Note: Descriptions are shown in the official language in which they were submitted.
W094/04467 21394~1 PCT/GB93/01762
PROCESS AND APPARATUS FOR NEUTRALISATION OF ACID
STREAMS.
This invention relates to a process for the
neutralisation of aqueous acid streams with calcium
hydroxide, and in particular to a process of
neutralising a hydrochloric acid stream with calcium
hydroxide. The invention relates in a further aspect
to an apparatus in which the process of the invention
may be effected.
Hydrochloric acid is produced on a wide scale
around the world, indeed it is produced as a
by-product in very many industrial processes. In
particular it is produced as a by-product in the
reaction of chlorine containing compounds with
hydrogen fluoride in which fluorine is eschanged for
chlorine, many of which are practised around the
world.
Although hydrochloric acid may be a commercial
product in itself, it is not practical to store large
quantities for lengthy periods of time and
consequently, if an immediate demand cannot be found,
then it is necessary to dispose of the hydrochloric
acid. Disposal of the acid involves first
neutralising the acid stream followed by discharge of
the neutralised effluent.
In the past, the neutralisation of such acid
streams has posed less of a problem than recently due
to the rather less stringent environmental controls
on the pH of the effluent discharge that have
prevailed hitherto. However, the pH control
requirements on the discharge of such neutralised
acid streams are becoming increasingly stringent and
pose particular problems for the control of pH during
the neutralisation of the acid streams.
W094/0~67 ~139~1 PCT/GB93/01762
. -
Acid streams are typically neutralised with abasic reagent containing sodium hydroxide or calcium
hydroxide. Each of these reagents offers certain
advantages and disadvantages. Thus, whilst sodium
hydroxide is convenient to use due to its high
solubility in water which allows it to bé employed in
the form of an aqueous solution, it is significantly
more expensive than calcium hydroxide and poses
problems of both pH measurement, due to the presence
of sodium ions which have a deleterious effect on the
operation of pH meters, and control, due to the
characteristic titration curve which shows a very
sharp change in pH over a very small change in sodium
hydroside addition in the neutral region. On the
~5 other hand, whilst being cheap, the use of calcium
(hydr)oside has posed various problems associated
with the low degree of solubility of calcium
hydroxide in water such that calcium hydroxide is
predominantly present in solid form when added to the
acid stream. In particular ~t poses control problems
in ensuring that the calcium hydroxide has
sufficiently reacted with the acid so that downstream
carryover of solid reagent and subsequent reaction
does not cause pH increase after the neutralisation
process.
The process of the present invention is based
upon the use of calcium hydroxide as the neutralising
agent.
In the past, control of the neutralisation
reaction has been achieved in dilute systems where
the acid concentration is usually about 5-10~.
However, the neutralisation of dilute acid systems
necessitates the use of very large vessels thereby
increasing the overall cost of the process. It has
also been proposed, in the belief that alkaline
W094/04467 PCT/GB93/01762
X1394~
-- 3
conditions promote the rate of dissolution of calcium
hydroxide in water, and therefore in order to achieve
greater control of the neutralisation process, to
neutralise the acid stream by first adding sufficient
calcium hydroxide to result in an alkaline solution,
i.e. overshoot of the neutral point and then to lower
the pH towards neutral by further addition of acid.
However, this approach increases the deposition of
solids, which is more common under alkaline
conditions, and which may have deleterious effects on
the performance of pH meters used to ensure that
accurate monitoring of the neutralisation process is
achieved.
These known processes are typically carried out
using a series of continuous stirred tank reactor
vessels (CSTR), each tank being provided with
feedback control by which the measured pH of the
solution within the tank controls the addition of
often both acid and alkali in order to ensure that
the pH is correctly adjusted in each vessel.
According to the present invention there is
provided a process for the neutralisation of an acid
stream which process comprises feeding the acid
stream and calcium hydroxide through a first reaction
zone to a second reaction zone so that the calcium
hydroxide partially neutralises the acid stream
during feed of the stream to the second reaction
zone, the second reaction zone being provided with
means for measuring the pH of the solution within the
second reaction zone and means for feeding aqueous
base to the second reaction zone.
The first reaction zone may comprise a conduit
to which the acid stream and a calcium hydroside
stream are fed, and the acid and calcium hydroxide
W094/04467 Z~3~1. PCT/GB93/01762
-- 4
streams may be combined to flow through the conduit
co-currently to the second reaction zone.
