Note: Descriptions are shown in the official language in which they were submitted.
(~ W094/04l09 21~ 2 8 71 PCT/US92/10672
Cross-Ref rence to Related Ap~lications
This application i a oontinuation-in-part
of our earlier filed pending U.S. application Serial
No. 07/769,155, filed Septe~ber 27, 1991, which :is a
continuation of U.S~ application S~rial No.
07/453,617, filed Dece~ber 20, 1989 (abandoned), which
is a divisional of U.S. application Serial No.
07/215,074, filed July 5, 1988 (U.S. Patent
4,915,950), which is a continuation-in-part of U.S.
application Serial No. 07/155r327, filed Februaxy 12,
1988 ~abandoned), all of which applications are
in~orporated herein by reference and to which
applications we claim priority under 35 U.S.C. 120.
Field o~ t~e lnve~tion
This invention relates generally to
transdermal delivery devices and to methods of making
and using such devices. More particularly the
- inYention rel~tes to transdermal nicotine delivery
: systems which include particular amounts of nicotine
within a matrix allowing the device to be worn for 24
hours but be depleted of nicotine to the extent that
nicotine is not further delivered to the patient after
a period of about 16 hours.
W094/04109 2 1 ~ 2 8 7 ~ PCT/US9Z/1067 ~
ackqround of the Iny nton
A variety of devi~es have been proposed or
used for administering drugs transdermally. These
devices ar~ generally in the form of a bandage or skin
patch that includes a reservoir that contains the drug
and a pressure-sensitive adhesive component by which
the device is attached to the skin. Depending upon
the inherent permeability of the skin to a particular
d~ug, the device may also include means for
coadministering a percutaneous bsorption enhancer or
an element, such as a membrane interposed between the
reservoir and the skin, that regulates the rate at:
which the drug or the percutaneous absorption enhancer
is administered to the skin.
The commercially available techniques for
manufacturing these devices involve conventional
casting and laminating processes. Actual
incorporation of the drug is typically effected by
(1) admixture of the drug with a compatible solvent,
(2) incorporation of the drug into the drug reser~oir
by immersion in the drug/solvent admixture, and
~ (3) evaporation of the solvent. ~n practice, this
-~ m~thod has proved to ha~e several disadvantages.
First, for many drugs, the solvent selected
is necessarily organic, rather than aqueous. As many
organic solvents are flammable and~or toxic, an
element of risk is thus introduced into device
fabrication and use. Another shortcoming is that with
volatile drugs or drugs that are sensitive to heat,
e~aporation of the solvent can either volatilize or
~- degrade the drug. The present invèntion is addressed
to these shortcomings, and provides a device ,
fabrication process which eliminates the necessity for
both organic solvents and high-temperature
evaporation. The process minimizes drug degradation
and loss to the environment, while eliminating the
possibility of contamination with organic residues
~ -WO94/04109 214 2 8 71 PCT/US92/1067~
which may be harmful to the skin, e.g., as irritants,
sensitizers, carcinogens, or the like.
Furthermore, conventional casting is done in
solid sheets or stripes. When laminated and die cut
5 ou~, the remaining web is left unusable and is
discarded. Highly exp nsive drugs are costly to
discard, as dangerous or controlled narcotic drugs can
be deli~ered for abuse or present o~her uncontrollable
hazards. By carrying out the method disclosed herein
lO it is possible to eliminate some of the disadvantages
of earlier methods and provide a system which can
include a precise amount of a drug which, after use,
will be depleted of the drug to the extent that
further drug could not be delivered to the patient,
15 thus reducing the costs, potential dangers and
potentials for abuse.
Most drug delivery devices are designed so
~hat they ~an be worn for 24 hours. The 24-hour wear
period provides for ~ood patient compliance in that
20 ~he patient can replace the patch each day at
approximately the same time. Further, most patches
are designed so as to continually~delive~ a particular
drug to the patient during the entire 24-hour period.
Although the once a day dosing regime is desirable,
25 the con~inuous delivery of drug to a patient over a
24-hour perîod is often not desirable. Problems
related to the continuous delivery of the drug to the
patient over a 24-hour period varied depending on the
type of drug. With respect to certain drugs the
30 problem becomes one of tolerance, i.e., the patient `
becomes less reactive to larger and larger amounts of -~
drugs in that the drugs are continually being
delivered. Tolerance is a problem with drugs such as t
narcotics and nitroglycerin used as a vasodilator.
