methyl or ethyl, in particular methyl.
Compounds I are additionally preferred in which m is 1, 2 or 3.
30 Compounds I are also preferred in which R3 is one of the following
groups: C(=O)Ra, C(=O)ORa, OC(=O)Ra, C(=O)NRaRb, C(=S)NRaRb,
NRaC(=O)Rb and NRaC(=O)ORb, Ra and Rb independently of one another
preferably being hydrogen or methyl.
35 Compounds I are furthermore preferred in which R3 is one of the
following groups: CRa=NRb, C(Ra)=NORb, C(XRa)=NORb and N=CRaRb, Ra
and Rb independently of one another preferably being methyl or
ethyl.
40 In addition, compounds I are preferred in which R3 is one to three
of the following groups: halogen, Cl-C4-alkyl, Cl-C4-haloalkyl,
Cl-C4-alkoxy, Cl-C4-haloalkoxy, Cl-C4-alkylthio and/or
C3-C6-cycloalkyl.
45 Compounds I are additionally preferred in which at least one R3
radical is phenyl or phenoxy.
- 8ASF Aktiengesellschaft 930627 O.Z. 0050/44487
` 15 21~7~18
other preferred compounds I are those in which one to three of
the R3 radicals are halogen atoms, in particular chlorine.
Compounds I are also preferred in which two R3 radicals bonded to
5 adjacent C atoms together are - CH=CH - CH=CH - .
Compounds I are particularly preferred in which Rl is methyl or
ethyl, R2 is methyl and R3 is halogen.
10 With respect to their biological action, particularly preferred
compounds I are compiled in the following tables; particular
importance is likewise ascribed to the corresponding compounds
having the Z configuration with respect to the preferred
preparation.
Table A
Compounds of the formula I.l, in which the combination of the
substituents Rl and R3m for one compound in each case corresponds
to one line of a column of the table.
CH3
CH20N ~3 R3m I . 1
~ ~ C(=NH)-SR
/ N
H3CO
30 Table B
Compounds of the formula I.2, in which the combination of the
substituents Rl and R3m for one compound in each case corresponds
to one line of a column of the table.
CH20N~ 3 R3m I.2
C ( =NH ) -SR
N-OCH3
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
2137018
16
Table
5 Column 1 Column 2
R3m Rl R3m Rl
-- (m=0) C2Hs 2-Cl C2H5
3-Cl C2H5 4-Cl C2H5
3,4-C12 C2H5 3,5-C12 C2H5
2,3,4-C13 C2H5 2,4,5-Cl3 C2H5
2-Br C2H5 3-Br C2H5
4-Br C2H5 2-NO2 C2H5
3-NO2 C2H5 4-NO2 C2H5
15 2-CH3 C2H5 3-CH3 C2H5
4-CH3 C2H5 2,4-(CH3)2 C2H5
3,4-(CH3)2 C2H5 3,5-(CH3)2 C2H5
2,5-(CH3)2 C2H5 2-CH(CH3)2 C2H5
20 3-CH(CH3)2 C2H5 4-CH(CH3)2 C2H5
2-c(CH3)3 C2H5 3-C(CH3)3 C2H5
4-C(CH3)3 C2H5 3-CH3, 4-C(CH3)3 C2H5
4-CH3, 3-C(CH3)3 C2Hs 2-OCH3 C2H5
3-OCH3 C2H5 4-OCH3 C2H5
2-OCH(CH3)2 C2H5 3-OCH(CH3)2 C2H5
4-OCH(CH3)2 C2H5 2-OC(CH3)3 C2H5
3-OC(CH3)3 C2H5 4-OC(CH3)3 C2H5
3-CF3 C2H5 4-CF3 C2H5
30 3-OCF3 C2H5 4-OCF3 C2H5
3-SC(CH3)3 C2H5 4-SC(CH3)3 C2H5
3-C(CH3)=NOCH3 C2H5 4-C(CH3)=NOCH3 C2H5
2-Cl (CH2)2CH3 3-Cl (CH2)2CH3
35 4-Cl (CH2)2CH3 3,4-C12 (CH2)2CH3
3,5-Cl2 (CH2)2CH3 2,3,4-Cl3 (CH2)2CH3
2,4,5-Cl3 (CH2)2CH3 2-Br (CH2)2CH3
3-Br (CH2)2CH3 4-Br (CH2)2CH3
2-NO2 (CH2)2CH3 3-NO2 (CH2)2CH3
4-NO2 (CH2)2CH3 2-CH3 (CH2)2CH3
3-CH3 (CH2)2CH3 4-CH3 (CH2)2CH3
2,4-(CH3)2 (CH2)2CH3 3,4-(CH3)2 (CH2)2CH3
3,5-(CH3)2 (CH2)2CH3 2,5-(CH3)2 (CH2)2CH3
2-CH(CH3)2 (CH2)2CH3 3-CH(CH3)2 (CH2)2CH3
4-CH(CH3)2 (CH2)2CH3 2-C(CH3)3 (CH2)2CH3
~ BASF Aktiengesellschaft 930627 O.z. 0050/44487
` ~ 17 21~7Q18
Column 1 Column 2
R3m Rl R3~ R1
3-C(CH3)3 (CH2)2CH3 4-C(CH3)3 (CH2)2CH3
5 3-CH3, 4-C(CH3)3 (CH2)2CH3 4-CH3, 3-C(CH3)3 (CH2)2CH3
2-OCH3 (CH2)2CH3 3-OCH3 (CH2)2CH3
4-OCH3 (CH2)2CH3 2-OCH(CH3)2 (CH2)2CH3
3-OCH(CH3)2 (CH2)2CH3 4-OCH(CH3)2 (CH2)2CH3
2-OC(CH3)3 (CH2)2CH3 3-oc(CH3)3 (CH2)2CH3
4-OC(CH3)3 (CH2)2CH3 3-CF3 (CH2)2CH3
4-CF3 (CH2)2CH3 3-OCF3 (CH2)2CH3
4-OCF3 (CH2)2CH3 3-SC(CH3)3 (CH2)2CH3
4-SC(CH3)3 (CH2)2CH3 3-C(CH3)=NOCH3 (CH2)2CH3
15 4-c(cH3)=NocH3 (CH2)2CH3 2-Cl CH3
3-Cl CH3 4-Cl CH3
3,4-C12 CH3 3,5-C12 CH3
2,3,4-Cl3 CH3 2,4,5-C13 CH3
20 2-Br CH3 3-Br CH3
4-Br CH3 2-N02 CH3
3-NO2 CH3 4-N02 CH3
2-CH3 CH3 3-CH3 CH3
4-CH3 CH3 2,4-(CH3)2 CH3
3,4-(CH3)2 CH3 3,5-(CH3)2 CH3
2,5-(CH3)2 CH3 2-CH(CH3)2 CH3
3-CH(CH3)2 CH3 4-CH(CH3)2 CH3
2-c(CH3)3 CH3 3-C(CH3)3 CH3
4-C(CH3)3 CH3 3-CH3, 4-C(CH3)3 CH3
4-CH3, 3-C(CH3)3 CH3 2-OCH3 CH3
3-OCH3 CH3 4-OCH3 CH3
2-OCH(CH3)2 CH3 3-OCH(CH3)2 CH3
35 4-OCH(CH3) 2 CH3 2-OC(CH3)3 CH3
3-OC(CH3)3 CH3 4-OC(CH3)3 CH3
3-CF3 CH3 4-CF3 CH3
3-OCF3 CH3 4-OCF3 CH3
40 3-SC(CH3)3 CH3 4-SC(CH3)3 CH3
3-C(CH3)=NOCH3 CH3 4-C(CH3)=NOCH3 CH3
2-Cl (CH2)3cH3 3-Cl (CH2)3cH3
4-Cl (CH2)3cH3 3,4-C12 (CH2)3cH3
3,5-C12 (cH2)3CH3 2,3,4-Cl3 (CH2)3cH3
2,4,5-Cl3 (CH2)3CH3 2-Br (CH2)3CH3
3-Br (CH2)3CH3 4-Br (CH2)3CH3
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
`` ~137018
. ..
