Note: Descriptions are shown in the official language in which they were submitted.
-ILE. Pll`l ~ t~N[~LU 21~172S3
.. TE~XT JRANS~ATION
Process for Treating Industrial Waste Waters
with Low Concentrations of Toxic Organic Pollutants
The invention relates to a process for effectively removing organic pollutants from waste water
flows. In particular, the invention relates to a process in which toxic organic pollutants are
adsorbed and concentrated on granul~r activated charcoal in a first step, and a second step
follows in which the organic pollutants are desorbed, so that the activated charcoal is
regenerated, and the pollutants are subsequently decomposed in a 3-phase oxidation reactor.
Technical Background of the Invention
Many waste water flows which originate in industrial activity contain organic pollutants that
are either toxic or extrenlely difficult to decompose biologically, so that no direct biological
treatment is possible. As an alternative in such cases, it can be necessary
~_, , 2197253
to use less common techniques, for example, chemical oxidation or wet-air oxidation, in order
to oxidize the organic material contained in the waste water. As a means for purifying waste
water, an alternative to non-catalytic wet-air oxidation is the oxidation of diluted hydrous
solutions of organic pollutants using oxygen via a solid-matter catalyst (J. Levec, Appl. Catal.,
63, 1990, p. 1; A. Pintar and J. Levec, J. Catal., 135, 1992, p. 345). In this process, organic
substances are oxidized to carbon dioxide at substantially lower temperatures and pressures
than in non-catalytic thermal processes. The key to effective catalytic oxidation, ho~ever, is
the catalyst. Recently, a catalyst has been developed that promotes oxidation of organic
substances in hydrous solutions below 200 C (DE 39 38 835 A1; EP 0 429 750 B1). With
these new catalysts, a large area of application opens up for using the oxidation process to
completely oxidize toxic organic substances in waste waters.
Adsorption with granulated activated charcoal is a proven method of purifying process water
(W.W. Eckenfehler Jr. et al., Chemical Engineering, 2 September 1985, p. 60). Activated
charcoal is especially effective in removing organic pollutants when the pollutants are dissolved
in the waste water in small quantities. Through the coal adsorption process, the organic
pollutants are also concentrated into the coal particles. Frequently, the coal exchange, the
removal of used coal and the supply of fresh coal are the most critical parameters in operating
the adsorption system and can be the determinant factors in operating costs. The in situ
regeneration of used activated charcoal with steam or hot gas is effective in regenerating fluid-
phase activated charcoal only to a limited extent. In the most frequently used
2197253
'._
regeneration technique, the used coal is heated in a controlled atmosphere in a multistage or
rotary kiln. Up to 10% of the coal is lost during such a reactivation process.
The process of the present invention permits activated charcoal to be regenerated within the
adsorber without the removal of the coal from the latter. This is achieved by recycling hot
water through the adsorber and the oxidation reactor at an increased temperature and increased
pressure. During recycling, most of the organic pollutants are desorbed by the coal and
released into the water; after this, they are oxidized through catalysis in a trickle bed reactor.
For optimum effect, the process according to the invention should be installed at the location
in an industrial facility where waste water is created.
Brief Description of the Invention
The process of the present invention consists of the following steps:
a) Adsorption step, in which waste water containing toxic organic pollutants and/or
organic pollutants difficult to decompose biologically is run through a column
(adsorber) loaded with activated charcoal until the coal bed is fully consumed, i.e., until
its adsorption capacity has been used up. The inflow concentration can fluctuateconsiderably without endangering the target outflow levels. Adsorption is
advantageously carried out at ambient pressure and ambient temperature. In fact,_ pressure has practically no influence on adsorption, although temperature undoubtedly
does. Higher temperatures should therefore be avoided.
. . . _ . . _ ~ . . .
