Note: Descriptions are shown in the official language in which they were submitted.
2 ~ 5~
HOECHST ARTIENGESELLSCHAFT HOE 94/F 039 Dr. D/wo
Description
Substituted cycloheYanol esters, their use for treating
diseases, and pharmaceutical preparations
The disease picture of diabetes is characterized by
elevated blood sugar values. In the case of insulin-
dependent or type I diabetes, the cause is the death of
the insulin-producing ~ cells of the pancreas; this
condition is therefore treated by administering insulin
(substitution therapy). By contrast, non-insulin-depend-
ent or type II diabetes is characterized by insulin
having a ~;m;n; shed effect on muscle tissue and fat
tissue (insulin resistance) and by an increased produc-
tion of glucose in the liver. The causes of these meta-
bolic disturbances are to a large extent still unclear.While the established therapy using sulfonylureas
attempts to compensate for the insulin resistance by
increasing the endogenous liberation of insulin, this
does not in all cases lead to normalization of the blood
sugar le~el and is not able to halt the progress of the
disease; many type II diabetics eventually become insu-
lin-dependent as a result of the ~ cells becoming
"exhausted", and suffer from late damage such as catar-
acts, nephropathies and angiopathies.
For this reason, no~el therapeutic principles for treat-
ing type II diabetes are desirable.
In the fasting state, the concentration of glucose in the
blood is determined by the glucose production of the
li~er. A variety of research groups have been able to
demonstrate that the increase in the blood sugar values
in type II diabetes is correlated with a proportionally
elevated output of glucose from the liver. The glucose
which is secreted by the li~er into the blood can be
formed both by degrading liver glycogen (glycogenolysis)
2142556
-- 2
and by gluconeogenesis.
Glucose-6-phosphate i8 the common end product of both
gluconeogenesis and glycogenolysis. The terminal step in
the hepatic liberation of glucose from glucose-6-phos-
phate is catalyzed by glucose-6-phosphatase (EC 3.1.3.9).
Glucose-6-phosphatase represents a multienzyme complex
present in the endoplasmic reticulum (ER). This enzyme
complex comprises a glucose-6-phosphate translocase which
is present in the ER membrane, a glucose-6-phosphatase
which is located on the luminal side of the endoplasmic
reticulum and a phosphate translocase ~for a review, see:
Ashmore J. and Weber G., "The Role of Hepatic Glucose-6-
phosphatase in the Regulation of Carbohydrate Metabo-
lism", in Vitamins and Hormones, Vol. XVII (Harris R.S.,
Marrian G.F., Thimann R.V., Edts.), 92 to 132, (1959);
Burchell A., Waddell I.D., "The molecular basis of the
hepatic microsomal glucose-6-phosphatase system", Bio-
chim. Biophys. Acta 1092, 129 to 137, (1990)]. The
extensive literature which is available shows that the
acti~ity of this multienzyme complex is also elevated
under all in~estigated conditions which, in animal
experiments, lead to elevated blood sugar values, e.g.
streptozotocin, alloxan, cortisone, thyroid hormone~ and
starvation. In addition to this, a large number of
investigations indicate that the elevated production of
glucose observed in type II diabetics is associated with
elevated glucose-6-phosphatase activity.
The importance of the glucose-6-phosphatase system for
normal glucose homeosta~is is also underscored by the
hypoglycemic symptoms of patients suffering from glycogen
storage disease type Ib, who lack the translocase compon-
ent of the glucose-6-phosphate system.
The use of suitable active compounds (inhibitors) to
diminish glucose-6-phosphatase acti~ity should result in
a decreased liberation of glucose from the liver. These
active compounds should be able to adapt the production
of glucose by the liver to the actual peripheral consump-
tion. In addition to this, the lower blood glucose values
231~556
thereby produced in type II diabetics in the fasting
state ought also to exert a preventive effect with regard
to late damage in diabetes.
A series of non-specific inhibitors of glucose-6-
phosphatase has been described in the literature, e.g.
phlorrhizin [Soodsma, J.F., Legler, B. and Nordlie, R.C.,
J. Biol. Chem. 242, 1955 to 1960, (1967)], 5,5'-dithio-
bis-2-nitrobenzoic acid lWallin, B.K. and Arion, W.J.,-
Biochem. Biophys. Res. Commun. 48, 694 to 699, (1972)~,
2,2'-diisothiocyanatostilbene and 2-isothiocyanato-2'-
acetoxystilbene lZoccoli, M.A. and Rarnowski, M.L., J.
Biol. Chem. 255, 1113 to 1119, (1980)]. The first thera-
peutically utilizable inhibitors of the glucose-6-
phosphatase system are proposed in European Patent
Applications No. 93 114 260.8 and No. 93 114 261.6.
The cycloheYAne derivatives which are characterized in
detail below are novel compounds which have not previ-
ously been described in the chemical and biological
literature. We have now found that esters of certain
cycloheYAnol derivatives, e.g. the compound according to
Example 4, are very good inhibitors of the glucose-6-
phosphatase system.
