Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
TS 0028 CAN
REMOVING HYDROGEN SULPHIDE FROM A GAS STREAM
The present invention relates to a process of
removing hydrogen sulphide from a gas stream. An example
of such a gas stream is a natural gas.
In International patent application publication No.
92/02 449 is described a process for removing hydrogen
sulphide from a gas stream, which proces:> comprises
contacting the gas stream at a temperature above the dew
point of elemental sulphur in the presence of free oxygen
with catalytic material in a first catalytic reactor to
obtain a partly purified gas stream and contacting the
partly purified gas stream at a temperature between the
dew point of water and the dew point of elemental sulphur
with catalytic material in a second cata7_ytic reactor to
obtain a purified gas stream.
IS In the known process air is supplied to the gas
stream upstream of the first catalytic rE;actor. Free
oxygen in the air oxidizes hydrogen sulphide directly to
elemental sulphur according to the react_lon:
H2S +1/2 022-_---> H20 + 1/x Sx.
Although the publication is silent about the amount
of free oxygen supplied to the first catalytic reactor,
this amount is in general stoichiometric or slightly
super-stoichiometric. That is to say the ratio between
the concentrations of oxygen and hydrogen sulphide is
0.5, the stoichiometric ratio, or more; l.he unit of this
ratio being mol/mol, or vol/vol at standard pressure and
temperature. In the specification and in the claims the
ratio between the concentrations of oxygen and hydrogen
sulphide will be called the "oxygen-hydrogen sulphide
ratio" .
~-~~91~J
- 2 -
Applicant now proposes to contact a gas stream with
catalytic material in a first catalytic reactor at a
temperature which is between the dew point of water and
the dew point of elemental sulphur so that elemental
sulphur is adsorbed on the catalytic material in the
first catalytic reactor. The first catalytic reactor is
used for bulk removal of hydrogen sulphide and the second
catalytic reactor is used for removing traces of hydrogen
sulphide from the gas leaving the first catalytic
reactor.
In the process of the present invention the first
catalytic reactor is used to remove the bulk of the
hydrogen sulphide from the gas stream, therefore the
largest part of the elemental sulphur formed is adsorbed
on the catalytic material in this catalytic reactor. The
adsorbed elemental sulphur improves the activity of the
catalytic material. However, when the amount of elemental
sulphur adsorbed on the catalytic material is above a
certain level, the conversion of hydrogen sulphide starts
to decrease and it decreases with the amount of sulphur
adsorbed on the catalytic material. Consequently a higher
amount of hydrogen sulphide leaves the first catalytic
reactor. In order to reduce the slip of hydrogen
sulphide, the catalytic material in the first catalytic
reactor has to be regenerated even befare the H2S-
conversion has dropped to below a predetermined level.
The time between two successive regenerations is thus
determined by the time it takes to reach in the first
catalytic reactor a sulphur-loading at which the H2S-
conversion has dropped to below a predetermined level.
It is an object of the present invention to increase
the cycle time.
To this end the process of removing hydrogen sulphide
from a gas stream according to the invention comprises
contacting the gas stream in the presence of free oxygen
- 3 -
at a temperature in the range of from the dew point of
water to the dew point of elemental sulphur in two
catalytic reactors with catalytic material to obtain
elemental sulphur which is deposited on the catalytic
material and a purified gas stream, wherein the gas
stream is contacted in the first catalytic reactor with
catalytic material in the presence of a sub-
stoichiometric amount of free oxygen to obtain a partly
purified gas stream, which partly purified gas stream is
contacted in the second catalytic reactor in the presence
of additional free oxygen with catalytic material to
obtain the purified gas stream.
The invention is based on the discovery that the
sulphur-loading of the catalytic material at which H2S-
conversion decreases depends on the oxygen-hydrogen
sulphide ratio. When this ratio is larger than or equal
to 0.5 the initial H2S-conversion is in general between
95 and 100, however, the sulphur-loading of the
catalytic material at which the H2S-conversion starts to
decrease is less than 400. When the oxygen-hydrogen
sulphide ratio is less than 0.5 the H2S-conversion is
less than 950, however, it was found that: the sulphur-
loading of the catalytic material at which the H2S-
conversion starts to decrease is about twice the sulphur-
loading at which the H2S-conversion starts to decrease
when the oxygen-hydrogen sulphide ratio i.s 0.5 or more.
This effect is shown in the following example.