2147253
. ~
Cooling at the ambient temperature or at the usual cooling water temperature is not
advisable, due to the required expenditure of energy. The possible temperature range
is between 10 and 30 C.
b) Desorption/regeneration step, in which the used activated charcoal is regenerated in
fluid-phase operation at temperatures from 80 to 200C and an increased pressure lying
abo~e the steam pressure of the fluid. If possible, an overpressure of 30 bar (31 bar
absolute) should not be exceeded. The flow of liquid within the adsorber can be
directed upwards or downwards in the adsorption operating mode as well as in thedesorption/regeneration operating mode.
c) Oxidative decomposition step, in which the desorbed organic pollutants are oxidized
to carbon dioxide in a trickle bed via a catalyst. An especially effective catalyst is
described in I)E 39 38 835 A1 and EP 0 429 750 B1 and contains 30-50% by weight
copper oxide, 40-50% by weight zinc oxide, 0-4% by weight chromium oxide and 5-
15% by weight aluminum oxide. Oxidation is carried out in the liquid phase at
increased pressure (up to 60 bar ovelpress~lre) and increased temperatures of at least
120 C to 250 C. In the oxidation reactor, the gaseous phase (air or oxygen) and the
liquid phase can each flow upward or downward in parallel flow. The oxygen partial
pressure should be at least 3 bar.
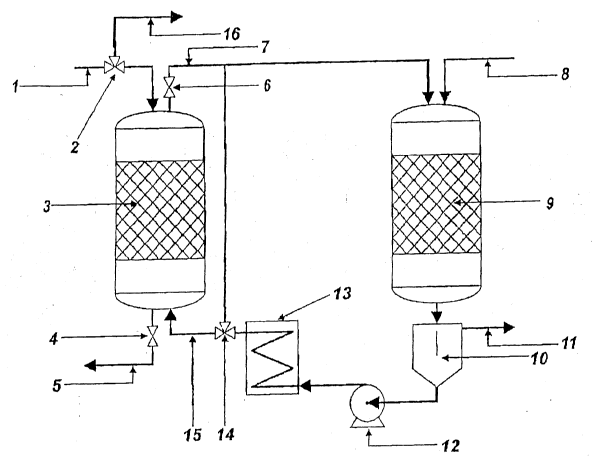
The process of the present invention is represented schematically in Figure 1. The waste water
which contains solute toxic organic pollutants is
..
. . .
21q7253
conveyed through a conduit 1 via a 3-way valve (inlet valve 2) into the adsorber 3, which is
loaded with granulated activated charcoal. The flow of liquid through the coal bed is forced
by a pressure gradient. The consumption of the coal bed (loading with pollutants) is
monitored through the appearance of the "breakthrough curve." After the coal bed becomes
saturated with organic substances and the inlet valve 2 and the outlet valve 4, which are
arranged in an outlet conduit 5 at the lower end of the adsorber 3, are closed, the adsorber 3
is heated to working temperature. At the same time, the preheater 13, which is connected to
the adsorber 3 via a hot water supply conduit 15 containing a 3-way valve, is switched on.
The adsorber 3 is connected at its upper end to a hot water extraction conduit 7, in which
there is a blocking valve 6 and which leads to a reactor 9. In turn, the reactor 9 is connected
at its lower end to the preheater 13 via a tube. As soon as the water temperature in the
adsorber 3 and the preheater 13 has reached the working temperature, the valves 6 and 14 are
opened, and the hot water begins to be circulated by means of a pump 12 through the
adsorber 3 and the reactor 9. After the hot circ~ ting water (recycling water) has reached the
reaction temperature (120-250C), a flow containing oxygen (e.g., air) is introduced through
a conduit 8 topside into the reactor 9. Advantageously, the pressure in both units (adsorber
3, reactor 9) is the same and measures, for example, 20 bar or even as much as 30 bar
(ove.pres~-lre). The reactor 9 works under 2-phase flow conditions, while only one phase
(liquid) occurs in the adsorber 3. Due to the increased temperature in the adsorber 3, the
organic substances which were adsorbed at the ambient temperature are now desorbed and
released into the hot recycling water; they are subsequently oxidized to carbon dioxide in the
trickle bed reactor 9. The flow of recycling water treated in this manner makes its way
behind the reactor 9 into a gas/liquid separator 10, so that the gas phase (especially
2I 4 72S3
` ,~,
the CO2 which has formed and the oxygen-containing gas which has not been consumed) can
be extracted through an exhaust conduit 11, while the pollutant-free recycling water is
conveyed back into the preheater 13 via the pump 12. During desorptive operation, the flow
of waste water to be purified is conveyed ~ia the 3-way valve 2 through the conduit 16 to a
second adsorption column (not shown) connected in a parallel hshion. The
desorption/regeneration and the associated oxidative reaction are ended as soon as the residual
content of organic substances in the recycling water drops below a certain concentration limit.
