Note: Descriptions are shown in the official language in which they were submitted.
WO94/0~16 21 4 0 7 7 3 PCT/AU93/00369
PROCESS FOR EXTRACTING ALUMINA FROM BAUXITE
Field of the Invention
This invention relates to processes for the production of
alumina from bauxite.
Ba~ o~ n~ of the Invention
r.n~al
Most alumina is pro~l~ce~ using the Bayer process. A high
volume of caustic liquor is circulated at a controlled
~o~entration. In the digestion part of the circuit, the caustic
is heated and reacted with bauxite to increase the alumina
co~c~ntration (pregnant liquor). After the bauxite residue is
separated by settling, the caustic liquor is cooled, ~ e~, and
alumina is precipitated as product. The spent liquor is
recirculated.
The quantity of circulating liquor can be as high as 20 m3/t
alumina proA~ce~. Expressed in terms of productivity, this is
only 50 g/litre. To increase production of existing plants or to
reduce the capital cost of new plants, it is important to achieve
high yield. Only a few alumina plants are achieving l80 g/l
yield today.
For high yield, the digestion step has to achieve a high
alumina cQ~-~ntration. A de~ e_ of supersaturation can be
tolerated during the residue separation step. Hos~e~e~, depen~ing
on certain factors, there is a limiting supersaturation above
which alumina will be lost in increasing amounts to the residue.
Usually the digestion system operates at the highest
flowrate that the equipment and steam heating will allow. Bauxite
is then ~ in a controlled manner to achieve a desired
pregnant liquor alumina cQnc~ntration.
The alumina ro~c~ntration is usually expressed as a ratio
on caustic, A/C. As an example, the pregnant liquor can be 0.72
A/C (gAl2O3/gNa2CO3) at say 105C for 200 g/l caustic expressed as
Na2CO3 (representing sodium hydroxide associated with the
dissolved alumina as well as free sodium hydroxide). The spent
liquor A/C will depend on precipitation conditions but can be say
0.37 A/C.
Free r ~ic
Usually the spent liquor to digestion is evaporated to some
degree and fresh caustic make-up added at this point, such that
W094/0~16 PCT/AU93/~369
3 2
the conc~ntration may be 240 g/l caustic at 0.36 A/C. The so
called free caustic of this liquor is defined as: 240 - 240 x
0.36 x 106/lOZ = 150 g/l. (representing sodium h~oxide)
Depen~; ng on the velocity and temperature of the liquor
heating to digestion, there will be an upper limit for free
caustic. For a high temperature process required to digest
boehmitic or AiAcporic bauxites the limit may be say 140 g/l free
caustic. Above this limit the heater tubes and piping may
erode/co G~e rapidly.
One way of dropping the free caustic is to-add bauxite that
will partially dissolve as the liquor is heated so as to raise
the A/C. Some modern high temperature plants use special slurry
heaters, or tube digestion as it is known, to ove - the free
caustic. Hcs~e~_ , most plants use shell and tube ~Y~h~n~Jc~s for
liquor heating, and ~dA;ng a slurry is not desirable.
Bau~ite ~.
Bauxites are minerals rich in alumina and low in silica.
The usual minerals contA;n;ng only alumina are gibbsite, boehmite
and diaspore. This is their respective order of solubility, for
example, to achieve 0.72 A/C at say 200 g/l caustic, the
digestion temperature required would be:
Gibbsite, Alz03.3H20 : 140 - 145C
(3.2 bar(a) vapour pressure)
Boehmite, Al203H20 : 260 - 265C
(41 bar(a) v~o~r pressure)
Diaspore, Al203H20 : impractical
The digestion L~ ,_ ature for boe~ ite can be raised higher
for higher A/C, hcwe~e., if the temperature for gibbsite is
raised higher, there is a risk of the dissolved alumina
precipitating out as boehmite, which is the stable species above
say 150C. This is particularly so if the gibbsitic bauxite also
contains boehmite to act as seed.
Mixed gibbsitic-boehmi.ic bauxi~es contAining above say 5%
as boehmite alumina are usually processed in a single digestion
step under boehmite extraction conditions.
Diqe~tion PloductivitY
The digestion productivity is a measure of the alumina
WO94/0~16 21 4 0 7 7 3 PCT/AU93/00369
co~c~ntration increase due to bauxite for a given flowrate of
liquor. If there is a limit to the flowrate, then potentially the
liquor can be e~d~p,ated so that the mass flow of caustic is
increased and more bauxite can be added in ~,v~-rtion.
