Note: Descriptions are shown in the official language in which they were submitted.
;
2i4~61
H 267 PCT / 26.05.19~3
~air treatment preparations
This invention relates to hair treatment prepara-
tions containing a special combination of active in-
gredients.
The washing and care of hair is an important part
5 of personal hygiene. Both the washing of hair with
shampoos and the decorative finishing of hair styles,
for example by coloring or permanent waving, are meas-
ures which affect the natural structure and properties
of hair. For example, the wet and dry combability of
hair, its hold and its body can be adversely affected
or the number of split ends can be increased by such
measures. In addition, the uniform distribution of dyes
applied with hair coloring preparations can often be
very problematical, particularly on hair which has been
seriously damaged.
Accordingly, it has long been standard practice
to subject the hair to a special aftertreatment. To
this end, the hair is treated, normally by rinsing, with
special active substances, for example quaternary
ammonium salts or special polymers. Combability, hold
and body are improved and the number of split ends are
reduced by this treatment, depending on the formulation
of the preparation used. Nevertheless, the results for
colored predamaged hair are still unsatisfactory.
It has now surprisingly been found that prepara-
tions containing a combination of representatives of two
specific classes of active substances each known for
their useability in hair treatment preparations have an
unexpectedly beneficial effect on the properties of
seriously predamaged, colored hair. The treatment of
seriously predamaged, colored hair with the preparations
in question produces above all a pronounced levelling
; ~ 21~58~1
H 267 PCT 2
effect with, surprisingly, no significant change in
color. Accordingly, the hair can be washed and after-
treated far more frequently before having to be recolor-
ed. In addition, the favorable biodegradability of the
combination of active ingredients provides for the
formulation of products which satisfy the ecological
requirement of ready degradability.
Accordingly, the present invention relates to the
use of a water-based preparation containing a combina-
tion of active ingredients consisting of
(A) alkoxylated wool wax alcohols and
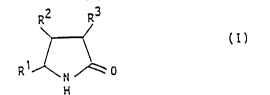
(B) a pyrrolidone carboxylic acid and/or pyrrolidone
carboxylic acid derivative corresponding to
15general formula (I)
R2 R3
>--~ (I)
1 /
R ~ ~ 0
in which at least one of the substituents Rl to R3
is a group -CooR4l where R4 is hydrogen, an alkali
metal ion, an alkaline earth metal ion or an
ammonium ion+NHR5R6R7, where R5 to R7 independently
of one another represent hydrogen, C~22 alkyl
groups, Cl4 hydroxyalkyl groups, C222 alkenyl
groups, C222 acyl groups or optionally substituted
C6l0 aromatic groups, and the remaining substitu-
ents Rl to R3 are hydrogen or Cl4 alkyl groups,
and typical cosmetic constituents for the treatment of
hair, more particularly for the washing and care of
hair.
Both the use of ethoxylated wool wax alcohols
(see, for example, H.P. Fie~ler, Lexikon der Hilfsstoffe
~ t ~l~iS~
H 267 PCT 3
fur Pharmazie, Kosmetik und angrenzende Gebiete, 2nd
Edition, Editio Cantor Aulendorf, 1981, page 724) and
the use of compounds corresponding to formula (I) (see,
for example, German patent application P 41 31 898.6)
for the treatment of hair are known.
DE-C2-33 30 628 (see in particular Example 2)
describes skin protection and skin care lotions contain-
ing ethoxylated wool fat alcohols and the sodium salt of
pyrrolidone carboxylic acid. However, there is nothing
in this document to suggest that the combination in
question has any effect on hair.
Wool wax alcohols, which are also known as
lanolin alcohols, are complex mixtures of sterols and
aliphatic alcohols which are obtained from wool wax.
The principal constituents of wool wax alcohols are
cholesterol, lanosterol and sterols derived therefrom,
Cl830 n-alcohols, C1626 isoalcohols, alkanediols and
isoalkanediols.
The reaction of these mixtures with alkylene
oxides, particularly ethylene oxide and propylene oxide,
gives the alkoxylated compounds which are known inter
alia as emulsifiers, solubilizers, moisturizers and
conditioners. These products are normally not uniformly
alkoxylated compounds, but instead are characterized by
a homolog distribution determined by the particular
alkoxylation process applied. The degree of alkoxyla-
tion mentioned is always based on the molar starting
quantities of wool wax alcohol and alkylene oxide.
Ethoxylated wool wax alcohols are preferred for
the purposes of the invention. Degrees of ethoxylation
of 1 to 50 and, above all, 2 to 30 are particularly
preferred.
The compounds (B) are derivatives of 2-pyrroli-
done. Preferred derivatives are 2-pyrrolidone-3-, -4-
and -5-carboxylic acid and salts thereof. Preferred
.~ ~ 214S861
H 267 PCT 4
salts of these compounds are the sodium, potassium,
calcium and magnesium salts and ammonium salts in which
the ammonium ion bears 1 to 3 Cl4 alkyl groups in
addition to hydrogen.
