Note: Descriptions are shown in the official language in which they were submitted.
21~0662
wo 94/02483 P ~ /GB93/OlS12
TETRACYCLIC COMPOUNDS PROCESS AND INTERMEDIATES FOR THEIR PREPARATION AND
THEIR USE AS ANTITUMOUR AGENTS
The ~.~ - ~ in~. i nn relatQ~ to h~tQrocyclic = A ~ which have
b~en found to have anti t activLty. More specifically, the
invention concc,..~ Pyrrolo [3,2-bl c~r~~~le~, lH-Benzofuro [3,2-fl
indol~s and lH-[l] BenzothiQno [2,3-f] indol~ ho~' for their
pr-paration, ph~ Lical f~ l~tinn- cont~inin7 them and their u-e
as anti L u ag~nts.
Re~earch in the area of cancer chamotherapy ha~ produced a variety of
anti t ~u agent~, which have differing da~ s of efficacy. Standard
cl;n;c~lly used agent~ include adriamycin, ac~ cin D, methotrex-
ate, 5-fluorouracil, ci~-platinum, vincristine and vinblastine. How-
ev~r, the~e ~..~ ly avaLlable anti t agents are known to have
various di~advantages, ~uch aa toxicity to healthy cells and
r-~istancQ to certain tumour typec.
Ther~ thus exists a cont; ntl i n7 need to develop new and i ~ vv.d
anti t ~u~ agents.
Xho~htariya et al, khim. G~t~rot~$kl. Soedin (1980), (2) 203-8,
disclosQ the synthesis of c~rtain indoloh~n~Q[b] thiGphenas.
Rhoshtariya et al, khim G~terot~ikl Soedin (1984), (10) 1366-70
di3close the ~ynthesi~ of c~rtain indolob~nro[b] furan~.
rishvili et al, khim G~terot-ikl Soe~;n (1985), (3) 355-8
di~clo~e the synthesi~ of certain derivativea of indolo[5,6-d~ and
indolo t5,4-d] benzotb] furan~
The patent ~pecification EP447,703 ~i~c~ose~ th~ ~ynthe~is of certain
benzo(5,6-b)benzofuran-2-carboxylates.
There have now been disc~v~.~d novel ~ which exhibit
anti t _ cell activity with low toxicity again-t normal cell lines.
~ 6 6 2
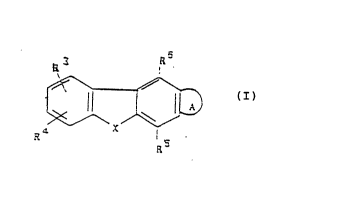
Thus, in a first aspect the present invemion provides a compound of the general fo;mula (1)
R6
R3
~ (I)
R4 RS
and salts and physiologically functional derivatives tnereof,
wherein A is
~R~ ~R2 Rl 2
X is 0, S, SO, S02, CH2, CO orNR7, wherein R7 is H, Cl 1o alkyl, aralkyl cont~inin~ 1 to
4 carbon atoms in the alkyl portion and up to 10 carbon atoms in the aryl portion, aryl
cont~ining up to 10 carbon atoms, alkenyl, Cl lo acyl, aLkynyl, or sulphonyl optionally
substituted by Cl lo alkyl, aryl co~ up to 10 carbon atoms or aralkyl cont~inin~ 1 to
4 carbon atoms in tne aLkyl portion and up to 10 Carbon atoms in the aryl portion;
Y is 0, S, SO, S02, CH2, CO or NR7;
Rl is COR8, COOR8, CHO, CH20H, CH20R9, CONH2, CONHNRlORl 1, CONHR10,
CONR1OR1 1, COO(CH2)nNRlOR1 1, wherein R8 is H, Cl 1o alkyl, aryl cont~ining up to
10 carbon atoms and optionally substituted by Cl lo alkyl, Cl lo alkoxy, halo, sulphinyl,
amino (optionally substituted by one or two Cl -l o alkyl groups), haloaLIcyl, sulphonyl or
cyano, or aralkyl co~ 1 to 4 carbon atoms in the alkyl portion and up to 10 carbon
atoms in the aryl portion, R9 is Cl lo acyl optionally substituted by C 1-10 aLkyl, Cl l o
alkoxy, halo, sulphonyl, amino (optionally substituted by one or two C l lo alkyl groups),
haloalkyl. sulphinyl or cyano, R1 0 and Rl 1 are independently hydrogen, C1 1 o alkyl or aryl
con~ining up to 10 carbon atoms, and n is 1 to 4 carbon atoms;
R2 is H, COOR8, Cl lo aLkyl, aryl cont~inin~ up to 10 carbon atoms, optionally substituted
by C 1-10 alkyl, Cl lo alkoxy, halo, sulphinyl. amino (optionally substituted by one or two
~ 0 ~ 6 2
Cl lo al}~yl groups), haloalkvl. sulphonyl or c~ano! or CH~CH~CO~Rl' wherein R12 is
C 1 1 o al~yl or aryl conr~inin~ up to 10 carbon atoms:
R3 and R4 are independently H, hvdroxv, C 1-10 aIkyl, haloalkvl. C 1-10 all~oxvt halo,
cvano, nitro, amino, aL~lamino, dialkylamino, alkyl substituted by C 1-10 all~yl, C 1-10
alkoxy, halo, sulphinyl, amino (optionally substituted by one or two C 1-10 alkyl groups),
haloalkyl, sulphonyl or cyano, carboxyl or CO~R12;
R5 is H, C1 -10 alkyl, optionally substituted by C 1-10 alkyl C 1-10 alkoxy, halo, sulphinyl,
amino (optionally substituted by one or two alkyl groups), haloalkyl, sulphonyl or cyano,
aralkyl cont~ining 1 to 4 carbon atoms in the alkyl portion and up to 10 carbon atoms in the
aryl portion, nitro, amino, halo, cyano, CHO, COOR8;
R6 is H, aryl cont~ining up to 10 carbon atoms, Cl 1o alkyl, aralkyl cont~inin~ 1 to 4
carbon atoms in the alkyl portion and up to 10 carbon atoms in the aryl portion, nitro,
halogen, CHO or CoR13 wherein R13 is C1 1o alkyl or aryl cont~ining up to 10 carbon
atoms;
with the proviso that
(i) when R2, R3, R4, R5 and R6 are all H and A is
~ Y~
R2
wherein Y is NH and X is O or S, then R1 is not C02H or C02Et;
and
(ii) when R2, R3, R4, R5 and R6 are all H and A is
~Rl
Il
`y~\R2
wherein Y is NH~ and X is O then Rl is not CHO;
and
~ ~1406fi2
(iii) Y is not O when X is O.
In yet a further aspect ~he present invention provides a compound of the general forrnula (1 )
above~ wherein A is
~R~ \Y~\R ~3~ or ~3~
X is O, S, SO, SO2, CH2, CO orNR7, wherein R7 is H, C1 1o alkyl, aralkyl cont~ining 1 to
4 carbon atoms in the alkyl portion and up to 10 carbon atoms in the aryl portion, aryl
cont~inin~ up to 10 carbon atoms, alkenyl, C1 1o acyl, akynyl or sulphonyl;
Y is O, S, SO, SO2, CH2, CO or NR7;
Rl is COOR8, CHO, CH20H, CH20R9, CONH2, CONHR10 OR CONRl0R11, wherein
R8 is H, Cl lo aLkyl, aryl cont~ining up to 10 carbon atoms, optionally substituted by Cl
10 alkyl, C 1 1 o alkoxy, halo, sulphinyl, arnino (optionally substituted by one or two C 1 1 o
alkyl groups), haloalkyl, sulphonyl or cyano or aralkyl Cont~inin~ 1 to 4 carbon atoms in
the alkyl portion and up to 10 carbon atorns in the aryl portion, R9 is C 1 1 o acyl optionally
substituted by C 1-10 aLkvl, Cl 1 o alkoxy, halo, sulphinyl, arnino (optionally substituted by
one or two Cl lo alkyl groups), haloalkyl, sulphinyl or cyano, and R10 and R11 are
independently Cl lo alkyl or aryl cont~inin~ up to 10 carbon atoms;
R2 is H, COOR8, Cl lo alkyL, aryl co~ g up to 10 carbon atoms, optionally substituted
by C 1-10 alkyl, C 1-10 alkoxy, halo, sulphinyl, ~mino (optionally substituted by one or two
Cl lo alkyl groups), haloalkyl, sulphonyl or cyano or CH2CH2CO2R12 wherein R12 is
C1 1o alkyl or aryl conr~ining up to 10 carbon atoms;
R3 and R4 are independently H, hydroxy, C l 1 o alkyl, haloalkyl, C 1 1 o alkoxy, halo,
cyano, nitro, amino, alkvlamino, dialkyl~ ino, alkyl substituted by Cl lo alkvl, Cl lo
alkoxy, halo, sulphinyl. amino (optionally substituted by one or two C 1-10 alkyl groups),
haloalkyl, sulphonyl or cvano, carboxyl or C02R12;
S~
~140~2
R~ is H~ Cl l o alkyl. optionally substituted bv Cl -10 alkyl, Cl lo alkoxy, halo~ sulphinyl,
amino (oplionally substituted by one or t~hO Cl lo alkyl groups~. haloalkvL sulphonyl or
cyano. aralkyl containing 1 to 4 carbon atoms in the alkyl portion and up to 10 carbon atoms
in the ar~l portion~ nitro~ halo, cyano CHO:
R6 is H, Cl lo alkyl, aralkyl cont~ining 1 to 4 carbon atoms in the alkyl portion and up to
10 carbon atoms in the aryl portion, nitro~ halo, CHO or CoR13 wherein R13 is Cl lo alkyl
or aryl containing up to 10 carbon atoms with the proviso described above.
Alkyl groups present in general formula (I) may be straight or branched chain alkyl groups,
and suitably contain 1-6 carbon atoms~ Examples of such alkyl groups include methyl,
ethyl, t-butyl and the like.
Acyl group may be straight or branched and suitably contain 1-6 carbon atoms. Examples
of suitable acyl groups include ethanoyl and propanoyl groups.
Alkoxy may be straight or branched and suitably contain 1-6 carbon atoms. Examples of
suitable alkoxy groups include methoxy, ethoxy and the like.
Aryl includes Both carbocyclic aryl groups and heterocyclic aryl groups. Carbocyclic aryl
group include, eg phenyl and naphthyl and contain at least one aromatic ring. Heterocyclic
aryl group include eg thienyl, furyl, pyridyl, indole and quinoline rings.
An aralkyl group may contain from 1 to 4 atoms in the alkyl portion and the aryl portion
may be a carbocyclic or heterocyclic aryl group.
Halogen represents fluoro, chloro, bromo or iodo~
In the compounds of formula (1)
X is preferably 0, S or NR7, wherein R7 is Hr Cl l o aLkyl, sulphonyl or toluene sulphonyl;
Y is preferably NR7;
Rl is preferably COR8, COOR8, CH20R9~ CONH2, CNHNR1OR1 1, CoNHR19,
CONRlORl 1, COO(CH~)nNRlORl 1, wherein R8 is H, Cl lo alkyl, aryl cont~inin~ up to
10 carbon atoms, optionally substituted bv Cl l o aLkyl, C l l o alkoxy, halo, sulphinyl,
S~
6 6 2
amino (optionally substituted by one or tw-o C 1-10 alkyl groups), haloalkyL sulphonyl or
cyano or aralkvl cont~inin~ 1 to 4 carbon atoms in the alkyl portion and up to 10 carbon
atoms in the arvl portion. R9 is C 1-10 acvl optionally subslimted by C 1-10 alkyl, C 1-10
alkoxy, halo, sulphinyl, amino (optionallv substituted by one or two Cl lo alkyl groups),
haloalkyl, sulphonyl or cyano and R10 and Rl 1 are independently hydrogen, Cl lo alkyl or
aryl cont~ining up to 10 carbon atoms and n is 1 to 4 carbon atoms;
R2 is preferably COOR8, Cl lo alkyl or CH~CH2C02Rl~ wherein R12 is Cl lo alkyl or
aryl cont~ining up to 10 carbon atoms;
R3 and R4 represents independently H, hydroxy, C1 1o alkyl, C1 1o alkoxy, halogen,
cyano, alkyl substituted by C 1-10 alkyl, C 1-10 alkoxy, halo, sulphinyl, amino (optionally
substituted by one or two Cl lo alkyl groups), haloalkyl, sulphonyl or cyano or carboxyl;
RS is preferably H or Cl 1o alkyl;
R6 is preferably H, C1 1o alkyl or aryl cont~ining up to 10 carbon atoms and salts and
physiologically functional derivatives thereo
X preferably represents S or NH, A is preferably
Rl
~R2
and Y preferably represents NH.
R1 is preferably COOR8, with R8 preferably being C1 1o allyl or aralkyl cont~ining 1 to 4
carbon atoms in the alkyl portion and up to 10 carbon atoms in the aryl protion.
R2 is preferably H or Cl l o alkyl.
R3 is preferably H, Cl 1o alkoxy or halo.
R4 is preferably H, C 1-10 alkoxy or halo.
RS is preferably C 1-10 alkyl and
~ W O 94/02483 ~ 1 ~ O ~ 6 2 P~/GB93/01512
R is prQferably n, al~u~y u~ Halo
i8 r_~af erably .J 1 ~-yl ~d
R ir pr-f~rably H
and ralt~ and phy~iologically functional d~rivativ~ thereof
Partic~lArly preferred :c ,~u-~ according to the p.~e~ert inv~ntion
include
3-Pyridyl 3,4-dimethylpyrrolo[3,2-b]carbazole-2-carboxylatQ
[(3-DimQthylamino)phenyl]3,4-dim~thylpyrrolot3,2-b~carbazole-2-carbo-
xylate
Benzyl 1,3,4-trimethylpyrrolot3,2-b]c~ rh- ~ol--2-carboxylate
PhQnyl 3,4-dimQthylpyrrolot3,2-b]c~ rb - ~ole-2-carboxylate
3,4-Dimethyl-2~ olylc~rhpnyl)pyrrolot3~2-klc~rh-~le
Ethyl 3, 4-dim~thylpyrrolo t3,2,-~]c~ r~- zole-2-csrboxylate;
Ethyl 3,4-dimethylbenzothienot4,5-f]indole-2-carboxylate;
B~nzyl 3,4-dimethylpyrrolot3,2-b]c~ rb- -ole-2-carboxylate;
BQnzyl 8-fluoro-3,4-dimethylpyrrolot3,2-~]c~ r~- ~ole-2-carboxylate;
Ethyl 8-fluoro-3,4-dimethylpyrrolot3,2-b]cArh-~ole-2-carboxylate
Benzyl 3,4,6-trimethylpyrrolot3,2-k]carbazole-2-carboxylate;
Ethyl 3,4,6-trimethylpyrrolot3,2-b]c~ rh~ ~ole-2-carboxylate;
8-Fluoro-3,4-dimQthylpyrrolot3,2-b]c~r~Azole-2-carboxylic acid
3,4-Dimethylpyrrolo[3,2-bl CA r~ ~ ~o le-2-carboxylic acid;
Ethyl 8-methoxy-3,4-dimethylpyrrolot3,2-b]c~rh~-ole-2-carboxylate;
3,4,6-~rimethylpyrrolot3,2-b]carbazole-2-carboxylic acid and
Benzyl 8-methoxy-3,4-dimethylpyrrolot3,2-~1c- rb- ~ole-2-carboxylat~;
and phy~iologically functional derivativQr thereof
W O 94/02483 8 P~/GB93/0151~
066~
C ~u - of the qen~ral fn 1 a ( I) have be~n to-ted again-t two
pecially ~lspe~ cell linss which are cloneo of thQ human
fibro~arcoma cell-line, HT1080~ One clone, HT1080Acc2, retainc the
tran-fr - L ' - ~y~ ~ of the parental line, whilst th~ other,
HT10801c, i~ a morphologically flat, non ~ igenic, r-vertant
Thu~, the effect~ of pot~ iAl anti L r ~ __-~n can be evaluated
on the ba-i- of thQir ability to effect d~tran-fc - ~gn in HT1080ccc2
CQll- .
~ of the p ac- ~ invention have been found to be particularly
effective in this a~ay ~y~tem
In addition, _~--n of the ~.e~snL invention have beQn found to be
effective again-t MCF7 human brea~t cancer cell-, A431 Epi~P~ id
carcinoma CQlls and A285 -1~- - cells
-
The c ~u l~ also exhibit low toxic$ty against normal cell-
= According to a further a~pect, the ~, -~ ~ invention also provides a
process for preparing c~ of general f~ 1 a ( I), which proces-
compri-e- cataly-ed ring clo_ ~ of _ -n of formula (IV) in the
p~ of a strong acid
~ 3 R
~X~ ~ R2 (T~
- Th~ p,e-~ L invention al-o provide~ for a ~.oc~c for preparing
_ - of f~ 1A (IV) which ~.~c~cc compri~ec either
= (a) Reaction of a _ ~u ' of the fc 1~ (II) with a - ~_ ' of the
f 1 A (III) to produce a - _ of the for~ula (IV), wherein
X,Y,R ,R ,R ,R and R are a- dofined her~ins-
WO 94/02483 21~ 0 6 6 2 PCI/GB93/01512
R5 '~ 3
R c ~
t~ R' (IV)
, a
c~J ~ ~.
