Note: Descriptions are shown in the official language in which they were submitted.
~ 94/08059 PCT/US92/08680
2t44772
REFRACTORY METAL SWAY COMPOSITION AND METHOD OF MAKING SAME
BACKGROUND OF THE INVENTION
This patent relates to a process to convert slivers
and fines (referred to as "SWARF" in the industry) from
refractory metal (titanium and zirconium metal) grinding
operations into a consolidated, safe to handle, raw
material suitable for aluminum, magnesium and iron
refractory metal alloys and master alloys, such as
aluminum-titanium alloys, magnesium-zirconium alloys,
aluminum-zirconium alloys, iron-titanium alloys, iron-
zirconium all~ys, aluminum-titanium-boron alloys, and the
like. At present, most refractory metal products are
produced from large ingots, which involve various hot
forging and rolling operations. Whenever refractory metals
are heated above about 700C. in air, refractory metal
oxides and nitrides are formed with large heat release
varying in thickness depending on both the temperature and
time. Most of the oxide is removed by mechanical means,
usually sand or bead blasting. However, some of the oxide
is in the form of pits which projects more deeply than
average into the base metal and is not removed by these
operations. The pits are usually removed by belt grinding
processes in which a silicon carbide, aliminum-zirconium
oxide, or other hard grit, typically about 60 mesh, bonded
to a belt is moved over the surface of the metal, removing
a mil to several mils of the surface per pass in the form
of small curved slivers of the refractory metal. The belt
W094/08059 PCT/US92/08680
21~772 2
grinding machine gouges the slivers of metal with each of
the individual grains of grit on the belt. These fine
slivers of refractory metal in bulk are called "SWARF" in
the industry. The amount of SWARF generated is perhaps on
the order of one percent of the weight of the metal piece
being processed, depending on the thickness of refractory
metal being ground. Belt grinding is also used to obtain
flat surfaces on refractory metal plates and slabs and to
obtain rounded surfaces on rods an other round objects.
Refractory metal particles are also produced by other
abrasive operations, such as grinding with abrasive wheels
or stone grinding using copious coolant. For purposes of
this invention, these refractory metal particles are
suitable for use in the present invention and will be
understood to be included within the term "SWARF". The
refractory metal particles produced from stone or abrasive
wheel grinding is typically sent to a settling tank with
the coolant and allowed to settle to the bottom of the
tank. The coolant is recycled.
At the present time, SWARF is considered a waste
product and is disposed of by burning in the open air at a
remote site. SWARF has a very low ignition point and is
highly pyrophoric; it combusts suddenly and violently with
the rapidity and brightness of a photographic flashbulb to
produce a very hazardous fire. Accordingly, SWARF must be
maintained under water or under a non-oxidizing environment
to reduce reaction with N2 and 2 in the air. This burn
operation generates a thick white smoke (Tio2) or (ZrO2) and
is receiving increasing scrutiny from regulatory agencies.
The existing grinding operations are conducted with
water or a non-flammable grinding fluid (collectively
"grinding fluid"). The SWARF is removed from the grinder
mechanically along with the grinding fluid. The SWARF and
grinding fluid frequently go through an initial screening
wherein the coarse SWARF is separated from the bulk of the
~ 94/08059 PC~r/US92/08680
.~1 4~7~2
fines SWARF and the grinding fluid. The SWARF fines and
grinding fluid are sent to a filter wherein the SWARF fines
are separated from the grinding fluid. The grinding fluid
is recycled back to the grinding operation. The coarse
SWARF and fine SWARF are combined and disposed of by
burning.
The grinding fluid usually contains components to aid
in continuously cleaning the SWARF from grinding media and
fire retardants. These components also unfortunately add
chemical impurities to the SWARF material tending to
further limit its value.
The refractory metals titanium and zirconium are made
from relatively cheap and plentiful ores. The extraction,
purification and consolidation of these metals is, however,
expensive. Thus the metallic value in the SWARF after its
removal during grinding is sufficient to warrant recovery
if it can be reprocessed to eliminate its hazardous,
pyrophoric nature and cleaned of components detrimental to
potential end uses.
SUMMARY OF THE INVENTION
The present invention is directed to a process for
producing a refractory metal product prepared from
refractory metals SWARF by treatment of the SWARF from the
initial stages of its production.
In one embodiment of the present invention, clean
comminuted refractory metals SWARF particles are prepared
from refractory metal grinding operations, typically strip
refractory metal grinding operations, comprising the steps
of:
conducting the refractory metal grinding operation
with suf~icient grinding fluid or coolant to prevent the
produced SWARF refractory metal slivers from exceeding a
temperature of some 650C.;
W094/08059 PCT/US92/08680 -
7 7 2
comminuting the SWARF with adherent coolant from the
grinding operation to reduce the SWARF refractory metal
elongated slivers to refractory metal SWARF particles
having length to width aspect ratios substantially less
than the elongated slivers, the reduction is accompanied by
a reduction in bulk volume of the SWARF and the release of
adherent coolant;
separating the refractory metal SWARF particles from
the released coolant; and
washing the refractory metal SWARF particles with
clean water to yield clean refractory metal SWARF
particles.
Preferably before the SWARF with its adherent coolant
is comminuted, the SWARF from the grinding operation is
separated from the excess coolant. Preferably the SWARF
and coolant are first screened to remove the coarse SWARF
with some residual coolant from the fine SWARF and the
excess coolant. The excess coolant is filtered from the
fine SWARF to separate the fine SWARF with some adhered
coolant from the bulk of the coolant which is recycled back
into the grinding operation.
During the comminuting stage, the bulk volume of the
SWARF is dramatically reduced to less than 50% of its pre-
comminuted volume. The comminuting also releases a
substantial amount of the adherent coolant, that is,
coolant adhering to the SWARF refractory metal elongated
slivers. The released adherent coolant separated from the
SWARF particles is preferably recycled back to the grinding
operation.
The comminuting operation also selectively fractures
and removes hard refractory metal material as a fine dust
from the SWARF particles. The hard refractory metal
material is produced by the embrittlement of the refractory
metal by oxygen, nitrogen and carbon from surface reactions
on the refractory metal prior to or during the belt
~ 94/08059 PCT/US92/08680
5 ~14~772
grinding, stone grinding or wheel grinding. Preferably the
comminution is carried out with a detergent, such as Calgon
0 dishwashing detergent, to solubilize oil present on the
SWARF. The detergent also wets the hard refractory
5 material fine dust to disperse the fine dust and prevent it
from settling out in the comminution slurry.
If the SWARF contains iron particles, which
surprisingly is not that uncommon, the iron particles are
preferably removed from the SWARF. Conveniently, a
lO substantial portion of the iron particles may be removed
during the comminution operation by inserting one or more
magnets into the comminuting slurry. The iron particles
and any other magnetic particles, are attracted to the
magnets. The magnets are withdrawn from time to time and
15 wiped free of particles and then inserted back into the
slurry. The operation is repeated until the magnets
attract little, if any, particles.
Preferably, the washing of the SWARF particles
comprises at least a displacement wash with water followed
20 by filtration. The displacement wash removes a substantial
amount of the remaining adherent coolant which can be
recycled back to the grinding operation if the SWARF has
not been treated with a detergent. If the SWARF is
collected from different grinding operations, the coolants
25 may not be compatible and the mixed coolant will not be
recycled back. In a preferred embodiment, the filtered
SWARF particles following the displacement wash are subject
to at least three counter-current washes with water. The
SWARF particles become cleaner with each succeeding wash
30 and the washing fluid becomes progressively more
contaminated with each wash. The washing fluids from the
counter-current wash can be used as a displacement wash
fluid.
If an ultra clean SWARF is required, after the washing
35 step, the cleaned SWARF particles can be acid etched with
W094/08059 PCT/US92/08680 -
21~4 17~ 6
a mineral acid, such as 10% hydrochloric acid, for a
sufficient period to etch metallic impurities, such as iron
particles, and then washed with water to remove the acid
and any metal chloride salts. In another preferred
embodiment of the present invention, the clean SWARF
particles after the final wash are pressed at elevated
pressures into SWARF briquettes or other formed body shapes
(collectively "briquettes") which reduces the SWARF void
volume by at least a factor of two (2). The pressing
operation is carried out with the SWARF in an undried state
to minimize a reaction of the clean SWARF surface with
oxygen and/or nitrogen and to prevent fire. After the
pressing operation, the SWARF briquettes can be dried in a
vacuum dryer indirectly heated. After the briquettes are
dried, they are individually wrapped to minimize oxygen
profusion into the briquettes and to prevent loose SWARF
particles from dropping out of the briquette. Fully
wetted, in contrast to moist, briquettes can also be
wrapped and boxed to prevent the loss of the water in the
briquette.