35 With other drugs, such as nicotine the continuous
delivery of the nicotine to the patient can result in
problems such as sleep disorders, skin irritation and
WO94/041~9 2 i 4 2 8 7 1 _4_ PCT/US9~/106 ~ -
the like (see D.M. Daughton et al., Arch. ~ntern. Med.
151:749-752 (l991) and K.O. Fagerstrom et al., J.
Smoking Related Dis., in press). The present
invention addresses these problems by loading specific
amounts of the drug into the device such that the drug
will be substantially depleted from the patch after a
given period of time, i.e., the drug is depleted from
the patch to the extent that drug is no longer
delivered to the patient even though it may remain in
the patch.
Because the fabrication process does not
involve the use of high temperatures, it is also
useful in incorporating volatile vehicles, excipients
or enhancers into transdermal delivery devices. In
addition, a device may be fabricated using the present
process so as to contain a volatile fragrance. Such a
device is designed to exude fragrance over a
.~ ~
protracted, predetermined period of time.
Summa~v o~ the ~ve~t~
.
Transdermal delivery devices are disclosed
which include a backing layer whic~ is substantially
impermeable to the drug, and a drug matrix layer which
may be in the form of a pressure-sensitive
pharmaceutically acceptable contact adhesive having
- the drug dispersed therein. The drug is loaded into
the drug matrix layer in an amount such that the drug
will, after about 14-18 hours (preferably 16 hours) of
contact with the patient, be depleted of the drug to
~! ~, ! 30 the extent that delivery of the drug to the patient is
slowed to a rate such that the effect of the drug on
the patient is negligible. To achieve this effect the
loading amount of the drug into the drug matrix layer
is clasely controlled and varies somewhat depending on
the particular drug in that different drugs have
different rates of delivery. For example, when the
system is designed to deliver nicotine, the nicotine
,
( - W094/04109 .~ 2 1 ~ 2 8 7 1 PCT/US92/10672
5--
is loaded into the drug matrix layer in an amount in
the range of about 0.70 to about 1.15, more preferably
0.75 to 0.95 mg/am2. By placing a drug delivery system
of the invention on a patient there is provided a
S method of drug delivery whereby the patch i5 placed on
the patient for a period of 24 hours during which time
the drug is delivered to the patient during only about
1~-18 hours (preferably about 16 hours) after which
the drug is depleted from the patch to the extent that
any further delivery to the patient is so
insignificant as to not have any detectable
pharmacological effect on the patient.
The delivery devices of the inve~tion are
ob~ained by a method comprising:
(a) laminating an adsorbent source layer to
a pressure-sensitive, pharmareutically acceptable
contact adhesive layer, the contact adhesive layer
comprised of a ~at~rial that is permeable to the drug
and which defines a basal surface for adhesion to
skin;
(b) depositing a drug in liquid form on one
face of the adsorbent source laye~;
(c) laminating an anchor adhesive layer to
the opposing face of the source layer; and
(d) applying a backing layer to the anchor
: adhesive layer which defines the upper surface of the
device and is substantially impermeable to the drug.
A preferred embodiment of the invention is a
transdermal drug delivery device for administering
nicotine to a human patient transdermally and
! continuously for a period of approximately 16 hours.
The device is comprised of a backing layer which is ;,
substantially impermeable to nicotine, which backing ,-
layer defines the upper surface of the device. The .s
device is further comprised of an anchor adhesive
layer which is adjacent to the backing layer and
laminated thereto. Thereafter, a layer of pressure-
~1428 71 ~ ~
W094/04109 PC~/US92/106
sensitiYe, pharmaceutically ~cceptable, contactadhesive which is permeable to nicotine is provided.
This pressure-sensitive adhesive layer defines the
basal surface of the device which is adhered to the
skin of the patient. The device is also preferably
comprised of an adsorbent source layer which is in
contact with and contained between the anchor adhesive
layer and layer of pressure-sensitive adhesive. The
nicotine is preferably dispersed uniformly throughout
the layer of pres~ure-sensitive ~ontact adhesive in an
amount such that the nicotine in the device will,
after 16 hours of contact with the patient, be
depleted to the extent that the delivery of the
nicotine to the patiant is slowed to a rate such that
the effect of the nicotine on the patient is
negligible, i~e., no pharmacological detectable
eff~ct. The amount of nicotine is preferably in the
range of 0.75 to 0.95 mg~c~ but can vary outside that
range in an amount of about 25% ~.