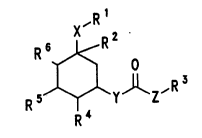
The invention therefore relates to cycloheYAnol esters of
the formula I X'R~
R6~R2
R s~Y~Z'R 3
- R~
in which the radicals have the following meAn;n~:
R1 is CoNHCoR15, CSNHR15, CoNHSo2Rl4, CSNHSo2R14 or
2 ~ 55(D
C~2NU.~o2Rl4, or
Rl i8 a radical Relected from the following formulae:
1N,W ~N~U O H
~N/ 1~ ~S ( O ) n 1 ~5 ( O ) n ' J~J S~
E~ ~N H ,[~1 0~ V--N
/S ( O ) n H o~ ~ ~N H ~N~U
R~4 U
U U~ H N~G~E ~
H H 11 H
~U U~ ~U _~N H U~ ~N H
=N ~ N~
U U
~ ' /N ~ ~ ~ OH and ~ U
in which V i~ N or CH, W i8 N or CH, U iQ O or S, E i~
NRl4, O, S or NH, G i~ -N=, -O-, -S- or ¦¦
-C-, M i~ NR14, NH, CH2
or CR8R9, and aromatic rings can be substituted once or
more than once by F, Cl, Br, I, OH, o-cl-c4-alkyl~ Cl-C4-
alkyl, CF3, NO2 or CN,
or R1 form~, together with R2, the ring
21~5S6
-- 5
U
~N H
\N~U
R ~ 6
R2 is C1-C1O-alkyl(Rll)n, O-C1-C1O-alkyl(Rll)n, C2-C10-
alkenyl(R11)n, O-C3-C10-alkenyl(Rll)n, C2-C10-
alkynyl(R11)n, O-C3-C10-alkynyl(Rll)n, S-C1-C10-
alkyl(R11)n, S-C3-C10-alkenyl(Rll)n, S-C3 -Clo~
alkynyl(R11)n, NH-C1-C1O-alkyl(Rll)n, NH-C3-C1o~
alkenyl(R11)n or NH-C3 -C10-alkynyl (Rll) n, where
Rll is optionally substituted by Rl2;
R3, R11 and R13 are alkyl having from 1 to 10 carbon
atoms, cycloalkyl having from 3 to 8 ring carbon atoms,
phenyl, naphthyl, ph~nAnthryl, pyridyl, thienyl, furyl,
pyrimidyl, indolyl, imidazolyl, coumarinyl, phthaliminyl,
quinolyl, piperazinyl, tetrazolyl, triazolyl, oxazolyl or
their thieno-fused, pyridino-fused, pyrimidino-fused or
benzo-fused derivatives, where the aromatic radical or
heteroaromatic radical can be substituted once or more
than once, identically or differently, by F, Cl, Br, I,
OH, -NO2, CN, cl-c4-alkoxy~ cl-c4-alkyl~ NR3R9, phenyl,
benzyl, thienyl, furyl, imidazolyl, pyridyl, O-phenyl or
O-benzyl, and R3, R11 and R13 are identical or different;
2 0 R4, R5 and R6 are H, OH, an OH group protected by custom-
ary alcohol protective groups, F, Cl or Br, or have the
m~An;ng8 given for R2, where R4, R5 and R6 are identical
or different;
R7 is C1-C4-alkyl, phenyl or benzyl;
25 R3 and R9 are H, cl-c4-alkyl~ cl-c4-alkanoyl or phenyl
which is optionally substituted by F, Cl, Br, I, OH, O-
C1-C4-alkyl, CF3, -NO2 or CN, where R8 and R9 are identi-
cal or different, or R3 and R9 form, together with the
nitrogen atom, a 4- to 10-membered, saturated
621~2~56
heterocyclic ring in which a CH2 group can be optionally
replaced by O, S or NRl0,
R10 is H, Cl-C4 -alkyl, phenyl or benzyl;
Rl2 is phenyl, naphthyl, p~enAnthryl, pyridyl, thienyl,
furyl, thiazolyl, pyrimidyl, indolyl, imidazolyl, coumar-
inyl, phthaliminyl, quinolyl, piperazinyl, tetrazolyl,
triazolyl, oxazolyl or their thieno-fused or benzo-fused
derivatives, where the aromatic radical or heteroaromatic
radical can be substituted once or more than once,
identically or differently, by F, Cl, Br, I, OH, CF3,
-NO2, CN, C1-C4-alkoxy, C1-C4-alkyl, NR8R9, phenyl,
benzyl, thienyl, furyl, imidazolyl, pyridyl, O-phenyl or
O-benzyl;
Rl4 is hydrogen, Cl-Cl0-alkyl, phenyl, naphthyl, phen-
anthryl, pyridyl, thienyl, furyl, thiazolyl, pyrimidyl,
indolyl, imidazolyl, coumarinyl, phthaliminyl, guinolyl,
piperazinyl, tetrazolyl, triazolyl, oxazolyl or their
thieno-fused or benzo-fu~ed derivatives, where the
aromatic radical or heteroaromatic radical can be substi-
2Q tuted once or more than once, identically or differently,by F, Cl, Br, I, OH, CF3, -NO2, CN, Cl-C4-alkoxy, C1-C4-
alkyl, NR8R9, phenyl, benzyl, thienyl, furyl, imidazolyl,
pyridyl, O-phenyl or O-benzyl, or R14 is a radical of the
formula
o
Il N N
H 3 C N H--~S~--
R15 is C3-C10-alkenoyl, C3-C1o-Al~noyl(R12), C1-C10-alkan-
oyl(R12), phenyl, naphthyl, p~nAnthryl, pyridyl, thien-
yl, furyl, thiazolyl, pyrimidyl, indolyl, imidazolyl,
coumarinyl, phthaliminyl, quinolyl, piperazinyl, tetrazo-
lyl, triazolyl, oxazolyl or their thieno-fused or benzo-
fused derivatives, where the aromatic radical or hetero-
aromatic radical can be substituted once or more than
- 21~2556
-- 7
once, identically or differently, by F, Cl, Br, I, OH,
CF3, -NO2, CN, Cl-C4-alkoxy, Cl-C4-alkyl, NR8R9, phenyl,
benzyl, thienyl, furyl, imidazolyl, pyridyl, O-phenyl or
O-benzyl;
R16 is Cl-C1O-alkyl(Rll)n, C3-ClOalkenyl(Rll)n or C3-C10-
alkynyl(Rll)n, where Rll is optionally substituted by Rl2,
X is (CH2) m ~ ~ CH=CH-, -C--C-, -CH2-O-CH2-, -CH2-S-CH2- or
-CH2-N-CH2-
R8
Y is (CH2)m, O, S or NR3,
Z is (CH2)m~ S~ O~ S-Cl-C10-alkyl, O-Cl-C1O-alkyl, CH=CH,
CH=CF, CH=CCl, CH=CBr, CH2-CO, CH2-CHF, CH2-CHCl, CH2-
CHBr, CH2-CHI, C3-C10-cycloalkylene, C3-C10-cycloalk-
enylene, where from 1 to 3 ring carbon atoms can be
replaced by sulfur atoms, oxygen atoms or nitrogen atoms,
CooR7, C_C, CH=C(Cl-C4-alkyl), CH=C(CN), CH=C(NR3R9),
CH=C(Cl-C4-alkanoyl), CH=C(Rl3) or NR8, and, if Y is
oxygen,
-C-z-R3-
can together be an amino acid residue, selected from the
group consisting of Ala, Arg, Asn, Asp, Cys, Gln, Glu,
Gly, His, Ile, Leu, Lys, Phe, Pro, Ser, Thr, Trp, Tyr and
their derivatives protected by customary protecti~e
groups,
n is zero, 1 or 2,
m is zero, 1, 2, 3 or 4.
