Note: Descriptions are shown in the official language in which they were submitted.
j - 21~6503
- 1 - 57,650
IMPROVI~D SUPERALLOY COATING COMPOSITIONS
~JD METHODS FOR USING THE SAME
FIELD OF THE INVENTION
The present invention relates to overlay coating
compositions used to protect metal substrates from oxidation,
corrosion, or both. Specifically, the invention relates to
the incorporation of aluminum oxide particles, preferably
Al2O3 particles, into the overlay composition.
BACKGROUND OF THE INVENTION
Protective coatings are commonly employed to extend
the operational life of metallic substrates used in combustion
sections of turbines. The metallic substrates are
conventionally superalloy materials that are either nickel,
cobalt, or iron based alloys, or combinations thereof, and
usually contain other elements in significant quantities such
as chromium, aluminum, titanium, and the refractory metals.
Various superalloys are shown in U.S. Pat. Nos. 4,933,239 and
3,754,902.
The superalloy substrates are exposed to oxidative
and corrosive environments during use in such applications as
gas turbine combustors, transitions, blades and vanes. This
harsh environment leads to shortened useful life of the
component as the structure, dimension and geometry of the
substrate is deteriorated over time.
Various coatings have been developed to protect the
surface of the superalloy substrate. One type of such
coatings are referred to as the "overlay" coatings. These
coatings are generally denoted as MCrAlY coatings where the
M represents such elements as Ni, Co, Fe, and combinations
2146503
- 2 - 57,650
thereof. These coatings derive their protective capability
from their ability to form a thin layer of alumina scale on
the outer exposed surface. This alumina layer has been found
to be quite beneficial in oxidation resistance. However, the
alumina scale has a tendency to spall and must be reformed
during use of the substrate. Additives such as yttrium,
hafnium, and silicon have been incorporated into such overlay
coatings to imp~ove coating overall performance, and the
alumina adherence to the substrate and to aid in the
regeneration of the alumina scale. The overlay coatings are
typically applied to the substrate surface through such
processes as low pressure plasma spraying, physical vapor
deposition, ion plating, and sputtering or slurry sintering.
Examples of such overlaying coatings are set forth in U.S.
Pat. Nos. 4,615,865; 4,585,481; 4,198,442; 4,101,715; and
3,754,903.
Another class of coatings for the oxidation and
corrosion protection of the substrate is the "aluminide"
coatings. These coatings are generated by an aluminizing
technique such as pack diffusion or chemical vapor diffusion.
These coatings are formed by interactions between an aluminum
source and the substrate surface. The aluminum forms cobalt
and nickel aluminide at the surface of the cobalt and nickel
based superalloy substrates, respectively. The coating
characteristics are largely affected by the substrate
chemistry and deposition process parameters. Examples of such
coatings are shown in U.S. Pat. No. 5,000,782.
Combinations of the two classes of coatings have
also been used to formulate protective coatings by aluminizing
an overlay coating as shown in U.S. Pat. Nos. 4,933,239;
4,910,092; and 4,897,315.
The dsscribed overlay and aluminide coatings
preferably contain yttrium as an additive element to aid in
the aluminide scale formation and retention. However, when
a substrate is exposed to harsh corrosive environments, such
as when fuel containing sulfur and other salt impurities is
oxidized in the turbine, the yttrium is essentially
2146503
.
- 3 - 57,650
deactivated. Thus, there currently exists a need to develop
a protective coating that contains an additive or additive
combination that can extend the oxidative and corrosive
properties of the substrate when fuels containing sulfur and
other salt impur:ities are oxidized with ingested impure air.
SUMMARY OF THE INVENTION
The present invention provides an improved overlay
coating composition which provides superior oxidation and
corrosion resistance when exposed to gaseous environments
containing sulfur compounds. The overlay coating composition
contains nickel, from 8-50% wt. chromium, from 6-40% wt.
aluminum, from 10-40% wt. cobalt, and from 0.1-10~ wt.
particulate aluminum oxide. The aluminum oxide is uniformly
distributed throughout the -particulate overlay coating
composition, and has been found to improve the protective
characteristics of the coating. The overlay coating
composition is deposited to a bare substrate, conventionally
comprised of a nickel or cobalt super alloy by conventional
techniques.