Particularly preferred compounds (A) are 2-
pyrrolidone-5-carboxylic acid and its salts. The sodium
salt is particularly preferred.
The preparations used in accordance with the
invention preferably contain the alkoxylated wool wax
alcohols (A) in quantities of 0.1 to 5% by weight, based
on the preparation as a whole. The compounds (B) are
also preferably present in quantities of 0.1 to 5~ by
weight.
Prepara~ions containing a polymeric compound in
addition to the compulsory components (A) and (B) are
particularly suitable for the treatment of hair. Pre-
ferred polymers are those from the group of cationic,
anionic and amphoteric polymers.
Suitable cationic polymers typically contain a
quaternary nitrogen atom, for example in the form of an
ammonium group. Preferred cationic polymers are, for
example,
- Quaternized cellulose derivatives of the type
commercially available under the names of Cel-
quat~ and Polymer JR~. The compounds Celquat H
100, Celquat L 200 and Polymer JR~ 400 are
preferred quaternized cellulose derivatives.
- Polysiloxanes containing quaternary groups,
- Polymeric dimethyl diallyl ammonium salts and co-
polymers thereof with esters and amides of
acrylic acid and methacrylic acid. The products
commercially available under the names of Mer-
quat~ 100 (poly(dimethyl diallyl ammonium chlo-
ride)) and Merquat~ 550 (dimethyl diallyl ammoni-
= ~ ~
'-- 21~61
H 267 PCT 5
um chloride/acrylamide copolymer) are examples of
such cationic polymers.
- Copolymers of vinyl pyrrolidone with quaternized
derivatives of dialkyl aminoacrylate and meth-
acrylate, for example vinyl pyrrolidone/dimethyl
aminomethacrylate copolymers quaternized with
- diethyl sulfate. Compounds such as these are
commercially available under the names of Gaf-
quat0 734 and Gafquat0 755.
- Vinyl pyrrolidone/methoimidazolinium chloride
copolymers of the type commercially available
under the name of Luviquat0.
- Quaternized polyvinyl alcohol
and the polymers containing quaternary nitrogen atoms in
15 the polymer main chain known under the following names:
- Polyquaternium 2,
- Polyquaternium 17,
- Polyquaternium 18 and
- Polyquaternium 27.
Examples of anionic polymers suitable for the
purposes of the invention are:
- Polyacrylic and polymethacrylic acids, salts
thereof, copolymers thereof with esters and
amides of (meth)acrylic acid and derivatives
thereof obtained by crosslinking with polyfunc-
tional agents. Compounds of this type are
commercially available, for example, under the
names of Carbopol0 934, Carbopol0 934P, Carbopol~
940, Carbopol0 950, Carbopol0 980 and ~ostacerin0
PN 73.
- Poly~xycarboxylic acids, such as polyketocar-
boxylic and polyaldehydocarboxylic acids and
salts thereof, such as for example PoC0 HS 5060
! ~ 2 1 ~ S 8 ~ 1
H 267 PCT 6
and POC~ AS 5060.
- Polymers and copolymers of crotonic acid with
esters and amides of (meth)acrylic acid, such as
vinyl acetate/crotonic acid and vinyl acetate/
vinyl propionate/crotonic acid copolymers.
Compounds of this type are commercially available
as Luviset~ CA-66 and Luviset~ CAP.
In the context of the invention, amphopolymers
10 are understood to be amphoteric polymers, i.e. polymers
which contain both free amino groups and free -COOH or
SO3H groups in the molecule and which are capable of
forming inner salts, zwitterionic polymers containing
quaternary ammonium groups and -COO~ or -SO3- groups in
the molecule and polymers containing -COOH or SO3H groups
and quaternary ammonium groups.
One example of an amphopolymer suitable for use
in accordance with the invention is the acrylic resin
commercially available as Amphomer~ which is a copolymer
of tert. butyl aminoethyl methacrylate, N-(1,1,3,3-
tetramethylbutyl)-acrylamide and two or more monomers
from the group consisting of acrylic acid, methacrylic
acid and simple esters thereof.
Other amphopolymers suitable for use in accord-
ance with the invention are the compounds mentioned in
GB--A--2,104,091, EP--A--47 714, EP--A--217 274, EP--A--283 817
and DE--A--28 17 369.
Preferred amphopolymers are polymers consisting
essentially of
(a) monomers containing quaternary ammonium groups
corresponding to general formula (II):
R8-CH=CR9-CO-Z-(CnH2n)-N(+)RlRllRl2 A() (II)
in which R3 and R9 independently of one another
21~5861
.~,
H 267 PCT 7
represent hydrogen or a methyl group and R10, R
and Rl2 independently of one another represent C1_4
alkyl groups, Z is an NH group or an oxygen atom,
n is an integer of 2 to 5 and A(-) is the anion of
an organic or inorganic acid
and
(b) monomeric carboxylic acids corresponding to
general formula (III)
R13_CH=cRl4-CooH (III)
in which R13 and R14 independently of one another
represent hydrogen or methyl groups.
