Note: Descriptions are shown in the official language in which they were submitted.
-
~ 94/26874 2138 2 5 4 PCT~S94/05392
IMPROVED OPTICAL DETECTION 8Y~TEN
~OR APPARATU~ TO CULT~RE AND
DETECT BACTERIA IN HUMAN TI~8UE
This application is a continuation-in-part of
application S.N. 07/887,627, filed May 22, 1992, pending,
and is also a continuation-in-part of application S.N.
07/638,481, filed January 4, 1991, also pending, which,
in turn, is a continuation-in-part of application S.N.
07,609,278, filed, November 5, 1990, now U.S. Patent No.
5,173,434, all of which prior applications are
incorporated herein by reference and made a part hereof.-
FIELD OF THE lNV~'~. .ION
This invention relates generally to analytical
apparatus for detecting the presence of bacteria in human
tissue, and is particularly directed to an improved
optical detection system for use in automated apparatus
for culturing and detecting viable bacteria in human
blood specimens.
BAC~GROUND OF THE INVENTION
Bacteremia -- the prolonged presence of one or more
viable bacteria in the blood -- is a serious and life-
threatening infection. The most common symptom of
2~ bacteremia is a fever of unknown origin. Accordingly,
hospitals routinely perform a large number of tests to
determine whether patients exhibiting this symptom have
bacteremia. Presently, the only way a definitive
diagnosis can be made is by isolating bacteria in the
blood by means of a so-called "blood culture." Because
bacteremia is life-threatening, positive specimens must
W094t26874 -- PCT~S94/05392
2138X~4
be detected as quickly as possible so that the patient
can be treated with the correct antibiotics.
Currently, there are several methods of detecting
positive blood cultures. The conventional manual method
involves inoculating bottles containing a growth medium
with blood specimens. The growth medium is formulated to
provide nutrients for bacterial growth. The bottles are
inspected daily for obvious signs of bacterial growth.
Samples from bottles suspected to be positive are then
o further cultured to obtain isolated bacterial colonies
which can then be identified. This method is very labor-
intensive and costly, since daily inspections and
subculturing of suspect bottles are required.
Various attempts have been made to improve the
conventional manual method. For example, culture bottles
have been made with added attachments containing solid
media. The user inverts the bottle each day, thereby
inoculating the solid media and enabling growth of
isolated bacterial colonies, which can then be
identified. Another improved process uses a "growth
indicator" which detects the buildup of gases in the
headspace of the bottle. A third method is to
concentrate organisms in the specimen by centrifugation
and then culture the concentrated bacteria on solid
media. Despite such improvements, these methods still
suffer from the drawback of being highly labor-intensive.
Attempts to automate the process of culturing blood
specimens have also been made. Most automated processes
rely on the fact that bacteria cultured in a medium
including a carbon source, such as glucose, break down
this carbon source to form CO2 as part of normal growth
and metabolism. Early efforts at automation used culture
V094/26874 PCT~S94/05392
~13~2~
bottles containing radioisotope-labelled media. Blood
specimens are inoculated into the bottle. Bacteria, if
present in the specimen, metabolize the carbon-containing
compounds in the media and give of radioactive-labelled
C2 as a waste product. Gas in the headspace of the
bottle is sampled by puncturing the seal at the top of
the bottle with a needle and removing a portion of the
gas. The radioactive C02 can then be detected by
conventional radiometry.
0 A number of drawbacks have been reported with such
systems. For example, EPO Patent Application No.
85302261.4, published October 16, 1985, states:
"Radioisotope labeled materials are expensive and require
special handling during storage, use and disposal.
15 Moreover, although the levels of radioactivity
encountered in using such systems are very low,
prospective users may be deterred by personal fears of
radioactivity." Moreover, some research has suggested
that radiometric detection systems are less accurate than
other methods and result in more false positive readings.
Second, such systems are "invasive," that is, they
require the use of a needle to puncture the bottle seal
to obtain gas for testing. Because sample gas must
actually be removed from the bottle, fairly complex
25 pneumatic systems are needed to handle the gas and return
it to the bottles. Further, if the needles are not
properly sterilized, the specimens can be contaminated
with bacteria on the needle, raising the potential for
"false positive" readings. In addition, because the
bottles are sampled and read invasively, automated
instruments are generally more complex mechanically,
since the bottles must be transported mechanically from
W094/26874 PCT~S94/05392
2~38Z5~
an "incubation" station, where the bottles are maintained
at the appropriate conditions for bacterial growth, to a
"reading" station, where the headspace gas is sampled and
read. Most significantly, the need to handle needles for
5 periodic testing is labor-intensive and, because the
culture bottles contain blood, increases the risk of
- disease transmission due to needle sticks and the like.
EP0 Application No. 83108468.6 (published August 27,
1983) summarizes the relative benefits of noninvasive
o sampling over invasive methods:
"there is no possibility of contamination caused by
needle or probe penetration of the vial septum;
the design of an automated apparatus is simplified,
in that there is no need to provide provisions for a
15 needle-carrying head assembly or other invasive sampling
apparatus;
the necessity of replacing flushed head space gas
with sterile culture gas is eliminated;
the use of special culture gases is not required;
faster vial sampling is possible, since only vial
positioning is involved;
no vertical head motion is necessary;
the cost of culture media raw materials is reduced
due to the elimination of any radiolabeled substrate; and
all radioisotopes are eliminated, which eliminates
the problems of shipping, handling and storing low level
radioisotopes."
(See EP0 Patent Application No. 83108468.5, pp. 8-9.)
Early attempts to improve automated instruments
30 focused on improving the detection system. Thus, EP0
Application No. 85302261.4 describes a system in which
radioisotope labelling has been replaced with direct
"094l26874 PCT~S94/05392
2 5 ~ -
detection of non-radioactive CO2 in the headspace gas by
means of infrared spectroscopy. While this alleviated
the problems associated with radiometric detection, the
shortcomings of invasive sampling remain. In addition,
5 the use of infrared spectroscopy requires that culture
bottles be made of special materials.
EPO Application No. 83108468.8 discloses a system
which detects CO2 levels in the headspace gas by taking
infrared readings directly through the culture bottle,
lo i.e., noninvasively. However, the instrument disclosed
is equipped with only a single light source and detector.
This, in turn, requires that the culture bottles be
periodically cycled past the detector for readings, thus
increasing the mechanical complexity of the instrument
15 and limiting the number of samples the instrument can
rapidly process. Finally, problems can occur in
calibrating the infra-red spectrometer to the many
bottles which must be read.
More recently, improved instruments with non-invasive
sampling systems have been developed. In these systems,
the culture bottle is incubated and read in the same
location within the instrument. Each bottle is held on a
rack inside the incubation chamber. The bottles are
periodically agitated (to increase the diffusion of CO2
25 and thereby shorten detection time) while being incubated
at approximately 35 C.
EPO Patent Application No. 89200554.7, published
September 20, 1989, describes the detection system used
in such instruments. A colorimetric sensor (pH
indicator) is adhered to the bottom inside surface of
each bottle. The sensor turns from green to yellow as
the level of CO2 within the media increases. Individual
W094/26874 PCT~S94/05392
~1382~
optical units are provided for each bottle. These
optical units include LEDs to illuminate the sensor,
photodetectors, and associated electronics and signal
conditioning equipment. The instrument periodically
5 "reads" each sensor using reflected light to monitor
changes in the transmission of the sensor at a specific
wavelength. When a level of CO2 consistent with
microbial growth is reached, the instrument alerts the
user of a positive blood culture.
lo While these improved systems have alleviated some of
the problems of conventional blood culture instruments,
several drawbacks still remain. First, these instruments
have been equipped with enclosed, "oven-like" incubation
chambers. This, in turn, requires that the instrument be
15 fairly large (particularly in height) to accommodate the
number of culture bottles typically processed in a
hospital laboratory. This is a significant disadvantage
in many laboratories, since floor and bench space is
typically at a premium. This arrangement is also
undesirable from the standpoint of the user, since the
topmost bottles may be out-of reach when the instrument
is placed on a laboratory bench.
Second, because the detection system is based on
changes in the light transmission of the sensor, the
25 light illuminating the sensor is the same wavelength as
the light reflected from the sensor. This makes it
possible for light which is not indicative of changes in
the sensor (e.g., light reflected from the bottom of the
glass or plastic culture bottle, as well as other
reflective surfaces) to reach the detector. Because the
detection system does not discriminate between light
reflected from the sensor and such unwanted "noise," the
~094/26874 213 ~ 2 ~ ~ PCT~S94/05392
dynamic range of detection is generally more limited. In
addition, it becomes critical to physically isolate the
illuminating light source from the detector, placing
further design constraints on the configuration of the
s optical system.
Accordingly, a need exists for an automated blood
culture instrument which is capable of incubating blood
specimens under the appropriate conditions, but which has
- a compact design, thereby reducing the laboratory floor
lo space it occupies and making it more convenient for use
by laboratory medical technicians. Further, a need
exists for an instrument which uses non-invasive sampling
and non-radiometric detection, but which has a highly
accurate and sensitive detection system, which does not
rely upon measuring changes in light transmission of
monochromatic light.
In copending application S.N. 07/887,627, filed May
22, 1992, an improved blood culture instrument, which is
designed to fulfill these needs, is disclosed. This
instrument includes a unique optical system designed to
optically interrogate a sensor located on the inside
bottom wall of a blood culture bottle. As noted in
copending application S.N. 07/887,627, it is preferred in
the practice of the invention to make~use of a sensor
made in accordance with the teachings of copending
applications S.N. 07/638,481, filed January 4, 1991, and
S.N. 07/609,278, filed November 5, l990 (now U.S. Patent
No. 5,173,434), both of which are entitled "Measurement
of Color Reactions by Monitoring a Change in
30 Fluorescence," are assigned to the assignee of the
present application, and are incorporated herein by
reference and made a part hereof.
W094/26874 PCT~S94/05392
21382~
,
In particular, it is preferred to use a sensor made
in accordance with the teachings of copending application
S.N. 07/609,278. As disclosed in that application, the
sensor preferably comprises a chromophore layer and a
s fluorophore layer. The chromophore layer preferably
consists of a pH sensitive chromophore encapsulated
within a gas permeable, hydrogen-ion impermeable matrix,
such as silicone. Positioned atop the the chromophore
layer (when the culture bottle is in its upright
lo position) is the fluorophore layer. The fluorophore
layer preferably consists of a fluorescent dye
encapsulated within a water and gas impermeable polymer,
such as an acrylic polymer. When the culture bottle is
placed within the blood culture instrument, the
15 chromophore layer is situated or sandwiched between the
fluorophore layer and an optical unit housed within the
instrument. The optical unit is designed to periodically
interrogate the fluorescent signal emanating from the
fluorescent layer, to determine whether bacterial growth
is occurring within the culture bottle.
Although an instrument designed in this manner
overcomes many of the drawbacks with conventional systems
noted above, further drawbacks were encountered. The
most significant problem was encountered in attempting to
2s use a commercial grade culture bottle in place of the
specially fabricated bottles typically used in automated
blood culture apparatus. Such specially fabricated
bottles are made by welding relatively flat pieces of
glass together, giving the bottle -- and, in particular,
the bottom wall of the bottle to which the sensor is
attached -- uniform and consistent optical
characteristics. While such specially fabricated bottles
--094/26874 PCT~S94/05392
~13~5~
are desirable from an optical standpoint, they are
generally more expensive than commercial grade bottles.
More importantly, because of the way in which they are
manufactured, they tend to be somewhat fragile.
5 Naturally, fragility is generally undesirable in this
context, since culture bottles contain human tissue
samples and bottle breakage could potentially result in
disease transmission from infected samples.
For these reasons, it was felt to be desirable to use
lo less expensive and sturdier commercial grade bottles
instead of the more conventional blood culture bottles.
Unfortunately, because of the way many commercial bottles
are made, the bottom wall of the bottle is not
substantially flat. Instead, this bottom wall is
15 generally concave on the outside and generally convex on
the inside, creating a convex raised area or "mound" on
the inside bottom wall. Since this is typically where
the sensor is located, difficulties were encountered in
the optical detection system. In particular, since the
20 sensor was now positioned a greater distance from the
optical detection unit and the curved glass had different
optical characteristics, one result was a significant
decrease in sensitivity. For two reasons, this made it
critical to reduce "noise" in the system. First, it was
25 necessary to maintain an acceptable signal-to-noise ratio
so that sensitivity would not be impaired. Second, the
increased noise made it difficult to construct relatively
simple algorithms to determine whether a specimen bottle
is truly "positive;" the noise would have made these
algorithms unduly complex.
In attempting to solve these problems, several
potential sources of noise were identified. A first
W094/26874 PCT~S94/05392
21~2~
source of noise was thought to be light entering from the
outside environment and reflecting off various optical
surfaces in the instrument and bottle. This noise
component seemed to be particularly great during the
5 early stages of bacterial growth. An additional noise
component was also encountered. When so-called
"hemolytic" organisms -- organisms which break down blood
cells -- were cultured in the bottle, a temporary
decrease in fluorescent emission was observed after
lo initial bacterial growth was detected. Because this
phenomenon was observed only in the case of hemolytic
organisms, it was felt that the source of this noise
component was reflected light interacting with the sample
itself. (As a result, this phenomenon was referred to by
15 the inventors as the "blood blip.") This noise component
was particularly troublesome, since it occurred during a
critical time period for detection of bacterial growth.
In addition to solving the sensitivity problems noted
above, it was felt desirable to overcome an additional
drawback encountered in automated apparatus for tissue
sample and culture. In a number of conventional
apparatus, when a bottle is placed in the instrument the
user must input information into the sytem computer to
alert the system that a bottle has been added in a
25 particular location. In laboratories where a large
number of tests are performed, this data entry process
can become cumbersome, since the laboratory technician
must either (a) maintain a separate list of where bottles
were placed and then enter all of the information into
the computer at once, or (b) alternate between entering
the information and placing bottles in the instrument.
