Note: Descriptions are shown in the official language in which they were submitted.
21~48~"~
1
Method for adjusting the S/Na ratio in a sulphate pulp
mill
The invention relates to a method for adjusting
sulfur concentration in a sulphate pulp mill by
removing cumulated sulfur from the chemical recovery
loop, wherein sulfur is removed from black liquor in
the form of sulfurous odor gases separated from the
black liquor during treatment.
In the production of sulphate pulp, wood is
treated with alkaline cooking liquor, whereby lignin
contained in the wood is dissolved in the liquor and
cellulosic fibres are released. After cooking, the
cellulosic fibres are separated from the liquor, and
the black liquor containing lignin is passed into
further treatment and back into the chemical recovery
loop. Black liquor is first concentrated by evaporat-
ing water from it, and the concentrated black liquor
is burned in a soda recovery boiler. Salt smelt
obtained from burning, containing mainly NazS and
NazC03, is dissolved in water, thus obtaining green
liquor. Green liquor is causticized with slaked lime,
that is, calcium oxide, thus obtaining white liquor
and CaC03, that is, calcium carbonate. White liquor is
recycled into pulp cooking, and calcium carbonate is
passed into a lime kiln, where it is again calcined
into calcium oxide needed in causticization.
Sulfur is introduced into the chemical recovery
loop and process of sulphate pulp e.g. from sulfuric
acid used in soap splitting. Part of the sulfur is
usually emitted with flue gases in the form of sulfur
dioxide and part remains in the chemical recovery
loop. For environmental reasons, attempts are made to
reduce the amount of sulfur compounds released with
flue gases by using e.g. a scrubber, as a result of
~1.~:~8~'~
2
which the amount of sulfur gathered in the chemical
recovery loop is correspondinly greater, as the
solution leaving the scrubber has absorbed sulfur, and
it is usually recycled into the cooking solution. The
amount of sulfur compounds in flue gases can be reduc-
ed in many ways. One way is to increase the solids
content of black liquor. Sulfur compounds are present
not only in flue gases but also in odor gases separ-
ated at different stages at the pulp mill and in flash
gases from the last concentration stage. Such sulfur
compounds include HZS, CHzHS, ( CH3 ) SZ, ( CH3 ) zS and
( CH3 ) zSz . These odor gases are usually burned in a
separate waste heat boiler or in a lime kiln. Burning
in the waste heat boiler results in sulfur dioxide,
which has to be removed from the flue gases of the
waste heat boiler by means of a separate scrubber.
Burning odor gases in the lime kiln requires a
separate odor gas burner, in addition to which the
reactions of sulfur and lime deteriorate the cal-
cination process of the lime kiln.
Attempts have been made to remove sulfur from
the sulphate cellulose process by using waste lime
obtained from lime reburning. This has been done by
treating the sulfur dioxide obtained in the burning of
odor gases with a NaOH solution, and the obtained
sodium bisulphite is treated with calcium oxide,
resulting in solid calcium sulphite and calcium
sulphate. This method disclosed in FI Patent Appli-
cation 920 531, a so-called dual-alkaline process, is
very complicated and difficult to realize.
The object of the present invention is to remove
sulfur from the chemical recovery loop of a pulp mill
in such a way that a desired S/Na ratio can be main-
tained. According to the invention, the odor gases are
passed into a power boiler at the pulp mill to be
3
burned there, calcium oxide produced in a lime kiln is
introduced into the power boiler from the kiln in an
amount such that at least sulfur compounds generated
from the odor gases and calcium oxide react forming
calcium sulphite and calcium sulphate so that they can
be removed from the flue gases as dry dust-like
reaction products.
The essential idea of the invention is that odor
gases containing various sulfur compounds are passed
into a power boiler to be burned there while intro
ducing calcium oxide from the lime kiln into the power
boiler. Calcium oxide binds sulfur compounds generated
in the boiler during burning into a solid reaction
product that can be separated in dust form. According
to one preferred embodiment of the invention, odor
gases containing sulfur compounds are first passed
into an evaporation plant and only then into the power
boiler, which allows the thermal energy of the odor
gases to be utilized in the evaporation plant.
An advantage of the method according to the
invention is that the desulfuration process can
utilize the existing equipment and material of the
pulp mill, and no separate auxiliary devices are
needed. Another advantage is that the method is simple
and easy to apply at existing pulp mills and its
investment costs are low.
The invention will be described more fully with
reference to the attached drawings, where
Figure 1 is a block diagram illustrating the
method according to the invention;
Figure 2 is a block diagram illustrating a
second embodiment of the method according to the
invention; and
Figure 3 illustrates a third embodiment of the
method according to the invention.
4
Figures 1 to 3 are block diagrams illustrating
sections of the pulp cooking process to such an extent
as is necessary for the understanding of the inven-
tion. In other respects, the sulphate pulp cooking
process is known per se and obvious to one skilled in
the art, and therefore it will not be described more
closely. In Figures 1 to 3, the same process sections
are indicated with the same reference numerals, which
will be explained mainly with reference to Figure 1.
In describing the other figures, the reference nu-
merals are further explained only when this is
necessary for the concerned embodiment of the inven-
tion.