In still another aspect of the invention, a
method and device similar to the aforementioned are
provided for the incorporation and~release of
fragrance. In such a case, the fragrance is initially
deposited onto the source lay~r and then released over
time through the adhesive and backing l~yers which are
selected so as to be permeable to the fragrance.
A key advantage of the present invention is
in the "printing" of the selected drug, drug-vehicle
combination, or other material, in liquid form, on the
adsorbent source layer in a particular amount. That
is, the material is loaded into the device by
substantially uniform deposition on the surface of the
source layer. For many materials, this one-step
deposition eliminates the need for organic solvents as
well a~ the need for heat treatment.
After loading of the drug onto the source
layer, the drug migrates into the underlying contact
94/04109 ~'~2~ ~ 71 PCT/~S92/10672
adhesive layer and, depending on the material selected
for the anchor adhesive layer, into that layer as
well. The release kinetics of the drug into the skin
from the contact adhesive layer are determined by the
degree of drug loadin~ (which can be at, above, or
below saturation ~ in this system) and the diffusivity
and solubility of the drug in the two adhesive layers.
The source layer thus serves to initially retain the
deposited drug which then migrates from the source
layer into one or both adhesive layers.
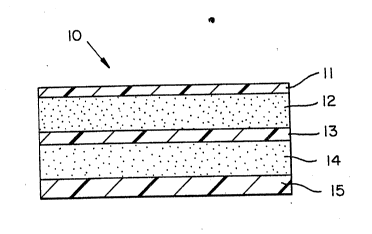
Brief Description of the Drawinqs
Figure l shows a partly schematic, sectional
view of a transdermal drug delivery device according
to the invention.
Figure 2 shows an apparatus which may be
used in fabricating a transdermal drug delivery device
according to the ~ethod of the invention.
Figure 3 shows the ln vitro permeation of
nicotine through human cadaver skin from a transdermal
drug delivery device fabricated according to the
pr~sently disclosed method.
Detailed Descriptio~ of the Invention
Before the present transdermal delivery
device, method of delivering drugs and method of
manufacturing such devices is described, it is to be
understood that this invention is not limited to the
particular devices, methods and processes described,
30 as such may, of course, vary. It is al50 to be
understood that the terminology used herein is for the
purpose of describing particular devices, methods and
processes only, and is not int~nded to be limiting
since the scope of the present invention will be
35 limlted only by the appended claims. 3
It must be noted that as used in this
specification and in the appended claims, the singular
W094/04109 ~ 21~2871 PCT/~S92/106 ~ -
-8
forms "a", "an" and "thel' include plural reference
unless the context clearly dictates otherwise. Thus,
for example, reference to ~'a drug permeation enhancer" .
includes mixtures o~ -~uch permeation enhancers, ~.
reference to "an adhesive'i includes ~ixtures of
adhesives and reference to "the method of delivery"
includes one or more methods of delivery of the
general t~pe described herein and o~ a type which
would be deduced by those skilled in the art upon
reading this disclosure.
Before providing a detailed description of
the invention, the following definition of terms will
- be provided.
l. Definitions:
lS By "printed" as used herein to describe the
method of incorporating a drug or other material into
the source layer is meant a substantially uniform
deposition of the drug, in liquid form, onto one
surface of the source layer. As the source layer
~ 20 comprises a porous material, the drug is initially
: ~ retained by that layer, i.e~, prior to equilibration,
and then diffuses into one or bot~ of the adjacent
layers. It will be appreciated by those skilled in
th~ art that a variety of techniques may b~ used to
effec~ substantially uniform deposition of material,
~.g., Gravure-type printing, extrusion coating, screen
coating, spraying, painting, or the like.
By a drug in "liquid form" as used herein is
meant either a drug that is itself a liquid or a drug
which is suspended, dissolved or disperse~ in a
~elected solvent. Solvents may or may not be aqueous,
depending o~ the parti~ular drug used, and mày include
commonly used liquid vehicles and skin penetration
enhancers. Preferred solvents are nonaqueous and
selected so that they can be incorporated into the
final syste~ without adverse effect.
. . .. . . .. .. .... , .. , ~. , ,,, ,.. .. , .,.. , .. ~ ~,i .. " . .. ,., ., , . . ,,. .
