Note: Descriptions are shown in the official language in which they were submitted.
.'VO 94/02577 21~ 0 2 (~ 8 PCI/US93/06149
ENZY~T:~C DBTERGENq~ COMPO8I~ION8 IN~IIB~TING
DYE: TRl~N8FER '.
~,.
=.
Field of the Invention
: :
- The present invention relates to dye transfer inhibiting
compositions containing enzymes.
More in particular, this invention relates to dye transfer
inhibiting compositions comprising polyamine N-oxide containing
poIymers and enzymes. ~
~.
;
Baakaround of the Invention ~`
Detergent compositions containing enzymes are well known in
art. It is equally well recognized that enzyme deactivation
occurs in detergent compositions formulated with enzymes.
,~
SU BSTlTUil'E SHEET
''"
~ .:
`~
W094/02577 21 4 0 2 ~ 8 2 PCT/US93/06149~-
The loss of detergent activity of enzymes is among others
depending on the presence of adjunct detergent ingredients.
One type of adjunct detergent ingredient that is added to
detergent ingredients are dye transfer inhibiting polymers. `
Said polymers are added to detergent compositions in order to
inhibit the transfer of dyes from colored fabrics onto other
fabrics washed therewith. These polymers have the ability to
complex or adsorb the fugitive dyes washed out of dyed fabrics -
before the dyes have the opportunity to become attached to
other articles in the wash.
Copending European Patent Application No. 92202168.8
describes polyamine N-oxide containing polymers which are very
efficient in eliminating transfer of solubilized or suspended
dyes. It has now been surprisingly found that certain polyamine
N-oxide polymers provide a stabilizing effect for enzymes
formulated in detergent compositions.
~.,,
In addition to this stabilizing effect, the dye transfer
inhibiting performance of the polyamine N-oxide containing
polymers are enhanced by the addition of certain type of
enzymes. This finding ailows to formulate detergent
compositions which exhibit excellent dye transfer inhibiting
properties while maintaining excellent enzyme activity.
According to another embodiment of this invention a process
is also provided for laundering operations involving colored
fabrics.
Polymers have been used within detergent compositions to
inhibit dye transfer. EP-A-O lQ2 923 describes the use of
carboxyl containing polymers within an aqueous compositions.
DE-A-2 814 329 discloses the use of N-vinyl-oxazolidone
polymers and FR-A-2 144 721 discloses the use of 15-35% of a
copolymer of polyvinylpyrrolidone and acrylic acid nitrile or
maleic anhydride within a washing powder. EP-265 257 describes
detergent compositions comprising an alkali-metal carboxy-metal
carboxymethylcellulose, a vinylpyrrolidone polymer and a
polycarboxylate polymer.
~ 1 4 0 ~ 8 8 ~
'O 94/02577 3 P ~ /US93/06149
'
Summary of the Invention
The present invention relates to inhibiting dye transfer
compositions comprising
~. .
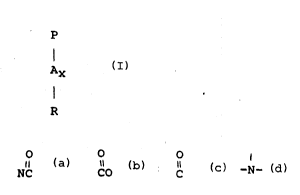
a) a polymer selected from polyamine N-oxide containing
polymers which contain units having the following structure
formula :
, .
P ~.
Ax `~
R
wherein P is a polymerisable unit, whereto the N-O group can !'`''~
be attached to or wherein the N-O group forms part of
the polymerisable unit.
,.
O o o ,
A is NC, CO, C, -O-,-S-,-N- ; x is O or l;
R are aliphatic, ethoxylated aliphatic, aromatic, `
heterocyclic or alicyclic groups whereto the nitrogen
of the N-O group can be attached or wherein the
nitrogen of the N-O group is part of these groups.
b) an enzyme.
Detailed descri~tion of the invention --
. ~
The compositions of the present invention comprise as an
essential element a polymer selected from polyamine N-oxide
containing polymers which contain units having the following
structure formula (I):
P ''
':
(I) Ax
~:.
R
SU BST17- UT~ ~;H ET
W094/0~577 2I10~88 ~ PCT/US93/06149 _ ~
wherein P is a polymerisable unit, whereto the R-N-O group can
be attached to OL- wherein the R-N-O group forms part of .;;
the polymerisable unit or a combination of both.
` ~
~ ;,
O o O , '
A is NC, ~O, C, -O-,-S-, -N- ; x is O or l;
R are aliphatic, ethoxylated aliphatics, aromatic, ~.
heterocyclic or alicyclic groups or any combination `
thereof whereto the nitrogen of the N-O group can be
attached or wherein the nitrogen of the~N-O group is
part of these groups
The N-O group can be represented by the following general
structures :
o O .~;.
-
(Rl)x -N- (R2)y -N- (Rl)x ?.~
(R3)z .
, `
wherein Rl, R2, R3 are aliphatic groups, aromatic, heterocyclic -"
or alicyclic groups or combinations thereof, x or/and y j`-
or/and z is 0 or 1 and wherein the nitroqen of the N-O ..
group can be attached or wherein the nitrogen of the N~
O group forms part of these gr`oups.
The N-O group can be part of the polymerisable unit (P) or `~
can be attached to the polymeric backbone or a combination of "``
both.
Suitable polyamine N-oxides wherein the N-O group forms part ~ !`
of the polymerisable unit comprise polyamine N-oxides wherein ~;~
R is selected from aliphatic, aromatic, ~alicyclic or
heterocyclic groups.
One class of said polyamine N-oxides comprises the group of.
polyamine N-oxides wherein the nitrogen of the N-O group forms ~.
'~:
' :
21402~
094/02577 5 PCT/US93/06149
part of the R-group. Preferred polyamine N-oxides are those `-
wherein R is a heterocyclic group such as pyridine, pyrrole,
imidazole, pyrrolidine, piperidine, quinoline and derivatives
thereof. -
Another class of said polyamine N-oxides comprises the ~roup
of polyamine N-oxides wherein the nitrogen of the N-O group is
attached to the R-group. -
Other suitable polyamine N-oxides are the polyamine oxides i`
whereto the N-O group is attached to the polymerisable unit.
Preferred class of these polyamine N-oxides are the polyamine i;
N-oxides having the general formula (I) wherein R is an
aromatic, heterocyclic or alicyclic groups wherein the nitrogen
of the N-O functional group is part of said R group.
Examples of these classes are polyamine oxides wherein R is a
heterocyclic compound such as pyridine, pyrrole, imidazole and
derivatives thereof.
Another preferred class of polyamine N-oxides are the polyamine
oxides having the general formula (I) wherein R are aromatic,
heterocyclic or alicyclic groups wherein the nitrogen of the N-
0 functional group is attached to said R groups.
Examples of these classes are polyamine oxides wherein R groups
can be aromatic such as phenyl.
Any polymer backbone can be used as long as the amine oxide
polymer formed is water-soluble and has dye transfer ihhibiting
properties. Examples of suitable polymeric backbones are
polyvinyls, polyalkylenes, polyesters, polyethers, polyamide,
polyimides, polyacrylates and mixtures thereof.
-
The amine N-oxide polymers of the present invention typically
have a ratio of amine to the amine N-oxide of l0:l to
l:lOOOOOO. However the amount of amine oxide groups present in
the polyamine N-oxide containing polymer can be varied by
appropriate co-polymerization or by appropriate degree of N-
oxidation. Preferably, the ratio of amine to amine N-oxide is -
from 2:3 to l:lOOOOOO. More preferably from l:4 to l:l000000,- -~
most preferably from l:9 to l:l000000. The polymers of the
SUE3S~IT~TE ~.r~EET
W O 94/02577 ~ 1 4 Q 2 8 8 - 6 PC~r/US~3/06149 "~
present invention actually encompass random or block copolymers
where one monomer type is an amine N-oxide and the other
monomer type is either an amine N-oxide or not. The amine oxide ;
unit of the polyamine N-oxides has a PKa < lO, preferably PKa < ~''
7, more preferred PKa < 6.