In either event, the process is often time-consuming and
'-'094/26874 PCT~S94/05392
._
~138254
11
labor-intensive, and has the potential for increasing
operator error.
In view of these additional drawbacks, a need exists
for an improved optical detection system which can be
5 used with sturdier and less costly commercial grade blood
culture bottles. A further need exists for an optical
system which is highly accurate and sensitive and which
reduces unwanted optical noise. Finally, a need exists
for an optical system which can automatically detect when
a bottle is placed in the instrument so that this
information need not be manually entered into the system
computer.
8UMMARY OF THE l~v~..lON
Accordingly, it is an object of the present invention
15 to provide an improved optical detection system for use
in an automated apparatus for culturing and detecting
bacteria in human tissue (in particular, blood) which
substantially reduces unwanted optical noise and thereby
has improved sensitivity.
It is a further object of the present invention to
provide an improved optical detection system which is
relatively inexpensive and which can be used with
sturdier and less expensive commercial grade blood
culture bottles.
It is a further object of the present invention to
provide an improved optical detection system which is
capable of automatically detecting when a bottle is
placed within a blood culture instrument, thereby
simplifying use of the instrument for laboratory
technicians.
W094l26874 PCT~S94/05392
21382Sg
;,
12
These and other objects are accomplished by providing
an instrument for detecting the presence of
microorganisms in human tissue in a specimen-containing
vessel which comprises means for holding one or more
s specimen-containing vessels and light emission means.
The light emission means is configured to permit emitted
light to impinge upon a sensor positioned on an inside
wall of a specimen-containing vessel held in the vessel
holding means. Light detection means are also provided
lo for converting light energy from the sensor into a
detectable signal. Finally, light blocking means are
provided for substantially covering all but a selected
portion of the sensor which is to be optically
interrogated. The light blocking means is designed to
substantially prevent light other than light from the
sensor from reaching the light detection means.
In another aspect of the invention, a control circuit
for use in detecting the presence of microorganisms in
tissue is also disclosed. The control circuit increases
the sensitivity of the optical detection system and makes
it possible for the system to easily and rapidly
determine automotacally whether a bottle has been placed
into a bottle-receiving opening in the instrument. The
control circuit comprises power supply means; light
emitting means for receiving power from said power supply
means and producing a light emission in response thereto;
modulator means for providing a time-variant signal to
said light emitting means causing the latter to replicate
said time-variant signal; light responsive means
responsive to a fluorophoric response produced by said
light emission and producing an electrical response
signal; demodulator means clocked to said time-variant
- 094/26874 PCT~S94/05392
213825~
signal to selectively apply a first level and a different
second level of amplification to said electrical response
signal dependent on the value of said time-variant signal
to provide a demodulated response signal; integrator
means receiving said demodulated response signal and
time-averaging this signal to provide an output voltage
signal; whereby said demodulator applies an amplification
to said response signal which is synchronized with and in
phase with said time-variant signal to selectively pass a
o portion of said electrical response signal to said
integrator, which passed signal portion is characteristic
of said time-variant signal.
The foregoing features and advantages of the present
invention will be more readily understood upon
consideration of the following detailed description,
taken in conjunction with the accompanying drawings, in
which:
BRIEF DESCRIPTION OF DRAWINGS
FIG. 1 is a perspective view of an automated blood
culture apparatus made in accordance with the present
invention;
FIG. 2 is a side view of the apparatus, showing one
of the specimen-holding drawers in its open position;
FIG. 3 is a view similar to that of FIG. 2 in
2s somewhat schematic form, with portions of the specimen-
holding assembly removed to show the system for heating
and circulating air within the specimen-holding drawers;
FIG. 4 is a side view of the specimen-agitating
assembly used in one embodiment of the present invention,
showing the specimen-containing racks in their lowermost
agitation position;
W094/26874 ~ PCT~S94/05392
2 ~g~4
FIG.. 5 is a view similar to that of FIG. 4, showing
the specimen-containing racks in their uppermost
agitation position;
FIG. 6 is a top view of one of the specimen-
5 containing drawers taken along the line 6--6 in FIG. 1;
FIG. 7 is a front view of one of an individual
specimen holder;
FIG. 8 is a cross-sectional view taken along the line
7--7 in FIG. 7;
o FIG. 9 is a side view of an alternative bottle-
gripping arrangement for retaining culture bottle within
the bottle holding racks;
FIG. 10 is a perspective view of an assembly for
moving the specimen-holding drawers between their closed
15 and open positions;
FIG. 11 is a perspective view of an assembly for
agitating the specimen-containing racks;
FIG. 12 is a dwell chart showing the relative
position of the specimen-containing racks during several
20 agitation cycles in graphical form;
.FIG. 13 is a graph of intensity as a function of
wavelength, showing schematically the optical properties
of the excitation light as well as the light emitted by
the fluorescent sensor; and
FIG. 14 is a side view of an alternative assembly
which may be used to agitate the specimen-containing
racks.
FIG. 15A is a view similar to that of FIG. 8, showing
an improved optical system and sensor used to detect the
growth of microorganisms in patient tissue specimens.
--~94/26874 ~1~ 8 2 ~ 4 PCT~S94/05392
FIG. 15B is an exploded perspective view of the
improved optical system, showing a spatial filter which
may be used to improve the sensitivity of the system.
FIG. 16 is a schematic diagram of a control circuit
5 which may be used in the practice of the present
invention.
FIG. 17 is a graph of voltage as a function of time
for various outputs of the control circuit depicted in
FIG. 16.
o DETAILED DESCRIPTION
Figs. 1 and 2 show the general arrangement of an
instrument 10 made in accordance with the present
invention. This Specification describes a preferred form
of the invention, in which the instrument is used to
lS culture and detect bacteria in human tissue and, in
particular, in human blood. However, although the
instrument is described as being used for detection of
microorganisms or bacteria in blood, it will be
understood that the instrument may be used to detect
microbial growth in any number of tissues, including
urine, cerebral-spinal fluid, synovial fluid, and others.
Fig. 1 illustrates the instrument of the present
invention generally. Instrument 10 includes a specimen-
handling module 12 under the control of a microcomputer
14, which is preprogrammed to follow certain specimen-
handling protocols in accordance with input from the
user. A detailed description of the general types of
software commands and processing steps which could be
- used to program the microcomputer to perform such
protocols is attached as an Appendix hereto.
- In the embodiment shown, each specimen-handling
module 12 includes a housing 32 and two slide-out drawers
W094/26874 ~ 5 4 PCT~S94/0~392
16, 18, each of which includes a plurality of racks 20,
22, 24, 26, 28, 30, which hold the specimen-containing
vessels or bottles for processing. In Fig. 1, drawer 16
is shown in its open position, while drawer 18 is shown
5 in its closed position.
As described in greater detail below, each of the
- slide-out drawers 16, 18 is equipped with a heating
system (see Fig. 3) designed to warm the drawers to the
appropriate temperature for bacterial growth and maintain
them substantially at that temperature. Each of the
drawers 16, 18 is also equipped with a mechanical
agitation system (see Figs. 4 and 5) for periodically
agitating the bottles. Such agitation is known to
shorten the time to detection by causing CO2 generated by
15 bacteria within the bottle to diffuse more rapidly to the
fluorescent sensor, which is preferably affixed to the
bottom inside of the bottle. Finally, the drawers 16, 18
are also equipped with an optical detection system,
including a plurality of optical units (see Figs. 7 and
20 8) which monitor CO2 production by optically
interrogating the fluorescent sensors on each of the
culture bottles. Optical readings for each bottle are
transferred via a data link (not shown) to the
microcomputer 14, where it is stored for later retrieval
25 and use.
As best seen in Figs. 1 and 2, in a preferred form a
blood culture instrument module includes at least one,
and preferably two or more, slide-out drawers 16, 18
slidably received in housing 32 for holding the blood
specimen-containing vessels or bottles during processing.
By configuring the instrument in this manner in
accordance with the present invention, the instrument has
-~94/26874 ~1 3 ~ ~ 5 4 PCT~Sg4/05392
sufficient bottle-holding capacity for hospital
laboratory use, while maintaining a compact size and a
small "footprint" desirable for most users. This is
because the bottles can be held within the instrument for
5 most processing steps, while still keeping them readily
available and within easy reach of the laboratory
technician upon opening the drawer. The compact size of
the instrument made in accordance with the present
invention is an important advantage in most settings,
o particularly hospitals, since laboratory space is
generally limited due to the large number of instruments
and pieces of equipment housed within a typical
microbiology laboratory.
Referring to Fig. 1, the front face of each drawer
5 includes an information panel/user interface for
displaying information relating to the specimens held
within that drawer and for enabling the user to control
certain functions pertaining to that drawer. (In Fig. 1,
the information panel for drawer 16 is designated by the
reference numeral 17.) Information which may be
displayed on the information panel by, for example, LED
or LCD displays, include the temperature within the
drawer, the number of specimen bottles which have been
read as "positive," and the number of available positions
2s for additional specimen bottles. Functions which may be
controlled by the user may include opening and closing
the drawer, as well as disabling an alarm designed to
signal, for example, a positive reading within the
drawer. However, it will be understood that other types
of information may be displayed on the panel and other
commands may be likewise be input from the user
interface, as desired.
W094/26874 PCT~S94/05392
'~l3~2~ ~ -
18
The system of the present invention is preferably
designed so that multiple specimen-handling modules may
be interfaced with a single microcomputer. In this way,
the specimen-holding capacity of the system may be
5 substantially increased, as desired. The modules are
also preferably designed so that they may be stacked one
atop another if desired, to minimize the amount of floor
space the system occupies.
Referring again to Fig. 1, the drawer 16 is slidably
lo received within housing 32. In a preferred arrangement,
a pair of integral slide extensions 34a, 34b are rigidly
affixed to the drawer 16 by means of screws, bolts or the
like at a position adjacent the top of drawer. The slide
extensions are slidably received within tracks 36a 36b.
Tracks 36a, 36b are themselves slidably received within
receiving guides (not shown) which are rigidly mounted to
the inside of housing 32. Conventional ball bearing
assemblies (not shown) permit the slide extensions 34a,
34b to slide freely within tracks 36a, 36b, and the
tracks 36a, 36b to slide freely within the receiving
guides. The slide extensions 34a, 34b, tracks, 36a, 36b,
and receiving guides are commercially available in the
form of a three-section ball bearing slide which permits
the drawer 16 to slide in and out of housing 32. Success
has been had with a three-section ball bearing slide
Model No. ESBB manufactured by Barnes Engineering Company
of Anaheim, California. Preferably, the slides are made
of hardened steel which has been electro-plated such that
they adequately support the drawers 16, 18 while
maintaining their corrosion resistance under the
temperature conditions prevailing within the drawers.
094/26874 PCT~S94/05392
~13825~1
Fig. 10 illustrates the manner in which the bottom of
drawer 16 is slidably mounted within the housing. A
single three-part ball bearing slide, positioned to lay
flat (i.e., rotated clockwise 90 degrees relative to the
slide extensions 34a, 34b of the three-part slides
illustrated in Fig. 1) is used to prevent the drawer from
"wobbling" from side to side within the housing. The
slide extension (not shown), is rigidly attached to the
underside of drawer 16 within a longitudinal recess lS0
o which runs substantially the length of the drawer 16.
This extension is received in a track 152, which, in
turn, is received within receiving guide 154 mounted to
the inside of the drawer housing. As in mounting the top
of the drawer, ball bearing assemblies are used to enable
the extension to slide freely within the track 152, and
the track 152 to slide freely within the receiving guide
154.
Although Figs. 1 and 10 illustrate one method of
slidably attaching the drawer to the housing 32 using
three-part ball bearing slides, it will be understood
that the drawers may be slidably mounted to the housing
using any suitable means, such as, by way of example,
conventional slides, tongue and groove configurations,
and the like.
In a preferred arrangement also illustrated in Fig.
10, means are also provided to move the drawer 16 under
power between a first, closed position, in which the
drawer and its contents are substantially enclosed within
the housing 32, and a second, open position, in which the
30 drawer and its contents are located substantially outside
the housing 32. The drawer is moved in response to a
command from the user, which can be input, for example,
W094/26874 - PCT~S94/05392
2~3~2,5~ ~ -
from microcomputer 14 or from the information
display/user interface 17 in Fig. 1. As shown in Fig.
10, motor M, under the control of the microcomputer,
powers an associated belt drive 156. The belt drive 156,
5 in turn, rotates a screw drive 158 which engages threaded
drawer extension 160. The drawer extension 160 is
rigidly attached adjacent a lower corner of the drawer
16. Upon actuation of the motor M, the rotating screw
drive moves the drawer under power in or out of the
o housing, as desired, in the directions of the double-
headed arrow. Appropriate flags are used to signal the
microcomputer to deactivate the motor M once the drawer
16 reaches its open or closed position.
It will also be understood that other means for
15 mechanically moving the drawer in or out of the housing,
such as, by way of example, belt drives, gear assemblies,
and the like, may also be used in practicing the present
invention.
Referring now to Fig. 2, the drawer 16 also includes
means for holding a plurality of specimen-containing
vessels. This vessel-holding means may take the form of
a plurality of racks 20, 22, 24, 26, 28, 30 which are
adapted to hold or retain the specimen bottles during
processing. Each rack has a plurality of bottle-
25 receiving openings 38 which are sized to accommodatespecimen bottles. As will be described in greater detail
below, at the base of each bottle-receiving opening 38 is
an optical unit 46 for taking optical readings of a
sensor affixed to the bottom inside of the bottle.