094/04109 ~`r~21 ~-~2 8 71 PCT/U592!l0672
By "pharmaceutically acceptable" material as
used herein is meant a material which does not
interfer~ with the biological effectiveness of the
drug administered and which is not for any reason
biologically or otherwise undesirable.
By a "permeable" adhesive is meant a
material in which the sele~ted drug has at least
moderate solubility and diffusi~ity, i.e., drug
solubility ont he order of 5 to 50 wt.%, preferably 10
to 30 wt.%, and diffusivity in the range of about 1 x
lQ~ to about 1 x l0l2 cm2/sec.
By "substantially impermeable" as used
herein to de~cribe th~ bac~ing layer is meant thal: an
effective amount of the selected drug will be
contained within the device without loss of any
substantial amount through the backing layer. It
should be noted that where the device is used for the
release of fragrance, however, the backing layer is,
by contrast, permeable to the fragrance. In such an
embodiment, ~he device thus allows for release of
fragrance into the atmosphere.
Desçx~pt~on ~ the ~ransdermal ~ru~ ~elivery ~evice
Referring now to Figure 1, the transdermal
drug delivery device provided by the present method is
;~ shown generally at lO. The device is designed
-- specifically for transd`ermal administration of a drug
- at controllable, therapeutically effective rates. The
device 13 is in the ~orm of a laminated composite that
is adapted t~ be adhexed to a predetermined area of
unbrokan skin or mucosal tissue. The individual
layers of the device include an upper backing or
"outer skin" layer ll, an anchor adhesive layer 12, a
source layer 13 onto which the drug and/or vehicles -~
are deposited initially,~a contact adhesive 14 which
is adapted to adhere to the skin or mucosa, and a
release liner 15.
2`1~2871
W094/~4~09 PCr/US9~/106 ~
--10-- I
The backing layer 11 functions as the
pri~ary structural element of the device and provides
the devica with much of its flexibility, suitable
drape, and, whare neces~ary, depending upon the
matexial incorporated into the device, occlusivity.
In the preferred embodiment in which the device serves
as a transdermal drug delivery system, the backing
layer also serve~ as a protective covering to prevent
lo~s of the drug (and/or vehicle, solubilizer or
~o permeation enhancer, if present) via transmission
through the upper surface of the device. (In the
alternative embodiment in which the device serves as a
fragrance patch, as noted above, the backing layer
will by contrast allow release of fragrance into the
atmosphere.) Backing layer 1~ may also be used to
impart the device with a desirable or necessary d~gree
of occlusivity which in turn causes the area of skin
on which the device is placed to become hydrated. In
su~h a case, a layer is selected that has a level of
water vapor transmissibility that makes the device
occlusive to the degree required to cause the area of
skin to be hydrated. It is then p~eferable that the
device provide at least about 90% hydration, more
preferably at least about 95% hydration of the skin,
-25 as m~aæured by a dielectric hydration probe available
`~from Dr. Howard Maibach, U.C.S.F., San Francisco,
California. Such occlùsivity is desirable when drugs
such as estradiol or other steroids are being
administered. If the drug being administered is such
that skin hydration is not necessary or desirable, it
is preferable to use layers that provide a composite
that is "breathable", i.e., transmits water vàpor from
the skin to the atmosphere. Such breathability
contributes to the nonocclusive nature o~ the
composite and lessens the likelihood that the area of
skin on which the composite is worn will become hi~hly
hydrated and irritated.
2142871 ~:
. . .
t ~wog4~o4log PCT/US92/10672
Backing 11 is preferably made o~ a sheet or
film of a preferably flexi~le elastomeric material
that is substantially impermeable to the selected
drug. The layer is preferably on the order of 0.0005"
to 0.003" in thickness, and may or may not contain
pigment. The layer is preferably of a material that
permits the device to mimic the contours of the skin
and be worn comfortably on areas of skin, such as at
joints or other points of flexure, that are normally
lo subjected to mechanical strain with little or no
likelihood of the device disengaging from the skin due
to differences in the flexibility or resiliency of the
skin and the device. Examples of elastomeric polymers
that are useful for making layer 11 are polyether
block amide copolymers (e.g., PEBAX copolymers), such
as NUERELL polymers, polyurethanes such as PELLATHANE
or ESTANE polymers, silicone elastomers, polyester
biock copolymers that are composed of hard and soft
segments (e.g., HYTREL polymers), rubber-based
polyisobutylene, styrene, and styrene-butadiene and
- styrene-isoprene copolymers. Polymers that are
flexible include polyethylene, pdqypropylene,
polyesters, e.g., polyester terephthalate (PET), which
may be in the form of films or laminates. The
preferred polymer used for the backing will depend on
the material or drug incorporated into the device and
on the nature of any vehicles, solubilizer~, or the
like that are used.