The wrap or coat is not necessary for sintered
briquettes because the sintering operation welds the
particles together in a compact mass. With pressed
briquettes, some of the SWARF particles in the briquette
are not tightly compressed into the mass and can drop out
of the formed or compressed mass. Individual SWARF
particles, because of their high surface to volume ratio,
are very susceptible to ignition from an open flame or a
spark and have relatively highly reactive surfaces. The
wrap or coating prevents the particles from dropping out of
the briquette and forms a barrier between the briquette and
the open flame, spark and air.
Conveniently the briquettes can be made in one (1)
pound sizes and wrapped with aluminum foil, such as kitchen
grade aluminum foil which is about two (2) mils thick. The
~ 94/08059 PC~r/US92/08680
~1~4772
briquette can be wrapped in aluminum foil a single layer
thick. The edges of aluminum foil are preferably over
lapped twice, both at the common seam and at the ends to
minimize air profusion into the briquette. Heavier
aluminum foil can be used and the briquette can be wrapped
with double or triple layers of foil. The aluminum foil
protects the briquette from sparks - aluminum is a
sparkless metal - and forms a flame barrier for the
briquettes. The briquettes also can be wrapped in a
plastic film such as polyethylene film, a Saran brand type
film, Cellophane brand type of film or other film which can
be used to wrap the briquette to prevent the escape of
individual SWARF particles and form a flame and spark
barrier. Alternatively, the briquettes can be coated,
either dip coated or spray coated, with paraffin wax, non-
pigmented lacquer, non-pigmented shellac, non-pigmented
varnish, non-pigmented polyurethane and the like to keep
the loose SWARF particles within the briquette and to form
an air, flame and spark barrier. For use with aluminum
refractory metal master alloy melts, the aluminum foil
appears to offer the most advantages.
The clean SWARF particles after the final wash can be
disposed of as SWARF is presently disposed of, that is, by
burning. Preferably, however, the clean/concentrated
refractory metal SWARF particles are pressed in
conventional pressing equipment into SWARF briquettes or
other formed body shapes (collectively "briquettes") which
reduces the SWARF void volume by at least a factor of two.
The SWARF briquettes can then be sintered by heating the
briquettes to temperature between 800C. and 1100C. under
a vacuum or under an inert gas atmosphere, such as a helium
gas atmosphere or argon gas atmosphere, for a period of
time, such as one-half hour, sufficient to sinter the
refractory metal in the briquettes to form sintered SWARF
briquettes. Preferably the sintering is done at about
W094/08059 PCT/US92/08680 -
21~4 172 8
950C. The briquettes have far less void volume and far
less surface area than the clean concentrated refractory
metal particles. The sintered briquettes are not
pyrophoric, they will not burn in the presence of air. It
was earlier thought that compacted SWARF sliver briquettes,
as distinguished from compressed sintered SWARF briquettes,
had to be stored under water or stored in an inert
atmosphere or vacuum to prevent dangerous combustion.
It has now been found that compacted SWARF briquettes
can be stored if the briquettes are packaged as described
herein and the SWARF is dry (0.05% moisture or less) or
fully wetted. Moist, in contract to wetted, refractory
metal SWARF can react with water in the presence of air to
produce hydrogen and refractory metal oxide. Hydrogen can
vigorously react with oxygen and can initiate combustion of
the refractory metal SWARF.
In another preferred embodiment of the present
invention, the clean concentrated refractory metal SWARF
particles are processed into a pyrophoric safe refractory
metal/salt briquettes. The clean concentrated refractory
metal SWARF particles, in a moist state, are mulled with an
alkali metal halide salt type to produce a refractory
metal/salt mixture. Sufficient alkali metal halide salt
type is employed in the mixture to render the refractory
metal/salt briquette product pyrophoric safe.
The refractory metal/salt mixture is pressed into
refractory metal/salt briquettes; and the refractory
metal/salt mixture briquettes are dried to produce dried
refractory metal/salt mixture briquettes.
The clean refractory metal SWARF particles are mixed
with about 30% to about 100~ by weight of the metal with
the alkali metal halide salt types. Thus, the dried
refractory metal/salt mixture briquettes will comprise from
about 23% to about 50% by weight salt with the balance
being the refractory metal.
W~94/08059 PCT/US92/08680
9 21~772
The alkali metal halide salt type can be an alkali
metal refractory metal halide, such as sodium titanium
fluoride, potassium titanium fluoride, sodium zirconium
fluoride, potassium zirconium fluoride or the like. The
salt type can also be an alkali metal halide, such as
sodium fluoride, potassium fluoride, sodium chloride,
potassium chloride, and the like, or a mix of an alkali
metal refractory metal halide and alkali metal halide. In
addition, the alkali metal halide salt type can be a sodium
boron fluoride, potassium boron fluoride and the like.
Preferably the alkali metal halide salt type is an alkali
metal refractory metal fluoride salt wherein the refractory
metal is the same as the refractory metal in the master
alloy. For example, if the master alloy is to be an
aluminum-titanium alloy, the preferred salt type would be
an alkali metal titanium fluoride salt. If the master
alloy contains boron in addition, preferably the alkali
metal halide salt type will be a mixture of salts wherein
one of the salts will be an alkali metal boron fluoride
salt. The weight ratio of boron to the refractory metal in
the salt mixtures should be the same weight ratio of the
boron to refractory metal in the master alloy.
Both the refractory metal Ti and Zr particles and the
Ti and Zr salts in the refractory metal/salt briquettes
will report to the aluminum master alloy. The salt appears
to serve as a flux which aids in the dissolution of the
refractory metal particles into the aluminum, magnesium and
iron master alloy. When the master alloy contains boron,
the refractory metal in the Ti salts reacts with the boron
salt _o form TiB2 alloy which reports to the aluminum master
alloy. The TiB2 alloy has grain refining properties in
aluminum metal. The boron salt must react with a
refractory metal salt to produce a TiB2 alloy. The bulk
refractory metal has mass transfer problems in forming the
W094/08059 PCT/US92/08680
7 1 ~ l o
TiB2 phase; thus the TiB2 phase is formed with titanium and
boron salts.
Thus, the pyrophoric safe refractory metal/salt
briquettes can be utilized to furnish the master alloy with
refractory metal or refractory metal and boron, if boron is
present in the master alloy. It appears that the SWARF
refractory metal in the briquette functions as a scavenger
for the iron master alloy by consuming oxygen and nitrogen
present in the alloy.
The refractory metal/salt mixture briquettes are dried
so that the briquettes can be safely added to the master
alloy. If appreciable moisture is retained in the
briquettes, the moisture in the briquettes upon contact
with the hot, molten master alloy reacts violently with the
molten metal to form hydrogen which is hazardous.
BRIEF DESCRIPTION OF THE DRAWINGS
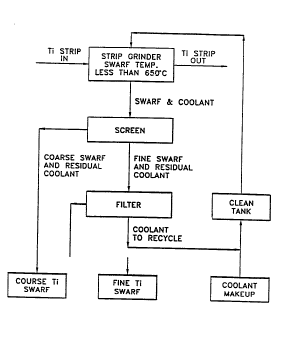
FIG. 1 is a block diagram showing a conventional
method of producing SWARF improved according to the present
invention;
FIG. 2 is a block diagram showing the improved method
of the present invention of producing clean SWARF
particles;
FIG. 3 is a flow sheet showing a process of the
present invention for treating SWARF;
FIG. 4 is a flow sheet showing the counter-current
washing step for the process of FIG.s 2, 3 and 5; and
FIG. 5 is a flow sheet showing an alternative
embodiment of the present invention for producing clean
SWARF particles.