.~ ~,s
followed by catalysed ring closure
The reaction is preferably carried out at room t ,- ~Lure in the
~ of a strong acid, eg p-toluene ~ulphonic acid or - ~ _illo-
nite ~10 clay a- a cataly-t to produce a _ -' of th- invention;
(b) Reaction of a c ,_ -~ of the formula (v) with a - ,-L ' of the
fs lA (III) to produce a _ , -' of the formula (IV) followed as in
(a) by cataly~ed ring closure
R ~/v/( Rl (v)
wherein L is a leaving group Examples of suitable leaving groups
include -OCOCH3, OET, -N Me3 and halo;
(c) A one step reaction p ocedu~e, reacting a c _- ' of the formula
(II) with a c ,-u~ of the fc 1~ (III) in the p.~ of a
catalyst, to produce a _ __L ' of the invention in a ingle step The
preferred cataly~t i3 ~ illonite ~10 clay;
In~ertion of the substituent R onto the ring system for e le
W O 94/02483 - ; PCT/GB93/0151 ~
- ' -- 10 --
2 ~ 2
(d) Carboxylation of a polyheterocyclic = ~u ~ uaing
(i) a carbonyl halide or
(ii) carbon ~i~Yi~-
According to known ~ (J March, AdvancQd Organic Chemi~try,2nd ed, r'~ Hill, N w York, 1977, p 497-498)
(e) Alt-rnatively one can prclu~ = - of the formula (I) where$n
R i~ CHO by method~ known to tho~e qki 1 le~ in the art, for - 1e2-
(i) The a~o~.iate aromatic polyheterocycle can be reacted with aformylating agent, ~uch a~ that ~ane ~ted by the reaction
bctu~ SnCl4 and Cl2CHOCH3 or equivalent reagent~
For example, according to the method of A Reiche et al, Chem
Ber 93, 88 (1960), or with other ctandard formylating
~ r ~/~.oced~ ~ known in the art, for ~ l-, the
Catterman-~och reaction (CO\HCl\AlCl3\CuCl), the Gatterman
r-action (HCN\HCl\ZnCl2), and the Vil~ -i~r reaction
(POCl3\PhN-(Me)CHO or POCl3\Me2NCHO) (J March, Vide Supra,
p 494-497); or
(ii) The a~ iate a~. -tic polyheterocycle, carrying a uitable
functional group, ~aid group being COh~_~ Led to an aldehyde
group by method~ known to thoce ~ki ~ le~ in the art SuLtable
functional group~ include CH8r2, CH3, COR , wher~in R is a
primary or ~e~ ry Cl_6 alkyl group, COOH or a derivative
= thereof such a~ an e~ter, amide, acid chloride or CN; or
(f) C __ - of the formula (I) wherein R i~ CONHR may al~o be
produced by the reaction of a c ~L ' wherein R i~ COOH or a
uitable reactiv~ acid derivativ~ thereof a- outlined in J Harch,
Vide ~upra For ~ ~ le an acid halide can be reacted with a _ _
NH2R in an in~rt ~olvent
W O 94/02483 2 1 i ~ C 6 ~ P ~ /GB93/01512
-- 11 --
:,
(g) Co ~_ ~ion of one : _su of formula (I) into ~not~ nd of
formula (I)
f th i jnn w - _in Rl is COOR and R i-, for le,
aralkyl can be co~v_L~ed to free acids wherein R is H by reduction in
the ~ ~ -e of H2 and a Pd cataly-t, or where R is, for le,
alkyl, by hydrolysis in tha ~_~ - of an a~p,op,iate ba-e e g
cae~ium c~ho~
It i~ thereafter poesible for the s~illed man to synthQsise e~ter and
amide - s~ '~ within the scope of the inv~ntinn by conv~ ~ion of the
free acid~ obtained, by known ~,oced~re~ (see J March, Vide Supra,
p363-365)
C __ ~ of the invention produced as described herein can be
conv~ Led to other -~ _SL ~- of the inv~ntin~ by electroFh~l~r
subet$tution at R and/or R , to introduce, for - ls, NO2, h-1~3
and CoRl3 wherein Rl is a~ defined herein
The above ~O~hRRe~ have been decr~bQd for ~ wherQin A iB
~'~, R '
_ Q
The ~ man will appreciate that these are egually appl i~hle when
A is
~ `Y R~ ~ Q
~' ~ `f ~ ` R
Y
In anothsr aspect the invention relat-s to novel inte ~-iatee of the
fo 1~- (II), (III), (IV) or (V)
W O 94/02483 - 12 - P ~ /GB93/0151~2~
21,~b6t
JJ The L_ ,~ '~ of the ~.~ Lnvention ar~s u~eful for th~ trer - L of
Ll ~ They may be employ~d in t --ti ng variou~ form~ of canc~r of
-l~ including carci- -~, for instancc of the stomach, pancr-a~,
brea-t, ut-ru~ and colon; ~ rci- -~, for in-t-nce of the lung
and colon; ~arcomas, for in-tanc~ fibro-arcomat 1~ ~r i-~ for
in-tance l~ -_yLiC L ~ and l~ , for in-tance myeloid
ly ~ - . .
The inv~ntion thus further prov$d~3 a method for the trer - ~ of
tumours in an i ~ 1 ~ ~ including - -l~, e~pecially human~, which
compri~es the a~ ini~tration of a clini~Ally useful amount of _ ~u l
of formula (I) or a ph- ~ceutically acceptable ~alt or
physiologically functional derivative in a rha -~suLically u~eful
form, once or ~-veral times a day or in any other app,o~iatc
uch~A~lle, orally, rectally, parenterally, or applied topically
In addition, there i~ provided as a further, or alternativQ, a-pect of
the invention, a ~ of formula (I) or a ph- -~eutLcally
acceptable ~alt or phy~iologi~lly functional derivat$ve thereof for
u~e in therapy, for ~Y~ le a~ an antitumour agent
The amount of ~ of fo1 la (I) required to be ~ffectivQ again-t
the afo~ ~ioned ~ ~ will, of cour~e, vary and i~ ultimately at
the di~cretion of the - ic~l or veterinary practitioner The factorn
to be con~iderlsd include the condition being treated, the route of
~ inintration, and nature of the formulation, the mammal'~ body
weight, ~urface area, age and genQral condition, and the par~
= __ ~ to be a~' i ni ~tered A ~uitable effective anti L dose is
in the rang~ of about 0 01 to about 100 mg/kg body weight, eg 0 1 to
about 100 mg/kg body weight, prQferably 1-30 mg/kg body weight Tho
total daily dose may be given a~ a ~ingle do~e, multiple dos~, e n ,
two to ~ix time~ per day or by int~avano~ infu-ion for ~ ct-d
duration For e~~ le, for a 75 kg mammal, the do~ range would be
about 8 to 900 mg per day, and a typical do-~ could be about 50 mg per
day If ~i r___ Le multiple do~e~ are indicated tr~atment might
21~0t~6Z
W O 94/02483 ~ P ~ /GB93/01512
- 13 -
typieally be 15 mg of a c _ ' of formula (I) qiven up to 4 tim~s
per day
Whilst it $~ pc~ihle for the aetive _ __ to be - ;ni ~ter~d
alone, it i~ re: ~ble to ~.~ t th~ aetiv - in a
Fb' ~~ Lic~l fc l~t,~n. ~ ti~n~ of th~ ~ i~n, for
- ~ i r- ~ USC, eomprise a __ __ ~ of f ormula (I) or a alt thereof
togeth~l with one or more ph- Lieally aeeeptable earriers and
opti~n-11y other th~ LLc ingredi~n~ff The carrier(s) should be
rh- -~eutieally acceptable in the sonse of being compatible with the
other ingredlents Of tha formulation and not deleteriou~ to the
recipient thureof
The ~.B-- ~ invention, therefore, further provide~ a ph- -~eutical
f~ l~tion compri~ing a _ __ -' of formula (I) or a ph~ -ceutically
a~eept~hle salt or physiologj~l1y funetional darivative th~r~of
together with a ph- -eQutically A~eert~hle carrier therQof
There i- al-o provid~d a method for the pr~paration of a
ph~ --eutical formulation eomprising bringing into association a
: _ ~L ' of formula (I) or a p~- ~reutieally aeeeptable ~alt or
physiologi~-11y funetional derivative therQof, and a rh- -eeutieally
~eept~h1e carrier thereof
.
Fc l~ti~n~ aeeording to tha ~- t i"~- i nn inelude thoce uitable
for oral, toFir~l~ reetal or parenteral (ineluding subeut~n~o~
intl ~eu1~r and int ~ -) r~ ini~tration Pr~ferr~d fo1 1~tions
are those ~uitable for oral or parenteral r ' i n i otration .
The f~ i ~n~ may conveniRntly be ~.~ e~ in unit do~age form and
may be prepared by any of the m~thods w~ll known in th2 art of
p~ y All methods in~ludo the ctep of bringing thQ aetive _ _
into ao~oeiation with a earrier whic~ con-titut~ one or mor~
~cr~sG~ y iny~. d~nt~. In genQral, the fs lrti~n~ ar~ prepar~d by
uniformly and inti -tely bringing the act~ve ~ u ' into ~s~ociation
f
: , ; `~ ,i, ' ,, "
W O 94/0,2483 - 14 - PCT/GB93/0151 J ~
~4066~
with a liquid c_rrier or a finely divid~d ~olid carrier or both and
then, if -~c-- y, sh-ri"g the product into desired fc lAtions
~ ;nn~ of the ~ ~r- ~ inventLon s~it~ for oral ~1 ;niRtration
may be ~ a~ discr-te units ~uch a~ s, cachet~, tabl-ts
or lo~ , each c~nt~ining a pred~ i s~ amount of th- active
: :_ '; as a powder or granule-; or a solution or su ~- ~in~ in an
aqueous or non a-l~.o~s liguid such a~ a syrup, an alixir, an lsi~n
or a draught
A tablet may be made by _ ~ssion or moulding, optinn~lly with one
or more Af eescry ingre~i~"ts C assed tablets may be prepared by
~ sing in a suitable -~hi n~ the active - __ ~ in a
free-flowing form such as a powder or granules, opt;o~-lly mixed with
a binder, lubricant, inert diluent, surfacQ active or disper~ing
agent ~n~ tablets may be mad~ by - l~ing in a suitablQ l rhi-- a
mixture of the ~_ ~- ed active A - ~ with any ~uitable carrier
A syrup may be made by adding the active - _ _ ' to a CQ r~LratQd,
aqu~_ ~ uolution of a sugar, for ~ le sucrose, to which may also be
added any a~cessory ingre~i~nts Such Ancess~_~ ingre~ients~-) may
in~ s flavourings, an agent to r-tard cryst~ -tion of the sugar
or an agent to increase the solubility of any other ingre~ie"ts, uch
as a polyhydric alcohol for ~ - le glycerol or sorbitol
Fc IAtin"~ for rectal ~ tration may be ~ e~- e~ as a
~rpos itory with a con~ LiQn-1 carrier uch as cocoa butter
F~ 1 ~ti nnn ~uitable for parenteral ~ ntration conv~niently
compri-e a stcrile aqueou~ preparation of the active c _~ ~ which is
preferably isotonic with the blood of the recipient Such fc 1 ati nn~
uitably comprise a solution of a ph- -nsutically and
ph~ -nologicaliy arceptahle acid addition salt of a c ~ o~ the
f~ 1A ( I) that is isotonic with the blood of the recipient
21406C2
WO 94/02483 PC.l /GB93/01512
- 15 ~
U~Qful f~ l~tinno al~o compri~e cQnr~ntrat-d solution~ or solido
cont~in;ng th~ _ ~u ~ of fc_ 1~ (I) whLch upon d$1ution with an
a~.op.iatQ ~olv~nt give a solution for parenteral -~ in;~tration a-
bovQ .
In addition to th~ afG,~ 5' 'ir ~' ing J'ir ~ th~ tions of
this i ~ i~n may furth-r in~lv~e on~ or more -cc~-- _y ingredient(~)
selected from ~;luAnt~, buff-r-, flaYouring ag~nts, bindQrs, surface
active ag~nt~, thirl a, lubricant~ v~Liv-~ (including
anti~YiA-nts) and the like
In a further aspect the p~3--L invention provides the uoe of a
. __ of fo 1 A ( I) or a rh- ~eutically accept-hle ~alt or
physiologically functional der$vative ther~of for the manufacture of a
~'i~- ~~ for the treatment of L
.
The invention will now be illu-trated by the following non-limiting
Example~z
All t - at~res are in dc~.~e- Celciu~ ( C)
IR spectra were ~eCOld~ ~ on a P~rkin-Elmer 257 grating ~E_L.oph~Lo-
meter or a B N k~r FS66 ~E_L.o~hoLc Ler
U V spectra were measured in ethanol on a Unicam SP800 spectrophoto-
m~t~r
lH NMR spectra were obtained on a Bruker WM 360-NMR ~poctrophotometer
at 360 MHz, or on a Bruker AC200 s~_L.~h~LomQter at 200 ~Hz J
values are given in Hz
Ma-s ~ctra w~re obtainQd on Varian cHSD(EI), Kratos CQ~L (EI) or
~ratos MsSO(FAB) inst.
W O g4/02483 ~ - 16 - P~/GB93/0151~ - ~
z~066~
Exam~le 1
PreDaration of Int~ e~t~
PreDaration of PYrrole~
Ethyl 4-~c~tyl-3,5-dim~thylpyrrole-2-carboxylate, b~nzyl 4-acQtyl-
3,5-dimethylpyrrole-2-carbo%ylate and ethyl 4-acetyl-3-ethyl-S-m~thyl-
pyrrole-2-carboxylatc) were pr~pared according to th2 method of
A.N.Johnson et al, J. Chem. Soc., 4254 (1958).
N-MethYlatLon of PYrroles - Gcneral PLoced~re
A mixture of the pyrrole (20 mmol) methyl iodide (50 mmol) and
po~ i c~bQn-te (50 mmol) was heat-d to r~flux in methyl ethyl
keton~ (50 ml) for 8 h. If TLC (toluQne/ethyl ac~tate 3:1) indicat~d
incomplete reaction, further aliguots of methyl iodide (50 mmol) and
pota~cium carbonate (50 mmol) werQ added and the mixture heated to
reflux for a further 6 h. After e~v.~ion $~ vacuo to d.y g~g, thQ
re~idue wa- tak n up in warm wat~r and extracted with ~thyl acetat~
(3xS0 ml). The combined extract~ w~re dried over ~- -Pium sulphat-
and LY~G~aLed ~ vacuo to leave a y~llow oil or solid which was
cry~ ed from ~ueous ethanol.
Ethvl 4-acetvl-1,3,5-trimethvlDyrrole-2-carboxYlate
obtained from ethyl 4-acetyl-3,5-dimQthylpyrrole-2-carboxylate a~
whit~ crystal- (2g; 41~) m.p. 61-62 C (Found: C, 64.17t H, 7.82~ N
6.16 C12H17NO3 require~ C, 64.55~ H, 7.68~ N, 6-27~) ~H(~ H61-DMSO)
4.25 (2H, g, CH2CH;), 3.70 (3H, ~, 1-CH3), 2.43 and 2.42 (2 x 3H, 2 x
3, 3-CH3 and COCH3), 2.38 (3H, ~, 5-CH3) and 1.25 (3H, t, CH2CH3)~ m/~
(~) 224(MH , 100), 208(40), 194(20), 178(40) and 133(20)(FAB)~ vmax
(~Br Di~c)/CM 2984, 1691 and 1651.
~ W 0 94/02483 _ 2 11~ 0 6 6 2 P ~ /GB93/01512
...~
,
Benzvl 4-acetvl-1.3.5-tri~ethvlDvrrole-2-carboxvlate
Obtained from benzyl ~ 1-3,5-dLmothyl-2-carboxylats a~ white
cry~tals m.p. 78-79 C IFounds C, 71.30s H, 6.74s N, 4-79S C17H1gN03
r-quir-~ C, 71.56S H, 6.715~ N, 4.91%); ~H ([ H61-DMSO) 7.52-7.27 (5H,
m, ArH), 5.30 ~2H, , Ç~2Ph), 3.73 (3H, s, 1-CH3), 2.42 (6H, s, 3-CH3
and COCH3) and 2.38 (3H, , 5-CH3); m/z (~) 285(76, M ), 270(87), 194
(S3), 178(23~, 151(36), 136(26) and 91(100); vmax (~Br DL-c)/cm
2974, 1693 and 1641.
Pre~aration of the 5-AcetoAv LhYl-4-acetYlDvrroles - General
u oc~dure.
To a cooled (O C) and tLrred ~ n of thn 4-acetyl-5-
methylpyrrole (0.02 mol) Ln dry diethyl ether (20 cm ) wa~ added,
dropwise over 15 min, freshly dLctLlled culfuryl chlorLde (2.2 cm ,
1.25 eguLv.). The reactLon mixture was stirred further and the
chloromethyl derivative crys~A~ ~d out lowly, filtration gave tho
5-chloromethyl derivative as colourless cryctals. The purity of the
chloromethylpyrrole wa- cl-Ac~Ad by H NMR pect.cs~ (90 MH ) and it
was used dLrectly without ~0 r-~A~ tion.
The above chloromethylpyrrole (0.01 mol) wa~ added to a ~olution of
sodium acetate (3 g) in acetLc acLd (50 cm ), the mLxture ntirred for
2 h and poured into ica w -~r (200 cm ). The resulting solid was
washed well with water until acid-free before drying.
Ethvl 5-acaLoA~ LhYl-4-acetYl-3-mothYll~Yrrole-2-carboxylate
cryct~ from ~ a- colourle-s n^e~l~s (1.87 g, 70~) m.p.
135.5-138 C (Found: C, 58.6; H, 6.45; N, 5.15.C~3H17NO~ require~ C,
58.4; H, 6.41; N, 5.24~ H(CDC13) 9.57 (1 H, br ~, NH), 5.40 (2 H,
z, CH20Ac), 4.35 (2 H, q, OCH2CH3), 2.6 (3H, s, 3-CH3) 2.5 (3 H, s,
WO 94/02483 - - - PCI/GB93/0151
- 18 -
066a
COCH3), 2.17 (3H, ~, OCOCH3) and 1.4 (3H, t, OCH2CH3); m/z (%)
267(83,M ), 224(46), 207(27), 178(100) and 162(42).
Benzvl 5-_cetoxvmethvl-4-acetY1 3-methvlDvrrole-2-carboxvlate
cry~t~ from methanol as co~our ess n~e~lo~ (2.34 g, 71~) m.p.
138-141 C (Found: C, 65.8; H, 5.95; N, 4.3 C18H1gNO5 requir~s C,
65.64; H, 5.81; N, 4.25~ H(CDC13) 9.44 (1 H, br s NH), 7.49-7.32 (5
B,m,ArH), 5.40 (2H,s,CH2OAc), 5.35 (2H,s,CH2Ph), 2.62 (3H,~;3-CH3),
2.49 (3H,s,CH3CO) and 2.14 (3H,~,OCOCH3); m/z (~) 329 (9,M ), 286
(13), 269(4), 178(19) and 91(100).
In the case of benzyl 5-acetoxymethyl-4-acetyl-3-(2-methoxycarbonyl-
ethyl)-pyrrole-2-carboxylate, there was no precip$t~tion when the
~olution wa~ poured into ic~-water. ExtractLon with chloroform (3 x
100 cm ), drying _nd removal of ~olvQnt under .~l~c.d pre-sure gave an
oLl which was cryn~ al li~ed from b~n~ - light pstrolsum to yi-ld
colourle~ n~e~les (2.69g, 67%) m.p. 97-100 C (Found C, 62.9; H 5.9; N
3.45. C21H23NO7 requires C,62.8; H, 5.78; N, 3.49~ H(CDC13) 9.15
(lH, br s,NH), 7.50-7.30 (5H, m, ArH), 5.35 (4 H, s, CH2Ph and
CH2OAc), 3.63 (3 H, s, OCH3), 3.37 (2 H, t, CH2CH2CO), 2-58 (2 H, t~
CH2CO), 2.51 (3 H,s,COCH3) and 2.15 (3 H,s,OCOCH3); m/z(~) 401(4,M ),
341(8), 268(6),250(60) and 91(100).