It must be emphasized that the organic substances do not have to be fully desorbed by the
activated charcoal or completely oxidized in the reactor 9. In such a case, the adsorption
capacity of the activated charcoal is simply reduced accordingly. As a result, the capacity of
the activated charcoal to remove organic substances at low concentrations in the adsorption
step can be reduced.
The process of the present invention, which consists of a combination of adsorption and
oxidative regeneration, utilizes the advantages of both of these individual processes. For
example, the regeneration of the coal can be carried out in situ without any loss of coal, so
that no coal preparation is needed. The discharged treated water is not polluted by metal
cations which might possibly wash out of the catalyst, because the cations remain in the
recycling water and also act there as a homogeneous catalyst. The deactivation of the catalyst
is significantly increased and the useful life of the catalyst is substantially lengthened in the
process according to the invention, compared to a process without prior adsorption (i.e., direct
oxida~ion). Furthermore, compared to direct oxidation, the prior concentration of organic
pollutants on activated charcoal results in higher oxidation rates and
2147253
'
thus in shorter catalyst beds.
Detailed Description of the Invention
~xample 1: Regeneration of Used Activated charcoal with Hot Pressurized Water
A hydrous phenol solution with a concentration of 1.0 g/l was used as the waste water in a
test. The solution was introduced at a flow rate of 3 I/h into a stainless-steel adsorption
column (inner diameter 34 mm), which was filled ~ith 125 g activated charcoal (bed height
380 mm) with a particle size of 1.6 mm. The consumption of the activated charcoal ~as
monitored by means of phenol concentration measurements (HPLC) in the adsorber outflow.
As soon as phenol was found in the outflow (concentration below 0.2 mg/l), the activated
charcoal was assumed to be saturated by the polluting substances. The adsorption process was
carried out at ambient pressure (1 bar absolute) and ambient temperature (c. 22 C).
Desorption/regeneration of the used activated charcoal was carried out by means of hot water
(150 C; 10 bar ovel~res~-lre) flowing through the adsorber at a rate of 3 l/h. The phenol
concentration in the discharged water was monitored by means of HPLC and is indicated in
the graph in Fig. 2 as a function of time (Curve I). After the phenol concentration dropped
below 1.5 g/l, the desorption/regeneration process was ended. Results showed that 26.7 g
phenol was adsorbed on the activated charcoal at room temperature; after the
desorption/regeneration step was carried out, 5.6 g phenol still remained adsorbed on the coal.
This means that under the conditions used approximately 80% of the original activated
charcoal capacity was again obtained. Catalytic treatment of the hot water loaded with phenol
according to the present invention was
21~7253
not yet carried out in this example.
xample 2: Regeneration of Used Activated charcoal with Hot Water and
Simultaneous Oxidative Decomposition of Desorbed Phenol
The activated charcoal bed saturated with phenol from Example 1 was regenerated by means
of hot water, which was circulated through the adsorber and the trickle bed reactor according
to the invention. The flow rate of the recycling water was set at 2 I/h. After the recycling
water had reached the predetermined temperature of 150 C, a flow of oxygen was introduced
into the trickle bed reactor at a rate of 2 I/min. The reactor (inner diameter 34 mm) was
loaded with 730 g of a catalyst (bed height 810 mm) as described in DE 39 38 835 A1 and EP
0 429 750 B1. The operating pressure in the adsorber and in the reactor was 10 bar
(ovel~ressllre). The hot water (2.51) circulated for a period of 10 h. During this time, the
phenol concentration in the hot recycling water, which was monitored by means of an HPLC
analyzer (high performance liquid chromatography) and a TOC analyzer kotal organic coal),
dropped to 0.8 g/l (Curve II in Figure 2), which corresponds roughly to a 90% restoration of
the original activated charcoal adsorption capacity. After 5 subsequent
adsorption/regeneration/oxidation cycles, the adsorption characteristics of the activated
charcoal had not changed. The concentration of leached-out copper and zinc ions in the
recycled water was measured at 20 and 32 mg/l, respectively, by means of AAS (atomic
absorption spectroscopy). In each case, the treated water discharged from the adsorption stage
contained neither phenol nor copper or zinc.