Note: Descriptions are shown in the official language in which they were submitted.
2 1'g 7 6 0 9
~ WO94/10177 -1- PCT/JP93/01505
DESCRIPTION
,
CEPHEM COMPOUNDS, AND THEIR PHARMACEUTICAL COMPOSITIONS
TECHNICAL FIELD
This invention relates to new cephem compounds and
pharmaceutically acceptable salts thereof which are useful
as a medicament.
BACKGROUND ART
Some cephem compounds have been known as described,
for example, in Japanese Kokai 62-36385.
DISCLOSURE OF INVENTION
The present invention relates to new cephem compounds
and pharmaceutically acceptable salts thereof. More
particularly, it relates to new cephem compounds and
pharmaceutically acceptable salts thereof, which have
antimicrobial activities, to processes for preparation
thereof, to pharmaceutical composition comprising the
same, and to a method for treating infectious diseases in
human being and animals.
Accordingly, one object of the present invention is
to provide the cephem compounds and pharmaceutically
acceptable salts thereof, which are highly active against
a number of pathogenic microorganisms.
Another object of the present invention is to provide
processes for the preparation of the cephem compounds and
salts thereof.
A further object of the present invention is to
provide pharmaceutical composition comprising, as an
active ingredient, said cephem compounds or their
pharmaceutically acceptable salts.
WO94~10177 PCT/JP93/01505
2~4~6o9
Still further object of the present invention is to
provide a method for treating infectious diseases caused
by pathogenic microorganisms, which comprises
administering said cephem compo~n~s to infected human
being or animals.
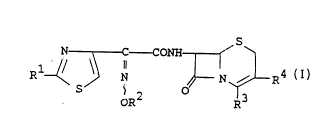
The object cephem compoùnds of the present invention
are novel and can be represented by the following general
formula (I) :
13
N ~ C - CONII ~
OR R
wherein Rl is amino or protected amino,
R is hydrogen, a hydroxy protective group,
lower alkyl or mono(or di or
tri)halo(lower)alkyl,
R3 is carboxy or protected carboxy, and
R4 is pyridylvinyl which may have suitable
substituent(s),
with proviso that
(i) when R is hydrogen and R4 is
3-pyridylvinyl, then
R3 is not carboxy, and
(ii) when R2 is tetrahydropyranyl and R4 is
3-pyridylvinyl, then
R3 is not benzhydryloxycarbonyl.
The object compound (I) of the present invention can
be prepared by the following processes.
WO94/10177 2 1 4 7 6 0 9 PCT/JP93/01505
Process (l)
2 1 ~
~ , , ~ N ~ R4
R3
(Il)
or its reactive derivative at the
l~ amino group, or a salt thereof
N C-COOH
Rl~
OR
(III)
or its reactive derivative
2D at the carboxy group,
or a salt thereof
N \ C - CO~I J 1 J
(I)
or a salt thereof
o
3~
WO94/10177 . PCT/JP93/015
-- 4
~4~609
Process (2)
S R ~ "j~ N ~" R
OR~ R
(la)
or a salt thereof
elimination reaction of the
hydroxy protective group
N ~ C - CON~
OH R
iIb)
or a salt thereof
Process j3)
N ~ C CONII r `
S S 2
OR COOH
~Ic)
~ d salt thereof
WO94/10177 2147 6 0 9 PCT/JP93/01505
esterification
.~ C CONH
S / ~ ~ 3
QR COOR
(Id)
or a s~lt thereof
wherein R1, R2, R3, and R4 are each as defined above,
R2 is a hydroxy protective group and
a9
~ is ester moiety of esterified carboxy
represented by a group of the formula :
-COOR9.
The starting compound (II) can be prepared by the
following processes.
Process (A)
R5 ~ S
N ~ CH2P~(R6)
(IV)
or a salt thereof
.
R7 - CHO
~v)
or a salt thereof
WO94/10177 . ~. PCT/JP93/0150
-- 6
609
R5 ~ CH~H-R7
~ ~3
~ VId)
or a salt thereof
Process (B)
~ 1.3
R
(Vla)
~r a salt thereof
7~
~limination reaction of
the amino protective group
H2N ~ 1 4
N ~- R
lR3
(II)
or a salt thereof
~ WO g4/10177 2 1 4 7 6 0 ~ Pcr/JPg3/olso5
-- 7
oc~
~ . .
,j N ~1 R
~1 3
(VIb)
or a salt thereof
1(~
elimination reaction of
the carboxy protective group
.
R5 ~-S~
~-N ~ ~- R~
COOH
,.r,
iVIc)
or a salt thereof
Process iD)
//-- N~ y
~ 3
~ VII)
or a salt thereof
3~
WO94/10177 PCT/JP93/015~
~,14rt609
(R )3Sn-R~
(VIII)
~r. a salt thereof
R5~ S~
lo ,L N ~ - R
R
~VI~
or a salt thereof
Process ( E )
R5 ~ S
G~ /I N~ 4
;~0~
('~Ic)
2~ or a salt thereof
esterification
214 7 6 0 9 PCT/JP93/01505
WO94/10177
_ g _
R
~OOR
(~Ie)
~r a salt thereof
wherein R3, R4 and R9 are each as defined above,
Ra is protected carboxy,
R is amino or protected amino,
Ra is protected amino,
R is lower alkyl or aryl,
l~ R7 is pyridyl which may have suitable
substituent(s),
R is lower alkyl,
X is acid residue and
Y is a leaving group.
~0
Regarding the compounds ;I), (Ia), (Ib), (Ic), (Id)
and (III), it is to be understood that said compounds
include syn isomer, anti isomer and a mixture thereof.
2i For example, with regard to the object compound (I),
syn isomer means one geometrical isomer having the partial
structure represented by the following formula :
N C-CO-
R~ -O-R2
(wherein Rl and R2 are each as defined above), and anti
isomer means the other geometrical isomer having the
3~ partial structure represented by the following formula :
PCT/JP93/015'
W094/10177 1 47609 - lo
N C-CO-
R ~ S ~ R2-O-N
(wherein Rl and R2 are eac~ as defined above), and all of
such geometrical isomers ànd mixture thereof are included
within the scope of this invention.
In the present specification and claim, the partial
structure of these geometrical isomers and mixture thereof
are represented for convenient sake by the following
formula :
N C-CO-
R~
S ~ 2
OR
(wherein R~ and R2 are each as defined above).
It is to be noted that the compound (I) and the other
compounds may include one or more stereoisomers due to
asymmetric carbon atom~s), and all of such isomers and
mixture thereof are included within the scope of this
invention.
In the above and subsequent descriptions of the
present specification, suitable examples and illustrations
of the various definitions which the present invention
include within the scope thereof are explained in detail
as follows.
The term "lower" is intended to mean l to 6 carbon
atom(s) unless otherwise indicated.
Suitable "lower alkyl" and "lower alkyl moiety" in
the term "mono~or di or tri)halo~lower)alkyl" may include
straight or branched one such as methyl, ethyl, propyl,
WO94/10177 2 1 4 7 6 0 9 PCT/JP93/01505
^ -- 11 --
isopropyl, butyl, t-butyl, ~entyl, hexyl, and the like, in
which more preferred one may be C1-C4 alkyl and the most
preferred one may be methyl, ethyl or propyl.
Suitable "protected amino" may include an acylamino
5 or an amino group substituted by a conventional protecting
group such as ar(lower)alkyl which may have suitable
substituent(s) [e.g. mono(or di or tri)phenyl~lower)alkyl
(e.g. benzyl, trityl, etc.), etc.] or the like.
