Note: Descriptions are shown in the official language in which they were submitted.
2139167
_
The present invention relates to an electrode used
in an electrochemical reaction and a fuel cell using
this electrode.
The background of the present invention will be
described with reference to a phosphoric acid fuel cell.
In the phosphoric acid fuel cell, two electrodes, i.e.,
a flat cathode at which oxygen reacts and a flat anode
at which hydrogen reacts oppose each other through an
electrolyte layer. Each electrode has a two-layered
structure consisting of a porous diffusion layer
consisting of carbon fibers and having a thickness of
about 1 to 2 mm and a porous catalyst layer formed on a
surface thereof, which faces the electrolyte layer, and
having a thickness of about 0.1 to 0.5 mm. A gas
passage is formed in the diffusion layer or a separator
adjacent to the diffusion layer. A reaction in the fuel
cell takes place particularly in the catalyst layer.
When the cathode and anode of the phosphoric acid
fuel cell are connected through an external circuit,
reactions represented by formulas (l) and (2) below
proceed in the cathode and anode, respectively.
Hydrogen ions H+ and electrons e- produced from hydrogen
H2 in the anode in accordance with formula (2) reach the
cathode through the electrolyte layer and the external
circuit, respectively, and react with oxygen ~2 to
produce water H2O. In this case, a flow of electrons
through the external circuit, i.e., a work of a current
213gl67
brings on energy extraction.
~2 + 4H+ + 4e- ~ 2H2O ... (1)
H2 ~ 2H+ + 2e- ... (2)
In these formulas, hydrogen ions H+ and electrons
e~ are present in an electrolyte and a solid substance,
respectively. For this reason, the reaction field is in
the solid/liquid interface. The reaction speed varies
depending on the types of solid substances. To obtain a
practical reaction speed, a catalyst mainly consisting
of a noble metal is generally required. In the
phosphoric acid fuel cell, platinum or an alloy contain-
ing platinum is very popular as the catalyst. To
increase the reaction area per volume, platinum or an
alloy containing platinum is used such that it is
granulated into a fine powder whose particle has a size
of several nm to several tens of nm, and the fine powder
is dispersed and carried on the surfaces of carbon
powder particles in practical applications. More
specifically, the reaction field is the interface
between the liquid electrolyte and the fine catalyst
powder in the catalyst layer, and the catalyst must be
wet with the liquid electrolyte.
~2 as an oxidant (reactant) appearing in
formula (1) and H2 as a fuel (reactant) appearing in
formula (2) are diffused as gases, dissolved in the
liquid electrolyte, and diffused to the surface of the
catalyst in the liquid. The diffusion rates of these
213gl67
substances in a liquid are much lower than those in a
gas. For this reason, to attain a quick reaction, the
diffusion distance in the liquid must be minimized.
To satisfy this requirement, there is proposed a
method of mixing polytetrafluoroethylene ( PTFE) powder,
which is water-repellent, and a carbon powder, which
carries a dispersed hydrophilic catalyst, and using the
resultant mixture as a material constituting the cata-
lyst layer. More specifically, after the catalyst-
carrying carbon powder and the PTFE powder are stirredand mixed, the surface of the diffusion layer is coated
or dusted with the resultant mixture. The resultant
layer is worked with a roller. To improve the disper-
sion degree of the PTFE powder, the worked layer is
heat-treated at a temperature of about 300~C to 390~C,
thereby forming a catalyst layer on the diffusion layer.
According to the concept of this method, although
the surface of the carbon powder particles which carries
the dispersed catalyst becomes wet with the liquid
electrolyte, the oxidant and fuel in a gaseous phase
penetrate into a region in which the PTFE powder
particles are combined or very close to each other, so
that the diffusion distance in the liquid can be
shortened. For this reason, according to the method
described above, to assure a gas passage in the catalyst
layer, the PTFE powder particles must be connected or
very close to each other. However, the connection or
2139167
the like of the PTFE powder particles is a stochastic
phenomenon because the carbon and PTFE powder particles
are almost uniformly distributed by stirring. To
increase the probability of connection or the like
between the PTFE powder particles, PTFE must be used in
a large amount. In this case, some of the catalyst-
carrying carbon powder particles are surrounded and
isolated by or covered with the PTFE powder particles to
increase the resistance to electron conduction or ionic
conduction or to decrease the surface area of the
catalyst, which actually contributes to the reaction.