Ethvl 5-acetoxvmethvl-4-acetvl-1,3-dimethYlPvrrole-2-carboxvlate
Crys~Alli~e~ from ethyl acetate/cycloh~YAn~ (61%) m.p. 100-101C.
(Found: C, 59.38; H, 6.73; N, 4.95. C14H1gNO5 requires C, 59.78; H,
6-81; N~ 4-98~ H(t H61-DMSO) 5.30 (2H, ~, C~2OAc), 4.29 (2H, q,
CH2CH3), 3.77 (3H, s, N-CH3), 2.43 and 2.42 (2 x 3H, 2 x ~, 3-CH3 and
CH3CO), 2.02 (3H, s, OCOCH3) and 1.31 (3H, t, CH2CH3); m/z (~) 281
(34,M ), 238(100), 222(48) and 192(52); ~ (KBr DL-c)/cm 1712 and
1697.
BenzYl 5-acetoxvmethvl-4-acetYl-1,3-dimetbvl~vrrole-2-carboxvlate
219û662
~ WO 94/02483 PCI`/GB93/01512
-- 19
Cryst~lli r ~ ~ from ~thyl acetate/cyc~ r~ . H ( t H ] 6-DMso)
7.51-7.32 ~SH, m, ArH), 5.34 and 5.32 ~2 x 2H, 2 x s, CH2Ph nd
CH2OAc), 3.78 ~3H, ~, N-CH3), 2.46 and 2.45 (2 x 3H, 2 x s, 3-CH3 and
CH3CO) and 2.04 (3H, s, OCOCH3); m/z (~) 343(5, M ), 284(100) and 91
(95).
' .~
EthYl S-acetoxvmethvl-4-ac~tvl-3-ethYlDYrrole-2-carboxvlate
Cryst~ e~ from ether/p~trol a~ fawn ~se~l~n (61~) with m.p. 97-98 C
~Found: C, 59.44; H, 6.78; N, 4.80. C14HlgNO5 r-quire-: C, 59.76; H,
6.81; N, 4.98~ H ~CDC13) 9.40 ~lH, br s, l-NH), 5.38 ~2H, s,
CH2OAc), 4.38 ~2H, q, J 7, CO2CH2), 3.10 (2H, q, J 7.5, 3-cH2), 2.54
(3H, Q~ COCH3), 2.18 ~3H, 8~ OCOCH3), 1.40 ~3H, t, J 7.5, CO2CH2C~3),
1.23 ~3H, t, J 7.5, 3-CH2C~3); m/z (~) 281 (42, M ), 238(61), 221(89),
206~58), 192(92), 175~95), 160(81), 147(59), 43(100); ~max (RBr
di~c)/cm 3277, 1738, 1674, 1657.
Svnthesis of the 3-lPYrrolvlmethYl)indole, 2-(PvrrolvlmethYl)benzo-
furan and 3-~PvrrolvlmethYl)benzothioDhene - General v~ocedure.
A solution of the 5-~cetoxymQthyl-4-acetylpyrrole (1.0 mmol) and
lndol- ~1.0 mmol) $n 1~2-Aich 1 oroethane (10 cm ) w~s h~ated at gentlQ
reflux and stirred with ~ illon~t- clay (1 g) for 1.5-2 h. After
filtration from clay and ~ hing WQll w~th 1,2-~hloroethane,
e~pGla~ion of the combined fLltrate~ under r~uced prQ--ure gave an
oil. Th~s oil was submitt~d to fla-h ch ~ -~G~ phy on ilica, eluting
with ~thyl acetate in light petroleum to giv~ 3-(3'-ac~tyl-5'-
ethoxycarbonyl-4'-methylpyrrol-2~-ylmethyl)indole. It gave colourless
cry-tals from ethyl acetate-light petroleum ~0.1465 g, 45~). m.p.
180-182 C (Found: C,70.5; H, 6.25; N, 8.65. ClgH20N2O3 requires C,
70.4; H, 6.21; N, 8.64~ H~CDC13) 8.78 (lH, s, ~y~ .), 8.27 ~1 H,
s, ind-NH)~ 7.45 ~lH, d, J7,4-H) 7.42 (lH, d, J7, 7-H), 7.25 (lH, t,
J7, 6-H~, 7.14 ~lH, t, J7, 5-H), 7.10 ~lH, s, 2-H), 4.45 ~2H, s,
3-CH2), 4.22 ~2H, q, OCH2CH3), 2.63 ~3H, ~, 4'-CH3) 2.53 ~3H, ,
CH3CO) and 1.2S (3 H, t, OCH2C~3); m/z (~) 324(100,M ) 309(48),
WO 94/02483 - PCI/GB93/Ol~il~
2~662
277(25), 263(54), 250(38),235(30),207(48), 139124),130(30),117~67) and
90~16); Vmax~CHC13)/cm 3490, 3430, 1680 and 1650.
Benzofuran ~1.0 mmol) wh-n u--d in-t-ad of indol-, after chromato-
graphy, gave 2-~3'-acetyl-S'-~thoxycarbonyl-4'-methylpyrrol-2~-
ylmethyl)benLoLu.~n ~0.106 g, i2~6~), m.p. 124-127 C ~Found: C, 70.1s
H, 6-1 N, 4.15 ClgH1gNO4 r-quLre~ C, 70.14s H, 5.89s N, 4.31~);
~H~CDC13) 9.25 (1 H, 8, NH), 7.50(1 H, d, J7.3, 4-H) 7.44 (lH, d,
J7.3, 7-H) 7.28-7.18 (2 H, m, 6-H and 5-H), 6.57 (1 H, , 3-H), 4.50
(2 H, , 2-CH2), 4.31 (2 H, q, OC~2CH3) 2.62 (3 H, 8, 4'-CH3) 2-50 (3
H, 3, CH3CO) and 1.35 (3 H, t, OCH2C~3); ~aturation of the ~inglet 3-H
at ~ 6.51 ~nh-n~ the signal~ due to 4H at ~7.50 (2.7~) and 2-CH2 at
~ 4.50 (0.8~); m/z (%) 325(100,M )~ 310(4),279(29), 264(17), 251(59),
236(27), 208(19), 193(9), 131(7) and 118~7); and the 2,3-bi-
~3'-acetyl-5'-ethoxycarbonyl-4'-methylpyrrol-2'-ylmethyl)benzofuran
~0.0238 g, 8.94%) m.p. 255-257 C; ~H ~CDC13) 10.09 ~1 H, s, NH) 9.95
~lH, ~, NH), 7.32 ~lH, d, J7.7, 4-H), 7.27 ~1 H, d, J7.7, 7-H), 7.17
~1 H, t, J7.7, 6-H), 7.08 ~1 H, t, J7.7, 5-H) 4.45 ~2 H, ~, 2-CH2),
4.40 (2 H, ~, 3-CH2), 4.36 (2H, q, OC~2CH3), 4.27 (2 H,q,OC_2CH3),
2.64~3 H,s,4'-CH3), 2.63~3H,s,4'-CH3), 2.58 ~3H, ~, CH3CO), 2.54 ~3 H,
~, CH3CO), 1.39 ~3H, t, OCH2CH3) and 1.31 ~3 H, t, OCH2CH3); m/z (~)
532~11,M ), 490~24), 444(9), 397(6), 324~100), 282(18), 278~27),
236~20), 209~28) and 162~28) ~Found: M ,532.2210. C30H32N2O7 require~
M, 532.2209).
When benzothioFh~n~ ~1.0 mmol) wa~ u~ed in the same way a~ $ndol2,
Ch ~ ~ - LG~. a~hY u~ing ethyl acetate in ~i~h 1 oromethane as eluent gave
colourle~s cry~tals of 3-~3'-acetyl-5'-ethoxycarbonyl-4'-methyl-
pyrrol-2'-ylmethyl)benzo~hiop~ 0.0963g, 28.2~) m.p.l25-128 C
~Found: C, 6675; H, 5.8; N,4.1 C1gHlgNO3S requires C, 66.84s H, 5.61s
N, 4.10~)s ~H(CDC13) 8.72 (1 H, br, s, NH), 7.88 (1 H, m, 4-H), 7.63
~1 H, m, 7-H), 7.37 (2 H, m, 6-H and 5-H), 7.20 (1 H, ~, 2-H), 4.54 (2
H, 8~ 3-CH2)~ 4-23 (2 H, q, OC_2CH3), 2.62 (3 H, ~, 4'-CH3~, 2.53 (3
H, s, CH3CO) and 1.28 (3 H, t, OCH2C~3); ~aturation of the 3-CH2
protons at ~ 4.54 ~nh~n~e~ the signal~ due to NH at ~ 8.72 ~3.3~), 4-H
WO 94/02483 21 - PCr/GB93/01512
at ~ 7.88 (7.7~), 2-H at ~ 7.2016~) and CH3CO at ~ 2.53 (1.3~); m/z
~) 341(100,M ), 326(9), 298(6), 295(20), 230(39), 267(46), 252(32),
224(27), 194~26) and 148(22); and th~ 2,3-bis (3'-ac~tyl-5'-ethoxy-
carbonyl-4'-methylpyrrol-2'-yLm thyl)~ ~thi~Fh~n~ as a pale y~llow
solid (0.0264 g, 9.6%), m.p. 206-209 C (Found: C, 65.6; H, 5.8; N 5.1
C30H32N206S r-quire- C, 65.67~ H, 5.88; N, 5.11%), ~H(CDC13) 9.77 (lH,
br, s, NH), 9.43 (lH, br, , NH), 7.70 (1 H, m, 4-H), 7.49(1 H, m,
7-H), 7.26 (2 H, m, 6-H and 5-H), 4.55 (2 H, s, CH2), 4.53(2 H, s,
CH ) 4.32 (2 H, q, OCH2CH3), 4.24 ~2 H, q, OC~2 3)
4'-CH3), 2-60 (3 H, 8r 4'-CH3~, 2.57 (3H, s, CH3CO) 2-49 (3 H, ~,
CH3CO), 1.35 (3H,t,OCH2C~3) and 1.28 (3H H,t,OCH2C~3)Sm/Z(%)
548(5,~ ), 530(11), 3~0(100), 294(27) and 162(10).
Exa~le 2
SYnthesi~ of 3-(PYrrolYlmethYl)benzo~hio~k~s~ and 3-(PYrrO1Y1-
methYl ) i n~nle3
a) 3-(3'-Acetvl-5'-benzvloxvcarbonyl-4'-methvlDvrrol-2'-vlmethYl~
h~n ~nthiQDhene
A solution of the 5-ac~toxymethyl-4-acetylpyrrole (0.33g; 1.0
mmol) and benzothioph~n~ (0.14 g; 1.05 mmol) in 1,2-~ hloro-
thane (10 cm ) was heated at reflux and stirred with
_illonite K10 clay (1 g) for 2.5 h. After cooling andfiltration from the clay, which wa- w ~ well with
1,2-~inhlorethanQ, the combined filtrates were J~o~aLed under
.~ ' ced ~,i~s-~re to leave a yellow oil. Flash ch.~ -~GJ~hy
on silica, eluting with di-thyl ether/light petroleum (1:2) gavQ
the title s __-' as a colourle~s solid.
8H(CDC13) 8.72 (lH, s, NH), 7.92-7.84 ~lH, m, 4-H), 7.69-7.58
(lH, m, 7-H), 7.43-7.16 ~8H, m, 2-H, 5-H, 6-H, ArH), 5.23 (2H,
, CH2Ph), 4.50 (2H, , 3-CH2), 2.61 (3H, s, 4'-CH3) and 2.50
W O 94/02483 - 22 - PCT/GB93/0151 ~
66~
(3H, 8, CH3C0); m/z (~) 403 (M+, 100); vmax (~8r Di-c)/cm
3290, 1690 and 1659.
b) 3-l3'-Acet~1-5'-ethoxr~rbonvl-i'-methvl~vrrol-2'-vlmethvl)-5-
cvanoindole
A ~olution of the 5-aceto~ hyl-4-acetylpyrrole (0.7 g, 2.6
mmol) and 5-cy~o;nA~1~ (0.41 g, 2.9 ~ol) in 1,2-~ichloroeth~ne
(S0 cm ) wa~ heated at r~flux and ~tirred w$th l~on~ illonit~
R10 clay (2.1 g) for 6 h. After cooling and fLltration from the
clay, which wa~ washed well with 1,2-~i~hloroethane, the
_ ' in~d filtrato~ were ~u~aLed undcr reduced pre~sure to
leave an orange solid. Cry~t~ tio~ from ~i~hloromethane/
thyl acetate yielded a ~mall amount of analytically pure title
_ ~ an cream crystals. The ~GL~ted mother liquor~ werQ
~ubmitted to fla~h chromatog~a~hy on ~ilica, luting with
oromethanQ/ethyl acetatQ (9:1) to give further ~od~L
(0-65g, 71%). m.p. 213-214 C (Found: C, 68.60; H, 5.46; N,
11.99. C20HlgN3O3 requirec C, 68.75; H, 5.48; N, 12.03~)
~H([ H61-DMSO) 12.0S (lH, s, 1'-NH), 11.38 (lH, ~, l-NH), 8.20
(lH, m, 4-H), 7.50 (lH, dd, J0.7 and 8.5, 7-H), 7.39 (lH, dd,
Jl.7 and 8.5, 6-H), 7.18 (lH, ~, 2-H), 4.32 (2H, 8, 3-CH2 4.27
(2H, q, CH2CH3), 2.33 (3H, ~, 4'-CH3), 2.51 (3H, 8, CH3C0) and
1-30 (3H, t, CH2CH3); m/z (~) 350(M , 70), 302(16), 279(18),
237(35), 208(100) and 181(20); Vm (RBr Di-c)/cm 3309, 2218
and 1665.
c) 3-(3'-Acetvl-5'-ethoxvcarbonv}-4'-methvlPvrrol-2'-vlmethYl)
indole-5-carboxvlic acid
A ~olution of the 5-acetoxymethyl-4-acetylpyrrole (0.74g, 2.8
mmol) and indole-5-carboxylic acid (O.Sg, 3 mmol) in toluene (50
cm ) was rtirred at room t - aLu~e with ~ illonite R10
clay (1 g) for 10 day~. After cooling and filtration from the
clay, which wa- wa~hed well with toluene, the combined filtrat-
~
2140662
WO 94/02483 PCI/GB93/01512
- 23 -
were e~GL~Lcd undcr L_' r-' pres~ure to laave an orange ~olid.
Cry~t~ tion from ethyl acetatQ/cycloh~Y~n~ yi-lded the title
_ _ ' as a gr-y powd~r (O.lSg, 15%). m.p. 227-228 C (Found:
C, 64.965 H, 5.58; N, 7.34. C20H20N2O5 reguir-- C, 65.21; H,
5-47; N~ 7.60%); ~H([ H6]-DMSO) 12.35 (lH, br, CO2H) 12.04 (lH,
, 1'-NH), 11.17 (lH, , 1-NH), 8.31 (lH, d, Jl.6, 4-H), 7.70
(lH, dd, Jl.6 and 8.7, 6-H), 7.37 (lH, d, J8.7, 7-H), 6.98 (lH,
s, 2-H), 4-36 (2H, ~, 3-CH2), 4-26 (2H~ g, C~2CH3), 2-51 and
2.31 (2 x 3H, 2 x s, 4'-CH3 and CH3CO) and 1.29 (3H, t,CH2CH3);
m/z (~) 369(22(M+1) ~, 351(37), .323(18), 305(19), 232(19),
208(60) and 181(20), 162(100); V (RBr Di~c)/cm 3359 and
1676.
d ) 3- ~ 3'-Acetvl-5'-ethoxvcarbonvl-4'-methvl~Yrrol-2'-vlmethYl)-5-
bromoindole
A ~olution of the 5-acetoxym~thyl-4-acetylpyrrole (1.3g, 4.9
mmol) and 5 ~,~ ~indole (1.09g, 5.6 mmol) in 1~2-~irhloroethane
(100 cm ) was heated at reflux and stirred with t~- ~,illo-
n$t~ K10 clay (3 g) for 5 h. After cooling and filtration from
the clay, which was w o~e~ wcll with 1,2-AichlsroethanQ~ the
combin~d filtrates were ~.~G,aLed und~r reduccd pr~ssure to
leave a yellow solid. Cry-t~ tion from ~irhloromethane/
light petrol~um/acetone yielded thc title ~ as cream
cry-tal~ (0.33 g, 17%). m.p. 181-183 C (Found: C, 56.24; H,
4.70; N, 6.86. C1gHlgBrN2O3 requir~s C, 56.59; H, 4.75; N,
6.95%); ~H([ H6]-DMSO) 12.00 (lH, s, 1'-NH), 11.00 (lH, s,
1-NH), 7.82 (lH, d, J1.9, 4-H), 7.31 (lH, d, J8.6, 7-H), 7.16
(lH, dd, J1.9 and 8.6, 6-H), 7.02 (lH, ~, 2-H), 4.30 (2H, s,
3-CH2), 4.28 (2H, g, CUHU2CH3),2.51 and 2.33 (2 x 3H, 2 x 5,
4;-CH3 and CH3CO) 1.31 (3H, t, CH2CH3) m/z (%) 404 and 402(100
M ), 389 and 387(24), 357(24), 330 and 328(32), 206(36) and
178(26); ~ma (RBr Di~c)/cm 3373 and 1672.
W O 94/02483 ` ' PCT/GB93/0151~ ~
,2,~,1~P~66~ .
Exam~le 3
a) E~hvl 3,4-~i~ethvlDvrrolo r 3.2.-blcarbazol~-2-carboxvlate
~, .
A solution of the 3-(pyrrolyLmQthyl)indole (0.108 g 0.33 mmol)
was hnated at gentle reflux in 1,2-~i~hloroQthanQ (10 cm ) and
tirrQd with ~ illonite R10 clay (1 g) for 2 h. TLC then
showed a single _ s ~ had been formed and that reaction wa-
complete. After filtration from clay and washing well with
1,2-~irh1oroethane, e.~.aLion of th~ combined filtrates under
reduced pressure gave a yellow solid which cry-t~llised from
ethyl acetate to give the pyrrolot3,2-b]-c~ ole a~ yallow
crystale (0.076 g; 75~), m.p. 209.5-211 C (Found: C, 74.6; H,
6.14; N, 9.03. C1gH18N2O2 requiras C.74.5; H, 5.92t N, 9.14~);
~H~ H6]-DMSO) 11.22 (lH, s, l-NH), 10.70 (1 H, ~, 5-NH), 8.06
(lH, d, J7, 9-H), 7.85 (lH, , 10-H), 7.40 (lH, d, J7, 6-H),
7.35 (lH, t, J7, 7-H), 7.08 (lH, t, J7, 8-H), 4.35 (2H, q,
OC~2CH3) 2.91 and 2.90 (2 x 3H, 2 x S, 3-CH3 and 4-CH3 and 1.35
(3 H, t, OCH2CH3). Saturation of the 10-H at ~ 7.85 ~nh~nred the
inglets due to l-NH at ~ 11.22 (3~) and 9-H at ~ 8.06 (4~);
m/z(%) 306(56,M ), 260(100), 323(39J, 205(15), 140(18) and
130(26); v (CHC13)/cm 3480 and 1700; Amax(EtOH)/nm 226~ 268
310sh, 327sh, 340, 390 and 410sh.
b) Ethvl 3,4-dLmethvl-lH-benzofuror3,2,-flLndole-2-carboxvlate
Toluene ~ eulfonic acid (50 mg) was added to a solutLon of the
2-(pyrrolylmethyl)benzofuran (0.100 g 0.31 mmol) in toluene (10
cm ), and the reaction mixture was heated under reflux for 3 h.