The polyamine N-oxide containing polymers can be obtained in
almost any degree of polymerisation. The degree of
polymerisation is not critical provided the material has the i~,
desired water-solubility and dye-suspending power. 'r','
Typically, the average molecular weight of the polyamine N- ;~
oxide containing polymers is within the range of 500 to ',',
lO00,000; preferably from 1000 to 30000, more preferably from ,~
3000 to 20000, most preferably from S000 to 15000. ''
The polyamine N-oxide containing polymers of the present
invention are typically present from 0.001% to 10%, more
preferably from 0.05~ to 1%, most preferred from 0.05% to 0.5 % ',
by weight of the dye transfer inhibiting composition.
The present compositions are conveniently used as additives to
conventional detergent compositions for use in laundry '-~
operations. The present invention also encompasses dye transfer ~-'
inhibiting compositions which will contain detergent
ingredients and thus serve as detergent compositions. ,''`
: : '
Metho~s for makina poly~nin~ N-oxide~
The production of the polyamihe-N-oxide containing polymers
may be accomplished by polymerizing the amine monomer and
oxidizing the resultant polymer with a suitable oxidizing
agent, or the amine oxide monomer may itself be polymerized to
obtain the,polyamine N-oxide. ~,
The synthesis of polyamine N-oxide containing polymers can be ,"
exemplified by the synthesis,of polyvinyl-pyridine N-oxide. j,~`
Poly-4-vinylpyridine ex Polysciences (mw. 50 000, 5.0 g.,
0.0475 mole) was predisolved in 50 ml acetic acid and treated . ,
with a peracetic acid solution (25 g of glacial acetic acid,
6.4 g of a 30% vol. solution of H202, and a few drops of H2S04 '
give 0.0523 mols of peracetic acid) via a pipette. The mixture `
S~E35~iTUTE SHEET
':'''
:
V094/02577 7 PCT/US93/06149 ~-
was stirred over 30 minutes at ambient temperat~re (32 c). The ~-
mixture was then heated to 80-85 c using an oil bath for 3
hours before allowing to stand overnight. The polymer solution ,~
then obtained is mixed with ll of acetone under agitation. The
resulting yellow brown viscous syrup formed on the bottom is ~i`
washed again with ll of aceton to yield a pale crystalline ~`
solid. .~
The solid was filtered off by gravity, washed with aceton and --
then dried over P2O5. -~
The amine : Amine N-oxide ratio of this polymer is l:4
(determined by NMR).
ENZYMES
The enzymes which are to be included in the detergent
formulations are detersive enzymes which can be used for a wide
variety of purposes including removal of protein-based,
carbohydrate-based, or triglyceride-based stains, for example,
and prevention of refugee dye transfer. The enzymes to be
incorporated include proteases, amylases, lipases, cellulases, `
and peroxidases, as well as mixtures thereof. Other types of
enzymes may also be included.
The enzymes may be of any suitable origin, such as vegetable,
animal, bacterial, fungal and yeast origin. However, their `
choice is governed by several factors such as pH-activity
andjor stability optima, thermostability, stability versus
active detergents, builders and so on. In this respect
bacterial or fungal enzymes are preferred, such as bacterial
~,
amylases and proteases, and fungal cellulases.
Enzymes are normally incorporated at levels sufficient to
provide up to about 5 mg by weight, more typically about 0.05
mg to about 3 mg, of active enzyme per gram of the composition.
Cellulase :
The cellulases usable in the present invention include both
bacterial or fungal cellulase. Preferably, they will have a pH
optimum of between 5 and 9.5. Suitable cellulases are disclosed
W094/02577 ~ ~ ~ 0 , 3 8 8 PCT/US93/06149fJ- ~
. .
in U.S. Patent 4,435,307, which discloses fungal cellulase
produced from Humicola insolens. Suitable cellulases are also
disclosed in GB-A-2.075.028 ; GB-A-2.095.275 and DE-OS-
2.247.832.
Examples of such cellulases are cellulases produced by a
strain of Humicola insolens (Humicola grisea var. thermoidea),
particularly the Humicola strain DSM 1800, and cellulases
produced by a fungus of Bacillus N or a cellulase 212-producing
fungus belonging to the genus Aeromonas, and cellulase
extracted from the hepatopancreas of a marine mollusc
(Dolabella Auricula Solander).
Other suitable cellulases are cellulases originated from
Humicola Insulens having a molecular weight of about 50KDa, an
isoelectric point of 5.5 and containing 415 amino acids. Such
cellulase are described in Copending European patent
application No. 93200811.3
Especiàlly suitable cellulase are the cellulase having color
care benefits. Examples of such cellulases are cellulase
described in European patent application No. 91202879.2,
Carenzyme (Novo). It has been found that cellulase enhances
considerably the efficiency of polyamine N-oxide containing
polymers in terms of color appearance.
Prote~se :
~ ."
Suitable examples of proteases are the subtilisins which are
obtained from particular strains of B.subtilis and r``'
B.licheniforms. Proteolytic enzymes suitable for removing
protein-based stains that are commercially available include
those sold under- the tradenames Alcalase , Savinase and
Esperase by Novo Industries A/S (Denmark) and Maxatase by
International Bio-Synthetics, Inc. (The Netherlands) and FN- `
base by Genencor, Optimase and opticlean by MKC.
Of interest in the category of proteolytic enzymes, ~
especially for liquid detergent compositions, are enzymes -
referred to herein as Protease A and Protease B. Protease A and
methods for its preparation are described in European Patent
Application 130,756. Protease B is a proteolytic enzyme which
SVE~Si~-~U~E Srz E~T :~;
,.
2 ~ 8 ~` `
~'094/02577 9 PCT/US93/~6149 ,~
. .,
differs from Protease A in that it has a leucine substituted
for tyrosine in position 217 in its amino acid sequence.
Protease B is described in European Patent Application Serial
No. 87303761.8. Methods for preparation of Protease B are also
disclosed in European Patent Application 130,756.
Amvl~s~: ^
Amylases include, for example, amylases obtained from a `;
special strain of B.licheniforms, described in more detail in
British Patent Specification No. 1,296,839 (Novo). Amylolytic
proteins include, for example, Rapidase, Maxamyl (International -
Bio-Synthetics, Inc.) and Termamyl,(Novo Industries). -~
Li~ase :
".
Suitable lipase enzymes for detergent usage include those
produced by microorganisms of the Pseudomonas group, such as
Pseudomonas stutzèri ATCC 19.154, as disclosed in British
Patent 1,372,034. Suitable lipases include those which show a
positive immunoligical cross-reaction with the antibody of the
lipase, produced by the microorganism Pseudomonas fluorescent
IAM 1057. This lipase and a method for its purification have
been described in Japanese Patent Application 53-20487. This
lipase is available from Amano Pharmaceutical Co. Ltd., Nagoya,
Japan, under the trade name Lipase P "Amano," hereinafter
referred to as "Amano-P". Such lipases of the present invention
should show a positive immunological cross reaction with the
Amano-P antibody, using the standard and well-known
immunodiffusion procedure according to Ouchterlony (Acta. Med.
Scan., 133, pages 76-79 (1950)). These lipases, and a method
for their immunological cross-reaction with Amano-P, are also
described in U.S. Patent 4,707,291. Typical examples thereof
are the Amano-P lipase, the lipase ex Pseudomonas fragi FERM P
1339 (available under the trade name Amano-B), lipase ex
Pseudomonas nitro-reducens var. lipolyticum FERM P 1338
(available under the trade name Amano-CES), lipases ex
Chromobacter viscosum, e.g. Chromobacter viscosum var.