It is, therefore, difficult to obtain a high output
density in the catalyst layer formed by this method.
Jpn. Pat. Appln. KOKOKU Publication No. 63-19979
discloses a gas-diffusing electrode material consisting
of a porous structure which has entirely continuous fine
pores. The structure comprises fine knots of a PTFE
resin and a large number of fine fibers of the PTFE
resin, which contain no conductive material powder
particle and which extend from the respective knots and
three-dimensionally couple the knots to each other.
According to this structure, the fine knots are
partially in contact with each other or are continuous
with each other. In addition, an electrolyte and/or
water hardly permeate a space constituted by only the
fine fibers of the PTFE resin, thereby providing a gas
diffusion passage therein.
2139167
In the structure proposed by No. 63-19979 set out
above, however, the problems as partial elimination of
the electrolyte necessary for the reaction, coverage of
the catalyst surface with the PTFE particles, and
degradation of electron conductivity cannot be solved
because the PTFE volume inside the fine knots un-
desirably increases. In addition, the moving distances
of ions, electrons, and gases increase due to the three-
dimensional gas diffusion passage, thereby increasing
the resistance.
The present invention has been made to solve the
conventional problems described above, and has as its
object to provide an electrode capable of obtaining a
higher output density than that in a conventional
example in the same amount of catalyst as in the
conventional example, and a fuel cell using this
electrode.
To achieve the above object according to the
present invention, the gas diffusion passage for
reactants (e.g., oxygen and hydrogen in the phosphoric
acid fuel cell) and/or reaction products produced by the
electrochemical reaction of the reactants is clearly
separated from the electrochemical reaction field.
More specifically, according to a first aspect of
the present invention, there is provided an electrode to
be used in an electrochemical reaction while being
arranged between a gas passage for flowing a gas as
2139167
a reactant and an electrolyte layer containing an
electrolyte, comprising:
a diffusion layer comprising a conductive porous
body in contact with the gas passage, for flowing the
gas from the gas passage along a direction of thickness
of the electrode; and
a catalyst layer arranged between the diffusion
layer and the electrolyte layer and in contact
therewith;
wherein the catalyst layer comprises
a plurality of agglomerate portions each comprising
a conductive porous body which extends in the direction
of thickness of the electrode and has pores containing
an electrolyte; and
a plurality of gap portions arranged alternately
with the agglomerate portions, each gap portion
defining a bore extending through the catalyst layer in
the direction of thickness of the electrode, and
including in the bore a plurality of liquid-repellent
fibers which connect two adjacent agglomerate portions.
According to a second aspect of the present
invention, there is provided a fuel cell in which an
electrochemical reaction of a gas as a reactant is used,
comprising:
an electrolyte layer containing an electrolyte;
cathode and anode electrodes facing each other via
the electrolyte layer, one of the electrodes comprising
2139167
an electrode according to the first aspect of the
present invention; and
a gas passage arranged in contact with the one of
the electrodes for flowing the gas.
When the electrode having the above arrangement
according to the present invention is used in an
electrochemical reaction, the gas flow gap portions are
independent of the agglomerate portions in which the
liquid electrolyte is impregnated. The rate of water
repellent in the agglomerate portions is greatly
reduced as compared with that in a conventional example.
As a result, coverage of the catalyst and water
repellency near the catalyst can be reduced, a suffi-
cient interface area between the catalyst and the liquid
electrolyte, which contributes to the reaction can be
assured, and the electron conduction resistance can be
reduced.