On cooling, the product cryst~ ed out, and after filtration
and w hin7 with ethanol gave the title _ ' as pale yellow
cryetal3 (0.084 g, 88.8~), m.p. 2~-265 C (Founds C, 74.25; H,
5-55) N, 4-6 ClgH17NO3 requires C. 74.25; H, 5.56; N, 4.56~);
~14066~
W O 94/02483 - 25 - P ~ /GB93/~l512
~H([ H61-DMSO) 11.52 (1 H, 8~ l-NH). 8.18 (1 H, d, J7.5, 5-H),
7.62 (1 H, d, J7.5, 8-H), 7.46 (1 H, t, J7.5, 7-H), 7.38 (lH, t,
J7.5, 6-H) 7.38 (1 H, , 10-H), 4.37 (2 H, q, OC~2CH3), 3.14 (3
H, , 4-CH3), 2.91 ~3; H, , 3-CH3) and 1.39 (3 H, t, OCH2CH3);
saturat$on ~of the 4-CH3 at ~ 3.14 ~nh-n~e~ the s$gnals due to
5-H at ~ 8.18 (4.5%) and 3-CH3 at ~ 2.91 (2.6%); m/z (%) 307
(53,M ), 261(100), 233(31) and 205(9); vm (Nu~ol)/cm 3350 and
1686; ~max(EtOH)/nm 240, 269,293,330 and 344.
c) EthYl 3,4-dimethvl-lH- r 1lbenzothieno r 2,3-f1indole-2-carboxYlate
Toluene p Lulfonic ac$d (45 mg) was added to the solution of the
3-(pyrrolylmethyl)benzothiophenQ (0.100 g, 0.29 mmol) in toluene
(10 cm ) and the reaction mixture was heated under reflux for 3
h. ~v~pG~tion of the olvent and w 8h~ ng the r~ulting solid
with ethanol gave th~ title ~ ' a- a pale yellow co~id
(0.0758 g, 80~), m.p. 191-193 C (Found: C,70.3; H 5.5; N, 4.2.
ClgH17NO2S requires C, 70.6; H, 5.30; N, 4-33%); ~H(~ H6]-DMSO)
11.64 (1 H, s, NH), 8.25(1 H, m, 9-H), 8.12 (1 H, s, 10-H) 7.95
(1 H, m, 6-H), 7.48 (2 H, m, 7-H and 8-H), 4.38 (2 H, q,
OC_2CH3), 2.87 and 2.85 (2 x 3H, 2 x S, 3-CH3) and 1.37 ~3 H, t,
OCH2C~3); m/z (~) 323(53,M ), 277(100), 249(33), 221(15), 139(7)
and 111(11); v (Nujol)/cm 3350 and 1686; ~ (EtOH)/ nm
240,269,293,330 and 344.
d) Benzvl 3,4-dimethYl-lH- r 1lbenzothieno r 2,3-flindole-2-carboxYl~te
~olhene ~ ~lFh~nic ac$d (40 mg) was added to the solut$on of
the 3-(pyrrolylmethyl)benzoth;oph~ne (0.100 g, 0.25 mmol) $n
toluene (12 cm ) and the react$on heated under reflux for 6 h.
~ ation of the solvent and w~hi ng the resultant solid with
ethanol gave the titl~ ' as a pale yellow solid (0.02 g,
20%) m.p. 203-204 C (Found: C, 74.7; H, 4.9; N, 3.6; C24H1gNO2S
requirec C, 74.8; H, 5.0; N, 3.6~ H ([ H61-DMSO) 11-64 (lH~
W O 94/024~ 2 PCT/GB93/0151
6 b - 26 -
, NH) 8.27-8.15 (lH, m, 9-H), 8.10 (lH, ~, 10-H), 7.90-7.89
(lH, m, 6-H). 7.60-7.30 (7H, m, 7-H, 8-H, ArH), 5.40 (2H, ,
CH2) and 2.87 (6H, s, 2 x CH3); mlz (%) 385(100~ M ), 277(89),
248(25), 221(15) and 91(28)7 max f~sr Di-c/cm 3331 and 1672.
e) E~hvl 8 cY~no 3.4-dim~thvlPYrolo r 3,2-blcarbazole-2-carboxvlate
A mixtur- of th- 3-~pyrrolylmQthyl)indol~ (0.6 g, 1.7 mmol) ~nd
~oni -_illonit~ K10 clay (2 g) in toluene wa~ Rtirred and heated
at reflux for 24 h. After cooling and filtration from the clay,
which was washed weIl with tolu~n~, the combined filtrat-s were
~a~oLated under reduced pressure to leave a brown solid which
wa8 ~ubmitt~d to flaah ch ~ - oJ-aphy on oilica, eluting with
cyclQhoY~no/ethyl acetate (3:1) to give a y~llow ~olid.
Crystalli~-tLon from cycloh~YAne/ethyl acetate yielded the titl~
~c __ ~ a~ a yellow powder (0.030 g, 5%). m.p. >240 C (Found;
C, 71.54; H, 5.18; N, 12.78, C20Hl7N3o2.o.2H2o require8 C,
71.71; H, 5.24; N, 12.54~) ~H([ H6]-DMSO) 11.39 (lH, s, l-NH),
11.29 (lH, s, 5-NH), 8.65 (lH, d, J1.7, 9-H), 8.01 (lH, ,
10-H), 7.71 (lH, dd, Jl.7 and 8.6, 7-H), 7.50 (lH, d, J8.6,
6-H), 4-36 (2H, q, CH2CH3), 2.91 and 2.89 (2 x 3H, 2 x s, 4-CH3
and 3-CH3) and 1.38 (3H, t, CH2C~3); m/z (~) 331 (52,N )
285(100), 256(32), 229~12), 167(14) and 149(40); v ax(~Br
Di~c)/cm 3414, 3550, 2212 and 1664.
f) 3,4-DimethYl-2-ethoxvcarbonYlPYrrolo r 3 2-blcarbazole-8-
~A boxvlic acid
A mixturc of the 3-(pyrrolylmethyl)indole (O.lg, 0.3 mmol) and
r- Lillonite R10 clay (0.34 g) in toluQne (15 cm3) was
tirred and heated at reflux for 6 h. After cooling and
filtration from the clay, which was washed well with toluene,
the combined filtrate~ were e~.pG. a~ed under reduced pros-urQ to
leaYe an orange ~olid. Cry~ ti~ from methanol/~{chloro-
~h~n~ yielded thQ title ~ a~ a yellow powder (0.027 g,
2140662
WO 94/02483 - 27 ; PCI/GB93/01512
28%). m.p. >260 C ~Found: C, 68.12; H, 5.19; N, 7.91
C20H18N2O4Ø 05H2O requir-~ C, 68.39; H, 5.19; N, 7.98~ H
( lH]6-DMSO) I2.42 (lH, br, COOH), 11.30 (lH, s, 1-NH), 11.09
(lH, s, 5-NH), 8.68 (lH, , 10-H), 8.07-7.93 (2H, m, 7-H _nd
9-H), 7.45 (lH, d, J9, 6-H), 4.38 (2H, q, CH2CH3), 2.94 and 2.90
(2 x 3H, 2 x o, 4-CH3 and 3-CH3) and 1.39 (3H, t, CH2CH3); m/z
(%) 350(100, M ), 304(100), 278(40), 232(35) and 181(38);
(XBr Di~c)/cm 3459, 1697 and 1674.
g) Ethvl 8 brr - 3,4-dimethvl~rrolo r 3 ~ 2-blcarbazole-2-carboxvlate
A mixture of the 3-(pyrrolylmethyl)indole (0.3g, 0.74 mmol) and
-_illonite R10 clay (1 g) in toluene was stirred and h~ated
at reflux for 6 h. After cooling and filtration from the clay,
which wa- wa~hed well with toluene, the combined filtrates were
_~pv.~ted under ~d~ced pres-ure to leave a brown colid which
was subm$tted to fla~h cl,.- -tGy~h~ on ~ilica, eluting with
~chloromethane/light petroleum (7:3) to give a yellow ~olid.
Cry~talli~ation from cycloh~YA -/ethyl acetate yielded the title
-~ au-' as a yellow ~awl~ (0.070 g, 24%). m.p. 204-205C
(~o~ Found: C,59.17S H, 4.43; N, 7.30. C1gH17BrN2O2
requires C, 59.23; H, 4.45S N, 7.27%); ~H([ H6~-DMSO) 11-26 (lH,
s, l-NH), 10.79 (lH, ~, 5-NH), 8.30 (lH, d, J2.2, 9-H), 7.92
(lH, s, 10-H), 7.47 (lH, dd, J2.2 and 8.8, 7-H), 7.35 (lH, d,
J8.8, 6-H), 4.36 (2H, q, C 2CH3), 2.89 and 2.88 (2 x 3H, 2 x ~,
4-CH3 and 3-CH3) and 1.38 (3H, t, CH2CH3); m/z (%) 386 and
384(100, M ), 340 and 338(70), 232(60) and 181(50); Vmax(RBr
D$~c)/cm 3350, 1705 and 1663.
W 94/0 ~ ~ 40662 28 - P ~ /GB93/0151
Exam~le 4
'~ ~
O,.e ~o~ Svntheai~ of the Pvrrolocarbazole~ - General ~ t . ~.
A solution of Lndol- (l.G mmol) and th~ 5_a~ ~A~ Lhyl-4-
acetylpyrrol- (1.0 mmol) in 1,2-~i rh loro-than~ (10 cm ) wa- heat-d
under gentle r~flux and ~tirred with ~--t - illonite ~10 clay (1 g)
for 3-4 h. The colour of clay turned light brown and th~ reaction wa~
followed to completion by ~LC. After filtration from clay and wa~hing
wQll with 1,2-dichloroethane, ev~po htion of the combined filtrat~
gave the pyrrolo[3,2-b]c~rh-~Qle~ which were obtained a~ yQllow
cry~tal~ after cry~t~ tion from ~i~hlsromethane or 2thyl acetate.
a) EthY1 3,4-dimethYlDvrrolo r 3.2.-blcarbazole-2-carboxvlate
(0.199 g, 6S%) wa- obtained from the reaction of indole and the
5-acetoxymethyl-4-~cetylpyrrole. It wa~ identical in all
.~~ecL~ to the pyrrolo[3,2-b]c~rh-~olQ from F le 1.
b) Benzvl 3.4-dimethvl~vrrolo r 3.2-blcarbazole-2-carboxYlate
(0.179 g, 48.8~) wa- obtained from the reaction ~ - indole
and the 5-acetoxymethyl-4-acetylpyrrole, it had m.p. 229-232 C
(Found: C, 78.2; H, 5.65; N, 7.8. C24H20N202 require~ C, 78.23;
H, 5.47; N,7-60%); ~H[ H6]-DMSO-d6) 11.29 (1 H, ~, l-NH), 10.65
(1 H, s, 5-NH),8.08 (1 H, d, J8, 9-H), 7.89 (1 H, ~, 10-H),
7.56-7.34 (7 H, m, ArH, 6-H and 7-H), 7.08 (1 H, t, J7, 8-H),
5.42 (2 H, 3, CH2Ph) and 2.92 (6H, ~, 3-CH3 and 4-CH3); m/z (%)
368(74,M ), 354(10), 260(100), 246(13), 231(20) and 91(31).
The pyrrolo[3,2-b~c~rh~~ole (0.166 g, 45%) wa~ also obtained
from the reaction of ~ndole and the 4-ac~tyl-5-(QthG~ - hyl)-
pyrrole.
- 29 -
c) Ethyl 8-methoxy-3,4-dimethylpyrrolo[3,2-b]carbazole-2-carboxylate
Was obtained from the reaction of 5-methoxyindole and the
5-acetoxymethyl-4-acetyl pyrrole, it had m.p. 119-122°C (Found:
C,71.6; H, 6.0; N, 8.05. C20H20N2O3 requires C, 71.4; H, 5.99;
N, 8.33%); .delta.H([2H6-DMSO) 11.20 (1 H, s, 1-NH), 10.38 (1 H, s,
5-NH), 7.85(1 H, s, 10-H), 7.62 (1 H, d, J 2.5, 9-H), 7.31 (1 H,
d, J9, 6-H), 7.01 (1 H, dd, J9 and 2.5, 7-H), 4.38 (2 H, q,
OCH2CH3), 3.88 (3 H, s, OCH3), 2.89 and 2.87 (2 x 3H, 2 x S,
3-CH3 and 4-CH3) and 1.39 (3 H, t, OCH2CH3); m/z (%) 336(60, M+),
290(100), 275(5), 262(4), 247(23), 219(8) and 145(9).
d) Benzyl 8-methoxy-3,4-dimethylpyrrolo[3,2-b]carbazole-2-carboxy-
late
(0.139 g, 35%) was obtained from the reaction of 5-methoxy
indole and the 5-acetoxymethyl-4-acetylpyrrole, it had m.p.
212-215°C (Found: C, 75.4; H, 5.55; N, 6.95. C25H22N2O3 requires
C, 75.4; H, 5.57; N, 7.03%); .delta.H([2H6]-DMSO) 11.29 (1 H, s,
1-NH), 10.38 (1H, s,5-NH), 7.88 (1 H,s,10-H), 7.65 (1 H, d,
J2.5, 9-H), 7.58-7.36 (5H, m, ArH), 7.32 (1H, d, J9, 6-H), 7.02
(1 H, dd, J9 and 2.5, 7-H), 5.43 (2 H, s, CH2Ph), 3.88 (3 H, s,
OCH3), 2.92 (3 H, s, 4-CH3) and 2.89 (3H, s, 3-CH3); m/z (%)
398(73,M+), 290(100), 262(10), 247(15), 219(7) and 91(17).
e) Ethyl 8-fluoro-3,4-dimethylpyrrolo[3,2-b]carbazole-2-carboxylate
(0.131 g, 40.5%) was obtained from the reaction of 5-fluoro-
indole and the 5-acetoxymethyl-4-actylpyrrole, it had m.p.
231-234°C (Found: C, 70.5; H, 5.3; N, 8.4. C19H17FN2O2 requires
C, 70.4;,H,5.28; N, 8.64%); .delta.H[2H6]-DMSO) 11.27 (1 H, s, 1-NH),
10.64 (1H, s, 5-NH), 7.93 (1 H, dd, J9 and 2.5, 9-H), 7.88 (1 H,
s, 10-H), 7.36(1 H, dd, J9 and 6,6-H), 7.19 (1 H, dt, J9 and
2.5, 7-H), 4.36 (2 H, q, OCH2CH3), 2.88 (6 H, s, 3-CH3 and
WO 94/024~ PCI/GB93/01512--
~,~4~ 30-
4-CH3) and 1.37 (3 H, t, OCH2CH3); m/z (%) 324(50,M ) 278(100),
250(31), 220(10), 139(8), 125(7) and 111(8)
. ~
f) R~vl 8-fluoro-3,4-d~QthvlvYrrolo r 3.2-blcarbazole-2-çarboxv-
late
(0.155 g, 40~) was obtained from 5-fluoroindol- and the 5-
ac-toxym4thyl-4-acetylpyrrole, it had m.p. 217-219 C (Found: C,
74.6; H, 4.95; N, 7.3. C24HlgFN202 r~quire~ C, 74.6; H, 4.96; N,
7.25); ~H[ H6~-DMSO) 11.36 (1 H, s, 1-NH), 10.86 (1 H, 8, 5-NH),
7.94 (1 H, dd, J9 and 2.5, 9-H), 7.89 (1 H, ~, 10-H), 7.56-7.38
(5 H, m, ArH), 7.39 (1 H, dd, J9 and 4,6-H), 7.21 (1 H, dt, J9
and 2.5, 7-H), 5.42 (2 H, 8, CH2Ph), 2.91 and 2.90 (2 x 3H, 2 x
S, 3-CH3 and 4-CH3); m/z (%) 386(68,M ), 278(100), 249(22) and
91(43)
.
g) Ethvl 3,4,6-trimethvl~vrrolo r 3,2-bl Ca ~h~ ~Qle-2-CarboXVlate
(0.206 g, 64.4%) wa~ obtained from the reaction of 7-methylin-
dole and the 5-acetoxymethyl-4-acetylpyrrole, it had m.p. 230 C
(decomp.) (Found: C, 74.9; H, 6.25; N, 8.65. C20H20N202 requir-s
C, 75.0; H, 6.29; N, 8.74%); ~H([ H6~-DMSO) 11.20 (1 H, ~,l-NH),
10.11 (1 H, s, 5-NH), 7.89(1 H, d, J7.5, 9-H), 7.84 (1 H, ,
10-H), 7.18 (1 H, d, J7.5, 7-H), 7.01 (1 H, t, J7.5, 8-H), 4.37
(2 H~ g, OC_2CH3), 2-98 (3 H, ~, 4-CH3), 2-91 (3 H, ~, 3-CH3),
2.58 (3 H, s, 6-CH3) and 1.34 (3 H, t, OCH2C~3); m/z (~)
320(54,M ), 274(100), 246(30), 230(5), 137(9), 123(7) and
109(6)-
h) Benzvl 3,4,6-trimethvl~vrrolor3,2-blcarbazole-2-carboxylate
(0.167 g. 43.7%) was obtained from the rcactLon of 7-methylin-
dole and the 5-aceto~ -thyl-4-acetylpyrrole, it had m.p. 222 C
(decomp.) (Found: C, 78.5; H, 5.9; N.7.25. C25H22N2O2 regu~res
C, 78.5; H, 5.80; N, 7 33~ H([ H6]-DMSO) 11-27 (1 H~ ,
~ WO 94/02483 21~ 0 6 6 2 P~/GBb3/01~12
- 31 - l i
1-NH), 10.11 (1 H, s, 5-NH), 7.89 (1 H, d, J7, 9-H), 7.85 (1 H,
s, 10-H), 7.56-7.35 (5 H, m, ArH), 7.18 (lH, d, J7, 7-H), 7.08
(lH, t, J, 8-H), 5.43 (2H, s, CH2Ph), 2.99 (3H, s, 4-CH3), 2.93
(3 H, , 3-CH3) and 2.59 (3 H, ~, 6-CH3); m/z (~) 382(71,M )
274(100), 246(19) and 91~22).