Suitable "acyl moiety" in the term "acylamino" may
include carbamoyl, aliphatic acyl group and acyl group
containing an aromatic or heterocyclic ring. And,
suitable examples of the said acyl may be lower alkanoyl
le.g. formyl, acetyl, propionyl, butyryl, isobutyryl,
valeryl, isovaleryl, oxalyl, succinyl, pivaloyl, etc.);
lower alkoxycarbonyl (e.g. methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl, tertiarybutoxycarbonyl, pentyloxycarbonyl,
hexyloxycarbonyl, etc.); lower alkanesulfonyl (e.g. mesyl,
ethanesulfonyl, propanesulfonyl, isopropanesulfonyl,
butanesulfonyl, etc.); arenesulfonyl (e.g.
benzenesulfonyl, tosyl, etc.); aroyl (e.g. benzoyl,
toluoyl, xyloyl, naphthoyl, phthaloyl, indancarbonyl,
etc.); ar(lower)alkanoyl (e.g. phenylacetyl,
phenylpropionyl, etc.); ar(lower)alkoxycarbonyl (e.g.
~5 benzyloxycarbonyl, phenethyloxycarbonyl, etc.), and the
like. The acyl moiety as stated above may have suitable
substituent(s) such as halogen (e.g. chlorine, bromine,
iodine or fluorine) or the like.
Suitable "protected carboxy" may include esterified
carboxy and the like. Suitable example of ester moiety of
esterified carboxy may be the ones such as lower alkyl
ester (e.g., methyl este~, ethyl ester, propyl ester,
isopropyi ester, butyl ester, isobutyl ester, t-butyl
ester, pentyl ester, t-pentyl ester, hexyl ester, etc.);
lower alkenyl ester (e.g., vinyl ester, allyl ester,
PCT/JP9~/01504
WO94/10177
2~ 4~ 60~9 - ~2 -
etc.); lower alkynyl ester (e.g., ethynyl ester, propynyl
ester, etc.);
lower alkoxyalkyl ester (e.g t. methoxymethyl ester,
ethoxymethyl ester, isoprop~Xymethyl ester, 1-methoxyethyl
ester, 1-ethoxyethyl este~, etc.); lower alkylthioalkyl
ester (e.g., methylthiomethyl ester, ethylthiomethyl
ester, ethylthioethyl ester, isopropylthiomethyl ester,
etc.); mono(or di or tri)halo(lower)alkyl ester (e.g.
2-iodoethyl ester, 2,2,2-trichloroethyl ester, etc.);
alkanoyloxy(lower)alkyl ester (e.g., acetoxymethyl ester,
propionyloxymethyl ester, butyryloxymethyl ester,
valeryloxymethyl ester, pivaloyloxymethyl ester,
hexanoyloxymethyl ester, 1-(or 2-)acetoxyethyl ester,
1-(or 2-)propionyloxyethyl ester, 1-(or 2-)butyryloxyethyl
ester, 1-(or 2-)isobutyryloxyethyl ester, 1-(or
2-)~aleryloxyethyl ester, 1-(or 2-)isovaleryloxyethyl
es~er, 1-(or 2-)pivaloyloxyethyl ester, 1-(or 2-)-
(3,3-dimethylbutyryloxy)ethyl ester, 1-(or 2-)~2-(or
3-)ethylbutyryloxy]ethyl ester, 1-(or 2-)[2-(or 3- or
~0 4-)methylvaleryloxy]ethyl ester, 1-(or 2-)[2-(or 3- or
4-)propylvaleryloxy]ethyl ester, etc.);
alkoxycarbonyloxy(lower)alkyl ester (e.g.,
methoxycarbonyloxymethyl ester, ethoxycarbonyloxymethyl
ester, propoxycarbonyloxymethyl ester, 1-(or 2-)-
[methoxycarbonyloxy]ethyl ester, 1-(or 2-)[ethoxycarbonyl-
oxy~ethyl ester, 1-(or 2-)[propoxycarbonyloxy~ethyl ester,
1-(or 2-)[isopropoxycarbonyloxy]ethyl ester, 1-(or
2-)[butoxycarbonyloxy]ethyl ester, 1-(or 2-)-
~isobutoxycarbonyloxy]ethyl ester, 1-(or 2-)~pentoxy-
carbonyloxy]ethyl ester, 1-(or 2-)[isopentoxycarbonyloxy]-
ethyl ester, 1-(or 2-)[1-(or 2-)ethylpropoxycarbonyloxy]-
ethyl ester, 1-(or 2-)[neopentoxycarbonyloxy~ethyl ester,
1-(or 2-)[1-(or 2- or 3-)methylbutoxycarbonyloxy)ethyl
ester, 1-(or 2-)[hexyloxycarbonyloxy]ethyl ester, l-(or
~5 2-)[3,3-dimethylbutoxycarbonyloxy~ethyl ester, 1-(or
WO94/10177 214 7 6 0 9 PCT/JP93/01505
~ - 13 - -
2-~ r i.sohexyloxycarbonyloxy]ethyl ester, 1-(or 2-)-
[i-(or 2- or 3-)ethylbutoxycarbonyloxy]ethyl ester,
1-(or 2-)[1-(or 2- or 3-)propylbutoxycarbonyloxy]ethyl
ester, etc.);
lower alkenyloxycarbonyloxy(lower)alkyl ester (e.g.,
ethenyloxycarbonyloxymethyl ester, 1-(or
2-)propenyloxycarbonyloxymethyl ester, 1-(or 2- or
3-)butenyloxycarbonyloxymethyl ester, 1-(or 2- or 3- or
4-)pentenyloxycarbonyloxymethyl ester, 1-(or
lu 2-)ethenyloxycarbonyloxyethyl ester, 1-(or 2-)[1-(or2-)propenyloxycarbonyloxy]ethyl ester, 1-(or 2-)[1-(or 2-
or 3-)butenyloxycarbonyloxy]ethyl ester, 1-(or 2-)[1-(or
2- or 3- or 4-)pentenyloxycarbonyloxy~ethyl ester, etc.);
lower alkoxycarbonyl(lower)alkenyl ester ~e.g., 1-(or 2-
1~ or 3-)methoxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3-)ethoxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3-)propoxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3-)butoxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3-)isobutoxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3-)pentoxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3-)isopentoxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3-)hexyloxycarbonyl-1-(or 2-)propenyl ester, 1-(or 2- or
3- or 4-)methoxycarbonyl-1-(or 2- or 3-~butenyl ester,
1-(or 2- or 3- or 4-)ethoxycarbonyl-l-(or 2- or 3-)butenyl
2~ ester, 1-(or 2- or 3- or 4-)propoxycarbonyl-1-(or 2- or
3-)butenyl ester, 1-(or 2- or 3- or
4-)butoxycarbonyl-1-(or 2- or 3-)butenyl ester, 1-(or 2-
or 3- or 4-)isobutoxycarbonyl-1-(or 2- or 3-)butenyl
ester, 1-(or 2- or 3- or 4-)pentoxycarbonyl-1-~or 2- or
3-)butenyl ester, 1-(or 2- or 3- or
4-)isopentoxycarbonyl-1-(or 2- or 3-)butenyl ester, 1-(or
2- or 3- or 4-)hexyloxycarbonyl-1-(or 2- or 3-)butenyl
ester, 1-(or 2- or 3- or 4- or 5-)methoxycarbonyl-1-(or 2-
or 3- or 4-)pentenyl ester, 1-(or 2- or 3- or 4- or
5-)ethoxycarbonyl-1-(or 2- or 3- or 4-)pentenyl ester,
` PCT/JP93/0150~
W094/10177 21 ~ 6 0 9
1-(or 2- or 3- or 4- or 5-)propoxycarbonyl-1-(or 2- or 3-
or 4-)pentenyl ester, 1-(or ~--or 3- or 4- or
5-)butoxycarbonyl-1-(or 2- -or 3- or 4-)pentenyl ester,
1-(or 2- or 3- or 4- or ~)isobutoxycarbonyl-1-(or 2- or