Insofar as they contain a carboxyl group, the novel
compounds of the formula I can form salts with inorganic
21~25~6
-- 8
or organic bases.
The invention also relates, therefore, to the physiologi-
cally tolerated salts of compounds of the formula I.
The novel compounds of the formula I contain a number of
stereocenters. The invention relates to all possible
enantiomers and diastereomer~. They are all represented
by the formula I.
Unless otherwise indicated, the following applies to the
statements made above and below:
The alkyl, Al kAnolyl and alkoxy radicals given under R1,
3 7 Rg R9 R10 R11 Rl2, R13, R15, R16 and Z are
straight-chain or branched.
The alkyl, alkenyl and alkynyl groups given under R2 and
R14 are straight-chain, branched or cyclic, it also being
possible for only a part of the radical to form a ring.
In addition, one of the CH2 groups can be replaced by 0,
S, S0, S02 or NR3, R11 can be substituted by R12, and,
when n is 2, the two radical~ R11 are identical or
different.
Unsaturated radicals are unsaturated once or more than
once.
Alcohol protective groups are:
Substituted ethers, such as
methoxymethyl, methylthiomethyl, t-butylthiomethyl,
benzyloxymethyl, p-methoxybenzyloxymethyl, t-butoxy-
methyl, siloxymethyl, 2-methoxyethoxymethyl, 1-ethoxy-
ethyl, allyl, benzyl, p-methoxybenzyl, 3,4-dimethoxy-
benzyl, o-nitrobenzyl, p-nitrobenzyl, p-halobenzyl, 2,6-
dichlorobenzyl, p-cyanobenzyl, p-phenylbenzyl, 2-picolyl
and 4-picolyl.
Protective groups for the amino acid are:
21~25S6
g
a) Carbamates, such as
methyl and ethyl, 9-fluorenylmethyl; 9-(2-sulfo)fluor-
enylmethyl, 9-(2,7-dibromo)fluorenylmethyl, 2,7-di-t-
butyl-l9-(10,10-dioxo-10,10,10,10-tetrahydrothioxan-
thyl)]methyl, 4-methoxyphenacyl, 2,2,2-trichloroethyl, 2-
trimethylsilylethyl, 2-phenylethyl, 1-(1-adamantyl)-1-
methylethyl, 1-dimethyl-2-haloethyl, 1,1-dimethyl-2,2-
dibromoethyl, 1,1-dimethyl-2,2,2-trichloroethyl, 1-
methyl-1-(4-biphenyl)ethyl, 1-(3,5-di-t-butylphenyl)-1--
methylethyl, 2-(2'- and 4'-pyridyl)ethyl, 2-(N,N-dicyclo-
hexylcarboxamido)ethyl, t-butyl, 1-adamantyl, vinyl,
allyl, 1-isopropylallyl, cinnamyl, 4-nitrocinnamyl, 8-
quinolyl, N-hydroxypiperidinyl, alkylthio, benzyl, p-
methoxybenzyl, p-nitrobenzyl, p-bromobenzyl, p-chloro-
benzyl, 2,4-dichlorobenzyl, 4-methylsulfinylbenzyl, 9-
anthrylmethyl and diphenylmethyl, t-amyl, S-benzylthio-
carbamate, p-cyanobenzyl, cyclobutyl, cyclohexyl, cyclo-
pentyl, cyclopropylmethyl, p-decyclobenzyl, diisopropyl-
methyl, 2,2-dimethoxycarbonylvinyl, o-(N,N-dimethyl-
carboxamido)benzyl, 1,1-dimethyl-3-(N,N-dimethylcarbox-
amido)propyl,1,1-dimethylpLo~y..yl,di-(2-pyridyl)methyl,
2-furanylmethyl, 2-iodoethyl, isobornyl, isobutyl,
isonicotinyl, p-(p'-methoxyphenylazo)benzyl, 1-methyl-
cyclobutyl, 1-methylcyclohexyl, 1-methyl-1-cyclopropyl-
methyl, 1-methyl-1-(3,5-dimethoxyphenyl)ethyl, 1-methyl-
1-(p-phenylazophenyl)ethyl, 1-methyl-1-phenylethyl, 1-
methyl-1-(4-pyridyl)ethyl, phenyl, p-(phenylazo)benzyl,
2,4,6-tri-t-butylphenyl, 4-(trimethylammonium)benzyl and
2,4,6-trimethylbenzyl.
b) Urea derivatives, such as
phenothiazinyl-(10)-carbonyl derivative~, N'-p-toluene-
sulfonylAm;nocArbonyl and N'-phenylaminothiocarbonyl.
c) Amides, such as
N-formyl, N-acetyl, N-chloroacetyl, N-trichloroacetyl, N-
trifluoroacetyl, N-phenylacetyl, N-3-phenylpropionyl, N-
picolinoyl,N-3-pyridylca-ho~-;de,N-benzoylphenylalanyl
derivatives, N-benzoyl and N-p-phenylbenzoyl.