Three experiments were carried out to determine the
H2S-conversion as a function of the sulphur-loading of
the catalytic material. In the experiments a nitrogen
stream saturated with water had been used to simulate a
methane stream saturated with water. The nitrogen stream
further contained 1 vol$ H2S, 1 volt C02, 200 ppmv (parts
per million by volume) COS and free oxygE:n. The amount of
free oxygen was so selected that the oxygen-hydrogen
~~ ~~~ ~.9
- 4 -
sulphide ratio was 0.6, 0.45 and 0.27. The stoichiometric
ratio of [02] to [H2S] is 0.5. The catalytic material
used was an activated alumina catalyst (A.201 from La
Roche). The pressure was 10 bar, the temperature was 150
°C, and the gas hourly space velocity was 2 000
vol/vol/hour. In Table 1 the results of the experiments
are shown in the form of the initial H2S-conversion and
the sulphur loading of the catalyst at which the
conversion starts to decrease as a function of the
oxygen-hydrogen sulphide ratio. The initial H2S-
conversion is defined as 1-[H2S]out/[H2S]ins wherein
[H2S]in is the concentration of hydrogen sulphide in the
gas stream entering into the catalytic reactor and
[H2S]out is the concentration of hydrogen sulphide in the
gas stream leaving the catalytic reactor. The sulphur-
loading of the catalyst is expressed in kg sulphur per kg
catalyst.
Table 1. Result of the experiments,
[02]/[H2S] Initial Sulphur-7_oading
H2S-conversion
0.6 1.0 0.30
0.45 0.9 0.70
0.27 0.5 0.75
Suitably the oxygen-hydrogen sulphide ratio in the
process according to the invention in they gas stream
entering into the first catalytic reactor is in the range
of from 0.40 to 0.49 and more suitably in the range of
from 0.45 to 0.49.
The second catalytic reactor is used to remove traces
of hydrogen sulphide, and to this end they partly purified
gas stream is contacted in the second catalytic reactor
with catalytic material in the presence c>f additional
free oxygen. The amount of additional free oxygen is so
2.~ ~~~~.~
- 5 -
selected that together with the initial amount of
hydrogen sulphide present in the gas stream the total
amount of oxygen is equal to or larger than the
stoichiometric amount.
The invention will now be described by way of example
in more detail with reference to the accompanying drawing
showing schematically the device for carrying out the
process according to the present invention.
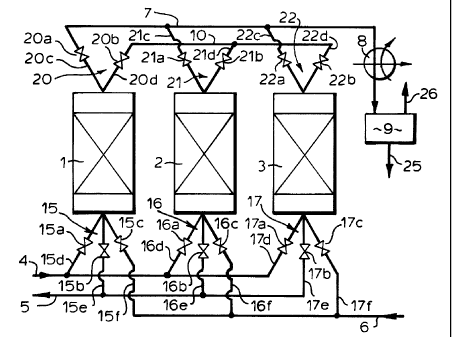
The device comprises three reactors containing
catalytic material referred to with reference numerals 1,
2 and 3, a feed supply conduit 4, an outlet conduit for
purified gas 5, a supply conduit for regeneration gas 6,
an outlet conduit for sulphur-containing gas 7, a
condensor 8, a storage tank for sulphur Via, and a
connecting conduit 10.
Each catalytic reactor 1, 2 and 3 has a lower
manifold referred to with reference numerals 15, 16 and
17. The lower manifold 15 includes valves 15a, 15b and
15c in conduits 15d, 15e and 15f, the lower manifold 16
includes valves 16a, 16b and 16c in conduits 16d, 16e and
16f, and the lower manifold 17 includes 'valves 17a, 17b
and 17c in conduits 17d, 17e and 17f. Conduits 15d, 16d
and 17d are connected to the feed supply conduit 4,
conduits 15e, 16e and 17e are connected 'to the outlet
conduit for purified gas 5, and conduits 15f, 16f and 17f
are connected to the supply conduit for :regeneration gas
6.
Furthermore each catalytic reactor l, 2 and 3 has an
upper manifold referred to with reference numerals 20, 21
and 22. The upper manifold 20 includes valves 20a and 20b
in conduits 20c and 20d, the upper manifold 21 includes
valves 21a and 21b in conduits 21c and 21d, and the upper
manifold 22 includes valves 22a and 22b in conduits 22c
and 22d. Conduits 20c, 21c and 22c are connected to the
- 6 -
outlet conduit for sulphur-containing gas 7, and conduits
20d, 21d and 22d are connected to connecting conduit 10.
During normal operation a gas stream including
hydrogen sulphide and a sub-stoichiometric amount of free
S oxygen is supplied through the feed supply conduit 4
through conduit 15d into catalytic reactor 1 for bulk
removal of hydrogen sulphide. A first purified gas having
a reduced hydrogen sulphide content is rf~moved from the
first catalytic reactor 1 through conduii~ 20d and it is
supplied via the connecting conduit 10 to the second
catalytic reactor 2 for trace removal of hydrogen
sulphide. Additional free oxygen is supplied to the
second catalytic reactor 2 via a separatE~ supply conduit
(not shown). Totally purified gas is removed from the
second catalytic reactor 2 through conduit 16e, and it is
removed for further use through the outlet conduit for
purified gas 5.
