Note: Descriptions are shown in the official language in which they were submitted.
213?755
1 75787-1
PALLIATION OF SICKLE CELL DISORDERS BY PHENYLUREA, BENZYLUREA, OR
PHENYLETHYLUREA OR BY A HOMOLOG RING-SUBSTITUTED WITH ONE
METHOXYL, METHYL, OR HYDROXYL RADICAL
This invention is directed to the novel use of arylurea
compounds and aralkylurea compounds consisting of phenylurea,
benzylurea, and phenylethylurea or by a mete-or pare-positioned,
ring-substituted homolog of each in which one hydrogen is
substituted by one methoxyl, methyl, or hydroxyl radical, for the
palliation of sickle cell anemia and other human sickling
diseases. (These compounds will sometimes be referred to
hereinafter as the aromatic ureas or the aromatic urea compounds.)
The invention is also directed to pharmaceutical formulations
containing these aromatic urea compounds.
This invention concerns the conception of and
const rust ive reduction to pract ice for therapeut is use of phenyl-
radical (C6H5-) or phenolic-radical (HOC6H4-) or methoxyphenyl-
radical (CH30C6H4-) or tolyl-radical (CH3C6H4-) unicyclic
compounds in which the radical is linked directly to urea, as N-
substituted aromatic urea alone or with one methyl (-CH2-) or one
ethyl (-C2H4-) linkage between the benzenoid nucleus and the urea
group. Such aromatic urea compounds are viewed as amphiphilic, by
possessing both the phenyl or phenolic (or methoxyphenyT or tolyl)
lipophilic or hydrophobic moiety and the urea hydrophilic moiety,
and they are conceived to have substantial inhibitory activity
against polymerization of deoxygenated or hypoxic HbS molecules
(sickle hemoglobin molecules) within intact human red cells in
vitro and in vivo, at relatively low concentrations between 1 and
12 or 22 mM and at normal human body temperature of 37oC and at
near-normal or normal pH.
2~377~~
2 75787-1
It is conceived that these lipophilic but somewhat water-soluble and
hydrophilic nonionized arylureas and aralkylureas will diffuse sufficiently
across the red-cell plasma membrane barrier to exert antigelling and
antisickling effects, by intracellular inhibition of deoxy HbS polymerization,
cell stiffness, and cell sickling. The mediated means of action is conceived
to be at least partly by aromatic-acting noncovalent chaeotropic competition
at the key site to deoxy HbS polymer formation, i.e. insertion of the beta 6
valine of one hemoglobin tetramer into the hydrophobic acceptor pocket
involving the beta 88 leucine and beta 85 phenylalanine group on an adjacent
tetramer, and at other hydrophobic sites of deoxy HbS molecules. Reference is
made to page 864 of Dean and Schechter, 1978, for definition of noncovalent
chaeotropic agent's and also to Ross and Subramanian, 1977, and Russu et al,
1986, for antisickling sites and also to Deuticke, 1968, for reference to non-
ionized amphiphilic agents which may alter detrimentally the shape or life of
human erythrocytes.
It is also conceived that these arylureas or aralkylureas will exert
greater antipolymerizing effect in deoxy, intact homozygous HbS cells and
deoxy HbSC red cells than will urea, methylurea, ethylurea, propylurea, and
butylurea as aliphatic ureas or alkylureas intracellularly (in vitro testing
of aliphatic ureas, see Chang et al 1983, and Elbaum et al, 1976) or with
purified deoxy HbS solutions (in vitro testing of aliphatic ureas, see
Poillon, 1980). Noncharged or non-eletrolytic aromatic urea and aralkylurea
compounds have not been tested to date for antipolymerizing effect on deoxy
HbS in cell-free solution or in intact deoxy HbS red cells. In in vitro
testing of noncharged aromatic ureas, this inventor has initially warmed or
heated to 45-65°C for several minutes water mixtures of only moderately
high
3 75787-1
millimolar concentrations of phenylurea, benzylurea, or
phenylethylurea compound or of their monosubstituted homologs to
enhance solubility, before test use at room temperature or 37°C.
Because these aromatic urea compounds display both
hydrophilic and moderately lipophilic properties, in contrast to
the very hydrophilic and quite lipophobic properties of urea,
itself, it is contemplated that these aromatic ureas will not
cause very substantial detrimental effects of crenation
(echinocytosis) of erythrocytes or hemolysis at the relatively low
levels, under 12 to 22 mM required to inhibit sickling.
It is also conceived that these amphiphilic arylureas or
aralkylureas will have much lower renal plasma clearances than
urea in man because of their much greater lipophilic nature and
the much greater passive tubular back diffusion of plasma-borne
aromatic urea compound delivered by renal glomerular filtration.
Therefore, the aromatic ureas will be lost by kidney excretion at
a much lesser rate than urea. Therefore, pharmacokinetically, one
or more of the arylureas or aralkylureas may be administered
effectively to human beings as antisickling drug daily by mouth or
by vein in divided doses of up to about 0.5 g/kg/24 hours. The
arylurea and aralkylurea compounds may display hypnotic effects
but for the purpose of the invention can lead to substantial
beneficial effects in the serious sickling disorders in doses low
enough to avoid severe hypnotic or other serious adverse effect.
To achieve a very efficacious antisickling blood plasma level of
arylurea or aralkylurea of about 5.0 mM, e.g. of m- or p-
methoxyphenylurea, the administration of about 0.50 g/kg of body
weight should be required acutely, if one assumes equal
distribution of arylurea or aralkylurea compound chiefly in most
body water at equilibrium and an average normal water content of
60$ of body weight and very
75787-1
poor renal excretion or loss of the arylurea or aralkylurea antisickling
agent. With cumulative effects from daily doses much lower than 0.50 g/kg,
human plasma levels of the order of magnitude of 3.5 to 5.0 mM may be readily
achieved in a few days if there is very poor daily renal excretion or body
loss of the compound.
Prior art includes the laboratory findings of non-aromatic aliphatic
urea-induced inhibition of deoxy HbS polymerization and gelation of purified
hemoglobin S in solution and in intact erythrocytes, with reduced red cell
Flexibility as tested by reduced filterability through 5 ~cm pore-diameter
membrane filters. -Of the aliphatic alkylureas, butylurea has been reported by
Chang et al (1983) to be the most potent and effective in vitro at 50 and 100
mM. However, no clinical trials have been reported to date with butylurea and
I have found that 50 to 100 mM levels cause HbSS hemolysis in vitro upon
37°C
incubation and standing for 1 to 2 hours at room temperature. Many other
chemicals and drugs have been tried since 1910 to prevent or treat the
sickling syndromes including sickle cell anemia and sickle cell vaso-occlusive
crises (see references by Chang et al in 1983, Aluoch in 1984, and Forget in
1992); these drugs include some other noncovalent inhibitory agents. However,
no noncovalent antipolymerizing drug has yet been developed with satisfactory
criteria of efficiency, safety, and dependability as therapy for the sickling
disorders.
Prior art has also shown that the solubility of deoxygenated HbS in the
presence of various nonelectrolyte aliphatic ureas correlates directly with
the individual partition coefficient of each agent (Abraham, 1982) and
therefore with its lipid, solubility (Davson, 1959). However, the relationship
of the inhibitory action of aromatic ureas and aralkylureas [upon hypoxic HbS
a
CA 02137755 2002-02-22
r
75787-1
red cell gelation and sickling] to the partition coefficient of
the aromatic urea at 37°C studied in vitro has not been
described before this invention.
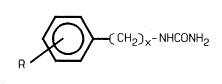
Specifically, the aromatic urea compounds of the
5 present invention may be represented by the following formula I
( CH2 )X-NHCONH Z ( I ~
R
wherein R is hydrogen, hydroxy, methyl or methoxy at the meta-
or para- position and x is 0,1 or 2.
The present invention includes: the use of the above
aromatic urea compounds of formula I in treating sickle cell
disorders in humans and in the preparation of compositions for
such treatment; pharmaceutical compositions containing the
compounds; and commercial packages containing the compounds
together with instructions for the treatment of sickle cell
disorders.
Administration of any of the arylureas and
aralkylureas for novel antipolymerizing use in serious sickling
disorders may be performed by well-known techniques including
oral administration, rectal administration and intravenous
injection. However, the present invention contemplates novel
pharmaceutical formulation for intravenous injection and also
for oral administration in tablet, capsule, and liquid form,
and for rectal administration in solid suppository form or in
retention enema liquid carrier form.