21~2~6
- 10 -
Compounds of the formula I are preferred in which
R1 is CoNHCoR15, CSNHR15, CoNHSo2R14, CSNHSo2R14 or
CH2NHSo2R14, or i8 a radical of the following formulae:
1--V
J~ IN , ~ or ,~
in which E is O, S or NH, Rl4 is H, U i8 O or S, V is N
or CH, W is N or CH, and aromatic rings can be substi-
tuted once or more than once by F, Cl, Br, I, OH, O-
Cl-C4-alkyl, C1-C4-alkyl, CF3, NO2 or CN,
or R1 forms, together with R2, the ring
U
~N H
\N~U
R 1 6
R2 has the following meAn;ngs: O-C1-C10-alkyl(Rl1)n, (n is
0, 1 or 2), where the alkyl moiety i~ unbrAn~he~
branched or cyclic, and R11 can be substituted by R12,
and, when n is 2, the two radicals R11 are identical or
different, O-C3-C10-alkenyl(R11)n, (n is 0, 1 or 2), where
the alkenyl moiety is unbrAnche~, brAnche~ or cyclic, and
21~25~
is unsaturated once or more than once, and Rll can be
substituted by Rl2,
O-C3-Cl0-alkynyl(Rll)n, (n is 0, 1 or 2), where the
alkynyl moiety is unbrAnche~, brAnche~ or cyclic, and is
unsaturated once or more than once, and Rll can be
substituted by Rl2,
R3 to Rl5 have the meAn;ngs given above,
and X, Y, Z and Rl6 have the following meAn;ngs:
X i8 (CH2)m, (m i8 O, 1, 2, 3 or 4), CH=CH, C_C, CH2OCH2
or CH2SCH2,
Y i8 (CH2)m, (m i8 O, 1, 2, 3 or 4), O, S or NR3,
Z i8 (CH2)m, (m i8 O, 1, 2, 3 or 4), S, O, S-Cl-Cl0-alkyl,
CH=CH, CH=CF, CH=CCl, CH=CBr, CH2-C(O), CH2-CHF, CH2-CHCl,
CH2-CHBr,CH2-CHI,C3-C10-cycloalkylene,C3-C10-cycloalken-
ylene, CoOR7, C_C, CH=C(Cl- C4- alkyl), CH=C( CN), CH=C( R13)
or NR .
R16 is Cl-Cl0-alkyl(Rll)n, C3-Cl0-alkenyl(Rll)n or C3-Cl0-
alkynyl(Rll)n, with n in each case being zero or one.
In formula I, the radicals have, in particular, the
following meAn;ngs:
Rl is CoNHCoRl5, CSNHRl5, CoNHSo2Rl4, CSNHSo2R14 or a
radical of the formulae:
~ N~W , ~ ~ R1~ ~ CN
in which E is O, S or NH, Rl4 is H, U is O or S, V is N
or CH, W is N or CH, and aromatic rings can be substi-
tuted once or more than once by F, Cl, Br, I, OH, O-
Cl - C4 - alkyl, Cl- C4 - alkyl~, CF3, NO2 or CN,
or Rl forms, together with R2, the ring
~1~2556
- 12 -
~N H
\N/~--U
R 1 6
R2 i8 0-Cl-C10-alkyl(Rll)n, (n is 0, 1 or 2), where the
alkyl moiety is unbrAncheA, brAnche~ or cyclic, and R
can be substituted by Rl2,
0-C3-C10-alkenyl(Rll)n, (n is 0, 1 or 2), where the
alkenyl moiety is unbrAncheA, brAnche~ or cyclic, and is
also unsaturated once or more than once, and Rll can be
substituted by Rl2,
0-C3-C10-alkynyl(Rll)n, (n is 0, 1 or 2), where the
alkynyl moiety is unbrAncheA, brAnche~ or cyclic, is
unsaturated once or more than once, and Rll can be sub-
stituted by Rl2,
R3 to Rl5 have the meAn;ngs given above,
X is (CH2)m, (m is 0, 1, 2, 3 or 4), CH=CH, C_C, CH20CH2
or CH2ScH2~
Y is (CH2)m, (m is 0, 1, 2, 3 or 4), 0, S or NR3,
Z is (CH2)m, (m is 0, 1, 2, 3 or 4), S, 0, S-Cl-C10-alkyl,
(unbrAnche~ or branche~), CH=CH, CH=CF, CH=CCl, CH=CBr,
CH2-C(0), CH2-CHF, CH2-CHCl, CH2-CHBr, CH2-CHI, C3-C10-
cycloalkylene, C3-C10-cycloalkenylene, CooR7, C_C,
CH=C(Cl-C4-alkyl) (unbr~nch~ or brAnche~), CH=C(CN),
CH=C(R13) or NRs
R16 is Cl-C10-alkyl(Rll) n~ C3-C10-alkenyl(Rll) n or C3-C10-
alkynyl(Rll) n~ with n in each case being zero or 1.
The following meAn~ngs of the radicals in formula I are
very particularly preferred:
Rl is CoNHS02R14 or a radical of the following formulae:
2142556
- 13 -
N V N/~
or \\ E
N
H
in which E i8 O, V is N and W is N, or Rl forms, together
with R2, the ring
U
~N H
\N~U
R 1 6
in which U i8 oxygen,
R2 is O-Cl-C6-alkyl(Rll) n~ (n is 1), where the alkyl
moiety is unbrAncheA~ branched or cyclic, or O-C3-C6-
alkenyl(Rll) n~ (n is 1), where the alkenyl moiety is
unbranched, br~n~heA or cyclic,
R3, Rl1 and Rl3 are phenyl, phenyl substituted by OH or
chlorine, imidazolyl or benzo-fused or pyridino-fused
imidazolyl, where R3, Rll and Rl3 are identical or differ-
ent.
R4, R5 and R6 are H or OH, where R4, R5 and R6 are identi-
cal or dif$erent.
R8 and R9 are Cl-C4-alkyl.
Rl4 is Cl-C4-alkyl, phenyl, naphthyl, thiazolyl or its
benzo-fused derivatives, where the aromatic radical or
heteroaromatic radical can be monosubstituted or disub-
stituted by chlorine, CF3, NO2, Cl-C4-alkoxy or NR3R9, or
Rl4 i~ a radical of the formula
21 12556
- 14 -
Il N N
H3C NH--~S~--
Rl6 is C1-C4-alkyl(Rll)n, with n being 1.
Insofar as they contain a carboxyl group, the novel
compounds of the formula I can form salts with inorganic
or organic bases. Salts with inorganic bases are pre-
ferred, particularly the physiologically harmless alkalimetal salts, especially sodium salts and potassium salts.
The compounds of the formula I inhibit the glucose-6-
phosphatase system of the liver in mammals. The compounds
are therefore suitable for use as pharmaceuticals. The
invention also relates, therefore, to pharmaceuticals
based on the compounds of the formula I, where approp-
riate in the form of the physiologically tolerated salts.
The invention furthermore relates to the use of compounds
of the formula I, or of the salts, for treating diseases
which are associated with an elevated activity of the
glucose-6-phosphatase system.
The invention also relates, therefore, to the use of
compounds of the formula I, or of the salts, for treating
diseases which are associated with an elevated production
of glucose from the liver.
The invention also relates to the use of compounds of the
formula I, or of the salts, for treating type II diabetes
(non-insulin-dependent or maturity-onset diabetes).
