Note: Descriptions are shown in the official language in which they were submitted.
214~2
WO 94/07470 PCr/US93/06 14,
5 ARTICLE CONTAINING A C~RE AND A COATING HAVING A NON CONSTANT THICKNESS
BACKGROUND OF THE INVENTION
This invention relates to articles for the sustained release of pharmAceuticAl
- co"~rosilions contained therein. More particularly, it relates to articles comprising a
core, containing said pharrnAceuticAls, at least partially coated with a coating of non-
10 uniform thickness and processes for preparing such articles.
Col lt,.,llecl release of a medicine or drug is important for several reasons. tn the
first place it serves to provide the body with medication over a long time and thereby
eliminates the need for ingesting standard dosAge forms, e.g., tablets, co~",~.,isi, l9 said
medications, at frequent intervals. The treatment of any ~iseAse with a medicine15 requires a fairly cor,~l~r,l high blood titre of the medicine. lf the medicine is
metA~o' ~ed or otherwise eliminated quickly from the body it would be necessA~y to
swallow an ordinary tablet quite often to maintain the desired blood level.
Some medicines have such a narrow therapeutic ratio that slightly more than is
necessAry, to achieve a therapeutic effect, will cause adverse toxic symptoms. lf an
20 ordinary tablet is taken, the rapid release of its medicai"erl conter,l may cause such
a high blood level thereof that undesirable side reactiolls will occur.
Other medicines are irritating to the alimentary mucosa and their rapid release
from ordinary dosage forms may cause damage to the alimentary organs. lt is,
therefore, desi.able that dosage forms release such me~icAtions at a very low,
25 pr~r~r~bly zero, initial rate and then at an exponentially inc~ased rate upon reaching
the stomach and/or i"te~li, .e as required.
Dosage forms have been prepared in the past which will control the release of
the contained medicine but they have not been entirely s~ r-~clo,y. Some of themhave been expensive to make either because of the ~ ,ensive ingredients or the
30 co",~ qd ~spparalus or processes to make them or they have been too large to
ingest becA~ ~se of the ~ ,eces59-- y additives to obtain the delayed release. Other tablets
have been u"snli~r~toly bec~se they have lacked a uniform release time although
made in exactly the same way. U.S. Patents 3,538,214 and 4,060,598 refer to a tablet
compi isi"g a coating compiisi, 9 a plastic rnalerial, insoluble in gastro-i"le~li"al fluids,
35 and a composition which is removable from the coating upon !contact with either the
stomach or illte~li"al fluids to form a dialytic memblane through which the me~icAtion
slowly diffuses.
2 1 ~ 3 2 ; r ~ r ~ ~
W094/07470 PCr/US93/06~S~, e
-2-
United States Patent 4,173,626 refers to a dosage form comprising a capsule
containing a mixture comprising pellets of a medication which are uncoated and pellets
of said medication coated with a slow dissolving material.
United States Patents 3,1 19,742 and 3,492,397 refer to dosage forms comprising
a mixture of groups of coated pellets of a medicament wherein said coating comprises
a slow dissolving material, with each group comprising a constant thickness of coating
which differs from that of the other groups. Alternatively, the '742 patent indicates that
each group may be coated with coating compositions of differing solubility rates.
The articles of the present invention employ inexpensive formulation materials
and achieve controlled release of the medicine. These articles can be made of
relatively small size. Furthermore, the total elapsed drug release time can be varied
and established by the practice of this invention.
SUMMARY OF THE INVENTION
The present invention provides articles which overcome the above
disadvantages. The articles may be so prepared that, by judicious choice of coating
material, erosion of the coating in the alimentary canal may be limited to a small, if any,
amount of erosion. The major portion of the medication will be released in the gastro-
intestinal tract and, depending on the choice of coating material, said release can be
limited to the stomach or intestine.
In accordance with the invention, a core, e.g., a tablet, or capsule, containingthe drug is made in a conventional manner and to at least a portion of it is applied an
erodible coating composition, in a nonuniform thickness, which will slowly be removed
from the surface of the core. This slow erosion action will occur because gastro-
intestinal fluids will slowly dissolve or disintegrate the coating to reach the drug in the
core.