~ 94/08059 PCT/US92/08680
~14~772
11
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The process of the present invention is applicable to
many different alloys of titanium and zirconium.
Refractory metals herein means titanium and zirconium metal
and/or alloys.
Referring to FIG. 1, a grinding belt having a flexible
fabric backing coated with silicon carbide or zirconium-
aluminum oxide grit, typically about 60 mesh, is used to
grind the surface of a refractory metal strip. SWARF can
also be produced when surfaces of refractory metal slabs or
plates are surface ground. The belt (not shown) is
typically two to four feet wide and is looped over two
rolls (not shown) approximately three inches in diameter,
one of which is powered to rotate the belt at high speed.
In operation the sheet, billet, or strip of refractory
metal is passed under the moving belt with an operator
controlling the pressure between the grinding media and the
metal. The point of contact between the grinding media and
the refractory metal surface is sprayed with grinding
fluid. The fluid is mostly water. Other components
include a water soluble oil, and other components such as
nitrates, phosphates, organic amines, etc. which aid in
keeping the grinding media clean, reducing the pyrophoric
nature of the SWARF, and reducing the surface tension of
the water.
The first step in the process of recovering SWARF,
according to the instant invention, is to prevent excessive
reaction of the SWARF with air in the grinding operation
itself, which would cause the formation of refractory metal
oxides and nitrides. The grinding operation can be a belt
grinding, abrasive wheel grinding or stone grinding
operation. Formation of the refractory metal oxides and
nitrides is due to reaction with air at elevated
temperatures experienced during the grinding operation.
The temperature at which reaction with oxygen and nitrogen
W 0 94/08059 PC~r/US92/08680 -
~, ~
4 r4 7 ~ 2 12
is rapid is 650C. One step of accomplishing this goal is
by using sufficient quantities of grinding fluid during the
grinding operation to act as a coolant to prevent SWARF
from reaching a temperature of 650C. or more. Preferably
sufficient grinding fluid (hereinafter "coolant") is used
to prevent the coolant from reaching its boiling point
temperature in the grinding operation. During the grinding
operation, the grinding belt, wheel or stone comes in
contact with a large area of the refractory metal sheet or
plate. The temperature of the SWARF in this contact area
varies depending upon a number of factors. The localized
temperature of the SWARF must be maintained below 650C.
This is best accomplished by flooding the grinding area
with grinding fluid to maintain the SWARF temperature below
650C. Sufficient quantities of coolant are used to keep
the SWARF particles awash in coolant and to prevent the
coolant temperature from reaching its boiling point
temperature within the grinding contact area. When the
coolant temperature in the grinding operation is kept below
its boiling point temperature, little, if any of the
refractory metal SWARF reaches a temperature of 650C. and
the refractory metal SWARF does not react with the coolant
water to produce hydrogen and refractory metal oxides.
Water based coolants are preferred due to their high
heat capacity. However, other types of coolants can be
used. Since the use of aqueous grinding fluid during the
grinding operation is current practice, the improvement
herein lies in using sufficient fluid as a coolant to
prevent the SWARF from reaching 650C. and reacting with
oxygen and nitrogen to form oxide and nitride refractory
metal impurities.
In a typical grinding operation, a refractory metal
strip is introduced to the grinder from a coiler/uncoiler
combination. These coils are typically 200 to over 1000
feet in length and vary in width from about two to four
W~94/08059 PCT/US92/08680
214A772
feet. The strip is ground on both sides in multiple
passes until, by visual inspection, the grinder operator
determines that surface flaws have been reduced to an
acceptable level. The coils are weighed before and after
grinding. Records of the weight changes are maintained.
During a belt grinding operation, the SWARF ~s
continuously removed from the grinder mechanically, falling
into troughs along with the coolant. The solid-liquid
mixture is moved down troughs by circulating rakes and the
excess coolant and SWARF are separated by filtrations. The
coolant is recycled to the grinding operation.
In a conventional SWARF belt grinding process (such as
shown in FIG. 1 without the temperature control during
grinding), the SWARF and excess grinding fluid are screened
to separate the coarse SWARF and residual grinding fluid
from the SWARF fines and excess grinding fluid. The SWARF
fines and residual fluid are separated from the excess
fluid by filtration. The excess grinding fluid is recycled
back to the grinding operation through a clean tank to
permit settlement of entrained solids. The grinding fluid
is moved from the tank to the grinding operation as needed.
Grindi~g fluid make-up is added as necessary. These steps
are s~own in FIG. 1.
SWARF collected from a number of separate grinding
operations will have different coolant compositions. In
these circumstances, the mixed recovered coolant is
disposed of as waste and not recycled.
Freshly produced SWARF retains appreciable amounts of
coolant and thus has a high moisture content, normally in
excess of 50%. Indeed, some moisture levels have been
measured at 66%. The present practice in the indus~y at
this point is to collect the SWARF in separate bins~ The
collected SWARF is periodically removed, transported to a
remote site, allowed to dry somewhat, and burned in
spectacular fires in the open. The burn is extremely rapid
W094/08059 PCT/US92/08680 -
', t
21~ 47 ~ 2 14
and violent, and it generates copious clouds of titanium or
zirconium oxide dust and combustion products of the agents
in the coolant.
Referring to FIG. 2, zirconium slab is fed to a slab
- 5 grinder wherein the surfaces of the slab are ground,
normally one at a time, with abrasive belts in the same
fashion as the titanium strip was ground in the strip
grinder of FIG. 1. The grinding operation is flooded with
sufficient coolant to keep the SWARF awash and to prevent
the coolant from reaching its boiling point temperature.
This flood cooling substantially prevents the Zirconium
SWARF from reaching 650C. during the grinding operation
and reacting with air to form oxide and nitride
cont~m;n~tes.
The SWARF and coolant are passed to a mill wherein the
SWARF is comminuted to reduce the size of the SWARF
slivers. The comminution reduces the SWARF bulk volume by
at least half. The excess coolant is separated from the
comminuted SWARF and recycled to the grinding operation via
the clean tank.
The comminuted SWARF which has residual coolant is
subject to a displacement wash with water or other wash
solvent to remove a substantial portion of the remaining
residual coolant. Conveniently the displacement wash is
conducted on the filter. In a displacement wash, the
comminuted SWARF is washed with an equal volume of water or
other wash solvent. Surprisingly, the wash fluid after
separation from the washed SWARF is slightly diluted
coolant which can be recycled to the grinding operation via
the clean tank. If the SWARF is collected from different
sources which have different coolant formulations, the wash
fluid from the displacement wash is normally not suitable
for recycle. When the displacement wash is conducted on a
filter, the wash fluid is separated from the washed SWARF
by filtration. The SWARF and wash fluid can be separated
~ 94/08059 PCT/US92/08680
2144~2
by other conventional means, such as settling and
decantation, centrifuge separation, screening and the like.
The comminuted SWARF, after separation from the
displacement wash fluid, can be counter-current washed as
described infra with regard to FIG. 4. The clean SWARF
particles can be treated as described below to produce
sintered SWARF briquettes or dried SWARF/salt briquettes.
Referring to FIG. 3, in the process of the present
invention, the SWARF fines after separation from the excess
coolant are combined with the coarse SWARF and subiected to
a comminution operation where the elongated s lvers of
refractory metal are broken up into shorter rods. This
operation is conveniently carried out with an intensive
mixer, such as a Morehouse - Cowles Direct Drive Disperser.
The average aspect ratio of the SWARF particles as
produced in the grinding operation is from 20:1 to 200:1.
An intensive mixer can readily reduce the aspect ratio
below 5:1. This operation results in a considerable change
in the bulk density of the SWARF by increasing the packing
volume. Whereas the SWARF as produced has a bulk density
similar to steel wool or a Brillo Pad, the comminuted SWARF
has a bulk density on the order of about 0.3g/cc and
assumes the characteristics of a metal sludge. This order
of magnitude change in bulk density greatly facilitates the
safe storage and shipping of the material, and as described
below, the recovery of coolant therefrom.
When the SWARF is in the low bulk density state, it
can hold up to twice its weight of coolant without having
any free liquid. In this state, it acts like a sponge. To
cover SWARF in this form, it takes about ten times its
weight in water or coolant. When the SWARF is comminuted,
a substantial portion of the adherent coolant is released.
once the SWARF has been converted to the high density
form as described above and drained of the freed adherent
c~olant, the retained coolant is about 40% by weight of the
W094/08059 PCT/US92/08680 -
. ;. ' 7 ` . . -,.
