Note: Descriptions are shown in the official language in which they were submitted.
28681-6
FIELD OF THE INVENTION
The present invention relates to a process for treat-
ing animal manure on small and large farm operations in order to
stabilize, deodorize, recover energy, arid add value to the
animal manure. In particular, the process involves the psychro-
philic anaerobic digestion of animal manure in intermittently
fed sequencing batch reactors. -
EACKGROUND OF THE INVENTION
Animal manure management practices, principally in
regions Where there is a surplus of manure are often detrimental
to the environment and also represent a potential hazard to
human and animal health. Animal manures can produce strong
odours, encourage fly breeding, induce weed problems and pollute
air, soil and water. For example, in some areas of Canada, the
drinking water source is polluted and water bodies cannot be
used for recreational purposes due to manure contamination. The
affected communities are expecting changes in manure management
from the farm industry. The National Workshop on Land Applica-
tion of Animal Manure, CARC (1991), recommended innovative
research that would allow farmers to adopt sustainable and
environmentally sound agricultural practices where animal manure
is integrated into the overall production systems. It was
further recommended that economical processes to stabilize,
deodorize; recover energy and add value toanimal manure be
developed:
Conventional anaerobic digestion 'of animal manure in
farm scaledigesters was attempted at several locations across
- 1 -
I
21~8Q~~
28681-6
Canada during 1975-1985. It was not successful for several
reasons (Van Die,-1987) as follows: 1) The digesters were
designed to operate at mesophilic (35oC) or thermophilic (60°C)
temperatures. Because of prolonged sub-freezing winter tempera-
tures in parts of North America,-digesters operating at these
temperatures during the winter used not only most of the gas
they produced but sometimes rerniired supplementary heating to
maintain the digester temperature. Fox :example, in a full scale-
anaerobic digester for cattle_ manure in Southern Ontario, more
energy was required to run the digester in winter months than
the energy generated in the biogas produced. 2) The anaerobic
digesters were not cost effective because they were designed to
produce electricity which made them even_more capital intensive.
3) The digesters were not practical for farm use because their
control and maintenance required skilled operators, increased
labour input, daily supervision and sometimes changes in farm
operational procedures. 4) The digesters were difficult to
control and had poor stability because they were pushed to the
limit to achieve maximum gas production.
Anaerobic digestion of municipal waste water-and
animal manures at low (psychrophilic) temperature has been.
reported in previous studies (O~Rourke, 1968; Stevens and
Schulte, 1977; Ke-Xin and Nian-Gua, 1980; Wellinger and Kauf-
mann, 1982; Chandler et al., 1983; Cullimore et al., 1985; Lo
and Liao, 1986; Sutter and Wellinger, 1987; Balsari and Bozza,
1988; and Safley and Westerman, 1992, 1994). Most of these
studies were aimed at biogas production while little considera-
- 2 -
2138~9~
28681-6
tion was given to odour reduction, waste stabilization or
increases in fertilizer value or.plant nutrient availability.
There was a vaide variation in the reported experimental results.
Some studies were successful in producing methane at tempera-
tures below 20oC while others were not.. The information pro-
vided in the above reports is inadequate to provide possible
reasons for these discrepancies. In most of these studies the
solids were separated from the liquid or the slurry solids
content was very low (less than 2~) compared to the typical
solids content of manure slurry at Canadian farms. It is
unlikely-that farmers would dilute manure slurry for anaerobic
digestion because it would require larger storage facilities and
increase substantially the volume of liquid manure to spread on
the land. Furthermore-; farmers are not interested in separating
the liquid and solid fraction of manure slurry as this necessi-
tates two different types of manure handling equipment, storage
and land application equipment, to handle both.the liquid and
solid fractions. Dague et al. (1992) indicated that the
sequencing batch reactor is highly suitable for anaerobic
digestion because: 1) It provides quiescent settling condition
for the anaerobic bacteria; and 2) The high food to micro-
organism ratio (F/M) at the beginning of the feed period and the
low (F/M) at the end of react period enhances the sludge
settling characteristics.
SUMMARY OF THE INVENTION
In view of the above, there is a need to develop a
process to treat animal manure that is low cost, is very stable,
2~~~Q9~.
28681-6
simple, easy to operate, requires minimum skill and does not
interfere With regular farm operations. Anaerobic digestion to-
treat animal manure under North American conditions is a viable
option as it would have a low capital and operational cost if it
could: 1) Make use of existing handling- equipment and storage
facilities at the farm; 2) Operate at relatively low tempera-
ture; 3) Require minimum handling; and 4) Does not require daily
maintenance and supervision.
The present inventors have developed a process for the
anaerobic digestion of animal manure that overcomes the draw-
backs ofthe prior art processes. In particular, the present
inventors have determined that psychrophilic anaerobic digestion
(PAD) of .animal manure in-intermittently fed sequencing batch
reactors (SBR), sterilized, deodorized, reduced pollution
potential, recovered energy, and increased plant nutrient -
availability from swine manure slurry.