Although in Fig. 2 the bottle receiving openings are
illustrated as being circular to accommodate a generally
cylindrical specimen bottle, it will be understood that
~94/26874 ~ 13 8 2 S ~ PCT~S94/05392
apertures having a variety of shapes (e.g., rectangular,
triangular, or polygonal), could also be used in
appropriate circumstances. In addition, although the
drawer 16 is illustrated with six racks accommodating 10
bottles each, it will be understood that other quantities
may also be held within the racks. Indeed, it is
preferred that each drawer accommodate as many bottles as
possible in order to maximize the capacity of each
module.
o When the drawer is in its closed position, the
vessel-holding means should be substantially enclosed
within, i.e., covered by, the housing. It will be
understood that the vessel-holding means need not be
completely enclosed within the housing, so long as the
vessels are substantially located within the housing,
thereby reducing the amount of space the instrument
module occupies. Likewise, when the drawer is in its
open position, the vessel-holding means should be located
substantially outside the housing, i.e., in a position in
which the vessels can be readily accessed or removed by
the instrument operator.
It will be understood that the racks may be fastened
together to form an integrated assembly, as illustrated
in the drawings, or may be fabricated as individual units
which can be removably attached within the drawer 16. It
may be desirable in certain circumstances for individual
racks to be removed so that specimen bottles can be
inserted offsite, and then the racks can be reinserted
into the instrument at a later time. It will be
understood that this can be accomplished in any number of
ways, including providing a frame within the drawer to
which the racks may be removably attached.
W094/26874 PCT~S94/05392
8~
The face of each bottle holding rack is equipped with
an LED (light emitting diode) panel 15, which includes an
array of LEDs 19, two of which are associated with each
bottle receiving opening 38. The LEDs associated with
5 each opening provide the user with information concerning
the status of the optical readings for the bottle
contained in that opening. For example, a red LED might
indicate a bottle testing "positive," while a green LED
might indicate a bottle which has as yet tested
o "negative." The panel 15 may take the form of a printed
circuit board which includes the array of LEDs for all of
the bottle receiving openings in that rack, as well as
associated circuitry for transmitting on/off information
and power to the LEDs under the control of the
lS microcomputer. The panel 15 may then be removably
mounted to its rack by means of Velcro~ fasteners or
other similar means.
Fig. 7 depicts a portion of one of the bottle-holding
racks in greater detail. Adjacent each bottle-receiving
aperture is gripping means adapted to removably grip the
specimen-containing vessel so that it may be repeatably
held at a predefined, substantially fixed depth within
the aperture. This depth is predefined and substantially
fixed to allow the optical unit to interrogate the sensor
affixed to the specimen-containing vessel from a well-
defined and repeatable position, thereby ensuring more
accurate optical readings when a vessel is removed and
then reinserted. The gripping means may comprise one or
more flexible arms positioned adjacent the periphery of
the aperture. The gripping means may take the form of
one or more arms. In Fig. 7, the gripping means includes
three outwardly extending fingers 40a, 40b, 40c
~94l26874 PCT~S94/05392
~13825~
positioned around the periphery of each cylindrical
opening 38 in order to repeatably position and support
the bottle within the rack. The fingers may be fastened
to the base of the rack (shown in Fig. 9) or formed
s integrally therewith so that they protrude upwardly
adjacent the opening. In one form of the invention, the
fingers 40a, 40b, 40c are molded integrally with the base
of each rack from a suitable thermoplastic resin, such as
an acrylonitrile-butadiene-styrene lABS) resin or an
o acetal resin (e.g., Delrin~, a registered trademark of
E.I. Du Pont de Nemours & Co.). Preferably, the fingers
40a, 40b, 40c are uniformly spaced at approximately 120
intervals around the periphery of the opening. Each of
the fingers 4Oa, 4Ob, 40c includes a recessed portion
41a, 41c (the recessed portion of finger 40b is not
visible in Fig. 7) which is shaped to engage an
engagement area on the outside surface of a specimen
bottle. A flanged end 42a, 42b, 42c on each finger is
designed to engage the shoulder of a culture bottle
inserted into the aperture 38.
Preferably, the fingers 4Oa, 4Ob, 40c are arranged to
form an opening which is smaller than the diameter of the
culture bottle. In that case, the fingers 4Oa, 4Ob, 40c
should also be capable of flexing or deforming outwardly
to admit the bottle and, in cooperation with the flanged
ends 42a, 42b, 42c, to engage the shoulder of the culture
bottle in a "snap-fittable" mechanical arrangement once
the bottle has been inserted to the pre-defined depth
within the aperture. Such an arrangement has several
advantages. First, it helps to properly position the
bottom of the bottle (and, as a result, the sensor
affixed to the bottle) securely and repeatably against
W094/26874 PCT~S94/05392
24
the optical unit 46 to ensure accurate and consistent
optical readings. Second, such an arrangement preferably
gives the instrument operator tactile and/or audible
feedback when the bottle is properly seated within the
5 opening, helping to reduce errors in loading and
positioning the bottles. In the absence of such tactile
feedback, the operator could insert the bottle into the
opening to varying degrees, causing inaccuracy and
inconsistency in the optical readings.
lo An alternative means of gripping the bottle within
the bottle receiving opening is illustrated in Fig. 9,
which shows a portion of one of the bottle holding racks.
In this embodiment, the bottle gripping means includes
springs 53 formed of a resilient metal, such as spring
15 stainless steel. Again, it is preferred that at least
three, and preferably four, springs 53 be provided for
each bottle and that they be equally spaced around the
opening. However, it will be understood that two or even
one spring could be used. The springs 53 are attached to
base plate 57 (which, in this embodiment is made from
aluminum or another suitable metal) by riveting, welding,
or other conventional means. Base plate 57 has a
plurality of apertures formed therein so that the sensor
(not shown) affixed to the inside of the bottle 120 can
be optically interrogated by the optical units 46. Each
spring 53 has a crimp 55 formed in one end for gripping
the bottle 120. The crimps 55 are shaped to engage a
corresponding engagement area taking the form of an
indentation or detent 47 in the bottle 120. The springs
are flexible and resiliently deformable so that when the
bottle 120 is inserted into the bottle receiving opening,
the springs 53 are resiliently deformed in an outward
V094/26874 21~ 8 2 S ~ PCT~S94/05392
direction to admit the bottle 120. Once the bottle is
fully seated at the appropriate depth within its
aperture, the springs 52 return substantially to their
original position and engage the detent 47 in the bottle
120. This is evident to the operator by the tactile and
audible feedback provided when the bottle "snap-fits"
into tight, mechanical engagement with the springs 53.
It will be understood by those skilled in the art
that other similar ways of removably holding the bottles
0 within the racks may also be used, such as, by way of
example, ball-spring plungers designed to engage a detent
in the bottle, a plurality of springs arranged within the
bottle receiving opening so as to grip the bottle, a
deformable plastic or rubber O-ring, or a cam and lever
gripping arrangement. Likewise, the engagement area on
the bottle may take any number of shapes, such as a
continuous detent around the entire circumference of the
bottle (as illustrated in Fig. 8) or a more localized
area. In this regard, as noted above, it is important to
keep in mind that the purpose of such arrangements is (1)
to hold the bottom of the culture bottle securely in a
pre-defined position adjacent to, and substantially
centered with respect to, the optical unit to help assure
greater accuracy and predictability in the optical
readings, (2) to provide the operator with some form of
tactile and/or audible feedback once the bottle is
properly seated within the rack, and (3) to assist the
operator in positioning the bottle within the rack in a
reproducible and repeatable fashion.
Fig. 9 also illustrates the manner in which the
optical units and related circuitry are attached to the
base plate 57 of the bottle holding racks. A plurality
WOg4/26874 PCT~S94/05392
~,~3~S 4 26
of PEM fasteners 59 are rigidly affixed to the base plate
57 at spaced intervals along its length. Each PEM
fastener has an annular base 54 and plurality of prongs
56 adjacent its opposite end. A plurality of optical
5 units 46 -- one for each bottle receiving opening -- are
attached along the length of a printed circuit board
(PCB) 41. The PCB 41 is equipped with the necessary
circuitry for providing power to the optical units and
for transmitting the optical readings (which, as
explained in greater detail below, are converted into a
voltage by the optical unit) to the microcomputer for
storage and later use. The PCB 41 also has a plurality
of holes formed along its length. To attach the PCB 41
to its bottle holding rack, the prongs 56 on the PEM
15 fasteners 59 are inserted into the holes in the PCB 41
until the PCB engages the annular bases 54. The prongs
56 deform inwardly so that they can pass through the
apertures in the PCB 41 and then spring back to their
original position so that they retain the PCB 41 in
20 engagement with the annular bases 54. In this way, the
PCBs 41 are easily assembled to the bottle holding racks,
and can easily be removed for repair or replacement.
As best seen in Figs. 1 and 2, the inside of each
drawer is preferably equipped with a bar-code reader 162
25 centrally positioned within a V-shaped channel 164, which
extends longitudinally across the drawer 16. The channel
164 is sized to accommodate specimen bottles which are to
be inserted into one of the bottle receiving openings 38.
Preferably, a bar-code label is placed on the side of
each specimen bottle to identify the patient from whom
the specimen was taken. It will be understood that many
hospitals now employ systems in which detailed
- ~o 94,26874 2 i 3 8 2 S ~ PCT~S94/05392
information about a patient is associated with a unique
bar-code for that patient. Labels containing that bar-
code are then used to track and identify treatments and
procedures pertaining to that patient. It is intended
that the instrument of the present invention should be
capable of interfacing with the hospital bar-code system,
if available. Alternatively, bar-code labels could be
generated solely for use with the instrument of the
present invention to track specimens and identify them as
o having come from a particular patient.
When the user wishes to insert a specimen bottle into
the drawer, he or she places the area of the specimen
bottle bearing the bar-code label in the V-shaped channel
164 and draws the bottle across the bar-code reader 162
to scan the patient information into the microcomputer.
The system automatically detects where the bottle is
placed within the drawer so that the patient information
can be associated with the optical readings for that
bottle. The optical readings and associated patient
information are stored for later retrieval and use.
As also seen in Figs. 1 and 2, the interior face of
the drawer is equipped with a second user interface/
information panel 166. This user interface enables the
user to perform certain additional operations, and
provides certain additional information, such as
instructions for inserting a new bottle into an available
bottle-receiving aperture.
Another significant feature of the present invention
is a system for controlling and maintaining the
temperature of the specimen bottles while they are being
held within the slide-out drawers of the instrument.
Because the optimal temperature for encouraging growth of
W094/26874 PCT~S94/05392
3~S~
28
many bacteria is approximately 35-37 C and, more
preferably, close to 35 C, for many blood culture
applications it is important to maintain the bottles near
or at this temperature so that any bacteria in the
s specimen will multiply as rapidly as possible, thereby
decreasing the time it takes to detect a positive
- culture. Accordingly, the present invention includes
means operably associated with the slide-out drawers for
(1) warming the interior of the drawer to an elevated
lo temperature suitable for encouraging growth of
microorganisms, and (2) maintaining the interior of the
drawer substantially at or near that elevated
temperature, when the drawer is in its closed position.
In a preferred form, such means comprises a forced air
convection system which will now be described in detail.
Fig. 3 illustrates the interior of one of the slide-
out drawers 16 with the bottle-holding racks removed.
Adjacent the interior front end of the drawer 16 is a
forward duct 60 positioned vertically within the drawer
16. The forward duct 60 is substantially hollow and open
at side 61, which faces the interior of the drawer 16.
Forward duct 60 is attached at its base to base plate 62,
which is positioned transversely to the forward duct 60
adjacent the interior bottom of the drawer 16. Adjacent
the interior rear end of the drawer 16 is a vertically
positioned rear duct 64, which is open at side 63 facing
the interior of the drawer 16 and which is also attached
to base plate 62. Preferably, the ducts are formed of
punched sheet metal, which is then bent and welded, or by
other conventional methods of metal forming. It will be
understood, however, that the ducts may be formed of
-'094/26874 PCT~S94/05392
21~8254
29
- other materials, such as molded plastic, and may be
formed in a variety of shapes and configurations.
- When the drawer 16 is in its closed position within
the specimen-handling module, the upper openings 63, 65
of the vertically extending forward and rear ducts 62, 64
are brought into alignment with corresponding openings in
upper duct 66, located within the module in the following
manner. Upper duct 66 forms a passageway which is
generally in the shape of an inverted U. When the drawer
lo 16 is closed, the vertical segments of this inverted U-
shaped passageway are brought into alignment with the
upper openings 63, 65 of the forward and rear ducts
located within the drawer 16, so that air may circulate
from this upper passageway into the forward and rear
ducts 60, 62.
Located within the upper duct 66 are a blower fan 68
and a heating coil 70. In response to direction from the
microcomputer, the fan 68 is energized and forces air in
the direction of the arrows in Figure 3. The air passes
over the heating coil 70, where it is warmed. The heated
air then passes downwardly in the direction of the arrows
into the interior of the drawer 16 through the upper
opening 65 in the rear duct 64 located within the drawer
16. The rear duct 64 is equipped with a plurality of
louvres 72, which are sloped in order to direct and
channel the heated air over, around, and across the
culture bottles held within the racks. The openings
between the louvres 72 coincide generally with the
position of the bottle-holding racks. (A representative
bottle, illustrated without its holding rack, is
identified by reference numeral 76 in Figure 3.)
W094/26874 PCT~S94/05392
~13~2S~ :
As shown in Fig. 3, the louvres also increase in size
(and, in particular, width) from the top to the bottom of
the rear duct 64. Because the air flow decreases at
greater distances from the fan 68, this configuration
5 assists in distributing the heated air in a substantially
equal manner to each of the bottle holding racks in the
drawer.