Anchor adhesive layer 12 adheres to backing
layer 11 and to source layer 13. The anchor adhesive
is preferably but not necessarily of a material in :
which ~he selected drug or v~hicle has moderate
solubility and diffusivity. In such a case, after
equilibration, the drug will have diffused not only
into the contact adhesive layer 14, but also into the
anchor adhesive. Diffusion into both adhesive layers
is useful insofar as regulation of release kinetics is
WO94/04104 2 1 4 2 8 7 1-l2- PCT/US92/106
concerned. That is, by careful selection of the
materials used for the anchor and contact adh~sive
layers~ the distribution of drug throu~hout the entire
system can be regulated. This is because the release
kinetics of the drug from the device can be control;ed
by the diffusivity and sslubility of the drug in both
of the adhesive layers as well a~ in backing layer 11.
When the drug is below saturation in all layers, the
total drug loading controls the release kinetics.
An important aspect of the invention is
providing for a specific range of drug loading which
will allow the drug to be delivered to the patient
over a period of about 14-18 hours (pre~erably 16
hours) even though the device is worn by the patient
for 24 hours~ This is accomplished by including a
particular conc2ntration of the drug into a layer such
as the pressure-sensitive contart adhesive layer. The
concentration of the drug within this layer will vary
somewhat depending upon the particular drug being
delivered. In connection with the present invention
it has been found that it is necessary to include
nicotine in a concentration withi~ the range of about
o.70 to about l.lS mg/cm2, preferably 0.75 - O.95
mg/cm2 and most preferably about 0.83 mg/cm2. By
including nicotine in the adhesive layer in this
concentration and placing the patch on the patient the
nicotine will be delivered to the patient for
approximately 16 hours, after which the drug will be
depleted from the delivery system to the extent that
the rate of delivery of the drug to the patient is
slowed to such an extent that the delivery of nicotine
to the patient becomes negligible, i.e., no detectable
pharmacological effect. The nicotine may be in the
form of a free base or a salt and a particularly
useful salt is nicotine monoacetate. The
concentration of any particular drug in the adhesive
layer will vary sorewhat depending on the permeability
.,
2I~2871
~ ~ ~0~4~041~9 ~ t;^ PCT/US92/10672
.
of that drug to human skin and also somewhat based on
the adhesive material.
Examples of suitable materials for anchor ~-
adhe ive layer 12 include polyethylenes,
polysiloxanes, polyisobutylenes, polyacrylates,
polyurethanes, plasticized ethyl ne-vinyl acetate
copolymers, low molecular weight polyether block amid~
copolymers (PEBAX copolymers), tacky rubbers such as
polyisobu ene, polystyrene-isoprene copolymers,
polystyrene-butadiene copolymers, and mixtures
thereof. The particular polymer~s) used for the
anchor adhesive layer will depend on the drug,
vehicle, enhancer, etc., selected. The thickness of
the anchor adhesive layer may vary but is typically in
~he range of about 0.0005" to about 0.005". In
the cas~ of a fragrance patch, the material serving as
the anchor adhesive layer æhould, like the backing
layer, be selected so as to be substantially permeable
to the fragrance incorporated into the patch.
Source layer 13 is a thin, flexible layer of
an adsorbent material which provides the surface on
which the drug is printed or othe~wise deposited. The
source layer allows the liquid drug (together with
vehicle, solubilizer or the like) to be printed on its
surface as a result of having surface properties not
found in either the contact or anchor adhesive layers.
During fabrication, the drug i5 deposited in liquid
form onto one face of this layer in a substantially
- uniform pattern. The drug must wet the surface in
such a way that squeezing of liquid to the periphery
of the device durin~ lamination is substantially
prevented. The material is selected so that the drug
is adsorbed, rathsr than absorbed, by the layer, since
the drug must be available to migrate into contact
adhesive layer 14 and preferably into anchor adhesive
layer 12 as well. The source layer is preferably of a
non-woven fabric, e.g., polyester, polyethylene,
WO94/04109 2 1 4 2 8 7 1 -14- PCT/US92/1067 ~ -
polypropylene, polyamides, rayon or cotton, and a
particularly preferred material for the source layer
is a lO0% non-wovan polyester. Woven fabrics,
however, can also be used if desired. The thickness
of the source layer may vary, but is preferably in the
range of about O.OOl" to O.OlO".
