Note: Descriptions are shown in the official language in which they were submitted.
W094t06585 2 1 4 5 1 6 1 PCT/US93/0883~
METHOD FOR MAKING A CERAMIC METAL COMPOSITE
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to ceramic-metal composite
materials, or cermets, and methods of producing the same.
More particularly, the invention relates to an efficient
method for producing ceramic-metal composites having
substantially continuous metal and ceramic phases which
permits a wide variety of metals and ceramic matrix
materials to be used and a wide variety of products to be
formed.
2. Description of Related Art
During the last few decades, ceramics have been
investigated for the purpose of introducing them into
structural applications, primarily in high temperature
environments. However, ceramic materials are not always
well suited since they are brittle, have a limited
ductility and low values of fracture toughness at low
temperatures. In addition, the fracture strength of
ceramics is not very reproducible since the average
strength usually varies from one lot of parts to the next,
which is attributed to the presence of processing flaws
which can initiate fractures. Because ceramic materials
have great potential for high temperature applications due
their creep and oxidation resistance, a great deal of
effort has been expended in an attempt to increase the
W094/06585 2 1 ~ 5 1 6 1 PCT/US93/08835
fracture reliability of ceramic materials and to develop
tough and creep-resistant composites.
One possible solution is the fabrication of a
metal/ceramic composite, commonly known as a cermet.
Traditionally, cermets have been obtained in one of two
ways; (1) by heating mixtures of ceramic and metal
materials to obtain a metal matrix having a discrete
ceramic phase, or (2) as disclosed in U.S. Patent No.
2,612,443 by Goetzel at al., issued September 30, 1952, by
forming green body substrates of either fibers, whiskers or
particles through pressing, injection molding, casting or
other techniques, sintering the green body and infiltrating
the porous body with a molten metal through use of squeeze-
casting or other means of applying pressure to force the
molten metal into the voids within the body.
Sintering green bodies to densify and increase grain
size is known in the art. Vapor phase sintering is also a
known method for increasing neck growth between grains
without densification in particulate green bodies. See,
for example, U.S. Patent No. 4,108,672 by Klug et al.,
issued September 22, 1978. Until recently however, vapor
phase sintering had not been used as a means of controlling
the total porosity and average pore size of ceramics. See
Readey et al., "Effects of Vapor Transport on
Microstructure Development", Ceramic Microstructures, pgs.
485-496 (1987) and Readey, "Vapor Transport and Sintering",
in Ceramic Transactions, Vol.7, pgs. 86-110 (1989).
~ 094/06585 2 1 4 5161 PCT/US93/0883s
Infiltration of molten metals into porous ceramic
preforms by squeeze casting and by applying pressure to the
molten metal are known, for example, see Verma and Dorcic,
"Performance Characteristics of Metal-Ceramic Composites
Made by the Squeeze Casting Process", Ceramic Engineering
Science Proc., Vol. 9, pgs. 579-5g6 (1988). The major
disadvantage of these methods, however, is that it is
difficult to achieve near complete infiltration of the void
space within the preforms without use of substantial
pressures. In addition, when ceramic preform materials
contain a high volume porosity, use of pressure in squeeze
casting techniques can crumble the delicate ceramic
structure.
Infiltration using vacuum furnaces and using
infiltration enhancers are also known. U.S. Patent No.
3,864,154 by Gaæza et al., issued February 4, 1975
discloses a method for infiltration of porous ceramics
under reduced pressure in a vacuum furnace. U.S. Patent
No. 5,004,035 by Burke et al., issued April 2, 1991,
discusses use of infiltration enhancers for infiltrating
aluminum alloys into alumina or silicon carbide preforms.
However, none of these references discuss processes to
control porosity and pore size of the preform over a broad
range in conjunction with infiltration.
Infiltration through capillary action has also been
utilized, as disclosed in U.S. Patent No. 3,310,427 by
Cheney et al., issued March 21, 1967 and Martins, "Modeling
of Infiltration Kinetics for Liquid Metal Processing of
.