As compared with the conventional structure of a
catalyst layer in which a water-repellent material and a
hydrophilic catalyst are present, the gas diffusion
passage can be properly assured because the gap portion
for flowing the gas consists of only a water-repellent
material. In addition, the water-repellent material is
fibrous to enhance the water-repellent effect in a small
volume as compared with a conventional example using a
spherical water-repellent material, thereby improving
the space efficiency in the catalyst layer.
2139167
Both the agglomerate portions and the gap portions
are formed to be linearly perpendicular to the electrode
surface. For this reason, the ions, electrons, and
gases move across the shortest linear distances, thereby
minimizing the resistance during their movement.
According to the electrode of the present
invention, these various effects can provide a higher
output density than a conventional example with the same
amount of catalyst.
This invention can be more fully understood from
the following detailed description when taken in con-
junction with the accompanying drawings, in which:
FIG. 1 is a sectional view showing an electrode
according to the first embodiment of the present
invention;
FIG. 2 is a schematic view of a fuel cell according
to the present invention;
FIG. 3 is an exploded perspective view of a
phosphoric acid fuel cell incorporating the electrode of
the first embodiment;
FIG. 4 is an exploded perspective view of a modifi-
cation of the phosphoric acid fuel cell shown in FIG. 3;
FIG. 5 is a graph showing the results obtained by
calculating the relationship between the catalyst layer
thickness and the fuel cell performance;
FIG. 6 is a graph showing the results obtained by
calculating the relationship between the widths of
213gl67
- 9 -
agglomerate and gap portions and the fuel cell
performance;
FIG. 7 is a graph showing the results obtained by
calculating the relationship between the agglomerate
portion porosity in a state wherein an electrolyte is
removed and the fuel cell performance;
FIG. 8 is a graph showing the results obtained by
calculating the relationship between the gap portion
porosity and the fuel cell performance;
FIG. 9 is a graph showing the current-voltage
characteristics of a conventional fuel cell and the fuel
cell using the electrode of the first embodiment of the
present invention; and
FIG. 10 iS a perspective view showing an electrode
according to the second embodiment of the present
invention.
The preferred embodiments of the present invention
will be described with reference to the accompanying
drawings.
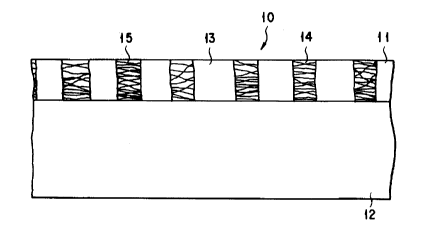
FIG. 1 is a sectional view showing an electrode
according to the first embodiment of the present
invention. FIG. 1 illustrates, on the basis of an
observation result with an electron microscope, an
electrode for a fuel cell as an application of the
electrode of the present invention, e.g., a phosphoric
acid fuel cell ( FIG. 3 or 4) constituted by, e.g., the
diagram shown in FIG. 2. The structure of a cathode
2139167
- 10 -
electrode is identical to that of an anode electrode,
and the electrode according to the present invention
will be described by using the cathode electrode as a
representative.
FIG. 3 is an exploded perspective view of the
phosphoric acid fuel cell incorporating the electrode of
the first embodiment. This cell comprises a flat
cathode electrode 10 and a flat anode electrode 20 which
are arranged to oppose each other through an electrolyte
layer 26. In the phosphoric acid fuel cell, oxygen
reacts at the cathode electrode 10, and hydrogen reacts
at the anode electrode 20. The electrode 10 (20) has a
two-layered structure consisting of a catalyst layer 11
(21) and a diffusion layer 12 (22). Separators 18 and
28 comprising dense carbon plates are formed on the outer
surfaces of the electrodes 10 and 20, respectively. A
plurality of grooves 17 and a plurality of grooves 27
are formed in that surfaces of the diffusion layers 12
and 22, respectively, which face in a reverse direction
to the electrolyte layer 26, thereby forming gas passages
between the diffusion layer 12 and the separator 18 and
between the diffusion layer 22 and the separator 28.