The novel formulation can include L-ascorbic acid as
supplemental ingredient in usual daily dose of about 10 mg/kg
for young children and of up to about 0.5 g to 1.0 g total
daily for
~1~'~'~
v..
s 75787-1
subjects weighing of the order of 50 or more kg. The
incorporation of L-ascorbic acid in the therapeutical preparations
if so three distinct purposes: 1) as antioxidant agent for
stabilization of the active arylurea or aralkylurea while within
the formulated state and when the active urea agent is initially
dispersed within the human body, 2) as concurrent nutritional,
antioxidant agent for symptomatic, stressful sickle cell disorders
while avoiding large or mega-therapeutic amounts of more than
about 1 g daily for adults (Pauling, 1974; Repka and Hebbel,
1991), and 3) as concurrent preventive agent to block possible N-
nitroso formation by blocking the reaction of the active aromatic
urea agent with-possible nitrite in the acid-catabolized
environment of the stomach - if the nitrite is contained in
ingested foods - or with possible nitrite arising endogenously
(Mirvish et al., 1972; Mirvish, 1975; Bartsch et al., 1988).
Ascorbic acid is known to prevent the formation of N-
nitroso product from the reaction of the secondary amine group in
aliphatic ethylurea with added nitrite at very low pH of about 2.5
(Synnott et al., 1975). Possible in vivo formation of N-nitroso
compound from reaction of the secondary amine group of the
aromatic ureas depicted in formula I through 3 with nitrite (even
by conversion of exogenous nitrate to nitrite by microorganisms)
or of the secondary amine group of the aralkylurea agents depicted
in formulas 4 through 9 is contemplated to be preventable by the
concurrent formulated use of ascorbic acid. Protective use of
ascorbic acid has been shown against hepatotoxicity induced by
combined oral administration of nitrite and aminopyrine, a
tertiary amine (Kamm et al, 1973). The rare possibility of
carcinogenic, mutagenic, or hepatotoxic effect from N-nitrosation,
or even C-nitrosation with the nitroso radical (-NO) arising from
reaction of the monohydroxy aromatic homologs in an acidic
6a 75787-1
environment with nitrite is contemplated to be preventable. This
is by concurrent administration of the ascorbic acid at pH near
5.0 to 7.0 in a liquid formulation, or in solid form of oral
tablet or capsule or of solid rectal suppository form.
213~~5~
7 75787-1
A novel elixir formulation containing about 4~ (v/v) n-butyl alcohol for
s
oral carrier use of the active aromatic urea ingredient and of ascorbic acid
is also contemplated. This is for the novel additional purpose of the
formulation containing the alcohol precursor of butyric acid, for likely
modest genic bone marrow stimulation of fetal globin production in some
individuals with serious beta-globin disorders including hemoglobin S
production. This is so that more hemoglobin F may be produced that is found
in the circulating erythrocytes. Butyric acid and precursor butyrate
derivatives, but not n-butyl alcohol, have been suggested for ameliorating
human sickling syndromes (Perrine et al., 1994).
Prior art shows that absorbed n-butyl alcohol is converted rapidly in
vivo to its oxidized product of butyric acid to apparently small accumulated
millimolar or sub-millimolar levels in circulating blood (Waugh, 1993). The
novel oral administration of an elixir containing about 4% 1-butanol is
therefore suggested for the dual functions of carrier of arylurea or
aralkylurea for direct antipolymerizing effect and precursor for end-product
butyrate stimulation of genic induction of fetal hemoglobin.
Alternatively, the novel elixir formulation can contain about 8% by
volume of 95% ethyl alcohol for oral carrier use of the aromatic urea
ingredient and for ascorbic acid delivery. Liquid formulation for rectal use
of retention enema by catheter insertion is contemplated to consist usually of
the aromatic urea medicament and supplemental ascorbic acid, modestly
buffered, in 2-times greater concentrations than used for oral elixir
formulation. The carrier vehicle consists of a mixture of water and about 10%
by volume propylene glycol. Retention enema dosage for a young child will be
about 10 to 30 ml two or more times daily.
CA 02137755 2001-06-27
75787-1
8
The desired clai:ly dose in tablet, capsule, or liquid
form is preferably presented as between two and four sub-doses,
given orally with water or liquid. Alternatively, the aromatic
urea or aral:kylurea antis:ickling agent may be injected slowly
by vein at appropriate intervals throughout the 24 hours. A
daily dose for a human weighing 50 kg or more generally is
contemplated to be 2 t:o 15 g of the aromatic urea or
aralkylurea compound; this amounts to no more than about
0.04 g/kg to 0.30 g/kg of the active antisickling ingredient
administered. The composition may be formulated in dosage unit
form wherein each unit: contains from 250 mg to 2.5 g or from
500 mg to 4.0 g of the active ingredient. Optimally, a human
daily dose i:~ contemplated to consist of two or three unit sub-
doses of 0.5 to 3 g each of the active urea agent ingredient
when administered to an adult subject by mouth or by vein only.
Daily dosage will. be dependent in part upon the
antipolymerizing potency of the aromatic urea ingredient, in
part upon it:~ pharmacokinetics, and in part upon the clinical
seriousness of the sick:ling syndrome in the given subject.
Present art c:l.assifies both the parent homologs,
phenylurea and benzylurE~a, as compounds of only moderate
toxicity by ingestion o:E hazard rating 2. Carcinogenic,
mutagenic, or hepatogen:i~~ serious adverse effects are not
listed (Sax'~~ 1992, vol. II & vol. III).
The invention is the use of the aromatic urea
compound, consisting of mono-aromatic phenyl-radical or the
meta-positioned or para--~?ositioned monohydroxyphenolic-radical
or monomethox.yphenyl-racf=ical or monomethylphenyl-radical
attached to ene nitrogen of the urea molecule, either directly
or with one methyl or ethyl group intervening, for substantial
noncovalent inhibition of deoxygenated hemoglobin S
polymerization inside human erythrocytes. This inhibitory
effect occurs when the aromatic urea agent is delivered at low
CA 02137755 2001-06-27
75787-1
9
millimolar concentrations into the extracellular fluid bathing
the red blood cells within physiologic ranges of blood pH and
temperature. An effective amount of the aromatic urea compound
is preferabl,~r sufficient to provide a blood concentration in
p the range of from 1 to 22 mM. The invention is for palliation
and beneficial therapeutic effect in sickle cell anemia and
other sick11l1g syndromes. Hemoglobin S (HbS) polymerization is
said to be the primary determinant of the hemolytic and
clinical severity of the sickling syndromes (Brittenham et al,
1985).
The novel and useful inhibitory parent homologs are
phenylurea, benzylurea, and phenyl.ethylurea. There are six
monohydroxy benzenoid homologs also for this novel and
beneficial u:~e. These six are meta-hydroxyphenylurea, para-
1~~ hydroxypheny7.urea, meta-hydroxybenzylurea, para-
hydroxybenzyl.urea, met.a-hydroxyphenylethylurea, and para-
hydroxyphenyl.ethylurea. There are also six monomethoxyl
benzenoid homologs and ;six monomethyl benzenoid homologs for
this novel arid benefici<~.1. use. These 12 are meta- and para-
methoxyphenyl.urea; mete- and pare-methoxybenzylurea; mete- and
pare-methoxyphenylethylurea; mete- and pare-methylphenylurea;
mete- and pare-methylbenzylurea; and mete- and para-
methylphenylethylurea.
Commercially available sources are available for some
of these arylureas and aralkylureas. Phenylurea is available
for purchase from Aldric:h Chem. Co. of Milwaukee, WIS, Pfaftz &
Bauer, Inc. of Waterbury, CT, and ICN Biomedicals, Inc. of
Irvine, CA. Benzylurea is available from Aldrich and Pfaltz &
Bauer. Mete-hydroxyphen~rlurea is available from Aldrich and
ICN Biomedicals. Mete- and pare-tolylu.rea are available from
ICN Biochemicals. The arylurea and aralkylureas may be
synthesized by known methods. Such a method is the Wohler
reaction with the intramolecular transformation of the aromatic
CA 02137755 2001-06-27
75787-1
9a
amine Cyanate as the :irr,mediate precursor into the urea. This
synthesis may be done with the aromatic amine free base and
equivalent amount of free mineral acid or use of
' 10 75787-1
hydrochloride or hydrosulfate precursor salt and mixing with aqueous potassium
cyanate in equimolar amounts, with filtration or evaporation to dryness and
purification from absolute alcohol (Buck, 1934; Buck, Hjort and de Beer,
1935). Aqueous sodium cyanate may be used instead. Precursor free bases of
meta- and para-methoxyaniline (m- and p-anisidine) may be purchased from Sigma
Chemical Co. of St. Louis, M0, from Aldrich, and from Pfaltz & Bauer. Meta-
and para-methylaniline, meta-methylaniline sulfate, and para-methylaniline
hydrochloride are obtainable from Pfaltz &~Bauer and para-methylaniline hydro-
chloride is also obtainable from Sigma. If the precursor chemicals are col-
ored, they may be purified by known method of use of organic solvent. A
precursor to make phenylethylurea.is phenylethamine hydrochloride, available -
_._
from Aldrich and the precursor para-hydroxyphenylethylamine hydrochloride
(tyramine HC1) may be purchased from Aldrich, Pfaltz & Bauer, and Sigma.