18
Column 1 Column 2
R3m Rl R3m Rl
2-NO2 (CH2)3cH3 3-NO2 (CH2)3cH3
5 4-NO2 (CH2)3cH3 2-CH3 (CH2)3cH3
3-CH3 (CH2)3cH3 4-CH3 (CH2)3cH3
2,4-(CH3)2 (CH2)3cH3 3,4-(CH3)2 (CH2)3cH3
3,5-(CH3)2 (CH2)3cH3 2,5-(CH3)2 (CH2)3cH3
2-CH(CH3)2 (CH2)3cH3 3-CH(CH3)2 (CH2)3cH3
4-CH(CH3)2 (CH2)3cH3 2-c(CH3)3 (CH2)3cH3
3-C(CH3)3 (CH2)3cH3 4-C(CH3)3 (CH2)3cH3
3-CH3, 4-C(CH3)3 (CH2)3CH3 4-CH3, 3-C(CH3)3 (CH2)3cH3
2-OCH3 (CH2)3cH3 3-OCH3 (CH2)3cH3
15 4-OCH3 (CH2)3cH3 2-OCH(CH3)2 (CH2)3cH3
3-OCH(CH3)2 (CH2)3cH3 4-OCH(CH3)2 (CH2)3cH3
2-OC(CH3)3 (CH2)3cH3 3-OC(CH3)3 (CH2)3cH3
4-OC(CH3)3 (CH2)3cH3 3-CF3 (CH2)3cH3
20 4-CF3 (CH2)3cH3 3-OCF3 (CH2)3CH3
4-OCF3 (CH2)3cH3 3-SC(CH3)3 (CH2)3cH3
4-SC(CH3)3 (CH2)3cH3 3-C(CH3)=NOCH3 (CH2)3cH3
4-C(CH3)=NOCH3 (CH2)3cH3 2-Cl CH(CH3)2
25 3-Cl CH(CH3)2 4-Cl CH(cH3)2
3,4-C12 CH(CH3)2 3,5-C12 CH(CH3)2
2,3,4-Cl3 CH(CH3)2 2,4,5-Cl3 CH(CH3)2
2-Br CH(CH3)2 3-Br CH(CH3)2
4-Br CH(CH3)2 2-NO2 CH(CH3)2
30 3-NO2 CH(CH3)2 4-NO2 CH(CH3)2
2-CH3 CH(cH3)2 3-CH3 CH(CH3)2
4-CH3 CH(CH3)2 2,4-(CH3)2 CH(CH3)2
3,4-(CH3)2 CH(CH3)2 3,5-(CH3)2 CH(CH3)2
35 2,5-(CH3)2 CH(cH3)2 2-CH(CH3)2 CH(CH3)2
3-CH(CH3)2 CH(CH3)2 4-CH(CH3)2 CH(CH3)2
2-c(CH3)3 CH(CH3)2 3-C(CH3)3 CH(CH3)2
4-C(CH3)3 CH(CH3)2 3-CH3, 4-C(CH3)3 CH(CH3)2
40 4-CH3, 3-C(CH3)3 CH(CH3)2 2-OCH3 CH(CH3)2
3-OCH3 CH(CH3)2 4-OCH3 CH(CH3)2
2-OCH(CH3)2 CH(CH3)2 3-OCH(CH3)2 CH(CH3)2
4-OCH(cH3)2 CH(CH3)2 2-OC(CH3)3 CH(CH3)2
3-OC(CH3)3 CH(CH3)2 4-OC(CH3)3 CH(cH3)2
45 3-CF3 CH(cH3)2 4-CF3 CH(cH3)2
3-OCF3 CH(CH3)2 4-OCF3 CH(CH3)2
i BASF Aktiengesellschaft 930627 O.Z. 0050/44487
``` 2137018
19
Column 1 Column 2
R3m Rl R3m Rl
3-SC(CH3)3 CH(CH3)2 4-SC(CH3)3 CH(CH3)2
5 3-C(CH3)=NOCH3 CH(CH3)2 4-C(CH3)=NOCH3 CH(CH3) 2
3,4-CH=CH-CH=CH- CH3 3,4-CH=CH-CH=CH- C2H5
The compounds I are suitable as fungicides.
The compounds I are distinguished by an outstanding activity
against a broad spectrum of phytopathogenic fungi, in particular
from the class of Ascomycetes and Basidiomycetes. They are
systemically active in some cases and can be employed as foliar
15 and soil fungicides.
They are of particular importance for the control of a
multiplicity of fungi on various crop plants such as wheat, rye,
barley, oats, rice, corn, grass, cotton, soybeans, coffee, sugar
20 cane, grapes, fruit and decorative plants and vegetable plants
such as cucumbers, beans and cucurbits, and on the seeds of these
plants.
They are specifically suitable for the control of the following
25 plant diseases: Erysiphe graminis (powdery mildew) in cereals,
Erysiphe cichoracearum and Sphaerotheca fuliginea on cucurbits,
Podosphaera leucotricha on apples, Uncinula necator on vines,
Puccinia species on cereals, Rhizoctonia species on cotton and
lawns, Ustilago species on cereals and sugar cane, Venturia in-
30 aequalis (scab) on apples, Helminthosporium species on cereals,Septoria nodorum on wheat, Botrytis cinerea (gray mold) on straw-
berries, vines, Cercospora arachidicola on groundnuts, Pseudocer-
cosporella herpotrichoides on wheat, barley, Pyricularia oryzae
on rice, Phytophthora infestans on potatoes and tomatoes, Fusa-
35 rium and Verticillium species on various plants, Plasmopara viti-
cola on vines, Alternaria species on vegetables and fruit.
The compounds I are applied by treating the fungi or the plants,
seeds, materials or the soil to be protected from fungal attack
40 with a fungicidally effective amount of the active compounds.
They are applied before or after the infection of the materials,
plants or seeds by the fungi.