These compounds may be used in accordance with
the invention both directly and in solid form, i.e.
after neutralization of the polymers, for example with
an alkali metal hydroxide. Full particulars of the
production of these polymers can be found in DE-A-39 29
973. Polymers in which monomers of type (a), where Rl,
Rl1 and R12 are methyl groups, Z is an NH group and A() is
a halide, methoxysulfate or ethoxysulfate ion, are most
particularly preferred. Acrylamidopropyl trimethyl
ammonium chloride is a particularly preferred monomer
(a). Acrylic acid is preferably used as monomer (b) for
the polymers mentioned.
The preparations according to the invention
preferably contain the cationic, anionic or amphoteric
polymers in quantities of 0.1 to 5% by weight, based on
the preparation as a whole.
According to the invention, it is most particu-
larly preferred to use preparations which contain a
polymer from the group of amphoteric polymers.
~. 21~61
H 267 PCT 8
Preferred preparations according to the invention
additionally contain at least one surfactant selected
from the group of cationic, anionic or nonionic surfac-
tants.
5In cases where the preparations according to the
invention are used in the form of hair rinses, it is
particularly to use surfactants from the group of
cationic surfactants.
Examples of cationic surfactants suitable for use
10in the hair treatment preparations according to the
invention are, in particular, quaternary ammonium
compounds, preferably ammonium halides, especially
chloride and bromides, such as alkyl trimethyl ammonium
chlorides, dialkyl dimethyl ammonium chlorides and
15trialkyl methyl ammonium chlorides, for example cetyl
trimethyl ammonium chloride, stearyl trimethyl ammonium
chloride, distearyl dimethyl ammonium chloride, lauryl
dimethyl ammonium chloride, lauryl dimethyl benzyl
ammonium chloride and tricetyl methyl ammonium chloride.
20Other cationic surfactants suitable for use in accord-
ance with the invention are quaternized protein hydroly-
zates.
Also suitable for use in accordance with the
invention are cationic silicone oils, such as for
25example the commercially available products Q2-7224 (a
stabilized trimethyl silyl amodimethicone; a product of
Dow Corning), Dow Corning 929 Emulsion (containing a
hydroxylamino-modified silicone which is also known as
amodimethicone), SM-2059 (General Electric), SLM-55067
30(Wacker) and Abil$-Quat 3270 and 3272 (Th. Goldschmidt;
diquaternary polydimethyl siloxanes, Quaternium-80).
Apart from a favourable conditioning effect,
alkyl amidoamines, particularly fatty acid amidoamines,
such as the stearyl amidopropyl dimethyl amine commer-
35cially available as Tego Amid$ S 18, are distinguished
~' 21458~1
H 267 PCT g
in particular by their ready biodegradability.
Quaternary ester compounds, so-called "ester-
quats", such as the dialkylammonium methosulfates and
methyl hydroxyalkyl dialkoyloxyalkylammonium methosul-
fates marketed as Stepantex~, also show ready biodegrad-
ability.
One example of a quaternary sugar derivative
suitable for use as a cationic surfactant is the commer-
cial product Glucquat~ 100 which bears the CTFA name
"Lauryl Methyl Gluceth-10 Hydroxypropyl Dimonium Chlo-
ride".
Particularly preferred cationic surfactants are
alkyl amidoamines, quaternary ester compounds and
quaternary sugar derivatives.
Shampoos used in accordance with the invention
preferably contain surfactants from the group of anionic
surfactants.
Anionic surfactants suitable for the preparations
according to the invention are any of the anionic sur-
factants which are suitable for use on the humar. body.They are characterized by a water-solubilizing anionic
group, for example a carboxylate, sulfate, sulfonate or
phosphate group, and a lipophilic alkyl group containing
approximately 10 to 22 carbon atoms. Glycol or polygly-
col ether groups, ester, ether and amide groups and alsohydroxyl groups may also be present in the molecule.
The following are examples of suitable anionic surfac-
tants in the form of the sodium, potassium and ammonium
salts and also the mono-, di- and trialkanolammonium
salts containing 2 or 3 carbon atoms in the alkanol
group:
- linear fatty acids containing 10 to 22 carbon
atoms (soaps),
- ether carboxylic acids corresponding to the
~ ~14~861
H 267 PCT 10
formula R-O-(CH2-CH2O)X-CH2-C00H, in which R is a
linear alkyl group containing 10 to 22 carbon
atoms and x = 0 or 1 to 16,
- acyl sarcosides containing 10 to 18 carbon atoms
in the acyl group,
- acyl taurides containing 10 to 18 carbon atoms in
the acyl group,
- acyl isethionates containing 10 to 18 carbon
atoms in the acyl group,
- sulfosuccinic acid mono- and dialkyl esters con-
taining 8 to 18 carbon atoms in the alkyl group
and sulfosuccinic acid monoalkyl polyoxyethyl
esters containing 8 to 18 carbon atoms in the
alkyl group and 1 to 6 oxyethyl groups,
- linear alkane sulfonates containing 12 to 18
carbon atoms,
- linear alpha-olefin sulfonates containing 12 to
18 carbon atoms,
- alpha-sulfofatty acid methyl esters of fatty
acids containing 12 to 18 carbon atoms,
- alkyl sulfates and alkyl polyglycol ether sul-
fates corresponding to the formula R-O(CH2-CH2O)X-
OSO3H, in which R is a preferably linear alkyl
group containing 10 to 18 carbon atoms and x = 0
or 1 to 12,
- mixtures of surface-active hydroxysulfonates
according to DE-A-37 25 030,
- sulfated hydroxyalkyl polyethylene and/or hy-
droxyalkyl propylene glycol ethers according to
DE-A-37 23 354,
- sulfonates of unsaturated fatty acids containing
12 to 24 carbon atoms and 1 to 6 double bonds
according to DE-A-39 26 344,
- esters of tartaric acid and citric acid with
alcohols which are adducts of approximately 2 to
~' 2145~1
H 267 PCT 11
15 molecules ethylene oxide and/or propylene
oxide with fatty alcohols containing 8 to 22
carbon atoms.