W094/06585 2 1 4 5 1 6 1 PCT/US93/08835
.. .. .. . .
Composites", Metallurgical Transactions B (1988). The
Cheney et al. patent discloses the infiltration of
refractory metal with a metal having a lower melting point.
Previously mentioned U.S. Patent No. 3,864,154 by Gazza et
al. also discloses infiltration of molten metals wherein a
cold-pressed compact of powder such as AlB,2 is placed
between two layers of metal and heated in a crucible.
More recently, other approaches for forming cermets
have been used due to a lack of success in obtaining
adequate control of cermet composition and forming with
traditional processes. For example, use of accelerated
oxidation reactions and "combustion wave" exothermic
reaction processes have been utilized to form cermets. The
patented LANXIDE process, as disclosed in U.S. Patent No.
4,853,352 by Newkirk et al., assigned to Lanxide Technology
Company, issued August 1, 1989, is a method for forming
cermets whereby a molten parent metal is oxidized, usually
in the presence of oxidation enhancing dopants, to create
a three-dimensional interconnected ceramic-metal composite
material which contains between 1 and 40% of the parent
metal by volume.
A number of other patents assigned to Lanxide
Technology Company, an example of which is U.S. Patent No.
4,824,622 by Kennedy et al., issued April 25, 1989,
disclose conducting an oxidation reaction within a bed of
filler material or other preforms to form macro- and micro-
composite materials.
W O 94/06585 . = ~ PC~r/US93/08836
2145161
Other patents that have been commonly assigned to
Lanxide Technology Company include U.S. Paten~ No.
4,828,008 by White et al. and issued on May 9, 1989. White
et al. disclose that in order to infiltrate aluminum metal
into a permeable mass of loose ceramic powder, such as
alumina, a nitrogen gas atmosphere must be used and
magnesium must be alloyed into the aluminum metal. U.S.
Patent No. 5,016,703 by Aghajanian et al. and issued on May
21, 1991, discloses a process for the spontaneous
infiltration of aluminum into a ceramic preform in the form
of particles, platelets, whiskers or fibers. An
infiltration enhancer, such as magnesium turnings, are
placed between the molten metal and the preform to permit
the infiltration.
U.S. Patent No. 4,868,143 by Newkirk et al. and issued
on September 19, 1989, discloses a process for making a
composite wherein an oxidation reaction product (e.g.
alumina) is formed with aluminum parent-metal
interconnected therethrough. The composite is contacted
with a molten second metal which then infiltrates the
interconnected parent metal by interdiffusion. The result
is a composite having a mixture of two metals
interconnected throughout the composite.
The difficulties with the LANXIDE processes and
similar processes are that only very limited control of
porosity is possible. In addition, in order to infiltrate
a metal different from the parent metal which was oxidized
to form the ceramic material, the remaining parent metal
W094/06585 ~ Sl 61 PCT/US93/08835
. t~ ~
~ -6-
must be removed from the three-dimensional interconnecting
pore system of the preform. Furthermore, in order to
insure near complete infiltration of the foreign metal,
pressure must be applied to the molten metal infiltrant or
an infiltration enhancer must be used which can alter the
composition of the composite. The LANXIDE ter-hn;que of
growing an oxidation layer from a molten parent metal by
application of oxidation enhancing dopants has some
utility, however, production of intricate geometric shapes
utilizing the LANXIDE process is extremely difficult.
Producing ceramic materials for infiltration through
exothermic combustion wave reactions, as disclosed in U.S.
Patent No. 4,988,645 by Holt et al., issued January 29,
l991, has been achieved to a limited extent to obtain
porous ceramic bodies for later infiltration with molten
metals. However, the applicability of this method of
producing cermets is limited to those materials which form
through exothermic reactions.
All of these processes have limitations on the control
of the metal and ceramic parameters thought to be important
for the development of a tough and creep-resistant ceramic-
metal composite material. Thus, there is a need for a new
method of producing ceramic-metal composites having
carefully controlled compositions. The present invention's
unique application of capillary action to infiltrate a
sintered ceramic preform with molten metal addresses this
need.