They can be converted into the customary formulations, such as
45 solutions, emulsions, suspensions, dusts, powders, pastes and
granules. The application form depends on the particular intended
use; they should in any case guarantee a fine and uniform
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
`.- 2137()18
~ 20
dispersion of the orthosubstituted benzyl ester of a cyclopro-
panecarboxylic acid. The formulations are prepared in a known
manner, eg. by extending the active compound with solvents and/or
carriers, if desired using emulsifiers and dispersants, it also
5 being possible to use other organic solvents as auxiliary sol-
vents when water is used as a diluent. Suitable auxiliary sub-
stances for this purpose are essentially: solvents such as aro-
matics (eg. xylene), chlorinated aromatics (eg. chlorobenzenes)~
paraffins (eg. mineral oil fractions), alcohols (eg. methanol,
10 butanol), ketones (eg. cyclohexanone), amines (eg. ethanolamine,
dimethylformamide) and water; carriers such as ground natural
minerals (eg. kaolins, clays, talc, chalk) and ground synthetic
minerals (eg. highly disperse silicic acid, silicates); emulsifi-
ers such as nonionic and anionic emulsifiers (eg. polyoxyethylene
15 fatty alcohol ethers, alkylsulfonates and arylsulfonates) and
dispersants such as lignin-sulfite waste liquors and methylcellu-
lose.
The fungicidal compositions in general contain from 0.1 to 95,
20 preferably from 0.5 to 90, % by weight of active compound.
Depending on the type of effect desired, the application rates
are from 0.01 to 2.0 kg of active compound per ha.
25 In seed treatment, active compound amounts of from 0.001 to
0.1 g, preferably 0.01 to 0.05 g, per kilogram of seed are in
general needed.
The compositions according to the invention can also be present
30 as fungicides together with other active compounds in the ap-
plication form, eg. with herbicides, insecticides, growth regula-
tors, fungicides or alternatively with fertilizers.
on mixing with fungicides, in many cases an increase in the fun-
35 gicidal spectrum of action is obtained here.
The following list of fungicides with which the compounds accord-
ing to the invention can be applied together should illustrate
the combination possibilities, but not restrict them:
sulfur, dithiocarbamates and their derivatives, such as ferric
dimethyldithiocarbamate, zinc dimethyldithiocarbamate, zinc
ethylenebisdithiocarbamate, manganese ethylenebisdithiocarbamate,
manganese zinc ethylenediaminebisdithiocarbamate, tetramethyl-
45 thiuram disulfide, ammonia complex of zinc (N,N-ethylenebisdi-
thiocarbamate), ammonia complex of zinc (N,N'-propylenebisdithio-
; BASF Aktiengesellschaft 930627 O.Z. 0050/44487
_ 21 2137018
carbamate), zinc (N,N'-propylenebisdithiocarbamate), N,N'-poly-
propylenebis(thiocarbamoyl) disulfide;
nitro derivatives, such as dinitro(1-methylheptyl)phenyl
5 crotonate, 2-sec-butyl-4,6-dinitrophenyl 3,3-dimethylacrylate,
2-sec-butyl-4,6-dinitrophenyl isopropylcarbonate, diisopropyl
5-nitroisophthalate;
heterocyclic substances, such as 2-heptadecyl-2-imidazoline ace-
10 tate, 2,4-dichloro-6-(o-chloroAn;l;no)-s-triazine, 0,0-diethyl
phthAl;m;dophosphonothioate, 5-amino-1-[bis(dimethylamino)
phosphinyl]-3-phenyl-1,2,4-triazole, 2,3-dicyano-1,4-dithio-
anthraquinone, 2-thio-1,3-dithiolo[4,5-b]quinoxaline, methyl
1-(butylcarbamoyl)-2-benzimidazole carbamate, 2-methoxycarbonyl-
15 aminobenzimidazole, 2-(fur-2-yl)benzimidazole, 2-(thiazol-4-yl)-
benzimidazole, N-(1,1,2,2-tetrachloroethylthio)tetrahydrophthal-
imide, N-trichloromethylthiotetrahydrophthalimide, N-trichloro-
methylthiophthAl; m; de;
20 N-dichlorofluoromethylthio-N',N'-dimethyl-N-phenylsulfamide,
5-ethoxy-3-trichloromethyl-1,2,3-thiadiazole, 2-thiocyanato-
methylthiobenzothiazole, 1,4-dichloro-2,5-dimethoxybenzene,
4-(2-chlorophenylhydrazono)-3-methyl-5-isoxazolone,
2-thiopyridine-1-oxide, 8-hydroxyquinoline or its copper salt,
25 2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiin, 2,3-dihydro-
5-carboYAn;l;do-6-methyl-1,4-oxathiin-4,4-dioxide, 2-methyl-
5,6-dihydro-4H-pyran-3-carboxanilide, 2-methylfuran-3-carboxani-
lide, 2,5-dimethylfuran-3-carboYAn;l;de, 2,4,5-trimethylfuran-
3-carboxanilide, N-cyclohexyl-2,5-dimethylfuran-3-carboxamide,
30 N-cyclohexyl-N-methoxy-2,5-dimethylfuran-3-carboxamide, 2-methyl-
benzanilide, 2-iodobenzanilide, N-formyl-N-morpho-
line-2,2,2-trichloroethyl acetal, piperazine-1,4-diyl-
bis(1-(2,2,2-trichloroethyl))formamide, 1-(3,4-dichloroAn;l;-
no)-1-formylamino-2,2,2-trichloroethane, 2,6-dimethyl-N-tridecyl-
35 morpholine or its salts, 2,6-dimethyl-N-cyclododecylmorpholine or
its salts, N-[3-(p-tert-butylphenyl)-2-methylpropyl]-cis-2,6-di-
methylmorpholine, N-[3-(p-tert-butylphenyl)-2-methylpropyl]piper-
idine, 1-[2-(2,4-dichlorophenyl)-4-ethyl-1,3-dioxolan-2-yl-
ethyl]-lH-1,2,4-triazole, 1-[2-(2,4-dichlorophenyl)-4-n-pro-
40 pyl-1,3-dioxolan-2-ylethyl]-lH-1,2,4-triazole, N-(n-pro-
pyl)-N-(2,4,6-trichlorophenoxyethyl)-N'-imidazolylurea,
1-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-triazol-1-yl)-2-bu-
tanone, l-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-triazol
-1-yl)-2-butanol, a-(2-chlorophenyl)-a-(4-chlorophenyl)-5-pyrimi-
45 dinemethanol, 5-butyl-2-dimethylamino-4-hydroxy-6-methylpyrimi-
dine, bis(p-chlorophenyl)-3-pyridinemethanol, 1,2-bis(3-ethoxy-
carbonyl-2-thioureido)benzene~ 1,2-bis(3-methoxycarbonyl-
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
22 ~ 7 0 1 8
2-thioureido)benzene~
and also various fungicides, such as dodecylguanidine acetate,
3-[3-(3~5-dimethyl-2-oxycyclohexyl)-2-hydroxyethyl]glutarimide~
5 hexachlorobenzene, DL-methyl-N-(2,6-dimethylphenyl)-N-2-furoyl
alaninate, DL-N-(2,6-dimethylphenyl)-N-(2~- methoxyacetyl)alanine
methyl ester, N-(2,6-dimethylphenyl)-N-chloroacetyl-D,L-2-amino-
butyrolactone, DL-N-(2,6-dimethylphenyl)-N-(phenylacetyl)alanine
methyl ester, 5-methyl-5-vinyl-3-(3,5-dichlorophenyl)-
10 2,4-dioxo-1,3-oxazolidine, 3-[3,5-dichlorophenyl-(5-methyl-5-
methoxymethyl]-1,3-oxazolidine-2,4-dione, 3-(3,5-dichlorophe-
nyl)-l-isopropylcarbamoylhydantoin, N-(3,5-dichlorophenyl)-
1,2-dimethylcyclopropane-1,2-dicarboximide, 2-cyano-[N-ethyl-
aminocarbonyl-2-methoximino]acetamide, 1-[2-(2,4-dichlorophenyl)-
15 pentyl]-lH-1,2,4-triazole, 2,4-difluoro-a-(lH-1,2,4-triazo-
lyl-l-methyl)benzhydryl alcohol, N-(3-chloro-2,6-dinitro-4-tri-
fluoromethylphenyl)-5-trifluoromethyl-3-chloro-2-aminopyridine,
l-((bis(4-fluorophenyl)methylsilyl)methyl-lH-1,2,4-triazole.