The invention furthermore comprises the use of comRounds
of the formula I, or of the salts, for preparing
pharmaceuticals for treating diabetes and other diseases
which are characterized by an elevated secretion of
glucose from the liver or by an elevated activity of the
214255~
- 15 -
glucose-6-phosphatase system.
The effect of the novel compounds on the glucose-6-
phosphatase system was investigated in an enzyme test
using liver microsomes.
Fresh livers from male Wistar rat:s were used for prepar-
ing the microsome fraction cont~;n;ng glucose-6-
phosphatase and were processed as described in the-
literature [Canfield, W.K. and Arion, W.J., J. Biol.
Chem. 263, 7458 to 7460, (1988)]. This microsome fraction
can be stored at -70C for at least 2 months without any
significant 1088 of activity.
The glucose-6-phosphatase activity was detected, as
described in the literature (Arion, W.J. in Methods
Enzymol. 174, Academic Press 1989, pages 58 to 67), by
determining the phosphate liberated from glucose-6-phos-
phate. 0.1 ml of test mixture contained glucose-6-phos-
phate (1 mmol/l), the test substance, 0.1 mg of microsome
fraction and 100 mmol/l HEPES buffer (4-(2-hydroxyethyl)-
piperazine-1-ethanesulfonic acid), pH 7Ø The reaction
was started by A~;ng the enzyme. After proceeding at
room temperature for 20 minutes, the reaction was stopped
by ~;ng 0.2 ml of phosphate reagent. The sample was
incubated at 37C for 30 minutes and the absorption (A)
of the blue color was then measured at 570 nm. The
inhibitory activity of the test substance was obtained by
comparing with a control reaction which did not contain
any test substance, according to the formula
A(control) - A(test substance)
Percent inhibition = ............................. x 100
A(control)
If necessary, the inhibitory activity of the test sub-
stance was determined as a function of the concentration
of the test substance employed and, from this, the
concentration was calculated which was reguired to
inhibit the enzyme activity by 50% (IC50).
2~556
- 16 -
The IC50 value was determined for the compounds listed
below:
Compound IC50 l~m]:
4 0.02
9 0~3
19 0.8
The invention also relates to a pharmaceutical which
contains one or more novel compounds of the formula I
and/or its/their pharmacologically tolerated salts.
The pharmaceuticals are prepared by processes which are
known per se and with which the person skilled in the art
is familiar. As pharmaceuticals, the novel, pharmacologi-
cally active, compounds (= active compound) are either
employed as such or, preferably, in combination with
suitable pharmaceutical auxiliary substances, in the form
of tablets, coated tablets, capsules, suppositories,
emulsions, suspensions, granules, powders, solutions or
preparations having a protracted release of active
compound, with the content of active compound advantage-
ously being from 0.1 to 95%.
Owing to his specialist knowledge, the person skilled inthe art is familiar with those auxiliary substances which
are suitable for the desired pharmaceutical formulation.
In addition to solvents, gel formers, suppository bases,
tablet adjuvants and other active compound excipients,
antioxidants, dispersants, emulsifiers, defoamers, taste
corrigents, preservatives, solubilizers or dyes can, for
example, also be used.
The active compounds may be administered topically,
orally, parenterally or intravenously, with the preferred
mode of administration depen~;ng on the disease to be
treated. Oral administration is preferred.
2142556
- 17 -
For a form for oral use, the active compounds are mixed
with the additives which are suitable for this purpose,
such as carrier substances, stabilizers or inert
diluents, and brought by customary methods into suitable
forms for administration, such as tablets, coated tab-
lets, hard gelatin capsules, aqueous, alcoholic or oily
suspensions, or aqueous, alcoholic or oily solutions.
Examples of inert excipients which can be used are gum
arabic, magnesium hydroxide, magnesium carbonate, potas-
sium phosphate, lactose, glucose or starch, in particularcorn starch. In this context, the formulation can be
effected as a dry granulate or as a wet granulate.
Suitable oily carrier substances or solvents are vege-
table or animal oils, such as sunflower oil or cod liver
oil.
For subcutaneous or intravenous administration, the
active compounds, or their physiologically tolerated
salts, are brought into solution, suspension or emulsion,
if desired together with the substances which are custom-
ary for this purpose, such as solubilizers, emulsifiersor other auxiliary substances. Examples of suitable
solvents are water, physiological sodium chloride solu-
tion or alcohols, e.g. ethanol, propanol or glycerol,
and, in addition, also sugar solutions such as glucose
solutions or mannitol solutions, or else a mixture of
different solvents.
Eyedrops, which contain the active compound in aqueous or
oily solution, are suitable pharmaceutical preparations
for topical and local use. Aerosols and spray~, and also
coarse powders, which are administered through the
nostrils by means of rapid inhalation, and especially
nose drops, which contain the active compounds in aqueous
or oily solution, are suitable for use on the nose.
The dosage of the active compound of the formula I to be
administered, and the frequency of administration, ~ep-n~
on the strength and the duration of the effect of the
2142556
- 18 -
novel compound used; also on the nature and severity of
the disease to be treated and on the sex, age, weight and
individual re~ponsiveness of the mammalian subject to be
treated. On average, the recommended daily dose of a
novel compound is, in the case of a mammalian subject -
most importantly a hl~mAn patient - of approximately 75 kg
in weight, in the range of from about 1 to 500 mg,
preferably from about 10 to 250 mg, it being possible,
according to requirement, for the administration to take
place in several do~es per day, and also, where approp-
riate, to be lower or higher.
The preparation of the novel compounds of the formula I
is elucidated by the examples. Room temperature denote~
a temperature of from 20 to 25C.
Example 1
H~ 0 H ~ O~,N~ ~
~o~3 Ç~-o~
~ 0~3
Preparation of compound 2 from 1
3.7 g (0.054 mol) of the carboxylic acid 1 (preparation
cf. EP Application No. 93 114 261.6, reaction scheme
method A, ~tructural element 68B) were dissolved in 36 ml
20 of anhydrous dimethylformamide, and 1.81 g (0.011 mol) of
N,N'-carbonyldi-(1,2,4-triazole) were added, at room
temperature and under argon, to this solution, which was
21q2556
- 19 -
then heated at from 50 to 60C for l.S hours. After it
had been cooled down, the 0.15 molar solution of 2 was
employed in the subsequent step without any $urther
working-up.