. ~ r ,V~
~1~4~7~ 16
metal. Thus over 70% of the coolant which is otherwise
lost with the SWARF under existing practice can be
recovered and recycled to the grinding operation if the
recovered coolant is not a coolant mix of two or more
coolant formulations. An additional amount of coolant, 15%
by weight of the metal, can be recovered by displacement
and counter-current washing of the comminuted SWARF.
Saving in the cost of coolant is significant and can
justify the processing of the SWARF, however, the
comminuted SWARF has other advantages. First, it has much
higher bulk density reducing the cost of storage and
shipping. Second, much less water is necessary to cover
the SWARF to eliminate the fire hazard.
After comminuting the SWARF, it is preferably further
processed in several wash steps in series, which removes
the organic matter and inorganic salts, such as nitrate or
phosphate salts, that are common components of the coolant
used in the conventional grinding operations. If not
washed off, the coolant presents a major source of oxygen,
nitrogen, and carbon in the SWARF product. Washing also
tends to remove some of the grinding media or grit which
has disengaged itself from the belt during the grinding
process. The washing is conveniently done with water
although aqueous solutions, organic solvents and the like
can be used.
The comminuted SWARF is first washed with a
displacement wash of water. The comminuted SWARF is
separated from the displacement wash water, usually by
filtration. This wash step is normally carried out in the
filtration apparatus. Surprisingly, the filtrate is
similar to undiluted coolant with respect to composition
and concentration and can be recycled to the grinding
operation if the recovered coolant is not a mix of two or
more coolant formulations.
~ 94/08059 PCT/US92/08680
~14~772
17
The counter-current wash is carried out in at least
three (3) stages. Referring to FIG. 4, the SWARF particles
from the displacement wash is passed to the first wash
stage wherein the SWARF particles are washed with the wash
solvent from the second wash stage. Preferably the washing
in each stage is intensive to remove contamination
entrapped in the SWARF matrix and the SWARF slivers. The
SWARF particles are separated from the wash solvent and
passed to the second stage where the particles are washed
with wash solvent-from the third stage. The wash solvent
from the third stage can be disposed of in an
environmentally sound way or, preferably, it can be passed
to the displacement wash stage wherein it is used as the
displacement wash. After the SWARF particles are washed in
the second wash stage, the particles are separated from the
wash solvent and passed to the third wash stage where the
particles are washed with fresh wash solvent. The wash
solvent from the second wash stage is passed to the first
wash stage. After the particles are washed in the third
wash stage, the clean SWARF particles are separated from
the wash solvent and burned as waste material or,
preferably, pressed into briquettes and sintered, or mixed
with an alkali metal halide salt type, pressed into
briquettes and dried. The wash solvent from the third wash
stage is passed to the second wash stage.
In counter-current washing, the SWARF particles as
they are cleaned from stage to stage are washed with
cleaner solvent. This type of wa~hing substantially
removes coolant components from the SWARF.
Following the washing process, it is necessary to
insure that all of the SWARF remains wet to prevent fire.
~he wet SWARF is preferably mixed with refractory metal
alkali metal fluoride salts in ratios consistent with the
ratios of refractory metal in the master alloy. For
example, if the master alloy contains 5% by weight titanium
W094/080~9 PCT/US92/08680 ~
1 2 18
and 2% by weight boron, the salt mixture would be
formulated to have a 5:2 weight ratio of titanium and
boron. For making aluminum-titanium or aluminum-titanium-
boron master alloys as an end product, then potassium
titanium fluoride, potassium boron fluoride, and titanium
SWARF are mixed in ratios appropriate to the end product,
as will be explained.
An analogous mixture can be made to produce aluminum-
zirconium, magnesium-zirconium, iron-titanium, and iron-
zirconium master alloys. For preparation of magnesium-
zirconium master alloys, mixtures of potassium zirconium
fluoride or sodium zirconium fluoride and zirconium SWARF
are useful. For producing products requiring the
refractory metal component only, mixture of the SWARF with
potassium aluminum fluoride for the aluminum master alloy
manufacturing process has been found effective.
Both alkali metal refractory metal fluoride salts and
bulk refractory metal, usually in the form of sponge or
scrap, is available for use with the SWARF. The instant
process permits the heretofore unused SWARF to be used in
the alloy industry as a getter for 2 I N2 and C and as a
grain refiner.
The SWARF is mixed with the alkali metal refractory
metal fluoride salts with sufficient mixing to insure that
the surfaces of the SWARF, which are quite extensive due to
the small average size of the SWARF, are completely wetted
with the salt. The wet, alkali metal refractory metal
fluoride salt-laden SWARF is then preferably compressed to
a convenient size. High compressive forces should be used,
such as 5000 pounds per square inch (psi) or more.
Preferably the briquettes are compressed at a sufficient
pressure to 'set' the compacted SWARF particles and form
briquettes having a density near the density of metal melt
which the briquettes are to be used in. If the briquettes
are compressed at insufficient pressure, the SWARF
~ 94/08059 PC~r/US92/08680
214~
19
particles are not sufficiently set in their compacted
configurations and the resulting briquettes are loose and
easily come apart. When the particles are sufficiently
compacted to set the SWARF particles, the briquettes are
firm and consolidated. The wet SWARF alkali refractory
metal fluoride mixture may be compacted with conventional
equipment. Useful devices include die and mold presses,
briquettes, and ~orrugated and smooth roll presses, and the
like. This step squeezes out much of the wash water from
the SWARF/salt mixture. Once compressed, the briquette has
some structural integrity due to the deformation and
interl~--king of the SWARF particles with each other.
The compressed SWARF/salt mixture forms a SWARF/salt
compacted mass unit which is preferably dried. The
compacted mixture can be dried in conventional equipment,
such as tray driers, belt driers, etc. Preferably, the
compressed SWARF/salt mixture is dried in a vacuum dryer
with indirect heating, such as steam coils. Although a
direct flame ~is preferably avoided, an indirect flame can
be used to dry as the compacted SWARF/salt mixture is not
flammable if it has 23% by weight salt on a dry basis and
will not sustain combustion even if heated to red heat
under a torch. For SWARF/salt compacted mass unit~ having
less than 23% by weight salt on a dry basis, burntng may
occur, but at a slow, controllable rate. The drying in
conventional equipment is heat transfer limited and no
"bound water" or "difficult to remove water" is observed.
As the SWARF dries, water leaving the mass unit leaves a
salt residue. Since surface tension acts to cause
collection of the liquids at points of closest contact
between the individual pieces of metal SWARF, the
evaporation of the water leaves salt "bridges" attached to
the closest points of metal. Salt bridging between very
close points of contact form sturdy bonds. Therefore,
these "salt bridges" strengthen the compressed briquettes.
W094/08059 PCT/US92/08680 -
4 47~ ~ 20
The SWARF can be mixed with salt over a wide weight
range, such as from 1:2 to 9:1 SWARF:salt on a dry basis.
The upper limitation for salt appears to be when the salt
content interferes with the structural integrity of the
SWARF/salt compacted mass unit and renders it friable or
easily broken. The lower limit for the salt content
appears to be when the salt present is insufficient to
prevent rapid ignition or combustion of the SWARF/salt
compacted mass unit. Ten percent salt by weight of the
mass unit appears to be around the lower limit.
The SWARF/salt compacted mass unit is surprisingly
superior in practice to the commonly used sponge or scrap
in master alloys. It dissolves more readily and in higher
yield into the molten metal and is more reactive with the
other components of a master alloy, for example, boron.
The SWARF/salt compacted mass unit having at least 30~
by weight salt is also surprisingly flame resistant and
therefore safely handled and stored in air. The
substantial a~d surprising degree to which the admixture of
alkali metal refractory metal fluorides salt and SWARF
suppress flammability appears to be due to several factors.
First, the alkali refractory metal fluorides arrest
the flame propagation reactions in combustion processes.