For a low temperature plant processing only gibbsitic
bauxite, more e~a~G,ation is required to keep the circuit in
h-l. e, since less fl~Ch ing o~u-~ on cooling than with a high
temperature digestion plant.
For gibbsite digestion, it is possible therefore to achieve
an A/C greater than 0.72 h~C~l~ce the caustic strength is above
200 g/l while remaining at 145~C. As well as the evaporation
aspect, indirect live steam heating is also used. For high
temperature digestion, indirect heating is not so easy and direct
steam injection is more common although counter-productive.
Free caustic is not such a ~onc~rn for liquor heating in a
low temperature plant. It becomes a cQn~rn in a high temperature
plant processing boehmitic bauxite in which the spent liquor is
heated separately and the bauxite added direct to the digester
(two stream process). In a two stream process, free caustic will
impose a limit to the productivity.
In a high temperature tube digestion design or similar
single stream process (liquor plus bauxite slurry heating) free
caustic is not a limitation to the productivity. Although tube
digestion has desirable process features, there are some
mech-n;cal limitations to pumping and slurry heating, and
co~s~,~,tly the production rate of such a unit is limited.
If the digestion A/C is higher than the degree of
supersaturation allowable for residue separation, then some spent
liquor trim can be added. this bypass of spent liquor around
digestion represents the productivity gain achieved.
One way of achieving digestion productivity with a boehmitic
bauxite is to add a gibbsitic bauxite downstream during flash
cooiing at say 17G - 150C, and to increase the achieved boehmite
A/C. This approach is known as "sweetening" and is practiced by
a number of plants in various forms. Sweetening can also
in~.~.ate spent liquor trim. Sweet~ning for some plants can be
a ~,lv~,ient retrofit, if the gibbsitic bauxite is available and
PCT/AU 9 3 / O 0 3 6 9
21~ 0 7 7 3 R~C~ 2 8 FEB 1~'''
handling of two bauxites is practical.
Irrespective of the source of alumina, the alumina
precipitates out as gibbsite, as the temperature is usually well
below 150C. with good residue settler and washer design and
operation, the degree of supersaturation can be as high as 25%
of equilibrium solubility of gibbsite. Some alumina loss will
occur (known as autoprecipitation) but is usually about
0.015 A/C.
For example, if the equilibrium solubility of gibbsite is
0.58 A/C (220 g/l caustic, 107C and impure plant liquor) then
the allowable A/C is 0.58 x 1.25 = 0.725 A/C. Allowing for
autoprecipitation, the digestion A/C = 0.725 ~ 0.015 = 0.74 A/C.
If the digestion unit can achieve higher than 0.74 A/C then trim
liquor has to be added. Usually the digestion conditions of
caustic strength and temperature have to be such that the
equilibrium solubility of boehmite or gibbsite from the bauxite
is
0.74 1 0.025 margin = 0.765 A/C.
SummarY of Invention and Object
The object of the invention is to provide an improved
process for extracting alumina from bauxite in which the problems
discussed above are at least ameliorated.
The invention provides a process for extracting alumina from
bauxite comprising the steps of combining caustic liquor with a
bauxite slurry to provide an alumina to caustic ratio (A/C) of
greater than about 0.70 in the slurry/liquor mixture, subjecting
the mixture to digestion at a temperature below that at which
reversion of dissolved gibbsite to boehmite may occur, to extract
alumina from the gibbsite bauxite in the slurry, separating the
high alumina pregnant liquor from the boehmite cont~ining
residue, ;Y;ng the residue with caustic liquor and subjecting
the mixture to a high temperature digestion process to extract
the rema;n;ng alumina from the residue, and separating the
alumina rich liquor, said alumina to caustic ratio of the
slurry/liquor mixture being achieved in part by the step of
evaporating spent caustic liquor supplied to the low temperature
digestion step.
IPI~A/AU
2140773 RECÉIVED 2~ FEB 1994
4a
If desired fresh caustic may be added to the slurry to
achieve the desired high A/C.
~PP.A/AI I
W094/OU16 PCT/AU93/00369
- 2140773
If desired, the bauxite slurry may be subjected to a known
predesilication process prior to heating. Alternatively, two
stage post-desilication using lime has been found to be
satisfactory.
The atmospheric settler or pressure decanter following the
high temperature digestion step is preferably operated at an A/C
which i8 lower than that of the low Le ,-rature decanter to
minimize auLG~.e_ipitation during subsequent residue w-shing.
The invention also provides alumina when ~,o.l...-~A by the
process defined in the pr~ce~ing paragraphs.