3- or 4-)pentenyl ester, 1-(or 2- or 3- or 4- or
~-)pentoxycarbonyl-1-(or 2- or 3- or 4-)pentenyl ester,
1-(or 2- or 3- or 4- or 5-)isopentoxycarbonyl-1-(or 2- or
3- or 4-)pentenyl ester, 1-(or 2- or 3- or 4- or
5-)hexyloxycarbonyl-1-(or 2- or 3- or 4-)pentenyl ester,
etc.]; phthalidylidene(lower)alkyl ester;
(5-(lower)alkyl-2-oxo-1,3-dioxol-4-yl)(lower)alkyl ester
[e.g., (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester,
(5-ethyl-2-oxo-1,3-dioxol-4-yl)methyl ester,
(5-propyl-2-oxo-1,3-dioxol-4-yl)methyl ester, 1-(or
2-)(5-methyl-2-oxo-1,3-dioxol-4-yl)ethyl ester, 1-tor
2-)(5-ethyl-2-oxo-1,3-dioxol-4-yl)ethyl ester, 1-(or
2-)(5-propyl-2-oxo-',3-dioxol-4-yl)ethyl ester, etc.];
cycloalkylcarbonyloxy(lower)alkyl ester which may have
suitable substituent(s) [e.g.,
cycloalkylcarbonyloxy(lower)alkyl ester which may have
lower alkyl (e.g., cyclopentylcarbonyloxymethyl ester,
cyclohexylcarbonyloxymethyl ester,
cycloheptylcarbonyloxymethyl ester,
1-methylcyclohexylcarbonyloxymethyl ester,
1-(or 2-)[cyclopentylcarbonyloxy]ethyl ester, 1-(or
2-)[cyclohexylcarbonyloxy]ethyl ester, 1-(or
2-)[cycloheptylcarbonyloxy]ethyl ester, etc.), etc.];
cyclo(lower)alkyloxycarbonyloxy(lower)alkyl ester which
may have suitable substituent(s) ~e.g.,
J O CyC lo(lower)alkyloxycarbonyloxy(lower)alkyl ester which
may have one or two lower alkyl (e.g.,
cyclopentyloxycarbonyloxymethyl ester,
cyclohexyloxycarbonyloxymethyl ester, 1-(or
2-)[cyclopentyloxycarbonyloxy]ethyl ester, 1-(or
2-)[cyclohexyloxycarbonyloxy]ethyl ester, 1-(or
2 1 ~ 7 6 0 9 PCr/JP93/01505
WO94/10177
~ - 15 -
2-) r 2-isopropyl-5-methylcyclohexyloxycarbonyloxy]ethyl
ester, etc.), etc.];
cyclo(lower)alkyl(lower)alkoxycarbon~,loxy(lower~alkyl
ester (e.g., 1-(or 2-)~cyclobutylmethoxycarbonyloxy]ethyl
5 ester, 1-(or 2-)~cyclopentylmethoxycarbonyloxy~ethyl
ester, 1-(or 2-)[cyclohexylmethoxycarbonyloxy]ethyl ester,
1-(or 2-)1[1-(or 2-)cyclobutylethoxy~carbonyloxy]ethyl
ester, l-(or 2-)[~1-(or 2-)cyclopentylethoxy]-
carbonyloxy]ethyl ester, 1-(or 2-)[[1-(or
2-)cyclohexylethoxy]carbonyloxy]ethyl ester, etc.);
lower alkanesulfonyl(lower)alkyl ester (e.g. mesylmethyl
ester, 2-mesylethyl ester,etc.);
ar(lower)alkyl ester, for example, phenyl(lower)alkyl
ester which may have one or more suitable substituent(s)
(e.g., benzyl ester, 4-methoxybenzyl ester, 4-nitrobenzyl
ester, phenethyl ester, trityl ester, benzhydryl ester,
bis(methoxyphenyl)methyl ester, 3,~-dimethoxybenzyl ester,
4-hydroxy-3,~-di-t-butylbenzyl ester, etc.);
aryl ester which may have one or more suitable
?û substituent(s) such as substituted or unsubstituted phenyl
ester (e.g., phenyl ester, tolyl ester, t-butylphenyl
ester, xylyl ester, mesityl ester, cumenyl ester,
4-chlorophenyl ester, 4-methoxyphenyl ester, etc.);
tri(lower)alkyl silyl ester; lower alkylthioester (e.g.
~5 methylthiGester, ethylthioester, etc.) and the like.
Suitable "hydroxy protective group" may include acyl
as mentioned above, phenyl(lower)alkyl which may have one
or more suitable substituent(s) ~e.g., mono(or di or
tri)phenyl(lower)alkyl (e.g., benzyl, trityl, etc.),
4-methoxybenzyl, etc.], trisubstituted silyl (e.g.,
trimethylsilyl, t-butyldimethylsilyl, etc.),
tetrahydropyranyl and the like.
Suitable "aryl" may include phenyl, naphthyl and the
- like.
Suitable "leaving group" may include acid residue and
w094/1017~ 1 47 6 0 9 PCT/JP93iO150
- - 16 -
the like
Suitable "halogen moie~y" in the term "mono(or di or
tri)halo(lower)alkyl" m~y lnclude fluorine, bromine,
chlorine and iodine.
', Suitable "acid residue" may include halogen ~e.g.,
fluorine, chlorine, bromine, iodine, etc.), acyloxy [e.g.,
~ulfonyloxy (e.g., benzenesulfonyloxy, tosyloxy, mesyloxy,
etc.), lower alkanoyloxy (e.g., acetyloxy, propionyloxy,
etc.), etc.] and the like.
Suitable "substituent" in the terms "pyridylvinyl
which may have suitable substituent(s)" and "pyridyl which
may have suitable substituent(s)" may include lower alkyl
(e.g., methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
t-butyl, pentyl, neopentyl, t-pentyl, hexyl, etc.), lower
1~ alkoxy (e.g., methoxy, ethoxy, propoxy, isopropoxy,
isobutoxy, t-butoxy, pentyloxy, neopentyloxy, t-pentyloxy,
he~ylo~, etc.), lower alkenyl (e.g., vinyl, 1-propenyl,
allyl, 1-methylallyl, 1 or 2 or 3-butenyl, 1 or 2 or 3 or
4-pentenyl, 1 or 2 or 3 or 4 or 5-hexenyl, etc.), lower
alkynyl (e.g., ethynyl, 1-propynyl, propargyl,
1-methylpropargyl, 1-methylpropargyl, 1 or 2 or 3-butynyl,
1 or 2 or 3 or 4-pentynyl, 1 or 2 or 3 or 4 or 5-hexynyl,
etc.), mono(or di or tri)halo(lower)alkyl (e.g.,
fluoromethyl, difluoromethyl, trifluoromethyl,
chloromethyl, dichloromethyl, trichloromethyl,
bromomethyl, dibromomethyl, tribromomethyl, 1 or
2-fluoroethyl, 1 or 2-bromoethyl, 1 or 2-chloroethyl,
1,1-difluoroethyl, 2,2-difluoroethyl, etc.), halogen
(e.g., chlorine, bromine, fluorine, iodine), carboxy,
3~ protected carboxy, hydroxy, protected hydroxy, aryl (e.g.,
phenyl, naphthyl, etc.), ar(lower)alkyl such as
phenyl~lower)alkyl (e.g., benzyl, phenethyl,
phenylpropyl, etc.), carboxy(lower)alkyl, protected
carbcxy~lower)alkyl, nitro, amino, protected amino,
~5 di(lower)alkylamino (e.g., dimethylamino, diethylamino,
2 1 ~ 7 6 0 9 PCr/JW3/01505
WO94/10177
- 17 -
dii~opropylamino, ethylmethylamino, isopropylmethylamino,
ethylmethylamino, ethylpropylamino, etc.),
hydroxy(lower)alkyl, protected hydroxy(lower)alkyl, acyl,
cyano, mercapto, lower alkylthio (e.g., methylthio,
e~hyllhio, propylthio, isopropylthio, butylthio, etc.),
imino, and the like.