The fluoride in the alkali metal fluoride salts and the
alkali metal refractory metal fluoride salts suppresses
free radical generation which is an important reaction in
the combustion process. Some refractory metal fluoride
compounds have, in the past, been used as fire retardants
in clothing.
Secondly, the alkali metal refractory metal and alkali
metal aluminum fluoride salts mentioned above have melting
points at around 650C., or just below the temperature at
which titanium and zirconium allow rapid diffusion of
oxygen necessary to sustain combustion. The highly
endothermic melting process of the alkali metal refractory
~ 94/08059 PCT/US92/08680
21 ~14~1772
metal fluoride salts removes heat from the SWARF, as the
salts melt at just below the combustion temperature of the
SWARF metal.
Thirdly, once melted, the molten alkali metal
refractory metal fluoride salt strongly wets the surface of
the SWARF metal with a molten salt film that severely
limits transport of oxygen and nitrogen to the metal to
support combustion.
Fourthly, the molten alkali metal refractory metal
fluoride salt forms a molten film which fills void spaces
in the SWARF/salt compacted mass unit which would otherwise
transport air to the interior of the compact and to those
sites inside the briquette which would otherwise have the
air metal mixture appropriate for reaction.
Table salt, NaCl, or any other alkali metal halide
salt, such as potassium chloride, potassium fluoride, may
be added to the wet SWARF/salt mixture prior to pressing to
enhance he economics of the resulting mixture since such
salts are cheaper than the refractory metal salt and can
assist in the reduction of the vapor pressure over the
aluminum refractory metal alloy melt during the addition of
the SWARF/salt compacted mass unit. In one embodiment, two
(2) moles of potassium chloride are added for each mole of
potassium fluotitanate. As can be deduced from the
mechanisms outlined above, the addition of an alkali metal
non-refractory metal halide salt will assist in
accomplishing some of the above objectives. Although
sodium chloride melts at about 801C., and potassium
chloride melts at about 776C., slightly above the
temperature where the refractory metals allow rapid
diffusion of oxygen, the presence of these salts still acts
to retard refractory metal combustion particularly as a
eutectic of the alkali metal halide and alkali metal
refractory metal halide salt mixture which melts at a lower
temperature than either salt.
W094/080~9 PCT/US92/08680 -
. ~1447.7~ 22
Where the manufacture of refractory metal-boron master
alloys is of importance, the alkali metal refractory metal
fluoride salt may be mixed in proportion with an alkali
metal fluoro borate salt. In this manner, the boron is
more easily added to the master alloy, and it serves to
reduce the flammability of the SWARF compacted mass unit,
along with the alkali metal refractory metal fluoride salt.
For example, potassium fluoroborate has a melting point of
about 350C., and would similarly melt below the
temperature at which oxygen diffusion into the SWARF metal
takes place. The melting of the potassium fluoroborate
would begin to pull any other salts present into its molten
solution early on, and thus perform some of the above
factors in an accelerated manner. The addition of
potassium titanium fluoride, potassium boron fluoride,
and/or potassium zirconium fluoride, optionally with
potassium fluoride, an intimate mixture is preferred for
preparation of aluminum master alloys.
Once the SWARF/salt compacted mass unit is dried, it
can be used in the production of alloys, master alloys
and/or re-alloying refractory metal. This unexpected
result occurs despite the fact that raw, refractory metal
with clean surfaces does not normally readily dissolve when
added to the alloy, master alloy or metal molten mass. The
salt rises to the top of the master alloy molten mass and
is easily drawn off. It appears the salt "fluxes" the
dissolution of the refractory metal SWARF with the molten
aluminum.
In another embodiment of the present invention, the
clean SWARF particles can be sintered. Once the SWARF is
washed, it can be compacted and dried without salt
addition. The SWARF particles are compacted at elevated
pressures, such as, at a pressure of 5,000 psi, preferably
higher, such as 20,000 psi. It may be heated rapidly and
briefly to between about 950C. and 1100C. to cause
~ 94/080~9 PCT/US92/08680
23 ~14~772
sintering. Sintering causes somè of the individual pieces
of the SWARF compacted unit mass to become bonded to each
other to form a mass having an even higher integrity than
the SWARF/salt compacted unit mass. In addition, the
surface area of the SWARF is highly reduced during
sintering. Sintering at 1000C. for four hours is
sufficient. However, temperatures between 950C. and
1100C. can be used to sinter the SWARF compacted mass
unit. The sintering is done under vacuum in an inert gas
atmosphere, such as under argon or helium. The resulting
refractory metal sintered SWARF briquettes can be used in
refractory metal metallurgy.
Referring to FIG. 5, an alternate embodiment of the
present invention is illustrated. SWARF with adherent
coolant which is a product of the SWARF fines with the
excess coolant removed and combined with the coarse SWARF
as described above, is combined with an aqueous detergent
and comminuted with a high intensity mixer, such as the
Morehouse - Cowles Direct Drive Disperser in a mixer tank.
The comminution operation reduces the average aspect ratio
of the SWARF particles to below 5:1 described above.
Preferably, the SWARF is mixed with a water and detergent
mixture prior to comminution. The water and detergent
mixture aids in cleaning the coolant off the surface of the
SWARF particles. The amount of water and detergent mixture
added is not critical, but preferably enough water and
detergent are added to cover the SWARF prior to
comminution. If the SWARF contains iron or other magnetic
material impurities, during the comminution step, magne~ic
rods or bars can be inserted into the comminution tank to
remove the magnetic particles. From time to time during
the comminution, the magnetic rods or bars are withdrawn
and the magnetic material is wiped off the rods and bars.
The rods and bars are then re-imme~~ed into the comminution
mix. This step is repeated until the magnetic bars or rods
W094/08059 PCT/US92/08680 -
2i~77~ 24
no longer collect magnetic material which indicates that
substantially all the magnetic particles have been removed.
During the comminution step, the intensive mixer breaks up
the SWARF particles and breaks off fine refractory metal
oxides, nitrides and carbides that form on the surface of
the SWARF material. After the comminution step is complete
and the magnetic treatment carried out, which is only
carried out if magnetic material is present, the
comminution is stopped and the mix is allowed to settle for
a minute or two. During the comminution step, the
immersion of a magnet into the comminution slurry will
quickly pick up particles of magnetic material if such
material is present. Thus the magnets can be used to
'test' for magnetic particles in the SWARF. The aqueous
slurry containing the refractory metal fines is decanted
off. The decanted floating slurry comprises the wash
fluid, a mixture of the coolant water and the detergent,
and the hard refractory metal fines comprising principally
refractory metal oxides, nitrides and/or carbides.
After the separation by decantation, the tank is
recharged with water and the mixing action is commenced
again to form a slurry. This step only takes a minute or
two. After the mixture has slurried up, the mixer is
stopped and the mix is allowed to settle for a minute or
two. The aqueous slurry is decanted off leaving a heavy
wet residual at the bottom of the tank comprising primarily
of clean SWARF particles. The decanted slurry comprises
primarily wash water contaminated with a slight amount of
detergent and coolant, and some refractory metal fines
comprising primarily of refractory metal oxides, nitrides
and/or carbides. The tank is preferably charged with water
again and once again slurried by starting up the mixer for
a brief period of time. The slurry is promptly pumped out
of the tank before it has had an opportunity to settle. An
additional charge of water may be added or needed to insure
~ 94/08059 PCT/US92/08680
~14~772
that all the clean SWARF is slurried and pumped out of the
tank. If there are any heavy non-refractory metal
materials in the initial charge of SWARF, such as metal
bolts or nuts or the like, they will remain in the bottom
of the tank since they will be too heavy to remain in the
slurry and it will be too heavy t~ be drawn up into the
pump. These heavy materials can be hand removed from the
tank.
The pumped slurry material is sent to a filter where
the slurry water is filtered off. The filtered SWARF
particles are displacement washed on the filter with
preferably at least three (3) displacement volumes of water
to thoroughly clean off the SWARF material. The clean
SWARF particles at this point can be burned as described
above, pressed and sintered as described above, or formed
into dried SWARF/salt briquettes as described above, or
pressed and packaged as herein described.