N-monosubstituted aromatic and aralkyl ureas may also be synthesized by
reacting the primary aromatic or aralkyl amine with silicon tetraisocyanate
according to the method of Neville and McGee, 1963.
The uti 1 i ty of the above 21 aryl ureas and aral kyl ureas as anti s i ckl i
ng
compounds is novel in that they are aromatic urea derivatives that are
essentially nonionized or nonelectrolytes at physiological human blood pH
ranges and in that they are amphiphilic with the characteristic of being
moderately both lipid soluble and water soluble at low millimolar levels at
25° and 37°C. The hydrophilic nature of these arylureas is
viewed as
important in the inhibitory intracellular action of these agents on hypoxic or
deoxygenated HbS tetramers in inhibiting polymerization, since their
inhibitory potency generally varies inversely with their octanol/saline or
oil/saline partition coefficient. However, the hydrophobic or lipophilic
213'~'~ ~ 5
11 75787-1
nature of these compounds is viewed as paramount to the benzenoid
antipolymerizing effect of these homologs. This conception for nonelectrolyte
amphiphilic aromatic ureas to require dual hydrophilic and hydrophobic
functions in order to exert substantial intracellular antipolymerizing
activity against deoxygenated HbS erythrocytes at low millimolar concentration
without serious adverse red cell side-effects is novel. This novel invention
applies particularly to the invented-use, monohydroxy-substituted and
monomethoxy-substituted arylamines possessing the phenolic-radical or the
methoxy-radical. Aromaticity is not a sufficient condition for inhibiting
polymerization of deoxy HbS even in cell-free solution. Prior art has shown
- ------ - that phenol by itself-enhances gelation and reduces the solubility
of - -
deoxygenated HbS in cell-free solutions (Noguchi & Schechter, 1978).
By laboratory reduction to practice, these antisickling arylureas and
aralkylureas are found not to be mostly bound to blood plasma proteins (see
Table 9 of Example 11); also, they were found not to cause detrimental, marked
crenation (echinocytosis) or hemolysis of human erythrocytes at low millimolar
levels. Importantly also, the monohydroxy and monomethoxy homologs of these
compounds are conceived as potentially causing less central nervous system
side-effects such as sedation or hypnosis when administered for antisickling
therapeutic effects, because of their more polar nature with one monohydroxyl
or monomethoxy group, which restricts diffusion of lipophilic molecules across
the b1 ood-brai n endothel i al vascul ar barri er. Past art i n mi ce wi th
phenylurea, para-hydroxyphenylurea, and para-methoxyphenylurea supports this
thesis (Buck et al, 1935).
Prior art is not known to me that details the pharmacokinetics of these
arylureas and aralkylureas in homeothermic animals or man. However, from
12 75787-1
reasoning of analogy with prior art concerning normal urea
kinetics and urea pharmacokinetics in man, much of the
pharmacokinetics I have conceived or predicted for two or more
potent antipolymerizing arylureas viz meta-hyroxyphenylurea and
benzylurea has been in vitro demonstrated as prototypical agents
for useful treatment doses in man. This has been demonstrated by
determinations of their percentage binding to plasma proteins
(which is modest or moderate, see Table 9 in Example 11) and by
determinat ions of their lipid/water part it ion coefficients ( see
Table 10 of Example 12).
The body of clearance of some of these monocyclic
arylureas, e.g. at least of meta- and para-methoxyphenylurea, and
some of these aralkylureas, e.g. at least of benzylurea, is
conceived to be almost entirely or chiefly by partial non-
reabsorption of kidney filtered arylurea or aralkylurea, similar
to renal excretion of urea after urea glomerular filtration. The
octanol/saline partition coefficients of meta-hydroxyphenylurea
and benzylurea average about 1.65 and 4.10, respectively (see
Table 10 of Example 12). Prior art reports that the octanol/water
part it ion coeff icient of urea is 0 . 011 and that the part it inning
between octanol and water for urea and aliphatic ureas gives
suitable agreement between biological and pharmacological results
(Abraham, 1982). The average relative octanol/water distribution
coefficient (ratio of aromatic urea to urea) calculates as
1.65/0.011 or about 150/1 for meta-hydroxyphenylurea and
calculates as 0.57/0.011 or about 52/1 for para-hydroxyphenylurea
and calculates as 4.10/0.011 or about 373/1 for benzylurea on
Example 12 of effective model. Therefore, it is conceived that
the renal tubular back-diffusion of filtered meta-
hydroxyphenylurea and para-hydroxyphenylurea is much greater than,
and perhaps at least 10 times greater than, back-diffusion of
filtered urea and perhaps at least about 10 to 20 times greater
for benzylurea than the tubular reabsorption of filtered urea.
213'~'~55
13 75787-1
Such kinetic relationships indicate that the renal excretion and
renal plasma clearance of meta-hydroxyphenylurea and para-
hydroxyphenylurea and similarly of meta- and para-
methoxyphenylurea average predictably much less than the urea
values and that the renal excretion and renal plasma clearance of
benzylurea also average much less than and perhaps no more than
about 10~ of the urea values in man.
Prior art has established that renal whole blood and
plasma clearances of urea in normal man at diuretic urinary flows
average about 1.1 ml/min/kg or about 1.6 liters/24 hrs./kg
("maximum" renal urea clearances) when glomerular filtration rate
is at normal average value of 1.6 ml/min/kg or about 2.3 liters/24
hr./kg of body weight in adults and children after 3 years of age.
Assuming body loss of administered arylurea is wholely or mainly
by renal excretion of the unchanged agent, maximal daily intakes
of about 0.8 mmoles of monohydroxyphenylurea or
monomethoxyphenylurea (0.1 x 3.5 mM x 2.3 liters) per kg and of
about 0.8 mmoles of benzylurea (0.1 x 3.5 mM x 2.3 liters per kg
are required to maintain blood plasma levels of about 3.5 mM for
these two arylureas and for benzylurea. The dosage gram amount of
0.8 mmoles/kg/24 hr. for monomethoxyphenylurea is 0.13 g/kg/24 hr
and the dosage gram amount of 0.8 mmoles/kg/24 hr for benzylurea
is 0.12 g/kg/24 hr. At a continued intake as high as 0.8
mmoles/kg/24 hr, accumulative rises to blood plasma and whole
blood levels of 3.5 mM or more is contemplated to result if blood
clearance of the administered aromatic urea anti-sickling agent is
mainly by renal loss and if the renal loss is substantially less
than an assumed rate of about 10~ of the normal renal clearance
rate for urea in man.
Prior toxicologic art reports that acutely injected LD50
for para-
14 75787-1
hydroxyphenylurea in mice is 6.0 mmoles/kg (0.91 g/kg) and that introduction
of a monophenolic hydroxy group caused the sedative and other hypnotic
properties of phenylurea to disappear (Buck et al, 1935). The acutely
injected LD50 for phenylurea in mice is reported variously to be 0.75 g/kg
(Buck et al, 1935) and to be 1.45 g/kg (Zirvi & Fakouhi, 1977). Prior art
also reports that at dose levels below 0.30 g/kg in mice, there is no
descernible central nervous system depressant activity. A subsedative
intraperitoneal dose of phenylurea is 0.20 g/kg in mice (Zirvi & Fakouhi,
1977). Prior art reports that the LD50 after acute injection into mice is 3.0
mmoles/kg (0.45 g/kg) for benzylurea and 3.0 mmoles/kg (0.49 g/kg) for
phenylethylurea (Buck et al, 1935). Prior art-reports that the LD50 after - --
.-
acute intraperitoneal injection into mice is 5.5 mmoles/kg (0.91 g/kg) for
para-methoxyphenylurea (Buck et al, 1935).