In accordance with the present.invention, there is
provided a process for treating organic waste comprising:
(a) feeding the waste to a digester containing a layer of
acclimatized anaerobic sludge wherein said digester is at a
temperature from about 5°C to about 25°C; and (b) allowing the
waste to react with the sludge.
Some of the advantages of the process according to the
present invention include:
- 4 -
28681-6
(1) The-process works at ambient temperatures ranging between 5
and 25°C. (Previous systems that were tried in Canada
worked at temperatures of 25 to 65°C.) As a consequence,
the. animal manure slurry does not need to be heated before
it is fed to the digester.
(2) The process makes use of Sequencing Batch Reactors (SBRs)
which were_not used previously with low temperature an-
aerobic digestion processes.
(3) The-process does not require continuous or daily feeding.
It can be intermittently fed only 1 to 3 times a week or
every two weeks. Because of intermittent feeding this
process does not need an expensive calibrated pump. It
will make use of existing handling equipment at the farm.
(4) The.process does not really require mixing. Although, it
is preferable to provide a minimum level of mixing up to 30
minutes per day. Mixing can be provided by biogas recircu-
lation (Previous systems were mixed continuously). Because
the a lurry and digester content does not have to be heated
and continuous mixing is not required, all the energy
produced will be available for on farm use.
(5) The process works very well with either short or long feed-
ing and reaction periods. Fill and react period lengths of
up to two months did not affect the.process stability and -
performance.
(6) The process is very stable when comparedto previous
systems. It was not affected by high concentration of
- 5 -
2138~9~
28681-6
volatile acids (6500 mg/L) and ammonia nitrogen
(3700 mg/L).
(7) Because this process works at low temperature, does not
require mixing and is not.affected by long fill and react
periods it can make use of existing manure slurry storage
at the farm.
(8) The process does not.require supervision because it is very
stable and also it does not interfere with regular farm
operations. This is because the digester is fed only dur-
ing normal manure removal operations and the farmer will
deal the effluent from the-digester: once a month or every
two months or even less often. (Previous systems required
daily supervision and farmers had to deal with the
digesters effluent on a daily basis:)
(9) The proces-s is-the only system that works satisfactorily
with pig manure-under Canadian climatic conditions.
(10) The process does not require manure slurry dilution and
solid/liquid separation. This process works well with
manure slurry that has a solids concentration between 1
and 10~.
(11) The process will work well at organic loading rate ranging
between 0.1 to 4.0 g COD per litre of digester volume per
day.
(12) The process very efficiently retained a high concentration
of slow growing microorganisms in the system.
- 6 -
213~~9~.
28681-6
(13) The-process will have low capital and operational costs.
Furthermore, if the energy recovered is used at the farm
the-process will be very cost effective.
BRIEF DESCP.IPTION OF THE DRAWINGS
Figure 1 is a schematic representation of a laboratory
scale sequencing batch reactor_
Figure 2A is a graph that illustrates the cumulative
methane production over time for various sequencing batch react-
ors at different organic loading rates.
Figure 2B is a graph that illustrates the soluble COD
production over time for various sequencing batch reactors at
different organic loading rates.
Figure 2C is a graph that illustrates the acetic. acid
production over time for various sequencing batch reactors at
different organic loading rates.
Figure 2D is a graph that illustrates the propionic
acid production over time for various sequencing batch reactors
at different organic loading rates.
Figure 3A-is a graph that illustrates the cumulative-
methane production over time for various sequencing batch
reactors -at different levels of mixing.
Figure 3B is a graph that illustrates the soluble COD
production over time for various sequencing batch reactors at
different levels of mixing.
Figure 3C is a graph that illustrates the acetic. acid
production over time for various sequencing batch reactors at
different. levels of mixing.
~~3$~~~
28681-6
Figure 3D is a graph that illustrates the propionic -
acid production over time for various sequencing batch reactors
at different levels of mixing.
Figure 4A is a graph that illustrates the cumulative
methane production over time for various sequencing batch react-
ors at different organic loading rates and different levels of
mixing.
Figure 4B is a graph that illustrates the soluble COD
production over time for various sequencing batch reactors at
different organic loading rates and different levels of mixing
Figure 4C is a graph that illustrates the acetic acid
production over time for various sequencing batch reactors at
different organic loading rates-and different levels of mixing.
Figure 4D is a graph that illustrates the propionic -
acid production over time for various sequencing batch reactors
at different.o~ganic loading rates and different levels of
mixing.-
Figure 5A is a graph that illustrates the cumulative
methane production over time for different feeding frequencies
at a cycle length of 28 days.
Figure 5B.is a graph that illustrates the soluble COD
production over time for different deeding frequencies at a
cycle length of-28 days.