i) Benzvl 3-~2-methoxYcarbonvl~thvl)-4-mQthvlDvrrolo r 3 2-blcarba-
zole-2-carboxvl~te
(0.230 g, 52.3~) wa~ obtained from indole and the 5-acetoxy-
m~thyl-4-acetylpyrrole, it had m.p. 211-213 C (Found: C, 73.7;
H, 5.6; N, 6.2. C27H24N2O4 requir~s C, 73.6; H, 5.49; N, 6.36%);
~H([ H61-DMSO) 11.51 (1 H, s, 1-NH), 10.71 (1 H, 8, 5-NH), 8.75
(1 H, d, J7.5, 9-H). 7.92 (1 H, ~, 10-H), 7.57-7.44 (7 H, m,
ArH, 6-H and 7-H), 7.18 (1 H, t, J7.5, 8-H), 5.43 (2 H, ~,
C~2Ph), 3.63 (3 H,s,OCH3), 3.59 (2 H, partially obscur-d, t,
CH2CH2CO), 2.88 (3 H, 3, 4-CH3) and 2.65 (2 H, t, CH2CO); m/z(~)
440(100,~ ), 332(20), 290(47), and 91(57).
j) Ethvl 3 4 5-trimethvlDvrrolo r 3 2-blcarbazole-2-carboxvlate
(0.140 g, 16%) was obta$nQd from th~ reaction (2.65 mmol scala)
~ N-methylindole and thQ 5-ac~toxymethyl-4-acetylpyrrole,
- it had m.p. 208 C (dQcomp.) (Found: C, 75.12; H, 6.40; N, 8.69,
C20H20N2O2 requir-s C, 74.98~ H, 6-29~ N, 8-74~ H(l H6]-DMSO)
11.28 (lH, ~, 1-NH), 8.08 (lH d, J7.9, 9-H), 7.88 (lH, s, 10-H),
7.44 (2 H, m, 6-H, 7-H), 7.07-7.17 (1 H, m, 8-H), 4.36 (2 H, q,
CH2CH3), 4-01 (3H, ~, 5-CH3), 3.13 (3H, s, 4-CH3), 2.90 (3H, ~,
3-CH3), and 1.38 (3H, t, CH2CH3); m/z (~) 320(72,M ), 274(100),
245(16), 149(28) and 137(12); v a (R8r Di~c)/cm 3329 and 1670.
k) BenzYl 3 4 5-tr~ethvlDvrrolo r 3 2-blcarbazole-2-carboxvlate
(0.220 g, 57%) was obtained from the reaction b~ - N-methyl
indole and the 5-acetoxymethyl-4-acetylpyrrole in toluene at
W 0 94/02483 ` ;~ ~ PCT/GB93/0151 ~
~ o
55 C, cataly~ed by tol. ~ 4 ~ ]ph~nir acid, $t had m.p.
228-229 C (Found: C, 77.17; H, 5.73; N, 7.09. C25H22N2O2Ø33
H2O require~ C, 77.31; H, 5.88; N, 7.21%); ~H(~ H61-DMSO) 11-28
(lH, ~ NH), 8.03 (lH, d, J7.5, 9-H), 7.88 (lH, s, 10-H),
7.56-7.34 (7H, m, Ar~, 6-H, 7-H), 7.15-7.07 (lH, m, 8-H), 5.40
(2H,~, Ç~2Ph), 4.02 (3H, , 5-CH~), 3.14 (3H, , 4-CH3) and 2.91
(3H, , 3-CH3); m/ (~) 382(72,M ), 291(4), 274(100) and 91(34)5
Vmax(KBr D$sc)/cm 3337 nd 1674.
1) ~thvl 1.3,4-trimethYlDvrrolo r 3.2-blcarbazole-2-carboxYlAte
(0.060 q, 7~) wa~ obtained from thc reaction (2.5 mmol scal~)
bet -- indole and the 5-_cetoxymethyl-4-acetylpyrrole, it had
m.p. 188-189 C (Found: C, 74.86; H, 6.32; N, 8.65), C20H20N2O2
requires C, 75.98; H, 6.29; N, 8.74%); ~H([ H6]-DMSO) 10-66 (1
H, s, 5-NH), 8.14 (1 H, d, J7.7, 9-H), 8.03 (lH, s, 10-H),
7.45-7.31 (2H, m, 6-H, 7-H), 7.06-7.15 (1 H, m, 8-H), 4.38 (2 H,
q, C_2CH3), 3-98 (3H, s, l-CH3), 2.91 (3H, ~, 4-CH3), 2.83 (3R,
, 3-CH3) and 1.38 (3H, t, CH2C_3); m/z (%) 320(M ,100),
306(10), 292(30), 247(8) and 231(10); v (~Br D$sc)/cm 3385
and 1657.
m) Benzvl 1,3,4-trimethYl~Yrrolor3,2-blcArbArole-2-carboxYlate
(0.240 g, 28%) was obtained from the reaction (2.7 mmol scal~)
~a~ _Ln indole and the 5-a~3~G~ Lhyl-4-acetylpyrrole, it had
m.p. 186-187 C (Found: C, 78.63; H, 5.83; N, 7.32, C25H22N2O2
require~ C, 78.51; H, 5.80; N, 7.32%); ~H([ H61-DMSO) 10-66 (lH,
~, 5-NH), 8.14 (1 H, d, J7.4, 9-H), 8.02 (1 H, ~, 10-H),
7.56-7.31 (7H, m, ArH, 6-H, 7-H), 7.06-7.15 (1 H, m, 8-H), 5.41
(2H, s, CH2Ph), 3-98 (3H, 8~ 1-CH3), 2.90 (3H, s, 4-CH3) and
2-83 (3H, s, 3-CH3); m/z (%) 382(M ,100), 338(10), 291(44) !
247(18) and 231(10); v a (~8r D$~c)/cm 3443 and 1697.
n) E~hvl 1,3,4,5-tetrame~hYl~vrrolo r 3,2-blcarbazole-2-carboxvlate
WO 94/02483 21 ~ 0 6 6 2 PCI/GB93/01512
- 33 -
i
.~ '
(0.220 g, 30t) wa- oht~ ~ from the roact$on (2.2 mmol cal-)
~L ~ r. ~hylindol- and th~ 5-r~~~GA~ Lhyl-4-acetylpyrrole,
~t had m.p. 165.5-167 C ~9- , . ) (Found: C, 75.50: H, 6.65; N,
8.30, C21H22N2O2 requlr-- C, 75.47; H, 6.63; N, 8.38t);
~H([ H6]-DNS0 8.1S (1 H, d, J7.5, 9-H), 8.07 (lH, ~, 10-H),
7.50-7.38 (2H, m, 6-H, 7-H), 7.09-7.19 (1 H, m, 8-H), 4.38 (2 H,
q, C~2CH3), 4.03 (3H, , 5-CH3), 3.96 (3H, ~, l-CH3), 3.14 (3H,
, i-CH3), 2.84 (3H, , 3-CH3) and 1.39 (3H, t, CH2C~); m/z (t)
334(100,M ), 306(18) and 245(6); ~ (RBr Disc)/cm 1690 and
1528.
o) BçnzYl 1 3 4 5-tetramethvlDvrrolo r 3 2-blcarbazole-2-carboxYlate
(0.070 g, 13%) was obtained from the reaction (1.4 mmol ~cale)
baL~ N-methylindole and the S-acetoxymethyl-4-acetylpyrrole,
it had m.p. 196-198 C (Found: C, 78.45; H, 6.16; N, 6.94,
C26H24N2O2 require~ C, 78.76; H, 6.10; N, 7-07t); ~H([ H6]-DMSO)
8.15 (1 H d, J7.8, 9-H), 8.07 (1 H, ~, 10-H), 7.59-7.29 (7H, m,
AsH, 6-H, 7-H), 7.10-7.20 (1 H, m, 8-H), 5.42 (2H, B, C~2Ph),
4.03 (3H, ~, S-CH3), 3.97 (3H, 3, 1-CH3), 3.13 (3H, ~, 4-CH3)
and 2.83(3H, ~, 3-CH3); m/z (%) 396(100,M ), 305(38), 245(16)
and 235(10);vma K8r Di~c)/cm 1696 and 1529.
p) Benzvl 3 4-dimethvl-5- ~ 4-toltlene~ ~honYL~ ~YSSO10 r 3 2-blcarba-
zole-2-carboxvlate
(0.012 g, 4t) was obtained from the reaction (0.6 mmol ~cale)
b~ N-(4- tol~en~tllphonyl)indole and the 5-acetoxymethyl-
4-acetylpyrrole, it had m.p. 270 C (Found: C, 70.83; H, 5.01; N,
S.23, C31H26N2O4S reguire~ C, 71-24; H, 5-01; N, 5-36~
H([ H61-DMSO) 11.58 (lH, ~, N-H), 8.28-8.08 (3H, m, 6-H, 9-H,
10-H), 7.66-7.21 (llH, m, ArH, T-H, 7-H, 8-H), 5.40 (2H, ~,
CH2Ph), 3.04 (3H, s, 4-CH3), 2.88 (3H, s, 3-CH3) and 2.20 (3H,
s, ~--CH3); m/z (%) 523(30,(M+1), 446(20), 367(30), 348(56),
33(100), 295(30) and 274(90); ~ma (~Br Di-c)/cm 3558 and 1666.
WO 94/02483 _ 34 _ PCI/GB93/0151~
,~40662
q) Ethvl 7-acetoxY-3,4-~imethYl-6-methG~yvv~olor3~2-blcarbazole
-2-carboxvlate
(7~) obtaLned from th r-action but ~- 6-acetoxy-7-mQthoxy-
indole and the s-acetoxymQthyl-4-acetylpyrrolQ~ it had m.p.
241-244 C. ~H (CDC13) 8.59 (lH, ~, br, NH), 7.78 (lH, s, br,
NH), 7.76 (lH, s, 10-H), 7.74 (lH, d, J8, 9-H), 6.88 (lH, d, J8,
8-H), 4-44 (2H, q, C_2CH3), 4.04 (3H, s, 6-OCH3), 2.96 and 2.92
(2 x 3H, 2 x 8, 4-CH3 and 3-CH3), 2.42 (3H, s, 7-CH3COO) and
1-46 (3H, t, CH2CH3); m/z (~) 394 (100, M )~ 352 (47), 348 (33),
306 (87), 263 (21) and 87 (73); ~maX(Nujol)/cm 3413, 3341,
1739 and 1675; ~max (MeOH)/nm 405, 386, 339, 325, 305, 269, 240
and 226. (Found: M , 394, 1529. C22H22N2O5 requires 394-1529)-
r) Ethvl 9-methoxv-3,4.5-trimethvlDvrrolo r 3,2-blcarbazole-2-car-
boxvlate
obt~in~ from the reaction between 4-methoxy-l-methy~in~ole and
the 5-acetoxymethyl-4-acetylpyrrole, it had m.p. 263-266 C
(Found: C, 71.84; H, 6.34; N, 7.91. C21H22N2O3 requir-~ C,
71.98; H, 6.33; N, 7 99%; ~H (CDC13) 8.60 (lH, ~, br, NH), 8.15
(lH, ~, 10-H), 7.40 (lH, t, J8, 7-H), 6.95 (lH, d, J8, 6-H),
6.66 (lH, d, J8, 8-H), 4.43 (2H, q, OCH2CH3), 4-10 and 4.04 (2 x
3H, 2 x s, N-CH3 and OCH3), 3.19 (3H, ~, 4-CH3), 2-98 (3H, ~,
3-CH3) and 1.46 (3H, t, OCH2CH3); m/z (%) 350 (74, M ), 304
(100), 276 (17), 223 (10) and 152 (19).
) Benzvl 8-chloro-3,4-dimethvl~vrrolo r 3,2-blcarbazole-2-carboxv-
~ate
(0.06g g; 17~ was obtain~d ~rom the reaction b_L
5-chloroindole and the 5-acetoxymethyl-4-acetylpyrrole, it had
m.p. 215-220 C. (decomp.). (Found: C, 71.42; H, 4.96; N, 7.11,
21~0662
WO 94/02483 - PCI/GB93/01~;12
- 35 -
C~4HlgClN2O2 r-quirc- C, 71.55; H, 4-75; N, 6.95~ H
( [H]6-DMSO) 11.39 (lH, , l-NH), 10.84 (lH, s, 5-NH), 8.17 (lH,
s, 9H), 7.93 (lH, s, 10-H), 7.54 (lH, d, J7, 7-H), 7.48-7.34
(6H, m, ArH and 6-H), 5.42 (2H, s, C~2Ph) and 2.88 ~6H, s, 3-CH3
and 4-CH3)5 m/z (%) 402 (30, m ), 358(5), 294 (65), 267 (25) _nd
91 (100). Tha cry-t~ tion li~uors were submitted to flash
cl~ -~o~ a~ky on sLl~ca. Elut~on with ethyl acetate/lLght
p trol-um y$elded furth-r titl- _~ which was cry-t~ d
from ethyl acetate (0.030g; 7~) and 3-~3'-acetvl-5'-benzYloxv-
c~rbonvl-4'-methvl~Yrrol-2'-YlmethYl)-5-chloroindole as cream
coloured crystals (0.152g; 36~) m.p. 141-143 C (Found: C, 68.20;
H~ 5-187 N~ 6-60 C24H21ClN2O3 requ$rQs C, 68-49 H~ 5.03; N~
6.65); ~H (CDC13) 8.72 (lH, s, l'-NH), 8.26 (lH, s, l-NH), 7.38
(lH, d, J2, 4-H), 7.35 (6H, m, ArH and 6-H), 7.18 (lH, dd, J8
and 2, 2-H), 7.09 (lH, d, J2, 2-H), 5.23 (2H, s, CH2Ph), 4.39
(2H, s, 3-CH2), 2-64 (3H, s, 4~-CH3) and 2-52 (3H, s, CH3CO);
m/z (~) 420 (20, M ), 405 (10), 311 (20), 151 (15) and 91 (100).
t) EthYl 3 4-d~methvl 8 h~ AV~Y~olor3 2-blcarbazole-2-carboxv-
late
Obtained from thQ reaction b~t 5 hydrv~yindole and thQ
5_Ar ~toA~ ~hyl-4-_CBtylpyrrOlR and cry~A~ ed from methanol,
~t had m.p. 250 C (decomp.) ~Hl H61-DMSO) 11.11 (lH, s, l-NH),
10.21 (lH, ~, 5-NH), 8.83 (lH, , OH), 7.73 (lH, s, 10-H), 7.37
(lH, d, J2.5, 9-H), 7.21 (lH, d, J8, 6-H), 6.87 (lH, dd, J8 and
2-5~ 9-H), 7-21 (2H, q, OC~2CH3), 2.87 (3H, s, 4-CH3), 2;84 (3H,
Q~ 3-CH3) and 1.38 (3H, t, OCH2C_3); m/z (~) 322 (69, M ), 276
(100), 248 (24), 220(3) and 138 (15); (Found: M , 322. 1322
ClgH18N2O3 require~ M, 322. 1317). Also isolated from the
reaction was 3-r3'-acQtYl-5'-ethoxYcarbonYl-4~-methyl~yrrol-2
ylm~thvl)-5 h~dL~Yindole,it had m.p. 99-102 C (Found: C, 66.89,
- H, 6.17; N, 8.03. ClgH20N2O4 requirss C, 67.04; H, 5.92; N,
8.23~) ~H(CDC13) 8.84 (lH, s, l'-NH), 8.14 (lH, s, l-NH), 7.20
(lH, d, J8, 7-H), 7.10 (lH, d, J 2.5, 2-H), 6.81 (lH, d, Jl.5,
W 0 9~/02483 : P ~ /GB93/0151 J
2 ~ 4 6 G ~ - 36 -
4-H), 6.79 (lH, dd, Jl.S and 8, 6-H), 5.60 (lH, , br, 5-OH),
4.31 (2H, ~, CH2), 4.21 (2H, q, OC_2CH3), 2.58 (3H, s, 4'-CH3)
2.48 (3H, ~, 3~-COCH3) and 1.27 (3H, t, OCH2C~3); m~z (%) 340
(100, M ), 325 (44), 293 (21), 279 (35), 266 (35), 2Sl (31), 223
(25), 196 (5), 147 (20) _nd 133 (36).
u) ~enzvl 3,4-dimethvl-7-fluGl~v~-olor3 2-blc~rb~zole-2-carboxv-
late and BenzYl 3,4-dimethYl-8-flu~ v vY~olor2.3-bl~J~h-~le-2-
carboxvlate
obtained a~ a mixture of i~ - s from the reactLon bLL
6-fluoroindole and the 5-acetoxymethyl-4-acetylpyrrolo.
Chromatographic ~eparation yielded the f3.2-bl$~omer (0.139g,
36~) m.p. 205 C (decomp.) ~H(l H6~-DMSO) 11.32 (lH, , l-NH),
10.85 (lH, s, 5-NH), 8.08 (lH, dd, J9 and 6, 9-H), 7.86 (lH, ,
10-H), 7.57-7.35 (5H, m, ArH), 7.12 (lH, dd, J10 and 2, 6-H),
6.90 (lH, dt, J9 and 2, 8-H), 5.43 (2H, s, C~2Ph), 2.91 (3H, ,
3-CH3) _nd 2-90 (3H, ~, 4-CH3); m/z (~) 386 (55, M ), 342(5),
295 (4), 278(100), 249(45), 236(20), 222 (25) and 91 (95);
(Found: MH , 387. 1509. C24H20FN2O2 require~ 387.1509); and the
r2.3-bli~omer m.p. 190-193 C ~H(CDC13) 8.54 (lH, s, br, l-NH),
8.10 (lH, dd, J9 and 6, 5-H), 7.87 (lH, ~, br, 9-NH), 7.51-7.34
(5H, m, ArH), 7.34 (lH, ~, 10-H), 7.22 (lH, dd, con~e~ by
10-H, 8-H), 6.93 (lH, dt, J2 and 9, 6-H), 5.41 (2H, s, CH2Ph),
3.20 (3H, 8, 4-CH3) and 3.00 (3H, ~, 3-CH3); m/z (~) 386 (100,
M ), 295 (12), 278 (96), 250 (27), 236 (7), 222(8) and 91 (59)~
(Found: M , 386.1433. C24HlgFN2O2 reguire~ 386.1431).
v) Ethvl 3.4-dimethYl-7-~luo,o~vr~olor3.2-blcarbazole-2-carbox~latc
Jn~ EthYl 3.4-dimethvl-8-fl~G vuvL~oler2.3-blc~rb~A7ole-2-~A~-
l~QxYlate
obtained as a mixture of ~ f_c~ the reaction b&tJ~--
6-fluoroindole _nd the S-acetoxymethyl-4-acetylpyrrole.