W094/06585 PCT/US93/08835
~ 21~Sl~l
The present invention results in ceramic-metal
composite materials which are particularly useful for
application as engine components, heat exchangers, kiln
furniture, cutting tools, abrasives, valve components, pump
components, bearings, seals, dies, diffusion tubes,
mufflers, tiles, and radiation barriers. The composites
are particularly useful in applications demanding creep
resistance and wear resistance where pure metals or
traditional cermets are insufficient.
SUMMARY OF THE INVENTION
The present invention is directed to a process for
forming a ceramic-metal composite. The process includes
the steps of contacting a porous ceramic body having a
continuous 3-dimensional pore structure with a molten metal
such that substantially all of the void space in the
ceramic is filled with metal.
In one embodiment, the ceramic matrix is formed by an
enhanced vapor phase sintering process. This process
advantageously permits independent control over the total
porosity and pore size of the ceramic material. In one
embodiment, the ceramic matrix comprises alumina and the
infiltrating metal comprises copper.
The present invention is also directed to a method for
making a ceramic-metal composite, comprising the steps of
forming an alumina ceramic body having substantially
interconnected porosity in the range of from about 10
W094/06S85 ~ PCT/US93/08835
2~ i6~ --
--8--
percent to about 80 percent, heating a metal comprising
copper metal to substantially melt the copper metal and
contacting the alumina ceramic body with the heated metal
in a vacuum to infiltrate the metal into the alumina
ceramic. In a preferred application, the alumina ceramic
body is a filter element and the copper comprising metal
infiltrates a portion of the filter element.
The present invention is also directed to a method for
sealing the end of a ceramic filter element, comprising the
steps of contacting the filter element with a molten metal
to infiltrate the metal into a portion of the filter
element and cooling the metal to form a filter element
having a composite portion. The method can also include
the step of brazing a seal ring to the composite portion.
The present invention is also directed to a method for
making a ceramic-metal gradient composite, comprising the
steps of forming a ceramic body having at least two
portions wherein at least one of the portions has a
different porosity than the other portion, heating a metal
to substantially melt the metal and contacting a porous
portion of the ceramic body with the heated metal to
infiltrate the metal into the ceramic. In one embodiment,
the ceramic comprises aluminum titanate.
The present invention also includes a process for
producing a sintered ceramic body, comprising the steps of
forming a green body comprising alumina powder, sintering
the green body at a temperature of at least about 1350C in
the presence of a reaction gas to promote the formation of
~yo94/~6585 PCT/US93/08835
. 21l~$l6l
an aluminum-containing transport gas species, wherein the
sintered ceramic body has a total porosity of from about 10
percent to about 80 percent and a substantially continuous
and interconnected pore structure.
The composites formed according to the present
invention are useful in many applications, particularly
those requiring high-temperature creep and toughness.
BRIEF DESCRIPTION OF THE DRAWINGS
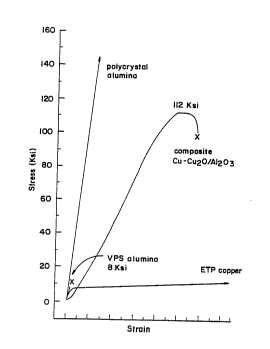
Fig. 1 is a stress-strain diagram comparing a
composite produced according to the present invention with
the component materials of the composite.
DESCRIPTION OF PREFERRED EMBODIMENTS
The present invention is directed to a method for
making a ceramic-metal composite material with
interconnecting and substantially continuous ceramic and
metal phases. The composite is formed by infiltrating
molten metal into a porous ceramic body having a
substantially interconnected continuous pore structure. In
one embodiment of the present invention, the ceramic is
formed by sintering a green body of ceramic powder using an
enhanced vapor phase sintering process. Vapor phase
sintering permits the total porosity and the average pore
size of the porous ceramic body to be carefully and
independently controlled.