The overlay coating has been found to provide
improved protective resistance to oxidation and corrosion by
incorporating haenium, silicon or mixtures thereof into the
composition. The combination of Al203 with silicon is
particularly preferred.
The overlay coating is useful in protecting a
metallic substrate. The coated metallic substrate is useful
as a part within a turbine or engine. Preferred uses of the
overlay coating are to extend the life of combustion or gas
turbine metallic substrate surfaces.
BRIEF DESCRIPTION OF THE DRAWING
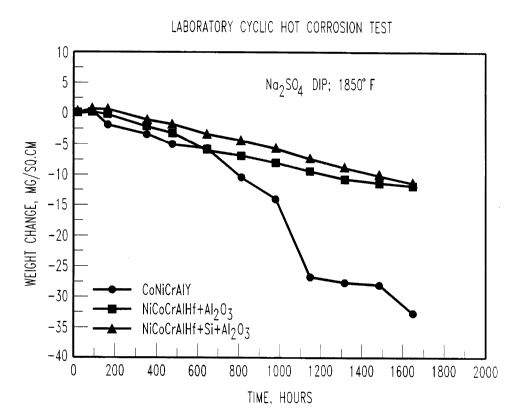
Fig. 1 is a graph of the experimental test results
of Example 1 showing the improved coating protection afforded
by the inclusi~n of Al2O3 particles into the coating
composition.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides improved coating
compositions fol use in extending the life of metallic
- 2146503
- 4 - 57,650
substrates used, for instance, in the hot sections of gas
turbines, combustion turbines, and aero-jet engines. Examples
of tubine components to which the coating compositions can be
applied include, for instance, gas turbine combustors,
transitions, blades and vanes. The coating compositions are
advantageously employed to coat and protect substrates that
are exposed to the combustion products of fuels containing
sulfur and other salt impurities. The coatings of the present
invention employ the use of aluminum oxide within the matrix
of the coating t~ enhance aluminum oxide scale formation on
the exposed surface of the coating and the adherence of that
formed scale to the substrate. Other inert metal oxides such
as, for example cerium oxides, scandium oxides, indium oxides,
and thorium oxides, can also be used in place of Al2O3 with
similar beneficial effects, with Al2O3 being preferred.
The base metallic substrate upon which the coating
composition is deposited can be any metallic substrate,
however the substrate is preferably a superalloy that is a
base alloy of nickel, cobalt, or combination thereof. The
nickel or cobalt based alloys can be in either the cast or
forged/wrought form. The nickel based superalloys derive
their high temperature mechanical strength primarily from
precipitation hardening processes. The major precipitates are
gamma prime with a composition of Ni3Al or Ni3AlTi type. The
nickel based superalloys are commonly used as substrates in
rotating blades used in turbines. The cobalt based alloys are
strengthened by solid solution hardening. The cobalt based
superalloys are commonly used as substrates in stationary
blades used in turbines. Other solution-strengthened Ni-based
or Fe-based al~oys can also be used. The superalloys
generally contain elements such as iron, boron, carbon, and
zirconium along with refractory elements such as tungsten,
tantalum, molybdenum and niobium for solid solution
strengthening. Examples of nickel-based superalloys are shown
in U.S. Pat. No. 3,754,902 which is herein incorporated by
reference in its entirety.
-
2146503
_ 5 _ 57,650
The superalloy substrate is then coated with an
overlay coating alloy. The coating alloy composition contains
sufficient amounts of chromium and aluminum to form protective
chromia and alumina scales during exposure to an oxidative
environment. The presence of the chromium reduces the amount
of aluminum necessary to form the alumina scale by the
"gettering" effect. The upper limit of the chromium in the
coating alloy composition is functionally restricted by
reduced oxidation resistance and the lower limit is restricted
by reduced corrosion resistance. Excess aluminum is
deleterious to the ductility of the coating and it also
reduces corrosion resistance, whiie a sufficient amount of
aluminum is necessary to enhance the oxidation performance.
The balance of these factors generally determines the
appropriate level of the chromium and aluminum in the coating
alloy composition. The chromium is present in an amount of
from about 8-50% wt., preferably from 15-3S% wt., and more
preferably from 20-30% wt. and the aluminum is present in an
amount of from about 6-40% wt., preferably 6-20% wt., and more
preferably from 8-12% wt. of the coating alloy composition.