W O 94/02~77 2 1 4 0 2 8 f~ 10 PC~r/US93/06149;,~``
r
lipolyticum NRRLB 3673, commercially available from Toyo Jozo
Co., Tagata, Japan; and further Chromobacter viscosum lipases
from U.S. Biochemical Corp., U.S.A. and Disoynth Co., The
Netherlands, and lipases ex Pseudomonas ~ladioli.
Especially suitable Lipase are lipase such as M1 Lipase (Ibis)
and Lipolase (Novo).
Peroxidase :
Peroxidase enzymes are used in combination with oxygen
sources, e.g. percarbonate, perborate, persulfate, hydrogen
peroxide, etc. They are used for "solution bleaching", i.e. to
prevent transfer of dyes of pigments removed from substrates
during wash operations to other substrates in the wash
solution. Peroxidase enzymes are known in the art, and include,
for example, horseradish peroxidase, ligninase, and haloperoxi-
dase such as chloro- and bromo-peroxidase. Peroxidase-
containing detergent compositions are disclosed, for example,
in PCT Internation Application ~O 89/099813, published Octobcr~
19, 1989, by O. Kirk, assigned to Novo Industries A/S, and in
European Patent aplication No. 91202882.6.
The peroxidases which may be employed for the present purpose
may be isolated from and are producible by plants (e.g. horse-
radish peroxidase) or micororganisms such as fungi or bacteria.
Some preferred fungi include ~strains belonging to the subdivi-
sion Deuteromycotina, class Hypho-mycetes, e.g. Fusarium, Humi-
cola, Tricoderma, Myrothecium, Verticillum, Arthromyces, Calda-
riomyces, Ulocladium, Embellisia, Cladosporium or Dreschlera,
in~particular Fusarium oxysporum (DSM 2672), Humicola insolens
Tricho-derma resii, Myrothecium verrucana (IFO 6113), Verticil-
luum alboatrum, Verticillum dahlie, Arthromyces ramosus tFERM
P-7754), Caldariomyces fumago, Ulocladium chartarum, Embellisia
allior Dreschlera halodes.
-Other preferred fungi include strains belonging to the sub-
~; division Basidiomycotina, class Basidiomycetes, e.g. Coprinus,
Phanerochaete, Coriolus or Trametes, in parti-cular Coprinus
cinereus f. microsporus (IFO 8371), Copri-nus macrorhizus,
. . .
SUBSTI~U ~ E S~ T ~
.
2140288
vo 94/02577 11 ` PCr/US93/06t49
Phanerochaete chrysosporium (e.g. NA-12) or Coriolus versicolor
(e.g. PR4 28-A).
- Further preferred fungi include strains belonging to the
subdivision Zygomycotina, class Mycoraceae, e.g. Rhizopus or
~Mucor, in particular Mucor hiemalis.
Some preferred bacteria include ~ strains of the order
Actinomycetales, e.g. Streptomyces spheroides (ATTC 23965),
Streptomyces thermoviolaceus~ (IFO 12382) or St~rep-toverticillum
; ~ ; verticillium ssp. vert~icillium. ~
Other preferred bacteria ~ inlude Bacillus pumillus (ATCC
905)~ Bacillus~ stearothermophilus,~ Rhododbacter sphae-roides,
Rhodomonas~ palustri, Streptococcus lactis, Pseudo-monas purro-
; ~ cinia;~(ATCC~iS958) or Ps~eudomonas fluarescens ~NRRL B-ll).
~ Other~potent~ial sources of~useful peroxidases are listed in
B.C. Saunders~et al~.~, op. ~oit.~, pp. ~41~-43. ~
Methods of producing enzymes to be used according to the
, ~ .
invention are described in the art, cf. ~for example FEBS
etters~;l625, ~ ~l`ied and~ Environmentàl~ Micro-b~iolo~,
Feb~ 1985~ pp.~ ;27~3_278~ pplied~ Mic biol.~ B o-technol._~26,
19~87,~. 158-16~3,~ ~Bi~otechnology~ è~ers~ 9(~5),~ 1987, pp~. 3-57-
360, ~Nature~32~6~ 2~ l987~ FEBS~ Letters 4270, 209(2),
p~ 321,~ EP~179 48~6,~EP 200 ~565, GB~ 2 ~167 42`1,~` EP 171 074, and
Aaric. Bio~ Chem.~50~ 1986,~ p. ~247~
Particùlarly~;preferred~pè`roxidases~;are those which are active
at ~the~typical~pH~ of washing~ rs, i.~e. at a ~pH of 6.5-10.5,
preferably 6~.~5-9.~5,~a`nd~ most`~preferably 7.5-9.5; Such enzymes
may be~isolatd~by~scrèe~ng~f~or~ the relevant ~enz~e~ production
alkal~ilic~ microorgan~sms~ e~.~g.~ using the ABTS assay
~cribed~ in~R.E,~ilds and;~W.G.~ Bardsley,~ 8iochem. J.145,
1975,~pp. ~93-103`~
Other p~referred peroxidases are those whioh exhibit a good
thermostability as well as a good stability towards commonly
us-d ~detergent comp`onents ~ such as ~non-ionic, cat-ionic, or
anionic surfacta~nts,~ d-terg-nt"builders~,~ phos-phate etc.
Another group o;f~useful ~peroxidàses~are haloperoxidases, such
as~chloro-~and bromoperoxidases.
The~ peroxidase-ensyme~may~ futhermore be one which is produ-
`cible ~by ~a method~ comprising cultivating a host cell trans-
W 0 94/02577 2140288 lZ PCT~US93/06149. ~
formed with a recombinant DNA vector which carries a DNAsequence encoding said enzyme as well as DNA sequences encoding
functions permitting the expression of the DNA sequence
encoding the enzyme, in a culture medium under conditions
permitting the expression of the enzyme and recover~ing the
enzyme from the culture.
A DNA fragment encoding the enzyme may, for instance, be
isolated by establishing a cDNA or genomic library of a micro-
organism producing the enzyme of interest, such as one of the
organisms mentioned above, and screening for positive clones by
conventional procedures such as by hybridization to oligonu-
cleotide probes synthesized on the basis of the full or partial
amino acid sequence of the enzyme, or by selecting for clones
expressing the appropriate enzyme activity , or by selecting
for clones producing a protein which is reactive with an
antibody against the native enzyme.
Once selected, the DNA sequence may be inserted into a
suitable replicable expression vector comprising appropriate
promotor, operator and terminator sequences permitting the
enzyme to be expressed in a particular host organism, as welI
as an origin of replication, enabling the vector to replicate
in the host organism in question.
The resulting expression vector may then be transformed into
a suitable host cell, such as a fungal cell, preferred examples
of which are a species of Aspergillus, most preferably Asper-
gillus oryzae or Aspergillus niger. Fungal cells may be trans-
formed by a process involving protoplast formation and trans-
formation of the protoplasts followed by regeneration of the
cell wall in a manner known per se. The use of Aspergillus as
a host micororganism is described in EP 238,023 (of Novo
Industri A/S).
Alternatively, the host organisms may be a bacterium, in
-particular strains of Streptomyces and Bacillus, or E. coli.
The transformation of bacterial cells may be performed
according to conventional methods, e.g. as described in T.
Maniatis et al., Molecular Clonina : A Laboratory Manual, Cold
Spring Harbor, 1982.
SUBS~ITU~E ~ ,E~ET
21402~8 `
'094/02577 13 ``i l~ pCT/US93/06149
The screening of appropriate DNA sequences and construction
of vectors may also be carried out by standard procedures, cf.