The ceramic matrix material can be chosen from any of
a number of metal oxides, carbides, nitrides or the like.
W094/06585 - - PCT/US93/08835
For instance, the ceramic matrix can comprise alumina
(Al2O3), titania (Tio2)~ zinc oxide (ZnO), zirconia (Zro2),
iron oxide (Fe2O3), magnesia (MgO), silica (SiO2), or any
other metal oxide. Further, non-oxide ceramics such as
silicon carbide (SiC), silicon nitride (Si3N4), aluminum
nitride (AlN) or titanium diboride (TiB2) can be used as the
ceramic matrix material. Preferred matrix materials
include alumina, aluminum titanate (Al2TiO5), silicon
carbide and silicon nitride.
The infiltrant metal can be selected from any metal
whose melting point is below the melting point of the
ceramic matrix material. For example, copper (Cu), nickel
(Ni), aluminum (Al) or alloys thereof can be used for the
metallic penetrating phase. Preferred metals include
copper, iron (Fe), stainless steel, nickel, titanium (Ti),
aluminum, magnesium (Mg), brass (Cu-Zn), bronze (Cu-Sn),
and nickel aluminide (NiAl). Further, high strength super
alloys and other high-grade metals can advantageously be
selected depending on the intended application of the
ceramic-metal composite material.
The ceramic matrix material is a sintered, coherent
body and should have an open and substantially continuous
pore structure to facilitate the infiltration of molten
metal into the matrix without the use of substantial
overpressure. Porous ceramics can be formed in a number of
ways known to those skilled in the art of ceramic
processing.
W094/06585 PCT/US93/08835
--11--
In one embodiment of the present invention, a green
body comprising a ceramic powder is formed and is sintered
in an enhanced vapor phase sintering process to form a
porous body that is particularly useful as the ceramic
matrix material. The porous ceramic body has a
substantially continuous and interconnected pore structure.
The total porosity and average pore size of the sintered
ceramic matrix can be controlled by controlling the
porosity of the green body and the sintering conditions.
Vapor phase sintering is a convenient process to produce
porous ceramics having controlled porosities and pore
sizes. As such, the porous ceramics form suitable matrices
for infiltration with molten metals to produce ceramic-
metal composites having interpenetrating three-dimensional
structures.
In this embodiment, a green body is preferably formed
comprising ceramic powder. In one embodiment of the
process, the powder has an average particle size of from
about 0.1 microns to about 2 microns. It is not believed
that the starting particle size is particularly critical to
the practice of the present invention, however, a smaller
average particle size can be used to produce a sintered
body having a lower average pore size.
The average particle size of the powder can
advantageously be reduced to a desired size by comminution
processes such as by using a ball mill or an attrition
mill. A ball mill is a hollow rotating cylinder or conical
cylinder partially filled with hard, wear-resistant media
W094/06585 PCT/US93/08835
2 ~ ~ S 1
-12-
that impacts the powder to reduce the particle size of the
powder. An attrition mill is a stirred-media mill wherein
a central shaft with arms rotates to mix the particles with
hard spherical media. The degree of reduction in particle
size can be controlled by controlling the amount of time in
the mill. Liquids can also be added to the mill charge to
assist in the comminution process and control agglomeration
of the particles.
According to one embodiment of the present invention,
it may be desirable to form agglomerates of the powder as
a means of controlling the porosity of a green body formed
from the powder. For example, aluminum hydroxide (Al(OH)3)
particles having a diameter of, for example, about 50
micrometers can be calcined to form alumina agglomerates
that have a porosity of about 50 percent. As used herein,
all percentages refer to volume percent, unless otherwise
noted.