In an alternative embodiment of the present invention,
the clean SWARF particles are pressed as described above to
form SWARF briquettes. Preferably the SWARF particles are
pressed at a sufficient pressure to give the SWARF
briquettes a density approximating the density of the
alloy, master alloy or metal melt that the briquette will
be utilized in. For example, titanium SWARF briquettes for
use in aluminum melts are compacted to a density of about
2.82, the density of molten aluminum. A compaction
pressure of about 20,000 psi will produce compact titanium
SWARF briquettes with a density of about 2.82. When the
briquette has a density about equal to the density of the
melt, the briquette submerges easily into the surface of
the melt and minimizes contact between air and the SWARF
refractory metal in the briquette ,which approaches the
temperature of the melt and increases the oxidation rate of
the refractory metal.
W094/08059 PCT/US92/08680 -
~ ; 26
Rather than sintering the briquettes, the briquettes
can be vacuumed dried to remove moisture to 0.05% moisture
or less. Preferably the vacuum dryer is indirectly heated.
The briquettes can also be fully wetted with water. Fully
wetted means saturated with water but drop free. After the
drying or wetting operation, the SWARF briquettes are
packaged to form a wrapped or coated dry SWARF briquettes.
The briquettes can be packaged individually or in packages
of two (2) or more briquettes. Conveniently, the
briquettes can be packaged into a roll of 5 briquettes.
The briquettes can be packaged with a variety of materials
to protect the SWARF from environmental effects.
Conveniently, the SWARF briquettes can be wrapped in
aluminum foil, such as kitchen grade two (2) mil aluminum
foil. Preferably the free ends of the foil wrapping are
folded over at least twice to form a relatively good seal
with the packaging material. The briquettes also can be
wrapped with wax paper or plastic films, such as Saran wrap
brand plastic film, cellophane brand wrapping film,
polyethylene film or polypropylene film and the like.
Optionally, the briquettes can be dipped or sprayed with a
coating material, such as a pigment-free lacquer, varnish,
polyurethane, paraffin wax, or other protective coating.
Preferably these coatings will be pigment free coatings so
as not to contaminate the SWARF. Wet briquettes are dipped
or sprayed with water compatible coating material.
Although a variety of wrapping material and coatings can be
used, it appears that when the SWARF briquettes are to be
used in a refractory metal aluminum alloy or master alloy,
the aluminum foil wrapping material appears to be the
material of choice for packaging the briquettes.
The wrapping material o~ coating material for the
dried briquettes accomplishes several objectives. The
packaging minimizes the reaction of the clean SWARF surface
with oxygen and nitrogen. The packaging keep wetted
~ 94/08059 PC~r/US92/08680
27 ~14d7~
briquettes wet - partially dry briquettes can be a fire
hazard because of the reaction of refractory metal with
water in the presence of oxygen to form hydrogen and
refractory metal oxide. This reaction is minimized with
dry or fully wet briquettes. The packaging also prevents
small SWARF particles from breaking loose from the
briquette. The small SWARF particles when separated from
the briquettes, are much more reactive and can be more
quickly heated than particles in a briquette. Thus, the
individual particles are far more dangerous and far more
likely to self ignite than the particles in a briquette.
By wrapping or coating the briquette, the particles are
kept with the briquette and are not allowed to fall off or
fall out of the briquette. The packaging also functions as
a spark protector for the briquette against a spark
initiating from the briquette or from landing into the mass
of SWARF particles making up the briquette. A spark will
have very little effect on the above wrapping materials and
coating materials, but a spark can ignite on a single
particle of SWARF. The packaging is also sparkless, that
is, the wrapped or coated briquette will not create a spark
if impacted against iron, flint, etc.
After the briquettes are packaged, they are boxed.
For purposes of this invention, the term "packaged
briquettes" and "packaged formed body of refractory metal"
will mean wrapped or coated dried refractory metal
briquettes. It is envisioned that the briquettes will be
boxed in amounts of from twenty-five (25) to fifty (50) one
pound br~uettes per box. However lighter or heavier
briquettes may be made and supplied in larger or smaller
boxes, bags, drums, bunches, tubes and the like.
Wet SWARF briquettes are dried, such as in a vacuum
dryer, to 0. 05% moisture or less before the briquette is
added to molten metal or molten metal alloy. Hot SWARF
vigorously rPacts with water as described above.
W O 94/08059 PC~r/US92/08680 -
-2;14477~ 28
Referring again to FIG. 3, there may be instances
where the SWARF surface has embedded small iron particles
that are not freed during the comminution and thus are not
captured by the magnetic separation. Optionally, such
SWARF can be treated with an acid leach to dissolve out the
magnetic material and leave the refractory metal SWARF.
Conveniently, the SWARF can be leached with 10%
hydrochloric acid. After the leaching operation which
normally only takes about a half-hour at a slightly
elevated temperature, such as a temperature of 100F or
higher, the acid leach solution is filtered off the SWARF
particles and the SWARF particles are washed with water to
remove all the acid and the metal acid salts formed by the
acid leach. The acid leach SWARF particles can be further
processed in the same manner as the clean SWARF particles.
That is, the clean SWARF particles can be burned, they can
be pressed and sintered, they can be formed into dried
SWARF/salt briquettes or they can be pressed and formed
into dry SWARF packaged briquettes.
The materials separated from the magnetic rods or bars
by the wiping operation contain an appreciable amounts of
refractory metal SWARF particles. This material can be
recycled back into the comminution steps to further
separate the magnetic material from the SWARF particles or
2 5 alternatively, this separated magnetic material can be
treated to an acid leach step similar to the acid leach
step described above to remove magnetic materials. The
separated magnetic material is treated with a dilute
mineral acid, such as 10% hydrochloric acid at a moderately
elevated temperature, such as 100F, for about a half-hour
to leach out the iron and other magnetic material and form
iron chloride and other metal chloride salts. The
refractory metal is resistant to the acid leach. After the
leaching operation, the acid is filtered off the refractory
metal slurry and the solids are treated to displacement
~ 94/08059 PCT/US92/08680
2~77~
29
washes on the filter to obtain clean refractory metal
particles. These SWARF particles can then be returned to
the comminution operation to further complete the cleaning
operation.
Traditional sources of refractory metal scrap for the
aluminum, magnesium and iron alloying markets have been
affected by the introduction of electron beam and plasma
beam melting. Previously titanium and zirconium turnings,
edge trims, and various other forms produced during the
conversion of ingot to finished parts for the aerospace and
nuclear markets had high enough impurity inclusion levels
to restrict their use as recycle materials. These
materials were sold to the alloy markets. Processing these
materials in a plasma or electron beam furnace eliminates
inclusions and allows them to be recycled to high ~uality
consolidated refractory metal which commands a higher price
than the traditional scrap markets. Thus the cost of
refractory metal feed materials for the alloy market has
risen. The re~fractory metal sintered SWARF bri~uettes can
be used as feed material in such processes.
Although the process steps disclosed herein are
generally applicable to refractory metal SWARF processing,
the specific examples given below outline the range of
application.
In any operation involving the handling or processing
of titanium or zirconium SWARF, safety is a paramount
concern. SWARF is classified as a hazardous material, by
virtue of its flammability. Flammability of dry titanium
SWARF is an important consideration in the design of any
r~covery process. Although the flammability
characteristics of SWARF has not been specifically studied,
some data has been accumulated on titanium powders by the
U.S. Bureau of Mines and is summarized in the following
paragraphs.
W094/08059 PCT/US92/08680 -
.. ~ - . . ..
21~47 12
Like many metal powders, titanium is capable of
forming explosive mixtures with air. The ignition
temperature of titanium dust clouds formed in laboratory
equipment with different samples of powder ranged from
330C. to 590C. The minimum explosive concentration
determined in tests was 0.045 ounces/cubic foot.
Measurements of maximum pressure produced in explosions of
powder in a closed bomb at a concentration of 0.5 oz/cu ft.
ranged from 46 to 81 lb/sq in. The average rate of
pressure rise in the explosion tests was 250 to 3400 lb/sq
in/sec and the maximum rate of pressure rise was 550 to
10,000 lb/sq in/sec. The minimum energy of electrical
condenser discharge sparks required for ignition of a dust
cloud was 10 millijoules and for an undispersed dust layer
the minimum value was 8 microjoules. Some samples of
titanium powder could be ignited by electric sparks in pure
carbon dioxide as well as in air. At elevated temperatures
in some cases titanium was found to react in nitrogen as
well as in carbon dioxide.1
Titanium powder in the form of sludge or in a wet
condition can be dried safely in a vacuum drier at a
temperature not exceeding 110C. Mixing or blending of dry
powder should be done in an inert atmosphere. Tests
indicate that the maximum values of oxygen allowed when
using different inert gases to prevent explosion of
titanium dust are given in TABLE I.