S Preferred anionic surfactants are alkyl sulfates,
alkyl polyglycol ether sulfates and ether carboxylic
acids containing 10 to 18 carbon atoms in the alkyl
group and up to 12 glycol ether groups in the molecule
and also sulfosuccinic acid mono- and dialkyl esters
containing 8 to 18 carbon atoms in the alkyl group and
sulfosuccinic acid monoalkyl polyoxyethyl esters con-
taining 8 to 1~ carbon atoms in the alkyl group and 1 to
6 oxyethyl groups.
Nonionic surfactants contain, for example, a
polyol group, a polyalkylene glycol ether group or a
combination of polyol and polyglycol ether groups as the
hydrophilic group. Examples of compounds such as these
are
- adducts of 2 to 30 mol ethylene oxide and/or 0 to
5 mol propylene oxide with linear fatty alcohols
containing 8 to 22 carbon atoms, with fatty acids
containing 12 to 22 carbon atoms and with alkyl-
phenols containing 8 to 15 carbon atoms in the
alkyl group,
- C122z fatty acid monoesters and diesters of
adducts of 1 to 30 mol ethylene oxide with
glycerol,
- C822 alkyl mono- and oligoglycosides and ethoxy-
lated analogs thereof and
- adducts of 5 to 60 mol ethylene oxide with castor
oil and hydrogenated castor oil.
The compounds containing alkyl groups used as
35 surfactants may be individual substances. However, it
~i` 214~861
H 267 PCT 12
is generally preferred to produce the compounds in
question from native vegetable or animal raw materials
so that mixtures of compounds differing in their chain
length according to the particular raw material used are
obtained.
The surfactants which are adducts of ethylene
and/or propylene oxide with fatty alcohols or deriva-
tives of such adducts may be both products having a
"normal-range" homolog distribution and also products
having a "narrow-range" homolog distribution. "Normal-
range" products are mixtures of homologs which are
obtained in the reaction of fatty alcohol and alkylene
oxide using alkali metals, alkali metal hydroxides or
alkali metal alcoholates as catalysts. By contrast,
narrow-range products are obtained when, for example,
hydrotalcites, alkaline earth metal salts of ether
carboxylic acids, alkaline earth metal oxides, hydrox-
ides or alcoholates are used as catalysts. It mey be
preferable to use narrow-range products.
The preparations used in accordance with the in-
vention preferably contain the surface-active compounds
in quantities of 0.5 to 30% by weight and, more
particularly, in quantities of 1 to 20% by weight, based
on the particular preparation.
The preparations used in accordance with the
invention preferably have a pH value of 2.5 to 7 and,
more particularly, in the range from 4.0 to 6Ø
Virtually any acid suitable for cosmetic purposes
may be used to establish this pH value. It may be
preferable to use an acid from the group of edible acids
which are understood to be those acids which are con-
sumed as part of the normal human diet and which have
positive effects on the organism. The acids in question
are, for example, acetic acid, lactic acid, tartaric
acid, citric acid, malic acid, ascorbic acid and gluco-
~ 21~58S~
H 267 PCT 13
nic acid. According to the invention, it is particular-
ly preferred to use citric acid and lactic acid.
The hair treatment preparations used in accord-
ance with the invention are preferably rinses, shampoos,5 hair tonics, coloring and tinting shampoos and creams.
However, the combination of active ingredients according
to the invention may also be used in hair coloring
preparations and in permanent wave preparations.
Depending on the intended purpose, the preparations are
preferably formulated as aqueous or aqueous/alcoholic
solutions, gels, creams or lotions.