As the core is coated in a nonuniform manner the various portions of the surfaceof said core are contacted by said gastrointestinal fluids at different times. The core
releases its pharmaceutical content at a rate which is proportional to the exposed
surface area and concentration of medication in the core. As the erosion of the coating
proceeds the exposed surface area of the core increases. During the same time, while
the exposed core surface area has been increasing the concentration of the drug, in
the core, has been decreasing. The overall effect of increasing exposed core surface
area and decreasing drug concentration in the core is to maintain an approximately
2 1 ~
,VO 94/07470 PCr/US93/06447
constant rate of drug release from the core. In the case of a core exhibiting constant
drug release, the increasing exposure of the core of the article causes the drug to be
released at an increasing rate.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a perspective view of a first embodiment of the invention.
Figure 2 is a front sectional view of the first embodiment along line 2-2 of Figure 1.
Figure 3 is a side sectional view of the first embodiment along line 3-3 of Figure 1.
Figure 4 is a front sectional view of a second embodiment of the invention.
Figure 5 is a right side sectional view of the second embodiment along line 5-5 of
Figure 4.
Figure 6 is a left side sectional view of the second embodiment along line 6-6 of Figure
4.
Figure 7 is a front sectional view of a third embodiment of the invention.
Figure 8 is a right side sectional view of the third embodiment along line 8-8 of Figure
7.
Figure 9 is a top sectional view of the third embodiment along line 9-9 of Figure 8.
Figure 10 is a front sectional view of a fourth embodiment of the invention.
Figure 11 is a side sectional view of the fourth embodiment along line 11-11 of Figure
10.
DETAILED DESCRIPTION OF THE INVENTION
The invention includes articles comprising coatings having shapes selected from
spheroids, ellipsoids, cylinders and rectangular prisms in combination with cores having
spheroidal, ellipsoidal, cylindrical or rectangular prismatic shapes with the proviso that
if the coating and core are both spheroidal or both are cubic they are not concentric.
The invention may be best illustrated with reference to the figures.
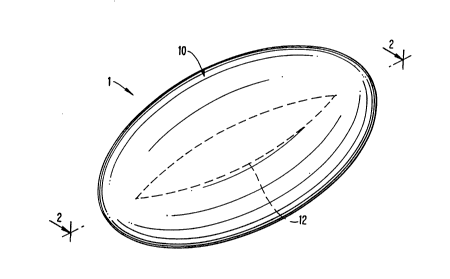
A first embodiment of the invention, designated by the numeral 1, is illustratedin Figures 1-3.
As shown in Figure 2, the article comprises an ellipsoidal core 11, comprising
a hollow cavity, containing the medication, surrounded by a wall 12 and an ellipsoidal
coating 10. The core and coating comprise major axes b - b and a - a and minor axes
b' - b' and a' - a', respectively. Other points on the surface of the core and coating are
indicated by c and c', respectively.
2142982 ~ ~
W O 94/07470 PC~r/US93/06447 -
After the device has been in contact with the eroding fluid for a period of timet1 the coating will have eroded, uniformly, to points b1, c1, and al. At a later time t2 the
coating will have eroded completely along major axis b - b thereby exposing the points
b' on the core and the surface of the core begins to dissolve thereby releasing the
medication into the contacting fluid. Erosion of the coating will continue until, at a time
t3, the surface of the core between points b' and points c' will have become exposed
thereby releasing additional medication into the eroding fluid. At that time a portion of
the coating between c' and a3, having a thickness a' - a3 will remain. During erosion
of the coating, after the first release of medication, the exposed surface area of, and
thereby the flux from, the core will increase. However, since the concentration of
medication in the core will be decreasing, during that time, the release rate will be
essentially constant until such a time when too little of the core wall remains and the
balance of the medication is immediately released into the fluid.
As the coating erodes it takes the shape of an ellipsoid truncated at both ends.If the core wall comprises a composition soluble in the eroding fluid, the wall of the
core will completely dissolve, after a time, and when said wall has dissolved and the
area of the openings of the the truncated ellipsoidal core is sufficiently large the balance
of contained medication will be released quickly in an uncontrolled manner.