Suitable pharmaceutically acceptable salts of the
object compound (I) are conventional non-toxic salts and
include a metal salt such as an alkali metal salt [e.g.
sodium salt, potassium salt, etc.] and an alkaline earth
metal salt ~e.g. calcium salt, magnesium salt, etc.],
an ammonium salt, an organic base salt ~e.g.
trimethylamine salt, triethylamine salt, pyridine salt,
picoline salt, dicyclohexylamine salt,
N,N'-dibenzylethylenediamine salt, etc.], an organic acid
salt ~e.g. formate, acetate, trifluoroacetate, maleate,
tartrate, methanesulfonate, benzenesulfonate,
p-toluenesulfonate, etc.], an inorganic acid salt ~e.g.
hydrochloride, hydrobromide, sulfate, phosphate, etc.],
a salt with an amino acid ~e.g. arginine salt, aspartic
acid salt, glutamic acid salt, etc.], and the like.
The processes for preparing the object and starting
compounds of the present invention are explained in detail
in the following.
Process tl)
The compound (I) or a salt thereof can be prepared by
reacting the compound (II) or its reactive derivative at
the amino group, or a salt thereof with the compound (III)
or its reactive derivative at the carboxy group, or a salt
thereof.
Suitable reactive derivative at the amino group of
the compound (II) may include Schiff's base type imino or
3~ its tautomeric enamine type isomer formed by the reaction
PCT/JP93/01505
WO94/10177
~4~609 18 -
of the compound ~II) with a carbonyl compound such as
aldehyde, ketone or the like; a silyl derivative formed by
the reaction of the compound~(II) with a silyl compound
such as bis(trimethylsil ~ acetamide,
; mono~trimethylsilyl)ace~a~ide [e.g. N-(trimethylsilyl)-
acetamide], bis(trimethylsilyl)urea or the like;
a derivative formed by reaction of the compound (II) with
phosphorus trichloride or phosgene, and the like.
Suitable reactive derivative at the carboxy group of
the compound (III) may include an acid halide, an acid
anhydride, an activated amide, an activated ester, and the
like. Suitable examples of the reactive derivatives may
be an acid chloride; an acid azide;
a mixed acid anhydride with an acid such as substituted
i5 phosphoric acid [e.g. dialkylphosphoric acid,
phenylphosphoric acid, diphenylphosphoric acid,
dibenzylphosphoric acid, halosenated phosphoric acid,
etc.~, dialkylphosphorous acid, sulfurous acid,
thiosulfuric acid, sulfuric acid, sulfonic acid ~e.g.
methanesulfonic acid, etc.], aliphatic carboxylic acid
[e.g. acetic acid, propionic acid, butyric acid,
isobutyric acid, pivalic acid, pentanoic acid,
isopentanoic acid, 2-ethylbutyric acid, trichloroacetic
acid, etc.] or aromatic carboxylic acid [e.g. benzoic
acid, etc.~; a symmetrical acid anhydride; an activated
amide with imidazoie, 4-substitued imidazole,
l-hydroxy-lH-benzotriazole, dimethylpyrazole, triazole or
tetrazole; or an activated ester [e.g. cyanomethyl ester,
methoxymethyl ester, dimethyliminomethyl [(CH3)2~=CH-]
ester, vinyl ester, propargyl ester, p-nitrophenyl ester,
2,4-dinitrophenyl ester, trichlorophenyl ester,
pentachlorophenyl ester, mesylphenyl ester,
phenylazophenyl ester, phenyl thioester, p-nitrophenyl
thioester, p-cresyl thioester, carboxymethyl thioester,
pyranyl ester, pyridyl ester, piperidyl ester, 8-~uinolyl
WO94/10177 21~ 7 6 0 9 PCT/JP93/01505
_ _ ~9 _
thioester, etc.], or an ester with a N-hydroxy compound
~e.g. N,N-dimethylhydroxylamine,
l-hydroxy-2-(lH)-pyridone, N-hydroxysuccinimide,
N-hydroxyphthalimide, l-hydroxy-lH-benzotriazole, etc.],
', and the like. These reactive derivatives can optionally
be selected from them according to the kind of the
compound (III) to be used.
The reaction is usually carried out in a conventional
solvent such as water, alcohol [e.g. methanol, ethanol,
etc.], acetone, dioxane, acetonitrile, chloroform,
methylene chloride, ethylene chloride, tetrahydrofuran,
ethyl acetate, N,N-dimethylformamide, pyridine or any
other organic solvent which does not adversely influence
the reaction. These conventional solvent may also be used
in a mixture with water.
In this reaction, when the compound (III) is used in
a free acid form or its salt form, the reaction is
preferably carried out in the presence of a conventional
condensing agent such as N,N'-dicyclohexylcarbodiimide;
N-cyclohexyl-N'-morpholinoethylcarbodiimide;
N-cyclohexyl-N'-(4-diethylaminocyclohexyl)carbodiimide;
N,N'-diethylcarbodiimide, N,N'-diisopropylcarbodiimide;
N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide,
N,N'-carbonyl-bis(2-methylimidazole); pentamethylene-
ketene-N-cyclohexylimine, diphenylketene-N-cyclohexyl-
imine; ethoxyacetylene; l-alkoxy-l-chloroethylene;
trialkyl phosphite; ethyl polyphosphate; isopropyl
polyphosphate; phosphorus oxychloride (phosphoryl
chloride); phosphorus trichloride; thionyl chloride;
3~ oxalyl chloride; lower alkyl haloformate [e.g. ethyl
chloroformate, isopropyl chloroformate, etc.];
triphenylphosphine; 2-ethyl-7-hydroxybenzisoxazolium salt;
2-ethyl-5-(m-sulfophenyl)isoxazolium hydroxide
intramolecular salt; l-(p-chlorobenzenesulfonyloxy)-6-
chloro-lH-benzotriazole; so-called Vilsmeier reagent
W094/1017~ ~ 4~ 609 - 20 - PCT/JP93/0150'
prepared by the reaction of N,N-dimethylformamide with
thionyl chloride, phosgene, trichloromethyl chloroformate,
phosphorus oxychloride~,-é`tc.; or the like.
The reaction m~ also be carried out in the presence
5 of an inorganic or organic base such as an alkali metal
bicarbonate, tri(lower)alkylamine (e.g. triethylamine,
diisopropylethylamine, etc.), pyridine,
N-(lower~alkylmorpholine, N,N-di(lower)alkylbenzylamine,
or the like.
The reaction temperature is not critical, and the
reaction is usually carried out under cooling to warming.
Process (2)
The compound (Ib) or a salt thereof can be prepared
by subjecting the compound (Ia) or a salt thereof to
elimination reaction of the hydroxy protective group.
Suitable method of this elimination reaction may include
conventional one such as hydrolysis, reduction and the
like.
(i) For Hydrolysis :
The hydrolysis is preferably carried out in the
presence of a base or an acid including Lewis acid.
Suitable base may include an inorganic base and an
organic base such as an alkali metal [e.g. sodium,
potassium, etc.], an alkaline earth metal [e.g. magnesium,
calcium, etc.], the hydroxide or carbonate or bicarbonate
thereof, trialkylamine [e.g. trimethylamine,
triethylamine, etc.], picoline, l,5-diazabicyclo[4.3.0]-
~0 non-5-ene, l,4-diazabicyclo~2.2.2]octane,
l,8-diazabicyclo~5.4.0]undec-7-ene, or the like.