After the heated air circulates within the closed
drawer, passing over the bottles and thereby warming the
o specimens and media contained inside, it passes under the
force of fan 68 into the forward duct 60. The air then
passes upwardly (in the direction of the leftmost arrows
in Figure 3) past a temperature probe 67 which monitors
the air temperature. Temperature information is conveyed
to the microcomputer, which is programmed to energize the
fan 68 and heating coil 70 as needed in order to maintain
the temperature of the interior of the drawer at about
35-37 C and, more preferably, at 35 ~2/-1 C, in order
to encourage bacterial growth within the specimen bottle.
Although this is the preferred temperature for most
microorganisms, it will be understood that the instrument
may be designed to maintain the internal temperature in
other appropriate temperature ranges. For example, the
preferred temperature for culturing many types of fungi
2s is approximately 31 C. In general, the instrument
should be designed to maintain a temperature which is
optimal for the particular type of microorganism to be
detected. It will also be understood that some
fluctuation in the temperature of the drawer interior is
permissible, as long as the temperature of the culture
vessels is kept within acceptable limits for encouraging
growth of microorganisms.
~094/26874 ~ 2 5 ~ PCT~S94/05392
To prevent heated air from escaping from the drawers
in substantial quantities, thereby permitting the bottles
to become unacceptably cool, means are also provided to
substantially seal the drawers from excessive air leakage
5 once they are in their fully closed position. This
sealing means is illustrated in Figure 6, which is a top
view of the specimen-handling module 12 taken along the
line 6--6 in Figure 1. It will be seen that the module
includes a bulkhead 78. The bulkhead 78 is fabricated of
lo aluminum or another suitable material, and may be lined
with an insulating material, such as a rubber pad. Each
end of the bulkhead 78 has an adjustment extension 80
which is attached to a corresponding support pillar 82
within the module housing by means of set screws 84.
15 Each set screw 84 passes through an elongated slot (not
shown) in the extension 80 and into a threaded receiving
aperture (not shown) in the corresponding pillar 82. In
this way, the bulkhead 78 may be adjusted at each end to
move toward or away from the drawer 16 which slides in
and out of the drawer receiving area 86.
By simultaneous reference to Figures 1 and 6, the
manner in which the bulkhead 78 functions can be seen.
By adjusting the bulkhead 78 so that it is moved inwardly
toward the drawer area 86, a seal is created between the
2s faces of the forward and rear ducts 60, 64 and the base
plate 62, on the one hand, and the bulkhead 78, on the
other hand, when the drawer 16 is moved inwardly into the
drawer receiving area 86. Because the bulkhead 78
travels along slots at each end, it can be adjusted to
optimize the seal, even when the drawer 16 does not
travel precisely in a perpendicular direction into the
drawer receiving area 86, or when the front and rear
W094/26874 PCT~S94/05392i
~ 1 3 ~ 2 32
ducts are not precisely aligned with the upper duct, due
to mounting tolerances and the like.
It will also be understood that the drawer 18 is
likewise equipped with a similar sealing arrangement
5 adjacent the left-most side of the drawer in Fig. 1. The
result is that a chamber which is substantially leak-
proof is created within the interior of the drawers
surrounding the bottle racks. In this way, the heat
generated by heating coil 70 can be substantially
lo confined to the interior of the drawer in which the
bottles are held and does not escape from the bottle-
holding drawers. It should be noted, however, that the
seal need not be completely airtight, as long as the
heated air is substantially confined within the interior
of the drawer. In this regard, it has been discovered
that once the bottles are heated to the appropriate
temperature, much of the heat is held within the liquid
media inside the bottles. Thus, once the media is heated
to the appropriate temperature, some amount of air
leakage can be tolerated. Likewise, the drawers may be
opened periodically for addition and removal of bottles
without undue heat loss. Indeed, in some instances, a
small amount of air leakage can help to more rapidly
lower the temperature in the drawers when the temperature
2s rises above the appropriate range.
It will be understood that while a preferred heating
system utilizes forced air convection to warm the
bottles, as described above, other means for warming the
drawer interior (and/or directly warming the specimen
bottles) may also be used. For example, the warming
means could comprise a heating element which warms the
bottle holding racks directly. The heat would then
rO94/26874 PCT~S94/05392
21382~4
indirectly warm the specimen-holding bottles and the
drawer interior. The heating means could also comprise a
radiator-type system, in which heated water is passed
through conduits within the drawer, thereby warming the
5 specimen bottles and the interior of the drawer
indirectly.
The instrument is also equipped with means for
periodically and cyclically rocking or agitating the
bottles while they are being held within the racks. It
o is known that such agitation assists in more rapidly
detecting microorganisms in the bottle by ensuring that
Co2 generated by the microorganisms diffuses throughout
the media and is thereby rapidly brought into contact
with the sensor affixed to the bottom of the bottle.
(Referring to Figure 8, the sensor is identified by
reference numeral 100.)
Referring first to ~igs. 4 and 5, the agitation
system will now be described in detail. Figs. 4 and 5
show three of the bottle holding racks 20, 22, 24 during
the agitation cycle. The racks 20, 22, 24 are each
pivotally attached to a first pair of rack supports 102a,
102b. Taking rack 20 individually, a pivot pin 106 and
bearing (not shown) are used to pivotally mount the first
racking support 102a to one side of rack 20 at a position
2s adjacent the back side 109 of the rack 20. A second
pivot pin and bearing are used to pivotally mount the
second racking support 102b to the opposite side of rack
20 in a similar manner. To prevent the racks from
accumulating a buildup of static electricity, which could
potentially interfere with the circuitry for the optical
units, bearings made of an electrically conductive
material, such as sintered metal, are preferred. In this
W094/26874 PCT~S94/05392
~13825~
34
way, the electronic circuitry for the optical units is
provided with a path to electrical ground.
Rack 20 is also attached to a pair of drive supports
112a, 112b at a position adjacent to the bottle-receiving
face 116 of rack 20. As with the racking supports 102a,
102b, the pivotal mounting of the drive supports 112a,
112b is accomplished by means of pivot pins and bearings.
Each of the other racks 22, 24 is likewise attached to
the racking supports 102a, 102b and the drive supports
lo 112a, 112b in a similar manner.
By means of a drive mechanism described in detail
below, drive support 112b is alternately and cyclically
driven in an upward direction (illustrated by the arrows
in Figure 5) and a downward direction (illustrated by the
arrows in Figure 4), thereby moving the attached racks
20, 22, 24 between a generally horizontal position (shown
in Figure 4) and an upwardly inclined position (shown in
Figure 5). This rocking motion agitates the bottles and
their contents to facilitate diffusion of C02 generated
by bacteria throughout the culture bottles and, in
particular, to the sensor affixed to the bottom of the
bottles.
Referring now to Fig. 11, the agitation drive
mechanism is illustrated in detail. Motor M rotates
shaft 170, which is supported on bearings 172a, 172b,
172c. Flexible coupling 174 absorbs any shock caused by
misalignment of the shaft 170 relative to the motor M. A
circular cam 176 is mounted at the end of shaft 170. Cam
follower 178 is rigidly mounted to the cam at a position
adjacent the outer circumference of the cam 176. The cam
follower 178, in turn, is slidably received within an
oblong slot 180 in arm 182. Arm 182 is rigidly attached
~094/26874 213 8 2 5 ll PCT~S94/05392
- to drive support 112b and conveys power thereto. Upon
actuation of the motor M, shaft 170, cam 176, and cam
follower 178 are caused to rotate. When cam follower 178
reaches the right side of the oblong slot 180 in arm 182,
5 it imparts a downward motion to arm 182 and, thus, to
drive support 112b. (The arm 182 displaced in a downward
direction is shown in phantom in Fig. 11.) Likewise,
when cam follower 178 reaches the left side of the oblong
slot 180 in arm 182, it imparts an upward motion to arm
o 182 and, thus, to drive support 112b. Continuous
rotation of shaft 170 thereby moves the racks i~ the
cyclical rocking motion illustrated in Figs. 4 and 5.
Braking means in the form of a conventional brake
assembly (not shown) operatively coupled to the vessel-
15 holding means (either directly or by acting on the motorM or the shaft 170) is used to stop the cyclical
agitation, when desired.
As shown in Fig. 12, an important feature of the
present invention is the type of cyclical rocking motion
imparted to the arm 182, drive support 112b, and racks
20, 22, 24. Fig. 12 is a dwell chart showing the
distance of a point P located on rack 20 from a fixed
reference point. The fixed reference-point is chosen as
the position of point P when the rack 20 is in its
25 lowermost position. As the shaft 170 rotates, the drive
mechanism of the present invention causes the distance of
travel of the point P from the fixed reference point to
increase and decrease in a substantially sinusoidal
fashion.
It will be seen that at positions substantially near
the maximum and minimum travel of point P (indicated by
brackets in Fig. 12), the slope of the sinusoidal curve
W094/26874 PCT~S94/0~392
~ 2 ~l~ 36
is relatively small. Since the slope of the curve is
proportional to the velocity of point P (and, therefore,
the velocity of the bottle holding racks), it can be seen
that the velocity of the racks near the maximum and
s minimum travel points is relatively low.
This has important consequences for the operation of
the instrument. Because optical readings must be taken
when the racks are at rest and in an inclined position
(to ensure that the sensor is completely covered with
liquid during optical readings), it is necessary to
periodically stop the bottles while they are in the
inclined position. Because the velocity of the racks is
lowest when they are in the inclined position (i.e., at
the maximum travel point), this provides a convenient
point at which to brake the rotating shaft (and, thus,
the racks) without imparting undue stress to the braking
assembly. Likewise, it is also desirable to stop the
racks when they are in their lowermost position (i.e.,
closest to horizontal) to permit the operator to have
ready access to the racks for removal and addition of
culture bottles. Once again, because the velocity of the
racks is also lowest when they are in their lowermost
position (i.e., at substantially the minimum distance of
travel point), this is another convenient point at which
to stop the rotating shaft. It is also desirable to re-
start the shaft rotating from these stopping positions,
since this minimizes the stress on the motor.
Such an arrangement also has the significant
advantage of reducing the cost of the motor and braking
means which can be used in the practice of the invention.
In particular, because the distance the racks travel for
a given angular movement of the shaft is small at
--~094/26874 ~1~ 8 2 ~ ~ PCT~S94/05392
positions near the maximum and minimum distances of
travel of the racks, greater leeway in stopping the
rotation of the shaft is allowed at these points.
Because the rotation need not be stopped to move exacting
s tolerances, relatively inexpensive motors and braking
systems may be used, thus reducing the total cost of the
instrument.
Fig. 14 illustrates an alternative mechanical
arrangement for imparting a substantially sinusoidal
lo pattern of motion to the bottle-holding racks. In this
arrangement, pivotal arm 190 is used to convey the
rotational motion of the cam 176 to the drive support
112b. A first end of the pivotal arm 190 is pivotally
mounted at pivot point 192 to the cam 176 at a location
15 near the circumferential periphery of the cam. A second,
opposite end of the pivotal arm 190 is pivotally mounted
at a pivot point 194 to drive support 112b. Upon
rotation of cam 176 in the direction of the arrow in Fig.
14, a sinusoidal pattern of motion is imparted to the
drive support 112b and, ultimately, the bottle-holding
racks. The position of the pivotal arm 190 after an
approximately 180 rotation of the cam 176 is shown in
phantom in Fig. 14.) It will also be understood that
various other systems for imparting a substantially
2s sinusoidal pattern of motion, such as gear assemblies and
the like, could also be used.
Another important aspect of the present invention is
the optical system for mechanically sensing changes in
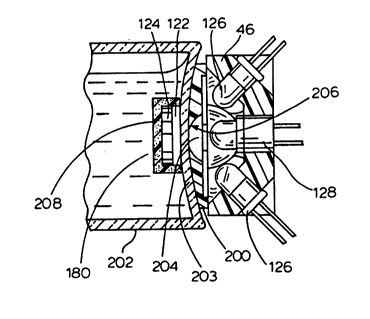
the Co2 sensor. As best seen in Fig. 8, the sensor 100
is affixed to the inside of the bottom wall of the
culture bottle 120. In a preferred form, the sensor is
made in accordance with the disclosures of copending U.S.
W094/26874 PCT~S94/05392
~,~3~
patent application S.N. 238,710, filed August 31, 1988
and/or copending U.S. patent application S.N. 609,278,
filed November 5, 1990, both of which are entitled
"Measurement of Color Reactions by Monitoring a Change of
5 Fluorescence," are assigned to the owner of the present
application, and which are incorporated herein by
reference and made a part hereof.
As disclosed in U.S. patent application S.N. 609,278,
the sensor preferably comprises a chromophore layer 122,
o which consists of a pH sensitive chromophore encapsulated
within a gas permeable, hydrogen-ion impermeable matrix,
such as silicone. Adjacent the chromophore layer 122 is
the fluorophore layer 124. The fluorophore layer 124
consists of a fluorescent dye encapsulated within a water
15 and gas impermeable polymer, such as an acrylic polymer.
The fluorophore layer 124 is preferably positioned above
the chromophore layer 122 when the bottle 120 is in an
upright position. When placed within an aperture in the
bottle-handling rack (see Fig. 7), the chromophore layer
20 122 is thereby situated or sandwiched between the optical
unit 46 and the fluorophore layer 124. In the form
illustrated in Fig. 8, the fluorophore layer 124 has a
plurality of radial cut-outs 121, which extend from a
position near the center of the fluorophore layer 124 to
25 its periphery. (These cut-outs 121 give the fluorophore
layer 124 an appearance similar to that of a "starfish"
when viewed from above.) The cut-outs 121 expose more of
the surface area of the chromophore layer 122 to the
liquid within the bottle, thereby permitting C02
generated by microorganisms within the bottle to diffuse
to the chromophore layer 122 more rapidly.