~h.. -LogrArh;r Jeparation yielded the r3.2-bli3omer wh$ch wa~
W 0 94/02483 21~ 0 fi 6 2 P~/GB93/01512
crystalli--~ from Airhlsromethane~ m.p. 231-234 C (Found: C,
70.45; H, 5.53; N, 8.66. ClgH17FN202 require~ C, 70.36, H,
5-28; N~ 8.64%); ~H([ H6]-DMSO) 11.27 (lH, ~, br, l-NH), 10.82
(lH, s, br, 5-NH), 8.90 (lH, dd, J9 and 6, 9-H), 7.85 (lH, s,
10-H), 7.12 (lH, dd, J10 and 2, 6-H) 6.89 (lH, dt, J2 and 9,
8-H), 4.37 (2H, q, OC_2CH3) 2.89 (6H, , 4-CH3), 1.39 (3H, t,
OCH2C~3); m/z (%) 324 (60, M ), 278 (100), 250 (34), 222 (10)
39 (7); (Found3 M , 324.1267. ClgH17FN2 2
324.1274); and the r2.3-blisomer m.p. 262-265 C; ~H(~ H6]-DMSO)
11.14 (lH, s, br, l-NH), 11.06 (1~, ~, br, 9-NH), 8.12 (lH, dd J
6 and 9, 5-H), 7.19 (lH, ~, 10-H), 7.15 (lH, dd J10 and 2, 8-H),
6.92 (lH, dt, J2 and 9, 6-H), 4.36 (2H, q, OCH2CH3), 3.13 (3H,
, 4-CH3), 2.93 (3H, 8, 3-CH3) and 1.39 (3H, t, OCH2CH3); m/z
(~) 324 (72, M ), 278 (100), 250 (39), 222 (9), 139(6) and
125(7); (Found: M , 324.1280. ClgH17FN2O2 requLre~ 324-1274)-
w) EthYl 3,4-dLmethvl 9 hY~ ~v~Y~olor3.2-blcarbazole-2-carboxv-
late and Ethvl 3.4-dimcthvl 5 hYd.o~Yv~olor3~2-blcarbazole-2
~-boxYlate
obtained as a mixture of i- - D from the reactLon bc~
q h~d ~A~Lndole and the 5-acetoxymethyl-4-acetylpyrrol~.
~h,~ -tographic separation yielded the r3.2-bli~omer whi~h was
- crystallL~ed from ethyl acetate/lLght petroleum, m.p. 260-262C('~~c - ) ~d(~ H6]-DMSO) 11.13 (lH, , l-NH), 10.56 (lH, ,
5-NH), 10.00 (lH, s, OH), 8.02 (lH, s, 10-H), 7.12 (lH, t, J7.5,
7-H), 6.83 (lH, d, J7.5, 6-H), 6.48 (lH, d, J7.5, 8-H), 4.39
(2H, q, O Q 2CH3), 2.87 (3H, s, 4-CH3), 2.85 (3H, ~, 3-CH3) and
1-38 (3H, t, OCH2CH3); m/z (~) 322 (61, M ), 276 (100), 248
(20), 219 (5) and 138 (11); (Found: M , 322.1305. ClgH18N2O3
r-quires 322.1317); and the r2.3-blisomer which was cryst~llire
from ethyl ac6tate, m.p. 251-254 C (~c~ H(~ H6]-DMSO)
10.95 (lH, ~, l-N~), 10.85 (lH, 5, 9-H), 9.89 (lH, ~, OH), 7.08
(lH, t, J7.5, 7-H), 7.07 (lH, s, 10-H), 6.77 (lH, d, J7.5, 8-H),
6.52 (lH, d, J7.5, 7-H), 4.32 (2H, q, OCH2CH3), 3.44 (3H, s,
WO 94/02483 - 38 - PCI/GB93/0151Z_
~4~6 4-CH3), 2.92 (3H, s, 3-CH3) and 1.37 (3H, t, OCH2C_3); m/z ~%)
322 (65, M ), 276 (100), 248 (88), 219 (15), 205(10), 191 (10),
178 (5), 165 (5), 138 (10) ~nd 115 (10); (Found:M , 322.1317.
C19H18N2O3 requ$r2s 322.1317).
x) Fthvl 6.9-dimethoxY-3 4-dim~thvlDYrrol-r3~2-blc~rb~Qle-2-
boxYlate and EthYl 5.8-dimethoxv-3.4-~i~methYl~Yrrolo r 2.3-bl
carbazole-2-carboxvlate
obtained a~ a mixture of i n- ~ ~ from the reaction bet/~-
4,7-dimethoxyindole and the 5-acetoxymethyl-4-acetylpyrrole.
Ch ~ -Lographic ~eparatLon yielded the r 3.2-bl$~omer (13.7%)
m.p. 256-258 C. (Found: C, 68.98; H, 6.23; N, 7.89. C21H22N2O4
requir~ C, 68.84; H, 6.05; N, 7-65~ H (CDC13) 8-58 (lH, ,
br, NH), 8.08 (lH, ~, 10-H),7.84 (lH, ~, br, NH), 6.82 (lH, d,
J8, 7-H), 6.50 (lH, d, J8, 8-H), 4.43 (2H, q, OC_2CH3), 4.05
(3H, s, 9-OCH3~, 3.98 (3H, s, 6-OCH3), 2.96 (3H, ~, 4-CH3), 2-92
(3H, s, 3-CH3) and 1.44 (3H, t, OCH2CH3); m/z (~) 366 (73, M ),
326 (100), 305 (11), 290 (11), 277 (23), 262 (15), 183(10), 160
(17), 152 (19) and 131(7); V (Nujol)/cm 3474, 3323 and
1674; ~ (MeOH)/nm 415, 387, 344, 330(sh), 305(sh), 266, 246
and 220; and the r2.3-bli~omer (9.3~) m.p. 193-195 C. (Found: C,
69.03; H, 6.29; N, 7.42. C21H22N2O4 require~ C, 68.84, H, 6.05;
N, 7.65%); ~H (CDC13) 8-44 (lH, ~, br, NH), 8.10 (lH, ~, br,
NH), 7.06 (lH, 8, 10-H), 6.82 (lH, d, J8, 7-H), 6.56 (lH, d, J8,
6-H), 4.40 (2H, q, OC_2CH3), 3-98 (3H, ~, OCH3), 3.97 (3H~ 8~
OCH3), 3-42 (3H, 8, 4-CH3), 3.00 (3H, ~, 3-CH3) and 1.43 (3H, t,
OCH2CH3); m/z (%) 366 (100, M ), 320 (82), 292 (20), 277 (24),
262 (10), 183 (14), 160 (28), 131 (3); V (Nujol)/cm 3457,
3345 and 1660; ~max (MeOH)/nm 381, 365, 293, 247 and 219.
y ) FthYl 7-methoxY-3, 4-dimethYl~vrrole r 3.2-blc~ ole-2-carboxY-
late ~n~ ~thYl 7-methoxv-3, 4-dimethvl~Yrrole r 2 3-blcarba~ole-2-
~rh-~Yylate
W O 94/02483 2 1 g 0 6 6 2 P~/GB93/01~12
obtained a~ a mixtur~ of iæ - ff from the r-aetion b_~
6-methoxyindol~ and th~ 5-ac~GA~ hyl-4-acetylpyrrole. Fla-h
C~ LG~PhY on silica, eluting with ethyl acQtate/cycloheYAn~
(lsl) yi~ld-d th- f3.2-blisom r which was cry-t~ from
thyl~ t~te/cyclr - -, m.p. 239-241 C (d~ H([ H61-
DMSO) 11.09 (lH, , l-NH), 10.49 (lH, , 5-NH), 7.91 (lH, d, J
8.7, 9-H), 7.73 (lH, , 10-R), 6.88 (lH, d, J2.3, 6-H), 6.68
(lH, dd, J 8.7 and 2.3, 8-H), 4.35 (2H, q, OC_2CH3), 3.84 (3H,
7-OCH3), 2-87 (3H, ~, 3-CH3), 2.86 (3H, s, 4-CH3) and 1.37
(3H, t, OCH2CH3); m/z (~) 336 (84, M ), 290 (100), 262 (32), 247
(16) and 219 (16); Vma (~Br D$sc)/cm 3342, 1674 and 1628; and
the r2.3-bli~omer which was cry~tallissd from ethyl
acetate/cycloh~Yans, m.p. 260 C (decomp.) ~H([ H6~-DMSO) 10.98
(lH, ~, l-NH), 10.74 (lH, s, g-NH), 8.00 (lH, d, J8.7, 5-H),
7.13 ~lH, ~, 10-H), 6.87 (lH, d, J2.7, 8-H), 6.70 (lH, dd, J8.7
and 2.7, 6-H), 4.34 (2H, q, OC_2CH3), 3.83 (3H, ~, 7-OCH3), 3.10
(3H, ~, 4-CH3), 2.91 (3H, g, 3-CH3) and 1.37 (3H, t, OCH2CH3);
m/z (~) 336 (56, M ), 290 (70), 262 (26), 145 (16), 129 (14);
Vma (RBr Disc)/cm 3379, 3339 and 1663.
z ) EthYl 3-ethYl-4-methYlDYrrolo r 3.2-blcarbazole-2-carboxvlate
(0.956g, 27%) wa~ obtained from the r-action (11 mmol scale)
~t indole and the Qthyl 5-acetoxymethyl-4-acetyl-3-ethyl-
pyrrol~-2-carboxylate, after ~ y~ t;~ from toluene, ~t
had m.p. 24~249 C (decomp.) (Found: C, 74.93; H, 6.35; N, 8.60.
C20H20N2O2 require~: C, 74.98; H, 6.29; N, 8.74~ H([2H61-
DMSO) 11.27 (lH, s, l-NH), 10.63 (lH, s, 5-NH), 8.09 (lH, d, J
8, 9-H), 7.93 (lH, s, 10-H), 7.31-7.47 (2H, m, 6-H, 7-H), 7.09
(lH, ddd, J 8, 5.5, 2, 8-H), 4.40 (2H, q, J 7, CO2CH2), 3.37
(2H, q, J 7, 3-CH2), 2.91 (3H, ~, 4-CH3), 1.41 (3H, t, J 7,
CO2CH2C~3), 1.30 (3H, t, J 7.5, 3-CH~CH3); m/z (~) 320(100, M ),
274(96);
~ma (RBr disc)/cm 3344, 3327, 1680, 1664, 1238.
WO94/02483 ,, ~ " PCI/GB93/01~1~
66~
EX~mD1~ 5
Pvrrolo r 3.2-blcarbazole-2-carboxvl$c Ac$ds General ~oc~du-e.
To a ~olution of tho benzyl pyrrolo[3,2-b~c~rb-~ole-2-carboxylate $n
dry tetral~dhoL~..n ~THF) (lO cm ) WA- add-d 10% ~1 on C (50 mg). The
react$on m$xture wa- hy~.3 ~ at one atmo~ u~e and room
t~ - ~Lu.~. Aftor uptake of H2 h-d cea~ad, the cataly~t wa~ d
by f$1trat$on through CelLte and wa~hed well w$th 1~, and the
comb$ned f$1trates were e~.~o,ated under reduced pre-~ur~.
Cry~ t$on of the re~ult$ng sol$d from acetone, methyl ethyl
ketone or aqueous methanol qave the
pyrrolo[3,2-blcarbazole-2-carboxylic acid~ a~ y~llow cryctals.
a) 3 4-Dimethvl~vrrolo r 3 2-blcarbazole-2-carboxvlic acid
(0.234 g, 84.3~) m.p. 237 C (decomp.); ~H(t H6]-DNSO) 12-74 (1
H, br, ~, CO2H), 11.13 (1 H, ~, l-NH), 10.60 (1 H, n, 5-NH),
8.05 (1 H, d, J7.5, 9-H), 7.87 (1 H, ~, 10-H), 7.42 (1 H, d,
J7.5, 6-H), 7.36 (1 H, t, J7.5, 7-H), 7.08 (1 H, t, J7.5, 8-H),
2.92 and 2.91 (2 x 3H, 2 x S, 3-CH3 and 4-CH3); m/z(%)
278(30,N ) 260(39), 234(100), 218(19), 204(8), 167(8) 149(16),
130(10) and 117(2S) (Found: M , 278.1060.C17H14N2O2 requ$re~
N,278.1055).
b) 8-Fluoro-3 4-dimethvl~vrrolo r 3 2-blcarbazole-2-carboxvl$c acid
(0.0845 g, 85.6~) m.p. 236-239 C, ~H([ H6]-DNSO) 12-80 (1 H,
br, ~, CO2H), 11.19 (1 H, ~, 1-NH), 10.60 (1 H, ~, 5-NH), 7.91
(1 H, dd, J9 and 2.5, 9-H), 7.86 (1 H, ~, 10-H), 7.37 (1 H, dd,
J9 and 4,6-H), 7.20 (1 H, dt, J9 and 2.5, 7-H) and 2.89 (6 H, ,
2xCH3); m/z (~) 296(51,M ), 278(71), 252(100), 250(37), 236(19),
222(13), 13g(22), 12~(36) and 111(28) (Found: N , 296.0960.
C17H13FN2O2 require~ M, 296.0961)
W O 94/02483 214 0 6 6 2 P ~ /GB93/01~12
- 41 -
c) 3.4.6-~rimethvl~Yrrolo r 3,2-blcarbazole-2-carboxvlic ac~d
o
(0.065 g, 85~) m.p. 230 C (d~comp.) (Found: C,74.2; H,5.55;
18 16 2 2 qu
~H([ H6~-DMSO) 12.80 (1 H, br, s, CO2H), 11.01 (1 H, s, l-NH),
10.08 (1 H, s, 5-NH), 7.90 (1 H, d, J7.5, 9-H), 7.82 (1 H, B,
10-H), 7.16 (1 H, d, J7.5, 7-H), 7.01 (1 H, t, J7.5, 8-H), 2.97
(3 H, s, 4-CH3), 2.92 (3H, s, 3-CH3) and 2.58 (3 H, s, 6-CH3);
m/z(%) 292(72,M ), 274(100), 246(50), 230(11),137(25), 122(24)
and 109(30).
d) 3-(2-Methoxvcarbonvlethvl)-4-methvlPvrrolo r 3.2-blcarbazole-2-ca-
rboxvlic acid
(0.0673 g, 84.6~) m.p. 255 C (decomp.) (Found: C, 68.4; H,5.3;
N,7.75.C2oH18N2O4 rcquir-- C, 68.6; H,5.18; N, 8.00%);
~H([ H6]-DMSO) 12.88 (1 H, br, , CO2H), 11.34 (1 H,g,l-NH),
10.65 (1 H, s, 5-NH), 8.06 (1 H, d, J 7.5, 9-H), 7.88 (1 H, ~,
10-H), 7.42 (1 H, d, J 7.5, 6-H), 7.36 (1 H, t, J7.5, 7-H) 7.07
(l H, t, J7.5, 8-H), 3.66 (3 H, s, OCH3), 3.63 (2 H, partLally
obscur~d t,CH2CH2CO), 2;89 (3 H, s, 4-CH3), 2.66 (2 H, t,
CH2CO)~ m/z(%) 350(100,M ), 332(17), 306(30), 290~63), 272(22),
259(32) and 233(47).
e) 1.3 4-TrLmethvlPvrrolo r 3.2-bloarbazole-2-oarboxvlio acLd
(0.060 g, 44~) m.p. 215-216 C (decomp.) (Found: C, 73.69; H,
5.51; N, 9.41; C18H16N2O2 requLre~ c, 73-95t H, 5.52; N, 9.58);
~H(~ H6]-DMSO) 12.94 (lH, br, s, COOH), 10.63 (lH, s, 5-NH),
8.13 (lH, d, J 7.9, 9-H), 8.00 (lH, ~, 10-H), 7.45-7.30 (2H, m,
- 6-H, 7-H), 7.14-7.04 (lH, m, 8-H), 3.99 (iH, s, l-CH3), 2.91
(3H, ~, 4-CH3) and 2.85 (3H, s, 3-CH3); m/z (~) 292(95,M ),
275(10), 247(40), 232(30), 180(100) and 135(100); vma (~8r
Dizc)/cm 3375, 2930 and 1709.
W O 94/02483 - 42 - PCT/GB93/01512 _
66~
f) 3.4,5-TrimethvlDYrrolo r 3,2-blcarbazole-2-carboxYlic acid
(0.015 g, 18%) m.p. 239-240 C (decomp.) (Found: C, 74.11; H,
5.38; N, 9.39; C18H16N202 r~quirec C, 73-95S H, 5-52; N, 9-58;
H61-DMSO) 11.15 (lH, ~ NH), 8.04 (lH, d, J 7.5, 9-H)
7.88 (lH, s, 10-H), 7.48-7.41 (2H, m, 6-H, 7-H), 7.17-7.06 (lH,
m, 8-H), 4.03 (3H, ~, S-CH3), 3;16 (3H, ~, 4-CH3) and 2.93 (3H,
8, 3-CH3); m/z (~) 292(90,M ), 274(75), 232(70), 197(35),
181(60), 149(30) and 130(100); u (XBr Di~c)~cm 3454, 2926
and 1670.
g) 1,3,4.5-Tetramethyl~yrrolo r 3.2-blcarbazole-2-carboxvlic acid
(0.030 g, 32%) m.p. 215-217 C (d~comp.) (Found: C, 74.44; H,
6-00; N, 9.14; ClgH18N2O2 requires C, 74.49; H, 5.92; N, 9.14);
~H([ H6)-DMSO) 12.98 (lH, br, 8, COOH), 8.14 (lH, d, J7.6, 9-H),
8.04 (lH, ~, 10-H), 7.48-7.38 (2H, m, 6-H, 7-H), 7.18-708 (lH,
m, 8-H), 4-01 (3H, ~, 5-CH3), 3.97 (3H, ~, l-CH3), 3-12 (3H, ,
4-CH3) and 2-84 (3H, ~, 3-CH3); m/z (%) 306(100,M ), 279(25),
232(38), 197(34), 181(80) and 149(25); vma (RBr Di~c)/cm 1935
and 1659.
h) 3.4-Dimethvl~Yrrolor3.2-blcarbazole-2-carboxYlic acid
The ethyl e~ter (500mg, 1.6mmol) in water (15 cm3) and methanol
(35 cm ) wa~ heated to reflux and ~ufficient methanol to achieve
di-colution was added. Cae~ium carbonate (5.32g; 16mmol) wa~
added and the mixture wa~ heated to reflux under nitrogen for
18h. After cooliny^, ~olvent wa~ .~ ved }~ vacuo to leave
approximatQly 20 cm of ~olution which wa~ ~ G~yhL to pH3 by th~
addition of 0.1~ h~d~ochloric acid wha,e pon the title ~ ~
pr~cipitated out. Filtration, ~- ~hin~ wLth water and drv$ng
under vacuum yielded analytically pure product (437mg; 96~)
which wa~ ~p~ctro~copically identical to that obtained in
r le 5a.