Suitable acid may include an organic acid [e.g.
formic acid, acetic acid, propionic acid, trichloroacetic
acid, trifluoroacetic acid, etc.] and an inorganic acid
[e.g. hydrochloric acid, hydrobromic acid, sulfuric acid,
2 1 ~ 7 6 0 9 pCT/JP93/0150~
WO94/10177
- 2t -
hydrogen chloride, hydrogen bromide, ammonium chloride,
e~c.]. The elimination using Lewis acid such as
trihaloacetic acid ~e.g. trichloroacetic acid,
trifluoroacetic acid, etc.] or the like is preferably
carried out in the presence of cation trapping agents
[e.g. anisole, phenol, etc.J.
The reaction is usually carried out in a solvent such
as water, an alcohol [e.g. methanol, ethanol, etc.],
methylene chloride, tetrahydrofuran, a mixture thereof or
any other solvent which does not adversely influence the
reaction. A liquid base or acid can be also used as the
solvent. The reaction temperature is not critical and the
reaction is usually carried out under cooling to warming.
(ii) For reduction :
Reduction is carried out in a conventional manner,
- including chemical reduction and catalytic reduction.
Suitable reducing agents to be used in chemical
reduction are a combination of a metal (e.g. tin, zinc,
~0 iron, etc.) or metallic compound (e.g. chromium chloride,
chromium acetate, etc.) and an organic or inorganic acid
(e.g. formic acid, acetic acid, propionic acid,
trifluoroacetic acid, p-toluenesulfonic acid, hydrochloric
acid, hydrobromic acid, etc.).
Suitable catalysts to be used in catalytic reduction
are conventional ones such as platinum catalysts te.g.
platinum plate, spongy platinum, platinum black, colloidal
platinum, platinum oxide, platinum wire, etc.), palladium
catalysts (e.g. spongy palladium, palladium black,
palladium oxide, palladium on czrbon, colloidal palladium,
palladium on barium sulfate, palladium on barium
carbonate, etc.), nickel catalysts (e.g. reduced nickel,
nickel oxide, Raney nickel, etc.), cobalt catalysts (e.g.
reduced cobalt, Raney cobalt, etc.), iron catalysts (e.g.
reduced iron, Raney iron, etc.), copper catalysts (e.g.
WO94/10177 ~ PCT/JP93/0150~
~41609
leduced copper, Raney copper, Ullman copper, etc.) and the
like. The reduction is usually carried out in a
conventional solvent which does not adversely influence
the reaction such as water, ~ethanol, ethanol, propanol,
, dioxane, tetrahydrofurar.~ N,N-dimethylformamide, or a
mixture thereof. Additionally, in case that the
above-mentioned acids to be used in chemical reduction are
in li~uid, they can also be used as a solvent.
The reaction temperature of this reduction is not
critical and the reaction is usually carried out under
cooling to warming.
The present invention includes, within the scope of
the invention, the case that the protected amino group in
~l is transformed into an amino group during this
reaction.
Process (3)
The compound (Id) or a salt thereof can be prepared
by subjecting the compound (Ic) or a salt thereof to
esterification reaction..
Suit2ble esterifying agent to be used in this
reaction may include a conventional one such as an alcohol
of the formula : HO-R9 (IX) (wherein R9 is as defined
above) or its reactive e~uivalent (e.g., halide,
sulfonate, sulfate, diazo compound, etc.) or a salt
thereof, or the like.
This reacticn is usually carried out in the presence
of a base.
Suitable base may include, for example, an inorganic
3~ base such as alkali metal hydroxide (e.g., sodium
hydroxide, potassium hydroxide, etc.), alkaline earth
metal hydroxide (e.g., magnesium hydroxide, calcium
hydroxide, etc.), alkali metal carbonate (e.g., sodium
carbonate, potassium carbonate, cesium carbonate, etc.),
alkaline earth metal carbonate (e.g., magnesium carbonate,
WO94/10177 21~ 7 6 0 9 PCT/JP93/01505
~ , ~
-alcium carbonate, etc.), alkali metal bicarbonate ie.g.,
sodium bicarbonate, potassium bicarbonate, etc.), alkali
metal acetate (e.g., sodium acetate, potassium acetate,
etc.), alkaline earth metal phosphate (e.g., magnesium
phosphate, calcium phosphate, etc.), alkali metal hydrogen
phosphate (e.g., disodium hydrogen phosphate, dipotassium
hydrogen phosphate, etc.) or the like, and an organic base
such as trialkylamine (e.g., trimethylamine,
triethylamine, etc.), picoline, N-methylpyrrolidine,
l9 N-methylmorpholine, l,5-diazabicyclo~4.3.0]non-5-ene,
l,4-diazabicyclo~2.2.2]octane, l,5-diazabicyclo~5.4.0]-
undecene-5 or the like.
This reaction is usually carried out in a solvent
such as benzene, N,N-dimethylformamide, tetrahydrofuran,
toluene, methylene chloride, ethylene dichloride,
chloroform or any other solvent which does not adversely
affect the reaction.
The reaction temperature is not critical and the
reaction is usually carried out under cooling to heating.
The compound (IX) or its reactive equivalent, or a
salt thereof can be prepared in the manner disclosed in
Preparations, similar manners thereto or a conventional
manner.
Process IA)
The compound (VId) or a salt thereof can be prepared
by reacting the compound (IV) or a salt thereof with the
compound (V) or a salt thereof.
This reaction is usually carried out in a solvent
such as water, alcohol le.g., methanol, ethanol, etc.),
benzene, N,N-dimethylformamide, tetrahydrofuran, toluene,
methylene chloride, ethylene dichloride, chloroform,
dioxane, diethyl ether or any other solvent which does not
adversely affect the reaction. These conventional solvent
may also be used in a mixture with water.
PCI/JP93fO150
WO 94/10177
z~4'1 609 . .~ -
The reaction temperature is not critical and the
reaction is usually carried out under cooling to heating.
The reaction is usually carried out in the presence
of an inorganic or an organ~ base such as an alkali metal
(e.g., sodium, potassium, etc.), an alkali metal hydroxide
(e.g., sodium hydroxide, potassium hydroxide, etc.), an
alkali metal hydrogencarbonate (e.g., sodium
hydrogencarbonate, potassium hydrogencarbonate, etc.),
alkali metal carbonate ~e.g., sodium carbonate, potassium
carbonate, etc.), tri~lower)alkylamine ~e.g.,
trimethylamine, triethylamine, diisopropylethylamine,
etc.), alkali metal hydride ~e.g., sodium hydride, etc.),
alkali metal ~lower)alkoxide (e.g., sodium methoxide,
sodium ethoxide, etc.), pyridine, lutidine, picoline,
dimethylaminopyridine, N-(lower)alkylmorpholine,
N,N-di(lower)alkylbenzylamine, N,N-di(lower)alkylaniline
or the like.
When the base and~or the starting compound are in
liquid, they can be also used as a solvent.
Process (B)
The compound (II) or a salt thereof can be prepared
by subjecting the compound ~VIa) or a salt thereof to
elimination reaction of the amino protective group.
This reaction can be carried out in a similar manner
to that of the aforementioned Process ~2), and therefore
the reagents to be used and the reaction conditions (e.g.,
solvent, reaction temperature, etc.) can be referred to
those of the Process ~2).
The present invention includes, within the scope of
the invention, the case that the protected carboxy group
in R3 is transformed into a carboxy group during this
reaction.