T. Maniatis et al., op. c_t.
The medium used to cultivate the transformed host cells may
be any conventional medium suitable for growing the ho~t cells
in guestion. The expressed enzyme may conveniently be secreted
into the culture medium and may be recovered therefrom by well-
known procedures including separating the cells from the medium
by centrifugation or filtration, precipitating proteinaceous
components of the medium by means of a salt such as ammonium
sulphate, followed by chromatographic procedures such as ion
exchange chromatography, affinity chromatography, or the like.
The screening of appropriate DNA sequences and construction
of vectors may also be carried out by standard procedures, cf.
T. Maniatis et al., op. cit.
The medium used to cultivate the transformed host cells may
be any conventional medium suitable for growing the host cells
in question. The expressed enzyme may conveniently be secreted
into the culture medium and may be recovered therefrom by well-
known procedures including separating the cells from the medium
by centrifugation or filtration, precipitating proteinaceous
components of the medium by means of a salt such as ammonium
sulphate, ~followed by chromatographic procedures such as ion
exchange chromatography, affinity chromatography, or the like.
At the beginning or during the process, H22 may be added,
e.g. in an amount of 0.001-5 mM, particularly 0.01-l mM. When
using Coprinus peroxidase, 0.01-0.25 mM H22 is preferred, and
with B. pumilus peroxidase 0.1-1 mM H202.
The hydrogen peroxide may be added as hydrogen peroxide or a
precursor thereof, preferably a perborate or percarbonate. The
level of hydrogen peroxide precursor that can be used is depen-
dent on the specific properties of the peroxidase chosen, e.g.
Coprinus peroxidase should be applied in a detergent composi-
tion which contains less than 5% perborate.
In the process of this invention, it may be desirable to
utilize an enzymatic process for hydrogen peroxide formation.
Thus, the process according to the invention may additionally
.
W094/02~77 2 1 4 0 2 8 8 14 PCT/US93/06149fc~
comprise adding an enzymatic system (i.e. an enzyme and a
substrate therefore) which is capable of generating hydrogen
peroxide at the beginning or during the washing and/or rinsing
process.
One such category of hydrogen peroxide generating ~systems
comprises enzymes which are able to convert molecular oxygen
and an organic or inorganic substrate into hydrogen peroxide
and the oxidized substrate respectively. These enzymes produce
only low levels of hydrogen peroxide, but they may be employed
to great advantage in the process of the invention as the
presence of peroxidase ensures an efficient utilization of the
hydrogen peroxide produced.
Preferred hydrogen peroxide-generating enzymes are those
which act on cheap and readily available substrates which may
conveniently be included into detergent compositions. An
example of such a substrate is glucose which may be utilized
for hydrogen peroxide production by means of glucose oxidase.
Suitable oxidases include those which act on aromatic compounds
such as phenols and related substances, e.g. catechol oxidases,
laccase. Other suitable oxidases are urate oxidase, galactose
oxidase, alcohol oxidases, amine oxidases, amino acid oxidase,
amyloglucosidase, and cholesterol oxidase.
The preferred enzymatic systems are alcohol and aldehyde
oxidases.
The more preferred systems for granular detergent application
would have solid alcohols, e.g. glucose whose oxidation is
catalysed by glucose oxidase to glucoronic acid with the
formation of hydrogen peroxide.
The more preferred systems for liquid detergent application
would involve liquid alcohols which could also act as, for
example, solvents. An example is ethanol/ethanol oxidase.
The quantity of oxidase to be employed in compositions
according to the invention should be at least sufficient to
provide a constant generation of 0.01 to 10 ppm AvO per minute
in the wash. For example, with the glucose oxidase, this can be
~V~S~ ~T~TE S~EET
21~2~8
'~ 94/02577 15 ~ PC~r/US93/06149 `
achieved at room temperature and at pH 6 to 11, preferentially
7 to 9 with 50-5000 U/l glucose oxidase, 0.005 to 0.5 % glucose `?'~
under constant aeration.
:,' '
The addition of another oxidisable substrate for the
peroxidase at the beginning or during the washing and/or
rinsing process may enhance the dye transfer inhibitory effect -
of the peroxidase employed. This is thought to be ascribable
to the formation of short-lived radicals or other oxidised
states of this substrate which participate in the bleaching or
other modification of the coloured substance. Examples of such
oxidisable substrates are metal ions, e.g. Mn+~, halide ions,
e.g. chloride or bromide ions, or organic compounds such as
phenols, e.g. p-hydroxycinnamic acid or 2,4-dich~orophenol.
Other examples of phenolic compounds which may be used for the
present purpose are those given in M. Kato and S. Shimizu,
Plant Cell Physiol. 26(7), 1985, pp. 1291-1301 (cf. Table 1 in
particular) or B.C. Saunders et al., op. cit., p. 141 ff. The
amount of oxidisable substrate to be added is suitably between
about 1 ~M and 1 mM. ~;
In the process of the invention, the peroxidase will typical-
ly be added as a component of a detergent composition and may ~-
be added in an amount of 0.01 - 100 mg enzyme per liter of wash
liquid. As such, it may be included in the detergent composi-
tion in the form of a non-dusting granulate, a liquid, in
particular a stabilized liquid, or a p~otected enzyme. Non-
dusting granulates may be produced, e.g. as disclosed in US
4,106,991 and 4,661,452 (both to Novo Industri A/S) and may
optionally be coated by methods known in the art. Liquid
enzyme preparations may, for instance, be stabilized by adding
a polyol such as propylene glycol, a sugar or sugar alcohol,
lactic acid or `boric acid according to established methods.
Other enzyme stabilizers are well known in the art. Protected
enzymes may be prepared according to the method disclosed in EP
238,216. The detergent composition may also comprise one or
more substrates for the peroxidase. Usually, the pH of a
solution of the detergent composition of the invention will be
preferably from 7-12, especially from 7.5 to 9.5. The wash pH `
W094/Ot577 2 1 4 0 2 ~ PCT/US93/06149 .ir..
is dependent on the peroxidase chosen, e.g. Coprinus peroxidase
should be applied in a wash pH below 9.5.
It has been found that peroxidases enhance considerably the
efficiency of polyamine N-oxide containing polymers in terms of
dye transfer inhibition.
.~
A wide range of enzyme materials and means for their
incorporation into synthetic detergent granules is also
disclosed in U.S. Patent 3,553,139. Enzymes are further
disclosed in U.S. Patent 4,101,457, Place et al, issued July
18, 1978, and in U.S. patent 4,507,219, Hughes, issued March
26, 1985, both incorporated herein by reference. Enzyme
materials useful for liquid detergent formulations, and their
incorporation into such formulations, are disclosed in U.S.
Patent 4,261,868, Hora et al, issued April 14, 1981.
For granular detergents, the enzymes are preferably coated or
prilled with additives inert toward the enzymes to minimize
dust formation and improve storage stability. Techniques for
accomplishing this are well-known in the art. In liquid
formulations, an enzyme stabilization system is preferably
utilized. Enzyme stabilization techniques for aqueous detergent
compositions are well known in the art. For example, one
technique for enzyme stabilization in aqueous solutions
involves the use of free calcium ions from sources such as
calcium acetate, calcium formate and calcium propionate.
Calcium ions can be used in combination with short chain
carboxylic acid salts, preferably formates. See, for example,
U.S. patent 4,318,818. It has also been proposed to use polyols
like glycerol and sorbitol. Alkoxy-alcohols,
dialkylglycoethers, mixtures of polyvalent alcohols with
polyfunctional aliphatic amines (e.g., such as diethanolamine,
triethanolamine, di-isopropanolamime, etc.), and boric acid or
alkali metal borate. Enzyme stabilization techniques are
additionally disclosed and exemplified in U.S. patent
4,261,868, U.S. Patent 3,600,319 and European Patent
Application Publication No. 0 199 405, Application No.