After a ceramic powder having a desired particle size
range has been obtained, the powder can be formed into a
green body. As used herein, the term green body refers to
an unsintered cohesive body comprising ceramic powder. For
example, the powder can be uniaxially pressed at a pressure
of from about 48 MPa to about 69 MPa (7 ksi to 10 ksi) or
isostatically pressed at similar pressures. In addition,
forming additives can be used to improve the mechanical
strength of the green body formed by pressing the ceramic
powder. Additives can include binders such as polyvinyl
alcohol, plasticizers such as polyethylene glycol, and
W094/06585 PCT/US93/08835
~ ` `*~ 5 1 6 1
-13-
lubricants such as aluminum stearate. In addition, other
forming methods such as injection molding, extrusion, slip
casting and the like can be used to form green bodies
according to the present invention.
Further, some green bodies with high levels of forming
additives may have sufficient strength to enable the green
body to be machined. Thus, intricate parts may
advantageously be formed by mach;n;ng processes while the
piece is in the soft green state.
According to the present invention, one method for
controlling the total porosity of the sintered ceramic
matrix is to control the total porosity of the green body.
This can be done, for example, by varying the pressing
pressure. Typically, green bodies formed by uniaxially
pressing finely-divided ceramic powder have porosities
ranging from about 50 percent to about 65 percent. The
total porosity can be increased by using agglomerated
powder, as discussed hereinabove. In this embodiment, the
agglomerates having a porosity of about 50 percent are
pressed into an arrangement yielding a void space between
agglomerates of 50 percent to 65 percent. Thus, the
compact may have a total porosity of from about 70 percent
to about 80 percent.
After forming the green body, the green body can be
sintered to obtain a sintered ceramic body. If organic
binders or other organic materials are used in the green
body forming process, these additives can advantageously be
removed prior to fully sintering the ceramic powder. This
W094/06585 ~6~ PCT/US93/08835
-14-
is commonly referred to as "binder burnout." For example,
the green body can be placed in a furnace and slowly heated
to a temperature of, for example, about 600C to volatilize
organic additives. Since these organic additives comprise
a large amount of carbon, it is preferable to volatilize
these materials under a flowing gas such as oxygen.
In one embodiment of the present invention, the green
body is presintered. Presintering is a convenient and
economical method of controlling the total porosity of the
final sintered body. Presintering conveniently lowers the
porosity of the green body to a range that is desirable for
the sintered body, since the vapor phase sintering
technique does not substantially affect the total porosity
of the sintered body.
Preferably, the presintering step is done at a
temperature that is slightly below the normal solid-state
sintering temperature of the ceramic material. For
example, alumina can be presintered at a temperature of
from about 1300C to about 1600C, more preferably from
about 1450C to about 1550C. The sintering atmosphere is
not critical and, therefore, air is preferred. However,
certain atmospheres may be undesirable due to reactions
between the atmosphere and the ceramic material at the
sintering temperature. The presintering step preferably
produces a presintered body having a total porosity of from
about 10 percent to about 50 percent. The total porosity
can be controlled by varying the time at the presintering
temperature, such as from about 1 minute to about 300
W094/06585 PCT/US93/08835
~ ~tl4~ 61
-15-
minutes. The presintering step can determine the total
porosity of the final sintered body, however, presintering
may not be necessary if the green body has the desired
total porosity for the final sintered product.
The presintered or green ceramic body is then sintered
to form a porous sintered ceramic body. According to one
embodiment of the present invention, the ceramic body is
sintered in an enhanced vapor phase sintering mode in order
to maintain control over the total porosity and average
pore size of the sintered body.