20 The compounds of the formula I are additionally suitable to con-
trol pests from the class of insects, arachnids and nematodes ef-
fectively. They can be employed as pesticides in plant protection
and in the hygiene, stored products protection and veterinary
sector.
The harmful insects include from the order of the butterflies
(Lepidoptera), for example, Agrotis ypsilon, Agrotis segetum,
Alabama argillacea, Anticarsia gemmatalis, Argyresthia
conjugella, Autographa gamma, Bupalus piniarius, Cacoecia
30 murinana, Capua reticulana, Cheimatobia brumata, Choristoneura
fumiferana, Choristoneura occidentalis, Cirphis unipuncta, Cydia
pomonella, Dendrolimus pini, Diaphania nitidalis, Diatraea
grandiosella, Earias insulana, Elasmopalpus lignosellus,
Eupoecilia ambiguella, Evetria bouliana, Feltia subterranea,
35 Galleria mellonella, Grapholita funebrana, Grapholita molesta,
Heliothis armigera, Heliothis virescens, Heliothis zea, Hellula
undalis, Hibernia defoliaria, Hyphantria cunea, Hyponomeuta
malinellus, Keiferia lycopersicella, Lambdina fiscellaria,
Laphygma exigua, Leucoptera coffeella, Leucoptera scitella,
40 Lithocolletis blancardella, Lobesia botrana, Loxostege
sticticalis, Lymantria dispar, Lymantria monacha, Lyonetia
clerkella, Malacosoma neustria, Mamestra brassicae, Orgyia
pseudotsugata, Ostrinia nubilalis, Panolis flammea, Pectinophora
gossypiella, Peridroma saucia, Phalera bucephala, Phthorimaea
45 operculella, Phyllocnistis citrella, Pieris brassicae, Plathypena
scabra, Plutella xylostella, Pseudoplusia includens,
Rhyacionia frustrana, Scrobipalpula absoluta, Sitotroga
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
-` _ 23 2137018
cerealella, Sparganothis pilleriana, Spodoptera frugiperda, Spo-
doptera littoralis, Spodoptera litura, Thaumatopoea pityocampa,
Tortrix viridana, Trichoplusia ni, Zeiraphera canadensis.
5 From the order of the beetles (Coleoptera), for example, Agrilus
sinuatus, Agriotes lineatus, Agriotes obscurus, Amph;~llus
solstitialis, Anisandrus dispar, Anthonomus grandis, Anthonomus
pomorum, Atomaria linearis, Blastophagus piniperda, Blitophaga
undata, Bruchus rufimanus, Bruchus pisorum, Bruchus lentis,
10 Byctiscus betulae, Cassida nebulosa, Cerotoma trifurcata,
Ceuthorrhynchus assimilis, Ceuthorrynchus napi, Chaetocnema
tibialis, Conoderus vespertinus, Crioceris asparagi, Diabrotica
longicornis, Diabrotica 12-punctata, Diabrotica virgifera,
Epilachna varivestis, Epitrix hirtipennis, Eutinobothrus
15 brasiliensis, Hylobius abietis, Hypera brunneipennis, Hypera
postica, Ips ~y~oy-aphus, Lema bilineata, Lema melanopus,
Leptinotarsa decemlineata, Limonius californicus, Lissorhoptrus
oryzophilus, Melanotus communis, Meligethes aeneus, Melolontha
hippocastani, Melolontha melolontha, Onlema oryzae,
20 Ortiorrhynchus sulcatus, Otiorrhynchus ovatus, Phaedon
cochleariae, Phyllotetra chrysocephala, Phyllophaga sp.,
Phyllopertha horticola, Phyllotetra nemorum, Phyllotetra
striolata, Popillia japonica, Sitona lineatus, Sitophilus
granaria.
From the order of the dipterous insects (Diptera), for example,
Aedes aegypti, Aedes vexans, Anastrepha ludens, Anopheles
maculipennis, Ceratitis capitata, Chrysomya bezziana, Chrysomya
hominivorax, Chrysomya macellaria, Contarinia sorghicola,
30 Cordylobia anthropophaga, Culex pipiens, Dacus cucurbitae, Dacus
oleae, Dasineura brassicae, Fannia canicularis, Gasterophilus
intestinalis, Glossina morsitans, Haematobia irritans,
Haplodiplosis equestris, Hylemyia platura, Hypoderma lineata,
Liriomyza sativae, Liriomyza trifolii, Lucilia caprina, Lucilia
35 cuprina, Lucilia sericata, Lycoria pectoralis, Mayetiola
destructor, Musca domestica, ~uscina stabulans, Oestrus ovis,
Oscinella frit, Pegomya hysocyami, Phorbia antiqua, Phorbia
brassicae, Phorbia coarctata, Rhagoletis cerasi, Rhagoletis
pomonella, Tabanus bovinus, Tipula oleracea, Tipula paludosa.
From the order of the thrips (Thysanoptera), for example,
FrAnkl;n;ella fusca, Frankliniella occidentalis, Frankliniella
tritici, Scirtothrips citri, Thrips oryzae, Thrips palmi, Thrips
tabaci.
BASF Aktiengesell~chaft 930627 O.Z. 0050/44487
-` 2137018
_ 24
From the order of the hymenopterous insects (Hymenoptera), for
example, Athalia rosae, Atta cephalotes, Atta sexdens, Atta
texana, Hoplocampa minuta, Hoplocampa testudinea, Monomorium
pharaonis, Solenopsis geminata, Solenopsis invicta.
From the order of the bed bugs (Heteroptera), for example,
Acrosternum hilare, Blissus leucopterus, Cyrtopeltis notatus,
Dysdercus cingulatus, Dysdercus intermedius, Eurygaster
integriceps, Euschistus impictiventris, Leptoglossus phyllopus,
10 Lygus lineolaris, Lygus pratensis, Nezara viridula, Piesma
quadrata, Solubea insularis, Thyanta perditor.
From the order of the plant-sucking insects (Homoptera), for
example, Acyrthosiphon onobrychis, Adelges laricis, Aphidula
15 nasturtii, Aphis fabae, Aphis pomi, Aphis sambuci, Brachycaudus
cardui, Brevicoryne brassicae, Cerosipha gossypii, Dreyfusia
nor~-nn;anae, Dreyfusia piceae, Dyasphis radicola,
Dysaulacorthum pseudosolani, Empoasca fabae, Macrosiphum avenae,
Macrosiphum euphorbiae, Macrosiphon rosae, Megoura viciae,
20 Metopolophium dirhodum, Myzodes persicae, Myzus cerasi,
Nilaparvata lugens, Pemphigus bursarius, Perkinsiella
saccharicida, Phorodon humuli, Psylla mali, Psylla piri,
Rhopalomyzus ascalonicus, Rhopalosiphum maidis, Sappaphis mala,
Sappaphis mali, Schizaphis graminum, Schizoneura lanuginosa,
25 Trialeurodes vaporariorum, Viteus vitifolii.
From the order of the termites (Isoptera), for example,
Calotermes flavicollis, Leucotermes flavipes, Reticulitermes
lucifugus, Termes natalensis.