86200586.5, Venegas. Non-boric acid and borate stabilizers are
,~
SU BSTITL; ~ E 5~ ET
~ 1 4 ~2 8 8
-;094/02577 17 PCT/US93tO6149
preferred. Enzyme stabilization systems are also described, for
example, in U.S. Patents 4,261,868, 3,600,319 and 3,519,570. -
DETERGENT ADJUNCT8 ~ ;
A wide range of surfactants can be used in the detergent ~;
compositions. A typical listing of anionic, nonionic,
ampholytic and zwitterionic classes, and species of these
surfactants, is given in US Patent 3,664,961 issued to Norris ~;
on May 23, 1972.
Mixtures of anionic surfactants are particularly suitable
herein, especially mixtures of sulphonate and sulphate
surfactants in a weight ratio of from 5:1 to 1:2, preferably
from 3:1 to 2:3, more preferably from 3:1 to 1:1. Preferred
sulphonates include alkyl benzene sulphonates having from 9 to
15, especially 11 to 13 carbon atoms in the alkyl radical, and
alpha-sulphonated methyl fatty acid esters in which the fatty
acid is derived from a C12-C18 fatty source preferably from a
C16-C18 fatty source. In each instance the cation is an alkali
metal, preferably sodium. Preferred sulphate surfactants are
alkyl sulphates having from 12 to 18 carbon atoms in the alkyl
radical, optionally in admixture with ethoxy sulphates having
- from 10 to 20, preferably 10 to 16 carbon atoms in the alkyl
radical and an average degree of ethoxylation of 1 to 6.
Examples of preferred alkyl sulphates herein are tallow alkyl ~-
sulphate, coconut alkyl sulphate, and C14_15 alkyl sulphates.
The cation in each instance is again an alkali metal cation, ~
preferably sodium. ~-
One class of nonionic surfactants useful in the present
invention are condensates of ethylene oxide with a hydrophobic
moiety to provide~ a surfactant having an average hydrophilic-
lipophilic balance (HLB) in the range from 8 to 17, preferably
from 9.5 to 13.5, more preferably from 10 to 12.5. The
hydrophobic (lipophilic) moiety may be aliphatic or aromatic in
nature and the length of the polyoxyethylene group which is `
condensed with any particular hydrophobic group can be readily ~`
: ;~
i "
:, '.
W094/02S77 2 1 ~ 0 2 8 8 18 PCT/US93/06149Js
adjusted to yield a water-soluble compound having the desired
degree of balance between hydrophilic and hydrophobic elements.
.,
Especially preferred nonionic surfactants of this type are
the Cg-Cl5 primary alcohol ethoxylates containing 3-8 ~oles of
ethylene oxide per mole of alcohol, particularly the C14-ClS .
primary alcohols containing 6-8 moles of ethylene oxide per
mole of alcohol and the C12-C14 primary alcohols containing 3-5
moles of ethylene oxide per mole of alcohol. `.
Another class of nonionic surfactants comprises alkyl .
polyglucoside compounds of general formula `.
RO (CnH2nO)tzx
wherein Z is a moiety derived from glucose; R is a saturated --
hydrophobic alkyl group that contains from 12 to 18 carbon
atoms; t is from 0 to 10 and n is 2 or 3: x is from 1.3 to 4, .:
the compounds including less than 10% unreacted fatty alcohol ,.
and less than 50% short chain àlkyl polyglucosides. Compounds .-~
of this type and their use in detergent are disclosed in EP-B 0
070 077, 0 075 996 and 0 094 118. -
Also suitable as nonionic surfactants are poly hydroxy fatty
acid amide surfactants of the formula
, . ,
R2 - C - N - Z,
Il I ',.'
O Rl ~
` ' `~`'
wherein R1 is H, or R1 is Cl 4 hydrocarbyl, 2-hydroxy ethyl, 2-
hydroxy propyl or a mixture thereof, R2 is Cs_31 hydrocarbyl,
and Z is a polyhydroxyhydrocarbyl having a linear hydrocarbyl I`
chain with at least 3 hydroxyls directly connected to the
chain, or an alkoxylated derivative thereof. Preferably, Rl is j:
methyl, R2 is a straight Cl1_15 alkyl or alkenyl chain such as !.
coconut alkyl or mixtures thereof, and Z is derived from a `~
SUBS ~ iT~TE ~HEET
. .
_ _ . . . , _.
~ 1 4 (~
~'094/02577 19 PCT/US93/06149
reducing sugar such as glucose, fructose, maltose, lactose, in
a reductive amination reaction.
~.~
The compositions according to the present invention may
further comprise a builder system. Any conventional~builder
system is suitable for use herein including aluminosilicate
materials, silicates, polycarboxylates and fatty acids,
materials such as ethylenediamine tetraacetate, metal ion
sequestrants such as aminopolyphosphonates, particularly -
ethylenediamine tetramethylene phosphonic acid and diethylene ^-
triamine pentamethylenephosphonic acid. Though less preferred
for obvious environmental reasons, phosphate builders can also
be used herein. `~
Suitable builders can be an inorganic ion exchange material,
commonly an inorganic hydrated aluminosilicate material, more
particularly a hydrated synthetic zeolite such as hydrated
zeolite A, X, B or HS.
Another suitable inorganic builder material is layered
silicate, e.g. SKS-6 (Hoechst). SKS-6 is a crystalline layered
silicate consisting of sodium silicate (Na2Si2O5).
Suitable polycarboxylates containing one carboxy group
include lactic acid, glycolic acid and ether derivatives
thereof as disclosed in Belgian Patent Nos. 831,368, 821,369
and 821,370. Polycarboxylates containing two carboxy groups
include the water-soluble salts of succinic acid, malonic acid,
(ethylenedioxy) diacetic acid, maleic acid, diglycollic acid,
tartaric acid, tartronic acid and fumaric acid, as well as the
ether carboxylates described in German Offenlegenschrift `
2,446,686, and 2,446,687 and U.S. Patent No. 3,935,257 and the
sulfinyl carboxylates described in Belgian Patent No. 840,623.
Polycarboxylates containing three carboxy groups include, in
particular, water-soluble citrates, aconitrates and
citraconates as well as succinate derivatives such as the
carboxymethyloxysuccinates described in British Patent No.
1,379,241, lactoxysuccinates described in Netherlands
Application 7205873, and the oxypolycarboxylate materials such
`".
:
W 0 94/02577 2 1 4 0 2 3 ~ 20 PCT/US93/06149~s
as 2-oxa-1,1,3-propane trlcarboxylates described in British
Patent No. 1, 387, 447 .
Polycarboxylates containing four carboxy groups include
oxydisuccinates disclosed in British Patent No. 1,~61,829,
1,1,2,2-ethane tetracarboxylates, 1,1,3,3-propane
tetracarboxylates and 1,1,2,3-propane tetracarboxylates.
Polycarboxylates containing sulfo substituents include the
sulfosuccinate derivatives disclosed in British Patent Nos.
1,398,421 and 1,398,422 and in U.S. Patent No. 3,936,448, and
the sulfonated pyrolysed citrates described in British Patent
No. 1,082,179, while polycarboxylates containing phosphone
substituents are disclosed in British Patent No. 1,439,000.
Alicyclic and heterocyclic polycarboxylates include
cyclopentane-cis,cis,cis-tetracarboxylates, cyclopentadienide
pentacarboxylates, 2,3,4,5-tetrahydrofuran - cis, cis, cis-
tetracarboxylates, 2,5-tetrahydrofuran -cis - dicarboxylates,
2,2,5,5-tetrahydrofuran - tetracarboxylates, 1,2,3,4,5,6-hexane
-hexacarboxylates and and carboxymethyl derivatives of
polyhydric alcohols such as sorbitol, mannitol and xylitol.
Aromatic polycarboxylates include mellitic acid, pyromellitic
acid and the phtalic acid derivatives disclosed in British
Patent No. 1,425,343.