WO94/10177 2 1 4 7 6 0 9 ~ PCT/JP93/01505
Process (C)
The compound (VIc) or a salt thereof can be prepared
by subjecting the compound (VIb) or a salt thereof to
elimination reaction of the carboxy protective group.
This reaction can be carried out in a similar manner
to that of the aforementioned Process ~2), and therefore
the reagents to be used and the reaction conditions (e.g.,
solvent, reaction temperature, etc.) can be referred to
those of the Process (2).
iO The present invention includes, within the scope of
the invention, the case that the protected amino group in
R5 is transformed into an amino group during this
reaction.
Process (D)
The compound (VI) or a salt thereof can be prepared
by reacting the compound (VII) or a salt thereof with the
compound (VIII) or a salt thereof.
This reaction can be carried out in the manner
disclosed in Preparation 16 or similar manners thereto.
Process (E)
The compound (VIe) or a salt thereof can be prepared
by subjecting the compound (VIc) or a salt thereof to
esterification reaction.
This reaction can be carried out in a similar manner
to that of the aforementioned Process (3), and therefore
the reagent to be used and the reaction conditions (e.g.,
solvent, reaction temperature, etc.) can be referred to
those of the Process (3).
Suitable salts of the object and starting compounds
and their reactive derivatives in Processes (l) ~ (3) and
(A) ~ (~) can be referred to the ones as exemplified for
the compound (I).
The object compound (I) and pharmaceutically
WO94/10177 PCT/JP93/01505
2 1 4~ 609 - 2~ - ~
acceptable salts thereof are novel and exhibit high
antimicrobial activity, inhibiting the growth of a wide
variety of pathogenic microor~ganisms including
Gram-positive and Gram-ne~ative microorganisms and are
5 useful as antimicro~ agents, especiaily oral
antimicrobial agents.
Now in order to show the utility of the object
compound (I), the test data on MIC (m;~;mA1 inhibitory
concentration) and the test data on urinary excretion of
each representative compound of this invention are shown
in the following.
(A) Minimal inhibitory concentration
Test method :
In vitro antibacterial activity was determined by the
~wo-fold agar-plate dilution method as described below.
One loopful of an overnight culture of each test
strain in Trypticase-soy broth (108 viable cells per ml)
was streaked on heart infusion agar (HI-agar)containing
graded concentrations of test compound, and the mi n;m~l
inhibitory concentration ~MIC) was expressed in terms of
~g/ml after incubation at 37C for 20 hours.
Test compound :
(l) 7~-L2-(2-Aminothiazol-4-yl)-2-hydroxyiminoacetamido}-
3-[(E)-2-(pyridin-4-yl)vinyl~-3-cephem-4-carboxylic
acid (syn isomer)
Test result :
MIC (~g/ml)
Test strain Test compound (l)
E. coli 31 ~ 0.025
WO94/10177 21~ 7 6 0 9
~_ 27 - .
(B) Urinary excretion
Test method :
Male JCL SD strain rats (age, 6-7 weeks) were used.
5 Test compound was suspended in 0.5% methyl cellulose
solution. The rats were starved overnight before dosing
with 20 mg ~*"free acid form of test compound" equivalent)
/kg. Urine samples were collected at 0 to 6 and 6 to 24
hours after oral administration. Antibiotic
concentrations were measured by the disc-plate diffusion
method using Bacillus subtilis ATCC 6633 as the test
organism and sodium citrate agar (0.8% sodium citrate,
0.5% polypeptone, 0.3% beef extract and 1.0% agar) as the
test medium. The diluents of "free acid form of test
compound" for the standard curves were prepared with 1/15
M phosphate buffer (pH 7.0). The plates were incubated at
37C for 18 hours and the zone of inhibition were
measured.
Test compound :
(2) 1-(Cyclohexyloxycarbonyloxy)ethyl
7~-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-
3-~(Z)-2-(pyridin-3-yl)vinyl~-3-cephem-4-carboxylate
dihydrochloride (syn isomer)
.5
Test result :
Urinary recovery in
Test compound 24 hours (%)
(2) 32.4
*"free acid form of Test compound" :
WO94~10177 PCT/JP9~/01505
~4169 - 28 - ~
7~-[2-(2-Aminothiazol-4-yl)-2-hydroxyiminoacetamido]-
3-[~Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
For therapeutic ad~inistration, the object compound
(I) and pharmaceutically acceptable salts thereof of the
present invention are used in the form of conventional
pharmaceutical preparation which contains said compound as
an active ingredient, in admixture with pharmaceutically
acceptable carriers such as an organic or inorganic solid
or liquid excipient which is suitable for oral, parenteral
and external administration.
The pharmaceutical preparations may be in solid form
such as tablet, granule, powder, capsule, or liquid form
such as solution, suspension, syrup, emulsion, lemonade
and the like.
In needed, there may be included in the above
preparations, auxiliary substances, stabilizing agents,
wetting agents and other commonly used additives such as
lactose, citric acid, tartaric acid, stearic acid,
magnesium stearate, terra alba, sucrose, corn starch,
talc, gelatin, agar, pectin, peanut oil, olive oil, cacao
butter, ethylene glycol, and the like.
While the dosage of the compound (I) may vary from
and also depend upon the age, conditions of the patient, a
kind of diseases, a kind of the compound (I) to be
applied, etc. In general, amounts between l mg and about
4,000 mg or even more per day may be administered to a
patient. An average single dose of about 50 mg, lO0 mg,
250 mg, 500 mg, lO00 mg of the object compound (I) of the
present invention may be used in treating diseases
infected by pathogenic microorganisms.
Especially, the object compound (I) wherein R3 is
esterified carboxy and a pharmaceutically acceptable salt
thereof are useful as prodrug of the object compound (I)
wherein R3 is carboxy and a pharmaceutically acceptable
salt thereof.
WO94/10177 2 1 ~7 6 0 9 PCT/JP93/01505
- 29 -
Preferred embodiments of the object compound (I) are
as follows.
Rl is amino, or protected amino [more preferably acylamino
or mono(or di or tri)phenyl(lower)alkylamino],
R2 is hydrogen, a hydroxy protective group [more
preferably acyl [more preferably lower alkanoyl] or
phenyl(lower)alkyl which may have one or more
suitable substituent(s) [more preferably mono(or di
or tri)phenyl(lower)alkyl, most preferably trityl]],
lower alkyl or mono(or di or tri)halo(lower)alkyl,
R3 is carboxy or esterified carboxy [more preferably
alkanoyloxy(lower)alkoxycarbonyl (more preferably
cl-C8 alkanoyloxy(lower)alkoxycarbonyl),
alkoxycarbonyloxy(lower)alkoxycarbonyl (more
preferably Cl-C7 alkoxycarbonyloxy(lower)-
alkoxycarbonyl), cy~loalkylcarbonyloxy(lower)-
alkoxycarbonyl which may have suitable
substituent(s) (more preferably
cyclo(C5-C7)alkylcarbonyloxy(lower)alkoxycarbonyl
which may have lower alkyl),
cyclo(lower)alkyloxycarbonyloxy(lower)-
alkoxycarbonyl which may have suitable
substituent(s) (more preferably cyclo(lower)-
alkyloxycarbonyloxy(lower)alkoxycarbonyl
which may have one or two lower alkyl),
cyclo(lower)alkyl(lower)alkoxycarbonyloxy(lower)-
alkoxycarbonyl, lower alkoxycarbonyl(lower)-
alkenyloxycarbonyl, lower alkenyloxycarbonyloxy-
(lower)alkoxycarbonyl or (5-(lower)alkyl-2-oxo-l,3-
dioxol-4-yl)(lower)alkoxycarbonyl] and
R4 is pyridylvinyl which may have lower alkyl,
with proviso that
(i) when R2 is hydrogen and R4 is 3-pyridylvinyl, then
R3 is not carboxy, and
(ii) when R2 is tetrahydropyranyl and R4 is
3-pyridylvinyl, then
R is not benzhydryloxycarbonyl.