WO 94/02483 2 1 4 0 6 6 2 PCI`/GB93/01512
ExamDle 6
.
Pvrrolor3.2-blcarbazole-2-cArboxYcliç Acid Est~r~ - General vLoced~re
The pyrrolo[3,2-b]c~rb--Ql~-2-carboxylic acid (1.0 mmol) and
N,N'-carbonyl ~ le ~1.1 mmol) w~rR di-~olved in fr~shly
distill-d tetrah~dror~ran under a nitrog-n ~ -_~ha,~. The re-ulting
~u~pen-ion wa- stirr-d at room t~ - ~t~r~ for at lea~t one hour, and
compl~te co..v~L~ion of the acid to the ~ rQlide ;- F - ' i Ate was
verified by ~LC. The alcohol or ph~nol (1.5-2.0 mmol, i.e. an
exce~) was added in one portion, and the r-sulting mixture was haat-d
to reflux until T~C showed complete con~ n of the i ;A~olide
int - ~tQ. ThQ product wa~ obt~inod by column ch-~ oJ,~ on
~ilica, followed by L~_LyS~ ~ tion.
a) Phenvl 3.4-dimethvl~Yrrolo r 3.2-blcarbazole-2-carboxYlate
wa- obtained from the r-action of the ~ olide ;nt~ -d~at~
with phQnol. ChL~ -tGJ-~phy (olutLng with 10~ aCRtone/90%
petrol) followed by L~ _~D~ tion from acetone ~ Lrol gave
orange crystals (0.230 g, 65~) m.p. ~230 C (dQcomp.) (Found:
C,78.17; H, 5.0g; N, 7.77. C23H18N202 requires C, 77.95; H,
5-125 N~ 7-90%); ~(1 H61-DMS) 11.55 (lH, s, l-NH), 10.64 (lH,
~, 5-NH), 8.10 (lH, d, J 7.5, 9-H), 7.94 (lH, s, 10-H),
7.30-7.58 (7H, m, PhH, 6-H, 7-H), 7.09 (lH, ddd, J 7.5, 5.5, 2,
8-H) and 2.97 and 2.95 (2 x 3H, 2 x , 3-CH3 and 4-CH3); m/~(%)
355(40,M ); ~ma (XBr Disc)/cm 3396, 1701 and 1180.
b) r ( 2-Dimethvlamino)ethvll 3.4-di~ethvl~vrrolo r 3.2-blcarbazole
- -2-carboxvlate
wa- obtainod from ~h~ r~action of th~ olide int- -'ia~
with (2-dimQthylamino)~thanol. ~hL~ ~ G~ a~hy (eluting with
10% - h-n~l/90% DCM) gave a yellow solid (0.350 g, 99~).
WO 94/02483 _ 44 _ PCI`/GB93/OlSl~
66?'
r- _y~ L~-t;~n of a portion from DCM gav ~ llow cry~tal-
with m.p. 174.0-175.7 C (decomp.) (Found: C, 70.46; H, 6.48S N,
11.76. C21H23N3O2Ø15CH2C12 ~quir-~ C, 70-295 H, 6-45; N~
11.55~ H(~ H61-DMSO) 11.~8~ , l-NH), 10.60 (lH, ~,
5-NH), 8.07 ~lH, d, J 8, 9-H), 7.89 (lH, s, 10-H), 7.30-7.43
~2H, m, 6-H, 7-H), 7.09 (lH, ddd, J 8, 6, 2.5, 8-H), 4.41 (2H,
t, J 6, OCH2), 2.91 (6H, , 3-CH3 and 4-CH3), 2.69 (2H, t, J
6.0, NCH2) and 2.27 (6H, ~, N(CH3)2), m/z(~) 350(46,(~+1) )~
261(68) and 133(100); vma~(RBr Di~c)/cm 3377, 1661 and 1238.
c) r (3-Dimethvlamino)~henY11 3 4-dimethvlDyrrolo r 3 2-blcarbazole-2-
carboxYlate
wa- obtained from the 0.95 mmol ~cale reaction of the ; ;~--o-
lide ; nt~ ~'; Ate with (3-dimethylamino)phenol. Ch.. - o~- h~hy
(eluting with 10~ ethyl acetate/90% tolu~n~) followed by
~,yD~A~ -tion from ethyl acetate gave yellow cry~tal~ (0.272
g, 72%) m.p. 240-242 C (decomp.) (Found: C, 75.37; H, 5.71; N,
10 36. C25H23N302 require~ C, 7S.55; H, 5.83; N, 10.57~; ~H
(~ H6]-DMSO) 11.49 (lH, , l-NH), 10.64 (18, s, 5-NH), 8.08 (lH,
d, J 8, 9-H), 7.91 (lH, ~, 10-H), 7.34-7.48 (2H, m, 6-H, 7-H),
7.27 (lH, t, J 8, 5'-H), 7.10 (lH, ddd, J 8, 6, 2, 8-H),
6.56-6.70 (3H, m, 2'-H, 4'-H, 6'-H), 2.96 (3H, ~) and 2.94
(9H,~) (3-CH3,4-CH3, N(CH3)2); m/z (~) 398(38, M+l) , 261(25),
232(21) and 217(100); vma (R8r Di-c)/cm 3350, 1674, 1610 and
1232.
d) (3-Pvr$dYl) 3,4-dimethvl~Yrrolo r 3 2-blcarbazole-2-carboxYlate
wa- obtAin-d from the r-action of the ; ~ olide ;nt~ - i At~e
with 3-hy~dcoAy~yLidine. Ch~ -tG~a~hy (eluting with 50~ ethyl
ac-tate/50% p trol) followed by ~ y~ -t~n from acetonc
gave yellow cry~tal~ (0.230 g, 65~) with m.p. ~270 C (decomp.)
(Found: C, 73.88; H, 4.76; N, 11.50. C22H17N302-o-2H2
roquir~: C, 73.61; H, 4.89; N, 11-71%); ~H ([ H61-DMSO) 11-59
21~066~
WO 94/02483 45 PCI/GB93/01512
, .
3 . , = ~ .
, .
(lB, o, 1-NH), 10.65 ~lH, s, 5-NH), 8.63 (lH, d, J 2, i~-H),
8.55 (lH, dd, J 4, 1, 6'-H), 8.10 (lH, d, J 8, 9-H), 7.90 (lH,
, 10-H), 7.86 (lH, ddd! J 8, 3, 1, 5'-H), 7.58(1H, dd, J 8, S,
4'-H), 7.32-7.45 (2H, m, 6-H, 7-H), 7.09(1H, ddd, J 8, 6, 2,
8-H) and 2.97 and 2.94 (2 x 3H, 2 x s, 3-CH3 and 4-CH3); m/z (%)
356(15, (M+l) ) ~max(~Br Di-c)/cm 3377, 1715 and 1173.
~) (4-r-~b~ovlDh~nYl) 3.4-~i~e~hvl~Yrrolo r 3 2-blcarbazole-2-
carboxvlate
was obtain~d from the r~action of the i ;~A~O1~e intr ~';atewith 3-h~d~oAy~ . Rec.~ -tion from ethanol gave a
YG11OW powder, and an impure residue. The latter material waz
further purified by column cl. -tGJ-~hy on oilica (eluted wLth
5~) methanol/95~ DCM then 10~ methanol/90% DCM) followed by
,Lc~y~L~ tion from ethanol. (0.262 g, 66%) m.p. >250 C
(~e- .) (Found: C, 71.72; H, 4.81~ N, 10.26. C24HlgN3O3Ø2H2O
reguir~s C, 71.88; H, 4.88; N, 10.48%); ~H ([ H6]-DMSO 11.56
(lH, s, l-NH), 10.63 (lH, o, S-NH), 7.90-8.12 ~SH, m, 9-H, 10-H,
3'-H, 5~-H, amide N-H), 7.33-7.49 (5H, m, 6-H, 7-H, 2~-H, 6'-H,
amide N-H), 7.09 (lH, ddd, J 8.5, 6, 1.5, 8-H) and 2.95 and 2.93
(2 x 3H, 2 x o, 3-CH3 Lnd 4-CH3); m/z (~) 398(10, (M+l) ),
279(100); ~ (RBr D$sc)/cm 3423, 1717, 1695 and 1171.
f) (PvridYl-4-methYl) 3 4-dimethvlPYrrolo r 3,2-blcarbazole-2-
carboxvlatQ
wa~ obtained from the reaction of the i ~ oli~v- intr~ ate
with 4-pyridylcarb$nol. ~I., - -_ a~h~ (~luting with thyl
~cst~te/petrol, gradient 60%, 80%, 100% ethyl aCQtatQ, then
methanol/Qthyl acetate, gradient 10~, 20~) follow~d by
_rs~ tion from t~trahydrofuran gave orange crystal~
(0.168 g, 46%) with m.p. >240 C(d~comp.) (Found: C, 72.16; H,
23 1gN302Ø7H2O requireo C, 72.31; H 5 38; N
11.00~ H(~ H6]-DMSO 11.31 (lH, o, 1-NH), 10.62(1H, o, 5-NH),
WO 94/02483 - 46 - PCI/GB93/0151~
~4~66~
8.62 (2H, dd, J 4.5, 0.5, 2~-H, 6~-H), 8.08 (lH, d, J 7.5, 9-H),
7.89 (lH, , 10-H), 7.53(2H, d, J 5.5, 3~-H, 5'-H), 7.32-7.43
(2H, m, 6-H, 7-H), 7.07 ~lH, ddd, J 8, 5, 1, 8-H), 5.45 (2H, ~,
ArCH2) and 2.94 and 2.92 ~(2 x 3H, 2 x 3-CH3 and 4-CH3); m/z (~)
369(27, (~+1) ), 327(10j) _nd 295(100); v a (RBr Di-c)/cm 3400,
1709 and 1232.
g) ~1 3-D$b~n~Ylo~Y~o~Y1-2~ 3.4-dimethvlDYrrolor3.2-blearbazole-2- carboxvlate
wa~ obtained from the 1.5 mmol scale rQaction of the i iA-~olide
te with (1,3-dibenzyloxy-2-propanol). ~hLI -tCyL~y
(eluting with 20% ethyl acetate/80~ toluene then 40% ethyl
acet_te/60~ toluene) followed by .-~_ y~allir~tion from ~thyl
acetate-etha. ~Lrol gave yellow cry~tal~ (0.776 g, 97~) m.p.
124.8-126 C (decomp.) (Found: C, 76.35; H, 6.07; N, 5.12.
C34H~2N204 require~ C, 76.67; H, 6.06; N, 5.26~;
~H([ H61-DMS0) 11.18 (lH, ~, 1-NH), 10.60 (lH, ~, 5-NH), 8.06
(lH, d, J 7.5, 9-H), 7.88 (lH, ~, 10-H), 7.22-7.42 (12H, m, 2 x
PhH5, 6-H, 7-H), 7.07 (lH, ddd, J 8, 6.5,1.5, 8-H), 5.44 (lH,
quintet, J 5, l'-H), 4.60 and 4.53 (2 x 2H, 2 x dd, J 12, 2 x
PhC~20), 3.77 (4H, d, J 5.5, OCH(C ~)2) and 2.91 _nd 2.89 (2 x
3H, 2 x ~, 3-CH3 and 4-CH3); m/z(~) 532(50,M ), 260(65) and
91(100); ~ (RBr Disc)/cm 3358, 1681 and 1234.
h) (4-Methvl~ulDhinvlDhenvl) 3.4-dimethvlPvrrolo r 3.2-blcarbazole-2-
rarboxvlate
= wa0 obtained from the r-action of the i ~ Qlide inte -'ia~e
with 4-mQthyl~ulphinylphQnol. Ch.~ -tog aphy (eluting with
ethyl acQtate/petrol, gradiant 90%, 95%, 98~, 100~ Qthyl
acetat-, thQn 10% m~thanol/ethyl ac~tat~) fo~ by
~6~ y~Lalli~-tion from tetrahydrofuran gave a yellow powdQr
(0.261 g, 63%) m.p. >230 C (decomp.) (Founds C, 68.40; H, 4.81;
N, 6.44. C24H2oN203SØ3H20 requir - ~ C, 68-32; H, 4-92; N~
21~0fi62
WO 94/02483 _ -47 _ PCI`/GB93/OlS12
6.64~; ~H([ H6]-DMSO) 11.59 (lH, s, 1-NH), 10.68 (lH, ~, 5-NH),
8.10 (lH, d, J 8, 9-H), 7.93 (lH, s, 10-H), 7.82(2H, d, J 9.5,
3'-H, 5'-H), 7.59 (2H, d, J 9.5, 2'-H, 6'-H), 7.33-7.45 (2H, m,
6-H, 7-H), 7.09 (lH, ddd, ~ 8, 6, 2.S, 8-H), 2.99 and 2.95 (2 x
3H, 2 x ~, 3-CH3 and 4-CH3) and 2.82 (3H, ~, CH3SO); m/z (~)
417(2,M+1 ), 261(100) and 233(75); ~max(XBr Di-c/cm 3427,
3288, 1717 and 1200.
i) Methvl 3 4-dimethYl~vrrolo r 3.2-blcarb_zole-2-carboxvlate
was obtained from the rQaction of the i ~ olide inte ~ te
with methanol. Chromato~aphy (eluting with 30% ethyl
acetate/petrol), follf ~' by ~ac.~Lalli~ation from ethyl
acetate gave a yellow powder (0.188g, 64%) with m.p. 211-213C
~ .) (Found: C,74.06, H, 5.49, N,9.42, C18H16N202 require~:
C, 73.95; H, 5.52; N, 9.S8~ H([ H6]-DMSO) 11-25 (lH~ s,
1-NH), 10.62 (lH, s, 5-NH), 8.08 (lH, d, J 8, 9-H), 7.89 (lH, ~,
10-H), 7.33-7.58 (2H, m, 6-H, 7-H), 7.09 (lH, ddd, J 8, 6, 1,
8-H), 3.92 (3H, 8, OCH3), 2.92 and 2.91 (2 x 3H, 2 x ~, 3-CH3
and 4-CH3); m/z (~) 292(68, M ), 260(100), 232(39); ~max (RBr
di~c)/cm 3342, 1684 and 1236.
; ) r t2-Methvl3ul~honvl)ethvl~3~4-dimethvlpvrrolor3~2-blcarbazole
~a~boxvlate
Was obtained from the reaction of the ; iA--olide ;nts -';ate
with (2-methyl~ulphonyl)ethanol. Chroma~og~a~h~ (gradi-nt
lution with ethyl acetate/petrol, 30~ - 100~) followed by
~ _ y~Lalli-ation from acaton~ gave f$ne yellow crystal~ (0.222
g, 58~) with m.p. 255-257 C (decomp.) (Found: C, 62.23; H, 5.25;
N, 7.08. C20H20N2O4S require~ C, 62.48; H, 5.24S N, 7.29~);
~[ H~]-DMSO 11.19 (lH, , l-NH), 10.60 (lH, s, 5-NH), 8.09 (lH,
d, J 7.5, 9-H), 7.8g (lH, 8, 10-H), 7.32-7.45 (2~, m, 6-H, 7-H),
7.09 (lH, ddd, J 7.5, 5.5, 3, 8-H), 4.69 (2H, t, J 5.5, OCH2),
3-69 (2H, t, J 5.5, SO2CH2), 3.12 (3H, 8, SO2CH2), 2.93 (6H, s,
.
WO 94/02483 PCI/GB93/0151
-- 48 --
Ct,~4~66~ +
3-CH3 and 4-CH~); m/z (%) 384 (17, M ), 260(13), 59(100);
(RBr di-c)/cm 3387, 1661, 1234.
k) Tert-butvl 3,4-dimethYl~yrrolo r 3,2-blcarbazole-2-carboxYlat~
The pyrrolo~3,2-blcr~ ~ cl~ 2-carboxylic ac$d (0.86 mmol) and
triphenyl,'- ~hLn~ 1 mmol, 1.05 oq.) w-r- di--olvcd Ln
fre~hly di~tillod tetrahy~,ofuran under a nitrogen a
Tertiarv-butanol (2.12 m~ol, 2.5 eq.) wa- added by zyrLnge, and
finally dLethyl ~o~ boxylate (0.95 mmol, 1.1 eq.) wa~ added
dropwi~e over 10 minute~. The re~ulting ~lL~ ion wa- stLrred
at room temperature for two hour-, by which time TLC ~howcd
complete con~_ Lion of the ~tarting acid. The title z~
wa~ obtaLned from the crude reaction mixture in ~overal ~tage~:
column chromatoy,a~hy on ~ilica, elutLng with 20% ether/80~
petrol then 50% ether/50~ petrol; column ch.~ -~o~,aphy on
silica (eluting with 25~ ether/75~ petrol then 40~ eth~r/60~
petrol); and finally, ~e_.y~ tion from DCM gave yellow
powder (0.030 g, 10~) m.p. 187-189 C (decomp.) (Found: C, 73.24;
22~H22N202Ø15CH2C12 requirQ~ C, 73.18; H
6-47; N~ 8 07%); ~H([ H6]-DMS0) 10.95 (lH, ~, l-NH), 10.57 (lH,
, 5-NH), 8.05 (lH, d, J 8, 9-H), 7.88 (lH, ~, 10-H), 7.29-7.43
(2H, m, 6-H, 7-H), 7.05 (lH, ddd, J 8, 6, 1, 8-H), 2.89 and 2.87
(2 x 3H, 2 x ~, 3-CH3 and 4-CH3) and 1.59 (9H, ~, C(CH3)3); m/z
(~) 355(62, M+l ), 278(90), 233(38), 126(32), 91(78) and
57(100); ~ (RBr Di~c)/cm 3337, 1664 and 1240.