The acid streams which are neutralised by the
process of the invention need not be diluted prior to
neutralisation and the acid streams will usually
therefore be concentrated streams, for example acid
streams with an acid concentration of at least lOZ by
weight, and especially acid concentrations of at
least 25Z by weight. Acid streams having a
concentration of up to 35Z or 40Z by weight may be
neutralised with satisfactory control by the process
of the invention. The acid stream is preferably that
of an acid, the calcium salt of which is soluble in
aqueous solution. Thus, the acid stream will usually
be a hydrochloric acid or nitric acid stream,
although small amounts of other acids, for example
sulphuric and hydrofluoric acid may also be present.
The calcium hydroside fed to the first reaction
zone is preferably in the form of an aqueous slurry
of calcium hydroxide. The aqueous slurry of calcium
hydroxide is preferably prepared from powdered
hydrated lime in order to reduce the amount of heat
liberated during the slurrying process and thus the
temperature of the reagent. The proportion of solids
in the slurry may vary within a wide range but
preferably the calcium hydroxide slurry contains from
about 102 solids by weight to about 40Z solids by
weight, especially from about 15Z by weight to about
25Z by weight.
The calcium hydroxide component of the slurry
preferably comprises calcium hydroxide solids
particles with a mean particle size in the range from
about 1 micron to about 10 micron with less than
about O.lZ of the particles having a size larger than
W094/04467 213~1 PCT/GB93/01762
-- 5
about 250 microns, in order to promote rapid reaction
of the calcium hydroxide with the acid.
Preferably the first reaction zone comprises a
Plug Flow reactor. The second reaction zone is
preferably a Stirred Tank Reactor.
Plug Flow and Stirred Tank Reactors are
conventional within the art and any design of these
reactors may be employed in the process of the
invention.
The reactors are preferably constructed of
materials resistant to the chemical streams with
which they are to be contacted. Thus, the reactors
may be constructed using rubber, per-fluorinated
copolymers, for esample polytetrafluoroethylene, or
from metal alloy systems also having resistance to
the chemical streams within them, for esample
suitable Hastelloy or Inconel alloys. The reactors
may also comprise for esample steel, hsving an inner
lining of these materials. In practice we prefer to
use rubber coated carbon steel.
We have found that the provision of a first
reaction zone comprising a plug flow reactor which
serves to maximise the estent of reaction for any
given reactor volume, allows the most efficient
reaction of the calcium hydroside and thus reduces
the reactor volume required for sufficient
conversions of the calcium hydroxide thus reducing
the tendency for any carry-over of unreacted calcium
hydroside from the neutralisation process. The
accelerated reaction of calcium hydroxide which we
have found under acid conditions is further enhanced
by the use of the plug flow reactor.
The plug flow reactor employed in the process
preferably comprises a static miser into which the
acid and calcium hydroside streams are fed followed
W094/0~67 PCT/GB93/01762
Z~
-- 6
by a length of pipe~sufficient to allow adequate
reaction of the calcium hydroxide. The optimum
capacity of the plug flow reaction zone depends upon
the properties of the acid stream and of the calcium
hydroside, in particular the particle size
distribution of the calcium hydroxide reigent.
Furthermore, may commercially available forms of
calcium hydroxide may contain calcium carbonate
impurities which may yield carbon dioxide during the
process. The presence of such carbon dioxide may also
influence the desired capacity of the plug flow
reactor. However, we generally prefer to employ
sufficient length of plug flow reactor to allow a
residence time within the reaction zone of at least
~5 30 seconds, preferably at least 35 seconds and
especially at least 40 seconds.
Preferably, the acid stream and calcium
hydroside are fed to the first reactor zone under
ratio feed-forward control in order to control the
degree of neutralisation which is effected in the
first reaction zone.
Ratio feed forward control of the streams may be
achieved by monitoring, for example, the density or
conductivity of the acid stream whereby to obtain the
acid concentration of the acid stream and by
monitoring the flow of the stream. The flow of
calcium hydroxide slurry theoretically required to
achieve a required first degree of neutralisation (as
described hereinafter) in the first reaction zone may
then be calculated and that flow of calcium hydroxide
may be fed to the first reaction zone.
The required degree of neutralisation in the
first reaction zone may vary within a wide range but
preferably sufficient calcium hydroxide is fed to the
first reaction zone tO effect as large a proportion
W094/04467 PCT/GB93/01762
2~3g441.
-- 7
of the total neutralisation as possible, thus
reducing to a minimum the amount of further aqueous
base required to be added to the second reaction zone
whilst allowing a substantial acid concentration to
be maintained throughout the first reaction zone and
minimising the chance of carry-over of calcium
hydroxide and hence pH overshoot following the
neutralisation. Preferably the amount of calcium
hydroxide fed to the first reaction zone is
sufficient to effect at least 802 of the
neutralisation, more preferably at least 902, and
especially about 952 of the neutralisation.