Prior art also reveals that after oral administration acutely, the LD50
for benzylurea is 2.50 g/kg, 4.41 g/kg, and 2.70 g/kg in mice, rats, and
rabbits (Gorianova et al, 1979); in chronic experiments, benzylurea at 1.0
mg/kg had no toxic effects.
I have shown in vitro that the sickling or deformation of deoxygenated
HbSS erythrocytes can be inhibited substantially at low levels between 3.5 to
7 mM of meta-hydroxyphenylurea, para-hydroxyphenylurea, para-methoxyphenylurea
and phenylurea, and between 10 to 12 mM of benzylurea; also, the inhibitory
effects are dose-dependent (see Tables 1, 2, 3, and 4 in Examples 3, 4, 5 and
I have also shown in vitro, that significant improvement in flexibility
of hypoxic HbSS erthrocytes, as measured by cell filterability studies
monitoring filtration pressure at constant flow rate, is caused by meta-
' 15 75787-1
hydroxyphenylurea at low levels under 11 mM (see Example 7), by benzylurea at
low levels under 23 mM (see Example 8), by para-hydroxyphenylurea (see Example
9), and para-methoxyphenylurea under 18 mM (see Example 10). Such preventive
antigelation effects in intact HbSS erythrocytes are shown to be dose-
dependent also (see Examples 7, 8 and 10). At such levels of these three
arylureas and one aralkylurea, microscopic examination showed no more than
slight membrane cremation (echinocytosis).
Prior art reveals that, in subjects with chronic sickle cell anemia, the
bones, joints, and spleen are mostly exposed to vaso-occlusive crises and to
painful syndromes even at rest. The prior art shows also that mean mixed
venous blood 02 gas tensions are-about 46.5 mm Hg even at rest in--these - - --
subjects, with profound, upper limit levels of physiological compensatory
mechanisms (Lonsdorfer et al, 1983). My invention shows that significant and
substantial inhibitory effects on cell stiffness and sickling, induced by low
P02 (partial oxygen pressure) levels in vitro that are comparable to those
found in mixed venous blood in prior art studies in vivo, are accomplished by
means of modest mM levels of select arylureas in vitro. Therefore, the data
contained in Tables 1 through 8 of the Examples is evidence that induced low
millimolar blood concentrations of antisickling aromatic urea agent will be
useful and beneficial in the palliation of human sickling disorders.
Improvement is contemplated to occur even at sustained modest blood levels as
low as 1 to 3 mM of the inhibitory urea compound in the circulating blood in
sickle cell disease in vivo. Prior art has shown that sustained and
irreversible red cell damage develops and anemia is intensified because of
continuous or cyclic hemoglobin S polymerization (sickling) and
depolymerization (unsickling) in the circulating blood in sickle cell disease
16 75787-1
as the blood is deoxygenated in peripheral tissues and re-oxygenated in
transit through the lungs (Zipursky et al, 1993). Low levels of 1 to 4 mM
inhibitory aromatic urea compound should decrease the magnitude of this
continuing adverse cyclic process in sickle cell disease.
The following Example 1 of Pharmaceutical Formulations is described in
illustration of the present invention; it should not be construed as in any
way constituting a limitation thereof.
EXAMPLE 1
Pharmaceutical Formulations
A. TABLET Composition:
'. ._ _ - Urea Compound __ 1:0 g _ _ _-
Ascorbic Acid 40 mg
Starch 150 mg
Sucrose 100 mg
Polyvinylpyrrolidone (PVC) 15 mg
Magnesium Stearate 15~ mg
Total 1.320 g
The urea compound, ascorbic acid, starch, and sucrose are mixed together
and then granulated with a solution of PVC in water. After drying the
granules, the magnesium stearate is mixed in and the tablets compressed at an
average weight of 1.32 g. Keep tablets in tightly closed bottles.
B. CAPSULE Composition:
Urea Compound 250 mg
Ascorbic Acid 10 mg
Starch 5 mg
Methylcellulose 400 cps 5 mg
Stearic Acid 5 mg
Total 275 mg
The urea compound, ascorbic acid, and starch are mixed together and then
granulated with a solution of the methylcellulose in water. After drying, the
1
21377~~
L
17 75787-1
granules are mixed with the stearic acid and the mixture filled into gelatin
capsules at an average fill weight of 275 mg. Keep in tightly closed bottles.
C. SUPPOSITORY Composition
Urea Compound 2.0 g
Ascorbic Acid 80 mg
Dibasic Sodium Phosphate 150 mg
Cocoa Butter 4.0 g
Total 6.23 g
Grind the urea compound, ascorbic acid, and one sodium salt to a
particle size below 200 ~c. Add the cocoa butter at 40 to 45°C. Mix to
give
a,uniform dispersion. Pour into suppository moulds and allow to cool.
D. INJECTION = S~-n,lc~e Dose. Intravenous:
Urea Compound 250 mg
Sodium Chloride 350 mg
Ascorbic Acid 10 mg
Dibasic Sodium Phosphate 30 mg
Monobasic Sodium Phosphate Monohydrate 10 mg
Total 650 mg
Water q.s. to 50 ml. vol.
Suspend the above solid compounds in about 45 ml of the water for
injection and warm to about 45°C with stirring gently to dissolve. With
cooling to 25°C, add sufficient water to required final volume of 50
ml.
Sterilize by passage through a sterile membrane filter of 0.2 micron pore
size. Fill under aseptic conditions into a light-resistant vial or ampul to
final volume of 50 ml per container. Store optimally at temperature between
15° and 30°C (59° - 86°F.). Inject the 50 ml
warmed solution of aromatic
compound of 0.5 g/dl slowly over 5 to 10 minutes.
18 75787-1
E. ORAL ELIXIR Composition; Sin 1e Dose:
Urea Compound 180 mg
. Ascorbic Acid 10 mg
Sucrose 200 mg
1-Butanol 1.00 ml
Water q.s. to 25.0 ml
The urea compound, ascorbic acid, and sucrose are mixed into a warmed
(to 45-65°C) volume of 4.0% (v/v) soln. of n-butyl alcohol, for
dissolution
of the solid compounds. Upon cooling, more 4.0% n-butyl alcohol soln. is
added to final volume of 25.0 ml per 0.18 g of urea compound. The final
solution should be stored in a light-resistant container, not exposed to air.
Single 25-ml doses orally should be followed by drinking about 50-ml or more
of water or-other liquid per 10 to 20 kg of child-body wt.
The following Examples 2 through 13 are provided in Table form in
illustration of the present invention. None of the Examples 2 through 13
should be construed as in any way constituting a limitation thereof.
EXAMPLE 2
Preparation of para-methoxyphenylurea
0.25 mole (16.93 g) of sodium cyanate was dissolved in distilled water
(150 ml). 0.25 mole (30.80 g) of purified para-Methoxyaniline as off-white
crystals (obtained as Grade I p-Anisidine free base from Sigma, FW 123.2) was
dissolved with stirring in 275 ml of distilled water, after prior addition of
21.43 ml concentrated hydrochloric acid (specific gravity 1.184) to yield a
solution containing equimoles (0.25 mole) of hydrochloric acid. The sodium
cyanate solution was added with stirring at room temperature to the formed
solution of para-methoxyaniline hydrochloride; whitish crystallization was
produced promptly. After standing at room temperature overnight (12 hr), the
reaction mixture was cooled to crushed-ice water temperature (2-3° C)
for a
19 75787-1
period of 30 min. The precipitated solid was separated by cold
filtration through a sintered glass funnel with suction. The
well-drained white product was added to 150 ml of warm absolute
ethyl alcohol, promptly heated at 70-72oC with stirring for ready
dissolution of the crystals. The alcoholic mixture was cooled and
kept at crushed-ice water temperature for two hours. The
recrystallized product was separated by cold filtration through a
sintered glass funnel with suction. The drained product was dried
by evaporation. Yield of para-methoxyphenylurea was 34.82 g
(83.9 of 0.25 mole) as white crystals devoid of cream color or
other chromatic hue, m.p. 165-166°C from absolute ethanol.
EXAMPLE 3
Antlsickling potency of amphiphilic ureas in vitro when
partial deoxygenation is induced by metabisulfite.