-rO94/26874 PCT~S94/05392
~13~2~
39
Figs. 7 and 8 illustrate the optical unit 46 in
detail. The unit includes at least one, and preferably
more than one, light emission means in the form-of a
light source. A plurality of light sources is preferred,
s since this helps to ensure excitation light impinges on
the area of the bottle where the sensor is located, even
when there are variations in the positioning of the
sensor on the culture bottle. In Figs. 7 and 8, four
light emitting diodes (LEDs) 126 serve as the light
o sources. As best seen in Fig. 8, each LED 126 has a
plastic lens 127 which defines the cone of light emitted
by the LED 126. It will be understood that it is
desirable to have as much light as possible directed to
the area of the bottle 120 where the sensor 100 is
located; the plastic lenses 127 assist in directing the
cone of light emitted by the LED to the vicinity of the
sensor and in minimizing stray light. Success has been
had with LEDs manufactured by Marktech International of
Menands, New York, bearing the designation MT 350 AK-UG.
These LEDs have an ultra-bright GaP green light emission
and use a T-l 3/4 water clear lens. According to the
manufacturer, these LEDs have the following maximum
ratings (Ta = 25 C): forward current, 25 mA; reverse
voltage, 5 V, power dissipation, 105 mW; peak pulse
2s current, 150 mA; operative temperature range, -50 to
approximately 100 C; storage temperature range, -50 to
approximately 100 C. According to the manufacturer,
these LEDs also have the following ele~Llo optical
characteristics (Ta = 25 C): forward voltage, typical
(2.2 V), maximum (2.5 V); reverse current, maximum (10
~A); luminous intensity, minimum (100/200 mod), maximum
(200/300 mod); peak wavelength, typical (565 nm); viewing
WOg4/26874 PCT~S94/05392
~ 1 3 8 2 5 4
angle, typical (30); spectral line half-width, typical
(30 nm)-
The LEDs 126 are positioned around a centrally-
located photodetector module 128, which is described in
greater detail below. The LEDs 126 are positioned so
that they fully illuminate the sensor 100 affixed to the
inside bottom of a culture bottle 120 placed in an
aperture 38. Preferably, the LEDs are also held within a
housing 130, which can be molded of a suitable plastic or
lo made by other conventional means.
The operation of the optical system is best
understood by reference to Fig. 8. LEDs 126 are selected
so that they emit light falling within an emission
wavelength range and, preferably, a generally
monochromatic light falling within a wavelength range
which will excite the fluorophore in the fluorophore
layer 124. For example, the commercially available LEDs
identified above emit a generally monochromatic light
having a peak wavelength of 565 nm and a spectral line
half width of about 30 nm. Light having these
characteristics is well-suited to excite the fluorophores
oxazine 1,7,0-perchlorate and oxazine 4-perchlorate,
which are preferred fluorophores in the practice of the
present invention.
Light from the LEDs impinges on the specimen bottle
(and, after passing through the bottle, on the sensor)
and excites the fluorophore encapsulated within the
fluorophore layer 124, causing it to fluoresce, i.e.,
emit radiation as it passes from a higher to a lower
electronic state. Light to be detected emanates from the
fluorophore within the specimen bottle. This sensor
emission light emanating from the sensor has different
~094/26874 PCT~S94/05392
82~
spectral characteristics from the excitation light, i.e.,
it has a different peak wavelength. Preferred
fluorophores emit light at peak wavelengths of
approximately 580-650 nm.
Any microorganisms cultured in the media 132 within
the bottle 120 produce C02, which diffuses into the gas
permeable chromophore layer 122, thereby causing a change
in pH within the chromophore layer 122. This pH change,
in turn, causes a change in the absorption spectrum of
lo the chromophore. Significant growth of microorganisms
results in additional production of CO2, which causes a
further change in the absorption of the chromophore. As
disclosed in copending U.S. patent application S.N.
609,278, the chromophore is preferably selected so that
15 its absorption spectrum overlaps with the excitation and
the emission spectrum of the fluorophore in the
fluorophore layer 124. In this way, changes in the
absorption spectrum of the chromophore -- which are
triggered by microbial growth -- will modulate (in a
preferred form, attenuate) the excitation light reaching
the fluorophore as well as the sensor light emitted from
the fluorophore. This attenuation in both the excitation
light reaching the fluorophore and the emission light
emanating from the fluorophore is measurable and can be
25 monitored by the optical module 128. The result is that
the growth of microorganisms within the bottle 120 can be
correlated to a measurable attenuation of fluorophore
excitation and emission.
A significant feature contributing to the success of
the optical detection system of the present invention is
the unique construction of the optical detection unit
128. The detection unit 128 includes light detection
W094/26874 PCT~S94/05392
~ 25 ~ 42
means for converting light energy emanating from the
sensor within the specimen bottle into a detectable
signal. In a preferred form, the light detection means
takes the form of a photodetector (not shown), which
5 converts light energy into an electric current. Success
has been had with a photodiode made by United Detector
Technology of Hawthorn, California, bearing the
designation HDT 455. The current generated by the
photodetector is transformed into a voltage by means of a
conventional transimpedance amplifier. The voltage,
which can be correlated to the amount of bacterial growth
in the bottle, is then measured by well-known means.
Significantly, the detection unit 128 is also
equipped with filter means optically interposed between
15 the LED light sources and the photodetector for
preventing substantially all light falling within the
wavelength range emitted by the LEDs from reaching the
photodetector. The filter means is carefully chosen in
order to achieve substantial isolation between the
20 spectrum of the excitation light and the emission
spectrum of the fluorophore. This spectral isolation is
best understood by reference to Fig. 13, which is a graph
showing schematically both the spectrum of the light
emitted by the LEDs and the emission spectrum of the
25 fluorophore. It will be seen that the spectrum of the
light emitted by the LEDs has a lower peak wavelength
than the sensor light emitted by the fluorophore
wavelength. However, because both the excitation light
and the emission light have a bandwidth, there is some
overlap of the spectra. This area of overlap, greatly
exaggerated, is represented by the single-hatched area in
Fig. 13. The filter means is chosen so that the
~094n6874 PCT~S94/05392
-- ~1382~'1
43
- photodetector receives a sufficiently strong fluorescent
signal, but the amount of overlap between the spectrum of
the excitation light and the spectrum of the fluorescence
emission light is minimized and, preferably, eliminated
5 altogether. In Fig. 13, only light having a wavelength
falling within the cross-hatched region is permitted to
reach the photodetector; the filter means prevents all
other light from reaching the photodetector. In this
way, an area of fluorophore emission is chosen in which
o the overlap of excitation light and emission light
(represented by the fully shaded region "a" in Fig. 13)
is minimal. It is preferred that the amount of such
overlap be less than about 20% of the total signal, more
preferably, less than about 5%, and still more
15 preferably, between 1 and 2% or less. In general, to
achieve substantial spectral isolation, the difference in
peak wavelength between the excitation light and the
fluorophore emission should be at least about 10-15 nm
and, preferably, 25-80 nm or more. It is also preferred
20 that any small amount of overlap be electronically
"subtracted" from the optical signal so that only
fluorescence emission is measured.
It will be seen that the filter means is selected in
order to substantially prevent light having a wavelength
25 other than that of the light emitted by the f luorophore -
- including substantially all of the excitation light
emitted by the LEDs 126 -- from entering the
photodetector. In this way, the detection system is, to
as great a degree as possible, substantially optically
"blind" to light having a wavelength other than the light
emitted by the fluorescing f luorophore, including the
excitation light emitted by LEDs 126.
W094/26874 PCT~S94/05392
~1382~
In a preferred embodiment, the light filter means
consists of a longpass filter which prevents light having
a wavelength smaller than a particular selected value
from entering the photodetector. When the fluorophore
5 chosen for the fluorophore layer 124 is oxazine 1,7,0-
perchlorate or oxazine 4-perchlorate, the longpass filter
is selected to prevent light having a wavelength of less
than about 645 nm from entering the photodetector. In
this way, light emitted by the fluorophore is detected by
o the photodetector, but light having a wavelength of less
than 645 nm (including substantially all of the
excitation light emitted by the LEDs 126, which has a
peak wavelength of approximately 565 nm) is not. Success
has been had with a glass longpass filter manufactured by
15 Schott Glass of Duryea, Pennsylvania, which bears the
designation RG 645. This filter can be attached to the
commercially available photodiode described above to form
an integrated unit having the optical characteristics for
use in practicing the present invention.
Such an optical arrangement has significant
advantages for use in a detection system for
microorganisms. This optical arrangement permits
substantially complete optical isolation between the
excitation light and the light emitted by the
2s fluorophore, thus significantly reducing background
noise. Comparable systems which rely on directly
monitoring monochromatic light transmitted by the sensor
have significantly more noise because light from the
light source can reflect off of other optical surfaces in
the instrument (including the bottom of the bottle) and
reach the optical system directly. In direct contrast,
because the detection system of the present invention is
'~094/26874 2 13 ~ 2 ~ 4 PCT~S94/05392
substantially optically "blind" to the excitation light,
the fluorophore can be inundated with excitation light,
thereby producing an exceptionally strong fluorescence
signal, without substantially increasing the noise
5 affecting the detection system. This helps to improve
the sensitivity of the system.
It should be pointed out that because an optical unit
is provided for each bottle-receiving opening, problems
encountered in calibrating a single detection unit are
o greatly reduced. This is because each unit is
effectively "self-calibrating" in that variables
affecting the signal generated can be read before
readings are taken and then "subtracted" from the signal
as successive readings are taken.
Although a particular optical arrangement has been
disclosed herein, it will be understood that other
arrangements may be employed, so long as the detector is
rendered substantially "blind" to light other than that
emitted by the fluorophore. For example, the location
and geometry of the exciting light source relative to the
photodetector could be changed to prevent substantially
all excitation light from reaching the photodetector.
Similarly, other types of optical devices and filters,
including interference filters and filters made from
materials other than glass, could also be used.
In particular, in adapting the optical system
described above to use with commercial grade specimen
culture bottles, it was found to be necessary to improve
the overall sensitivity of the system even further. This
was due to the fact that the bottom wall of a commercial
grade bottle is generally convex, so that a sensor placed
on the bottom wall is located a greater distance from the
W094/26874 PCT~S94105392
C~i38æ5~
46
optical system than in more conventional bottles, in
which the bottom wall is relatively flat. Because the
fluorescence signal decreases as the sensor is placed
farther away from the detection system, previously
5 acceptable levels of optical "noise" in the system became
unacceptable when the preferred commercial grade bottles
were used. In addition, when hemolytic organisms were
cultured within the bottle, a "blood blip", or sudden
decrease in the fluorescence signal, created additional
lo noise, thereby further reducing sensitivity.
As best seen in Figs. 15A and l5B, it was
unexpectedly discovered that the level of optical noise
in the system could be significantly reduced by more
completely isolating the detection system and sensor from
15 external sources of light, such as light from the ambient
environment and light from the patient sample and culture
medium. This reduction in noise permitted the use of
sturdier, but less optically consistent and desirable,
commercial glass culture bottles. It is believed that
such a system may also permit the use of polymeric
culture bottles, which are even sturdier and less costly
than commercial glass bottles.
Referring now to Fig. 15A, a preferred form of the
improved optical system of the present invention is
2s illustrated. It will be seen that the optical unit ~6 is
identical to that described above, except that a
specially designed spatial filter 200 is interposed
between the optical unit 46 and the culture bottle 202.
Note that the bottle 202 illustrated in Fig. 15A has a
convex bottom wall 203 and that the sensor 100, being
attached to this bottom wall, is therefore positioned
'~0 94/26874 ~13 ~ 2 5 4 PCT/US94/05392
47
somewhat further away from the optical unit 46 than when
a conventional culture bottle is used.
The spatial filter 200 has an aperture 204 formed in
the center thereof and is preferably made of a light-
5 absorbent material, such as a molded, black polymericmaterial. However, it will be understood that other
light blocking materials may be used, as long as the
spatial filter substantially blocks the passage of light
into the optical unit except through the aperture 204.
lo It is preferred that the filter 200 be made of a material
which absorbs as much light as possible so that it
performs the function of preventing light from external
sources from reaching the optical unit 46 and, in
particular the photodetector 128. In this way,
15 excitation light emanating from the LEDs 126 is
preferably directed only onto the sensor and, still more
preferably, on a selected portion of the sensor. As
shown in Fig. 15A, the wall 206 which defines the
aperture 204 is arcuate or tapered to help concentrate as
20 much of the excitation light as possible onto the sensor.
As described in greater detail below, in addition to
focusing the excitation light onto the sensor, the
spatial filter 200 also helps to substantially prevent
light other than light from the sensor 100 -- such as
25 ambient light and light from the bottle media and sample
-- from reaching the optical detection system and, in
particular, the photodetector 128.
Referring now to Fig. 15B, the spatial filter 200 is
illustrated in greater detail. As noted above, the
30 spatial filter 200 is preferably molded from a light-
absorbent polymeric material, such as black Delrin0. In
addition, the upper surface of the spatial filter 200 is
W094/26874 PCT~S94/05392
~1~825~
48
preferably tapered and contoured to generally conform to
the shape of the outside surface of the bottom wall of a
specimen culture bottle. However, it will be understood
that the filter may be made of any number of materials
5 and may be formed in many conventional ways, as long as
it has the effect of creating an optical aperture
- directed solely at the bottle sensor or, preferably, a
selected portion thereof. For example, the spatial
filter could be formed or extruded in many different
o shapes with any number of different aperture shapes and
configurations. The filter could also be made of a
material which selectively absorbs certain wavelengths of
light most associated with noise in the system or which
otherwise prevents selected light from impinging on the
15 optical detection system.