It should be pointed out that the source
layer does not serve as a drug reservoir; the drug is
only transiently adsorbed by the source layer pending
equilibration, i.e., migration intu one or both of the
adjacent adhesive layers.
Alternatively, the inner surface of either
the anchor or contact adhesive layers may be treat:ed
and thus itself serve as the source layer for purposes
of drug deposition. Still another alternative is to
u~e a contact or adhesive layer that has a porous
surface, enabling the drug to be printed "into" the
surface pores. Contact adhesive layer 14, which
plays the principal role in dstermining the rate at
which drug is released from the device, is a pressure-
sensitive skin contact adhesive comprised of a
pharmaceutically acceptable materi~l. Like source
layer 13, it must be chemically and physically
compatible with the dru~ and with any enhancer used.
Further, the drug selected must have at least moderate
solubility and diffusivity in this layer, since the
drug must be able to readily migrate from source layer
13 into and through contact adhesive layer 14 and to
the skin. The thickness of the contact adhesive layer
is preferably in the range of about 0.0005" to about
b~oo5~.
Suitable materials for contact adhesive
layer 14 include those enumerated for anchor adhesive
12. It is possible (in some cases) to usa materials
for the contact adhesive layer that are relatively
impermeable to the drug, e.g., where the diffusivity
of the drug through skin is quite high. In the case
-, 21~2871 ~
WO94/04109 ~ PCT/US92/10672
-~5-
of a fragrance patch, contact adhesive layer 14 may or
may not be permeable to the fragrance. In any
particular device abricated according to the present
process, the materials chosen for the contact and
anchor adhesive layers may be the same or different.
Prior to use, device 10 includes a release
liner 15. Just prior to use, this layer is removed
from the device to expose contact adhesive layer 14.
~he release liner will normally be made from a
drug/vehicle/enhancer i~permeable material that i~
inherently "strippable" or rendered so by techniques
such as silicone or fluorocarbon treat~ent.
Device 10 need not include a means for
controlling the rate at which either the drug or the
enhancer is administered to skin. Instead, the
relea~e kinetics of the drug from the bandage can be
controlled by the materials selected for the anchor
and contact adhesive layers and by ~he degree of drug
loading~ Either the contact adhesive layer or the
source layer could be rate-controlling, depending on
the drug and materials ~elected. Alternatively, the
drug and/or vehicle microenaapsul~ted to provide
controllad release could be deposited on the source
layer prior to lamination, i.e., instead of deposition
of the drug in "liquid form" as previously defined.
Typically, over the effect~ve li~etime of the device,
the drug is presented to the skin at a rate in excess
of the rate ~hat the treated area of skin is able to
absorb. It will be appreciated, however, that
depending upon the particular drug (and enhancer when
one is needed) that is being administered, that it may
be nece~sary or desirable to include an element in the
device that will control the release rate of the drug
and/or the enhancer. Such elements are known in the
art. The most common is a polymer membrane having
appropriate drug/enhancer permeability properties
W094/04109 ~ ~ -16- PCT/US92/1067
interposed between the source layer and the contact
adhesive lay~r.
The term "drug" as used to describe the
principal a~tive ingredient of the device intends a
biologically active compo~nd or mixture of compounds
that has a therapeutic, prophylactic or other
beneficial pharmacological and/or physiological effect
on the wearer of the device. Examples of types of
drugs that may be used in the inventive device are
anti-inflammatory drugs, analgesics, antiarthriti~
drugs, tranquilizers, narcotic antagonistis,
antiparkinsonism agents, anticancer drugs,
immunosuppression agents, antiviral agents, antibiotic
agents, appetite suppressants, antiemetics,
anticholinergics, antihistaminics, antimigraine
agents, coronary, cerebral or peripheral vasodilators,
anti-anginals, e.g., calcium channel blockers,
hormonal agents, contraceptive agents, antithrombotic
agents, diuretics, antihypertensive agents,
cardiovascular drugs, chemical dependency drugs, and
tha like. The appropriate drugs of such types are
capable of permeating through the ~kin either
inherently or by virtue of treatment of the skin with
a percutaneous absorption enhancer.