WO94/10177 PCT/JP93/01505
~4~609 - 30 -
The following Preparations and Examples are given for
the purpose of illustrating-the presen~ invention in more
detail. ;
; Preparation 1
To a mixture of diphenylmethyl 7~-tert-butoxy-
carbonylamino-3-triphenylphosphoniomethyl-3-cephem-4-
carboxylate iodide (100 g), methylene chloride (1000 ml)
and saturated sodium chloride (170 ml) was added lN sodium
hydroxide solution ~173 ml) with stirring at room
temperature. After the mixture was stirred at the same
temperature for 45 minutes, the separated organic layer
was washed with brine, dried over magnesium sulfate. To
this organic layer was added 3-pyridinecarbaldehyde (24.6
g) at room temperature, and the mixture was stirred at the
same temperature for 20 hours. The solvent was evaporated
in -Jacuo, and the residue was chromatographed on silica
gel to give diphenylmethyl 7~-tert-butoxycarbonylamino-3-
~(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (21.03
g) ~Compound A) and diphenylmethyl 7~-tert-butoxycarbonyl-
amino-3-r(E)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(7.81 g) (Compound B).
Compound A
IR (Nujol) : 1765, 1700 cm 1
NMR tDMSO-d6, ~) : 1.41 (9H, s), 3.30 and 3.56 (2H,
ABq, J=17.5Hz), 5.21 (lH, d, J=4.8Hz), 5.56 ~lH,
dd, J=8.3Hz, 4.8Hz), 6.49 (lH, d, J=12.2Hz),
6.55 (lH, d, J=12.2Hz), 6.79 (lH, s), 7.27-7.65
(12H, m), 8.08 (lH, d, J=8.3Hz), 8.38 (lH, m),
8.43 (lH, m)
Compound B
IR (Nujol) : 1770, 1700 cm 1
NMR (DMSO-d6, ~) : 1.42 (9H, s), 3.75 and 4.05 (2H,
2147609 PCT/JF93/01505
W094/10177
~~ - 31 -
~Bq, J=17.5Hz), 5.21 (lH, d, J=4.8Hz), 5.58 (lH,
dd, J=8.8Hz, 4.8Hz), 7.12 (lH, s), 7.18 (lH, d,
J=16.2Hz), 7.27-7.58 (13H, m), 8.12 (lH, d,
J=8.8Hz), 8.45 (2H, m)
Preparation 2
The following compounds were obtained according to a
similar manner to that of Preparation 1.
Diphenylmethyl 7~-tert-butoxycarbonylamino-3- E ( z ) - 2 -
(pyridin-4-yl)vinyl]-3-cephem-4-carboxylate (Compound A)
and
Diphenylmethyl 7~-tert-butoxycarbonylamino-3-[(E)-2-
(pyridin-4-yl)vinyl~-3-cephem-4-carboxylate (Compound B~
Compound A :
IR (Nujol) : 1770, 1720, 1695 cm 1
NMR (DMSO-d6, ~) : 1.41 (9H, s), 3.28 and 3.59 (2H,
ABq, J=17.9Hz), 5.23 (lH, d, J=4.8Hz), 5.58 (lH,
dd, J=4.8Hz, 9.OHz), 6.46 (lH, d, J=12.lHz),
6.60 (lH, d, J=12.lHz), 6.80 (lH, s), 7.11-7.50
(12H, m), 8.07 (lH, d, J=9.OHz), 8.48 (2H, m)
Compound B :
IR (Nujol) : 1775, 1705 cm 1
NMR (DMSO-d6, ~) : 1.42 (9H, s), 3.74 and 4.05 (2H,
ABq, J=17.6Hz), 5.22 (lH, d, J=4.9Hz), 5.60 (lH,
dd, J=8Hz, 9Hz), 7.01 (lH, d, J=16.6Hz), 7.04
(2H, m), 7.08 (lH, s), 7.25-7.67 (llH, m), 8.13
(lH, d, J=8.9Hz), 8.46 (2H, m)
Preparation 3
To a solution of diphenylmethyl 7~-tert-butoxy-
carbonylamino-3-~(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylate (70.8 g) in formic acid (280 ml) was added
PCT/JP93/OlS0
WO94/10177
~4~ 609 3~ _ ~
~ropwise conc. hydrochloric acid (54.8 ml) at room
temperature. After being stirred at room temperature for
2 hours, the mixture was~S.added dropwise to a mixture of
acetone (2.1 ~) and et~yl acetate (4.2 Q) under
J ice-cooling. The res~lting precipitate was collected by
fil.ration to give 7~-amino-3-[(Z)-2-~pyridin-3-yl)vinyl]-
3-cephem-4-carboxylic acid dihydrochloride (34.27 g).
IR (Nujol) : 1760, 1700 cm 1
NMR (DMSO-d6, ~) : 3.54 and 3.67 (2H, ABq,
J=17.3Hz), 5.17 (lH, d, J=5.0Hz), 5.36 (lH, d,
J=5.0Hz), 6.78 (2H, s), 7.93 (lH, m), 8.34 (lH,
m), 8.77 (lH, m), 8.83 (lH, m)
Preparation 4
The following compound was obtained according to a
similar manner to that OL Preparation 3.
7~-Amino-3-[(E)-2-(pyridin-3-yl)vinyl~-3-cephem-4-
carboxylic acid dihydrochloride
IR (Nujol) : 1760, 1695 cm
NMR (DMSO-d~, ~) : 3.86 and 4.13 (2H, ABq,
J=17.2Hz), 5.18 (lH, d, J=5.2Hz), 5.24 (lH, d,
J=5 2Hz), 7.30 (lH, d, J=16.5Hz), 7.72 (lH, d,
J=16.5Hz), 7.99 !lH~ m), 8.57 (lH, m), 8.77 (lH,
m!, 8.99 (lH, m)
Preparation 5
To a solution of diphenylmethyl 7~-tert-
butoxycarbonylamino-3-[(E)-2-(pyridin-4-yl)vinyl]-3-
cephem-4-carboxylate (1.6 g) in methylene chloride (4.8
ml) and anisole (1.6 ml) was added trifluoroacetic acid
(3.2 ml) under ice-cooling. After being stirred at room
temperature for 2 hours, the mixture was poured into
diisopropyl ether. The resulting precipitate was
collected by filtration to give 7~-amino-3-[(E)-2-
214760i9
WO94/10177 PCT/JP93/01505
- 33 -
~pyridin-4-yl)vinyl]-3-cephem-4-carboxylic acid
bis(trifluoroacetate) (1.-49 g).
IR (Nujol~ : 1780, 1660, 1600 cm
NMR (DMSO-d6, ~) : 3.8Ç and 4.15 (2H, ABq,
J=17.5Hz), 5.27 (lH, d, J=5.2Hz), 5.34 (lH, d,
J=5.2Hz), 7.09 (lH, d, J=16.6Hz), 7.27 (lH, d,
J=16.6Hz), 7.78 (2H, d, J=6.4Hz), 8.73 (2H, d,
J-Ç.4Hz)
Preparation 6
To a solution of 3-pentanol (1.76 g) in
dichloromethane (18 ml) were added pyridine (1.62 ml) and
;-cnloroethyl chloroformate ~2.16 ml) at 5C. The mixture
was stirred at 5C for 30 minutes and then at room
temperature for 3.5 hours. The reaction mixture was
diluted with dichloromethane, washed with water, with lN
hydrochloric acid, with aqueous sodium hydrogencarbonate
solution and with brine, dried over magnesium sulfate, and
evaporated in vacuo to give 1-chloroethyl 1-ethylpropyl
carbonate (3.66 g).