Preferably the ratio of the flow of calcium
hydroxide slurry and acid to the first reaction zone
is also adjusted by a feedback control loop from the
~ proportion of aqueous base added to the second
reaction zone whereby to compensate any variations in
the concentration of the calcium hydroxide feed to
the first reaction zone and so as to maintain the
desired proportion of calcium hydroxide being added
to the first reaction zone.
The product of the first reaction zone is then
fed to a second reaction zone which comprises a
Stirred Tank Reactor, preferably a Continuous Stirred
Tank Reactor (CSTR), to which further aqueous base is
added, preferably under feedback control from means
for monitoring the pH of the solution within the
CSTR, in an amount sufficient to achieve a second
required degree of neutralisation. The amount of
aqueous base necessary to achieve the required second
degree of neutralisation will depend upon the amount
of calcium hydroxide fed to the first reaction zone,
but in order that a reliable reading may be taken
from the pH meter, the amount of aqueous base added
is preferably such as to result in a solution which
W094/04467 2~3~441 PCT/GB93/0176?.
may be recovered from the second reaction zone having
a pH of at least 1, preferably at least 1.5. Further,
in order to minimise any carry-over of calcium
hydroxide or other aqueous base in the solution
recovered from the second reaction zone, the amount
of aqueous base added may be such as to produce a
solution having a pH of not greater than 4,
preferably not greater than 3.
The aqueous base which is added to the second
reaction zone is preferably calcium hydroxide since
the use of calcium hydroxide in the second reaction
zone as well as the first reaction zone promotes the
buffering effect previously described. However, other
aqueous bases may be employed if desired, for esample
sodium hydroxide.
According to a preferred embodiment of the
invention there is provided a process for the
neutralisation of an acid stream which comprises the
steps of (a) feeding to a first reaction zone under
ratio-feed-forward control the acid stream to be
neutralised and sufficient calcium hydroxide whereby
to perform a first required degree of neutralisation
(as hereinbefore described), wherein said first
reaction zone comprises a plug flow reactor, (b)
feeding the product from step (a) to a second
reaction zone comprising a stirred tank reactor
provided with means for measuring the pH of the
solution within the tank and feeding calcium
hydroYide thereto, wherein a feedback control loop
from the pH measuring means controls the addition of
sufficient calcium hydroxide to the tank to perform a
second required degree of neutralisation and (c)
recovering a solution from the second reaction zone
having a pH of from about 1 to about 3.
WO 94/04467 2~3944~ PCI`/GB93tO1762
g
The solution recovered from step (c) may then be
passed to a third reaction zone in which fine
adjustment of the solution to a pH of between 6 and 9
is carried out by the addition of small amounts of an
aqueous base under feedback control. The aqueous base
employed may be calcium hydroxide, or it may be
another aqueous base, for example sodium hydroxide.
We have found that achieving the required pH control
in the third reaction zone in order to meet discharge
requirements is facilitated by a strong buffering
effect (reduced sensitivity of pH to concentration
variations) observed when employing calcium hydroxide
as the neutralisation reagent in the first reaction
zone in which the major part of the neutralisation is
effected, and preferably also as the aqueous base
which is added to the second reaction zone. This
buffering effect may be exploited in the third
reaction zone by the use of calcium or sodium
hydroside. ~e particularly prefer to employ sodium
hydroside since only very small-amounts are added to
achieve the required pH adjustment, and controlled
addition of such small amounts is facilitated by the
use of an aqueous solution of a base (sodium
hydroxide) rather than an aqueous slurry (calcium
hydroxide).
During the process of the invention, there is an
increase in the temperature of the solution flowing
through the system due to the highly exothermic
reactions taking place and the absence of substantial
dilution. Indeed, the temperature of the solutions
flowing through the system, may rise to a temperature
of about 80C. In order to maintain the full benefits
of reliable control and minimal plant provided by
carrying out the neutralisation at high
concentrations, the pH in the third reaction zone is
W094/0~67 2'~3~41 PCT/GB93/01762
- 10 -
preferably controlled to a value we have determined
by investigation of the chemical characteristics of
the calcium hydroxide/acid system, to give a pH on
solids removal and cooling which is within the
5 regulatory pH limits allowed for effluent discharge.