From the order of the orthopterous insects (Orthoptera), for
example-, Acheta domestica, Blatta orientalis, Blattella
germanica, Forficula auricularia, Gryllotalpa gryllotalpa,
Locusta migratoria, Melanoplus bivittatus, Melanoplus femur-
35 rubrum, Melanoplus mexicanus, Melanoplus sanguinipes, Melanoplusspretus, Nomadacris septemfasciata, Periplaneta americana,
Schistocerca americana, Schistocerca peregrina, Stauronotus
maroccanus, Tachycines asynamorus.
40 From the class of the Arachnoidea, for example spiders (Acarina)
such as Amblyomma americanum, Amblyomma variegatum, Argas
persicus, Boophilus annulatus, Boophilus decoloratus, Boophilus
microplus, Brevipalpus phoenicis, Bryobia praetiosa, Dermacentor
silvarum, Eotetranychus carpini, Eriophyes sheldoni, Hyalomma
45 truncatum, Ixodes ricinus, Ixodes rubicundus, Ornithodorus
moubata, Otobius megnini, Paratetranychus pilosus, Dermanyssus
gallinae, Phyllocoptruta oleivora, Polyphagotarsonemus latus,
BASF Aktiengesellschaft 930627 0.Z. 0050/44487
`- 2137018
_ 25
Psoroptes ovis, Rhipicephalus appendiculatus, Rhipicephalus
evertsi, Sarcoptes scabiei, Tetranychus cinnabarinus, Tetranychus
kanzawai, Tetranychus pacificus, Tetranychus telarius, Tetra-
nychus urticae.
From the class of the nematodes, for example, root gall
nematodes, eg. Meloidogyne hapla, Meloidogyne incognita,
Meloidogyne javanica, cyst-forming nematodes, eg. Globodera
rostochiensis, Heterodera avenae, Heterodera glycines, Heterodera
10 schachtii, Heterodera trifolii, stem and leaf eelworms, eg.
Belonolaimus longicaudatus, Ditylenchus destructor, Ditylenchus
dipsaci, Heliocotylenchus multicinctus, Longidorus elongatus,
Radopholus similis, Rotylenchus robustus, Trichodorus primitivus,
Tylenchorhynchus claytoni, Tylenchorhynchus dubius, Pratylenchus
15 neglectus, Pratylenchus penetrans, Pratylenchus curvitatus,
Pratylenchus goodeyi.
The active compounds can be applied as such or in the form of
their formulations or the application forms prepared therefrom,-
20 eg. in the form of directly sprayable solutions, powders,suspensions or dispersions, emulsions, oil dispersions, pastes,
dusts, scattering compositions or granules by spraying, atomiz-
ing, dusting, scattering or pouring. The application forms depend
entirely on the purposes of use; they should in any case as far
25 as possible guarantee the finest dispersion of the active com-
pounds according to the invention.
The active compound concentrations in the ready-for-application
preparations can be varied within substantial ranges.
In general, they are from 0.0001 to 10%, preferably from 0.01 to
1% .
The active cv...pounds can also be used with success in ultra-low
35 volume processes (ULV), where it is possible to apply for-
mulations contA;n;ng more than 95% by weight of active compound
or even the active compound without additives.
The application rate of active compound for controlling pests un-
40 der outdoor conditions is from 0.1 to 2.0, preferably from 0.2 to
1.0 kg/ha.
For the preparation of directly sprayable solutions, emulsions,
pastes or oil dispersions, mineral oil fractions of medium to
45 high boiling point, such as kerosene or diesel oil, and also coal
tar oils and oils of vegetable or animal origin, aliphatic,
cyclic and aromatic hydrocarbons, eg. benzene, toluene, xylene,
BASF AXtiengesellschaft 930627 O.Z. 0050/44487
2137018
26
paraffin, tetrahydronaphthalene, alkylated naphthalenes or their
derivatives, methanol, ethanol, propanol, butanol, chloroform,
carbon tetrachloride, cyclohexanol, cyclohexanone, chlorobenzene,
isophorone, strongly polar solvents, eg. dimethylformamide, dime-
5 thyl sulfoxide, N-methylpyrrolidone and water are suitable.
Aqueous application forms can be prepared from emulsion
concentrates, pastes or wettable powders (oil dispersions) by
addition of water. For the preparation of emulsions, pastes or
10 oil dispersions, the substances can be homogenized in water as
such or dissolved in an oil or solvent, by means of wetting
agents, tackifiers, dispersants or emulsifiers. However, the
concentrates consisting of active substance, wetting agent,
tackifier, dispersant or emulsifier and possibly solvent or oil
15 can also be prepared, which are suitable for dilution with water.
Suitable surface-active substances are alkali metal, alkaline
earth metal and ammonium salts of lignosulfonic acid, naphthal-
20 enesulfonic acid, phenolsulfonic acid, dibutylnaphthalenesulfonicacid, alkylarylsulfonates, alkylsulfates, alkylsulfonates, fatty
alcohol sulfates and fatty acids and their Al kAl; metal and alka-
line earth metal salts, salts of sulfated fatty alcohol glycol
ethers, condensation products of sulfonated naphthalene and naph-
25 thalene derivatives with formaldehyde, condensation products ofnaphthalene or of naphthalenesulfonic acid with phenol and form-
aldehyde, polyoxyethylene octylphenyl ether, ethoxylated iso-
octylphenol, octylphenol, nonylphenol, alkylphenol polyglycol
ether, tributylphenyl polyglycol ether, alkylaryl polyether alco-
30 hols, isotridecyl alcohol, fatty alcohol ethylene oxide conden-
sates, ethoxylated castor oil, polyoxyethylene alkyl ethers,
ethoxylated polyoxypropylene, lauryl alcohol polyglycol ether
acetal, sorbitol esters, lignin-sulfite waste liquors and methyl-
cellulose.
Powders, scattering compositions and dusts can be prepared by
mixing or joint grinding of the active substances with a solid
carrier.
40 The formulations in general contain from 0.01 to 95% by weight,
preferably from 0.1 to 90% by weight, of the active compound. The
active compounds are employed here in a purity of from 90% to
100%, preferably 95% to 100% (according to NMR spectrum).
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
2137018
- _ 27
Examples of formulations are:
I. S parts by weight of the compound No. 7 are intimately
mixed with 95 parts by weight of finely divided kaolin. A
dust which contains 5% by weight of the active compound
is obtained in this way.
II. 30 parts by weight of the compound No. 11 are intimately
mixed with a mixture of 92 parts by weight of powdered
silica gel and 8 parts by weight of paraffin oil which
has been sprayed on the surface of this silica gel. A
preparation of the active compound having good adhesion
is obtained in this way (active compound content 23% by
weight).