Figure-5C is a graph that illustrates the acetic acid
production over time for different feeding frequencies at a
cycle length of 28 days.
g -
r ~1~~~91
28681-6
Figure 5D is a graph that illustrates the propionic
acid production over time for different.feeding frequencies at a
cycle length of 28 days.
Figure 6A is a-graph that illustrates the cumulative
methane production over-time for different feeding frequencies
at a cycle length of 14 days.
Figure 6B is a graph that illustrates the soluble COD
production over time for different feeding frequencies at a
cycle length of 14 days.
Figure 6C is a graph that illustrates the acetic acid
production over time for different feeding frequencies at a
cycle length of 14 days.
Figure 6D. is a graph that illustrates the propionic
acid productionover time for different-feeding frequencies at a
cycle length of 14 days.
Figure 7A is a graph that illustrates the cumulative
methane production over time for different cycle lengths with
the sequencing batch reactors fed three times a week.
Figure 7B is a-graph that illustrates the soluble COD
production over time for different cycle lengths with the
sequencing batch reactors fed three times a week_
Figure 7C is a graph that illustrates the acetic acid -
production over time for different cycle lengths with the
sequencing batch reactors fed three times a week.
Figure 8A is a graph that illustrates the cumulative
methane production over time for different cycle lengths with
the sequencing batch reactors fed once a week.
_ g _
~
~ 238091
28681-6
Figure 8B is a graph that illustrates the soluble COD
production over time for different cycle lengths with the
sequencing batch reactors fed once a week.
Figure 8C is a graph that illustrates the acetic acid
production over time for different cycle lengths with the
sequencing batch reactors fed once a week.
Figure 9 is a graph illustrating the effect of cycle
length on cumulative methane production.-
Figure 10 is a graph illustrating the effect of cycle
length on daily methane production.
Figure 11 is a graph illustrating total daily methane
production from two sequencing batch reactors operated simul-
taneously.
Figure 12A is a graph that illustrates the cumulative
methane production over time in various sequencing batch
reactors -for different acclimatization times.
Figure 12B is a graph that illustrates the soluble COD
production over time in various sequencing batch reactors at
different acclimatization times.
Figure 12C is a graph that illustrates the acetic acid
production over time at various sequencing batch reactors at
different.acclimatization times
Figure 12D is a graph that illustrates the propionic
acid production over time at various sequencing batch reactors
at different acclimatization times.
- 10 -
~
213~Q91
28681-6
Figure 13A is a graph illustrating the cumulative
methane production over time for four-successive cycles in
sequencing batch reactors-numbers 9-10.
Figure 13B is a graph illustrating the cumulative
methane production over time for four successive cycles in
sequencing batch reactors numbers 11-12.
DETAILED DESCRIPTION OF THE INVENTION
The present inventors conducted a thorough study into
the feasibility of using psychrophilic anaerobic digestion to
stabilize and deodorize swine manure and'to recover biogas for
energy. The inventors conducted two test runs, each test run
consisting of twelve sequencing batch reactors. Various para-
meters of the process were altered such as organic loading
rates, fill and react period lengths, mixing intensity, feed
frequency and sludge age.
Manure slurry was obtained from gutters under a par- -
dally slatted floor in a growing-finishing barn at a commercial
swine operation. 'The manure was up to four days old at the time
of collection. It was screened to remove particles larger than
3.5 mm toprepare SBR feed samples. These large particles tend
to create. operational problems with small scale laboratory
digesters. The feed samples were ~ tored in a freezer at -15°C
to prevent biological activity. Manure feed samples were heated
to the digester operating design temperature (20°C) prior to
feeding.
- 11 -
CA 02138091 2000-07-18
29184-2(S)
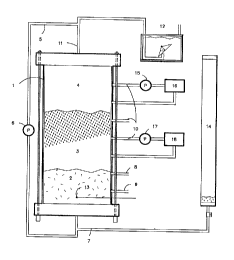
Figure 1 is a schematic illustration of a laboratory
scale sequencing batch reactor according to the present inven-
tion. Each of the reference numerals refer to the following:
1. 300 mm diameter plexiglass digester
2. sludge bed zone, 8.0 L
3. variable volume zone, 28.0 L
4. head space zone, 6.0 L
5_ biogas recirculation line
6_ gas pump
7. influent line
8. effluent line
9. sludge sample port, also used for sludge wastage
10. mixed liquor or supernatant sampling port
11. gas outlet
12. gas meter
13. thermocouple
14. feeder tube
15. gas pump
16. hydrogen gas monitor
17. liquid pump
18. dissolved hydrogen gas monitor
In the SBR shown in Figure 1, the manure is loaded
into the feeder (14) and fed to the digester (1) through influ-
ent line (7). The manure is fed through the bottom of the
digester which has been pre-inoculated with an anaerobic sludge
(2). In certain test runs the reactor was mixed by recirculat-
- 12 -
23.38092
28681-6
ing the biogas produced in the headspace (4) through the biogas
recirculation line (5) using a gas pump (6).