Enhanced vapor phase sintering has been studied for
some ceramic materials. According to this process, the
ceramic is sintered in the presence of a volatile transport
gas at a high partial pressure. Preferably, the partial
pressure of the transport gas is at least about 10-4 atm at
the sintering temperature and more preferably at least
about 10-3 atm. It has been found that for some ceramic
materials, the vapor phase sintering process may be
enhanced by the presence of a reaction gas, particularly a
gas comprising a halide, in the sintering atmosphere. For
example, vapor phase sintering of magnesia can be enhanced
by the addition of hydrogen chloride (HCl) gas:
Mgo ( s) + 2HCl (g) ~ ~ MgC12 (~) + H2 o(~
W094/06585 ~ ~6~ PCT/US93/08835
-16-
In one embodiment of the present invention, an
alumina-containing body is sintered in the presence of
hydrogen chloride gas (HCl), thereby promoting the
reaction:
Al203 (~" + 6HC1 (g) ~ 2Al Cl3 (g) ~ 3H20(,;" (2 )
Alternatively, alumina may be sintered in the
presence of hydrogen fluoride gas (HF) in which case the
vapor phase transport occurs primarily via the process:
Al203(s, + 6HF(g) ` 2AlF(g) + 3H20(g) (3)
The reaction gas (e.g., HCl or HF) can be added to the
sintering furnace directly in the form of commercially
available bottled gas. In this embodiment, the gas should
be dry and contain minimal residual moisture. Residual
water (H2O) can drive the reaction back to, for example,
alumina formation and inhibit formation of the vapor
transport species. Preferably, the partial pressure of the
reaction gas is at least about 0.25 atm and is more
preferably from about 0.4 atm to about 1 atm.
Alternatively, the gas may be formed in-situ within
the sintering furnace. For example, aluminum fluoride
(AlF3) powder can be placed in a closed furnace. As the
furnace is heated, hydrogen gas is added to the furnace to
promote an in-situ reaction to form hydrogen fluoride gas
over the alumina. This procedure is particularly
W094/06585 PCT/US93/08835
~ 2 ;1 4 ~ 1 6
advantageous when dangerous gasses such as hydrogen
fluoride are used.
Sintering temperatures can vary depending on the
ceramic material being sintered For example, alumina
powder is preferably sintered at a temperature from about
1400C to about 1600C to form a sintered ceramic body.
Iron oxide may be sintered at 1300C or less. The pore
size and pore size distribution can be controlled by
adjusting the amount of time that the body is sintered at
the sintering temperatures. Table 1 lists the mean pore
diameter for alumina compacts sintered at 1600C for
varying amounts of time under 1 atm HCl. For each sample,
the porosity of the sample remained at about 50 percent
regardless of the sintering time.
TABLE 1
TIME MEAN PORE SIZE
lo min. 2.1 microns
80 min. 3 microns
250 min. 4 microns
201080 min. 7.5 microns
As Table 1 illustrates, as the sintering time
~ increases, the average pore diameter also increases.
However, the total porosity remains unchanged.
The ceramic body may be sintered in any system in
which the partial pressure of the reaction gas, and hence
W094/06585 PCT/US93/08835
2 1~5~
-18-
the transporting gas can be controlled. For example, a
simple tube furnace having a sealed end with an inlet for
the reaction gas may be provided.
It has also been observed that the sintered bodies
formed according to the present invention may have a thin
(e.g., about 1 grain thick), uniform skin of dense ceramic
on their surface. The formation of this skin can be
advantageous when the sintered bodies are used in filter
applications or if molten metal is infiltrated into the
pores to form a composite. The composite would thus have
a thin layer of ceramic on the surface and a base
comprising a thermally or electrically conductive metal.
Such a composite would be particularly useful as a
substrate for electronic applications. The density of the
skin appears best at higher sintering temperatures, such as
at about 1600C.
When a porous ceramic matrix having the desired total
porosity and pore size is obtained, molten metal can be
infiltrated into the void space of the ceramic matrix. In
a preferred embodiment of the present invention, the
ceramic is brought into contact with the molten metal and
infiltrates the ceramic by capillary action without the
assistance of substantial pressure. Thus, the molten metal
enters the pore structure of the ceramic and fills
substantially all of the void space. Preferably, the use
of infiltration aids that can alter the composition and
affect the properties of the composite are not used.
W094/06585 PCT/US93/08835
21~51~
--19--
In order to fill substantially all of the void space
in the ceramic matrix, it is necessary that the ceramic
matrix material have a three ~ime~ional, interconnecting
pore structure. Capillary action will pull the metal into
the ceramic and thereby fill substantially all of the void
space. Although the ideal pore size will vary depending on
the ceramic matrix material and metal being infiltrated, it
is generally desirable that the average pore size be from
about 1 micrometers to about 10 micrometers.