Of the above, the preferred polycarboxylates are
hydroxycarboxylates containing up to three carboxy groups per
molecule, more particularly citrates.
Preferred builder systems for use in the present compositions
include a mixture of a water-insoluble aluminosilicate builder
such as zeolite A or of a layered silicate (sks/6), and a
water-soluble carboxylate chelating agent such as citric acid.
, ~ .
A suitable chelant for inclusion in the detergent
compositions in accordance with the invention is - `~
ethylenediamine-N,N'-disuccinic acid (EDDS) or the alkali ~`
metal, alkaline earth metal, ammonium, or substituted ammonium ~-
salts thereof, or mixtures thereof. Preferred EDDS compounds `
SU BSTITUTE SH ET ```
. .
- vog4/02577 2l - PCT/US93/06149
are the free acid form and the sodium or magnesium salt
thereof. Examples of such preferred sodium salts of EDDS
include Na2EDDS and Na4EDDS. Examples of such preferred
magnesium salts of EDDS include MgEDDS and Mg2EDDS. The
magnesium salts are the most preferred for inclusion in
compositions in accordance with the invention.
Especially for the liquid execution herein, suitable fatty acid
builders for use herein are saturated or unsaturated C10-18
fatty acids, as well as well as the corresponding soaps.
Preferred saturated species have from 12 to 16 carbon atoms in
the alkyl chain. The preferred unsaturated fatty acid is oleic
acid.
Preferred builder systems for use in granular compositions
include a mixture of a water-insoluble aluminosilicate builder
such as zeolite A, and a watersoluble carboxylate chelating
agent such as citric acid.
Other builder materials that can form part of the builder
system for use in granular compositions the purposes of the
invention include inorganic materials such as alkali metal'
carbonates, bicarbonates, silicates, and organic materials such
as the organic phosphonates, amiono polyalkylene phosphonates
and amino polycarboxylates.
Other suitable water-soluble organic salts are the homo- or co-
polymeric acids or their salts, in which the polycarboxylic
- acid comprises at least two carboxyl radicals separated from
'each other by not more than two carbon atoms.
Polymers of this type are disclosed in GB-A-1,596,756.
Examples of such 'salts are polyacrylates of MW 2000-5000 and
their copolymers with maleic anhydride, such copolymers having
a molecular weight of from 20,000 to 70,000, especially about
40,000.
Detergency builder salts are normally included in amounts of
from 10% to 80% by weight of the composition preferably from
20% to 70% and most usually from 30~ to 60% by weight.
Detergent ingredients that can be included in the detergent
compositions of the present invention include bleaching agents.
, `.~
W094/02s77 211~2~8 22 PCT/US~3/06149~
These bleaching agent components can include one or more oxygen
bleaching agents and, depending upon the ble~ching agent
chosen, one or more bleach activators. When present bleaching
compounds will typically be present at levels of from about 1%
to about 10%, of the detergent composition. In ~eneral,
bleaching compounds are optional components in non-liquid
formulations, e.g. granular detergents. If present, the amount
of bleach activators will typically be from about 0.1% to about
60%, more typically from about 0.5% to about 40% of the
bleaching composition.
The bleaching agent component for use herein can be any of
the bleaching agents useful for detergent compositions
including oxygen bleaches as well as others known in the art.
In a method aspect, this invention further provides a
method for cleaning fabrics, fibers, textiles, at temperatures
below about 50C, especially below about 40C, with a detergent
composition containing polyamine N-oxide containing polymers,
optional auxiliary detersive surfactants, optional detersive
adjunct ingredients, and a bleaching agent.
The bleaching agent suitable for the present invention can be
an activated or non-activated bleaching agent.
-One category of oxygen bleaching agent that can be used
encompasses percarboxylic acid bleaching agents and salts
thereof. Suitable examples of this class of agents include
magnesium monoperoxyphthalate hexahydrate, the magnesium salt
of meta-chloro perbenzoic acid, 4-nonylamino-4-oxoperoxybutyric
acid and diperoxydodecanedioic acid. Such bleaching agents are
disclosed in U.S. Patent 4,483,781, U.S. Patent Application
~- 740,446, European Patent Application 0,133,354 and U.S. Patent
4,412,934. Highly preferred bleaching agents also include 6-
nonylamino-6-oxoperoxycaproic acid as described in U.S. Patent
4,634,551.
Another category of bleaching agents that can be used
encompasses the halogen bleaching agents. Examples of
hypohalite bleaching agents, for example, include trichloro
isocyanuric acid and the sodium and potassium
dichloroisocyanurates and N-chloro and N-bromo alkane
.~.
SUBSTITUTE S!!~EET
21~0288
~094t02577 23 PCT/US93/06149
sulphonamides. Such materials are normally added at 0.5-10% by
weight of the finished product, preferably 1-5% by weight.
Preferably, the bleaches suitable for the present
invention include peroxygen bleaches. Examples of s~uitable
water-soluble solid peroxygen bleaches include hydrogen
peroxide releasing agents such as hydrogen peroxide,
perborates, e.g. perborate monohydrate, perborate tetrahydrate,
persulfates, percarbonates, peroxydisulfates, perphosphates and
peroxyhydrates. Preferred bleaches are percarbonates and
perborates.
The hydrogen peroxide releasing agents can be used in
combination with bleach activators such as
tetraacetylethylenediamine (TAED), nonanoyloxybenzenesulfonate
(NOBS, described in US 4,412,934), 3,5,-
trimethylhexanoloxybenzenesulfonate (ISONOBS, described in EP
120,591) or pentaacetylglucose (PAG1, which are perhydrolyzed
to form a peracid as the active bleaching species, leading to
improved bleaching effect. Also suitable activators are
acylated citrate esters such as disclosed in Copending European
Patent Application No. 91870207.7.
.~. .
The hydrogen peroxide may also be present by adding an
enzymatic system (i.e. an enzyme and a substrate therefore)
which is capable of generating hydrogen peroxide at the `~`
beginning or during the washing and/or rinsing process. Such
enzymatic systems are disclosed in EP Patent Application
91202655.6 filed October 9, 1991. `
..
Other peroxygen bleaches suitable for the present `~
invention include organic peroxyacids such as percarboxylic
acids. ~-
i ..
Bleaching agents other than oxygen bleaching agents are
also known in the art and can be utilized herein. One type of ¦
non-oxygen bleaching agent of particular interest includes
photoactivated bleaching agents such as the sulfonated zinc ``
~ ~ T~ t~
'.~'
2140288
W O 94/02577 24 PC~r/US93/06149 ~
and/or aluminum phthalocyanines. These materials can be
deposited upon the substrate during the washing process. Upon
irradiation with light, in the presence of oxygen, such as by
hanging clothes out to dry in the daylight, the sulfonated zinc
phthalocyanine is activated and, consequently, the subs~rate is
bleached. Preferred zinc phthalocyanine and a photoactivated
bleaching process are described in U.S. Patent 4,033,718.
Typically, detergent compositions will contain about 0.025% to
about 1.25~, by weight, of sulfonated zinc phthalocyanine.
Other suitable detergent ingredients that can be added are
enzyme oxidation scavengers which are described in Copending
European Patent aplication N 92870018.6 filed on January 31,
1992. Examples of such enzyme oxidation scavengers are
ethoxylated tetraethylene polyamines. Especially preferred
detergent ingredients that can be added are technologies which
also provide a type of color care benefit. Examples of these
technologies are metallo catalysts for color maintance
rejuvenation. Such metallo catalysts are described in copendinq
European Patent Application No. 92870181.2.
In addition, it has been found that the polyamine-N-oxide
containing polymers eliminate or reduce the deposition of the
metallo-catalyst onto the fabrics resulting in improved
whiteness benefit.
