Note: Descriptions are shown in the official language in which they were submitted.
v ~:1.3~2ZD
.. .
-:'JVO 94/00533 PCT/AU93/00317
. ;, ;
.. i
TREATMENT OF WASTE
:.
The present invention relates to the treatment of
inorganic solid waste.
In particular, the present invention relates to the
treatment of inorganic solid waste which is commonly
referred to as "dusts" .
The term "dusts" as used herein is understood to mean
any relatively finely divided particulate material and
includes but is not limited to:
(a) metallic or metallic oxide containing material
substantially collected by gas cleaning systems
operated, for example, as a component of pyro-
metallurgical production and processing; and/or
s,
(b) inorganic residues arising from the combination
or incineration of toxic, hazardous and non- ',
hazardous wastes, including fly ash, bottom ash
and particulate material collected by gas
s
cleaning systems.
In many instances, for a range of;environmental and
! materials handling reasons it is difficult and expensive to
dispose of dusts. For example, dusts often contain
hazardous compounds and reQuire particular processing
before disposal. In addition, dusts often contain
components which, whilst valuable, cannot be recovered
economically.
It is an object of the present invention to provide a
method of treating dusts which alleviates the disadvantages
described in the preceding paragraphs. '"
According to the present invention there is provided a
method of treating inorganic solid waste in a bath of
molten metal contained in a vessel which has a space above
the bath and a waste gas outlet, the method comprising:
(a) injecting waste into the bath to form a primary
reaction zone in which there are reactions
between the waste and the bath or in which the
waste undergoes a change of phase to convert the
1
waste into more readily recoverable or disposable
products; and
(b) injecting oxygen-containing gas towards the
surface of the bath to form a secondary reaction
zone in a section of the space above the bath
through which oxidisable products released from ..
the primary reaction zone flow to reach the waste
Qas outlet in the vessel and in which the
oxid~isable products are oxidised and the heat
released by such oxidation is transferred into
the bath.
It is understood that references herein to "a bath of '
a
molten metal" cover a bath containing molten metal and slag
as well as a bath containing molten metal only.
'30 The present invention is based partly on the
realisation that a molten metal bath provides a suitable
u~ environment, both in terms of temperature and composition,
.< for converting inorganic solid waste, particularly dusts,
E :.'.
213v 2~. ~ =--
v'~WO 94/00533 PCT/AU93/00317
into more readily disposable components.,_ The present
invention is also based partly on the realisation that the
use of a secondary reaction zone for oxidising any
oxidisable products released from the molten metal bath
provides a means of minimising the energy input to maintain
the temperature of the molten metal bath.
It is preferred that the method further comprises
injecting a Qas into the bath to cause splashes andlor
droplets of molten metal to be ejected upwardly from the
bath into the secondary reaction zone or into a section of
the space above the bath which is between the secondary
reaction zone and the waste gas outlet to facilitate
efficient heat transfer to the bath and scrubbing of
volatilised species and any particulate material in the
products released from the primary reaction zone and/or
produced in the secondary reaction zone.
It can readily be appreciated that the combination of
the oxidation of any oxidisable products in the secondary
reaction zone and the scrubbing effect provided by the
splashes and/or droplets of molten metal in the secondary
reaction~zone or downstream thereof provides a high level
of assurance against unreacted or partially reacted
inorganic solid waste short-circuiting treatment altogether
i and reporting in the exit gas stream from the vessel. This
is achieved by providing at least two separate reaction v
zones through which unreacted or partially reacted
inorganic waste must pass before exiting the vessel.
It is particularly preferred that the method further y
comprises injecting carbonaceous material into the bath to,
form a carburising zone in which the carbon in the
r
carbonaceous material dissolves into the bath and is
_,,:
_..
_.
.._:::
~~.3~~22,
WO 94/OOS33 PCT/AU93/Of~r=r~'
4
available for reaction with waste in the primary reaction
zone.
The term carbonaceous material is herein understood to
include: solid carbonaceous fuels such as coke and coal;
liquid fuels such as oil, light fuel oil, diesel oil and
heavy fuel oil; and gaseous fuels, such as natural gas,
methane, ethane, propane, butane; or any mixtures of the
fuels.
It is preferred that the carbonaceous material be
selected from one or more of the group comprising coal,
spent pot linings from aluminium smelting furnaces, and
sewage sludge. It is particularly preferred that the
carbonaceous material comprises coal.
In the above described embodiment the heat transferred
to the bath from the secondary reaction zone contributes to
balancing the heat loss from the bath as a consecruence of
endothermic reactions in the carburising and primary
reaction zones.