Brief descriPtion of the drawings
In order that the invention may be more readily understood,
two preferred emho~; - ts of the invention will now be described
with refe,~,ce to the accompanying drawings in which:
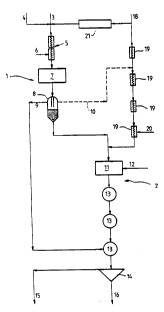
Figure 1 is a schematic flow chart of a bauxite digestion
~ess ~mhodying the invention, and
Figure 2 is a similar schematic flow diagram of a modified
bauxite digestion process embodying the invention.
DescriPtion of Preferred Embodiments
The preferred ~o~ se5 are applicable to mixed bauxites
cont~ining a high ~.G~o-Lion of gibbsite together with less
soluble boehmite and diaspore. The proces~es can also be applied
to a mixed bauxite of boehmite and diaspore. The ~-~--~sses can
also be applied to gibbsite bauxite where an over-charged
digestion (for high A/C) can be followed by a re-digestion for
complete extraction. The following description relates to
gibbsitic-boehmitic bauxite and refers to Figs. 1 and 2 of the
drawings, but the ~G~e-S is equally applicable to other
bauxites.
The preferred process involves two sequential bauxite
digestion steps 1 and 2 to extract gibbsite at low temperature
and high caustic, followed by boehmite at high temperature and
low caustic to produce alumina at high liquor productivity. The
process is particularly suited to retrofitting a two stream high
t~m~erature digestion plant, as will be described further below.
The low temperature digestion step 1 is designed to extract
essentially all the gibbsite from the bauxite feed, and the
WO94/0~16 PCT/AU93/00369
~4~'l3 6
process is therefore particularly beneficial to bauxites having
a high ~-o~o-Lion of gibbsite (eg Comalco bauxite containing
about 75% of available alumina as gibbsite). The conditions are
designed to achieve a very high A/C liquor~with minimal eve-~ion
and without free caustic limitations.
In this step 1, bauxite slurry 3 is fed with spent liquor
18 and caustic make-up 4 to a heat ~vch~nger 5, fed with flash
and low pressure live steam 6. The low temperature digestion
conditions are controlled to limit the temperature to below 150C
to avoid eve~ion of dissolved gibbsite to boehmite. To achieve
the required high A/C of greater than 0.70 A/C strong caustic
liquor is required. This is achieved by evaporation 21 of the
spent liquor 18 and by indirect heating in 5. Caustic make-up 4
can also be a~e~.
The strong spent liquor after evaporation can then be heated
up to the free caustic limitation, which depPn~ng on heater
design can range from 130 - 150C. If for example the limitation
is 130C, then the spent liquor can be heated from 85C say to
130C in shell and tube heat PY~h-n~srs (not shown) using flash~
steam. The bauxite slurry at 70C say can be heated up to a
similar temperature in slurry heaters. Both streams can then be
combined for ru~ L~e~ heating using flash and indirect low
pressure live steam (not shown). Alternatively, both liquor and
bauxite slurry can be combined for all the heating as a single
stream in suitable heaters 5 as shown.
The heated slurry from the heat eY~h~ngers 5 is held at
temperature in a well stirred vessel 7 or holding tube to achieve
the required al~ ; n~ extraction.
Depending on the nature of the bauxite, some predesilication
may be necessary prior to heating the slurry. This requires
heating the ground bauxite caustic liquor slurry up to say 100C
and holding it in stirred vessels (not shown) to allow any silica
such as kaoiinite to dissolve and then to reprecipitate as
desilication product (sodium aluminium silicate). Without
predesilication, there is the risk of desilication product
scaling out and fouling the heaters. As indicated in the Example,
the ~lo~ess has been successfully operated using a post-
WO94/0~16 21 ~ 0 7 7 3 PCT/AU93/00369
desilication step in which about 96% of the silica is ,.- ~ed.
As well as for gibbsite extraction, some of the digestion
holding time may be required for further desilication so as to
avoid excessive silica levels rem~;n;ng in liquor.
Immediately following the low temperature digestion, the
boehmite con~ining residue is ~eparated in a pressure decanter
8 at digestion temperature using a suitable synthetic flocculent.
Such a device has been described in US Patent 5080803 and
el~ew~-e. H$gh underflow density and good overflow clarity can
be achieved.
The underflow at high density and temperature is pumped with
suitable pumps (not shown) to the high temperature digestion step
2.
The pressure decanter overflow 9 at high A/C pregnant liquor
is flash cooled in flash tanks 13 to atmospheric boiling point
for dilution, filtering, cooling and se~;ng to produce alumina
product. If required, post-desilication can be carried out on the
overflow at temperature before flash cooling.