The inventors altered various parameters of the
process keeping in mind that for results to be applicable to
farm conditions the laboratory tests should simulate as closely
as possible the actual farm operation. At a typical farm,
manure is-generally removed from the barn one to three times a
week. Therefore the SBR should be intermittently fed one and
three times a week. The fill cycle should not be longer than a
month in. order to limit the volume of the SBR. The settling
period should be long enough to provide complete solids/liquid
separation. The react period should be long enough to produce
almost odourless effluent with reduced pollution potential and
increased fertilizer value. For the PAD in SBR to be cost
effective, it is very important that the.operational cost is
kept very low. The operation of SBR at ambient temperatures and
the reduction or elimination of mechanical mixing would substan-
tially reduce the energy input and increase. the energy effi-
ciency of the SBR because all the energy produced will be
available for on farm utilization.
Table 1 gives the SBRs operating conditions that were.
used in this study. Test run No. 1 and 2 investigated the
effect ofloading rates, mixing intensity, fill-react period
length, feeding frequency and sludge age on the performance of
PAD of swine manure slurry in SBR.
Digesters 5 to 12 were inoculated in a previous run
(Masse et. al. 1993). Digesters 1 to 4 were. also inoculated with
- 13 -
29184-2(S)
CA 02138091 2000-07-18
the same mixture of anaerobic sludges. In particular, the
digesters were started using 7.5 L of granulated anaerobic
sludge obtained from the Agropur dairy wastewater treatment
plant at Notre-Dame du Bon Conseil in Quebec province and an
additional 2 L of anaerobic non-granulated sludge obtained from
the municipal wastewater treatment plant of the City of Ottawa
in Ontario. In general, the anaerobic sludge to manure ratio
should be within 0.5 to 1Ø
A mixed liquor sample was withdrawn (through a sampl-
ing port (10) from each SBR at the beginning of the experiment
and once a week during the experimental run. At the end of the
test, after the sedimentation period, additional samples were
withdrawn from the supernatant (3) and sludge bed zones (2).
The samples were analysed for pH, alkalinity, solids, volatile
acids, total Kjeldahl nitrogen (TKN), ammonia nitrogen, total
chemical oxygen demand (TCOD) and soluble COD (SCOD). Some of
the samples were further analyzed to determine concentration of
C, H, N and other elements. The biogas production was monitored
daily and its composition analyzed weekly. All the analytical
tests carried out on the mixed liquor were also performed weekly
on samples of swine manure slurry fed to the SBRs.
Soluble COD was determined by analyzing the super-
natant of centrifuged slurry. The pH, alkalinity, and solids
were determined using standard methods (APHA, 1992). TKN and
ammonia nitrogen were determined using an auto-analyzer.
Volatile acids and biogas composition were determined by gas
chromatography. Metal concentrations (K, Ca, Mg, Cu, Zn, Na,
- 14 -
2~38~9~
28681-6
Hg) weredetermined by the inductively coupled plasma (ICP)
methods (APHA, 1992).
Table 2 gives compositions of the swine manure and
inoculum sludges used in the experimental runs. The total
solids content of the manure slurry was high. It was around
4.1~ (weight basis). The fresh slurry had a neutral pH and very
high concentrations of TCOD, SCOD, TKN, NH3-N and volatile acids
and alkalinity. The concentration of inorganic elements such as
calcium, magnesium, potassium, sodium, zinc and copper were also
quite high.
The main characteristics of the Agropur granulated
sludge was that it had a vary high TS, TCOD, SCOD, TKN and cal-
cium content. The municipal sludge is less concentrated than
the granulated Agropur-sludge but it has a higher fibre content
on a dry weight basis and also has a lower alkalinity than dairy
sludge. Both of these sludges came from digester operated at
35°C.
All the SBRs maintained an alkalinity around 12000 mg
(CaC03)/L and a pH between 7.5 and 8.0 during experimental runs 1 -
and 2. Both the pH and alkalinity decreased slightly during the
feed period due to volatile acids-(VA) accumulation and they
both increased slightly during the react period due to the VA
utilization.
Figure 2-shows the typical response of the SBR fed
with different organic loading rates. During the four-week fill
period the cumulative biogas production was identical for the
three organic loading rate. T7~e reason for this might be that
- 15 -
2I38~~~.
28681-6
the three set of digesters had about the same population of
methane formers at the start of the test and the methane produc-
tion rate was not limited by the substrate availability but was
rather controlledby the growth rate of methane formers_ During
the subsequent four-week react period the digesters with the
lowest organic loading rate (0.81 g COD/1-d) stopped producing
methane. This was because most of the soluble COD and volatile
acids were consumed during the fill period. The digesters with
the intermediate organic loading rate (1.22 g COD/1-d) stopped
to producegas midway through the react period for the same
reasons.