If, however, the core wall is insoluble (e.g., an insoluble, osmotically permeable
wall) or the core is a matrix tablet the structure of the core will maintain itself for a
longer time, and, in the case of the osmotic tablet, it will not lose its form.
In Figures 1-3, the article illustrated is one wherein the thickness of the coating
along the minor axis is less than, and continually increases to, that along the minor
axis. It is to be understood that the reverse is also within the scope of the invention,
i.e., where the thickness along the major axis is greater than along the minor axis.
Furthermore, although the shape of the device along the minor axis, as shown in
Figures 1-3, is circular it can also be ellipsoidal, rectangular, square, etc.
The composition of the coating will be chosen, as required, to be erodible by
any one, or more, of the fluids in the esophagus, stomach and intestine.
If the coating is erodible by the esophageal fluids and it is desired that the
contained medication not be released except in the stomach or tntestine, the minimum
coating thickness will be chosen so that sufficient coating remains on the core to
prevent release of the medication until the article passes through the esophagus. The
t -
~WO 94/07470 2 1 4 ~ ~ 8 2 PCI/US93/06447
article then passes into the stomach where the surface coating begins, or continues if
erosion had commenced in the esophagus, to erode if the coating is sensitive to the
acidic stomach fluids. If the coating is, instead, sensitive to the basic intestinal fluids
the article will pass from the stomach into the intestine where erosion will continue or
commence. In some cases, erosion of the coating with concomitant release of the
medication will occur in the stomach and intestine.
Figures 4-6 illustrate a second embodiment of the invention, comprising a
medication containing hollow cylindrical core 13 comprising a water insoluble, water
permeable plastic wall 17, partially covered, at its end portions, by a cylindrical coating
having non-uniform thicknesses. As shown in Figure 4, the central portion 16 of the
wall 17 of the core 13 is uncoated. The left portion of the coating comprises a
longitudinal section 14, adjacent the uncoated portion 16 of the core wall 17, and a
transverse end section 20 of unequal thicknesses. The right portion of the coating
comprises atransverse end section 21 and a longitudinal section 15 disposed between
the uncoated section 16 of the core surface and said end sections.
In Figures 4-6 the thicknesses of all of the coating sections 14, 15, 20 and 21
are shown to be unequal. The thicknesses of the coating sections increase according
to the order: is 15 < 14 < 21 < 20. In the practice of the invention it is only necessary
that two of the coating sections 14, 15, 20 and 21 be of unequal thickness.
After being placed in the eroding fluid the medication is first released throughthe uncoated portion 16 of the core wall 17. After a period of time, the coating section
15 will have eroded coimpletely thereby exposing additional surface on the core. The
resultant increased surface area of the exposed portion of the core will result in an
increased drug release rate. After an additional period of time, the coating section 14
will have completely eroded, the total exposed surface area will have again increased
and the release rate of the medication will also have increased again. The above will
continue until coating section 21 and then section 20 will have completely eroded. With
proper choice of coating composition and thicknesses, core wall composition and
medication concentration various combinations of release rate and time for complete
release can be achieved.
A first aspect of a third embodiment of the invention~comprising a circular
cylindrical core 22 and a rectangular prismatic matrix coating 27 is illustrated in Figures
7-9. As shown in Figures 8-9 all six of the coating sections, i.e., sections 23, 24, 25,
21~2~8~
WO 94/07470 PCr/US93/06447
26, 27 and 28, are of unequal thicknesses. This embodiment functions in the samemanner as the first and second embodiments, i.e., as one section of coating is eroded
the underiying portion of the core is exposed to the eroding fluid thereby permitting the
medication to be released with concomitant reduction of the concentration of themedication in the tablet. As a result of the increasing exposed surface area anddecreasing medication concentration in the core, the medication is released into the
fluid at a sustained and approximately constant rate. Thus, by proper choice of coating
composition, coating thicknesses, matrix binder composition and medication
concentration in the tablet articles having differing drug release rates and times for total
medication release may be prepared.
In a second aspect of the third embodiment, illustrated in Figures 10 and 11, the
coating 29 comprises an ellipsoid and the core 30 a cylinder. This article functions in
a similar manner to that of the first aspect of this embodiment.