Preparation of compound 3 from 2
0.057 g (0.006 mol) of meth~ne~ulfonamide was dissolved
in 3 ml of anhydrous dimethylformamide, and 0.02 g
(0.0066 mol) of sodium hydride (80% in oil) was added at
room temperature. The suspension was stirred at from 50
to 60C for 45 minutes. 3.1 ml (0.00047 mol) of the
0.15 molar triazolide solution 2 were then added dropwise
at this temperature. The reaction mixture was stirred at
60C for 1 hour. It was then added to a saturated solu-
tion of ammonium chloride, whereupon the product 3
precipitated out as an amorphous solid. The precipitate
was filtered off with suction and then washed with
distilled water; the solid thus obtained was then dried
over calcium chloride at 10-2 Torr and 40C for 3 hours.
0.248 g of compound 3 was obt~ine~.
Preparation of compound 4 from 3
0.24 g (0.000316 mol) of cyclohexylidene ketal 3 was
initially introduced in 10 ml of dioxane, and 1.6 ml
(0.0032 mol) of 2 molar hydrochloric acid were added at
room temperature while stirring vigorously. The clear
solution was stirred at from 50 to 60C for 2 hours. The
reaction solution was then cooled down to from 10 to 20C
and titrated with 1 molar sodium hydroxide solution to pH
3; the reaction mixture was then diluted with 20 ml of
distilled water and concentrated in vacuo until no
further dioxane distilled off. On being stirred up with
water, a precipitate slowly crystallized and was filtered
off with suction and washed with water. After drying at
40C under high vacuum, 0.18 g of compound 4 was obtained
as a colorless solid.
21~25~6
- 20 -
In this manner, the following compounds of the formula I
were synthesized:
Ho, C\S
~ /~
HO OH
Cl 4
MS (FAB): m/z = 680 (M + H+)
,_~0~/
N ~ 5 ~
~ ~ ~
HO OH
Cl 5
MS (FAB): m/z = 843 (M I H+)
21425~
- 21 -
~N 2 N~
~ ~ b,l
HO OH
Cl 6
MS (FAB): m/z = 855 (M + H+)
CH
H3C--N
~^~0~
HO OH
Cl 7
MS (FAB): m/z = 835 (M + H+), 418 (M + 2H+)
- 22
H3C ~ ~ N~
C ~ H
MS (FAB): m/z = 807 (M + H+)
~ HO OH
MS (FAB): m/z = 792 (M ~ H+)
2142S56
- 23 -
N~
Cl~O 05 10
MS (FAB): m/z = 787 (M + H+)
"S ~`OH
MS (FAB): m/z = 630 (M + H+)
12~\~3
MS (FAB): m/z = 668 (M + H+)
2142556
- 24 -
Example 2
~; _ ~_
O~ ~ O O O~ ~ O H
~ 16 ~ 17
<N~3 C~ N~)
c~6 ~0 OH ~ ~3
2142556
- 25 -
Preparation of compound 14 from 13:
5.0 g (0.012 mol) of lactone 13 (preparation cf. EP
Application No. 93 114 261.6, reaction scheme method A,
structural element 68B) were dissolved in 80 ml of
anhydrous toluene, and 10 ml (0.012 mol) of a 1.2 molar
solution of diisobutylaluminum hydride in heYAne were
added dropwise at -78C and under an argon atmo~phere.
After 1 hour at -50C, hydrolysis wae carried out using
a ~aturated ~olution of ammonium chloride. The mixture
was extracted with ethyl acetate and the combined organic
phases were washed with a saturated solution of sodium
chloride and dried using magnesium sulfate. The organic
phase was concentrated in vacuo and the lactol 14 thus
obtained was employed in the subsequent step without any
further purification.
Preparation of compound 15 from 14:
4.6 g (0.011 mol) of lactol 14 and 0.761 g (0.011 mol) of
hydroxylamine hydrochloride were dissolved in 50 ml of
methanol. 750 mg (0.014 mol) of potassium hydroxide were
added. This solution wa~ stirred at room temperature for
1 hour. 300 ml of methyl tert-butyl ether were then ~
to the solution, and this was followed by w~h; ng with
water and with a saturated solution of sodium chloride;
after drying with magnesium sulfate, the organic pha~e
was concentrated in vacuo. The residue was purified by
chromatography on silica gel (eluent: ethyl acetate/n-
heptane 1:2). 3.6 g of oxime 15 were obtained as a
colorless oil.
Preparation of compound 16 from 15:
20.0 g (0.046 mol) of oxime 15 were initially introduced
in 200 ml of anhydrous dichloromethane, and 23.0 g
(0.14 mol) of N,N'-carbonyldiimidazole were added. There
followed a powerful evolution of gas. After 14 hours at
room temperature, 100 ml of methanol were added to the
2142556
- 26 -
reaction solution, and the mixture was heated under
reflux for a further 4 hours. For the working-up, the
solution was brought to dryness by rotary evaporation and
the residue was taken up in methyl tert-butyl ether. The
organic phase was washed with a mixture of water/0.1 M
solution of potassium hydrogen sulfate, dried using
magnesium sulfate and` concentrated in vacuo. The residue
was purified by chromatography on silica gel (silica gel
particle size: 35 to 70 ~m, eluent system: ethyl acetate/
n-heptane 1:5, towards the end, the proportion of n-
heptane was decreased: 1:3). 12.9 g of nitrile 16 were
obtained as a colorless oil.
Preparation of compound 17 from 16:
12.9 g (0.0286 mol) of nitrile 16 were dissolved in
250 ml of anhydrous toluene and heated at 110C. 5.89 g
(0.0286 mol) quantities of trimethyltin azide were added
at 24-hour intervals over a period of three days. The
reaction solution was then concentrated in vacuo, and
50 ml of 10 molar sodium hydroxide solution and 20 ml of
tetrahydrofuran were added to the residue while stirring
vigorously. The resulting sodium salt of 17 was filtered
off with suction and then suspended in distilled water
and this suspension was acidified with 2 molar acetic
acid. It was extracted with ethyl acetate and the
combined organic phases were dried using magnesium
sulfate and concentrated in vacuo. 7.7 g of tetrazole 17
were obt~; ne~ .