Figure 2 also illustrates the soluble COD, acetic and
propionic acids concentrations as a function of time. As ex-
pected, the concentration of volatile acids and SCOD in the SBR
increased with an increase in organic loading rate. This in-
creased in volatile acids indicates that hydrolysis and acidi-
fication-were occurring and that utilization of acetic acid by
the acetoclastic methane formers was the rate limiting step.
This accumulation in volatile acids and-SCOD was typical of all
experimental run_
For the lowest loading rate (0.81 g COD/1-d) there was
no propi.onic acid accumulation in the SBR while acetic acid con-
centration stayed below S00 mg/L. For the SBRs with the highest
loading rate (1.63 g COD/L-d) acetic and propionic acids were
both present and-their respective concentrations reached maximum
values o~3000 and 900 mg/L at the end of the fill period. For
each loading rate the volatile acids were completely utilized at
- 16 -
238091
28681-6
the end of the react period. From these results it can be con-
chided that the SBRs were very stable at these loading rates.
The lowest loading rate would not be recommended because no
treatment occurs during the react period. A loading rate of
1.63 g COD/L-d-should be recommended. As shown in Figure 2 at
this loading rate the react period is utilized to its maximum.
Complete=utilization of both volatile acids and soluble COD
occurred at the end of this period.
Figures 3 and 4 compare the SBR performance for
different intensity of mixing at loading ratesof 1.22 and 1.63
gCOD/L-d, respectively, Figure 3 shows that intermittent mixing
slightly increased the production rate of methane and the util-
ization rate of volatile acids, but did not have an effect on
soluble COD. Because the SCOD and VA concentration were the
same at the beginning and end of the cycle for the intermittent-
ly mixed and non-mixed SBRS, the methane production and VA
utilization should have been the same. These differences in
methane production and VA utilization could be due to a slightly
different organic loading rate.
Figure 4 shows that for digesters fed a higher organic
loading rate (1.63 g COD/L-d) there was-rio difference in process
performance between the intermittently mixed and non-mixed
digesters. Mixing of a full scale digester-consumes large
amounts of energy, and based on these experimental results, SBR
mixing may not necessary for full-scale farm digesters. This
would simplify the operation of the SBR, reduce maintenance cost
as well as possible mechanical problems.
- 17 -
28681-6
Figures 5 and 6 compare the typical response of SBR to
feeding frequency of 1 to 3 times a week with the same total
weekly organic loading for all digesters. Figure 5 shows that
frequency of feeding had no significant effect on SBRs with a
feed-react cycle length of 28 days. The SBRs fed once a Week
produced 13~ more gas and had about the same effluent soluble
COD concentration as reac'tbrs fed 3 times/week. These results
indicate-that the SBRs fed once a week were also very stable and
treated the swine manure slurry adequately.
For the SBRS with a cycle length of 14 days the feed-
ing fre-quency had no effect on SCOD, acetic and propionic acids
accumulation. Only the cumulative methane production was 14~
higher. These experimental results indicate that both one and
three times a week feeding frequency may be acceptable for farm
scale SBRs.
Cycle length is an important parameter in the design
of SBR because it controls the size of the digester, the treat-
ment efficiency as well as the frequency that the farmer has to
deal with SBR effluent removal.
Figures 7 and 8 show that the cycle length has only a
small effect on the distribution of SCOD and acetic acid concen- .
trations.- The SBR with the shorter feed-react period had twice
the number of cycles compared to the. longer feed react period
over the 56 days of operation. As a result, there was more
fluctuation in the SCOD and acetic acid concentration. Figure 7
and 8 also show that the cycle length has no effect on process
performance. Final concentration of SCOD and acetic acid were
- 18 -
213891
28681-6
the same: after 56 days of SBR operation using either the two-
week or four-week cycle-
Figures 9 and 10-show the effect of cycle length on
cumulative and daily methane production. For both cycle lengths
the maximum daily methane production occv.rred at the end of the
fill period and the=minimum at the end of the react period
(Fig. 10). As expected. the SBRs with the shorter cycle length
(14 days) showed more variation in weekly methane production:
Figure 9 shows that the total cumulative methane production
after 56 days was the same for both cycle lengths. Therefore
the total amount of-energy recovered by PAD in SBR was not
affected by the cycle length investigated in this study.
At the farm a steady and constant production of
methane gas would be preferable in order to develop an adequate
biogas utilization strategy. A minimum of two SBRs would be
required to process the swine manure slurry at a farm.
Figure 11 shows the total daily methane production from two SBRs
operated simultaneously at fill/react cycle-lengths of 14 and 28
days. By comparing Figure lI with Figure 1D, it is obvious that
a pair of SBRs provides a more-constant supply of methane than a
single SBR. Figure 11-also shows that with a pair of SBRs the
cycle length did net have a significant effect on the total
daily methane production. Therefore at the farm, SBRS with
cycle length of either 14 or 28 days would be acceptable.