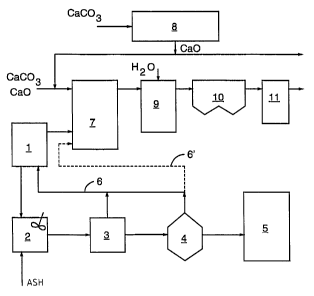
Figure 1 illustrates an evaporation plant 1,
into which black liquor is introduced in order that
water could be removed from it, thus concentrating the
black liquor. From the evaporation plant the black
liquor is passed into a mixing tank 2, where ash is
mixed with it. From the mixing tank 2, the black
liquor is further passed into a final concentration
stage 3, where the dry solids content of the black
liquor is increased by up to 80$ and even more at a
pressure exceeding atmospheric pressure and at a
temperature exceeding 100°C. From the final con-
centration stage 3, the black liquor is further passed
into a flash tank 4, where it is allowed to expand so
that more steam and sulfurous gases will be released.
From the flash tank 4, the black liquor is passed into
a soda recovery boiler 5, where it is burned so as to
recover chemicals.
Steam and odor gases leaving the final con-
centration stage 3 and the flash tank 4 can be carried
through a line 6 to the evaporation plant, where their
thermal energy is utilized in the evaporation of black
liquor. The steam and the odor gases entrained in it
~~4~$2'~
may also be fed directly into the power boiler through
a line 6' indicated with a broken line in the figure.
Odor gases from the evaporation plant 1 are also
carried into the power boiler 7 to be burned there,
5 whereby the gases produce sulfur oxides, mainly sulfur
dioxide, in the power boiler 7. The power boiler 7 may
be of any type, including various circulating fluid-
ized bed boilers, fluidized bed boilers or grate
boilers, which usually burn wood bark, a fuel abund-
antly available at a pulp mill, or some other suitable
material.
Sulfur oxides produced in the power boiler 7 can
be removed in different ways. According to one embodi-
ment of the invention, calcium oxide produced in a
lime kiln 8 and, if required, also additional calcium
carbonate is introduced into the power boiler 7 in
order to remove oxides generated in the burning of
sulfur introduced together with in the fuel and odor
gases. In the power boiler 7, calcium oxide or the
calcium oxide formed in the burning of calcium
carbonate react partly with sulfur oxides, which
yields calcium sulphite or calcium sulphate. From the
power boiler 7 they are carried with flue gases e.g.
into a separate desulfuration reactor 9, where water
is introduced into the flue gases. Calcium oxide that
has not reacted in the boiler is thus hydrated into
calcium hydroxide and it reacts actively with the
remaining sulfur oxides at the same time. The dust-
like reaction product leaving the desulfuration
reactor 9 with the flue gases is separated by an
electrostatic precipitator 10. If required, rest of
the flue gases can be passed into a gas scrubber 11,
where they are washed with a NaOH solution and then
passed into a chimney.
The essential aspect of the invention is that
214827
6
odor gases from all process steps are passed into the
power boiler to be burned there and the resulting
sulfur oxides are removed from the flue gases by
introducing calcium oxide produced in the lime kiln
into the power boiler. In this way the thermal energy
of the flue gases can be utilized in different ways,
in addition to which the entire process can directly
use material that the mill anyway produces, i.e.
calcium oxide. No separate waste heat boiler is thus
needed nor does the desulfuration disturb the burning
process in the lime kiln, as the lime required for the
neutralization of sulfur is taken from the lime kiln
only after lime reburning and no substances disturbing
the process are introduced into the kiln.
Figure 2 in turn illustrates another embodiment
of the invention, which deviates from that shown in
Figure 1 only with respect to the section after the
power boiler 7. In this embodiment, calcium oxide from
the lime kiln 8 is mixed with water in a separate
mixing tank 12 so that calcium hydroxide is obtained.
Calcium hydroxide solution in turn is passed into the
gas scrubber 11, into which flue gases from the power
boiler 7 are also passed. In this embodiment, dust
contained in the flue gases can also be removed with
the calcium hydroxide solution, so that no separate
dust filter shown in Figure 1 is necessarily needed.
After the scrubber, the flue gases can be passed
directly into the chimney.
Figure 3 shows a third embodiment according to
the invention, where a separate thermal treatment
stage 13 for black liquor is added to the arrangement
shown in Figure 1 after the final concentration stage
3. In this thermal treatment stage, black liquor is
treated at a pressure exceeding atmospheric pressure
and at a temperature exceeding the cooking temperature
CA 02144827 2004-07-16
7 .
before it is introduced into the flash tank 4. Tn this
embodiment" the amount of sulfur to be removed can be
adjusted by varying the temperature and duration of
the therma:L treatment. This allows desulfuration to be
optimized in view of the sulfur accumulation of the
process. The thermal treatment as such is well-known
e.g, from FI Patent Application 921 444. In this
embodiment of the invention it is also possible to
use.. the ca:Lcium hydroxide wash illustrated-in Figure
2 in place of the separate desulfuration reactor.
The :Lnvention has been described above and shown
in the drawings by means of example, and it is not in
any way restricted to it. It is essential that odor
gases generated in the process are passed into the
power boiler to be burned therein, and sulfur oxides
formed in the power boiler are neutralized by intro-
ducing calcium oxide produced in the lime kiln into
the power boiler. In addition, calcium oxide from the
lima kiln is possibly used in the form of calcium
hydroxide solution for washing flue gases. It is to be
noted that when the power boiler is a circulating
fluidized bed boiler, desulfuration in the power
boiler is s:o efficient that no separate humidification
reactor is needed. If desired, this embodiment may
also use washing with calcium hydroxide solution for
the final washing of flue gases.