TABLE I
Carbon Dioxide 0% Oxygen
Nitrogen 6% Oxygen
Argon 4% Oxygen
Helium 8% Oxygen
U.S. Bureau of Mines. RI 3722. RI 4835.
~ 4/08059 PCT/US92/08680
~1i44.772 '
31
Heretofore, SWARF had been labeled as too contaminated
to be useful in the metal alloy market. The oxidation of
the SWARF slivers by air during their removal in the
grinding operation and observation of the tendency of
SWARF, even when compacted, to float on top of molten
aluminum, magnesium, and iron baths and further oxidize,
strengthened this belief. Chemical analysis of the SWARF
usually showed it to be high in oxygen, nitrogen, and
carbon. Surprisingly, the present inventor found that if
sufficient coolant was used during the grinding step, that
the SWARF slivers themselves remained substantially free
from oxygen, carbon, and nitrogen contamination, and that
the SWARF could be freed from the majority of contamination
by these elements by washing the coolant off the SWARF with
water. By using the process disclosed herein, the coolant
could be economically recovered for reuse. The cleaned
SWARF could be consolidated for use in alloying markets.
EXAMPLE 1
Eight hundred grams of as-produced titanium SWARF,
having the consistency of steel wool from a sheet grinding
operation in which all of the free moisture was drained,
was placed in a food processor with a chopping blade
turning at 3600 RPM to comminute the material. In two
minutes the SWARF was converted from a low bulk density
steel wool-like material to a metal particle slurry. The
liquid in the slurry had been entrained in the well-drained
SWARF even though it appeared to be reasonably dry.
Separate drying tests showed pre-comminuted SWARF to
contain 66~ volatiles indicating even more coolant is
present since the coolant was only 94% water and the
coolant additives were non-volatiles.
After the comminuted SWARF slurry had settled, 350
grams of coolant was drained off, having been liberated or
freed by the comminution. The remaining SWARF slurry (440
W094/08059 PCT/US92/08680 -
2 i 4 32
grams) was placed in a Buchner filter and given a
displacement wash which removed an additional 116 grams of
the coolant. The coolant was removed at essentially full
strength and was suitable for recycle to the grinding
operation. The remaining SWARF was then intensively washed
with two liters of distilled water. The analysis of the as
produced titanium SWARF which was dried at 110C. and the
comminuted, washed SWARF which was dried at 110C. is shown
in the following Table II.
TABLE II
AS PRODUCED CLEANED
SWARF SWARF
Percent Ti 96.4% 99.7%
Percent Si 0.45% 0.12%
Percent C 0.85% 0.16%
Percent O 1.9% 0.38%
Percent N 0.42~ 0.026%
These data show that the majority of contamination in
SWARF produced with plenty of coolant, can be removed by
comminuting and washing with water.
EXAMPLE 2
The wet, washed titanium SWARF obtained from Example
1 was divided into four samples. Separate samples were
mixed with 10%, 30% or 50% by weight of wet (about 15%
moisture) potassium titanium fluoride and 50% by weight
potassium aluminum fluoride. Each sample was compacted
into ten pellets each measuring nominally 1/2" diameter X
1/2" tall. Ten similar pellets were made of comminuted and
washed zirconium SWARF and potassium zirconium fluoride.
Blank pellets of both comminuted and washed titanium and
zirconium SWARF were also prepared. The pellets were
prepared using a Carver press. The compaction into pellets
resulted in the expulsion of most of the water in the
pellets. The pellets without salt had a residual 15%
moisture in the pellet when pressed at 20,000 psi in a 1/2"
94/08059 ' ' 1~ ~PCT/US92/08680
33 2 14 47 72
diameter die. The pellets with salt addition retained 4-
10% moisture depending on the amount of salt in the
mixture. The pellets were dried in an oven at 105C. All
of the pellets reached constant weight in thirty minutes
and none of the pellets showed any indication of bound
water by the shape of the drying curve.
Several of the pellets of each sample were subjected
to flame tests which were conducted by holding the pellets
in a neutral O2-C2H2 flame of sufficient intensity to heat
10a 1" X 1" X 1/2" thick steel plate to full red heat in 45
s,econds. The test was conducted by placing the test pellet
on an 8" wide piece of 316C stainless steel flat bar and
pushing it under a fixed torch burning under constant
conditions.
15Under these conditions, loose, washed and dried
titanium and zirconium SWARF ignited immediately in a
bright photo flash fashion. Since considerable heat
release occurs during the burning process, any significant
accumulation of washed and dried SWARF would be extremely
dangerous.
Compacts of uncomminuted, washed and dried, zirconium
and titanium SWARF ignited on the order of one second and
burned in a self-sustaining fashion in about 5 seconds.
Compacted, uncomminuted, washed SWARF is a dangerous
material in any significant accumulation and must be stored
under water.
Both zirconium and titanium pellets with mixed salt
were much less flammable. Potassium titanium fluoride with
titanium SWARF, potassium aluminum fluoride with titanium
SWARF, potassium zirconium fluoride with zirconium SWARF
all significantly improved flammability resistance.
Pellets containing about 50% by weight salt took about lO
seconds to reach a temperature where reaction with air
began to occur as evidenced by the white color in the
pellet flame. In this case, combustion would not sustain
W094/080S9 ~ 4 4 7 ~ PCT/US92/08680 -
itself when the torch flame was removed. The 30% by weight
salt pellets were borderline in their ability to sustain
reaction with air when the torch flame was removed, and
those with 10% by weight salt sustained reaction with the
torch removed, but burned in a controllable fashion and far
less readily than the pellets with no salt. Compacted
SWARF/salt pellets, containing at least 10% by weight
alkali metal refractory metal halide salt could be stored
without a water cover. Preferably the SWARF pellets would
contain at least 30% by weight salt.
Zirconium and titanium SWARF pellets with 30% and 50%
by weight salt of the types given above, were immersed in
molten aluminum at 700C. and held under the surface with
a graphite tool. The pellets readily reacted and dissolved
into the molten metal. Similar tests run on SWARF compacts
without salt addition tended not to dissolve and would
simply rise to the surface of the melt when the graphite
tool was raised. The salt obviously helped "flux" the
dissolution of refractory metal into the aluminum.
EXAMPLE 3
Several of the titanium and zirconium SWARF pellets
prepared in Example 1 without salt were placed in a vacuum
furnace and heated to 1000C. for four hours. The
resulting pellets were reduced in volume by about 40~ and
had a density or 90% of theoretical. These pellets did not
sustain combustion in the torch test described above.
These pellets did not readily dissolve in aluminum at
700C. until a layer of potassium titanium fluoride was
added to the top of the molten aluminum which led to ready
dissolution of the pellets and also reaction of the
titanium salt. These pellets have desirable handling
characteristics for charging into titanium melting
furnaces.
94/08059 PCT/US92/08680
3~ 2~ 4772
EXAMPLE 4
A visual examination of coarse SWARF shows the
material to be highly agglomerated in the form of entangled
slivers of titanium metal. The unwashed SWARF has a dull,
non-lustrous appearance which is improved by washing. The
SWARF sliver length appears to be from about 0.02
millimeters to about 2 or 3 millimeters. The cross
sectional dimension appears to be relatively uniform and is
estimated to be less than about O.Olmm in width.
Grains of dark SiC grit are visible under
magnification. The grit is dark, lustrous, irregularly
shaped but tending to an oblate spheroid. They do not
exhibit sharp facets or fracture surfaces. The grit in the
SWARF falls roughly into three categories, including (1)
Free grit, (2) Grit that is mechanically trapped in the
SWARF tangles, and (3) Grit that appears to be bound to the
titanium sliver. The SiC grit may be attached to the
titanium by a reaction of the titanium with the SiC.