Simultaneously the catalytic material in catalytic
reactor 3 is being regenerated, to this End hot
regeneration gas is supplied through the supply conduit
for regeneration gas 6 via conduit 17f into catalytic
reactor 3. The temperature of the hot gaa is above the
dew point of elemental sulphur. Regeneration gas loaded
with sulphur from the catalytic material in catalytic
reactor 3 is removed from the catalytic reactor 3 through
conduit 22c and supplied through the out:Let conduit for
sulphur-containing gas 7 to the condenso:r 8 where
elemental sulphur is condensed. The condensed sulphur is
stored in storage tank 9 from which it can be removed via
outlet conduit 25, and sulphur-free gas :is removed from
the storage tank 9 via outlet conduit 26.
In this first phase catalytic reactor 1 is used for
bulk removal of hydrogen sulphide, catalytic reactor 2
for trace removal and the catalytic material in catalytic
reactor 3 is being regenerated.
2~39I9~
The position of the valves in the first phase is
given in Table 2.
When the catalytic material in catalytic reactor 1 is
loaded to a such a level that the conversion starts to
decrease, the catalytic material in catalytic reactor 1
is regenerated, bulk removal of hydrogen sulphide is now
done in catalytic reactor 2 and trace removal is done in
catalytic reactor 3 of which the catalytic material had
been regenerated in the first phase. To properly direct
the flow some valves have to opened and other valves have
to be closed, the position of the valves in this second
phase is given in Table 2. Additional free oxygen is
supplied to the third catalytic reactor :3 via a separate
supply conduit (not shown).
When the catalytic material in catalytic reactor 2 is
loaded to a such a level that the conversion starts to
decrease, the catalytic material in catalytic reactor 2
is regenerated, bulk removal of hydrogen sulphide is now
done in catalytic reactor 3 and trace rernoval is done in
catalytic reactor 1 of which the catalytic material had
been regenerated in the second phase. Additional free
oxygen is supplied to the first catalytic reactor 1 via a
separate supply conduit (not shown). To properly direct
the flow some valves have to opened and other valves have
to be closed, the position of the valves in this third
phase is given in Table 2.
Subsequently the process is continued with the first
phase.
_ g _
Table 2. Position of the valves, "op" means open and "cl"
means closed.
15 20 16 21 17 22
a b c a b a b c a b a b c a b
phase op cl clcl op clop cl clop cl cl opop cl
I
phase cl cl opop cl opcl cl clop cl op clcl op
II
phase cl op c1cl op clcl op opcl op cl clcl op
III
The below hypothetical example of the first phase
further illustrates the present invention.. Catalytic
reactors 1, 2 and 3 are filled with 66.8 m3 catalytic
material in the form of activated alumina particles
having a particle size of 4 mm.
A feed consisting of natural gas with 1.0 volt H2S,
100 ppmv (parts per million by volume) COS, 1 volt C02
and 0.48 volo of 02 is passed at a pressure of 60 bar and
a temperature of 100 °C into the first catalytic reactor
1 through conduit 15d. The flow rate is 170 000 Nm3/h
(standard cubic meter per hour). The gas hourly space
velocity in the reactor is 2 500 vol/vol/hour. In the
first catalytic reactor 1 95~ of the H2S is converted to
elemental sulphur which is adsorbed on the catalyst
particles. The catalyst particles are loaded to 75 wt~ in
16.6 hours, at which moment the second phase starts.
Because the reaction is exothermic first purified gas
leaves the first catalytic reactor 1 at a temperature of
150 °C, this gas is supplied with an additional amount of
250 ppmv 02 into the second catalytic reactor 2 through
conduit 21d, and purified gas leaves reactor 2 at about
the same temperature. The hydrogen sulphide concentration
in the purified gas is less than 30 ppmv H2S, and the
~-~ ~~1 ~,~
- 9 -
amount of elemental sulphur in the purified gas is less
than 3 ppmv.
Catalyst particles in the third catalytic reactor 3
are regenerated by passing gas at a pressure of 60 bar
and at a temperature of 400 °C through the third
catalytic reactor 3. For a gas flow rate of 28 129 Nm3/h
regeneration of the catalyst particles takes about 13
hours. The regeneration gas is suitably a side stream of
the purified gas leaving the second catalytic reactor 2.
The sulphur-free gas leaving the storage tank for sulphur
9 through conduit 26 can be added to the purified gas in
conduit 5.