Depending on the intended purpose and the partic-
ular formulation, the preparations according to theinvention may contain any of the cosmetic additives
typically used for the particular purpose envisaged,
namely:
- amphoteric surfactants such as, for example, N-
alkyl glycines, N-alkyl propionic acids, N-alkyl
aminobutyric acids, N-alkyl iminodipropionic
acids, N-hydroxyethyl-N-alkylamidopropyl gly-
cines, N-alkyl taurines, N-alkyl sarcosines, 2-
alkylaminopropionic acids and alkylaminoacetic
acids all containing approximately 8 to 18 carbon
atoms in the alkyl group,
- zwitterionic surfactants such as, for example,
the so-called betaines and 2-alkyl-3-carboxymeth-
yl-3-hydroxyethyl imidazolines,
- nonionic polymers such as, for example, vinyl
pyrrolidone/vinyl acrylate copolymers, polyvinyl
pyrrolidone and vinyl pyrrolidone/vinyl acetate
copolymers,
- substantive dyes,
- so-called coupler and developer components as
oxidation dye precursors,
~' 21~5861
H 267 PCT 14
- reducing agents such as, for example, thiogly-
colic acid and derivatives thereof, thiolactic
acid, cysteamine, thiomalic acid and ~-mercapto-
ethane sulfonic acid,
- oxidizing agents, such as hydrogen peroxide,
potassium bromate and sodium bromate,
- thickeners, such as agar agar, guar gum, algin-
ates and xantham gum,
- structurants, such as glucose and maleic acid,
- hair-conditioning compounds, such as phospholi-
pids, for example soya lecithin, egg lecithin and
cephalins, and also silicone oils,
- protein hydrolyzates, more particularly elastin,
collagen, keratin, milk protein, soya protein,
almond protein, pea protein, potato protein, corn
protein and wheat protein hydrolyzates, condensa-
tion products thereof with fatty acids and
quaternized protein hydrolyzates,
- perfume oils, dimethyl isosorbide and cyclodex-
trins,
- solubilizers, such as ethanol, isopropanol,
ethylene glycol, propylene glycol, glycerol and
diethylene glycol,
- dyes,
- antidandruff agents, such as Piroctone Olamine
and Zinc Omadine,
- other substances for pH adjustment,
- active substances, such as panthenol, allantoin,
plant extracts and vitamins,
- light stabilizers,
- consistency regulators, such as sugar esters,
polyol esters or polyol alkyl ethers,
- fats and waxes, such as spermaceti, beeswax,
montan wax, paraffins and fatty alcohols,
- fatty acid alkanolamides,
~ ~ 214~861
H 267 PCT 15
- complexing agents, such as EDTA, NTA and phos-
phonic acids,
- swelling and penetration agents, such as glycer-
ol, propylene glycol monoethyl ether, carbonates,
hydrogen carbonates, guanidines, ureas and
primary, secondary and tertiary phosphates,
- opacifiers, such as latex,
- pearlescers, such as ethylene glycol monostearate
and distearate,
- propellents, such as propane/butane mixtures, N2O,
dimethyl ether, CO2 and air and
- antioxidants.
The combination of active ingredients according
to the invention is preferably used in hair treatment
preparations which are rinsed off from the hair after a
certain contact time of, generally, a few seconds to
about 20 minutes.
Accordingly, the present invention also relates
to a process for the treatment of hair in which, after
treatment with a preparation as claimed in any of claims
1 to ll, the hair is rinsed with water or with a prepar-
ation largely containing water.
The following Examples are intended to illustrate
the invention.
E x a m p l e s
I. Colorimetric tests to determine color retention
in colored hair
Method
A levelling tress (natural white, 2 g, approx. 15
cm long; obtainable from the Kerling company) was tied
halfway up. To simulate seriously damaged hair, the
21~861
H 267 PCT 16
lower part of the tress was alternately cold-waved twice
and ultra-bleached twice. Cold waving was carried out
by treatment with an aqueous solution of a cold-wave
preparation based on ammonium thioglycolate (30 minutes)
and subsequent fixing with potassium bromate solution
(10 minutes). Super bleaching was carried out with an
aqueous solution of hydrogen peroxide and ammonium
peroxydisulfate. To simulate lightly damaged hair, the
upper part was only ultra-bleached once. The entire
tress was then colored with Poly Diadem Pflege Intensiv
Tonung (shade: mahogany coral), a product of Henkel
KGaA. The coloring mixture was used in a quantity of 4
g per g tress. After a contact time of 30 minutes, the
tresses were rinsed with warm water (30-C) and dried
with a blow dryer. The colored hair tresses were then
stored for 4 days at room temperature.
After colorimetric determination of the zero
values, the hair tresses were treated with the test mix-
ture 6 times for 1 minute, the tresses being thoroughly
rinsed with water (30C) after each treatment and dried
with a blow dryer. The tresses were then colorimetric-
ally evaluated using a Datacolor Texflash with D65 light
(daylight). The sample to be evaluated was clamped to
the spectral photometer and the remission values were
measured over the visible light range from 390 to 700 nm
at intervals of 10 nm and processed by computer. The
computer program determined the standard color values
according to the CIE system (Commission Internationale
de L'Eclairage), corresponding to DIN 5033, and conver-
ted them into color intervals according to DIN 6174.
The measured values shown in the following are all
averages of 4 measuring points per tress half.
The followlng values were determined:
Total color interval DE (relative to the zero
~ 214~61
H 267 PCT 17
value) of the seriously
damaged part of the tresses
Levelling L (difference between the to-
tal color intervals DE
after the treatment of
seriously damaged and
lightly damaged hair)
Percentage color strength S of the seriously damaged
part of the tresses (zero
value = 100%).