To improve capillary action between the ceramic and
the molten metal, it may be desirable to modify the wetting
or spreading characteristics of the ceramic and metal. One
way to do this is to coat the ceramic with a coating that
is more easily wetted by the molten metal. For instance,
the surfaces of a magnesia or alumina ceramic can be
modified by vapor phase coating the ceramic with nickel
oxide. Similarly, the surface of an alumina ceramic can be
modified by vapor phase coating the ceramic with copper
oxide. The result of the above surface modifications is
that the interfacial free energy of the ceramic is reduced
and the metal can penetrate the pores more easily.
Another way of enhancing the wetting characteristics
is to alter the chemical composition of the molten metal.
This is typically accomplished by doping the molten metal
with a dopant. For instance, molten copper can be doped
with from about 2 weight percent to about 5 weight percent
oxygen to form copper oxide (Cu2O) or copper can be doped
with from about 4 atomic percent to about 8 atomic percent
W O 94/06585 2 1 4 5 1 6 1 PC~r/US93/08835
,.
. . . ...
-20-
titanium (Ti). Doping reduces the interfacial free energy
between the metal and the ceramic.
After one or more of the surface modifications and
chemical alterations noted above, if necessary, the molten
metal will wet the ceramic and infiltrate substantially all
of the void space of the ceramic through capillary action.
In a preferred embodiment, the metal infiltration step
is performed in a vacuum atmosphere. As used herein,
vacuum atmosphere refers to an atmospheric pressure of
about 10 millitorr or less. The evacuation of air from the
ceramic void space reduces the likelihood that air pockets
will form in the metal infrastructure.
The temperature at which infiltration takes place is
dependent on the ceramic and molten metal used. In one
embodiment, an alumina ceramic with a copper oxide coating
and a 3 micrometer average pore size is infiltrated with
copper doped with about 3 weight percent oxygen at about
1275C. The total time required for infiltration is very
short and can occur in less than about l minute in most
cases.
The ceramic-metal composites produced by the present
invention have relatively high strengths and toughness. In
one embodiment, a composite comprising about 65 percent
alumina and about 35 percent copper/copper oxide. The
composite has a strength of at least about llO ksi.
According to one preferred embodiment of the present
invention, the sintered ceramic matrix material has a
porosity gradient. That is, the porous ceramic matrix has
W094/06S85 ~ ;. PCT/US93/08835
-21- ~i~ S1 61
regions of different porosity. For example, one portion of
the ceramic matrix can be substantially 100 percent dense
while another portion can have a high porosity, for example
about 60 percent or greater. When the porous end is
contacted with molten metal, the metal will infiltrate
throughout the ceramic porosity, resulting in an article
having a dense ceramic portion and a composite portion.
The porosity gradient may be a gradual through the material
or it may include one or more abrupt changes in porosity,
such as a ceramic matrix formed by layers of material
having different porosity characteristics. The advantages
of a gradient composite material can include the
alleviation of the effects of an abrupt thermal expansion
gradient, the ability to attach the composite to a variety
of materials and the ability to have an article with a
dense ceramic surface intimately attached to a composite
surface.
The ceramic-metal composites produced according to the
present invention are particularly useful as materials for
high temperature applications where creep resistance and
high toughness are needed. For example, in automotive
components such as valves, exhaust port liners, and seal
faces, turbine blades, electrical contacts, armor, boiler
tubes, and the like.
One particular application where the process of the
present invention has found particular utility is in the
field of filters. Ceramic filters comprise long
cylindrical bodies of porous ceramic, such as alumina. The
W094/06585 2 ~ 5161 PCT/US93tO8835
-22-
cylinders have a plurality of channels parallel to the
cylindrical axis for receiving the material (i.e. a liquid)
to be filtered. Since the opposite end of the cylinder is
sealed or recirculates the liquid to the front end, the
liquid is forced through the porous ceramic and is thereby
filtered. An example of such a filter is illustrated in
U.S. Patent No. 4,069,157 by Hoover et al., which is
incorporated herein by reference in its entirety.