The core can be of any type known in the art including soluble capsules, such
as gelatin, porous capsules made of materials such as cellulose acetate and matrix
tablets. The specific core type will be selected by the user in accordance with his
requirements, e.g., compatibility with the medication.
The thickness and composition of the coating will be so chosen that erosion willoccur, as required, in the esophagus, stomach or intestine or any combination thereof.
The coating composition comprises substances which are selectively, but readily
soluble in, or disintegratable by, the stomach fluids or intestinal fluids. If it is intended
that the medicament be released in the stomach, the coating composition must be one
that will be removed by the acid fluids of the stomach. On the other hand, if it is
intended that the medicament be released in the intestines (i.e. the article is to pass
through the stomach substantially intact) the coating composition must be acid
resistant and one that will be removed under the alkaline condition of the intestines.
For removal in the stomach, suitable coating compositions are polyvinyl
pyrrolidone and solid polyethylene glycols, poly (ortho ester), poly (~-caprolactone),
poly (acrylic acid), poly (vinyl alcohol), hydroxypropylmethyl cellulose, dextran, gelatin,
polyacrylamide, polysaccharides, gum arabic, polyphosphates, Eudragit (trademark)
E100 (a copolymer of dimethylaminoethyl methacrylate and methacrylic acid ester), a
copolymer of glutamic acid and ethyl glutamate, polyglycolic acid; polylactic acid, a
copolymer of lactide and ~-caprolactone and a terpolymer of lactide, glycolide and
21 ~2~82
94/07470 PCr/US93/06447
~- caprolactone. For removal in the intestines, suitable coating compositions include
cellulose acetate phthalate, hydroxypropylmethylcellulose acetate succinate,
hydroxypropyl-methylcellulose phthalate and Eudragit (trademark) L 100 (a copolymer
of methacrylic acid and methacrylic acid ester).
The desired rate of erosion may sometimes be achieved by a combination of
materials from both groups.
The coating composition is applied to the tablet or capsule core, the thickness
being dependent upon the desired rate of release of the medication from the tablet.
In practice, the range of thickness of the coating may be varied in accordance with the
medicament employed and the amount of control of release desired by the practitioner
hereof.
To apply the coating composition, conventional tablet coating practices are
used. They include use of a tumbling barrel, for the dose forms, into which the coating
composition is sprayed or fluidized column techniques in which the coating
composition is sprayed upwardly through the bed of dose forms.
Heretofore, it has been the practice to apply an enteric type coating to
pharmaceutical tablets to insure non-lesion inducing passage through the stomach.
This enteric-type coating resists disintegration by stomach fluids but is fully
disintegrated or dissolved by the intestinal fluids during its passage through the
intestine. The present invention normally obviates the necessity for any such enteric-
type coating. That is because the coating only dissolves slowly, if at all, in the
stomach fluids and prevents or delays release of the medicinal agent in the stomach
in accordance with the user's requirements. It allows slow release of the medicinal
agent from the tablet into either the stomach or the intestines, depending on the user's
needs.
The coating of the invention not only restricts the access of the gastro-intestinal
fluids to the medicinal agent of the matrix core, but it moreover serves to position or
space the medicinal agent itself away from the gastro-intestinal mucosa so that a large
concentration thereof is not permitted to reach a comparatively small area of the gastro-
intestinal mucosa.
In the practice of this invention, it is possible to provide-a final overcoating to
improve the appearance, taste or stability of the tablet. They may contain sugar, or a
film former in combination with dyes or pigments, or even other medicaments. This
21~2~82
W O 94/07470 .~ . PC~r/US93/06447
Iatter medicament may, for example, be one which is to be administered with the drug
in the tablet core but which should not be in contact with each other in the complete
tablet. This may be because of the incompatibility of the two or because it is desired
that the medicine in the outer coating be released rapidly and that the drug in the core
5 be released slowly.
The following examples are illustrative of the present invention and are not to
be construed as limiting.