Aliquots of heparinized blood from 8 patients with
homozygous sickle cell disease (HbSS) (1 male, 7 females) were
used. Median patient age was 9 years (range 5 to 18). Sickling
was induced by addition of sodium metabisulfite to 8.1 mM to
duplicate 200 u1 aliquots of whole blood diluted 22/78 (v/v) with
isotonic buffered saline-glucose solution containing no urea agent
or containing amphiphilic aromatic urea agent at 3.5 to 10 mM, or
butylurea at 100 mM final concentration as known antisickling
agent. The samples, at pH 7.0 - 7.1, were incubated for 30-
minute periods at 37°C. Cells were then fixed by anaerobic
addition of 400 ~1 of 3.7~ formaldehyde in buffered isotonic
saline. Aerated control blood aliquots with 22/78 (v/v) additions
of isotonic saline-glucose solution without sodium metabisulfite
were concurrently incubated at 37oC and then fixed with the 3.7~
formaldehyde-saline solution. Red cell shape was examined
microscopically at
~~3~~~~
20 75787-1
705 X magnification by counting the shape of 400 erythrocytes. Induced
sickling was counted as sickled/bizarre/ovoid red cells newly formed during
the 30-minutes of hypoxia. Counting results of red cells were expressed as
percentages. Tests with 3.5 mM meta-hydroxyphenylura and with butylurea were
carried out with blood from only 6 and 7 patients, respectively. The results
are given in Table 1. These results show that meta-hydroxyphenylurea,
phenylurea, and benzylurea are potent inhibitory agents at concentrations much
lower than found with 100 mM butylurea.
2~~~7~~
21 75787-1
Table 1. Effects of amphiphilic ureas as inhibitors of sickling at 37°C
induced by
partial deoxygenation, with metabisulfite added to 8.1 mM
Percent newly Percent relative
Condition sickled cellsab inhibitory activityb
(%)
Control sta te 56.8 3.7 0.0
m-Hydroxy- 37.4 4.0c 31.4 3
5c
phenylurea, 3.5 mM .
m-Hydroxy- 33.6 3.9c 39.6 4
7c
phenylurea, 7.0 mM .
Phenylurea, 3.5 mM 44.1 3.0c 22.3 2.9c
Phenylurea, 7.0 mM 40.5 2.7c 29.4 3.0c
Benzylurea, 10 mM 46.4 3.8c 17.5 2.3c
Butylurea, 100 mM 36.8 4.3c 36.7 3.5c
aNewly sickled cells were averages of newly formed sickled/bizarre/ovoid red
cells during 30-minute periods of induced hypoxia done in duplicate (pH 7.0 -
7.1).
Control aerated state sickling was 19.5 ~ 4.0%.
bValues are means ~ SEM in paired determinations in HbSS blood from 8
patients,
except for 3.5 mM-OH-phenylurea and for butylurea, where n was 6 and 7,
respectively. Mean hematocrit was 21.1 ~ 1.5%.
cMean differences from new sickling of control hypoxic state are significant
at
P-value < 0.001.
22 75787-1
EXAMPLE 4
Inhibitory potency of amphiphilic aromatic urea compounds and butylurea
in vitro on sickling induced by hypoxia by means of low oxygen gassing.
Aliquots of heparinized whole blood from 9 patients with homozygous
sickle cell disease (6 males, 3 females) were used. Median patient age was 8
years (range 5 to 19). Sickling was induced by change of gas phase above the
liquid samples from room air to 45-minute periods of gassing with humidified
5% 02/95% N2. Used were duplicate aliquots, located in series, of blood
'' _ .diluted 22/78 (v/v) with isotonic buffered saline-glucose solution
containing
no urea agent (control) or containing as the urea agent, meta-
hydroxyphenylurea, meta-tolylurea (meta-methylphenylurea), phenylurea,
benzylurea, or 50 mM butylurea. Gassings of 1.0-ml sample mixtures were
performed in 7-ml siliconized glass bottles (each containing one small
stainless steel ball) positioned on a platform oscillating at 51 times/min
immersed in a water bath at 37° C. Aerated (room air) sample mixtures
were
incubated concurrently. Control blood sample mixtures were similarly
incubated with room air aeration or with 5% 02 gassing, for sample gas oxygen
tension and pH measurements at the end of the incubation periods. Red cell
fixation in the blood-solution mixtures at the end of the test periods was
performed by addition of 400 ~1 of 3.7% formaldehyde-buffered saline
solution. This was carried out during continued 5% 02/95% N2 gassing of the
75787-1
hypoxic samples. Subsequently, microscopic counting of the shape of 400
erythrocytes was carried out for each test sample, as described in Example 3
above. Red cell counting results were similarly expressed as percentages.
Tests with meta-tolylurea and butylurea were performed with blood from only 6
and 8 subjects, respectively. The results are given in Table 2. The
inhibitory potency results indicated that 5 mM meta-hydroxyphenylurea was
greater than 12 mM benzylurea, which was approximately equal to 50 mM
butylurea, which was greater than 5 mM meta-tolylurea, which in turn was
approximately equal to 5 mM phenylurea in inhibitory activity.
4
- 24 75787-1
Tabl a 2. Inhi bi tory effects of aromati c ureas and butyl urea on si ckl i
ng i nduced by
hypoxi a to 1 ow oxygen pressures of 44. 6 ~ 1. 2 mm Hg, by gassi ng wi th
humi di f i ed 5~
02/95% N2 at 37°C
Percent newly Percent relative
Condition sickled cellsab inhibitory activityb
(%) (%)
Control state 27.9 ~ 4.9 0.0
m-Hydroxy- 12.5 ~ 3.1c 57.4 ~ 5.6d
phenylurea, 5.0 mM
,, Benzylurea, 12 mM 16.5 ~ 3.9c 44.7 ~ 6.4d
Phenylurea, 5.0 mM 23.4 ~ 4.9c 17.9 ~ 5.9c
m-Tolylurea, 5.0 mM 21.9 ~ 7.0c 24.1 ~ 7.1c
Butylurea, 50 mM 16.7 ~ 3.9c 44.9 ~ 4.3d
aNewly sickled cells were averages of newly sickled/bizarre/ovoid red cells
duri ng 45 mi nutes of hypoxi a done i n dupl i cate . Control aerated s i ckl
i ng was 13 . 6
~ 3.0% at P02 of 143.9 ~ 2.3 mm Hg and pH of 7.25 ~ 0.02; pH was 7.39 ~ 0.02
(n =
9) after 5% 02 gassing for 45 minutes.
bValues are means ~ SEM in paired analyses of homozygous HbS blood from 9
subjects, except for m-tolylurea and butyl urea, where n was 6, and 8,
respectively.
Mean hematocrit was 23.0 ~ 1.9%.
cMean differences from new sickling of control hypoxic state are significant
at
P-value < 0.01.
dMean differences from new sickling of control hypoxia state are significant
at
P-value < 0.001.
25 75787-1
EXAMPLE 5
Inhibitory effects of meta-hydroxyphenylurea, para-hydroxyphenylurea,
and para-hydroxyphenylethylurea on sickling induced by hypoxia by means of
low oxygen gassing.
Aliquots of heparinized whole blood from 5 subjects with homozygous
sickle cell anemia (3 males, 2 females) were used. Median patient age was 12
years (range 6 to 15). Red cell sickling was induced as described in Example
4. The procedures used were also as described in Example 4, but with blood
_._'_,- __-_ _ diluted 22/78 (v/v) with isotonic buffered saline-glucose
solution containing
no urea agent (control) or containing meta-hydroxyphenylurea, para-
hydroxyphenylurea, or para-hydroxyphenylethylurea as the urea agent. Red
cell counting results were similarly expressed as percentages in these paired
tests. The results are given in Table 3. The inhibitory potency results
showed that 7 mM meta-hydroxyphenyl urea was greater than 7 mM para-
hydroxyphenylurea, which was more effective than 7 mM para-
hydroxyphenylethylurea, and showed that the inhibitory potency was dose-
related. Table 3 also shows that the inhibitory activity of para-
hydroxyphenylurea on hypoxic sickling was substantial at concentrations as
low as 3.5 mM.
26 75787-1
Table 3. Inhibitory effects of meta-hydroxyphenylurea, para-hydroxyphenylurea,
and
para-hydroxyphenylethylurea on sickling increased by hypoxia to low oxygen
pressures
of 40.9 ~ 0.5 mm Hg, by gassing with humidified 5% 02/95% N2 at 37°C
Percent newly Percent relative
Condition sickled cellsab inhibitory activityb
(%) (%)
Control state 45.5 ~ 7.6 0.0
m-Hydroxy-
phenylurea, 7.0 mM 16.2 ~ 3.4df 65.0 ~ 3.0e
p-Hydroxy-
phenylurea, 7.0 mM 27.4 ~ 5.5df 42.7 ~ 4.6e
_ _.____.__ . p=Hydroxy- - _ __ __ ._. _ . _ _. _ . _ _. ____ __ .._.. ._ _ .