O _ ~ O
~O H ~N' `?
21425S6
- 27 -
Preparation of starting compound B from A:
274 mg (0.001 mol) of carboxylic acid A (preparation cf.
EP Application No. 93 114 261.6, method I) were dis-
solved, under an argon atmosphere and at room tempera-
ture, in 20 ml of anhydrous dimethylformamide, and180.4 mg (0.0011 mol) of N,N'-carbonyldi-(1,2,4-triazole)
were added. The reaction solution was stirred at 60C for
1 hour. The resulting solution of compound B was employed-
in the subsequent step without any further working-up.
Preparation of compound 18 from 17:
3.0 g (0.00652 mol) of compound 17 were dissolved, under
an argon atmosphere, in 30 ml of anhydrous dimethylform-
amide, and 0.70 g (0.023 mol) of sodium hydride (80%
dispersion in oil) was added at room temperature. After
1 hour, 157 ml (0.0078 mol) of an 0.5 molar solution of
B in dimethylformamide were added dropwise, and the
mixture was stirred once again at room temperature for 1
hour. The reaction solution was subsequently added to a
saturated ~olution of ammonium chloride, and this mixture
was extracted with ethyl acetate. The combined organic
phases were washed with a saturated solution of sodium
chloride, dried over magnesium sulfate and concentrated
in vacuo. The crude product was purified by chromato-
graphy. (Silica gel particle size: 35 to 70 ~m, eluent
system: ethyl acetate/n-heptane/methanol/glacial acetic
acid 30:10:2:1). The e~ter 18 was obtA;ne~ as an amor-
phous solid.
Preparation of compound 19 from 18:
3.8 g (0.0053 mol) of cyclohexylidene compound 18 were
taken up in 150 ml of dioxane, and 10 ml (0.02 mol) of 2
molar hydrochloric acid were added while stirring. This
solution was heated at 60C for 2 hours. The pH of the
reaction solution was then adjusted to 3 using 18 ml of
lN molar sodium hydroxide solution and the solvent was
- 21425~i6
- 28 -
remo~ed by rotary e~aporation. The residue was taken up
in ethyl acetate and the precipitate which developed was
filtered off. The filtrate was concentrated in vacuo and
the residue was purified by chromatography on silica gel
(silica gel particle size: 35 to 70 ~m, eluent system:
ethyl acetate/methanol/water/glacial acetic acid
4:1:1:0.5). 2.5 g of compound 19 were obtA;ne~ as a
colorless amorphous solid.
In this manner, the following compounds of the formula I
were synthesized:
H`.. /\ - H
'N~N
~N H <N~3
~ ~ o~3
O H ~ g
MS (FAB): m/z = 627 (M + H+)
C I
~ O
H O ~ oJ~
OH 2 O OH
MS (FAB): m/z = 515 (M I H')
21~2~S6
- 29 -
Example 3
H N H
~OH ~/ ~OH N~
H O OH
21 22 23
H H
XC H H, C C H ~ ~3
24 25
O~N
--HO
26
Preparation of compound 22 from 21:
2.26 g (0.01 mol) of the ketone 21 which i~ known from
the literature (cf. J.C. Barrier et al., Helv. Chim. Acta
66, 296 (1983)) and 4.29 g (0.025 mol) of 3-phenylpropyl-
amine hydrochloride were initially introduced, under an
argon atmosphere, in 5 ml of methanol and 3 ml of
distilled water. The mixture was cooled to 0C and a
solution of 1.63 g (0.025 mol) of potassium cyanide in
4 ml of distilled water was added dropwise. The reaction
mixture was stirred at 0C for 4 hours and at room
21~2556 `
- 30 -
temperature for 1 hour and was then added, while stirr-
ing, to ice/water; this mixture was extracted three times
with ethyl acetate. The combined organic phases were
wa~hed three times with distilled water and once with a
saturated solution of sodium chloride and then dried
using magnesium sulfate and concentrated in vacuo. 5.0 g
of crude product 22 were obt~;n~A, which product was
employed in the subsequent step without any further
purification.
Preparation of compound 23 from 22:
3.7 g (0.01 mol) of cyano compound 22 were dissolved in
8 ml of glacial acetic acid, and a solution of 1.62 g
(0.02 mol) of potassium cyanate in 4 ml of distilled
water was added at room temperature and while stirring.
The reaction solution was stirred at room temperature for
75 minutes and then added to a mixture of ice and water;
thi~ new mixture was extracted twice with ethyl acetate,
and the combined organic phases were washed once with
distilled water and once with a saturated solution of
sodium chloride. After the organic phase had been dried
with magnesium sulfate, it was concentrated in vacuo; the
oily residue thus obtained was dissolved in 4 ml of
dioxane, and 10 ml of 2 molar hydrochloric acid were
added to this solution while stirring. After stirring at
55C for one hour, the reaction mixture was poured onto
an ice/water mixture and the whole was extracted three
times with ethyl acetate. The organic phases were w~e~
three times with water and once with a saturated solution
of sodium chloride, dried over magnesium sulfate and
concentrated in vacuo. The oily residue was purified by
chromatography on silica gel (silica gel particle size:
35 to 70 ~m, eluent: ethyl acetate/n-heptane/methanol/
glacial acetic acid 20:10:2:1), and 0.36 g of product 23
was obtained.
21~2556
- 31 -
Preparation of compound 24 from 23:
0.36 g (0.00106 mol) of compound 23 was dissolved in
1.09 ml (0.0106 mol) of dimethoxypropane and 20 ml of
anhydrous dichloromethane. 26 mg (10 mol %) of pyridinium
para-toluenesulfonate were added. The mixture was heated
at 40C for 45 minutes. The reaction solution was then
added to a saturated solution of sodium hydrogen sulfate
and this mixture was extracted with ethyl acetate; the
combined organic phases were dried using magnesium
sulfate. The residue was purified by chromatography on
silica gel (silica gel particle size: 35 to 70 ~m, eluent
system: ethyl acetate/n-heptane 2:1), and 0.24 g of
compound 24 was obtained as a colorless solid.