EXAMPLE 1
SOFT GELATIN CAPSULE WITH A NON-UNIFORM ERODIBLE COATING
Soft gelatin capsules (~6 oblong) of Procardia~ (10 mg nifedipine) were coated
with hydroxypropylmethylcellulose acetate succinate in a Hi-Coater (trademark). The
coating level was thinner at the two ends of the capsule as illustrated in Figure 1. The
coated and uncoated capsules were placed in a USP dissolution apparatus containing
simulated intestinal fluid (U.S.P. XXII test solution adjusted to pH 7.0) at 37C and
15 stirred at 50 rpm. Aliquots of the test fluid were removed, from the apparatus,
periodically and assayed for released medication at 225 nm in a UV spectrophotometer.
The in vitro release of nifedipine at pH 7.0 was monitored at 225 nm using a UV
spectrophotometer. The erosion of the capsules in water started at the ends of the
20 capsule where the coating was thinner. The in vitro release profile as shown in Figure
5 was fairly linear over 6 hours. For uncoated Procardia$ capsules, nifedipine was
released at pH 7.0 in 2 hours.
As shown in Table I the coated capsules released the medication at an
approximately constant rate over a period of about six hours. On the other hand, more
25 than half of the contained medication was released from the uncoated capsules within
the first hour and the balance within the second hour.
21~2~?
WO 94/07470 PCI/US93/0644
TABLE I
% NIFEDIPINE % NIFEDIPINE
RELEASED FROM RELEASED FROM
TIME (HOURS) UNCOATED CAPSULE COATED CAPSULE
0 0.0 0.0
0.5 4.6 4.6
1.0 . 61 4.6
2.0 100 17.8
4.0 1 00 50.8
6.0 100 100
8.0 100 100
EXAMPLE 2
OSMOTIC CAPSULE WITH A NON-UNIFORM ERODIBLE COATING
Pseudoephedrine HCI (210 mg) was placed in a capsule made of a porous
cellulose acetate membrane. The capsule was coated with 10 mg Eudragit (trademark)
L100 at one end and 20 mg at the other, leaving the center of the capsule uncoated.
This is illustrated in Figure 2.
The coated and uncoated capsules were placed in a USP dissolution apparatus
containing simulated gastric fluid (U.S.P. XXII test solution, without enzyme, adjusted
to pH 2.0) at 37C and stirred at 50 rpm. Aliquots of the test fluid were removed, from
the apparatus, periodically and assayed for released medication by HPLC using a Nova
- Pak (trademark) C 18 column at a 254 nm detection wavelength.
Table ll shows that very little of the medication was released by the coated
capsules during the first three hours after exposure to the simulated gastric fluid. About
one fourth of the medication was released during the next hour after which the
medication was released at a sustained rate whereby only about 80% of the contained
medication was released after a total exposure time of about fifteen hours. On the other
hand, the uncoated capsules rapidly released their contained medication with about a
third of the medication being released in the first two hours aftQr exposure to the test
fluid. The uncoated capsules then released the balance of their contained medication
at a sustained rate which was greater than that for the coated capsules. As a
2142~82 .
WO 94/07470 PCI/US93/0644
-10-
consequence, most of the medication had been released from the uncoated capsules
in about ten hours.
TABLE ll
% PSEUDOEPHEDRINE % PSEUDOEPHEDRINE
HCI RELEASED FROM HCI RELEASED FROM
UNCOATED OSMOTIC COATED OSMOTIC
TIME (HOURS) CAPSULE CAPSULE
O O O
11.3
2 35.0 1.6
3 51.1 6.2
4 61.2 27.8
72.7 36.1
6 80.1 45.5
7 88.8 51.9
8 91.4 58.3
9 92.9 63.0
94.0 66.6
11 70.4
20 12 73.1
13 75.5
14 77.2
78.7
~, 2 1 4 2 ~ ~ 2 PCr/US93/06447
EXAMPLE 3
MATRIX TABLET WITH A NON-UNIFORM ERODIBLE COATING
Matrix tablets comprising flat faced circular discs of 5/8 inch diameter and 1/8inch thickness were prepared comprislng the following composition:
Inqredients mq/tablet
Acrylic acid copolymer 330
Lactose 100
Pseudoephedrine HCI 120
Magnesium Stearate 11
Total 561
The tablet was annealed at 110C for 10 minutes to become nondisintegrating
in water at pH 2Ø The tablet was coated with an erodible polymer, Eudragit
(trademark) L30D (a copolymer of methacrylic acid and methacrylic acid esters), at
different levels on each side. For example, 6.9 mg and 13.8 mg on flat surfaces of the
15 tablet and 1.9 mg on the edge side of the tablet. This is illustrated in Figure 3. The
tablet was placed in stirred water (pH 2.0) at 37C. The in vitro release profile as
shown in Figure 7 was fairly linear over 14 hours.