Another optional ingredient is a suds suppressor, exemplified
by silicones, and silica-silicone mixtures. Silicones can be
generally represented by alkylated polysiloxane materials while
silica is normally used in finely divided ~orms exemplified by
silica aerogels and xerogels and hydrophobic silicas of various
types. These materials can be incorporated as particulates in
which the suds suppressor is advantageously releasably
incorporated in a water-soluble or water-dispersible,
substantially non-surface-active detergent impermeable carrier.
Alternatively the suds suppressor can be dissolved or dispersed
in a liquid carrier and applied by spraying on to one or more
of the other components.
A preferred silicone suds controlling agent is disclosed in
Bartollota et al. U.S. Patent 3 933 672. Other particularly
SUBSTI~JTE ~ ET
21402~8
-`'094/02577 25 PCT/US93/06149
useful suds suppressors are the self-emulsifying silicone suds
suppressors, described in German Patent Application DTOS 2 646
126 published April 28, 1977. An example of such a compound is
DC-544, commercially available from Dow Corning, which is a
siloxane-glycol copolymer. Especially preferred suds
controlling agent are the suds suppressor system comprising a
~mixture~ of silicone oils and 2-alkyl-alcanols. Suitable 2-
alkyl^alcanols are 2-butyl-octanol which are commercially
ayailable~un~er the trade name Isofol 12 ~R.
Such suds suppressor system are described~in Copending European
~Patent application N 92870l74.7 filed~l0~November, 1992.
Especi~a~lly ~preferred sillcone suds controlling agents~ are~
described~ln~Copending European~Patent application N-9220l649.8
Said~compositions can comprise a~ silicone/silica ;mixture in
combination~with~fumed~nonporous silica such as AerosilR.~
The suds suppressors described above are normally employed at`
leve~ls~ of from 0.00~l~ to 2% by weight of the composition,
referably~from~o~.~ol% to~l% by weight.~
Other ~ components~ uséd ~in ~detergent`~ compositions may be
employed~ such~as~soil-suspending~agents soil-release agents,
: ~iGa~ brighteners,~ abrasive~s,~ bactericides, tarnish
i ~ bitors~ coloring~ agents à~nd~ encapsulated and/or non~
encapsulatêd~perfumes~
Antiredeposition~and;~soil~ suspension;~agents; suitable herein
~-?~ nclude~ cellulose~ derivat~ives~ sùch~ as ~methyIcellulose,
¢arboxyme ~ lcellulose~ and~ hydroxye ~ lcellul~ose, and homo- or
co-polymeric~po~lycàrboxylic~ àcids or~-;their salts. Polymers of
th~ls~ type~ include~the~;` polyac ~ ates~`~and maleic anhydride-
acrylic~acid- copolymers ~previously mentioned as builders, as
well as copoIymers of maleic anhydride with eth~lene,
methylvinyl~ ether or ~methacrylic acid, the~maleic anhydride
constituting at least 20 mole~percent of the copolymer. These
; materials ~are~ normally~used~at levels of from 0.5% to 10% by
wéight,~more~p Nf-rably~from 0.75%~to~8%, most~preferably from
1% to 6%~by~weight of the composition.;
. ...
W094/02577 2 1 4 0 2 8 ~ 26 PCT/US93/06149-
Preferred optical brighteners are anionic in character,
examples of which are disodium 4,41-bis-(2-diethanolamino-4-
anilino -s- triazin-6-ylamino)stilbene-2:21 disulphonate,
disodium 4, - 41-bis-(2-morpholino-4-anilino-s-triazin-6-
ylaminostilbene-2:21 - disulphonate, disodium 4,41 - bis-(2,4-
dianilino-s-triazin-6-ylamino)stilbene-2:21 - disulphonate,
monosodium 41,411 -bis-(2,4-dianilino-s-triazin-6
ylamino)stilbene-2-sulphonate, disodium 4,41 -bis-(2-anilino-4-
(N-methyl-N-2-hydroxyethylamino)-s-triazin-6-ylamino)stilbene- `
2,21 - disulphonate, disodium 4,41 -bis-(4-phenyl-2,1,3-
triazol-2-yl)-stilbene-2,21 disulphonate, disodium 4,41bis(2- `
anilino-4-(1-methyl-2-hydroxyethylamino)-s-triazin-6- ;
ylamino)stilbene-2,21disulphonate and sodium 2(stilbyl-4
(naphtho-11,21:4,5)-1,2,3 - triazole-211-sulphonate.
Other useful polymeric materials are the polyethylene j-
glycols, particularly those of molecular weight 1000-10000, ~
more particularly 2000 to 8000 and most preferably about 4000. P
These are used at levels of from 0.20% to 5% more preferably
from 0.25% to 2.5% by weight. These polymers and the previously
mentioned homo- or co-polymeric polycarboxylate salts are -
valuable for improving whiteness maintenance, fabric ash
deposition, and cleaning performance on clay, proteinaceous and
oxidizable soils in the presence of transition metal
; ~,
mpurltles. . ,~
Soil release agents useful in compositions of the present
invention are conventionally copolymers or terpolymers of
terephtha}ic acid with ethylene glycol and/or propylene glycol
units in various arrangements. Examples of such polymers are
disclosed in the commonly assigned US Patent Nos. 4116885 and `
4711730 and European Published Patent Application No. 0 272
033. A particular preferred polymer in accordance with EP-A-0
272 033 has the formula
(CH3(pEG)43)o.75(poH)o.25~T-po)2.8(T-pEG)o.4]T(po-
H)0.2s((pEG)43cH3)o.75 ~-
:.
where PEG is -(OC2H4)O-,PO is (OC3H6O) and T is (pcOC6H4CO).
SUBSTITU~ S~3~T
~3.4~h~
~'094/02577 27 i PCT/US93tO6149
,
Also very useful are modified polyesters as random copolymers
of dimethyl terephtalate, dimethyl sulfoisophtalate, ethylene
glycol and 1-2 propane diol, the end groups consisting
primarily of sulphobenzoate and secondarily of mono esters of
ethylene glycol and/or propane-diol. The target is to obtain a
polymer capped at both end by sulphobenzoate groups,
"primarily", in the present context most of said copolymers
herein will be end-capped by sulphobenzoate groups. However,
some copolymers will be less than fully capped, and`therefore
their end groups may consist of monoester of ethylene glycol
andjor propane 1-2 diol, thereof consist "secondarily" of such
species.
.:,.
The selected polyesters herein contain about 46% by weight of
dimethyl terephtalic acid, about 16% by weight of propane -1.2
diol, about 10% by weight ethylene glycol about 13% by weight
of dimethyl sulfobenzoid acid and about lS% by weight of
sulfoisophtalic acid, and have a molecular weight of about
3.000. The polyesters and their method of preparation are
described in detail in EPA 311 342.
~ i
The detergent compositions according to the invention can be
in liquid, paste, gels or granular forms. Granular compositions
- ~according to the present invention can also be in "compact
form", i.e. they may have a relatively higher density than
conventional granular detergents, i.e. from 550 to 9SO g/l; in
such case, the granular detergent compositions according to the
present invention will contain a lower amount of "inorganic
filler salt", compared to conventional granular detergents;
typical filler salts are alkaline earth metal salts of
sulphates and chlorides, typically sodium sulphate; "compact"
detergents typically comprise not more than 10% filler salt.
The liquid compositions according to the present invention can
also be in "concentrated form", in such case, the liquid
detergent compositions according to the present invention will
contain a lower amount of water,compared to conventional liquid
detergents. Typically, the wàter content of the concentrated
liquid detergent is less than 30~, more preferably less than
l, .
W094/02577 ~1 4 0 2 8 ~ 28 PCT/US93/0614
20~, most preferably less than 10% by weight of the detergent
compositions. Other examples of liquid compositions are
anhydrous compositions containing substantially no water.