The compositions of the mixtures tested and the
results obtained are set out in Table 1 below.
Table 1 rOuantities in parts bY weiqht]
Component / mixture C1 C2 C3 Il I2
Texapon~N 251 42.0 42.0 42.0 42.0 42.0
Euperlan~PK 4052 8.0 8.0 8.0 8.0 8.0
Akypo~RLM 100 NV3 5.0 5.0 5.0 5.0 5.0
Edenor~KPK+9.2 Eo4 2.0 2.0 2.0 2.0 2.0
Dehyton~K5 10.0 10.0 10.0 lo.0 lo.0
Polymer P1 according to
DE 39 29 973* 1.2 1.2 1.2 1.21.2
Plantaren~12006 2.0 2.0 2.0 2.02.0
Polychol~5' - - 1.0 1.0
Polychol~2 o3 - - - - 1 . O
Ajidew~N 509 - 0.8 - 0.80.8
Perfume oil, preservative,<------- ad 100 ->
sodium chloride, citric acid,
water
pH 5.0 4.7 4.9 5.15.1
DE (seriously damaged hair) 16.9 17.5 17.7 10.4 15.8
L 11.2 9.8 8.8 1.9 6.9
Color strength % (seriously
damaged hair) 34.5 26.7 27.0 53.8 31.0
~' 214S8~1
H 267 PCT 18
Sodium lauryl ether sulfate (approx. 28% active
substance; CTFA name: Sodium Laureth Sulfate)
(HENKEL)
2 Fatty alcohol ether sulfate pearlescing mixture
(18.5% active substance; CTFA name: Sodium Laureth
Sulfate (and) Glycol Distearate) (HENKEL)
3 Cl214 fatty alcohol+l0 ethylene oxide acetic acid
sodium salt (22% active substance; CTFA name:
Sodium Laureth-ll Carboxylate) (CHEM-Y)
0 4 Fatty acid polyglycol ester (HENKEL)
5 Fatty acid amide derivative, betaine structure,
corresponding to the formula R-CONH(CH2)3N+(CH3) 2-
CH2COO- (approx. 30% active substance; CTFA name:
Cocoamidopropyl Betaine) (HENKEL)
6 C12-16 alkyl glucoside, degree of oligomerization
1.4 (approx. 50% active substance; CTFA name:
Lauryl Polyglycoside) (HENKEL)
7 Lanolin alcohol + 5 ethylene oxide (CTFA name:
Laneth-5) (CRODA)0 8 Lanolin alcohol + 20 ethylene oxide (CTFA name:
Laneth-20) (CRODA)
9 DL-2-pyrrolidone-5-carboxylic acid sodium salt
(approx. 50% active substance; CTFA name: Sodium
PCA) (AJINOMOTO)
* Acrylamidopropyl trimethyl ammonium chloride/acry-
lic acid copolymer, neutralized with sodium hy-
droxide
II. Application ExamPles
The quantities in the following Examples are %
by weight.
1) Hair rinse
Stenol~16181 1.8
~ ` 214~61
H 267 PCT 19
Tegoamid~S 1811 1.6
1,2-Propylene glycol l.O
Citric acid 0.6
Polychol05 0.5
Ajidew0N 50 0.6
Perfume oil, water ad lOO
C16/Cl8 fatty alcohol (HENKEL)
11 N,N-dimethyl-N'-stearoyl-1,3-diaminopropane (CTFA
name: Stearamidopropyl Dimethylamine) (GOLD-
SCHMIDT)
2) Hair rinse
Stenol~1618 , 1.8
Stepantex0VS go12 1.8
1,2-Propylene glycol 0.7
Citric acid O.9
Polychol05 0.5
Ajidew~N 50 0.8
Perfume oil, dyes wdter ad lOO
12 N-methyl-N-(2-hydroxyethyl)-N,N-di-(tallowacyloxy-
ethyl)-ammonium methosulfate (9O~ active substance
in isopropanol) (STEPAN)
3) Hair rinse
Stenol~1618 2.5
Eumulgin0B 113 l.O
Eumulgin~B 214 l.O
Cutina~CP15 l.O
Eutanol~G16
Polawax~GP 2Oo17 0.75
Abil~Quat 327013 0.5
Dow Corning~929-Emulsion19 2.6
Polychol~5 l.O
21~8Sl
~`
H 267 PCT 20
Ajidew~N 50 1.5
Citric acid 0.02
Water ad 100
13 Cetostearyl alcohol containing approx. 12 mol EO
(CTFA name: Ceteareth-12) (HENKEL)
4 Cetostearyl alcohol containing approx. 20 mol EO
(CTFA name: Ceteareth-20) (HENKEL)
15 Ester of saturated long-chain fatty alcohols and
fatty acids, primarily palmitic acid cetyl ester
CTFA name: Cetyl Palmitate) (HENKEL)
16 Condensation product of saturated liquid fatty