One of the problems associated with manufacturing
these filters is sealing the ends of the filter.
Typically, the end of the filter must form a seal with a
metal component, such as a stainless steel ring. According
to one embodiment of the present invention, the end of the
filter is infiltrated with metal to assist in sealing the
filter. For example, the metal can be selected from copper
or a reactive braze such as a copper/silver/titanium alloy.
When copper is used to infiltrate into an alumina filter,
the resulting composite can be nickel coated to promote
adhesion to a stainless steel ring using, for example, a
Cu/Ag braze. When using a reactive braze, no brazing
material is necessary and the stainless steel ring can be
adhered to the filter by heating the components while in
contact.
Another application of the present invention is in the
area of engine component liners, such as exhaust port
liners for diesel engines. See, for example, the port
liners described in U.S. Patent No. 5,066,626 by Fukao et
al., which is incorporated herein by reference in its
W094/06585 2 1 -~ ~l 61
-23-
entirety. Aluminum titanate is a preferred ceramic
material due to its resistance to corrosion and its low
thermal expansion characteristics. One of the problems
associated with these port liners is that a compliant layer
between the metal (e.g. aluminum or cast iron) and the
aluminum titanate is necessary to absorb stresses resulting
from the contracting metal.
It is believed that the problem can be reduced by
infiltrating an aluminum titanate material having a
gradient porosity. The metal composite gradient will
assist in alleviating the stresses caused by the
contraction of the metal. For example, aluminum could be
infiltrated into the aluminum titanate porous body.
ExamPles
An alumina powder (CERALOX HPA, Ceralox Corp., Tucson,
AZ) is obtained having an average particle size of about
1.0 microns. The alumina powder is formed into a green
body by uniaxially pressing the powder at a pressure of
about 50 MPa to obtain a green body having a porosity of
about 55 percent.
The green body is then presintered in air at a
temperature of about 1500C for about 3 minutes. The
presintered body has a porosity of about 35 percent.
The presintered body is then placed in a sintering
furnace that comprises an alumina tube. The temperature of
the furnace is raised as the furnace is evacuated. Before
reaching about 800C, the furnace is purged with argon gas
W094/06585 21~ 51 61i PCT/US93/08835
-24-
to remove impurities in the furnace atmosphere. At about
800C, the furnace is filled with HCl gas having a pressure
of about 1 atm. The alumina body is then sintered under
HCl gas at a temperature of about 1600C for about 80
minutes.
The sintered alumina ceramic has a total porosity of
about 35 percent and has an average pore size of about 3
micrometers. The alumina forms a continuous three-
dimensional structure and there is substantially no closed
porosity.
~Thereafter, the alumina ceramic is contacted with a
molten copper bath at a temperature of about 1275C. The
bottom surface of the alumina ceramic is contacted with the
molten metal and the molten metal infiltrates through the
alumina matrix via capillary action. The composite is then
cooled. The composite comprises about 65 percent of a
substantially continuous alumina phase and about 35 percent
copper/copper oxide.
The stress strain diagram for the sample is
illustrated in Figure 1. The composite has a strength of
about 112 ksi.
To demonstrate the feasibility of infiltrating
aluminum titanate, three disks of a porous aluminum
titanate material were obtained. Three metal beads of 1)
copper, 2) iron and 3) stainless steel were placed on an
aluminum titanate disk and heated to just above the melting
point of the metal. Each of the metals infiltrated the
W094/06585 1 PCT/US93/08835
2I~5l6i
-25-
aluminum titanate and formed a ceramic-metal composite with
the aluminum titanate.
While various embodiments of the present invention
have been described in detail, it is apparent that
modifications and adaptations of those embodiments will
occur to those skilled in the art. However, it is to be
expressly understood that such modifications and
adaptations are within the spirit and scope of the present
lnvention.