FIG. 4 is an exploded perspective view of a modifi-
cation of the phosphoric acid fuel cell shown in FIG. 3.
This cell comprises the pair of electrodes 10 and 20
arranged to oppose each other through the electrolyte
layer 26, and the pair of separators 18 and 28. The
2139167
electrode 10 (20) comprises the catalyst layer 11 (21)
and the diffusion layer 12 (22) as in the cell shown in
FIG. 3. However, in this modification, the grooves 17
and 27 for forming the gas passages are not formed in
the diffusion layers 12 and 22, but in the separators 18
and 28.
In the embodiment shown in FIG. 1, the catalyst
layer 11 comprises a porous layer having a thickness of
about 0.1 mm (100 ~m). The diffusion layer 12 comprises
a porous layer having a thickness of about 1.6 mm. The
thickness of the catalyst layer 11 preferably falls
within the range of 0.05 to 0.5 mm (50 to 500 ~m), and
more preferably 0.05 to 0. 3 mm.
FIG. 5 is a graph showing the results obtained by
calculating the relationship between the thickness of
the catalyst layer 11 and the fuel cell performance. As
shown in FIG. 5, it is apparent that the cell output is
greatly lowered when the thickness of the catalyst layer
11 is less than 0.05 mm, while the cell output does not
increase if the thickness of the catalyst layer 11
exceeds a certain value. In other words, the cell out-
put is lowered when the thickness of the catalyst layer
11 is less or greater than an optimum thickness range of
the catalyst layer 11 wherein the cell output has a
peak. As shown in FIG. 3, when the grooves 17 are to be
formed in the diffusion layer 12 itself, the thickness
of the diffusion layer 12 preferably falls within the
2139167
-
range of 1 to 2 mm. Alternatively, as shown in FIG. 4,
when the grooves 17 are to be formed in the separator
18, the thickness of the diffusion layer 12 preferably
falls within the range of 0.1 to 1 mm.
The catalyst layer 11 mainly comprises a conductive
porous body whose skeleton consists of carbon as the
major component and a small amount of dispersed
polytetrafluoroethylene ( PTFE). More specifically,
unlike a conventional electrode catalyst layer, the
catalyst layer 11 is constituted by a large number of
agglomerate portions 13 and a large number of gap por-
tions 14 which are alternately and almost parallelly
arranged in the form of stripes. The width of each
stripe of both the portions 13 and 14 is a few ~m, pref-
erably about 1 ~m. Each stripe extends in a direction
perpendicular to the drawing surface of FIG. 1. Note
that the agglomerate portion 13 represents an agglomer-
ate formed by holding an electrolyte in the conductive
porous body and the gap portion 14 represents a portion
connecting two adjacent agglomerate portions 13 by
fibers of polytetrafluoroethylene ( PTFE) and being
essentially free from the electrolyte.
The agglomerate portion 13 has a porous lump or
body wherein fine carbon powder particles on which an
alloy catalyst containing platinum as a major component
is dispersed and carried are combined to each other.
The pores of the agglomerate portion 13 substantially
2139167
communicate with each other. PTFE particles each having
a diameter of about 0.3 ~m are very sparsely present
inside the porous body or lump, and the porous body is
less water-repellent. For this reason, during an
operation, most of the spaces, i.e., pores between the
fine carbon powder particles of the agglomerate portion
are filled with the electrolyte, and consequently the
electrolyte serves to bind the fine carbon powder parti-
cles together. The envelopes of the outer surfaces of
the agglomerate portions 13 are substantially linear in
the direction of thickness of the electrode. In a state
wherein the electrolyte is removed from the agglomerate
portions 13, the agglomerate portions 13 have a porosity
of 50% or more. In this embodiment, the agglomerate
portions 13 have a porosity of 55 to 75%.