The coating alloy composition also contains cobalt
and nickel. The content of the cobalt and nickel will depend
upon whether the substrate base alloy is a nickel or cobalt
based alloy. If the overlay coating is to be used with a
nickel based substrate, then the cobalt is present in an
amount of from about 10-40% wt., preferably from 15-35% wt.,
and more prefer~bly from 20-30% wt. of the coating alloy
composition. The nickel would then constitute essentially the
balance of the nickel based overlay coating, excluding the
stated additive elements or compounds set forth herein. If
the overlay coating is to be used with a cobalt based
substrate, then the nickel is present in an amount of from
about 10-40% wt., preferably from 15-35% wt., and more
preferably from 20-30% wt. of the coating alloy composition.
The cobalt would then constitute essentially the balance of
the cobalt based overlay coating, excluding the stated
additive elements or compounds set forth herein.
~146503
- 6 - 57,650
The way by which overlay coatings such as the
NiCoCrAl type protect alloy substrates is by thermally forming
a tenacious, uniform and slowly forming thin protective oxide
scale, usually an alumina scale. The alumina scale acts as
s a barrier for further diffusion of Al and 2~ thereby slowing
down the formation of alumina scale to a low steady terminal
rate. The thickness of this scale increases over time upon
exposure to process conditions. The scale is not very
ductile, and ultimately cracking and spallation occurs during
the thermal cycling process. Reformation of the scale occurs
at the spalled locations at higher rates thus contributing to
fast depletion of Al from the coating. When the Al is
depleted from the coating, non-protective oxide scales of the
base metal form and scale penetration occurs resulting in a
lack of protection for the base alloy substrate.
The coating alloy composition of the present
invention further includes aluminum oxide for the enhancement
of the oxidation and corrosion resistance, especially wherein
the substrate is to be used in an atmosphere containing the
combustion products of a fuel and air containing sulfur and
other salts or where gaseous SO2 or S03 (SOx) compounds are
present. The inert oxide is present in the coating alloy
composition in an amount of from about 0.1-10% wt., preferably
0.1-5% wt., and more preferably from 0.5-3% wt. The preferred
oxide is aluminum oxide, Al2O3.
The coating alloy composition can also contain other
additives useful in adhering the alumina scale to the surface
of the overlay ~oating. Representative additives include
hafnium and silicon. The hafnium can be present in an amount
of from 0.01-4% wt., preferably from 0.1-2% wt.; the silicon
can be present in an amount of from 0.01-5% wt., preferably
from 0.1-2.5% wt.; all basea upon the total overlay coating
alloy composition. Another element, cerium, can also be added
in the coating alloy composition in an amount of from 0.01-10%
wt., preferably from about 0.1-5% wt., and more preferably
from 0.1-2.5% wt. Further, rhenium can be added to improve
oxidation -resist:ance in amounts of about 0.01-40% wt.,
- ~14650~
- 7 - 57,650
preferably from about 0.01-8~ wt., and more preferably from
0.01-4% wt.
Variou; other additives can be incorporated into the
overlay coating alloy composition such as Sc, La, Gd, and
combinations thereof. These additives can be present in an
amount of from 0.1-10% wt. individually, however the total of
these additives is preferably below about 20% wt., more
preferably below about 15% wt., of the coating composition.
Yttrium can also be added to the coating composition
in an amount of from about 0.01-10% wt., preferably from 0.1-
4% wt., however it is not desired for uses where sulfur
containing fuels are employed. It is believed that if the
yttrium is oxidized, then it is not resistant to sulfation and
the aluminum oxide scale adhesion is correspondingly
decreased.
The thickness of the overlay coating is generally
at least about 0.002 inches (0.005 cm), preferably at least
about 0.003 inches (0.0076 cm), more preferably from about
0.003 inches (0.0076 cm) to about 0.015 inches (0.038 cm), and
even more preferably from about 0.004 inches (0.01 cm) to
about 0.01 inches (0.025 cm) in thickness. The aluminum oxide
is present throughout the thickness of the coating as
disperoids, and not just on the surface, and is preferably
present in a substantially uniform amount, even more
preferably present in a uniform homogeneous amount, throughout
the thickness of the overlay coating.