III. 10 parts by weight of the compound No. 5 are dissolved in
a mixture which consists of 90 parts by weight of xylene,
6 parts by weight of the addition product of 8 to 10 mol
of ethylene oxide to 1 mol of oleic acid N-monoethanol-
amide, 2 parts by weight of calcium salt of dodecyl-
benzenesulfonic acid and 2 parts by weight of the addi-
tion product of 40 mol of ethylene oxide to 1 mol of cas-
tor oil (active compound content 9% by weight).
IV. 20 parts by weight of the compound No. 10 are dissolved
in a mixture which consists of 60 parts by weight of
cyclohexanone, 30 parts by weight of isobutanol, 5 parts
by weight of the addition product of 7 mol of ethylene
oxide to 1 mol of isooctylphenol and 5 parts by weight of
the addition product of 40 mol of ethylene oxide to 1 mol
of castor oil (active compound content 16% by weight).
V. 80 parts by weight of the compound No. 5 are well mixed
with 3 parts by weight of the sodium salt of diisobutyl-
naphthalene-alpha-sulfonic acid, 10 parts by weight of
the sodium salt of a lignosulfonic acid from a sulfite
waste liquor and 7 parts by weight of powdered silica gel
and the mixture is ground in a hammer mill (active com-
pound content 80% by weight).
VI. 90 parts by weight of the compound No. 7 are mixed with
10 parts by weight of N-methyl-~-pyrrolidone and a solu-
tion is obtained which is suitable for application in the
form of very small drops (active compound content 90% by
weight).
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
` _ 2137018
28
VII. 20 parts by weight of the compound No. 11 are dissolved
in a mixture which consists of 40 parts by weight of
cyclohexanone, 30 parts by weight of isobutanol, 20 parts
by weight of the addition product of 7 mol of ethylene
oxide to 1 mol of isooctylphenol and 10 parts by weight
of the addition product of 40 mol of ethylene oxide to
1 mol of castor oil. By pouring the solution into and
finely dispersing it in 100,000 parts by weight of water,
an aqueous dispersion is obtained which contains 0.02% by
weight of the active compound.
VIII. 20 parts by weight of the compound No. 10 are well mixed
with 3 parts by weight of the sodium salt of diisobutyl-
naphthalene-~-sulfonic acid, 17 parts by weight of the
sodium salt of a lignosulfonic acid from a sulfite waste
liquor and 60 parts by weight of powdered silica gel and
the mixture is ground in a hammer mill. By finely dis-
persing the mixture in 20,000 parts by weight of water, a
spray liquor is obtained which contains 0.1 % by weight
of the active compound.
Granules, eg. coated, impregnated and homogeneous granules, can
be produced by binding the active compounds to solid carriers.
Solid carriers are eg. mineral earths, such as silica gel,
25 silicic acids, silicates, talc, kaolin, attapulgite, limestone,
lime, chalk, bole, loess, clay, dolomite, diatomaceous earth,
calcium sulfate and magnesium sulfate, magnesium oxide, ground
synthetic materials, fertilizers, such as eg. ammonium sulfate,
ammonium phosphate, ammonium nitrate, ureas and vegetable
30 products, such as grain meal, tree bark, wood and nut shell meal,
cellulose powder and other solid carriers.
Oils of various types, herbicides, fungicides, other pesticides
and bactericides can be added to the active compounds, if
35 appropriate also just immediately before application (tank mix).
These agents can be admixed to the compositions according to the
invention in the weight ratio 1:10 - 10:1.
Synthesis examples
The procedures presented in the synthesis examples below can be
utilized with appropriate modification of the starting compounds
to obtain further compounds I.
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
29 2 1 3 7 0 1 8
Example 1
Preparation of (Z)-2-methoxyimino-2-(2'-methylphenyl)acetonitrile
5 188 g (1.68 mol) of dry potassium tert-butoxide were initially
introduced into 2 l of dry toluene and treated rapidly with 200 g
(1.53 mol) of ortho-methylbenzyl cyanide and 173 g (1.68 mol) of
tert-butyl nitrite in 200 ml of toluene. The mixture warmed to
about 70 C, and was subsequently stirred for 2 h and treated with
10 1 l of methyl tert-butyl ether. The yellow potassium salt was
filtered off with suction, washed with methyl tert-butyl ether
and dried at 50 C under reduced pressure.
290 g (1.46 mol) of this salt were initially introduced with 20 g
15 (0.15 mol) of potassium carbonate into 2.5 l of dry acetone.
While 234 g (1.65 mol) of iodomethane were added dropwise, the
temperature rose to about 35 C. The mixture was subsequently
stirred overnight and then partitioned between water and methyl
tert-butyl ether. The organic phase was washed with water, dried
20 over Na2SO4, concentrated and distilled under reduced pressure
(b.p. = 95 - 100 C / 0.3 - 0.1 mm). 229 g (78%) of the title com-
pound were obtained as a poorly mobile liquid.
lH-NMR (CDCl3): ~ = 2.53 (s, 3H); 4.22 (s, 3H);
7.2 - 7.4 (m, 3H); 7.54 (dd, lH) ppm.
Example 2
Preparation of (Z)-2-methoxyimino-2-(2'-bromomethylphenyl)aceto-
nitrile
48 g (0.28 mol) of (Z)-2-methoxyimino-2-(2'-methylphenyl)aceto-
nitrile and 54 g (0.30 mol) of N-bromosuccinimide were initially
introduced into 250 ml of carbon tetrachloride. The mixture was
irradiated for 40 min from outside using an Hg vapor lamp
35 (300 W). The succinimide was filtered off with suction and the
solution evaporated in vacuo. 62 g (88~) of the title compound
remained (purity according to lH-NMR spectrum: about 80~).
lH-NMR (CDCl3): ~ = 4.27 (s, 3H); 4.80 (s, 2H)
7.4 - 7.5 (m, 3H); 7.69 (dd, lH) ppm.
BASF AXtiengesellschaft 930627 O.Z. 0050/44487
-- 30 2137018
Example 3
Preparation of (Z)-2-(methoxyimino-2-[2~-(E)-(1~-(3"~,5~-di-
chlorophenyl)-1"-methyl)iminooxymethyl]phenylacetonitrile
6 g (0.25 mol) of sodium hydride were initially introduced into
800 ml of acetonitrile under protective gas and 47 g (0.23 mol)
of 3,5-dichloroacetophenone oxime were added in portions at room
temrerature. The mixture was warmed to 70 C for 1 hour. After
lO cooling to S0 C, 74 g (0.23 mol) of (Z)-2-methoxyimino-2-(2'-
bromomethyphenyl)acetonitrile were added dropwise in 200 ml of
acetonitrile. The mixture was then stirred for 8 hours, poured
onto cold 2M hydrochloric acid and extracted with methyl tert-
butyl ether. The combined organic phases were washed with water,
15 dried over Na2SO4 and concentrated. After purifying by column
chromatography on silica gel (methyl tert-butyl ether/n-hexane)
and triturating with diisopropyl ether, 55 g (64% yield) of the
title compound were obtained as light yellow crystals of melting
point 73 to 75-C.
H-NMR (CDCl3): ~ = 2.26 (s, 3H); 4.22 (s, 3H); 5.49 (s, 2H);
7.32 - 7.69 (m, 7H) ppm.