In a preferred form of the invention, the spatial
filter 200 is equipped with a plurality of downwardly
extending tabs 208a, 208b, 208c spaced around its
periphery. In assembling the spatial filter 200 to the
optical unit 46, these tabs 208a, 208b, 208c are inserted
into corresponding recesses 210a, 210b, 210c in the
housing of the optical unit 46. (A fourth tab and
corresponding recess are not visible i-n Fig. 15B.) The
spatial filter 200 is then welded to the polymeric
2s housing of the optical module 46 by melting together the
polymeric tabs and the polymeric material surrounding the
recesses. In this way, the spatial filter 200 is welded
to, and becomes integral with, the optical module. It
will be understood, however, that the spatial filter may
be molded or formed integrally with the polymeric housing
of the optical module or may be attached to the module by
~o 94,26874 ~ 1 3 8 2 5 ~ PCT~S94/05392
other means, such as by using adhesives or mechanical
locking arrangements.
In addition to the spatial filter, the improved
optical system of the present invention includes a
5 further aspect. Referring again to Fig. 15A, an
important additional feature is the addition of a light-
blocking third layer to the sensor 100. (This third
layer is illustrated by the reference numeral 208 in Fig.
15A.) The purpose of the third layer 208 is to
lo substantially optically isolate the fluorophore layer 124
and the chromophore layer 122 of the sensor 100 from
external sources of light other than excitation light
from the LEDs 126 which passes through the aperture 204
of the spatial filter 200. These external sources of
light include, for example, light from the ambient
environment and, importantly, light emanating or
reflected from the sample and media in the bottle.
In a preferred form of the invention, the third layer
208 is made of a light-absorbent material which is gas-
permeable so that carbon dioxide generated bymicroorganisms within the bottle can diffuse through the
third layer 208 and, eventually, to the chromophore layer
122. Success has been had with a third layer made of
silicone into which a fine powder of activated carbon
(carbon black) has been uniformly dispersed. The
activated carbon makes the third layer black in color and
light-absorbent, in order to give it the desired optical
properties needed to prevent external light from entering
the other layers of the sensor. It will be understood,
however, that a variety of materials could be used in the
manufacture of the third layer as long as the layer
substantially prevents external light from impinging upon
W094/26874 PCT~S94/05392
~138`~4
the other layers of the sensor, while still permitting
metabolic products produced by microorganisms in the
specimen, such as carbon dioxide, to diffuse through the
third layer and, ultimately, into the chromophore layer.
5 For example, a wide variety of light-absorbent materials
could also be dispersed in silicone, including charcoal
substitutes, powdered obsidian, or other inert, dark-
colored materials, so long as the material does not
interfere to a great extent with the diffusion of carbon
o dioxide to the chromophore layer. Similarly, other gas
permeable, proton impermeable materials could also be
used to encapsulate the light-absorbent material.
As illustrated in Fig. 15A, the third layer 208
substantially envelopes or covers the outer exposed
15 surfaces of the sensor 100, that is, all surfaces other
than the portion affixed to the inside surface of the
bottom wall 203 of the bottle 202 through which the
sensor is optically interrogated. In this way, a light-
absorbent optical barrier substantially surrounds all
20 surfaces of the sensor except the bottom surface through
which excitation light passes.
It will be readily understood that the third layer
208 of the sensor 100 and the spatial filter 200
cooperate to form a light blocking means or light barrier
25 which helps to prevent a substantial portion of external
light, including light from the outside environment and
light emanating or reflecting from the interior of the
bottle, from reaching the optical unit and, particularly,
the photodetector. In this way, it is possible to
substantially isolate the detection system from
interfering light from sources other than the fluorescent
~094/26874 PCT~S94/05392
~138~5~
emission of the fluorophore layer 124, as modulated by
the chromophore layer 122.
Of course, it will be understood that the third layer
208 could also be expanded so that it covered the entire
inside bottom surface of the bottle, thereby also
performing the function of the spatial filter 200.
However, it is generally preferable to use a mechanical
filtering means like the spatial filter, since it is
relatively easy to include a mechanical structure within
the apparatus and to mold the third layer as part of the
sensor prior to insertion in the bottle. On the other
hand, adding material to the inside surfaces of the
bottle often becomes difficult and costly.
In making the three-layer sensor of the present
invention, it will be understood that in general the
methods set forth in prior applications S.N. 07/638,481
(particularly those set forth in Examples 2 and 3
thereof) and 07/609,278 (now U.S. Patent No. 5,173,434)
may be used, with the addition of the third light-
absorbent coating layer described above. The third layeris made by uniformly dispersing activated carbon into
silicone elastomer and catalyst, degassing the resultant
mixture by exposing it to vacuum, and then coating the
appropriate surfaces of the two sensor layers with the
mixture. The three-layer sensor is then inserted into
the bottle and affixed to the bottom wall thereof. (It
will be understood that the sensor may be formed with a
protrusion or knob on its top surface so that automated
or semi-automated insertion apparatus can "grip" the knob
to facilitate insertion into the bottle.)
W094/26874 PCT~S94/05392
213g~5g
The following example further illustrates the manner
in which the three layer sensor of the present invention
can be made and inserted into the specimen bottle:
E~AMP~E -- THREE-LAYER BEN80R MANUFACTURE
The sensor is preferably comprised of three layers;
the fluorophore layer, the chromophore layer, and the
light blocking, "black" third layer. The fluorophore
layer is made using an opthalmic grade of clear
lo polycarbonate plastic (GE OQ21), which is combined with a
laser dye (Eastman Kodak, Oxazine 4 Perchlorate). In the
combination process, the dye is dissolved in an organic
solvent and the feedstock plastic pellets are uniformly
coated with the dye solution. Following the coating of
15 the pellets, the plastic is melted, mixed, and reextruded
to form feedstock. The resultant material is molded by
melting it and then injecting it at high pressure into an
eight-cavity mold. Since the fluorescent dye has been
found to be highly sensitive to heat, extremely close
control of time/temperature profile of the material must
be maintained. The mold is shaped to form the material
into the "starfish"-shaped layers described above. In
addition, as described in additional detail below, the
mold may be designed to form a "knob," which protrudes
25 from the center of the fluorophore layer.
The chromophore layer is a gas permeable silicone
which has a pH sensitive dye mixture incorporated
therein. The dye mixture is designed to change its
absorbance characteristics with respect to passage of
light in the 567 nm to 650 nm ranges. The feedstock
materials for the chromophore layer are silicone (Wacker
SilGel 601), bromothymol blue salt, sodium hydroxide, and
-~094/26874 21~ ~ ~ 5 ~ PCT~S94/05392
,~ , ,.
53
ethylene glycol. The pH sensitive dye mixture is mixed
with the silicone base material and degassed using a
vacuum chamber. The material is then injection molded
using a Kuntz liquid injection molding machine. In the
5 injection molding process, the mold holds the fluorophore
layer while the absorbance layer is injected and cured,
thereby encapsulating the fluorophore layer. (It will be
understood that if the fluorophore layer is formed with a
protruding knob, as described above, this knob extends
lo down into the chromophore layer and, when the sensor is
affixed to the inside wall of the culture bottle,
protrudes downward so that it is closer to the optical
detection system. Such an arrangement can be useful,
since the protruding knob containing fluorescent dye can
15 be more readily detected by the optical detection system
described herein. This, in turn, can make it easier to
detect when a bottle is inserted into a bottle-receiving
opening.) In the process, the thickness and uniformity
of the absorbance layer on the fluorophore layer is of
20 the utmost importance, as small variations in thickness
will impact both initial optical values and sensitivity
of the system. Current goals are for the chromophore
layer to be .015 inch ~/- .001 inch.
The third layer is molded to the back of the
25 previously described subassembly. This layer is made of
the same silicone used for the absorbance layer doped
with 3~ by weight carbon black. (The resultant material
is also degassed, as described above.) The method of
manufacture follows that of the chromophore layer, except
that a different mold, which accepts the two-layer
subassembly described above, allows the molding of a .030
inch black layer on the surfaces which are not to be
W094/26874 PCT~S94/05392
~13825g
54
affixed to the inside wall of the culture bottle. In
addition, as noted above, a knob or protrusion may be
formed in this black layer to facilitate insertion of the
sensor into the culture bottle. The sensor may be
affixed to the inside bottom wall of the bottle by
conventional means, such as by using silicone as an
adhesive.
Although the improved optical system and sensor
described above are adapted to substantially reduce noise
in the system and thereby improve sensitivity, further
improvements in the processing of the resulting signal
are also possible. Accordingly, Figs. 16 and 17 in
conjunction illustrate another feature of the optical
detection system which is designed to further reduce
noise and thereby improve the sensitivity of detection.
Viewing Fig. 16, it is seen that the instrument 10
includes a control circuit for the optical units 46,
which control circuit is generally referenced with the
numeral 400. This control circuit 400 includes a power
supply 402, which may receive power from a common 110
volt alternating current source, for example. The power
supply 402 provides direct current (DC) voltage and
current via a power line 404 at appropriate levels for
the operation of the LED's 126, as will be explained
further. The line 404 supplies the DC power to a
modulator 406 which provides chopped square-wave DC power
via a branched power line 408, the voltage wave form for
which is depioted as line 410 on the voltage-time graph
of Fig. 17. One branch of the power line 408 ~upplies
the chopped DC power to the LED's 126 of the optical
units 46, so that these LED's produce a light output
signal (indicated with arrow 412 leaving the LED 126 on
~094/26874 213 ~ ~ 5 ~ PCT~S94/05392
"_ ~
Fig. 16) substantially replicating the wave form
indicated by line 410 of Fig. 17.
As will be well understood in view of the explanation
above, the light 412 illuminates the sensors 100, so that
these sensors produce a fluorophoric response to the
incident excitation light 412. This light produced by
the fluorophoric response of the sensors 100 is indicated
on Fig. 16 with the arrow 414. The light represented by
arrow 414 is incident on the photo diodes 128 of the
o optical units 46, so that these photo diodes produce a
voltage response to this incident light. Unfortunately,
the light 414 is not the only light incident on the photo
diodes 128. When the drawers 16, 18 are open, as they
must be for the insertion of additional culture bottles
120, the ambient room light also affects the photo diodes
128. This effect from ambient room lighting may include
natural sun light, but generally will have the wave form
of a sixty-cycle flicker. Also, electronic noise from
various sources in the environment of the instrument 10
may also be present in the output signal from the photo
diodes 128.
The resulting combination output signal from a photo
diode 128 of one of the optical units 46 is indicated on
the graph of Fig. 17 with the voltage-time line 416.
This output signal appears on an output line 418 from the
photo diode 128. Each of the photo diodes 128 has a
separate output line 418 connecting with the
microcomputer 14, as will be further explained. As can
be seen from a comparison of the lines 410 and 416 of
Fig. 17, the output signal 416 from the photo diodes has
a significant component 420 which is attributable to the
WOg4/26874 PCT~S94/05392
~13~2~
56
excitation light 412 from the LED's 126, and has a
frequency like the voltage wave form 410.
Respective output lines 418 from each photo diode 128
connect to a band pass filter 422, which has a passage
5 frequency generally matching the frequency of the signal
410, and the component 420 of signal 416. This band pass
filter removes any direct current component of the signal
416, and provides rough discrimination of the signal
component 420 from other noise components. The filtered
o output from band pass filter 422 is provided via a line
424 to an amplifier 426. This amplifier 426 increases
the level of the signal to a more easily measurable
level, which is provided on a line 428. The voltage wave
form for the signal on line 428 is depicted on Fig. 17
with the line 428'. As can be seen, at this point the
signal has a generally sinusoidal wave form with unequal
positive and negative values, and some rounding of the
wave form by capacitances in the circuits. While not
depicted on Fig. 17, those ordinarily skilled in the
pertinent arts will recognize that the wave form 428'
includes noise components which may be of various forms.
Next, the signal on line 428 is subjected to the
operation of a clocked demodulator 430. The demodulator
430 is clocked in synchronization with the output 410 of
the modulator 406 by a branch connection of the power
line 408. This demodulator 430 has an amplification
function depicted as line 432 of Fig. 17. The
amplification function of demodulator 430 has a negative
one (-1) value while the voltage level of signal 410 is
zero. That is, a negative one for the amplification
function is meant to indicate that the signal is
inverted. When the voltage level of signal 410 raises
--~094/26874 213 ~ 2 ~ ~ PCT~S94/05392
above zero to produce a light output signal from the
LED's 126, the demodulator 430 has an amplification value
of positive one (+1). In this case, with a positive one
amplification factor, the signal is passed through
s substantially without change.
Consequently, the signal 428' is rectified so that
all of the signal becomes positive. Noise signals,
similarly, pass through the demodulator 430 full wave
rectified. That is, the half of the noise signal which
is in phase with the signal portion 420 will continue to
have a positive value, while the other half of the noise
signal will have been inverted by the -1 amplification of
the demodulator 430. Moreover, signals of interest are
rectified such that they make a positive contribution to
an integral. Signals which are not of interest are
rectified such that they cancel upon integration. Thus,
noise signals cancel one another.
Consideration of Fig. 17 will quickly show that the
signal 410 and the amplification value of demodulator
430, as represented by line 432 on Fig. 17, are
synchronized and in phase with one another. That is, the
driving signal to the light emitting diodes 126, and the
amplification level applied to the response signal from
the light responsive photo diodes increase and decrease
in phase with one another. The output of the demodulator
430 is provided by a line 434 to an integrator 436. On
Fig. 17, this demodulator output is represented with line
434'. As can be easily understood, the positive and
negative portions of the noise signal cancel one another
when they are integrated. On the other hand, the signal
- portion 420 when integrated, provides an output voltage
(Vout), indicated with arrow 438 on Fig. 16. This output
W094/26874 PCT~S94/0~392
~13825~
signal is provided to the microcomputer 14. Also, the
output "Vout", from each of the photo diodes 128 is
provided by a similar signal processing channel to the
microcomputer 14 so that this computer can receive the
s fluorophoric response from the sensor 100 in any opening
38 of the racks 20-30.