alcohols, primarily decyl alcohol, prepared by the
Guerbet reaction (CTFA name: Octyl Dodecanol)
(HENKEL)
7 Stearyl alcohol/polyethylene glycol stearate mix-
ture (CTFA name: Stearyl Alcohol (and) PEG-
Stearate) (CRODA)
18 Diquaternary polymethyl siloxane (CTFA name:
Quaternium-80) (GOLDSCHMIDT)
9 Aminofunctional polydimethyl siloxane (35% active
substance) (DOW CORNING)
4) Hair rinse
Rewoquat~W 750 02 1 .1
Lanette o2~ 3.0
Eumulgin~B 1 0.8
Eumulgin~B 2 1.6
Cutina~GMS22 0.5
Eutanol~G 1.0
Polychol~5 2.0
Ajidew~N 50 1.5
Water ad 100
1-Methyl-2-nortallow alkyl-3-tallow fatty acid
~`` 214~861
H 267 PCT 21
amidoethyl imidazolinium methosulfate (approx. 75%
active substance) (REWO)
21 Mixture of higher saturated fatty alcohols, pri-
marily cetyl alcohol and stearyl alcohol (CTFA
name: Cetearyl Alcohol) (HENKEL)
22 Glycerol monostearate (CTFA name: Glyceryl
Stearate) (HENKEL)
5) Shampoo
Texapon~N 25 43.0
Dehyton~K 10.0
Plantaren~-1200 4.0
Euperlan~PK 300023 1.6
Arquad~31624 0.8
Polychol~20 3.0
Ajidew~N 50 4.0
Glucamate~DOE 12025 0.5
Sodium chloride 0.2
Water ad 100
23 Liquid dispersion of pearlescers and amphosurfac-
tant (approx. 62% active substance; CTFA name:
Glycol Distearate (and) Glycerin (and) Laureth-4
(and) Cocoamidopropyl Betaine) (HENKEL)
24 Tri-C16-alkyl methyl ammonium chloride (AKZO)
Ethoxylated methyl glucoside dioleate (CTFA name:
PEG-120 Methyl Glucose Dioleate) (AMERCHOL)
6) Shampoo
Texapon~N 7026 21.0
Plantaren¢-1200 8.0
Genamin~DSAC27 1.2
Cutina~EGMS2B 0.6
Polychol~20 2.0
Ajidew A 10029 2.8
~` 2145~61
H 267 PCT 22
Antil~1413 1.3
Sodium chloride 0.2
Magnesium hydroxide ad pH 4.5
Water ad 100
26 Sodium lauryl ether sulfate (approx. 72% active
substance) (HENKEL)
27 Dimethyl distearyl ammonium chloride (HOECHST)
28 Ethylene glycol monostearate (approx. 25-35% mono-
ester, 60-70% diester; CTFA name: Glycol Stearate)
(HENKEL)
29 DL-2-pyrrolidone-5-carboxylic acid (CTFA name:
PCA) (AJINOMOTO)
30 Polyoxyethylene propylene glycol dioleate (40%
active substance: CTFA name: Propylene Glycol
(and) PEG 55 Propylene Glycol Oleate) (GOLD-
SCHMIDT)
7) Shampoo
Texapon~K 14 S31 50.0
Dehyton~K 10.0
Akypo~RLM 100 NV 4.5
Polymer Pl acc. to DE 39 29 973 0.6
CUtina~AGS32 2.0
D-Panthenol 0.5
Glucose 1.0
Salicylic acid 0.4
Sodium chloride 0.5
Polychol~20 2.5
Ajidew~N 50 2.0
Water ad 100
31 Sodium lauryl myristyl ether sulfate (approx. 28%
active substance; CTFA name: Sodium Myreth Sul-
fate) (HENKEL)
~` 214~86 1
H 267 PCT 23
32 Ethylene glycol stearate (approx. 5-15% monoester,
85-95% diester; CTFA name: Glycol Distearate)
(HENKEL)
5 8) Shampoo
Texapon~K 14 S 25.0
Texapon~SB 333 7.5
Eucarol~TA34 12.0
Akypo~RLM 100 NV 9.0
Dehyton~AB 3035 8.3
Polymer Pl acc. to DE 39 29 973 0.6
Elfacos~GT 282S36 0.5
Polychol020 4.0
Ajidew A 100 2.8
Sodium chloride 0.5
KOH ad pH 5.0
Water ad 100
33 Sulfosuccinic acid semiester based on an alkyl
polyglycol ether, di-Na salt (approx. 40% active
substance; CTFA name: Disodium Laureth Sulfosuc-
cinate) (HENKEL)
34 Lauryl alcohol+7 ethylene oxide tartrate sodium
salt (approx. 25% active substance; CTFA name:
Sodium Laureth-7 Tartrate) (AUSICHEM)
Fatty amine derivative, betaine structure (approx.