Preparation of compound 25 from 24:
230 mg (0.0006 mol) of hydroxy compound 24 were dissolved
in 10 ml of anhydrous dimethylformamide, and 55 mg
(0.00184 mol) of sodium hydride (80% dispersion in oil)
were added at room temperature and under an argon atmos-
phere. After 30 minutes at room temperature, 22 ml of a
0.5 molar solution of B in dimethylformamide were added
dropwise. After a further 30 minutes at this temperature,
a clear solution was obt~;n~A; a saturated solution of
ammonium chloride was added to this clear solution,
whereupon the product 25 precipitated out as an amorphous
solid. This solid was filtered off with suction and dried
in vacuo. 310 mg of compound 25 were obt~;ne~.
Preparation of compound 26 from 25:
290 mg (0.00047 mol) of compound 25 were dissolved in
30 ml of dioxane, and 4 ml (0.008 mol) of 2 molar hydro-
chloric acid were added, at room temperature and whilestirring vigorously, to this solution. After stirring at
50C for two hours, the reaction solution was cooled down
to from 10 to 20C and titrated to pH 3 using 1 molar
sodium hydroxide solution. The solution was concentrated
21~2556
- 32 -
in vacuo and the oily residue was taken up in isopropan-
ol; the precipitate of salt was filtered off and the
filtrate was concentrated onee again in vaeuo. The
residue was stirred up with methyl tert-butyl ether and
the amorphous preeipitate was filtered off with suetion.
After drying in vaeuo, 140 mg of eompound 26 (end produet
of the formula I) were obt~ineA.
H
Nr~
26
MS (FAB): m/z = 581 (M + H')
Example 4
N~
O HO~S
~OTHP O--OTHP
0~0 ' _ 0~0
27 28
2142~56
- 33 -
Cl
~ N~
C 1~ ~--OTHP
X
29
~ ~--O T H P ~ ~--O H
C I \/ C I \/
31 O 32 O
- ~'~
C I HO OH
33
Preparation of compound 28 from 27:
26.4 g (14.5 ml, 0.161 mol) of 2-bromothiazole were
dissolved, under an argon atmosphere, in 500 ml of
anhydrous diethyl ether, and 107.5 ml of n-butyllithium
in hexane (1.5 molar solution) were added dropwise at
-78C. The mixture was stirred at -78C for 30 minutes
and a solution of 25.0 g (0.081 mol) of ketone 27 (cf. EP
Application No. 92 114 260.8, formula scheme 4, compound
21~2556
- 34 -
23B) in 50 ml of anhydrous tetrahydrofuran was then added
dropwise. The reaction solution was allowed to heat up to
-30C within the space of 30 minutes. After that, the
reaction solution was added to a~monium chloride solu-
tion, and this mixture was extracted with ethyl acetateand the combined organic phases were washed with a
saturated solution of sodium chloride and dried using
sodium sulfate. The organic phase was concentrated in
vacuo and the residue was purified by chromatography on
silica gel (eluent: ethyl acetate/heptane 1:2, particle
size: 35 to 70 ~m). 23.3 g (77%) of compound 28 were
obtained as a viscous oil.
Preparation of compound 30 from 28:
15.0 g (0.038 mol) of alcohol 28 were dissolved in 250 ml
of anhydrous dimethylformamide, and 1.5 g (0.05 mol) of
sodium hydride were added at from 0 to 10C. The mixture
was stirred at 10C for 1.5 hours and then cooled down to
0C, when 13.2 g (0.057 mol) of cis-3-(4-chlorophenyl)-
propenyl bromide (29), dissolved in 30 ml of anhydrous
dimethylformamide, were added dropwise. The reaction
solution was allowed to warm to room temperature and was
stirred at thi~ temperature for 2 hours. The reaction
solution was subsequently added to a saturated solution
of Ammonium chloride and this mixture was extracted with
ethyl acetate; the combined organic phases were washed
with a saturated solution of sodium chloride. After
having been dried using sodium sulfate, the organic phase
was concentrated in ~acuo and the residue was purified by
chromatography on silica gel (eluent: ethyl acetate/n-
heptane 1:2, particle size: 35 to 70 ~m). 19.0 g of
thiazole 30 were obtA;ne~ as a ~iscous oil.
Preparation of compound 31 from 30:
56.6 ml of a 1.1 molar solution of diethylzinc in toluene
were added dropwise, at 0C and under an argon atmos-
phere, to 250 ml of anhydrous dichloroethane, and 9.0 ml
2142SS~
- 35 -
(0.125 mol) of chloroiodomethane were then added at 0C.
The reaction solution was stirred at the same temperature
for 30 minutes and, after that, 17.0 g (0.031 mol) of
olefin 30, dissol~ed in 30 ml of anhydrous dichloro-
ethane, were added dropwise. The mixture was allowed towarm slowly to room temperature. After 2 hours, the
reaction solution was added to a saturated solution of
ammonium chloride and this mixture was extracted with
ethyl acetate; the combined organic phases were washed
with a saturated solution of sodium chloride. After the
organic phase had been dried using sodium sulfate, it was
concentrated in vacuo and the residue was stirred
thoroughly with methyl tert-butyl ether. The precipitate
was filtered off (methylation of the nitrogen in the
thiazole ring took place as a side reaction) and the
filtrate was concentrated once again. 4.2 g (24%) of
compound 31 were obt~;ne~ as a viscous oil.
Preparation of compound 32 from 31:
4.2 g (0.008 mol) of 31 were dissolved in 100 ml of
methanol and 150 ml of dichloromethane, and 0.7 g
(0.003 mol) of pyridinium p-toluenesulfonate was added at
room temperature. The clear solution wa~ allowed to stand
at room temperature for 14 hours and 20 ml of a 1 N
solution of sodium hydrogen carbonate were then added to
it; this mixture was concentrated until only the aqueous
phase remained. This phase was extracted with ethyl
acetate and the combined organic phases were washed with
a saturated solution of sodium chloride, dried using
sodium sulfate and concentrated in vacuo. The residue was
purified by chromatography on silica gel (eluent: ethyl
acetate/n-heptane 1:1, particle size: 35 to 70 ~m).
1.82 g (51%) of compound 32 were obtained as a colorless
oil.
Preparation of compound 33 from 32:
In analogy with the preparation of compound 19 from
21~2S56
- 36 -
compound 17, as described in Example 2, compound 33 of
the formula I was obt~;ne~, as an amorphous solid, from
32 in 2 stages.
MS (FAB): m/z = 542 (M I H~)