Having observed the structure of the control circuit
400 depicted in Fig. 16, attention may now be given to
two particularly advantageous methods of operation with
this circuit. As can be easily understood, when the
drawers 16, 18 are closed, and the presence of
microorganisms in media 132 is to be detected, the LED's
126 are illuminated and the responses of the photo diodes
128 is received and analyzed by the microcomputer 14.
S For this operation, a very high level of discrimination
between various levels of response of the fluorophore
124, as well as discrimination of changes in response
levels of the fluorophore 124 as moderated by the
chromophore 122, can be detected using a comparatively
long integration time for each specimen bottle 120. That
is, a three second integration time conducted at the
integrator 436 for each of the specimen bottles 120 in
the racks 20-30 will allow the computer 14 to make very
fine distinctions between levels of response at the
2s sensors 100. Thus, the presence or absence of
microorganism growth in the media 132 is easily
detectable.
On the other hand, with the drawers 16 or 18 open for
the insertion of additional specimen bottles 120 to the
racks 20-30, another mode of operation of the control
circuit 400 has particular advantage. That is, when a
technician is to insert additional specimen bottles 120,
~-'094/26874 PCT~S94/05392
-- 21382~1
the optical units 46 of each of the vacant cylindrical
openings 38 in the racks 20-30 can be rapidly scanned in
sequence by the microcomputer 14 in order to identify the
openings which receive new specimen bottles, and those
5 openings 38 which remain vacant. Thus, the technician
may scan a specimen bottle 120 along channel 162 past the
bar code reader 164 so that the computer 14 is informed
not only of the bar-coded information concerning patient
identification, but is also informed that a new specimen
o bottle is about to be loaded into one of the vacant
openings 38. Under these circumstances, each of the
optical units 46 for the vacant openings 38 is scanned
with an integration time of about ten milliseconds each.
This scan is looking simply for some level of
characteristic response like that represented by portion
420 of line 416 in Fig. 17. Of course, because the
vacant openings 38 do not contain a specimen bottle with
its sensor 100, the vacant openings can be identified by
the absence of the characteristic response from the
associated photo diodes.
When the microcomputer 14 detects such a response at
a previously vacant opening, two items of information are
established for the inventory control portion of the
computer program. First, the computer is informed that
the specimen bottle just scanned by the technician has
been placed in an identified opening 38, and the computer
thereafter identifies the particular patient with any
future results from that specimen. Secondly, the
computer now knows that the previously vacant opening 38
into which the new specimen bottle has been placed is no
longer on the list of vacant openings which need to be
W094/26874 2~ S 4 PCT~S94/05392
scanned to identify where subsequent specimen bottles are
placed by the attending technician.
The result of this second mode of operation for the
control circuit 400 is that a technician attending the
5 instrument 10 need not identify from the keyboard and
screen of the microcomputer where specimen bottles are to
be inserted into the racks 20-30. The technician can
simply scan the specimen bottles along channel 162 past
the bar code reader 164, wait briefly for an
o acknowledgment signal, which may be visual or audible,
and then insert the specimen bottle in any vacant opening
38.
The computer 14 by interface with control circuit 400
will identify the opening into which the new specimen
15 bottle has been placed. With a mere 10 millisecond
integration time being sufficient to discriminate vacant
from newly occupied openings 38, this identification
process can be completed by the computer-so quickly that
even if the racks 20-30 are empty, or nearly so, the
20 technician can simply scan and insert specimen bottles at
a rate of one bottle about every two seconds, or faster
if desired. Consequently, loading the instrument 10 with
specimen bottles is both considerably speeded up by use
of the control circuit in its second mode of operation,
25 and chances of error because of placing specimen bottles
in the wrong openings 38 is virtually eliminated. This
second mode of operation also has a corollary advantage
when specimen bottles are to be removed from the racks
20-30. The newly vacated openings 38 are quickly
identified by the computer and added to the list of
vacant openings to be scanned for newly-inserted specimen
bottles.
~094126874 ~1 3 8 2 ~ ~ PCT~S94/05392
The following is a sample protocol which further
illustrates the manner in which the instrument of the
present invention can be used in detecting the presence
of bacteria in human blood. A detailed description of a
5 software program which may be used to preprogram the
microcomputer to monitor and control the functions
performed by the instrument in this protocol is found in
the Appendix hereto.
~AMPLE INSTRUMENT PROTOCOL
Blood drawn from a patient exhibiting symptoms of
bacteremia is drawn and brought to the hospital
microbiology laboratory, where it is inoculated into a
culture bottle containing media conducive to bacterial
growth and labelled with a bar code containing
15 information linking that sample to the patient. By means
of the user interface on the front of one of the drawers
of the module, the instrument operator initiates a
command to the minicomputer to open the drawer. If the
bottle holding racks are being agitated at that time, a
command is sent to stop agitation when the bottle holding
racks are near their lowermost agitation position.
Alternatively, if optical readings are being-taken at
that time, the readings are completed before the drawer
is opened. The system is preferably preprogrammed so
25 that the agitation, heating, and optical reading
functions are disabled (and cannot be restarted) while
the drawer is open.
The microcomputer then signals activation of the
drawer-opening motor in order to open the drawer. Once
the drawer is opened, the operator draws the bar-code on
- the culture bottle across the V-shaped channel and bar-
W094126874 PCT~S94/05392
21`3&2~
code reader located inside the drawer, and the bar code
information is scanned into the system. This information
is transmitted to the microcomputer, which sends a signal
to the inside information panel prompting the operator to
5 place the bottle in an available bottle-receiving
opening. The operator inserts the bottle into the proper
opening until it "snap-fits" into engagement with the
bottle-retaining means. The control circuit described
above rapidly identifies the opening into which the new
lo specimen bottle has been placed. Thereafter, optical
readings for that bottle are associated with the patient
information which has been scanned into the system.
By means of the temperature control subsystem
illustrated in Fig. 3, the temperature inside the drawers
15 and, thus, the temperature of the bottles, is kept at the
preferred temperature for microbial growth. The
microcomputer signals activation of the fan and heating
elements, as required, in order to maintain that
temperature within specified limits. Periodically, at
regular intervals, the microcomputer signals the
agitation motor to agitate the bottles using the system
illustrated in Figs. 4, 5, and 11. Also at regular
intervals, agitation of the bottle holding racks is
stopped when the bottles are in their uppermost agitation
25 position. While the racks are in this position, optical
readings are taken. The system is capable of
distinguishing between empty openings and openings which
contain bottles by the nature of the optical signal. The
optical readings are transmitted to the microcomputer
where they are associated with the appropriate patient
information and stored for later retrieval and use.
~13 8 2 ~ ~ PCT~S94/05392
If the optical reading for a particular ~pecimen
exceeds a predetermined threshold, the microcomputer
treats that sample as a "positive" and transmits that
information to the instrument. An audible alarm is
5 activated to signal this information to those present in
the laboratory. The microcomputer also sends a command
to illuminate the appropriate LED adjacent that bottle to
identify the positive culture for the operator. That
bottle can then be removed and subcultured so that the
o infecting bacterium can be identified, and an appropriate
treatment regimen (including appropriate anti-microbial
agents) can be prescribed for that patient.
Since the microcomputer is also programmed to store
and manipulate the data pertaining to the specimens, it
15 iS also possible to generate print-outs of the data in
various formats, including tables, graphs, and the like.
While the invention has been described in connection
with certain presently preferred components and
arrangements, those skilled in the art will recognize
many modifications to structure, arrangement, portions,
elements, materials, steps and components which can be
used in the practice of the invention without departing
from the principles thereof.
WO 94/26874 64 PCT/US94/OS392
213~2S4
APPENDlX
Terms Used
The following terms are used throughout this document. They
are presented below for reference.
Boolean
Having two possible states - binary.
Comm~nd
A message, sent from the host PC to the instrument, which
commands the instrument to perform some action, return some data,
set some parameter, etc.
Comm~nd Processor
A software module which accepts commands (from the host or
from other modules) and either acts on those c-omm~nds, or relays
them to other module(s) for action.
Context
A frame of reference. In multi-tasking systems, the context is the
memory (code, data, stack) and processor state (i.e. register contents)
which "belong" to a given task.
Context-switch
Task-switch.
Event
The name of an edge in a state diagram. Events cause states
changes to occur.
Message
A block of information passed between tasks. In this case,
messages are forwarded between tasks by ~X. Commands from the
host and responses to the host are a special class of messages.
213~2S4
'~ro 94/26874 65 PCT/US94/05392
MMX
An operating system software component which relays messages
between tasks.
Module
A logically grouped portion of software. Generally, a module will
be contained within a single compilation unit, and will contain
functions and data which implenet a particular portion of the
requirements for the software system.
Pseudocode
An informal, structured F,ngli.~h representation of program
coding.
Response
A message sent from the instrument to the host which relays the
results of a previous command.
Task
An independent thread of execution. The operating system
provides facilities to make multiple tasks, or threads, appear to
execute simultaneously.
Variable
A piece of data.
WO 94/26874 ~ 13 ~ 66 PCT/US94/05392
Messa~e Passin~ Fundamentals
The Blood Culture Operating System, and MMX in particular,
provide me~h~ni.~mc for transporting messages between independent
tasks. MMX provides what can be described a sa many-to-many
message system. This means that many tasks may send a particular
message, and many tasks may receive a particular message.
Passing messages involves (at least) three steps in MMX: First,
tasks must declare which message they are "interested" in receiving.
To send a message, a task "Posts" the message to MMX, which, in turn,
delivers it to all other tasks which have declared an intent to receive
that message. To receive a message, a task "Pends" for one. The
execution of the task is suspended until a message is available for the
task.
Variations on the basic case outlined above include a "pend with
time-out," which limits the amount of time a task will remain
suspended when waiting. Message may be "held" - reception deferred
until later, while allowing other messages to be passed. Messages
which have been hold must eventually be "released" and received.
Z13~4
'''O 94/26874 67 PCT/US94/05392
S~stem Overview
The Reller Operating System will be composed of the software
components rles~rihed at the Blood Culture System Design Review
held previously. Though the operating system plays a key role in
system operation, it will not be discussed extensively in this document.
Instead, this document will focus on the software component generally
called "the application." VVhile every software component contributes
to the overall functionality of the instrument, it is the application that
p~rform.~ those functions that are most important to the host computer,
and eventually, the user. In short, the application is the component
that makes the Blood Culture instrument be a Blood Culture
instrument.
The application will be composed of seven modules, and ten tasks:
A Coordinator task, responsible for application start-up and time
m~rking; a host communication task, responqih~e for providing a
standard command processor interface and response path for tasks; an
access task which handles the actions necessary for letting users into
drawers; an analog reading task, which allmini~ters the hardware for
analog reading; two bottle reading tasks, responsible for handling the
~iming of and storage of bottle reads; two temperature control tasks,
which control the temperature into drawers; and lastly, two agitation
tasks, which are responsible for the timing and initiation of bottle
agitation within d~awels. The bottle reading, temperature control,
and agitation tasks will be written so the main code body will be
reentrant, with independent data segments.
WO 94/26874 ~ 68 PCT/US94/05392
Global Requirements
* For each bottle, the m~chine must read the sensor in bottle and
store the inform~tion periodically contingent upon the drawer being
open and agitation in progress.
* The m~chine must allow user access to either drawer (but only one
drawer) at any time. The access times and duration must be
stored.
* The m~hine must maintain and record temperature on some
periodic basis.
* M~rhine must report stored data to host computer on request.
* Time of user accesses, and duration of opern drawer will be
recorded.
* Termperature in each drawer will be recorded.
* All bottle readings will be recorded.
Global Assumptions
* The photoboards sent to ICAAC will comprise the photosystem,
with the addition of the lock-in detector.
* We will use the Garrand CPU. 1/0 architecture not .~ignific~ntly
changed from the version as of this date.
* Heaters will be installed in each drawer, and the software will be
expected to control them.
* New motors will be installed to control the agitation and drawer
movement. There will only be crude control of these motors (i.e.
Motor on and direction).
* Optos will be relocated and will privde feedback on: drawer closed,
drawer open. Optionally a third opto might be provided to indicate
that a drawer is parked (closed all the way).
* The functionality of the agitation home optos will be unchanged.
* Communication will be serial RS-232 with a yet-to-be-determined
PC.
~'0 94/26874 ~13 ~ 2 ~ 4 69 PCT/US94/05392
Conventions used throu~hout the document
Most modules are described using the format below:
¦ ---A function name (TaskName)--- ¦
START
Overview of TaskName.
requirements---
Design requirements of TaskName.
assumptions---
Implicit, or practical desig assumptions used during task design.
cornmands supported---
Comm ~n d function name (parameter).ideal flow---
"Structured~ engli.~hevents---
EventName:
[
Pseudocode. -- Comment
]
<Non-MMX generated event>
[
Pseudocode.
]
~Non-event based code block~:
[
WO 94/26874 ~ 3 2 ~ q ~ 70 PCT/US94/05392
Pseudocode.
]
END
---A function name (TaskName)---
In the above example task, comma7lds supported--- indicates
which functions will be provided by the module's command processor.
(The command processor pseudocode is not described in this
document.)
The ideal flow--- section of a module description will describe in
"structured english," the ideal sequence of operations for major
functional requirements of the module.
Lastly, the events--- section describes the functions that each task
will perform when a particular event occurs in the system.
Special code blocks, that are neither command processors, nor
directly related to supporting a system event are indicated by braces.
Please note that where an asterisk ("*") appears in any identifier,
the asterisk may be substituted with either an "R" or an "L". The
asterisk is used for tasks that will run to a specific drawer.