_. ___
phenylurea, 3.5 mM 29.1 ~ 5.4d 36.9 ~ 2.5e
p-Hydroxyphenyl-
ethylurea, 7.0 mM 32.4 ~ 6.1c 29.g + 4.7d
p-Hydroxyphenyl-
ethylurea, 14.0 mM 27.6 ~ 5.5c 40.7 ~ 5.1e
aNewly sickled cells were averages of newly sickled/bizarre/avoid red cells
duri ng 45 mi notes of hypoxi a done i n dupl i cate . Control aerated s i ckl
i ng was 15 . 4
~2.9%atP02of141.6~1.5mmHgandpHof7.21~O.Ol;pHwas7.34~0.01 (n=
5) after 5~ 02 gassing for 45 minutes.
bUalues are means ~ SEM in paired analyses of homozygous HbS blood from 5
subjects. Mean hematocrit was 22.5 ~ 1.29.
cMean differences from new sickling of control hypoxic state are significant
at
P-value < 0.02.
dMean differences from that of control hypoxic state are significant at
P-value < 0.01.
eMean differences from that of control hypoxic state are significant at
P-value < 0.001.
fMean difference between values for the two ureas is significant at P-value
< 0.02.
i
27 75787-1
EXAMPLE 6
Inhibitory effects of para-methoxyphenylurea,and para-methoxybenzylurea
on sickling induced by hypoxia by means of low oxygen gassing.
Aliquots of heparinized whole blood from 6 patients with homozygous
sickle cell disease (2 males, 4 females) were used. Median patient age was
12 years (range 4 to 15). Red cell sickling was induced as described in
Example 4. The procedures used were also described in Example 4, but with
blood diluted 15/85 (v/v) with isotonic buffered saline-glucose solution
_ _ __ containing_no urea_agent (control) or containing para-methoxyphenylurea
or
para-methoxyphenylethylurea. Red cell counting results were similarly
expressed as percentages in these paired tests. The results are given in
Table 4. The inhibitory potency results showed that para-methoxyphenylurea
was more effective than para-methoxyphenylethylurea, that the effectiveness
was dose-related, and that para-methoxyphenylurea was significantly
inhibitory at concentrations as low as 4.2 mM.
~i~~7~~
28 75787-1
Table 4. Inhibitory effects of para-methoxyphenylurea and para-
methoxybenzylurea
on sickling induced by hypoxia to low oxygen pressures of 45.2 ~ 1.3 mm Hg
at pH 7.27 ~ 0.01, by gassing with humidified 5% 02/95% N2 at 37°C
Percent newly Percent relative
Condition sickled cells inhibitory activity
%~
Control state 47.9 ~ 8.4 0.0
p-Methoxy-
phenylurea, 8.5 mM 33.5 ~ 8.8a 37~7 ~ 10.2c
p-Methoxy-
phenylurea, 4.2 mM 39.9 ~ 8.7b 24.6 ~ 8.3d
p-Methoxy-
benzylurea, 8.5 mM 38.4 ~ 8.9a 25,7 ~ 8.6d
Control aerated sickling was 12.2 ~ 4.7% at P02 of 150 ~ 1.4 mm Hg and pH of
7.19 ~ 0.01 at 37°C, at the end of the concurrently performed hypoxic
gassing
periods of 45 minutes.
Values are means ~ SEM in paired experiments in vitro using mixtures with 15%
homozygous HbS whole blood from 6 subjects. Mean hematocrit was 22.6 ~ 1.3%.
aMean differences from new sickl ing of control hypoxic state are significant
at
P-value < 0.001.
bMean difference from that of control hypoxic state is significant at P-value
< 0.005.
cMean difference from that of control hypoxic state is significant at P-value
< 0.02.
dMean differences from that of control hypoxic state are significant at P-
value
< 0.05.
- ,
29 75787-1
EXAMPLE 7
Inhibitory effects of meta-hydroxyphenylurea on hypoxic
increases in filtration pressure at constant flow through small
pore-diameter filters, as a deformability index of hypoxia-induced
hemoglobin SS polymerization.
Aliquots of heparinized whole blood from 6 patients with
homozygous sickle cell anemia (4 males, 2 females) were used.
Median pat lent age was 6.5 years (range 5 to 11). Hypoxia was
induced in duplicate aliquots, located in series, of blood diluted
11/89 (v/v) with isotonic buffered saline-glucose solution
containing no urea agent (control) or containing meta-
hydroxyphenylurea at two different mM concentrations. Gassings
were carried out as described in Example 4 and then constant flow
perfusions of aliquot mixtures were carried out 2-3 minutes after
aerated periods or after 45-minute periods of hypoxic gassings.
The constant flow perfusions were performed in plastic membrane
devices (Pop-Top holders) each containing a 5 um pore-diameter
Nucleopore membrane filter at 37°C. The filtration pressure
resulting from constant flow perfusion and filtration was measured
closely upstream from the filter holder. Pressure measurements
were used at 42 seconds of perfusion-filtration at a flow rate of
0.55 m1/42 seconds, as an inverse index of the flexibility or
deformability of the red blood cells to pass pore openings,
somewhat smaller than the average diameter of normal human
erythrocytes. Prior art has shown that the deformability of red
cells
30 75787-1
containing hemoglobin SS varies inversely with the degree of hemoglobin SS
polymerization, which varies directly with the amount of induced
deoxygenation. The results are given in Table 5. The results showed that 10
mM meta-hydroxyphenylurea markedly inhibited the hypoxic stiffness of HbSS
red cells and that the inhibitory activity was dose-related, 5 mM being
effective to a lesser extent.
~ 13'~'~ ~ ~
31 75787-1
Table 5. Inhibitory effects of meta-hydroxyphenylurea on hypoxic increases in
filtration pressure at constant flow through 5 ~m pore-diameter filters, as
erythrocyte deformability index of hemoglobin SS polymerization
* Hypoxic Gain
Condition Filtr P, Filtr P, Percent Relative
(No of subjects) Oxy Deoxy-Oxy Inhibitory Activity
(mm Hg) (mm Hg) (%)
Control state 4.3 ~ 0.6 65.5 ~ 5.6 0.0
(n = 6)
m-Hydroxyphenyl-
urea, 5 mM 3.2 ~ 0.6 55.6 ~ 4.2ac 19.7 ~ 2.lbc
(n = 4)
m-Hydroxyphenyl-
'' urea, 10 mM 4.6 ~ 0.8 30.1 ~ 4.8bc 55.6 ~ 4.6bc
Yal ues are means ~ SEM at 42 seconds wi th HbSS whol a b1 ood-reagent mi
xtures i n
11:89 ratios (v/v) perfused at 0.55 m1/42 sec through 5 ~m pore-diameter
Nucleopore
membrane filters at 37°C. Constant perfusions of aliquots were done 2-3
minutes
after aerated periods (oxy) or 2-3 minutes after 45-minute hypoxic periods
(deoxy) .
Perfusate hematocrits were 2.4 ~ 0.1% (n = 6).
*Fi 1 tr P represents fi 1 trati on pressure c1 osely upstream from the fi 1
ter holder
device (Pop-Top), positioned at 20° angle at 37°C.
aMean difference from that of paired control state is significant at P-value
< 0.02.
bMean differences from that of paired control state are significant at P-value
< 0.005.
cMean differences between paired values at the two concentrations of agent are
significant at P-value < 0.01 (n = 3).
32 75787-1
EXAMPLE 8
Inhibitory effects of benzylurea on hypoxic increases in
filtration pressure at constant flow through small pore-diameter
filters, as a deformability index of hypoxia-induced hemoglobin SS
polymerization.
Aliquots of heparinized whole blood from 4 patients with
homozygous sickle cell disease (3 males, 1 female} were used.
Median subject age was 10 years (range 6 to 19). Hypoxia was
induced as described in Example 7 and procedures used were similar
to those described in Example 7 but with the test blood mixtures
containing benzylurea at two different concentrations. Results
are shown in Table 6. The inhibitory activity of benzylurea on
hypoxia-induced stiffness of homozygous hemoglobin S red cells was
dose-related, at low mM concentrations.