Consequently, cooling may be carried out on the final
neutralised stream without the need for subsequent pH
adjustment on the cooled stream and without the need
for pre-cooling of the acid stream and/or the calcium
hydroxide stream~ all of which would require the use
of equipment constructed from exotic materials having
resistance to the acid media being cooled within
them. Cooling may be carried out, for example by
evaporative cooling.
}5 Our investigations have shown that the pH of the
final neutralised solution recovered from the third
reaction zone should be lower than that desired fo-r
the cooled solution to be discharged due to our
observed decrease in pH with increasing temperature.
Preferably, the pH sensing means employed in the
third reaction zone is pre-set to compensate for the
effect of the temperature reduction (cooling) on the
pH. The pH of the pre-cooled solution may therefore
be from sbout 6.S to about 7.5, such that after
cooling, the neutralised stream to be discharged has
a pH within the range from about 7.5 to about 8.5.
According to a further aspect of the invention
there is provided an apparatus for the neutralisation
of an acid stream with calcium hydroxide which
comprises a first plug flow reaction zone provided
with means for feeding the acid stream and calcium
hydroxide thereto under ratio feed-forward control,
and a second reaction zone comprising a continuous
stirred tank reactor provided with means for
measuring the pH of the solution therein, an aqueous
W094/0~67 2 ~ 3g ~ 1. PCT/GB93/01762
._
base feed and feedback control means for controlling
the addition of aqueous base thereto.
Preferred embodiments and further features of
the apparatus of the second aspect of the invention
are as described in relation to the first process
aspect of the invention.
The invention is illustrated but not limited by
the following figure which is a schematic flow
diagram of an apparatus in which the process of the
invention may be operated.
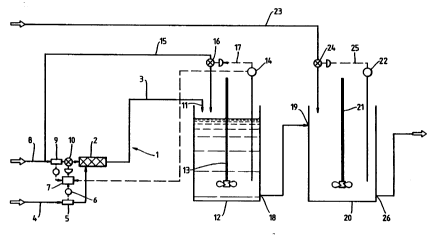
In the figure, a plug flow reactor 1 comprises a
static miser 2 and a pipeline 3 constructed from
rubber-lined carbon steel.
A hydrochloric acid feed line 4 to the static
miser 2 is provided with a conductivity meter 5 and
flow meter 6. The outlets from the conductivity and
flow meters are connected to a ratio feed-forward
control unit 7. A calcium hydroxide feed line 8 to
the static miser 2 is provided with flow meter 9 and
valve 10. Flow meter 9 and valve 10 are connected to
the outlet from the ratio feed-forward control unit
7.
Outlet 11 from the pipeline 3 enters a
Continuous Stirred Tank Reactor (CSTR) 12 which i8
provided with an agitator 13, p~ meter 14 and calcium
hydroside feed line 15. Calcium hydroside feed line
15 is provided with a valve 16 controlled by feedback
circuitry 17 connected to the pH meter 14. Outlet 18
from the CSTR 12 is connected to inlet 19 of a
Continuous Stirred Tank Reactor (CSTR) 20 which i8
provided with an agitator 21, p~ meter 22 and sodium
hydroside feed line 23. Sodium hydroside feed line 23
3S is provided with a valve 24 controlled by feedback
W094/0~67 PCT/GB93/01762
Z1394~1
- 12 -
circuitry 25 connected to the pH meter 22. Outlet 26
from the CSTR 20 is connected to downstream cooling
and solids removal equipment (not shown).
In operation of the apparatus, the hydrochloric
acid feed stream is fed through line 4, conductivity
meter 5 and flow meter 6 to the static miser 2. The
conductivity and flow measurements are fed to the
ratio feed-forward control unit 7 which performs a
mathematical ratio function based on the
theoretically re~uired amount of calcium hydroxide
required to neutralise an acid stream having the
measured flow and concentration and controls the
valve 10 and flow meter 9 to provide a flow of
calcium hydroxide through line 8 to the static miser
2 which is sufficient to provide 95~ of the
theoretical amount of calcium hydroside
required to perform the neutralisation. The calcium
hydroxide and hydrochloric acid streams are mixed in
the static miser 2 and flow through the pipeline 3
which is of sufficient capacity to allow a 40 second
residence time within the pipe. The mixed stream then
enters the CSTR 12 where it is mixed by agitator 13.