ExamPle 7
PYrrolo r 3.2-blcarbazole-2-carboxvlic Acid AmLde~
a) 3,4-DimethYl-2-(1-i id~rolYcarbonvl)Pvrrolor3.2-blc~rb~zole
3,4-Dimethylpyrrolo~3,2-blc~rh-~ole-2-carboxylic acid (0.280 g,
1.0 mmol) and N,N'-carbonyl~ le (0.164 g, 1.0 mmol) w re
di~olved in freshly diotilled tetrahydrofuran (5 cm ) under a
214U662
W O 94/02483 P ~ /GB93/01512
- 49 -
nitrogen - -_~h~a. The re~ulting ~ p~n~i n~ was atirr-d at
room t - atu~ for two hour~, and complete co..~- sion of the
acid to the ; ;~--ol;~~ was verified by TLC. The T~r wa-
~ d and the ~ e ,.c_y~ from ~thyl acet~ta togive the ~ vdu~L a- a ~ ow olid (0.125 g, 38~) m.p. 252C
( -~ .) (Founds C, 73.17; H, 4.87; N, 16.80, C20H16N4O
r~quir--s C, 73.15; H, 4.91; N, 17.06%); ~H([ H6~-DMSO) 11-53
(lH, s, l-NH), 10.20 (lH, , 5-NH), 8.30 (lH, ~, 2'-H), 8.12
(lH, d, J8, 9-H), 7.94 (lH, s, 10-H), 7.79 (lH, 8, 5'-H),
7.33-7.47 (2H, m, 6-H, 7-Hl, 7.19 (lH, ~, 3'-H), 7.09 (lH, ddd,
J8, 6, 2, 8-H), 2.95 (3H, s, 3-CH3), 2.73 (3H, 8, 4-CH3~; m/z
(~) 261 (40); V (~8r Disc)/cm 3427, 1699 and 1242.
b) Ethvl 3,4-dimethvl~vrrolo r 3 2-blcarbazole-2-ca,L- ~
3,4-Dimethylpyrrolo[3,2-b]c~rh-~ole-2-carboxylic acid (0.278 g,
1.0 mmol) wa- d$-solv~d in dimethoxyethane (10 cm ) to give a
yellow rolut$on. To this were added dii~o~,opylethylamine
(0.260 g, 2.0 mmol), ethylamine l-~I,ochloride (0.245 g, 3.0
mmol) and the tetrafluo,bG~ alt of O-benzotrlazolyl-N,~,N',
N'- tetramethyluronium (TBTU) (0.482 g, 1.5 mmol) to give a
white u-p~nnion in the ~ DW solution. The reaction mixture
wa~ stirred at room t - ~Lu, for 24 h by which time TrC
howed no ~. -ining acid. The ~olvent was ,~ ~3d !n vacuo to
give a y~llow bLc ,. ~olid. This wa~ subjected to ~olumn
chromato~,a~hy on silica eluting firstly with DCM and then with
10~ EtOAc/90~ DCM to give the ethylamide product as a yellow
solid (0.240 g, 79~). To remove a trace impurity, a portion was
~ y~L~ 9~ from ~irhl~rorethane/petrol to give the
analyt~cally pure a~ a y~llow powder with m.p. 23S C (decomp.)
(Found: C, 73-21; H, 6.10; N, 13.33. ClgH19N3OØ1C2H4C12
requir-~: C, 73.15; H, 6.20; N, 13-32~ H([ H6]-DMSO) 10-72
(lH, 8, l-NH), 10.57 (lH, 8, 5-NH), 8.09 (lH, d, J8, 9-H), 7.93
(lH, t, J5, amide N-H), 7.83 (lH, 8, 10-H), 7.27-7.41 (2H, m,
6-H, 7-H), 7.06 (lH, d, J8, 8-H), 3.35 (2H, q, J 7.5, CH2CH3),
W O 94/02483 PCT/GB93/01~1 ~
,6?~
2.89 and 2.71 (2 x 3H, 2 x s, 3-CH3 and 4-CH3), 1.18 (3H, t, J
7.5, CH2C~3); m/z (~) 305 (65,M ), 260 (100); ~max (~Br
D~sc)/cm 3314, 1603 and 1545.
.. . .
c) 3,4-Dimethvl~Yrrolo r 3 2-blc~rb~role-2-ca.L- idP
3,4-Dimethylpyrrolo[3,2-~]c~r~~~olr 2-carboxylic acid (0.556 g,
2.0mmol) waz di--olv-d in ~ h.-_~' h-n~ (20m1) to give a
y llv-- ~olution. To thi~ were added ~ o~o~ylethylam$ne
(0.520 g, 4.0 mmol), - i hydcochloride (0.321 g, 6.0mmol)
and the tetrafl~o.LG-ate salt of O-benzotriazolyl-N,N,N',N',
-tetramethyluronium (TBTU) (0.963 g, 3.0mmol) to give a white
eu~pen~ion in the yellow ~olution. The reactLon mixture wa~
stirrQd at room t~ - ature for 24 hours, by which time TLC
howed no -i n i ng acid. The solvent was ~ vacuo to
give a y~llo/ b.. ., solid. This was subjected to column
ch.~ hy on silica (eluting with ethyl acetate/DCM,
gradient 10%-30%) to give thc amide product a~ a ysllow ~olid
(0.350 g, 63%). To remove a trace impurity, a portion wa-
.~_.ysLallised from ethyl acetate/petrol and then purifLed by
preparative HPLC (column size 25 cm x 2.12 cm i.d., packed wLth
C8 Zorbax, gradient elution: 5~ acetonitrile/9S~ water to 95~
acetonitrilQ/water; d~tected at 340 nm) to gLve a yellow powder
with m.p. 240 C (decomp.) ~H([ H61-DMSO) 10.82 (lH, s, l-NH),
10.54 (lH, B, 5-NH), 8.08 (lH, d, J 7.5, 9-H), 7.84 (lH, 8,
10-8), 7.29-7.43 (4H, m, 6-H, 7-H, NH2), 7.07 (lH, ddd, J 8,
5.5, 2, 8-H), 2.89 and 2.85 (2 x 3H, 2 x s, 3-CH3 and 4-CH3)t
m/z (%) 277 (62, M ), 260 (100), 232 (44); ~max (RBr di~c)/cm
3317, 1628, 1595; (Found: M+, 277.1205, C17H15N30 requires
277.1215).
d) Phenvl 3.4-dimethYlDvrrolor3,2-blc~rb~ole-2-caL ~. ~d~
3,4-Dimethylpyrrolo~3,2-b]c~r~-~Qle-2-carboxylic acid (0.278 g,
1.0 mmol) wa~ di~olved in dLm~thoxyethane (lOml) to give a
2190662
W O 94/02483 - 51 - PCT/GB93/01~12
y~llow solution. To this w~re _dd~d ~ii r_~ v~ thylamine
~0.130 g, 1.0mmol), ni1 jno ~0.190 g~ 2.0~~ ol) and the
tetrafl~olbGl~Le salt of O-benzotriazolyl-N,N,N',N',-tetra-
m~thyluron$um ~TBTU) ~0.482 g, l.Smmol) to g$ve a wh$t~;~n $n thQ ~ n~ ~lution. Th~ react$on mixture wa-
t$rred _t room t- L~.- for 42 hours, by which tims TLC
howod no .. -in~ng ac$d. Th- solv~nt was ~ n vacuo to
give a ~ ol$d, wh$ch wa- d$--olv-d in ethyl Ar~ and
the rnsulting solution w ~ w$th water. Ths organic layer
wa~ dried over MgSO4, concentrated, and subjectQd to column
ch.. o~.a~hy on ~ilica, eluting with EtOAc/petrol ~gradient
lution 5%-100%) followed by ;~C~yD~ tion from acetone to
give the phenylamide product a~ a yellow ~o~ 0.10 g, 30~)
w$th m.p. 260 C ~dQcomp.) (Found: C, 77.79; H, 5.26; N, 11.64.
C23HlgN3O requires: C, 78.16; H, 5.42; N, 11.89%); ~H~[ H6]-
DMSO) 11.10 ~lH, ~ NH), 10.59 ~lH, ~, S-NH), 9.96 ~lH, s,
am$de N-H), 8.10 ~lH, d, J 7.5, 9-H), 7.89 (lH, ~, 10-H), 7.79
~2H, d, J 9, 2'-H, 6'-H), 7.29-7.45 (4H, m,6-H, 7-H, 3'-H,
5'-H), 7.00-7.14 (2H, m, 8-H, 4'-H), 2.93 and 2.88 (2 x 3H, 2 x
s, 3-CH3); m/z ~%) 353 ~46, M ), 260 ~100); vmax ~RBr disc)/cm
3310, 1614, 1595 and 1317.
c) 3~4-Dim~thvl-2-~hvdrazinocarbonvl)~vrrQlo r 3.2-lcarbazole
Ethyl 3,4-dimethylpyrrolot3,2-b ] CA r~h- -ole-2-carboxylate ~500 mg)
and 95~ hydrazinc ~5 cm ) w~re ~t$rred and ~--t~ at 120 C for
6h $n a Readi-Vial. Tho mixture was ~llau ' to ~tand ovsrnight,
cooled in ice and f$1tnr~d. Th- re-ult$ng y~llow sol$d wa~
w-~d car~fully with water and driod. Yield of titlc _
- 350mg ~73%), no ~harp m.p. but ~B~ n~ at 285 C. ~Found: C,
69sl9; H, 5.57; N, 19.38. Calc. for C~Hi N40. 0.lH_O roquir~-
C, 69.42; H, 5.55; N, 19.05~ Hl H6]-DMSO) 10.B0 (lH, ~,
~Y~h-n~j -hl8, NH), 10.S5 ~lH, s, AY~h~ -hlo, NH), 9.20 ~lH, ,
~ ye-hls, NH), 8.06 (lH, d, J7.5 9-H), 7.81 (lH, ~, 10-H),
W O 94/02483 PCT/GB93/01~1
- 52 -
7.42-7.28 (2H, m, 6-H nd 7-H) 7.12-7.01 (lH, m, 8-H, 4.5 (2H,
br, s, . -n7e-hle~ NH2), and 2.4 and 2.3 (2 x , 4-CH3 and
3-CH3), m/z 293 (M+1) , FAB)l.
.
ExamPle 8
2-Acetvl-3.4-dimeth~l~Yr~olo r 3.2-blc~rb~ole
Ste~ 1
2,4-DiacQtyl-3,5-dimethylpyrrole was prepared from acetylaceton~
and hyd~oA~lamine-O-sulphonic acid according to the ~.ocedure of
Y.Tamura, S. ~ato and M.Ikeda (Chem L Ind., 1971, 767).
SteD 2
~-AcetoxYmethvl-3.5-diacetYl-4-methvl~Yrrole
To a ~tirred mixture of 2,4-~; A - eLyl-3,5-dimethylpyrrole (1-0
g), ~i~hloromethane (35 cm ) and pota~sium carbonat- (7.73 g) at
0-5 C was added a solution of s-~lFh~yl chloride (0.79 g) in
~ichlo~, -th-n~ (15 cm ). The t~ Lure of th~ mixture wa-
~ i ntA i n~ at 0.5 C during the addition by extQrnal cooling andthen the mixturQ was stirred at thi~ t- - aL~re until ad~udg-d
complete by t.l.c. (ca 2h). The mixture was then filter~d and
_.~u~aLed to give crude 2-chloromethyl-3,5-diacetyl-4-m2thyl-
pyrrole. This matarial wa- dissolv~d in acetic acid (10 cm ),
odium acetate (1.83g) addQd, and then more ac~tic acid (10
cm ) added. The mixture was stirred overnight at room
t~ _- a~u ~, s~.polaLQd i~ V4CuO, and the residue tirred with
ice-cold water for 2h. A solid was collect-d by filtration and
the filtrate extract~d twice w~th ethyl a~ te. The extract-
werQ dried (MgS04), &~a~v ~Led and the residue combined w$th the
solid abov~, to giv~ the crudQ ~od~cL. Ch~ oJ-a~l.y on
silica ~luting with ~thyl acetate h_Aana ( 1: i ) gave 0.075 q. of
pure ~.~d~cL a- an off-white solid m.p. 112.5-114.5 C; m/z 238
~ W O 94/02483 21 4 0 6 6 2 P ~ /GB93/01512
- 53 -
(M +1, FA8), ~H~ CDC13~ 2.16 (3H, s, OCOCH3), 2.50 (3H, s, CH3),
2.53 (3H, s, CH3), 2.62 (3H, s, CH3), 5.38 (2H, B~ OCH2)-
Ste~ 3
To a solution of 2-ac-toxymethyl-3,5-diacetyl-4-methylpyrrole
(0.200 g) and indole (0.098 g) in dichloro2thane (90 cm ) was
added I illonito ~10 clay (0.30 g). The m$xture waa
stirred and heated at reflux for 80 h. After cool~ng the clay
was ll v~d by filtration and the filtrate concentrated to ca 20
cm in vacuo. The crude product wa~ ~ v d by filtration and
then chromato~Laphed on silica. Elution with chloroform-
methanol (60:1) yielded 0.08 g of the title ~ - a~ a yellow
~olid m.p. 258-260 C, m/z (EI) 276 (M ) ~H(l H6]-DMSO) 2.58
(3H, 8, COCH3), 2-88 (3H, 8, CH3), 2.92 (3H, 8, CH3), 7.05 (lH,
m, 8-H), 7.38 (2H, m, 6-H, 7-H), 7.85 (lH, s, 10-H), 8.08(1H,
J, 8 Hz, 9-H), 10.6 (lH, ~, NH), 11.17 (lH, s, NH). (Found: C,
77.0; H, 5.74; N, 9.76; C18H16N2O. 0.14 EtOAc require~ C, 77.2;
H, 5.98; N, 9.70%.)
Exam~le 9
Ethvl 1.5-dihvdroindeno r 2 1-flindole-2-carboxvlate
Ste~ 1
~thvl 2-azido-3-fluoren-2-Ylacrvlate
SoAi (1.7eq) was added to absolute ethanol stirred under
nitrogen at room t~ - a~u~e. When di~solution was complete the
reaction was cooled to -10C and fluG ~ - 2-c-rh~Y~l~-h~d~ (log)
and athyl ~ o-cQtat~ (3eg) d$-solved togeth~r in the ; ni
of tetrahyd ofuran were added dropwise. The mixture was
tirred at -10 C for 20h and then ~ y the addition of
W O 94/ 2483 P ~ /GB93/0151 ~
~4~66~ - 54 -
water and ~ichloromethane. The combined organic extraet~ were
dried (HgS04) and :v~o~ated in vacùo. Flash chromatc~.a~hy
y$elded the pure Product (37~) v (cHcl3)/cm 2120 and 1765.
Ste~ 2
Ethyl 2-azido-3-fluoren-2-ylacrylate ~us~ $n dry toluene
wa- heated at reflux for lh, and the resulting solution was th-n
e~GLaLed to dryne~s n vacuo. The resulting mixture of ethyl
1,5-dihydroindinol2,1-f~indole-2-carboxylate and ethyl
1,10-dihydroindino[1,2-g]-indole-2-carboxylate wa~ crystallised
from ethanol, thus remo~ing mo~t of the [1,2 ~] i~omer and
leaving the title comDound (contr in-te~ with approximately 30%
of the ~1,2-g~isomer) in the mother liquor~ which wære
~va~GL~ted to dryness. ~H (CDC13) 9.11 (lH, s, br, l-NH),
7.82-7.76 (3H, m), 7.56-7.52 (lH, m), 7.37 (H, dd, J 1 and 7),
7.34-7.28 (lH, m), 7.25 (lH, dd, J 1 and 2), 4.45 (2H, q,
OCH2CH3), 3.97 (2H, s, CH2) and 1.46 (3H, t, OCH2C~3).
ExamPle 10
Effect of ~ of the invention in detransformation
("flattening") a~ay using HTl0800cc2 and HT10801c cell line~.
Cell Lines and Culture Conditions
Transformed and revertant HT1080 sub-line~, HT1080scc2 and HT10801c
were obtained from the In~titute of Cancer Re~earch, Che~ter 8eatty
Laboratorie~, Fulham Road, London. They were ~intained routinely in
Dulhecco's Modified Eagle's MD~; (DMEM) ~uppl~ ~ ted with 10% foetal
calf ~erum (FCS) and 1% p~ni~ n/-trept~ ycin ~olution cont~;
10,000 units per ml. All .~e~ were obtained from Gibco Ltd.
~140fi62
W O 94/02483 P ~ /GB93/01512
- 55 -
..
Cells were incubat-d in ti~-ue culturc grade plastic ~ els at 37 C
in 5 ~- CLn~ CO2 in air.
Asaavs for ~. 1 activitv
A-says for c~ll proliferat$on/~-oLo~ity w re carri~d out in tissu~
culture gradQ 96 w~ll microtitre plates (Co-tar). CQ11~ in log growth
were addQd to tho plates at ~ c~n~rtration of lxlO c~lls per well on
day 0 and ~erially dilut~d ~ '- wore th~n added on day 1. Plates
wQre then incubated at 37 C Ln 5* CO2 in air for a further 4 days.
For quantitation of cell growth, the methylQne blue biomaso ~Aini
method was used, the test boing read on a Multiscan plate reader at
wavoiength of 620nm. The s,phology of the cell~ was ch~
microscopic~lly under pha~ contrast i -'iatoly before the fixation
and st~1nin~ with mQthylone blue, and by ordinary light mi~ copy
therQaftor. ICS0 values for active ~ worQ obtained using the
computer ~y~ ~, GS1 and dos--rQsponse slopes wer~ also plotted.
When _ ,- ~ wore tested for act$v$ty in a colony f~ i ng a~say th~
methods used were i~onticAl to thosQ d~scribed earlier except that
ser$ally dilut-d ~_ _ ' w-- added to th~ loppy agar when the test
was SQt up, and replenish~ at the same con~ rat$on on day 7. The
te~t results were read on day 14.
Result~
ComDarative arowth and moroholo~v of ~T1080scc2 and HT10801c
Crowth rates in terms of cell number woro si ~ for both lin~s to
day 4 but t~ ter ~T1080scc2 cells continued to divide to roach
saturat$on densities approYi - Q~ y 2 to 3 times higher than HT10801c
by day S.
W 0 94/02483 - 56 - PCT/GB93/0151 ~
?,~.4~66?
Pl3noLy~$c diff~ _nce~ ba~s~-- the 2 lin~a wsre cl~arly Qvid-nt
HT10801c c~lls di~playad a much flatt~r - ~hology than the
tran-formud c~lls and only a f-w mitotic cell~ w-re een in confluont
areas of th~Q cultur-s HT1080Jce~ c~ conti -~ to divide
with - ~s mitotic cell- visible after confluence
Grown under ancho~age i-' , -'- ~ conditions in soft agar, HT1080~Qcc2
p,~d~c_d everal large col~ni~ w -~ HT10801c cQll~ failed to
produce any colonie~ ter than O lmm in diam~ter
Effects of ~elec~ed c _nds
A number of ~ Q of the invention were evaluated again~t the cQll
lines
The ~ of the invention exhibited low toxic$ty with IC50 value~
in the range 50-lOO~M
Below the re-ults of thQ "flattening" as~ay for ~ ~u '~ of cell
invention are howns-
~sLr' SCC2 flattenin~ (~M)
1~ 3 0 04
r l. 4(a) 0~04
r 1~ 4(b) 0 04
r le 4(f) 0 8
le 4(e) 0 8
le 4(h) 25
Exampl~ 4(g) 25
The - _ '- are effectiv- at .~hi~ ~ing ~flattening~ ie de-tran-form-
ation, at levels significantly balow their toxicity l-vel
Th~ a~e c~ ~ A- werQ al~o t-sted in a-says u-ing MCF7 human br-a~t
cancer cell-, A431 ~pi~ -id carcinoma cells and A285 -l~- - cell-
In all ca~- the = _ '- wQre effectiv~ in the range 1-5~M