-'~O 94/26874 ?~ 2 .j 4 PCT/US94/0~392
¦ ---Agitation Control (AgCtrl*)--- ¦
START
The agitation control task attempts to agitate the bottles in "its"
drawer as close as possible to the set agitation period.
requirements---
Agitation must
* occur periodically.
* occur for a fixed duration.
* not interrupt a read in progress.
* disallow reading to occur while agitating.
* be of higher initiation priority than bottle reading.
assumptions---
* Agitation period or duration may change.
* ~rking time in five second increments is sufficient temporal
resolution to begin an agitation cycle.
commands supported---
Set agitation period: time (increments of 5 seconds).
Get agitation period.
Set agitation duration: time (increments of 5 seconds).
Get agitation time.
Enable agitation.
Disable agitation.
Force agitation start.
Force agitation stop.
wo 94126874 72 PcT/uss4/05392
~i3~2~4
ideal flow---
Initialization.
For forever do:
After Agitation Period 5-second time intervals pass,
then stop bottle reads in this drawer (ReadInhibit*).
After bottle reads have stopped (ReadInhibited*),
start the agitation motor.
If, while agitating, ~gTnhihit* is received, stop
agitation;
otherwise
continue agitation for AgDuration.
Stop Agitation.
Reset the AgPeriod counter.
End for.
variables---
AgPeriod : Number of FiveSecTicks between agitation
starts.
AgDuration : Number of FiveSecTicks to agitate bottles.
AgitatingFlag : Boolean indicating that agitation isunderway.
~gTnhihit : Integer where non-zero indicates that
agitation should be stopped, and no agitation
should commence.
TimeCounter : Number of Time_tick periods to go until
commencing next agitation phase (on or off).
- O 94/26874 21~ 8 2 5 4 73 PCT/US94/05392
events---
~gTi`,n~hle*:
Reenable agitation.
[
if ~gTnhihited flag <> O then
[
decrement ~gTnhihited flag.
EVENT: Agitation Enabled (DEBUG, time, drawer);
]
]
~gTnhibit*:
Something in the system is going to happen that should preclude
agitation. If we're agitating, we'll stop. We will inhibit any further
agitation.
[
If AgitatingFlag set
then
[
Stop agitating, and home agitator.
Clear AgitatingFlag.
Send ReadEnable*.
Reset PeriodCounter to AgPeriod.
]
EVENT: Agitation Inhibited a~EBUG, time, drawer).
Increment ~FTnhihited flag.
Send ,AETnhibited*~
Pend with no timeout.
]
WO 94/26874 74 PCT/US94/0~392-
;~13~2~4
FiveSecTick:
A standard system time interval has expired. We need to see if
it's time to start an agitation cycle.
[
if TimeCounter c~ O then
decrement TimeCounter.
if TimeCounter = O then
if AgitatingFlag set then
[
Stop Agitation, and home agitator.
Send ReadEnable*.
Reload TimeCounter with (AgPeriod-AgDuration).
]
else
[
Set AgitatingFlag.
Send ReadInhibit*.
]
]
ReadInhibited*:
If ReadInhibited* was received, we sent a ReadInhibit* message
to Bottle*. That means that we intended to commence agitation, and
now upon receiving this message, have gotten the go-ahead to do so.
[
If AgitatingFlag --Did an .AgTnhihit* sneak in?
then --Nope. It's cool to go ahead.
[
EVENT: Agitation Started (EVENT, time, drawer).
Start Agitation.
Reload TimeCounter with AgDuration.
]
]
END
¦ ---Agitation Control (AgCtrl*)--- ¦
' 'O 94/26874 ~ ~ 8 2 ~ ~ PCT/US94/05392
¦ ---Temperature Control (TempCtrl*)--- ¦
START
Maintain the temperature in the drawer.
requirernents---
* Temperature must be controlled in drawer with .1 degree C
accuracy. (This is not addressed in this document.)
* Heater duty cycle must be adjusted periodically.
* Time/Drawer stamped temperature readings must be stored
periodically.
* Temperature control will be disabled from time to time.
Assumptions---
* The control algorithm will be able to time-normalize both
readings, and control information.
* The time needed to obtain a temperature reading is
dependent upon the duration needed for a slow lock-in.
commands supported---
Set temperature target: target
Get temperature target: target
Get current temperature. (Both drawers reported.)
Enable heating.
Disable heating.
Fetch next temperature readings: number
Fetch temperature reading count.
ideal flow---
At every one second interval, attempt to request a temperature
reading from this drawer.
Wait for the analog reader task to respond.
When the analog reader task responds, compute the new heater
duty cycle, and communicate it to the heater ISR.
If we have taken no readings, store the temperature in a buffer.
WO 94/26874 76 PCT/US94/05392
uariables---
SkipCounter : number of temperature readings to skip before placing
reading into circular buffer
HeatPeriod : Total control period of drawer heater
DutyCycle : Current intended duty cycle of heater
TickCounter : Number of main clock ticks to remain in present
heater state
HeatDisable : non-zero means that the heater will not be activated
-'1 94/26874 ~? 1 3 ~ 2 5 ~ 77 PCT/US94/05392
events---
OneSecTick:
We will try to read the temperature every second.
If TempInProgress not set then send ReadAnalog (temperature*).
*DrawerChannelRead:
A temperature read has completed.
Decrement SkipCounter.
If SkipCounter=0 then
store temperature reading, time, drawer in bufEer.
reset SkipCounter.
Compute new heater duty cycle.
Update DutyCycle.
DrawerOpen*:
Set HeatDisable ~ag.
Turn offheater.
DrawerClose*:
Clear HeatDisable flag.
WO 94/26874 ~ 1 3 ~ 2 5 4 78 PCT/US94/05392
SR, tied into main VRTX clock ISR}:
If HeatDisable flag not set then
Decrement TickCounter.
If TickCounter = O then
heater is on then
Turn of ~ heater.
Reset TickCounter with (HeatPeriod-DutyCycle).
else
Turn on heater.
Reset TickCounter with DutyCycle.
]
END
¦ ---Temperature Control (TempCtrl*)--- ¦
94/26874 ~ 82~i PCT/US94/05392
¦ ---Bottle Reader (Bottle*)--- ¦
START
Read bottles in drawer.
requirements---
* For each bottle reads must occur periodically.
* Bottle readings will be disabled from time to time.
* Time/Cell stamped readings will be stored.
? The read will be stored in a mystery format that m~ximi7~esthe amount of time the system can operate without
offloading data to a host computer.
assumptions---
* calibration (cell standardization) will be a manual process.
* The bottle reader task will be told which bottles to read via a
host comm~nd.
* Reading norm~li7.~tion will occur on the host.
operating assumption---
* Uncalibrated cells will not be assigned.
commands supported---
Enable a cell (Cell number).
Disable a cell (Cell number).
Get number of readings.
Get next readings (number).ideal flow---
At each five second interval, a table of active bottles is checked.
If an active bottle is encounted, it is checked to determine if the
read period has expired for it.
If a read is required a read is requested from the analog reader
task.
Wait for the analog reader to respond.
When the analog reader task responds with the reading, the
reading is stored in a circular buffer.
WO 94/26874 80 PCT/US94/0~392
~38~
variables---
ReadInProgress : Flag that indicates that a bottle read is
underway.
NoReads : Flag that indicates that no readings should be
started.
Bottle : Array of (number of bottles in drawer) of records.
Active : Flag indicating that this cell should be sampled
regularly.
PeriodCount : Number of time periods remaining until next
read.
events---
ReadIn_ibit*:
[
Set NoReads flag.
If ReadInProgress flag not set then
Send Rea-lTnhihited*.
]
ReadEnable*:
[
Clear NoReads flag.
]
BottleRead*:
[
Store time, cell, bottle reading, in circular buffer.
Reset Bottle[cell] PeriodCount to ReadPeriod.
If NoReads flag set then
send Rear1Tnhihited*.
]
~13~2~
'~0 94/26874 81 PCT/US94/05392
FiveSecTick:
[
index = lowest bottle number (this drawer).
repeat
with Bottle[index] do
if (Active flag set) and PeriodCount non-zero then
decrement PeriodCount.
if (BottlePeriod = O) and (NoReads ~lag not set) then
[
Set ReadInProgress flag.
Send ReadBottle*(index).
]
]
increment index.
] until (index = (highest bottle number (this drawer)+1) or
(ReadInProgress flag set).
]
END
¦ ---Bottle Reader (Bottle*)--- ¦
wo 94126874 82 PCT/US94/05392
5 ~ -
Analog Reader (AnaRead)--- ¦
START
Perform all the operations necessary to perform analog readings.
requirements---
* The availability of the analog read circuitry must be
m~ximi7.ed.
* No operations concerning the any analog measurement must
be allowed to interfere with one another.
assumptions---
* There is one VCO. VCO measurement may be initiated atany time.
* There are two drawers. Only one analog measurement may
occur in a particular drawer at a time.
* There is only one slow lock-in amp. Performing an analog
measurement with the lock-in amp takes a long time.
* Analog readings from a drawer will use the lock-in detect or
not.
cornmarlds supported---
read a cell (Cell number, drawer number). --uses slow lock-in
amp
read a drawer channel (cell number, drawer).--measures DC only.
read a drawer channel with gain (cell num, drawer, gain) --
DC*gain
read a MUX channel. --read a Mux
channel
set X switch (direction).
set gain (gain).
set mux (mux).
read VCO (time).
read full VCO. --returns time to fill VCO counter.
--'O 94/26874 ~ 1 ~ 8 ~ ~ ~ 83 PCT/US94/05392
ideal flow---
For a VCO read:
MUX is selected.
Wait for DC settle time.
Set up VCO timer counter.
Integrate.
After integration, read VCO counter.
Provide VCO reading with 2.5 and 0.0 volt references.
For a drawer channel read:
Drawer channel is selected.
MUX is switched to appropriate drawer.
Wait for DC settle time.
Set up VCO timer counter.
Integrate.
After integration, read VCO counter.
Provide VCO reading with 2.5 and 0.0 volt references.
For a bottle read:
Drawer channel is selected.
Gain is set.
X-Switch is set appropriately (for slow lock-in detect).
Wait for slow lock-in.
MUX is switched to slow lock-in.
Wait for DC settle time.
Set up VCO timer counter.
Integrate.
After integration, read VCO counter.
Provide VCO reading with 2.5 and 0.0 volt references.
WO 94/26874 84 PCT/US94/05392
2 5 ~
variables---
Delay : Number of VRTXticks to delay.
DelayInProgress : A delay is in progress. A better name would be
'Lo~kTnTnProgress'.
Lasttime : The last time we started a pend (in VRTXtime)
~Pend routine~
In this task, there is a single pend point which might pend with a
time-out, depending on whether a lock-in is in progress. If a lock-in
has been started, we want to be able to service non-competing
requests, so we pend for them here. If a request is received, we'll
service it, then return here, determine the rem~ining time to lock-in,
and pend with a timeout with that value.
[
If DelayInProgress flag set then
[
Set Delay to ( (current VRTX ticktime) - Lasttime )
If Delay < O then
[
Reset DelayInProgress flag.
Reset Delay to 0.
go execute <time-ouP.
]
else if Delay <> O
[
Set DelayInProgress flag.
]
-- ~ 94/26874 ~ 1 85 PCT/US94/05392
If Delay <> O then
[
Save current VRTX ticktime as LastTime.
Pend with timeout Delay.
]
else
Pend with no timeout.
- - Pend takes place.
If pend timed-out then
[
Reset DelayInProgress flag.
Reset Delay to 0.
go execute ~time-out>.
]
else
go execute appropriate event.
]
euents---
<Pend Time-out>:
[
Set MUX to slow-lock-in channel.
Delay for minimum 5ms.
Set up VCO counters.
Take VCO reading.
send [ResponseTypel (vco reading, cal values).
-~13 8 2 ~ 4 8G PCT/US94/05392
If ResponseType is BottleReadL then
[
Release LeftDrawer message class.
Release BottleReadL.
]
else
[
Release RightDrawer message class.
Release BottleReadR.
]
]
ReadBottleL(bottle):
[
Holdoff ReadLDrawerCh.
Holdoff ReadBottleR.
Select bottle within left drawer.
Set X-switch for slow lock-in on left drawer.
Select gain.
Set Delay to SlowLockTime.
Set ResponseType to BottleReadL.
]
ReadBottleR(bottle):
-
Holdoff ReadRDrawerCh.Holdoff ReadBottleL.
Select bottle within right drawer.
Set X-switch for slow lock-in on right drawer.
Select gain.
Set Delay to SlowLockTime.
Set ResponseType to BottleReadR.
]
--~ 94/26874 87 PCT/US94/05392
_ ~1382~4
ReadRDrawerCh(channel):
[
Select channel within right drawer.
Set MUX to right drawer channel.
Delay for minimum 5ms.
Set up VCO counters.
Take VCO reading.
send RDrawerChRead(vco reading).
]
ReadLDrawerCh(channel):
[
Select channel within left drawer.
Set MUX to left drawer channel.
Delay for minimum 5ms.
Set up VCO counters.
Take VCO reading.
send LDrawerChRead(vco reading).
]
ReadAnalog(channel):
[
Set MUX to channel.
Delay for minimum 5ms.
Set up VCO counters.
Take VCO reading.
send AnalogRead(vco reading, cal values).
]
END
¦ ---Analog Reader (AnaRead)~
WO 94/26874 88 PCT/US94/05392 `-
~1382~
¦ ---Access Control (Access)--- ¦
START
Design yet to be recorded.
The access task is responsible for prim~ly two major operations:
(1) Notifying the bottle reader tasks, and agitation tasks that a
particular drawer is going to be opened, and (2) handling all
appropriate drawer parking and movement.
END
¦ ---Access Control (Access)--- ¦