The coated and uncoated tablets were placed in the a USP dissolution
apparatus containing simulated gastric fluid (U.S.P. X~(ll test solution, absent enzyme,
20 adjusted to pH 2.0) at 37C and stirred at 50 rpm. Aliquots of the test fluid were
removed, from the apparatus, periodically and assayed for released medication byHPLC using a Nova - Pak C 18 column at a 254 nm detection wavelength.
Table lll shows that very little of the medication was released by the coated
tablets within the first hour after exposure to the simulated gastric fluid whereas about
25 one third of the medication will have been released from the uncoated tablets during
that time. The coated tablets then continued to release the medication at a sustained,
approximately constant rate during the next fourteen hours. At that end of that time
only about 60% of the contained medication had been released. On the other hand,after the initial rapid release of medication the uncoated capsules had released the
30 medication at a sustained non-constant rate which was greater than that of the coated
tablets. As a consequence, about 86% of the medication origtnally contained in the
uncoated tablets was released within ten hours.
21~2~82
W O 94/07470 PC~r/US93/064~7
. . -12-
TABLE lll
% PSEUDOEPHEDRINE % PSEUDOEPHEDRINE
HCI RELEASED FROM HCI RELEASED FROM
TIME (HOURS) UNCOATED DISK COATED DISK SHAPED
SHAPED MATRIX MATRIX TABLET
TABLET
O O O
1 34.9 4.4
2 52.3 8.6
3 61.9 13.3
4 71.0 18.1
76.5 23.0
6 79.3 29.6
7 80.0 33.0
8 85.2 38.4
9 83.4 45.5
86.1 45.5
11 48.9
12 51.5
13 54.3
14 56.9
59.7
EXAMPLE 4
MATRIX TABLET WITH A NON-UNIFORM ERODIBLE COATING
The tablet composition of Example 3 was compressed into oblong shaped
tablets. These tablets were then annealed at 100C for 10 minutes to become non-
disintegrating in water at pH 2Ø The tablets were then coated with 2% (w/w) Eudragit
(trademark) L100, in a Hi Coater (trademark). Because of the shape of the tablets, a
coating of non-uniform thickness was obtained as illustrated in Figure 4. The tablet was
placed in stirred water (pH 2.0) at 37C. The in vitro release profile as shown in Figure
8 was fairly linear over 12 hours.
WO 94/07470 2 1 ~ 2 9 8 2 PCr/US93/06447
-13-
The coated tablets and their controls were treated as in Example 3. As shown
in Table IV, after about one hour of exposure to the test fluid the coated tablets
released their contained medication at a sustained, approximately constant rate with
only about 64% of the medication being released in at about twelve hours. On the other
5 hand, the uncoated tablets released about one-third of their contained medication within
the first hour of exposure. The balance of the contained medication was released at a
slower but non-constant rate with almost all of the medication having been released
within twelve hours.
TABLE IV
% PSEUDOEPHEDRINE % PSEUDOEPHEDRINE
HCI RELEASED FROM HCI RELEASED FROM
TIME (HOURS) UNCOATED OBLONG COATED OBLONG
SHAPED MATRIX SHAPED MATRIX
TABLET TABLET
O O O
30.1 2.1
2 47.5 9.8
3 58.6 17.1
4 67.4 25.0
75.3 31.7
6 79.9 37.8
7 86.2 43.7
8 89.6 48.8
9 92.5 53.3
93.3 56.2
11 95.3 60.3
25 12 96.4 63.8
Although specific forms and types of articles have been illustrated it is to be
understood that combinations of all types and forms known to the art may be used in
preparing the articles of the invention.
Furthermore, it is to be understood that the above proposed theory of operation
of the devices of the invention is not a part of the invention.