Example 4 -
25 Preparation of (Z)-2-methoxyimino-2-[2'-(E)-(1"-(3"',5"'-di-
chlorophenyl)-1"-methyl)iminooxymethyl]phenylthioacetamide
Hydrogen sulfide was introduced as a gas into a solution of 20 g
(0.053 mol) of (Z)-2-methoxyimino-2-[2'-(E)-(ln-(3"',5n~_
30 dichloro-phenyl)-ln-methyl)iminooxymethyl]phenylacetonitrile and
2.0 g (0.027 mol) of diethylamine in 200 ml of dimethylformamide
at 50 C until it was saturated. The mixture was subsequently
stirred for 30 minutes and then poured into water and extracted
with methyl tert-butyl ether. The combined organic phases were
35 washed with water, dried over Na2SO4 and concentrated. After
triturating with methyl tert-butyl ether/n-hexane, 21.7 g (100%
yield) of the title compound were obtained as light yellow
crystals of melting point 108 to llO-C.
40 1H-NMR (CDCl3): ~ = 2.21 (s, 3H); 4.05 (s, 3H); 5.41 (s, 2H);
7.33 - 7.58 (m, 7H); 7.83 (br s, lH) ppm.
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
2 1 37018
31
Example 5
Preparation of S-methyl (Z)-2-methoxyimino-2-[2'-(E)-(1"-
(3"',5"'-dichlorophenyl)-1"-methyl)iminooxymethyl]phenylthio-
5 acetimidate
3.0 g (0.020 mol) of trimethyloxonium tetrafluoroborate were
added to a solution of 8.2 g (0.020 mol) of Z-2-methoxyimino-
2-[2'-(E)-(1"-(3"',5 n ~ -dichlorophenyl)-1"-methyl)iminooxymethyl]-
lO phenylthioacetamide in 60 ml of methylene chloride. The mixturewas stirred at room temperature for 1 hour and was diluted with
water and extracted with methylene chloride. After drying
(Na2SO4), concentrating the organic phase in a rotary evaporator
and then triturating with methyl tert-butyl ether/n-hexane, 6.3 g
15 (74% yield) of the title compound were obtained as light yellow
crystals of melting point 81 to 83-C.
H-NMR (CDCl3): ~ = 2.25 (s, 3H); 2.42 (s, 3H); 4.04 (s, 3H);
5.46 (s, 2H); 7.32 - 7.51 (m, 7H) ppm.
Example 6
Preparation of (E,E)-2-methoxyimino-2-[2'-(1"-(3"', 5 n ~ -di-
chlorophenyl)-1 n -methyl)iminooxymethyl]phenylthioacetamide
25 5 ml of a saturated ethereal hydrogen chloride solution were
added to a solution of 8.2 g (0.020 mol) of (Z)-2-methoxy-
imino-2-[2'-(E)-(1 n _ ( 3 n ~ ~ 5 n ~ -dichlorophenyl)-1 n -methyl)iminooxy-
methyl]phenylthioacetamide in 100 ml of toluene and the mixture
was allowed to stand at room temperature for 16 hours. After
30 addition of methyl tert-butyl ether, it was washed with saturated
NaHCO3 solution and then with water until neutral, and the organic
phase was separated off, dried over Na2SO4 and concentrated. 5.4 g
(66~ yield) of the title compound were obtained as light yellow
crystals of melting point 138 to 140-C.
H-NMR (CDCl3): ~ = 2.18 (s, 3H); 3.99 (s, 3H); 5.10 (s, 2H);
7.12 - 7.51 (m, 7H); 8.13 (br, lH) ppm.
Example 7
40 Preparation of S-methyl (E,E)-2-methoxyimino-2-[2'(1 n _ ( 3"" 5 n ~ _
dichlorophenyl)-ln-methyl)iminooxymethyl]phenylthioacetimidate
1.6 g (11 mmol) of trimethyloxonium tetrafluoroborate were added
to a solution of 4.5 g (1 mmol) of (E,E)-2-methoxyimino-2-[2'-
45 (ln-(3n~,5n'-dichlorophenyl)-ln-methyl)iminooxymethyl]phenylthio-
acetamide in 40 ml of methylene chloride and the mixture was
stirred at room temperature for 1 hour, diluted with water and
BASF Aktiengesellschaft 930627 O.Z. OOSO/44487
32 21~7018
extracted with methylene chloride.-After drying (Na2SO4) and
concentrating the organic phase in a rotary evaporator, 4.4 g
(94% yield) of the title compound were obtained as a light yellow
oil.
H-NMR (CDCl3): ~ = 2.14 (s, 3H); 2.37 (s, 3H); 3.99 (s, 3H); 5.07
(s, 2H); 7.10 - 7.54 (m, 7H) ppm.
Example 8
lO Preparation of (Z)-2-methoxyimino-2-[2'-(E)-(1"-(4 n ~ -chloro-
phenyl)-l"-methyl)iminooxymethyl]phenylacetonitrile
5.6 g (0.23 mol) of sodium hydride were initially introduced into
1000 ml of acetonitrile under protective gas and 36 g (0.21 mol)
15 of 4-chloroacetophenone oxime were added in portions at room
temperature. The mixture was warmed to 70 C for 1 hour. After
cooling to 50 C, 67 g (0.21 mol) of (Z)-2-methoxyimino-2-(2'-
bromomethylphenyl)acetonitrile were added dropwise in 200 ml of
acetonitrile. The mixture was then stirred for 8 hours, poured -
20 onto cold 2M hydrochloric acid and extracted with methyltert-butyl ether. The combined organic phases were washed with
water, dried over Na2SO4 and concentrated. After purifying by
column chromatography on silica gel (methyl-tert-butyl
ether/n-hexane) and triturating with diisopropyl ether, 57 g (79%
25 yield) of the title co,..pound were obtained as a light yellow
powder of melting point 54 to 56 C.
H-NMR (CDCl3): ~ = 2.28 (s, 3H); 4.20 (s, 3H); 5.47 (s, 2H);
7.30 - 7.69 (m, 8H) ppm.
Example 9
Preparation of (Z)-2-methoxyimino-2-[2'-(E)-(1"-(4"'-chloro-
phenyl)-ln-methyl)iminooxymethyl]phenylthioacetamide
35 Hydrogen sulfide was introduced as a gas into a solution of 57 g
(0.167 mol) of (Z)-2-methoxyimino-2-[2'-(E)-(1 n - ( 4"'-chloro-
phenyl)-ln-methyl)iminooxymethyl]-phenylacetonitrile and 5.8 g
(0.079 mol) of diethylamine in 500 ml of dimethylformamide at 50 C
until it was saturated. The mixture was subsequently stirred for
40 30 minutes and then poured onto water and extracted with
methylene chloride. The combined organic phases were washed with
water, dried over Na2SO4 and concentrated. After triturating with
methyl tert-butyl ether/n-hexane, 60 g (96~ yield) of the title
compound were obtained as a light yellow powder of melting point
45 65 to 67-C.
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
33 ~ 1 3 7 0 1 8
H-NMR (CDCl3): ~ = 2.23 (s, 3H); 4.03 ~s, 3H); 5.40 (s, 2H);
7.29 - 7.64 (m, 8H); 7.81 (brs, lH) ppm.