Both aqueous and non-aqueous liquid compositions can be
structured or non-structured.
The present invention also relates to a process for
inhibiting dye transfer from one fabric to another of
solubilized and suspended dyes encountered during fabric
laundering operations involving colored fabrics.
The process comprises contacting fabrics with a laundering
solution as hereinbefore described.
The process of the invention is conveniently carried out in
the course of the washing process. The washing process is
preferably carried out at 5C to 75C, especially 20 to 60, but
the polymers are effective at up to 95-C and higher
temperatures. The pH of the treatment solution is preferably
from 7 to ll, especially from 7.5 to 10.5.
The process and compositions of the invention can also be
used as detergent additive products.
Such additive products are intended to supplement or boost the
performance of conventional detergent compositions.
The detergent compositions according to the present invention
include compositions which are to be used for cleaning ~`
substrates, such as fabrics, fibers, hard surfaces, skin etc.,
for example hard surface cleaning compositions ~with or without
abrasives), laundry detergent compositions, automatic and non
automatic dishwashing compositions.
....
The following examples are meant to exemplify compositions of
the present invention , but are not necessarily meant to limit
or otherwise define the scope of the invention, said scope
being determined according to claims which follow.
A liquid detergent composition according to the present
invention is prepared, having the following compositions :
SUBSTITUTE S~E'r
-'0~4/02577 29 P~T/US93/06149
,'.
% by weight of the total detergent composition
.
Linear alkylbenzene sulfonate l0
Alkyl sulphate 4
Fatty alcohol (Cl2-Cl5) ethoxylate 12
Fatty acid ~o
Oleic acid 4
Citric acid l M
NaOH 3.4
Propanediol l.5 -i
Ethanol l0 ~
Table I `
, . ..
EXAMPLE I :
The extent of dye transfer from different colored fabrics
was studied using a launder-o-meter test that simulates a 30
min wash cycle. The launder-o-meter beaker contains 200 ml iof a
detergent solution, a l0cmxlOcm piece of the colored fabric and
a multifiber swatch which is used as a pick-up tracer for the
bleeding dye. The multifiber swatch consists of 6 pieces
(1.5cmx5cm each) of different material (polyacetate, cotton,
polyamide, polyester, wool and orlon) which are sewn together.
.
The extent of dye transfer is assessed by a Hunter Colour `~
measurement. The Hunter Colour system evaluates` the colour of a
fabric sample in terms of the ~E value which represents the
~change in the Hunter L, a, b,values which are determined by
reflecting spectrometrie. The ~E value is defined by the
following equation: ;`
~E = ((af -ai)2 + (bf-bi)2 + (Lf-Li) ) /
~ -
where the subscripts i and f refer to the Hunter value before
and after washing in the presence of the bleeding fabric,
respectively. The least significant difference is l at 95%
confidence level.
~ S~ S
21 ~ 0~8
W094/02577 30 PCT/US93/06149
Ex~erimental conditions: ;
. ~
Example I demonstrates the increased dye transfer inhibiting
performance of the combination of polyamine-N-oxide containing ~;
polymers (PVNO : poly(4-vinylpyridine-N-oxidej which has an
average molecular weight of about lO,OOO and an amine to amine
N-oxide ratio of l:lO (determined by NMR)) and peroxidase.
~..
The extent of dye transfer from different colored fabrics was ,-~
studied using a launder-o-meter test that simulates a 30 min
wash cycle. The launder-o-meter beaker contains 0.7% of the
detergent composition, a lOcmxlOcm piece of the colored fabric
and a multifiber swatch which is used as a pick-up tracer for
the bleeding dye. The multifiber swatch consists each of
cotton.
A set of two realistic bleeding fabrics (50 cm2 of each) were
washed together with a multifiber pick-up tracer in a -
launderometer for 30 min. In a first launderometer pot (Test
A), the detergent solution did not contain any dye transfer
inhibiting agent. The second pot contained lO ppm PVNO (Test
B). The third pot contained 7 peroxidase (ex-Novo) Units/ml of
wash-solution (Test C). Also added are lO ppm glucose and O.l
units of Glox/ml to generate oxygen which is necessary to
activate the Peroxidase. Finally the fourth pot contains the
peroxidase system and PVNO (Test D).
Table : Level of dye transfer reduction by PVNO, Peroxidase and
the combination (~E values).
pH = 7.8 / Washing temperature 40 C
: ',
,,
Bleeding fabric Bleeding fabric A B C D
compo ~ `
_ _ _ _ ~ .
. :~
SUBS~ITUTE S~EET ;~
.~
~`
"O94/02577 31 PCT/US93/06149
EXAMPLE II
A liquid detergent composition according to the present
invention is prepared, having the following compositions :
~ .
. ~
% by weight of the total detergent compo~ition
A B C D
:;
Linear alkylbenzene sulfonate 10 10 10 10
Alkyl sulphate 4 4 4 4 ~;Fatty alcohol (C12-C15) ethoxylate 12 12 12 12
Fatty acid 10 10 10 10 '~
Oleic acid 4 4 4 4 -`
Citric acid
Diethylenetriaminepentamethylene 1.5 1.5 1.5 1.5
Phosphonic acid ~
NaOH 3.43.4 3.4 3.4 ~`
Propanediol 1.51.5 1.5 1.5 '
Ethanol 10 10 10 10
Ethoxylated tetraethylene pentamine 0.7 0.7 0.7 0.7 `-
Poly(4-vinylpyridine)-N-oxide 0-1 0-1 0-1 0-1
Thermamyl 0.13 - 0.13 0.13
Carezyme 0.01 0.01 - 0.01 `
FN-Base 1.8 1.8 1.8 - `~`
Lipolase 0.14 0.14 0.140.14 -
Endoglucanase A 0.53 0.53 - 0.53
Suds supressor (ISOFOLr) 2.5 2.5 2.5 2.5 ~
Minors up~to 100 ~;
, .
: `~
EXAMP~
` ;~''
A compact granular detergent composition according to the !`' '
present invention is prepared, having the following `
formulation: ~
W O 94/02577 2 1 4 0 2 8 S 32 PCI`/US93/06149~
% by weight of the total detergent compo~ition `
A B C D
Linear alkyl benzene sulphonate11.40 11.40 11.40 11.40 ~.
Tallow alkyl sulphate 1.80 1.80 1.80 ~ 1.80 ~,~
C45 alkyl sulphate 3.00 3.00 3.00 3.00
C45 alcohol 7 times ethoxylated4.00 4.00 4.00 4.00
Tallow alcohol 11 times ethoxylated 1.80 1.80 1.80 1.80
Dispersant 0.07 0.07 0.07 0.07
Silicone fluid 0.80 0.80 0.80 0.80 `~`
Trisodium citrate 14.0014.00 14.00 14.00 ~;
Citric acid 3.00 3.00 3.00 3.00 `
Zeolite 32.5032.50 32.50 32.50 .:~
Maleic acid acrylic acid copolymer 5.00 5.00 5.00 5.00 ~.
Perborate 0.5 0.5 0.5 0.5
Cellulase (active protein) 0.03 0.2 - 0.2 -`~
Alkalase/BAN 0.60 - o.6 0.6 ~.
Lipase 0.36 0.36 0.36
Peroxidase 0.4 - 0.4 0.4
Sodium silicate 2.00 2.00 2.00 2.00
Sodium sulphate 3.50 3.50 3.50 3.50
Poly(4-vinylpyridine)-N-oxide 0-1 0-1 0-1 0-1 .
Minors up to 100 :~
.
The above compositions (Example II and III) were very good .`
at displaying excellent cleaning and detergency performance .
with outstanding color-care performance on colored fabrics and
mixed loads of colored and:white fabrics. `-
;'~
SUBSTITUTE SHEET