Because the size of the device is limited
for patient acc~ptance reasons, the preferred drugs
are those which are effective at low concentration in
the blood stream. Examples of specific drugs are
steroids such as estradiol, progesterone,
norethindrone, norethindrone acetate, levonorgestrel,
. ethynodiol diacetate, norgestamate, gestadene,
desogestrel, 3-keto desogestrel, demegestone,
promegestrone, testosterone, hydrocortisone, and their
esters~ nitro compounds such as amyl nitrate,
nîtroglycerine and isosorbide nitrates; amine
compounds such as nicotine, chlorpheniramine,
terfenadine and triprolidine; oxicam derivatives such
094/04109 21 ~2 8 71 PCT/US92/1067
-17~
a piroxicam; mucopolysaccharidases such as
thio~ucase; opioids such as buprenorphine, fentanyl
and fentanyl derivatives or analogs, naloxone,
codeine, dihydroergotamine, pizotiline, slabutamol and
terbutaline; prostaglandins such as those in the PGA,
PGB, PGE and PGF series, e~g., misoprostol and
enprostil, omeprazole, imipra~ine; benzamides such as
metoclopramine and scopolamine; peptides such as
growth releasing factor, growth factors tEGF, TGF,
PDGF and the like), and ~omatostatin; clonidine;
dihydropyridines such as nifedipine/ verapamil,
diltiazem, ephedrine, propanolol, metoprolol and
spironolactone; thiazide~ such as hydrochlorothiazide
and flunarizine; cydononimines such as molsidomine;
sulfated polysacchari~es such as heparin fractions;
and the salts of such compounds with pharmaceutically
acceptable acids or bases, as the case may be.
It should be noted that the present method
and device are suitable for use with volatile drugs
and excipients, as no heat treatment step is involved
or necessary. Thus, the present invention is useful
with drugs such as nicotine, nitr~glycerin, amyl
nitrate, and scopolamine. The present device is also
useful with drugs such as fentanyl, which will
: 25 typically be incorporated into the patch using
nona~ueous, volatile vehicles and/or enhancers which,
: . becau~e they volatilize during heat treatment, have
proven difficult to incorporate into a transdermal
delivery device by conventional means.
Since the inherent permeability of the skin
to some drugs, such as steroids, is too low to permit
therapeutic levels of such drugs to pass through a
reasonably sized area of unbroken skin, it is
necessary to coadminister a percutaneous absorption
35` enhancer with such drugs. Accordingly, in such a
case, a percutaneous absorption enhancer will be
present in the device along with the drug, i.e., will
:
WO94/04109 4~ 18- PCT/US9?t1067
be initially deposited on source layer 13 together
with the drug. In addition to affecting the
permeability of the skin to the drug, the enhancer may
also increase the diffusivity of the drug in the
souxce layer and in the adhesive layers, thus
increasing the permeability of th~ device as a whole
to the drug. ~ny number of the many percutaneous
absorption e~hancers known in the art may be used in
conjunction with the present invention. For examples
of suitable enhancers, ~ee U.S. Patents Nos.
3,996,93~; 4,460,372; 4,552,~72; 4,557,934 and
4,568,343 and the patents referenced therein.
When the inventive device is used to
administer drugs to which the permeability of the skin
1~ is inherently too low to allow passage of therapeutic
amounts of the drug, enhancers will be included in the
device, "printed" onto the source layer along with the
drug or incorporated into one or both of the adhesive
layers. Correlatively, when the device is used to
admini~ter a drug to which the permeability of t~e
skin is inherently ~ufficient to pass therapeutic
amounts, it is not necessary to 30administer an
enhancer. Thus, in general terms, the inclusion of an
enhancer in the device is optional, depending on the
particular drug that is being administered.