The gap portions 14 are formed to be porous by a
large number of PTFE fibers 15 connecting the agglomerate
portions 13 to each other. In this embodiment, the
diameters of the PTFE fibers 15 fall within the range of
about 0.05 ~m to 0.2 ~m. The diameter of each PTFE
fiber 15 may be larger than about 0.2 ~m if it satisfies
other various conditions. Most of the PTFE fibers 15
extend into the agglomerate portions 13 and combined
with the body of the agglomerate portion 13. However,
the PTFE fibers in the adjacent gap portions 14 are mostly
kept separated from each other and extend into different
portions of the agglomerate portion 13 to constitute the
213gl67
- 14 -
skeleton of the catalyst layer 11. The gap portions 14
have a porosity of 20% or more. In this embodiment, the
gap portions 14 have a porosity of 60 to 90%.
The fibers 15 must have repellency with respect to
the electrolyte and/or water under operating conditions.
From this point of view, the fibers 15 preferably
consist of a compound having a covalent bond of fluorine
and carbon.
The diffusion layer 12 consists of carbon paper
having a substantially uniform fiber distribution. The
diffusion layer 12 has a porosity of about 70%.
FIG. 6 is a graph showing the results obtained by
calculating the relationship between the fuel cell
performance and the widths of the agglomerate and gap
portions 13 and 14. Reference symbols Ll, L2, L3, and
L4 in FIG. 6 denote the widths of the gap portions 14,
i.e., the distances between the agglomerate portions 13
which are 1 ~m, 5 ~m, 10 ~m, and 20 ~m, respectively.
As shown in FIG. 6, the gap portion 14 preferably has a
narrow width and is desirably set to have a width of
10 ~m or less. Note that a narrower width of the gap
portion 14 causes an increase in gas diffusion
resistance and at the same causes the outer surfaces of
the agglomerate portions 13 to communicate with each
other through the electrolyte and/or water as a reaction
product, thereby clogging the gap portions 14.
Therefore, the width of the gap portion 14 is preferably
2139167
- 15 -
set to O.s ~m or more.
As shown in FIG. 6, the width of the agglomerate
portion 13 has an optimal range, which varies depending
on other factors such as the width of the gap portion
14. To obtain satisfactory performance, the width of
the agglomerate portion 13 is preferably set to be 10 ~m
or less. As previously described, ~2 in the cathode
or H2 in the anode in accordance with formula (1) or
(2) diffuses through the electrolyte from the outer
surface of each agglomerate portion 13 into the inside
thereof. Further, H2O in the cathode in accordance
with formula (1) diffuses through the electrolyte from
the inside of each agglomerate portion 13 to the outer
surface thereof. Therefore, where the width of each
agglomerate portion 13 is larger than the value set out
above, the diffusion resistance of the gases and water
increases to degrade the cell performance.
The optimal porosity of the agglomerate portions
13 from which the electrolyte is removed is determined
by the electron conduction resistance of the fine
carbon powder particles constituting the agglomerate
portions 13 and the ionic conduction resistance of the
electrolyte filled in the spaces between the fine carbon
particles. In general, since the former resistance is
lower than the latter resistance, a higher porosity is
preferred. FIG. 7 is a graph showing the results
obtained by calculating the relationship between the
2139167
-
porosity of the agglomerate portions 13 in a state
wherein the electrolyte is removed and the fuel cell
performance. As shown in FIG. 7, to obtain satisfactory
performance, the porosity of the agglomerate portions 13
free from the electrolyte is preferably set to 50% or
more.
FIG. 8 is a graph showing the results obtained by
calculating the relationship between the porosity of the
gap portions 14 and the fuel cell performance. As shown
in FIG. 8, when the porosity of the gap portions 14
becomes less than 20%, and particularly less than 10%,
the output degradation of the fuel cell becomes typical
due to an increase in gas diffusion resistance. To
obtain satisfactory performance, the porosity of the gap
portions 14 is preferably set to 20% or more.
The catalyst layer 11 shown in FIG. 1 can be
prepared by, e.g., the following method. The process
performed in an actual experiment will be described
below.