One particularly preferred embodiment comprises
locating the carburisinQ zone directly below the secondary
reaction zone.
It ie preferred that the bath comprises at least 10%
metal. It is particularly preferred that the bath
cos<priaes at least 70% metal. It is more particularly .'
preferred that the bath comprises at least 80% metal.
It is preferred that the metal be selected from one or
more from the group comprising iron, ferroalloys nickel,
tin, chromium. silicon, and copper, and mixtures thereof.
z ~ ~ ~ z z~ ~~~,
..
,:.-.;
~'y,NO 94/00533 PCT/AU93/003i7
It is particularly preferred that the metal comprises iror_.
It is preferred that the gas injected into the b~.th to
cause molten metal and slag splashes and/or droplets to be
5 ejected upwardly into the secondary reaction zone be
selected from one or more of an inert gas, recycled process
gas, natural gas, C02, propane, or butane, or mixtures of
the gases. It is particularly preferred that the inert gas
be nitrogen.
It is preferred that the oxygen-containing gas be
selected from the group comprising oxygen, air and steam.
It is particularly preferred that the air be preheated. It
is more particularly preferred that the air.be preheated to
temperatures in the range of 900 to 1600°C.~
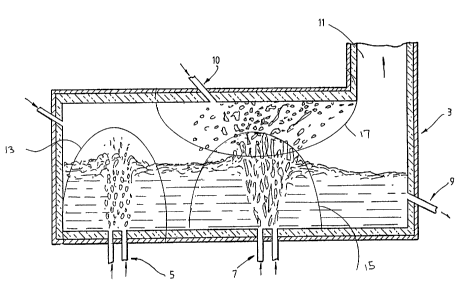
The present invention is described further with
reference to the accompanying figure which is a schematic
illustration of a preferred embodiment of a method of
treating inorganic solid waste in accordance with the
present invention.
The preferred embodiment of the method of the present
invention is described hereinafter in the context of
treating dusts although it is understood that the present
invention is not so restricted and extends to the treatment t
of solid inorganic waste generally.
The preferred embodiment of the method is carried out
in a vessel generally identified by the numeral 3.
1
The vessel 3 may be of any suitable known design of
metallurgical vessel with refractory lined internal walls
and an outer metal shell. In the arrangement shown in the
~..
. ,
., f~.'.r'..
WO 94/00533 PCi'/A~U93/00~:' ;.~ ~~
6
figure tre vessel 3 is a generally cylindrical shape
j
disposed horizontally and has bottom tuyeres 5, 7, a 1
slag/metal tap 9, an air injection port 10, and an upper
off-gas outlet 11 at one end of the vessel 3. Typically, ' ;
the ratio of the length and the diameter of the vessel is
3:1.
The vessel 3 contains a volume of molten metal which
comprises at least 10°% iron and a layer of slag at a
temperature of 1400°C. The other metals in the bath may be
selected as required and, by way of example, may comprise
one or more of ferroalloys. tin, nickel, silicon and
copper.
The preferred embodiment of the method comprises
injecting dusts entrained in a suitable carrier, such as an
inert Qas, through the bottom tuyeres 5 into the bath to
form a primary reaction zone indicated schematically by the
line identified by the numeral 13 which is located at the
end of the vessel 3 remote from the off-gas outlet 11. The
dusts undergo a range of reactions and phase changes in the
primary reaction zone 13 depending on the composition of
the dusts. Typically, the metal oxides in the dusts are
reduced an8 the metal values report into the bath or in
some cases are volatilised. Other components of the dusts t
may be broken down or volatilised and released directly
into the gas space above the bath.
The method also comprises iajecti: pre-heated air,
s
typically at a temperature in the rax~ of 900 to 1600°C,
or any other suitable oxygen-contain. _~ gas through
, injection port 10 towards the surface of the bath adjacent
the primary reaction zone 13 to form a secondary reaction
zone indicated schematically by the line identified by the ,
2132
;v~'~ W~ 94!00533 fCT/AU93/00317 ~~.::
7
numeral 17 in the section of the space above the bath that
is located between the section that is directly above the
primary reaction zone 13 and the off-gas outlet 11."
The method also comprises simultaneously injecting
nitrogen or any other suitable gas through tuyeres 7 into
the bath immediately below the secondary reaction zone 17
to cause eruption of molten metal and slag in splashes
and/or droplets from the surface of the bath into the
secondary reaction zone 17. Typically, the nitrogen is
injected in an amount greater than or equal to 0.1 Nm3
min"1 tonne-1 of molten metal in the bath.