Processes of Nakina the Transdermal_Device~
The device of the present invention is
readily manufactured as follows. As illustrated by
Figure 2, anchor adhesive 12 may be roll-coated onto a
backing layer 11 of a commercially available film at a
coating weight in the range of about 0.2 ~g/cm2 to 15
mg/cm2, more preferably in the range of about 1 mgJcm2
to 10 mg/cm2. Similarly, the pressure-sensitive skin
contact adhesive 14 may be coated onto release liner
lS at a coating weight in the range of 0.2 mglcm2 to 15
mg/cm2, more preferably 1 mg/cm2 to 10 mg/cm2. The
; ~- WO94/04109 -i9' ~ ~ 2871 PCT/US92/10672
source layer 13 is then deposited onto either contact
adhesive layer 14 or onto anchor adhesive 12,
preferably onto the contact adhesive. The selected
drug in liquid form (optionally admixed with
enhancer), is then printed onto the exposed surface of
source layer 13 using conventional printing
techniques. In n alternative embodiment of the
invention, the drug is initially contained in one or
both of the anchor and contact adhesive layers (e.g.,
by incsrporation of the drug into the layers prior to
lamination), and enhancer and/or vehi~le is printed
onto the source layer.
EXAMPLES
The following examples are put forth so as
to provide those of ordinary skill in the art with a
complete disclosure and description of how to make the
transdermal drug delivery devices of the invention and
are not intended to limit the scope of what the
inventors regard as their invention. Efforts have
been made to ensure ac~uracy with respect to numbers
used ~e.g., amounts, temperature,~concentrations,
etc.) but some experimental errors and deviations
should be accounted for. Unless indicated otherwise,
part~ are parts by weight, molecular weight is weight
average molecular weight, concentrations are
: milligrams per square centimeter of device,
temperature is in degrees centigrade and pressure is
: at or near atmospheric.
Example 1
A bandage for delivering nicotine`
transdermally for approximately 16 hours was prepared
as follows. The anchor adhesive was coated onto a
facestock of about 0.0015" flexible polyester laminate
at a coating weight of 6.5 mg/cm2. The composition of
the anchor adhesive was approximately 1:5:2
W094/04109 ' 4~ 20- PCT/US92/1067~'
polyisobutylene, m.w. l. 2 X 106/ polyisobutylene, m.w.
35,000/ polybutene blend, m.w. 2300. The pressure- ¦
sensitive contact adhesive having the same composition
as the anchor adhesive layer was coated, also at
6.5 mg/cm2, onto a 0.003'~ siliconized polyester relea~e
liner. ~he source layer, a 100% non-woven polyester
fabric at 4.2 mg/cm2, was then laminated to the anchor
adhesive. Nicotine free base was deposited, neat,
onto the source layer using a fine mist airbrush in a
uniform pattern, at about 0.9 mg/cm2. The contact
adhesive/release liner composite was then laminated
onto the exposed surfac2 of the drug reservoir,
forming a laminate of the final de~ice as shown in
Figure l. Individual devices were die cut from the
laminated product. The resulting in vitro skin
permeation over 13 hours is shown in Figure 3.
Example 2
- Example l was repeated, except that prior to
2~ deposition the nicotine was diluted with freon to a
concentration of lO wt.% to facilitate dispersal in
the source layer. After depositi~n, the freon is
removed by blowing warm air (about 30C) over the
laminate for about 2 minutes.
Exam~le 3
A bandage for delivering nitroglycerine was
made in a manner similar t~ that described in Example
l for the nicotine bandage. The nitroglycerine. was
deposited onto the source layer as a 10% solution in
ethanol using polyethylene glycol monolaurate (PGML)
as carrier. The ethanol was allowed to evaporate and
the final laminate was prepared as described in
Example l.
~ 2 ~
WO94/0410g ! `~ ~ PCT/US92/10672
21;-i
Example 4
A transdermal device for delivering nicotine
monoacetate transdermally for approximately 16 hours
was prepared as follows. A first subassembly PIB
adhesi~e was coated onto a facestock of a 12.5 micron
flexible polyester film at a coating weight of 4.0
mg/~m2. The ~IB adhesive was coated, also at 4.0
mg/cm2, onto a 0.003" siliconized polysster release
liner to pro~ide a second subassembly. A 100%
polyester non-woven fabric at 35 g/yd2 was then
laminated to the PIB adhesive of the first assembly.
Nicotina monoacetate was deposited, neat, onto thle
~ fabric in a uniform pattern, at about l.l mg/cm2. The
second subassembly composite was then laminated o:nto
the exposed surface of the drug-contaîning fabric
forming a five-layer laminate. Individual devices
were die cut from the laminated composite.
The instant invention has been shown and
described herein in what is considered to be the most
2Q practi al, and preferred embodiments. It is
recognized, however, that departu~es may be made
therefrom which are within the scope of the invention,
and that obvious modifications will occur to one
: skilled in the art upon reading this disclosure.
i