In the start-up run, digesters-were inoculated with
fresh anaerobic sludge at 35°C and either fed with milk pro-
cessing plant wastewater sludge or municipal sludge. It was
- 19 -
y 2~~~~~~
28681-6
expected-that the inoculum sludge in the SBR would acclimatize -
with operating time.
Figure 12 compares the SBRs response to sludge age.
Anaerobic sludge in digesters 7 and 8 in the start-up run (Masse
et al. 1993) were exposed to swine manure slurry and low
temperature for thefirst time. During this run there was a
long lag phase in the biogas production during the.feeding
period. In test run number one, the same sludge that had
already been exposed to swine manure slurry and low temperature
for a period of three months. Both were fed about the same
organic loading rate. The SBRs with an older acclimatized
sludge had: 1) a shorter lag phase and a substantially higher
methane production rate; and 2) substantially lower concentra-
tion of soluble COD, acetic and propionic acids at the end of
the react period. These experimental results indicate that
sludge age has a significanti.nfluence on the process response.
Figure 13 compares the cumulative methane production
for each.consecutive cycle during test run number two. These
figures clearly indicate that the initial methane production
rate and the total cumulative methane production for each cycle
increased after each successive cycle and the lag phase at the
beginning of the cycle decreased as the test progressed. These
results clearly indicatethat micro-organisms' acclimatization
to low temperatures and swine manure slurry was taking place.
The biogas produced in test runs one and two was of
high quality with a methane concentration between 75 and 80~.
Table 3 gives the methane production as a function of unit mass
- 20 -
~~.380~~
28681-6
of volatile solids fed to the digester_ The CHa production
ranged from 0.48 to0.66 L/g VS'for most of the experimental
run. Methane productions obtained in this study were substan-
tially higher than methane production from swine manure obtained
by digestion at 35°C in continuous flow digesters by Kroecker
et al. (1979) who reported methane production of 0.45 L CH,/g VS
added for a loading rate of 2.5 kg VS/m'-day, and by Hashimoto
(1983) who reported 0.42 L CH4/g VS added for a loading rate of
2.5 kg VS/m'-day.
The higher methane production per gram of volatile
solids -fed to the SBRs obtained in this study could be due to
the lower organic loading rate and longer hydraulic residence
time. Another possible reason could be the lower operating
temperature and the absence of. mixing which maintain a higher
concentration of hydrogen and carbon dioxide gases in the liquid
phase. As a result more carbon dioxide can be reduced to
methane by the hydrogen-utilizing methanogens. A high rate of
methane production was not the main objective of this work but
it is very useful to asses the system performance and stability.
The steady production of methaneper unit mass of volatile -
solids fed indicates that anaerobic digestion of swine manure at
20°C in the laboratory-scale SBR digesters was a stable process.
Table 3 also gives the level of removal of TCOD, SCOD
and volatile solids for all runs. The total COD removal ranged
from 41 to 83~ and the volatile solids removal ranged from 46 to
84~. Results for volatile solids and total COD were highly
- 21 _
2~.38Q91
28681-6
variable due to sampling variation caused by rapid settling of
heavy particulates_ Some samples had less solids than others.
This affected the VS and TCOD determination as well as the
calculated methane production per gram of VS.
The soluble COD test results were consistent. High
SCOD renmoval was achieved during most of:the experimental runs.
Its removal ranged from 798> to 9.3~ except in a few runs dis-
cussed below. Experimental runs that achieved 70 to 93~ SCOD
removal and complete utilization of VA's, produced treated
manure that was relatively odourless compared to the raw manure.
SBRS-1, 2, 3 and 4 in test run.No. 2 had very low
energy recovery and reduction in SCOD. These SBRs were started-
up in test run 1 and their organic loading rate was doubled in
test run-2. This rapid increase in organic loading rate caused
their total failure.
Theanaerobic sludge had excellent settling charact-
eristics: In the SBR that were not mixed, there was a clear
interface between the liquid and sludge bed zones. A thick
layer of-sludge was observable at the bottom of the digester.
At the end of the react period where the.biogas production was
very low, the demarcation between the liquid and solids was even
more evident. In the SBRs that were mixed there were no dis-
tinguishable supernatant and sludge zones. For these SBRs, when
mixing was stopped at the end of react period, It would take
about 2 to 6 hours for a zone settling or liquidlsolids inter-
face to form and another 24 to 48 hrs for the sludge blanket to
completely settle at the bottom of the SBR. Therefore the SBR
-- 2 2 -
2138~9~
28681-6
provides excellent settling conditions to retain the slow grow-
ing-microorganisms when enough time-is allowed for the settling
period.
Another very important feature of a SBR is that it
does not required continuous feeding. Therefore, in farm
applications, PAD in SBR will not interfere with regular farm
operations as previous systems did. It could be loaded during
normal manure removal operations and thefarmer would not have
to deal daily with the digester effluent. At the farm the SBR
effluent will need to be handled once every one or two months,
depending on the operating conditions. Because of intermittent
feeding the SBR could make use of existing manure handling
equipment at the farm and also because SBR will not interfere
with farm operation, it will increase substantially the interest
in anaerobic digestion to treat animal manure on small and large
farm operations.