33 75787-1
Table 6. Inhibitory effects of benzylurea on hypoxic increases in filtration
pressure at constant flow through 5 ~cm pore-diameter filters, as erythrocyte
deformability index of hemoglobin SS polymerization
* Hypoxic Gain
Condition Filtr P, Filtr P, Percent Relative
(No of subjects) Oxy Deoxy-Oxy Inhibitory Activity
(mm Hg) (mm Hg) (%)
Control state 18.3 ~ 4.2 66.3 ~ 8.5 0.0
(n = 4)
Benzylurea, 15.1 mM 16.7 ~ 3.1 57.8 ~ 9.9a 14.6 ~ 6.2ad
(n = 4)
Benzylurea, 22.1 mM 16.0 ~ 2.8 49.7 ~ 9.2c 27,3 ~ 6,3bd
(n - 4)
Values are means ~ SEM in paired experiments at 42 seconds with HbSS whole
blood-reagent mixtures in 11:89 ratios (v/v) perfused at 0.55 m1/42 sec
through 5
um pore-diameter Nucleopore membrane filters at 37°C. Constant
perfusions of
al iquots were done 2-3 minutes after aerated periods (oxy) or 2-3 minutes
after 45-
minute hypoxic periods (deoxy). Perfusate hematocrits were 2.2 ~ 0.2% (n = 4).
*Fi 1 tr P represents f i 1 trati on pressure c1 osely upstream from the f i 1
ter hol der
device (Pop-Top), positioned at 20° angle at 37°C.
aMean differences from that of control state are significant at P-value <
0.05.
bMean difference from that of control state is significant at P-value < 0.025.
cMean difference from that of control state is significant at P-value < 0.001.
dMean difference between values at the two concentrations of agent is
significant
at P-value < 0.025.
.~ 34 75787-1
EXAMPLE 9
Inhibitory effects of para-hydroxyphenylurea and para-
hydroxyphenylethylurea on hypoxic increases in filtration pressure at
constant flow through small pore-diameter filters, as a deformability index
of hypoxia-induced hemoglobin SS polymerization.
Aliquots of heparinized whole blood from 4 patients with homozygous
sickle cell disease (2 males, 2 females) were used. Median subject age was
12 years (range 6 to 15). Hypoxia was induced as described in Example 7, and
similar procedures were used, but with the test blood mixtures containing
equimolar concentrations of 21 mM para-hydroxyphenylurea or para-hydroxy-
phenylethylurea. Results are shown in Table 7. Para-hydroxyphenylurea was
substantially more effective than para-hydroxyphenylethylurea in preventing
hypoxia-induced stiffness of homozygous hemoglobin S red cells.
- .
__. 2137'55
35 75787-1
Table 7. Inhibitory effects of equimolar concentrations of para-
hydroxyphenylurea
and para-hydroxyphenylethylurea on hypoxic increases in filtration pressure at
constant flow through 5 ~m pore-diameter filters, as erythrocyte deformability
index of hemoglobin SS polymerization
* Hypoxic G*in
Condition Filtr P , Filtr P Percent Relative
(No of subjects) Oxy Deoxy-0xy Inhibitory Activity
{mm Hg) (mm Hg) (~)
Control state 7.3 ~ 1.0 79.3 ~ 9.1 0.0
{n = 4)
p-Hydroxy- -
_ _ phenxlurea, 21.0 mM 6.3 ~ 0.9 45.0 ~ 7.7bc 44.0 ~ 4_7bc
{n - 4) . ..
p-Hydroxyphenyl-
ethyl urea, 21.0 mM 6 . 7 ~ 0 .8 65. 3 ~ s , 7bc 16 . 2 ~ 3. Oac
(n = 4)
Values are means ~ SEM in paired experiments at 42 seconds with HbSS whole
blood-reagent mixtures in 11:89 ratios {v/v) perfused at 0.55 m1/42 sec
through 5
~m pore-diameter Nucleopore membrane filters at 37°C. Constant
perfusions of
al iquots were done 2-3 minutes after aerated periods (oxy) or 2-3 minutes
after 45-
minute hypoxic periods (deoxy). Perfusate hematocrits were 2.5 ~ 0.1% (n = 4).
*Filtr P represents filtration pressure closely upstream from the filter
holder
device (Pop-Top), positioned at ZO° angle at 37°C.
aMean difference from that of control state is significant at P-value < 0.02.
bMean differences from that of control state are significant at P-value <
0.005.
cMean differences between values for the two areas are significant at P-value
< 0.01.
2~3'~'~55
36 75787-1
EXAMPLE 10
Inhibitory effects of para-methoxyphenylurea on hypoxic
increases in filtration pressure at constant flow through small
pore-diameter filters, as a deformability index of hypoxia-induced
hemoglobin SS polymerization.
Aliquots of heparinized whole blood from 6 subjects with
homozygous sickle cell disease (2 males, 4 females) were used.
Median subject age was 12 years (range 4 to 15). Hypoxia was
induced as described in Example 7 and similar procedures were
used, except for control and test blood-reagent mixtures used in a
15:85 ratio (v/v) and perfusion-filtration was performed at a
different rate of 0.60 m1/22 sec through the 5 um pore-diameter
Nucleopore membrane filters at 37°C. Pressure rise measurements
at 20 seconds of perfusion-filtration were used as an inverse
index of the flexibility or deformability of the red blood cells
to pass pore-openings somewhat smaller than the average diameter
of normal human erythrocytes. The results are given in Table 8.
The results show that the significant inhibitory activity of para-
methoxyphenylurea is dose-dependent in preventing hypoxia-induced
stiffness of homozygous hemoglobin S red cells. Comparison with
the results in Example 8 suggest that para-methoxyphenylurea is
more effective than benzylurea at equimillimolar blood
concent rat ions .
213'~"~55
37 75787-1
Table 8. Inhibitory effects of para-methoxyphenylurea on hypoxic increases
in filtration pressure at constant flow through 5 ~m pore-diameter filters,
as erythrocyte deformability index of hemoglobin SS polymerization
* Hypoxic Gai*
Condition Filtr P, Filtr P, Percent Relative
(No of Subjects) Oxy Deoxy-Oxy Inhibitory Activity
(mm Hg) (mm Hg) (%)
Control State 14.3 ~ 3.3 80.0 ~ 13.6 0.0
(n = 6)
p-Methoxyphenyl- 10.6 ~ 2.5b 65.8 ~ 14.3a 22.2 ~ 7.0a
urea, 12.0 mM
(n = 6)
p-Methoxyphenyl- 9.1 ~ 1.9a 57.0 ~ 11.8c 30.7 ~ 4.8d
urea, 17.0 mM
(n = 6)
Val ues are means ~ SEM at 20 seconds wi th HbSS whol a b1 ood-reagent mi
xtures i n
15:85 ratios (v/v) perfused at 0.60 m1/22 sec through 5 ~,m pore-diameter
Nucleopore
membrane filters at 37°C. Constant flow perfusions were done 2-3
minutes after
aerated periods (oxy) or 2-3 minutes after 45-minute hypoxic periods (deoxy).
Initial perfusate hematocrits were 3.4 ~ 0.2%.
*Filtr P represents filtration pressure closely upstream from the filter
holder
device (Pop-Top), position at 20° angle at 37°C.
Oxy state mean perfusate P02 was 150.7 ~ 1.7 mm Hg and mean pH was 7.18 ~ 0.02
(n = 6) . Deoxy state mean perfusate P02 was 44.8 ~ 1.1 mm Hg and mean pH was
7.26 ~
0.02 (n = 6).
aMean differences from that of control state are significant at P-value <
0.05.
bMean difference from that of control state is significant at P-value < 0.02.
cMean difference from that of control state is significant at P-value < 0.005.
dMean difference from that of control state is significant at P-value < 0.001.
213'~'~ ~ ~
38 75787-1
EXAMPLE 11
Percentages of nonprotein-bound and ultrafiltrable
amphiphilic arylureas and aralkylurea in sickle cell blood plasma
after exogenous additions.
In order to measure the extent of plasma protein binding
of prototypical amphiphilic antisickling aromatic urea compounds
at low millimolar levels, test were carried out in vitro.
Aliquots of heparinized blood plasma obtained from both male and
female young patients with homozygous sickle cell disease were
used. The blood plasmas were mixed in a volume ratio of 70/30
with control 40 mM phosphate-buffered isotonic saline reagent of
pH 7.3 containing no urea compound or mixed in a 70/30 volume
ratio with similar reagent solution also containing meta- or para-
hydroxyphenylurea or benzylurea. Plasma protein-binding or
ultrafiltrability was determined by the percentage of the aromatic
urea compound which passed through Centricon-10 centrifugal
concentrator devices, of nominal molecular weight membrane cut-
off of 10,000 (Amicon, Inc., Beverly, MA, U.S.A.). the
centrifugal separations were carried out at 37°C. Measurements of
the concentrations of aromatic urea compounds that were recovered
in the ult raf ilt rates were done by ult raviolet spect rophotomet ry
at 255 nm or by spectrophotometry at 450 nm using a modification
of a colorimetric method for arylamines (Waugh & Beall, 1974y.