Some of the desilicated decanter overflow may be diverted
to the remaining 1~nçonç~ntrated spent liquor via a free caustic
trim line lO to control the free caustic to allow high
temperature heating for the high temperature digestion step 2.
The low temperature digestion pressure decanter underflow
is pumped direct to a high temperature digester vessel ll, with
or without further heating. Alternatively it can be mixed with
the incoming ~lnconçentrated spent liquor after an a~ Gpriate
spent liquor heating and final pumping stage. The single stream
can then be further heated in suitable heaters by flash steam and
high pressure live steam or other heat transfer medium (not
shown). High pressure steam 12 is added direct to the digester
as required.
The temperature and time of the high temperature digestion
vessel ll, or holding tube, is selected so as to extract ~he
~ ~;ning boehmite, at the caustic conç~ntration prevailing to
achieve a medium to normal pregnant liquor A/C.
Slurry from the high temperature digestion vessel ll is
flash cooled in flash tanks 13 to atmospheric conditions for
WO94/0~16 PCT/AU93/00369
2~4~13
residue removal by settling in a settler or separator 14 followed
by w~h;ng and disposal of red mud via 16. The settler overflow
pregnant liquor 15 is diluted, filtered, cooled and seeded in a
known manner not shown for alumina production.
Alternatively, as shown in Fi-gure 2, a pressure decanter 17
can be applied at the appropriate temperature down the flash
train 13. The overflow can then 3Oin the overflow of the main low
temperature digestion decanter for common flash cooling.
The pressure decanter underflow of final residue or "red
mud" can be cooled indirectly or directly by wash water prior to
hi ng and disposal.
The high temperature digestion pressure decanter 17 will
nG~ -lly be at a lower A/C than for the main decanter. This will
minimise au~G~,ecipitation during subsequent red mud wAshing.
As a further refinement (not shown), the high A/C and normal
A/C pregnant liquor streams 9 and 15 can be cooled and diluted
separately for a more optimised precipitation of alumina.
If necessary, flash tank sweetening and spent liquor trim
can still be applied to the high temperature digestion step (not
shown).
The above embodiments of the invention are particularly
suitable for retrofitting of existing alumina plants processing
a high gibbsite, moderate boehmite bauxite using a two stream
digestion process.
The bulk of the digestion is carried out at low temperature
freeing up the high temperature equipment for potentially greater
p~;ty and efficiency.
A large ~.o~o~ion of the previous high pressure live steam
heating is replaced by low pressure steam. this allows better
utilization and rApAçity of existing boilers and electrical
generators.
The high A/C pregnant liquor from the low temperature
digestion has the potential to replace part or all of any
sweetening bauxite previously used.
2140773
WO94/02416 PCT/AU93/00369
-
~L~
Laboratory digestion tests were carried out using Weip
bauxite and synthetic Bayer liquor at various caustic strengths,
temperature, time and bauxite charge, without Predesilication.
The aim was to find conditions for high A/C from gibbsite
dissolution with minimal e~c ~ion (auLo~ e_ipitation) to
boehmite or gibbsite. The following results were obt~ine~ from
one of the tests:-
Bauxite: 54.6% Al20" 11.4% Fe20" 2.59% Ti02
5.92% Si02 (incl. 1.3% quartz),
25.19% LOI (loss on ignition)
Distribution
of bauxite
Al20,: 3.93% in kaolini'~
0.48% in iron minerals
40.34% in gibbsite
9.85% in boehmite
Synthetic
spent
liquor: 346.5 g/l caustic soda as Na2C03 (C)
0.394 A/C
0.845 C/S (caustic/(caustic + carbonate)
2.5 g/kg Si02/C
Digestion
conditions: 140C
2 minute digestion time after 5-minute heat up
290 g dry bauxite/litre spent liquor
Pregnant
liquor
result: 301.2 g/l caustic soda (C)
0.778 A/C
0.843 C/S
28.6 g/kg Si02/C
Kaolinite
reacted: 93%
Gibbsite
reacted: 92%
Post
Desilication: Because of the high residual silica in pregnant
liquor, post desilication was necessary. A 2-
stage procedure was carried out using DSP seed
followed by lime, both at 140C.
W O 94/02416 P(~r/AU93/00369
~4~ 3
Pregnant
liquor after
DSP s~e~;ng: 313.0 g/l caustic soda (C)
0.750 A/C
0.843 C/S `;`
5.3 g/kg SiO2/C
Pregnant
liquor after
lime
treatment: 306.0 g/l caustic soda (C)
1.5% CaO/ 0.735 A/C
bauxite 0.848 C/S
3.5 g/kg SiO2/C