It will be appreciated to one skilled in the art that
various modifications can be made to the above described system
without departing from the scope and spirit of the invention.
For example, the size of the SBR and the fill and react periods
will depend largely on the size of the farm as well as the
personal choice of the operator. The process can also be used -
to treat other ypes oforganic waste such as slaughter house
waste water, food processing plant waste-water and high strength
waste water produced by other types of industries.
- 23 -
2138~9~
28681-6
REFERENCES
1. Alpha, (1992). Standard Method for the Examination of
Water and Wastewater, 18th. ed. American Public Health
Association, Washington, D.C.
2. Balsari, P. and E. Bozza, (1988). Fertilizers and Biogas
Recovery Installation in a Slurry Lagoon. In Agricultural
Waste Management and Environmental Protection. Proceedings
of the 4th International Symposium of CIEC, ed. E. White
and I. Szabolcs, 71-80.
3. CARC, 1991. Proceedings of the National Workshop on Land -
Application of Animal Manure. Eds. Leger,D.A.,--Patni,N.K.,
and Ho,S.K., Canadian Agricultural Research Council,
Agriculture Canada, Ottawa, ON, 176 pp.
4. Chandler, J.A., S.K. Hermes, and K.D. Smith, (1983). A Low
Cost 75 kW Covered Lagoon-Biogas System. Presented at
Energy from Biomass and Waste VII, Lake Buena Vista, FL. -
23 pp.
5. Cullimore, R.R.,- A. Maule, and N. Mansui, (1985). Ambient _
Temperature Methanogenesis from Pig Manure Waste Lagoons.
Thermal Gradient Incubator Studies, Agricultural Waste,
12:147-157.
6. Dague, R_R., C.E. Habben, and S.R. Pidaparti; (1992).
Initial Studies on the Anaerobic Sequencing Batch Reactor,-
Water Science and Technology, 26: Nb. 9-11, 2429-2432
7. Hashimoto, A.G. (1983), Thermophilic and Mesophilic An-
aerobic Fermentation of Swine Manure, Agricultural Wastes,
Vol. 6, 175-191.
- 24 -
~ 2~.3~f~9~
28681-6
8. Ke-Xin, I. and L. Nian-Guo, (I980). Fermentation Tech-
nology for Rural Digesters in China. Proceedings Bioenergy
80, Bio-Energy Council, New York, 440-442.
9. Kroeker, E.J., D.D., Schulte, A.B., Spading and H.M. Lapp,
(1979), Anaerobic Treatment Process Stability. Journal
Water Pollution Control Federation, Vol. 51, 718-27.
10. Lo, K.V. and P.H., Liao, (1986), Psychrophilic Anaerobic
Digestion of Screened Dairy Manure. Energy in Agriculture,
5:339-345
11. Mas s, D.I., R.L. Droste, K. Kennedy and N.K. Patni, 1993.
Psychrophilic Anaerobic Treatment of Swine Manure in Inter-
mittently Fed Sequencing Batch Reactors. Presented at the
1993 International Winter Meeting of the American Society
of Agricultural Engineers, ASAE Paper No. 93-4569, St-
Joseph, MI, 49085-9659-.
12. O'Rourke, J.T. (1968), Kinetics of Anaerobic Waste Treat-
ment at Reduced Temperature. Ph_D. Thesis, Stanford
University, Cali-forma, US.
13. Safley, L.M., and P.W. Westerman, (1992). Performance of a
Dairy Manure Anaerobic Lagoon, Bioresource Technology,
42:43-52 -
14. Safley, L.M., and P.W. Westerman, (1994). Low Temperature
Digestionof-Daizy and Swine Manure Bioresource Technology,
47:165-171
15. Stevens, M.A., and D.D. Schulte, (1977). Low Temperature
Anaerobic Digestion of Swine Manure. American Society
- 25 -
21~~~~~
28681-6
Agricultural Engineers, Paper 77-1013, St-Joseph, MI_ 19
pp_
16. Sutter, K:, and A., Wellinger, (1987). ACF-System: A New
Low Temperature-Biogas Digester. Tn Proceedings of the 4th
International Symposium ofCIEF, 11-14 March 1987, Braun-
schweig-Volkenrode, Germany.
17. VanDie, P: (1987). An Assessment of Agriculture Canada's
Anaerobic Digestion Program. Engineering and Statistical
Research Centre_ Contribution No. I-933, Agriculture and
Agri-Food-Canada, Ottawa, Ontario, K1A OC6.
18. Wellinger; A., and R., Kaufmann, (1982). Psychrophilic
Methane Production from Pig Manure.' Process Biochemistry,
17:26-30
- 26 -
2~3809~
28681-6
Table 1. SBR Operating Conditions
RUN DIGESTERLOADING FEEDING MIXING""FILL. REACT N0.