The coating alloy composition is prepared by first
making an alloy melt of the elements and compounds in the
coating alloy composition except for the aluminum oxide. This
melt portion of the coating alloy is then spray atomized to
form a particulate alloy using conventional techniques such
as argon spray atomization.
The particulate alloy is then blended with
particulate aluminum oxide to form a homogeneous particulate
coating alloy composition. The average particle size of the
atomized alloy portion is preferably from about S to about S0
microns, and the average particle size of the aluminum oxide
~146503
-
- 8 - 57,650
is from about 0.5 to about 3 microns. This particle size
distribution may vary according to the selection of
application method and process parameters.
The particulate coating alloy is then deposited onto
the substrate surface. The deposition process is preferably
a low pressure plasma spraying (LPPS) process. Other thermal
spray methods including high velocity oxy fuel (HVOF) and
sputtering can also be used to apply the overlay composition
coating of the present invention.
The present inventive coatings can also be used as
a bond coat or base coat below any top thermal barrier coating
with and without any tie coat or intermediate coating.
Example
Cyclic hot corro:3ion/oxidation studies
Tests were performed to analyze the behavior of
NiCoCrAl coatings containing additions of Y or Hf with Alz03
and Al203/Si. The CoNiCrAlY coating was used as a base
reference coating. The base substrate alloy was Inconel
IN738LC which has a composition of, in weight percent, 16% Cr,
8.5% Co, 3.4~ Al, 3.4% Ti, 1.6% Mo, 1.6% Ta, 2.5% W, 1% Nb,
and 0.1~ C with the balance Ni. The three overlay coatings
applied to the base alloy are set forth in Table 1.1 in a
weight percent basis except Al203 which is given in volume
percent.
--- 2146503
~ 9 ~ 57,650
Table l.l
Overlay Coating Composition~
Constituent A B C
Co(wt.%) 38 23 23
5 Cr(wt.%) ~l 20 20
Al(wt.%) 8 ll ll
Y(wt.%) ().4
Hf(wt-%) ~ 0.6 0.6
Al2O3(vol-%) ~ 2.0 2.0
lO Si(wt.%) - - 0.7
Ni(wt.%) Bal Bal Bal
The IN738LC substrate was prepared free from dirt
and other contaminants. The three different coatings were
applied using a l;ow pressure plasma spray process (LPPS). The
apparatus used for the coating process was a Model EPI03C8
supplied by Elec1:ro Plasma Inc.; the substrate was coated in
a closed chamber maintained at a pressure of about 35 torr of
argon. A mixture of argon and helium was used to generate
plasma. Powder of NiCoCrAlHf and NiCoCrAlSiHf was
homogeneously pre-mixed with Al2O3 powder by ball milling.
The particle size distribution of the base coating powders and
the Al2O3 powder was from 10-40 ~m and from 0.6-2.5 ~m,
respectively. The coating thickness applied to the substrate
was uniformly 6 mils + l mil.
Cyclic hot corrosion testing was performed using a
laboratory electric furnace maintained at about 1850F. The
coated substra~s had a layer of about l mg/cm2 Na2SO4
deposited upon 1:hem by dip-dry process. The thus coated
samples were then exposed to the furnace conditions. The
samples were thermally cycled three times a day - they were
removed from the furnace, fan cooled to about 350F, and
reintroduced into the furnace. Periodically, about once every
~1~6503
- - 10 - 57,650
168 hours, the s~mples were removed from the furnace, cooled
to ambient, weighed, coated with fresh Na2S04 and placed back
into the furnace
The results of the testing are shown in Fig. 1 where
the x-axis is the hours of exposure with the thermal cycling
and the y-axis is the weight change of the sample. The weight
change represent-; the difference between the weight gain due
to alumina oxide scale formation and the weight loss due to
scale cracking, ~nd scale/coating spallation. Lower weight
changes represent:less of a reaction rate and scale spallation
indicative of a longer coating life and higher degree of
protection afforded by the coating. As can be seen from the
Fig. 1, the weight change was marked improved by the
replacement of the Y and the addition of the Al203 either with
or without the Si, however the addition of the Si further
improved the coating life characteristics.