Example 10
5 Preparation of S-methyl (Z)-2-methoxyimino-2-[2'-(E)-(1"-(4~
chlorophenyl)-l"-methyl)iminooxymethyl]phenylthioacetimidate
3.9 g (0.027 mol) of trimethyloxonium tetrafluoroborate were
added to a solution of 10 g (0.027 mol) of Z-2-methoxyimino-2-
10 [2'-(E)-(1"-(4"'-chlorophenyl)-1"-methyl)iminooxymethyl]phenyl-
- thioacetamide in 50 ml of methylene chloride and the mixture was
stirred at room temperature for 1 hour, diluted with water and
extracted with methyl tert-butyl ether. After drying (Na2SO4) and
concentrating the organic phase in a rotary evaporator, and then
15 triturating the residue with n-hexane, 7.6 g (73% yield) of the
title compound were obtained as light yellow crystals of melting
point 56 to 58-C.
lH-NMR (CDCl3): ~ = 2.27 (s, 3H); 2.42 (s, 3H); 4.04 (s, 3H);
5.42 (s, 2H); 7.30 - 7.59 (m, 8H); 9.58
(sbr, lH) ppm.
Example 11
Preparation of S-methyl (Z)-2-methoxyimino-2-[2'-(E)-(1~-(4
25 chlorophenyl)-l"-methyl)imino-oxymethyl]phenylthioacetate
25 ml of 10% strength hydrochloric acid were added to a solution
of 5.0 g (0.013 mol) of S-methyl (Z)-2-methoxyimino-2-[2'-(E)-
(1"-(4"'-chlorophenyl)-1"-methyl)iminooxymethyl]phenylthioacetim-
30 idate in 200 ml of tetrahydrofuran. The mixture was allowed tostand at room temperature and was diluted with water and
extracted with methyl tert-butyl ether. The combined organic
phases were washed with saturated NaHCO3 solution and then with
water until neutral, and the organic phase was separated off,
35 dried over Na2SO4 and concentrated. After filtration through
silica gel (methyl tert-butyl ether/n-hexane), 4.0 g (80% yield)
of the title compound were obtained as a yellow oil.
lH-NMR (CDCl3): ~ = 2.28 (s~ 3H); 2.43 (s, 3H); 4.03 (s, 3H);
5.45 (s~ 2H); 7.30 - 7.61 (m, 8H) ppm.
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
- _ 34 2137~18
Example 12
Preparation of (Z)-2-methoxyimino-2-[2'-(E)-(1"-(4 n ~ -chloro-
phenyl)-l"-methyl)iminooxymethyl]phenylacetic acid
monomethylamide
2.0 g (5.1 mmol) of S-methyl (Z)-2-methoxyimino-2-[2-(E)-(l~-
(4 n ~ -chlorophenyl)-l n -methyl)iminooxymethyl]phenylthioacetate
were dissolved in 200 ml of tetrahydrofuran, and the solution was
treated with 50 ml of 40% strength aqueous monomethylamine
lO solution and stirred at room temperature for 2 hours. It was then
concentrated, the residue was taken up in methyl tert-butyl
ether, and the organic phase was extracted with water, dried over
sodium sulfate and concentrated again. 1.8 g (94% yield) of the
title compound were obtained as a beige powder of melting point
15 111 to 113-C.
H-NMR (CDCl3): ~ = 2.23 (s, 3H); 2.80 (d, 3H); 4.04 (s, 3H);
5.39 (s, 2H); 6.69 (s, br, lH); 7.30 - 7.55
(m, 8H) ppm.
Example 13
Preparation of (E,E)-2-methoxyimino-2-[2'-(1 n _ ( 4 n ~ -chlorophenyl)-
l"-methyl)iminooxymethyl]phenylacetic acid monomethylamide
25 50 ml of a saturated ethereal hydrogen chloride solution were
added to a solution of 8.4 g (0.022 mol) of (Z)-2-methoxyimino-
2-[2'-(E)-(1"-(4 n ~ -chlorophenyl)-l n -methyl)iminooxymethyl]phenyl-
acetic acid monomethylamide in 300 ml of toluene and the mixture
was allowed to stand at room temperature for 4 hours. After
30 addition of methyl tert-butyl ether, it was washed with saturated
NaHCO3 solution and then with water until neutral, and the organic
phase was separated off, dried over Na2SO4 and concentrated. After
purifying by column chromatography on silica gel (methyl tert-
butyl ether/n-hexane), 5.4 g (65% yield) of the title compound
35 were obtained as colorless crystals of melting point 119 to 121 C.
H-NMR (CDCl3): ~ = 2.17 (s, 3H); 2.86 (d, 3H); 3.94 (s, 3H); 5.11
(s, 2H); 6.71 (sbr, lH); 7.19 - 7.55 (m, 8H)
ppm.
Example 14
S-Methyl (Z)-2-methoxyimino-2-{2'-(E)-1 n _ [ 2-naphthyl]-1"-methyl-
iminooxymethyl}phenylthioacetimidate (Rl=CH3, R2=CH3,
R3m=3,4-CH=CH-CH=CH) was prepared correspondingly and has a
45 melting point of 106 to 108-C.
~ BASF Aktiengesellschaft 930627 O.Z. 0050/44487
_ 35 21~7018
Use Examples
1. It was possible to show the insecticidal action of the
compounds of the general formula I by the following tests:
The active compounds were prepared
a) as a 0.1% strength solution in acetone or
10 b) as a 10% strength emulsion in a mixture of 70% by weight of
cyclohexanol, 20% by weight of Nekanil~ LN (Lutensol$ AP6,
wetting agent with emulsifier and dispersant action based on
ethoxylated alkylphenols) and 10% by weight of Emulphor~ EL
(Emulan~ EL, emulsifier based on ethoxylated fatty alcohols)
and accordingly diluted to the desired concentration with acetone
in the case of a) or with water in the case of b). After
conclusion of the tests, the lowest concentration was determined
in each case at which the compounds still caused an 80 - 100%
20 inhibition or mortality in comparison with untreated control
tests (activity threshold or m; n; mllm concentration).
2. It was possible to show the fungicidal action of the
compounds of the general formula I by the following tests:
The active compounds were prepared as a 20% strength emulsion in
a mixture of 70% by weight of cyclohexanol, 20% by weight of
Nekanil$ LN (Lutensol$ AP6, wetting agent having emulsifier and
dispersant action based on ethoxylated alkylphenols) and 10% by
30 weight of Emulphor~ EL (Emulan~ EL, emulsifier based on
ethoxylated fatty alcohols) and diluted with water according to
the desired concentration.
After conclusion of the tests, the attack on the treated plants
35 was assessed in relation to the untreated plants.
Erysiphe graminis var. tritici (wheat mildew)
Leaves of wheat seedlings (Fruhgold variety) were first treated
40 with the aqueous preparation of the active compounds. After about
24 h, the plants were dusted with spores of wheat mildew
(Erysiphe graminis var. tritici). The plants treated in this way
were then incubated for 7 days at from 20 to 22 C and a relative
atmospheric humidity of 75 - 80%. The extent of fungal
45 development was then determined.
BASF Aktiengesellschaft 930627 O.Z. 0050/44487
' ~ 36 2 1 3 7 Ot.8
In this test, the plants treated with 63 ppm preparations of the
compounds Examples No. 5 and No. 7 showed an attack of 15% and
less, while the untreated plants were attacked to 65%.