N0. NO.. RATE FREQUENCY PERIODPERIOD of
CYCLE
g COD/feedg COD"/L-d(Per (WEEK)(WEEK)
week)
1 1-2 14.25 0.81 3 N 4 4 1
3-4 14.25 0.81 3 Y 4 4 1
5-6 21.40 1.22 3 N 4 4 1
7-8 21.40 1.22 3 Y 4 4 1
9-10 28.50 1.63 3 N 4 4 1
11-12 28.50 1.63 3 Y 4 4 1
2 1-2 28.50 1.63 3 N 4 4 1
3-4 85.50 1.63 1 N 4 4 1
5-6 28.50 1.63 3 N 2 2 2
7-8 85.50 1.63 1 N 2 2 2
9-10 28.50 1.63 3 N 1 1 4
11-12 85.50 1.63 1 N 1 1 4
* Equivalent loading rate if the swine manure would have been
fed continuously.
** SBR was intermittently mixed by biogas recirculation.
Mixing lasted 10 minutes every thirty minutes.
- 27 -
y 213~~91
28681-6
Table 2 Compositioa of S~wiae Manure Slurry (Substrate) and
Inoculum Anaerobic Sludges
CONSTITUENT SWINE AGROPUR MUNICIPAL
MANURE SLUDGE SLUDGE
Total Solids (TS), ~ 4.1 11 2.6
Volatile Solids (VS), ~ 2.7 5.6 1.26
Soluble COD (SCOD), g/L 28 10 3
Total COD (TCOD), g/L 57 73 8.2
TKN, g/L 6.8 7.9 1.8
NH4-N, g/L 5.0 1.3 1.0
pH 7.3 7.6 7.3
Alkalinity, g 13.5 16 6
CaC03 /L
Acetic Acid, g/L 5.3 0.0 0.0
Propionic Acid, g/L 1.7 0.0 0.0
Butyric Acid, g/L 2.2 0.0 0.0
Cellulose, ~ TS 2.43 0.70 0.84
Hemicellulose, ~ TS 4.15 0.73 3.98
Lignin, ~ TS 1.31 1.56 2.88
Total Carbon, ~ VS 38.18 48.4 55.9
Total Nitrogen, ~ VS 4.69 9.64 10.6
Hydrogen, ~ VS 6.10 7.54 8.48
Calcium, mg/kg TS 54800 84720 46800
Copper,- mg/kg TS 960 80 630
Magnesium, mg/kg TS 8600 1770 2600
Mercury, mg/kg TS NA NA 2420
Potassium, mg/kg TS 42800 6160 10000
Sodium, mg/kg TS 13900 7060 400
Zinc, mg/kg TS 450D 1240 600
- 28 -
28681-6
M H
W ~ W ~ ('~ (' . . I
I
I I
V ~ I 1 I I
M
uon
t(7VOri H N 07
~ o
Ub a ,a~ ~ ~ ~ - . . .
N ,-i~ . ~n.
In ~
~W cn N N 07 N W ~
A
p ~ Ino Irt,-IN Inoo.-im m o,
N U InN N ,-IO O ~ pt~ M O N
r7~ E ~ ~ InIn ut Lntoio t~t~m
O~
ero
H
l~ HtrltD ~ tf7N InH N L~N N M H o
x ~ ~ ~ r mo ,-i.-1 mnInm
~
wn
U o n o 0 0
-
~ 0 0 0 0 0 0 0
~
N
~a~
N m
ya
m
cnb .a
rl O H H v-Iv-Iri ei v-Iv-IN N V~~I
U
z~
~ o
o m
Hq
U
U
N ri W dl~pW cMcH ~ '~~ N N v-Iv-I
~
-I
-ri ~
ri '
1~ W
+~
ro
Id A
r-I
~ o ao
H ~ dl~ ~ cn ~ ~ V~N N ~-I.-I
F4
ww~
w ~
o ca c~
z
H z ~ z s z s. z z z z z z
r3
H
~'.
N
C9
U
z~
H
ri
M M M M M M M H M e-IM n-I
W
a
w~
w
+~
~o
H H N N M M M M M M M M
O O
q J N N N ~O ~O tptpt0 ~Otpt0
U
O O e-iN r-Iv-I fiN N e-Iv-Iv-i
l~
bl
v O
z
ro
H
N
~ -H Q w u,Ino 0 0 0 0 0 0 0 0
0
N N ~ chIn In In117tf1u'11t7tt7
c~ a
A cn~n.-i.am m m N m ~ o ,n
UO n-IrlN N N N N W N N N of
b1
17
~ U bl
N ~
oro
> a~
FC ~ N ~ ~OOJO v-I N V~t0 07O
~I .
u-I
W 1 I I I '1 I I I 1 1 ~ I
O
Ue riM N f~y -I ~-IM tttI~I v-I
z
p H
m
ri
~ ,~ N
z
E
- 29 -