The p8 of the solution within the CSTR is
continuously monitored by pH meter 14 and the
addition of calcium hydroxide to the CSTR is
controlled by feedback circuitry 17 from pH meter 14
to valve 16, to produce a solution in the CSTR with a
pH of about 2. The solution flows under gravity from
outlet 18 of CSTR 12 through inlet 19 of, and into
CSTR 20. The pH of the solution in the CSTR 20 is
then adjusted to pH 7.5 by the addition of sodium
hydroxide through line 23 under the control of
feedback circuitry 25 provided from pH meter 22 to
valve 24 on the sodium hydroxide feed line 23. The
neutralised stream from outlet 26 then passes to
W094/0~67 z~3~ PCT/GB93/01762
_ 13 -
downstream processes in which residual solids are
removed and the stream is cooled, with a consequent
increase in pH to about pH 8.
The invention is further illustrated by the
following example which was conducted on a laboratory
apparatus described below.
The apparatus comprised a 6~ spiral helix
plastic mixer (supplied by RS Components Ltd, Ref:
503/385) set inside a glass tubing of length 15cm and
diameter lOmm, and provided with hydrochloric acid
and calcium hydroxide feed inlets, each having a
peristaltic pump. A two metre length of rubber tubing
1-5 was attached in fluid flow communication with the exit
from the glass tubing, the glass and rubber tubing
together providing the plug flow reaction zone and
the rubber tubing having provided therein a vent for
carbon dioside generated during the neutralisation
reaction.
The outlet from the rubber tubing fed into a
50ml vessel provided with a pH feedback control
system comprising a pH meter set at pH 1, and
electronic circuitry connected to the calcium
hydroxide feed line to control the flow rate of
calcium hydroxide into the glass tubing (this
arrangement providing a small-scale, short-lifetime
equivalent to a ratio-feed-forward control of the
calcium hydroxide feed from the acid flow).
Below the 50ml vessel, there is provided a
stirred tank of capacity 3.5 litres provided with a
pH feedback control system comprising a pH meter set
at pH 2 and a calcium hydroxide feed line. The pH
control system controls the feed of calcium hydroxide
to this stirred tank to achieve a measured pH of 2
WO 94/04467 PCI/GB93/0176"
X~39A~
_ 14
for the solution in the tank. The outlet from the
tank is connected to a second stirred tank of
capacity 3.25 litres and provided with a sodium
hydroxide feed line and pH feedback control system
set at pH 6.1. The pH control system controls the
feed of sodium hydroxide to this stirred tank to
achieve a measured pH of 6.1 for the solution in the
tank. The outlet from this second stirred tank is
connected to a third vessel provided with a pH meter
and in which the p8 of the product neutralised
solution is measured.
In a continuous run over a 5 hour period, a 33Z
w/w solution of hydrochloric acid was fed to the
glass tubing at a flow rate of 3.6 kg/hour together
with 10.6 kg/hour of a lOZ w/w lime slurry,
corresponding to 85Z of the theoretically calculated
amount of lime required to achieve the neutralisation
and the flow rates being such that a residence time
of 40 seconds was provided in the plug flow reaction
zone.
The process stream flowed continuously into the
50ml vessel in which the pH of the solution therein
was measured in order to control the flow rate of the
calcium hydroxide to the glass tubing to achieve a pH
of 1 for the solution in this vessel. The overflow
from the vessel flowed into the first stirred tank
beneath the vessel and in which the solution had a
residence time of 15 minutes and to which further
(about l.lkg/hour) calcium hydroxide was added under
feedback control from the pH meter in order to
achieve a measured pH for the solution exiting the
tank of pH 2. The solution exiting the first tank
flowed continuously into the second stirred tank in
which the solution had a residence time of 15 minutes
and to which sodium hydroxide was added under
W094/04467 PCT/GB93/01762
;~ 4~
- 15 -
feedback control from the pH meter in order to
achieve a measured pH for the solution exiting the
tank of pH 6.1. The solution exiting the second
stirred tank flowed into a third tank in which the pH
of the product neutralised stream was measured.
Over the 5 hour period, a neutralised stream was
produced in which the pH of the product varied
between 6.9 and 7.4.
It will be readily apparent from the foregoing
description and example that a concentrated acid
stream may be neutralised without first diluting the
stream, thus directly reducing the required reactor
volume and associated reactor costs, whilst providing
the required control to meet the increasingly
stringent requirements of environmental legislation
on the pH control permitted for the neutralised
discharge. The rate of reaction of calcium hydroside
in the aqueous solution is in fact promoted by acid
conditions and this effect may be esploited to
further decrease the required reactor volumes and
costs. Moreover when employing calcium hydroxide
derived from typical high calcium lime, significant
advantage is obtained by a buffering effect which
allows effective and reliable control of pH to
neutrality.