The distinguishing features of fine SWARF and sludge
with respect to coarse sludge is simply the particle size
and the absence of large SiC particles. Fine SWARF shows
no large, discrete particles of SiC grit. Silicon analysis
of this material shows appreciable amounts of silicon.
Accordingly, the fine SWARF material and sludge apparently
contains silicon carbide fines as well as the titanium
fines. The fine SWARF and sludge sample particles are at
least one order of magnitude and smaller in size than the
coarse material.
EXAMPLE 5
A 1,000 gallon tank is filled with the contents of
nine (9) 55 gallon drums containing SWARF particles
generated by abrasive wheel grinding. The tank is charged
with 500 gallons of a water detergent mixture (Calgon
dishwashing detergent; fifteen (15) pounds of detergent).
W O 94/08059 PC~r/US92/08680 -
~i ~ 36
The tank is fitted with a Morehouse - Cowles Direct Drive
Disperser with a 50 hp motor drive at about 1,200 RPM. The
mixer is started and allowed to operate for ten (10)
minutes. The mixer blade breaks up the SWARF particles and
breaks the surface coatings of hard refractory metal
oxides, nitrides and carbides to a fine dust which is
easily dispersed in the slurry mixture. The whirling
mixing blade has sufficient agitation to entrain the SWARF
particles in the aqueous slurry mixture. Larger, heavy
materials, like nuts, bolts, cutting tools, and the like,
fall to the bottom of the tank. The agitation is not
sufficient to slurry these materials and they remain on the
bottom and are easily removed by hand after the operation.
During the comminution employing the intensive mixer,
a magnetic rod is inserted into the tank to determine if
there are any magnetic materials, such as iron in the tank.
The magnetic materials are attracted and adhere to the rod,
after a minute within the tank, the rod is withdrawn. If
there are adhered particles on the rod, the rod is wiped
off with a cloth or gloved hand and reinserted into the
tank. This operation is continued until the rod remains
substantially clean of all particles which indicates that
all magnetic particles having a density similar to that of
the SWARF particles have been removed from the slurry.
After the intensive mixing has run its course,
normally in about ten (10) minutes, the intensive mixer
motor is stopped and the slurry is allowed to settle for a
minute or two. The aqueous slurry is then decanted off.
The decanted aqueous slurry comprises principally of water,
detergent, coolant and fine hard particles of refractory
metal oxides, nitrides and/or carbides. After the
decantation step, the tank is charged with a fresh charge
of clean water (approximately 500 gallons) and the
intensive mixing action is commenced again for a minute or
two. The intensive mixer is stopped, the aqueous slurry is
~ 94/~8059 PCT/US92/08680
37 214477~
allowed to settle for two (2) minutes and then the aqueous
slurry is decanted off leaving a residual at the bottom of
the tank comprising principally of clean SWARF particles.
The aqueous slurry decanted off contains principally water,
a very small amount of detergent and coolant and fine hard
fines refractory metal oxide~, nitrides and/or carbides.
The tank is charged a third time with clean water and
the intensive mixer is started up again and allowed to run
for one (1) minute. After the mlxer is stopped, the
aqueous slurry is pumped out of the tank and on to a
filter. After all the aqueous material is pumped out of
the tank, the tank is charged a fourth time with fresh,
clean water, the intensive mixer is run again and the
aqueous slurry is pumped out of the tank. The intensive
mixture is turned off when the slurry level falls below the
mixer blade. This operation substantially removes all the
clean SWARF particles from the tank. If there are any
heavy metal particles in the initial SWARF, such as iron or
steel bolts, nuts, tool, tool parts or the like, they w~ll
remain in the bottom of the tank and can be clean out of
the tank by hand. On the filter, the pumped slurry is
filtered and washed three (3) times on the filter with
equal volumes of water to leave clean SWARF particles on
the filter.
The wet, clean SWARF particles are removed from the
filter and pressed at 25,000 psi in a press to produce
SWARF briquettes measuring 1 1/2 inches in thickness and
about 3 1/2 inches in diameter. Each briquette weighs
approximately one (1) pound dry. A charge of several
hundred wet briquettes are placed in a sealed vacuum dryer
which is indl~ectly heated with steam coils at
approximately 300F. The dryer is evacuated under vacuum
to about ten Torrs or less and the briquettes are dried
over a period of several hours to a moisture content of
less than 0.05%. The briquettes are allowed to cool down,
W094/08059 PCT/US92/08680 -
-`2tl~4772 38
under vacuum, in the dryer until they reach ambient
temperature. The briquettes are removed from the dryer and
each is wrapped individually in aluminum foil, the ends of
the aluminum foil being folded over twice. The wrapped
briquettes are packed into cardboard boxes, 48 briquettes
per box.
EXAMPLE 6
The tank of Example 5 is charged with the contents of
nine (9) 55 gallon drums containing titanium SWARF
particles produced by stone grinding. The SWARF contains
about 5% iron particles. The tank is charged with 500
gallons of an aqueous detergent mixture and the resulting
mixture is comminuted in the tank using the intensive
mixer. During the comminution step, a highly magnetic rare
earth oxide magnet rod measuring 1/2 inch by 3 feet,
manufactured by Ford Motor Company, is inserted into the
tank for about 30 seconds and then withdrawn. The rod is
covered with magnetic metallic particles. The rod is wiped
clean with a gloved hand and inserted back into the
agitated tank. This step is repeated until the rod no
longer picks up magnetic metallic particles. The magnetic
treatment lowers the iron content of the slurry to less
than 1/10%. The comminuted slurry following the magnetic
treatment is treated in the same manner as the SWARF
particles of Example 5 to yield clean SWARF particles.
EXAMPLE 7
The clean SWARF particles of Example 6 in the undried
state are compacted at 20,000 psi in a press to produce one
pound wet briquettes having a density of about 2.8 grams
per cc. The briquettes are fully wetted with clean water
and packaged as a roll of 5 briquettes with 4 mil. aluminum
foil. Ten rolls (50 briquettes total) are boxed together
in a moisture barrier box to keep the packaged briquettes
wet. Before the briquettes are added to a molten metal
~ 94/08059 PC~r/US92/08680
2~4~7~2
39
melt, the briquettes are dried under vacuum to a moisture
content of at least 0.05~ or less.
EXAMPLE 8
The clean SWARF particles of Example 6 are added to an
acid leach tank containing 10% hydrochloric acid. The
slurry is agitated for 1/2 hour at a temperature of
approximately 120F. The agitation is stopped, the slurry
is allowed to settle and the leach liquor is decanted off
the leach tank. The residual SWARF particles remaining in
the tank are mixed with water, slurried and pumped to a
filter as a slurry. The charge of SWARF on the filter,
after filtration of the water, is washed three (3) times on
the filter with equal displacements of wash water to y~ld
clean acid leached SWARF particles which are virtually fr~e
of iron. The acid leach SWARF particles can be utilized to
produce the wrapped briquettes of Examples 5 or 6, the
SWARF pellets of Example 2 or the sintered SWARF pellets of
Example 3.
EXAMPLE 9
The separated magnetic metallic particles of Example
6 wiped off the magnetic rod are inserted into the
comminution tank of Example 6. These particles contain 25
to 40% iron with the balance being titanium. These
particles are treated in the same manner as unprocessed
titanium SWARF to recover the titanium particles and
separate the magnetic metallic particles. Alternatively,
these particles can be treated to the acid leach step of
Example 7 to dissolve out ~he iron leaving the titanium
particles. The leach step is carried out by using at least
a stoichiometric amount of dilute hydrochloric acid, such
as 10% hydrochloric acid, at an elevated temperature, such
as 110F, for a sufficient period to cause a dissolution of
W094/08059 PCT/US92/08680 ~
~1~47~2 40
iron into iron chloride salts. After the leaching step,
the remaining titanium particles can be recycled back into
the process of Examples 1, 5 or 6 to thoroughly clean the
titanium particles as described herein.
EXAMPLE 10
The clean SWARF particles of Example 8 are compacted
at 20,000 psi in a press to produce 5 pound briquettes
measuring 2 1/2 inches thick and 5 inches in diameter. The
briquettes are fully wetted with water and packaged in
polyethylene film (3 mil). Ten packaged briquettes are
boxed in a moisture proof box.