The results are given in Table 9. These results revealed average
plasma protein bindings of these organic urea compounds of between
about 7 to 14~
~137~~~
39 75787-1
Table 9. Percent nonprotein-bound and ultrafiltrable meta-hydroxyphenylurea,
para-
hydroxyphenylurea, and benzylurea in sickle cell blood plasma at 37°C
m-Hydroxy- p-Hydroxy-
phenylurea, phenylurea, Benzylurea,
4.2 mM 4.2 mM 13.5 mM
Percent ultrafiltrable
86.1 ~ 1.5* 92.7 ~ 3.0* 88.8 ~ 2.0*
[89.7 ~ 1.6] [96.4 ~ 3.1] [92.5 ~ 2.2]
Values are means ~ SEM in determinations in HbSS blood plasma from 8, 6, and 7
_'_ ___ -_ subjects, respectively. The actual plasma protein concentrations
used during the
analyses were 70% of the i n i ti al plasma protei n val ues of 7.82 ~ 0. 29,
7 . 42 ~ 0. 21,
and 7.74 ~ 0.23 g/dl, respectively.
*Listed ultrafiltrable, recovery values are corrected for plasma water volume
displacement by protein using 0.730 as the mean specific volume of plasma
proteins.
The values listed in brackets are found values before correction for the water
displacement effects.
EXAMPLE 12
Partition coefficients of select amphiphilic urea analogues which
posess antisickling activity.
In order to determine the relative solubility of some amphiphilic
aromatic urea compounds at low millimolar concentrations and of butylurea at
40 mM, analyses were carried out at 37° C using 1-octanol and vegetable
oil
as lipid solvents and buffered isotonic saline solution of pH 7.3 as water
solvent. One volume part of the water solvent solution containing one of the
213755
40 75787-1
organic urea compounds in the dissolved state was added to one volume part of
the lipid solvent and the mixture mixed vigorously at 37° C for 3
minutes by
means of a vortex mixer. Water/lipid solvent phase separations were then
done by centrifugations at high speed. Concentrations of the organic urea
compounds in the water phase before and after mixing with the lipid phase
were determined spectrophotometrically. Concentrations in the lipid solvent
phases were determined by found differences in the water phases before and
after mixing. Distributions were expressed as ratios of the solute concen-
trations in the two phases. Results are shown in Table 10. The
octanol/saline partition coefficients were highest for meta-tolylurea, pheny-
lures, and benzylurea, with average partition coefficients of 19.2, 7.3 and
about 4.0, respectively, for these three aromatic urea compounds.
213'~7~~
41 75787-1
Table 10. Partition coefficients of amphiphilic urea analogues at
37° C*
Partition Coefficients
Analogue Concn
(mM) Octanol/Saline Oil/Saline
Meta-hydroxy- 2.0 1.72 0.06 0.014 0.003
phenylurea 10.0 1.60 0.02 0.014 0.001
Para-hydroxy- 4.2 0.57 0.03 0.009 0.009
phenylurea
Phenylurea 2.0 7.26 0.28 0.211 O.U10
Meta-tolylurea 2.0 19.2 0.40 0.211 0.016
Benzylurea 2.0 4.30 0.46 0.060 0.005
10.0 3.89 0.09 0.032 0.003
Para-hydroxy- 4.2 1.26 0.02 0.022 0.009
phenylethylurea
Butylurea 40 1.64 0.28 0.660 0.151
*Distributions were performed at 37°C with 1-octanol and oil as lipid
solvents and buffered saline soln. of pH 7.3 as water solvent (1 part lipid
solvent to 1 part saline soln.). Data are expressed as mean ~ SEM of 6
experiments with each pair of solvents. The oil used was soybean oil (Wesson
brand); the saline soln. consisted of (in mM): NaCI 82, KC1 3.7, Na2HP04 32,
NaH2P04 8.0, and MgS04 0.6.
- ,
213~7~5
42 75787-1
EXAMPLE 13
Toxicity Data
Para-methoxyphenylurea was administered orally to 3 male
and 3 female rabbits in gelatin capsules acutely to total agent
dose of 0.35 g/kg. The compound was given in a formulation of
1.00 g para-methoxyphenyluera per 0.040 g L-ascorbic acid per
0.010 g sucrose and per 0.010 g stearic acid. Ingestion of the
capsules did not result in acute hypnotic effects or other
apparent adverse effects. Over a post-dosing observation period
of 14 days, all rabbits remained active and healthy in appearance.
Rabbit body weight averaged 3.04 + 0.44 kg before the acute dosing
and averaged 3.10 + 0.41 kg (mean + S.E.M.) 14 days later.
213'~'~ ~ 5
' 43 75787-1
Listed Disclosed References
OTHER PUBLICATIONS
1. Abraham, D.J., Blood Cells, vol. 8, pp. 345-355, 1982.
2. Aluoch, J.R., Trop. Geograph. Med., vol. 36, pp. S1-S26, 1984.
3. Bartsch, H. et al, Mutation Res., vol. 202, pp. 307-324, 1988.
4. Brittenham, G.M. et al, Blood, vol. 65, pp. 183-189, 1985.
5. Behe, M.J. and Englander, Biochem., vol. 18, pp. 4196-4201, 1979.
6. Buck, J.S., J. Am. Chem. Soc., vol. 56, pp. 1607-1608, 1934.
7. Buck, J.S. et al, J.P.E.T., vol. 54, pp. 188-212, 1935.
8: Chang, H. et al, Blood, vol. 61-, pp. 693-704; 1983. - - - --- - -
9. Davson, H., Textbook of General Physiol., 2nd. edition, pp. 225-226,
1959.
10. Dean, J. et al, New Engl. J. Med., vol. 299, pp. 752-763, 804-811, 863-
870, 1978.
11. Deuticke, B., B.B.A., vol. 163, pp. 494-500, 1968.
12. Elbaum, D. et al, Blood, vol. 48, pp. 273-282, 1976.
13. Forget, B.G. in Vol. 1, Cecil Textbook of Medicine, 19th edition, pp.
889-893, 1992.
14. Goriainova, A.N. et al, Gig. Sanit., vol. 44, pp. 68-70, 1979 - cited by
Database entry on Toxl. BRS Search Mode from 1965 - Sept. 1992.
15. Ivankovic, W. et al, Naturwissenschaften, vol. 60, p. 525, 1973.
16. Lonsdorfer, J. et al, Bull. Europ. Physiopath. Resp., vol. 19, pp. 339-
344, 1983.
_ ,
44 75787-1
17. Mirvish, S.S. et al. Science, vol. 177, pp. 65-68, 1972.
18. Mirvish, S.S., Toxicol. Applied Pharmacol., vol. 31, pp. 325-
351, 1978.
19. Neville, R.G. et al., Canadian J. Chem., vol. 41, pp. 2123-
2129, 1963.
20. Noguchi, C.T. et al., Biochem., vol. 17, pp. 5455-5459, 1978.
21. Pauling, L., Proc. Nat. Acad. Sci. USA, vol. 71, pp 4442-
4446, 1974.
22. Perrine, S.P. et al., Am. J. Ped, Hematol, Oncol., vol. 16,
pp. 67-71, 1994.
23. Poillon, W.N., Biochem., vol. 19, pp. 3194-3199, 1980.
24. Poillon, W.N., Biochem., vol. 21, pp. 1400-1406, 1982.
25. Repka, T. and Hebbel, R.P., Blood, vol. 78, pp. 2753-2758,
1991.
26. Ross, P.D. et al., B.B. Res. Comm., vol. 77, pp. 1217-1223,
1977.
27. Russu, I.M. et al., Biochem., vol. 25, pp. 805-815, 1986.
28. Sax's Dangerous Properties of Industrial Materials, 8th
Edition, Lewis R.J. Sr. ed., vol. 2, p. 414, and vol. 3, p.
2777, 1992.
29. Waugh, W.H., Am. J. Emerg. Med., vol. 11, pp. 20-27, 1993.
30. Waugh, W.H. and Beall, P.T., Kidney International, vol. 5,
pp. 429-436, 1974.
31. Zipursky, A., et al., Am. J. Ped. Hematol. Oncol., vol. 15,
pp. 219-225, 1993.
32. Zirvi, K.A. et al., Argneimittel-Forschung, vol. 27, pp.
1194-1198, 1977.