30% active substance; CTFA name: Coco-Betaine)
(HENKEL)
36 Tallow alcohol+60 ethylene oxide myristyl ether
(CTFA name: Talloweth-60 Myristyl Glycol) (AKZO)
9) Tonic pack (removable by rinsing)
Stenol~1618 3.0
Eumulgin~B 1 0.5
Eumulgin~B 2 0.5
~ ~ 21~5861
H 267 PCT 24
Cutina~CP 1.0
Eutanol~G 1.5
Hostacerin~PN 7337 0.004
Dow Corning~929 Emulsion 2.9
Polychol~5 1.5
Ajidew~N 50 2.0
Water ad 100
37 Polyacrylic acid sodium salt copolymer (HOECHST)
10) Tonic pack (rem~; n; ng on the hair)
Celquat~L 20038 0.6
Luviskol~K3039 0.2
D-Panthenol 0.5
Polymer Pl acc. to DE 39 29 973 0.6
Dehyquart~SP40 1. 0
Nutrilan~I4l 1. 0
Natrosol~250 HR42 1.1
Polychol~5 1.5
Ajidew~N 50 2.0
Water ad 100
36 Quaternized cellulose derivative (95% active sub-
stance; CTFA name: Polyquaternium-4) (DELFT
NATIONAL)
39 Polyvinyl pyrrolidone (95% active substance; CTFA
name: PVP) (BASF)
Aqueous solution of hydroxyethyl alkylammonium
phosphate (approx. 50% active substance; CTFA
name: Quaternium-52) (HENKEL)
41 Collagen hydrolyzate (approx. 39% active subs-
tance; CTFA name: Hydrolyzed Collagen) (HENKEL)
42 Hydroxyethyl cellulose (AQUALON)
21~5861
H 267 PCT 25
11) Dyeing cream
C12 l8 Fatty alcohol 1.2
Lanette O 4.0
Eumulgin~B 2 0.8
Cutina~ KD 1643 2.0
Sodium sulfite 0.5
L(+) Ascorbic acid 0.5
Ammonium sulfate 0.5
1,2-Propylene glycol 1.2
Polymer JR~40044 0.3
p-Aminophenol 0.35
p-Tolylene diamine 0.85
2-Methyl resorcinol 0.14
6-Methyl-m-aminophenol 0.42
Polychol~5 0.5
Ajidew0N 50 1.2
Ammonia 1.5
Water ad100
20 43 Fatty acid mono/diglyceride emulsifier mixture
(CTFA name: Tallow Glyc~rides (and) Glyceryl
Stearate (and) Potassium Stearate) (HENKEL)
44 Quaternized hydroxyethyl cellulose (Union Carbide)
25 12) Developer dispersion for dyeing cream 11)
Texapon~N 25 2.1
Hydrogen peroxide (50%) 12.0
Turpinal~SL45 1.7
Latekoll~D46 12.0
Polychol~20 0.9
Ajidew~N 50 0.3
Water ad100
l-Hydroxyethane-l,1-diphosphonic acid (60% active
substance; CTFA name: Etidronic Acid) (HENKEL)
21~S8~1
. ~
H 267 PCT 26
46 Acrylate/methacrylic acid copolymer (25% active
substance) (BASF)
The dyeing cream had a pH value of 10Ø It gave
the hair a strong red tint.
13) Tinting shampoo
Texapon~N 70 14.0
Dehyton~K 10.0
Akypo~RLM 45 NV47 14.7
Plantaren~-1200 4.0
Polymer Pl acc. to DE 39 29 973 0.3
Cremophor~RH 4048 0.8
Dye C.I. 12 719 0.02
Dye C.I. 12 251 0.02
Dye C.I. 12 250 0.04
Dye C.I. 56 059 0.03
PHB ester 0.25
Perfume oil q.s.
Polychol~20 1.6
Ajidew~N 50 1.2
Water ad 100
47 Lauryl alcohol+4.5 ethylene oxide acetic acid
sodium salt (20.4% active substance) (CHEM-Y)
48 Castor oil, hydrogenated + 45 ethylene oxide (CTFA
name: PEG-40 Hydrogenated Castor Oil) (BASF)
When the hair was washed with this tinting
shampoo, it was left bright light blond in color.
14) Permanent wave cream
Wave cream
Plantaren~-80049 5.0
Thioglycolic acid 8.0
2145~1
.
H 267 PCT 27
Turpinal~SL 0.5
Ammonia (25%) 7.3
Ammonium carbonate 3.0
Cetostearyl alcohol 5.0
Guerbet alcohol 4.0
Polychol~5 3.0
AjidewXN 50 2.0
Perfume oil q.s.
Water ad 100
49 C8-Clo alkyl glucoside, degree of oligomerization
1.6 (approx. 60% active substance) (HENKEL)
Fixing solution
Plantaren~-800 5.0
Hydrogenated castor oil 2.0
Potassium bromate 3.5
Nitrilotriacetic acid 0.3
Citric acid 0.2
Merquat~5505 0.5
Polychol~20 1.5
Ajidew~A 100 0.5
Perfume oil q.s.
Water ad 100
Dimethyl diallylammonium chloride/acrylamide co-
polymer (8% active substance; CTFA name: Poly-
quaternium 7) (MOBIL OIL)