In the secondary reaction zone 17 the pre-heated air
oxidises any oxidisable products from the primary reaction
zone 13. Furthermore, the heat released by such oxidation
is efficiently transferred to the splashes and/or droplets
of molten metal and slag and subsecguently into the bath
when the splashes and/or droplets fall downwardly to the
surface of the bath. The splashes and/or droplets also
scrub volatilised species and any particulate material from
the primary reaction zone 13 and/ar formed in the secondary
reaction zone 17 and transfer the scrubbed values to the
bath.
It is preferred that the carbonaceous material be
selected from one or more of the group comprising coal,
spent pot linings from aluminium smelting furnaces, and
sewage sludge. It is particularly preferred that the
carbonaceous material comprises coal.
It is noted that in effect the splashes and/or droplets
of molten metal and slag form a curtain which is an
effective and efficient means of transferring heat to the
8
bath and scrubbing volatilised species and particulate
material from products from the primary reaction zone 13
and/or secondary reaction zone 17. ~ .
Typically, the temperature in the secondary reaction
zone 17 is controlled to be at least 200°C higher than that
of the molten metal. Typically, the temperature in the
secondary reaction zone 13 varies between 1500°C and
2700°C.
It can be readily appreciated from the foregoing that
in the preferred embodiment of the method the secondary
reaction zone 17 has three important functions.
Specifically, the secondary reaction zone 17:
(a) oxidises any oxidisable products from the primary
reaction zone 13;
(b) ensures that the heat released by such oxidation
is transferred to the bath; and
(c) scrubs any volatilised species and any
particulate material from the primary reaction
zone l3 and/or formed in the secondary reaction
zone 17. v
The preheated air may be injected into the secondary
reaction zone 17 by any suitable means such as top-blowing
single or multiple tuyeres or lances with one or more'
..
openings.
~ In many instances, the reduction of metal oxides in the
dusts to metal values will be a dominant reaction in the .
primary reaction zone 13. As a consequence, in such
v':
.1392~~
v:WO 94/00533 ., PCT/AU93/00317
9
situations, in order to maintain a level of carbon in the
bath to reduce efficiently the metal oxides in the dusts,'
the method also comprises injecting carbonaceous material
such as coal into the bath through tuyeres 7 to form a
carburisation zone indicated schematically by the line
identified by the numeral 15. The volatiles in the coal
are thermally cracked and the carbon dissolves in the iron
and disperses through the bath and in particular into the
primary reaction zone 13.
It is noted that the heat transfer to the bath is
important since reduction reactions in the primary reaction
zone I3 and the carburisation zone 15 are essentially
endothermic and it is important to balance the heat loss
due to such reactions to maintain the temperature of the
bath at an effective operating level.
It can be readily appreciated from the foregoing.that
the preferred embodiment of the method of the present
invention is an efficient means by which solid inorganic
waste, particularly dusts, can be converted into component
parts which are non-hazardous and comparatively straight- ,
forward to recover. '
In addition, it can be readily appreciated that the use
1
of two separate reaction zones in the preferred embodiment
provides a high level of assurance against unreacted dusts.
which may include hazardous components, short-circuiting
x:.
r:~:.
treatment altogether.
9.
J
t
Many modifications may be made to the preferred .,
embodiment of the method of the present invention without
departing from the spirit and scope of the present
invention.
In this regard, whilst in the preferred embodiment the
inorganic solids and coal are injected into the batkr to
form separate, essentially macro-sized, reaction and
carburisation zones in the bath, it can readily be
appreciated that the present invention is not so limited
and the injection of the constituents into the bath can be
controlled to form arrays of separate essentially micro-
sized primary reaction and carburisation zones.
Furthermore, whilst the preferred embodiment includes
the location of the,secondary reaction zone 17 immediately
above the ca~burisation zone 15, it can readily be
appreciated that the present invention is not so limited
aad the secondary reaction zone 17 may be located above a
section of the bath that is adjacent to the carburisation
zone 15.
Furthermore, whilst~the preferred embodiment comprises
injecting nitrogen or any other suitable gas into the bath
to cause eruption of molten metal and slag splashes and
droplets to form a curtain in the secondary reaction zone ,
17, it can readily be appreciated that the present
invention is not so limited. By way of example, the v
curtain of splashes and droplets of molten metal and slag w
may be projected into a section of the bath which is
between the secondary reaction zoae 17 and the waste gas
outlet 11 so that products, gaseous or solid, flowing from
the secondary reaction zone 17 are reQuired to pass through
the curtain before reaching the waste gas outlet 11. As a
consecZuence. the curtain enables heat transfer back to the :,
bath and scrubbing of volatilised species and any
particulate material flowing from the secondary reaction
zone 17.
-,