A liquid containing dispersed
polytetrafluoroethylene ( PTFE) was mixed in a solution
obtained by mixing a binder, a solvent, and fine carbon
powder particles on which an alloy catalyst mainly
containing platinum was dispersed and carried. The
resultant mixture was dried. In this case, the weight
ratio of the fine carbon powder particles on which the
catalyst is dispersed and carried to the PTFE was
2139167
-
10: 1.
An intermediate product upon drying is compressed
and stretched to prepare a sheet element. A plurality
of such sheet elements were stacked, compressed, and
worked with a roller to form a sheet. A uniaxial
tension was applied to the resultant sheet to obtain a
100-~m thick sheet, i.e., a catalyst layer 11. The
sheet-like catalyst layer 11 was adhered to the surface
of a 1.6-mm thick diffusion layer consisting of carbon
fibers. The resultant structure was heat-treated at
about 200 to 300~C for an hour to obtain an electrode 10.
As the above binder, an acrylic resin-based
binder, polyvinyl alcohol, polyvinyl butyral, paraffin,
or a cellulose-based binder were tried. As the above
solvent, xylene, toluene, cyclohexane, butanol, acetone,
methyl ethyl ketone, kerosine or water were tried. In
view of dispersion properties, adhesion strength,
remaining amount upon volatilization, and the like, a
combination of an acrylic resin-based binder and xylene
was used in this embodiment.
In stacking the roller-worked sheet elements, they
were aligned in the compression-stretched direction,
i.e., in the pulling direction from the roller in one
case. In other case, the sheet elements were stacked so
that their stretched directions were perpendicular to
each other. In view of formability, sheet elements
were stacked so that their stretched directions were
2139167
- 18 -
perpendicular to each other in this embodiment.
As a method of applying a unidirectional tension to
the sheet, this embodiment employed a method in which
the sheet was brought into contact with the end face of
a plate-like 100-Hz vibrator having a width of 20 cm
which was slightly larger than the width of the sheet,
and the sheet was slowly moved to shift the position
where the sheet was in contact with the vibrator. As
any other method, a method of holding the front and rear
ends of the sheet with clamps and pulling them, a method
of clamping the sheet with front and rear pairs of
rollers and changing the rotational speeds of the front
and rear pairs of rollers, or the like can be used.
The structure of the catalyst layer 11 shown in
FIG. 1 can be obtained by the method using the vibrator
due to the following reason.
In general, when a tension is applied to a polymer
sheet, fine cracks called crazes containing thin fibers
called fibrils, which extend parallel to the direction
of tensile stress, are known to be formed inside the
sheet at a predetermined stress or more. The details
of this mechanism are unknown, but crazes are experimen-
tally known to be easily formed at a lighter stress with
a higher distortion rate. This embodiment is different
form the experimental care in that a sheet obtained by
mixing a polymer and a fine carbon powder is used in
this embodiment. It is, however, estimated that the
2139167
-
- 19 -
stripe-like crazes having a very small size, i.e., the
gap portions 14 are formed by a similar mechanism with a
very high distortion rate caused by the vibrator.
The resultant electrode 10 was used as a cathode
electrode to form a phosphoric acid fuel cell, and a
power generation experiment was performed. In this
experiment, as shown in FIG. 2, a matrix layer 26 having
a thickness of about 100 ~m and containing silicon
carbide having a porosity of about 50% was applied to
the surface of the catalyst layer 11. In addition,
an anode electrode having a thickness of about 1.7 mm
and consisting of a catalyst layer 21 and a diffusion
layer 22 and a diffusion layer 22 formed in the same
manner as the electrode 10 was adhered to the opposite
surface of the matrix layer 26. In this case, the
matrix layer 26 and the catalyst and diffusion layers 21
and 22 of the anode electrode were impregnated with an
aqueous phosphoric acid solution serving as an
electrolyte in a predetermined amount in advance, so
that this catalyst was contained in the pores of
the agglomerate portions 13 of the catalyst layer 11 of
the cathode electrode 10 upon assembly.
A fuel cell consisting of the layers 11 to 26
formed as described above was set in a holder 32 such
that hydrogen was supplied to the anode electrode side
and air was supplied to the cathode electrode side. A
voltmeter 34 was connected between the anode and cathode
2139167
- 20 -
electrodes of the finished phosphoric acid fuel cell,
and the power generation characteristics were examined.
FIG. 9 shows, as a result of the above experiment,
a current-voltage characteristic curve Lll of the
phosphoric acid fuel cell using the electrode 10 of the
present invention and a current-voltage characteristic
curve L12 of the conventional phosphoric acid fuel cell
in which the platinum filling amount per unit area is
equal to that of the cell of the present invention.
Both the fuel cells were operated at about 8.4 atm. and
about 205~C with a utilization ratio of 70%. As can be
apparent from FIG. 9, a decrease in voltage of the
phosphoric acid fuel cell of the present invention with
an increase in current was more moderate than that of
the conventional phosphoric acid fuel cell. At
400 mA/cm2, the voltage of the phosphoric acid fuel cell
of the present invention was 0.77V which was higher than
the phosphoric acid fuel cell of the conventional
structure by 40 mv. The output density of the
phosphoric acid fuel cell of the present invention was
0.30 W/cm2.
Upon the power generation experiment, the electrode
10 was observed with an electron microscope and energy
dispersion type X-ray analyzer. The fibers 15 in the
gap portions 14 were kept in a good condition, and
ingress of the liquid electrolyte was rarely observed.
In addition, a large amount of phosphorus was uniformly
2139167
detected inside the agglomerate portions 13, and it is
assumed that the agglomerate portions 13 were suffi-
ciently impregnated with the liquid electrolyte during
the operation.
As an electrode according to the second embodiment
of the present invention, an electrode using a catalyst
layer divided into a plurality of parts on a plane was
used due to the following reason. In a large electrode
in practical use, if small catalyst layer sheets can be
arranged next to each other, the manufacturing apparatus
can be made compact, and the manufacturing cost can be
reduced. To examine the performance of an electrode
having such an arrangement, the following two electrode
samples were prepared. One of which was an electrode
sample obtained by arranging four square catalyst layer
segment sheets lla each having a side of 5 cm on a
square diffusion layer 12 having a side of 10 cm so as
to form a 2 x 2 matrix, as shown in FIG. 10. The other
was an electrode sample obtained by arranging one square
catalyst layer sheet having a side of 10 cm on a square
diffusion layer 12 having a side of 10 cm. Phosphoric
acid fuel cells using these samples were formed
following the same procedures as described above, and
the power generation characteristics of the resultant
fuel cells were examined. It was found that the power
generation characteristics of these samples were almost
equal to each other.
2139167
-
As an electrode according to the third embodiment
of the present invention, a catalyst layer having the
same structure as in the first embodiment except that a
Nafion (tradename available from DuPont) as an ion-
exchange resin was used in place of the binder of thefirst embodiment was prepared. In this case, the width
of each gap portion was set to about 4 ~m. The heat
treatment was performed at 120~C for an hour. Note
that Nafion as the ion-exchange resin serves as an
electrolyte of a solid polymer contained in the
agglomerate portions 13.
It is possible to use the electrode of the present
invention to form cathode and anode electrodes, and
to use a Nafion film serving as an electrolyte layer
sandwiched between these electrodes to prepare a solid
polymer electrolyte fuel cell.
The present invention has been applied to a
phosphoric acid fuel cell and a solid polymer electro-
lyte fuel cell. However, the present invention is
not limited to this. The present invention is also
applicable to the electrode of a cell of a different
type, a primary cell or a secondary cell. The present
invention is further applicable to electrodes of various
electrochemical reaction apparatuses for performing
reactions in which reactants and/or reaction products
contain gases. These electrodes can be exemplified by
an electrode for electrolysis, a sensor electrode, and
2139167
the like.
As described above, according to a fuel cell using
an electrode of the present invention, a higher output
density than that in a conventional fuel cell can be
obtained with the same amount of catalyst.