IR (Film) : 1750 cm 1
NMR (CDCl3, ~) : 0.93 and 0.94 (total 6H, t, J=7Hz),
1.5-1.7 (4H, m), 1.84 (3H, t, J=6Hz), 4.66 (lH,
p, J=6Hz), 6.44 (lH, q, J=6Hz)
Preparation 7
The following compounds were obtained according to a
similar manner to that of Preparation 6.
3Q (1) 1-Chloroethyl [(lR,2S,5R)-2-isopropyl-5-
methylcyclohexyl]carbonate
IR (Film) : 1740 cm 1
NMR (CDCl3, ~) : 0.8-1.2 (llH, m), 1.3-2.2 (6H, m),
1.83 (3H, d, J=6Hz), 4.59 (lH, m), 6.43 (lH, q,
J=6Hz)
21 ~ 7 6 0 ~ 34 - PCT/JP93/0150~
(23 l-Chloroethyl 4-methyipentyl carbonate
IR (Film) : 1745 cm 1
~MR (CDCl3, ~) : 0.9~0 t6H, d, J=7Hz), 1.1-1.3 (2H,
m), 1.4-1.8 (3~`, m), 1.84 (3H, d, J=6Hz), 4.19
(2H, t, J=7Hz), 6.43 (lH, q, J=6Hz)
Preparation 8
To a solution of l-chloroethyl l-ethylpropyl
carbonate (3.60 g) in benzene (16.2 ml) were added
tetrabutylammonium bromide (119 mg) and
trimethylbromosilane (3.67 ml). The mixture was stirred
at 80C for 23 hours. After cooling to the room
temperature, the reaction mixture was evaporated in vacuo.
The residue was dissolved in dichloromethane. The
solution was washed with water and with brine, dried over
magnesium sulfate, and evaporated in vacuo to give
l-bromoethyl l-ethylpropyl carbonate (3.42 g).
IR ~Film) : 1745 cm
NMR (CDCl3, ~) : 0.93 and 0.94 (total 6H, t, J=7Hz),
1.5-1.7 (4H, m), 2.04 (3H, d, J=6Hz), 4.67 (lH,
p, J=6Hz), 6.62 (lH, q, J=6Hz)
Preparation 9
The following compounds were obtained according to a
similar manner to that of Preparation 8.
(1) l-Bromoethyl 3,3-dimethylbutyrate
IR (Film) : 1750 cm 1
NMR (CDCl3, ~) : 1.06 (9H, s), 1.99 (3H, d, J=6Hz),
~0 2.24 (2H, s), 6.72 (lH, q, J=6Hz)
(2) l-Bromoethyl [(lR,2S,5R)-2-isopropyl-5-
methylcyclohexyl]carbonate
IR (Film) : 1745 cm
NMR (CDCl3, ~) : 0.7-1.1 (llH, m), 1.4-2.2 (6H, m),
WO94/10177 21 ~ 76 0 9 PCT
- 35 -
2.03 (3H, d, J=6Hz), 4.60 (lH, m), 6.62 (lH, q,
J=6Hz)
(3) 1-Bromoethyl 4-methylpentyl carbonate
IR ~Film) : 1745 cm 1
NMR ~CDCl3, ~) : 0.90 (6H, d, J=7Hz), 1.1-1.3 (2H,
m), 1.4-1.8 ~3H, m), 2.03 (3H, d, J=6Hz), 4.20
(2H, t, J=7Hz), 6.61 (lH, q, J=6Hz)
Preparation 10
To a mixture of n-hexyl acrylate (18 g) and
1,4-diazabicyclo[2,2,2]octane (1.29 g) was added dropwise
propionaldehyde (12.47 ml). After stirring at room
temperature for 2 weeks, the reaction mixture was diluted
with ethyl acetate, washed with water and brine, dried
over magnesium sulfate and evaporated in vacuo. The
residue was distilled under vacuum ta give n-hexyl
2-(1-hydroxypropyl)-2-propenoate (10.38 g).
IR (Neat) : 3400, 1695 cm 1
NMR (DMSO-d6, ~) : 0.80-0.90 (6H, m), 1.28-1.36 (8H,
m) ! 1.51-1.71 (2H, m), 4.09 (2H, t, J=6.3Hz),
4.25-4.33 ~lH, m), 4.g5 ~lH, d, J=5.1Hz), 5.82
~lH, m), 6.09 (lH, m)
Preparation 11
The following compounds were obtained according to a
similar manner to that of Preparation 10.
(1) n-Butyl 2-(1-hydroxypropyl)-2-propenoate
IR (Neat) : 3400, 2920, 1700 cm 1
NMR (DMSO-d6, ~) : 0.69-0.93 (6H, m), 1.23-1.34 (3H,
m), 1.51-1.71 (3H, m), 4.10 (2H, t, J=6.4Hz),
4.24-4.33 ~lH, m), 4.96 (lH, d, J=5.0Hz),
5.81-5.83 (lH, m), 6.09-6.10 (lH, m)
214~ 6 0 - 36 - PCT/JP93/0150C
(2~ 3-Methylbutyl 2-(1-hydroxypropyl)-2-propenoate
IR (Neat) : 2930, ~710 cm 1
NMR (DMSO-d6, ~) ~ 0.82 ~3H, t, J=7.4Hz), 0.89 (6H,
d, J=6.4Hz), 1.17-1.73 (5H, m), 4.13 (2H, t,
J=6.7Hz), 4.25-4.33 (lH, m), 4.96 (lH, d,
J=5.1Hz), 5.82-5.83 (lH, m), 6.09 (lH, m)
(3) 3-Methylbutyl 2-(1-hydroxyethyl)-2-propenoate
IR (Neat) : 3350, 2900, 1690 cm
NMR (DMSO-d6, ~) : 0.90 (6H, d, J=6.4Hz), 1.18 (3H,
d, J=6.4Hz), 1.46-1.74 (3H, m), 4.17 (2H, t,
J=5.4Hz), 4.43-4.48 (lH, m), 5.03 (lH, d,
J=4.8Hz), 5.84-5.86 (lH, m), 6.05-6.06 (lH, m)
Preparation 12
To a n-hexyl 2-(1-hydroxypropyl)-2-propenoate (6 g)
was dropwise added 47% hydrobromic acid (8.21 ml) under
ice-cooling. After a few minutes, conc. sulfuric acid
(8.05 ml) was added thereto dropwise and the reaction
G 0 mixture was allowed to reach room temperature. After
stirring at ambient temperature overnight, the resulting
reaction mixture was transferred into a separatory funnel,
the lower layer was discarded and the upper layer was
extracted twice with ether. The com~ined ether extracts
were washed with saturated sodium bicarbonate solution,
water and brine, and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give
(Z)-2-(n-hexyloxycarbonyl)-2-pentenylbromide (4.05 g).
IR (Neat) : 2910, 1700, 1635 cm
NMR (CDCl3, ~) : 0.90 (3H, t, J=6.4Hz), 1.13 (3H, t,
J=7.5Hz), 1.34 (6H, m), 1.66-1.73 (2H, m),
2.24-2.39 (2H, m), 4.15-4.19 (2H, m), 4.23 (2H,
s), 6.95 (lH, t, J=7.7Hz)
2147~09
WO94/10177 ~ PCT/JP93/01505
_ - 37 -
Pr~paration 13
~ he following compounds were obtained according to a
similar manner to that of Preparation 12.
(1) (Z~-2-(n-Butoxycarbonyl)-2-pentenylbromide
IR (Neat) : 1700 cm 1
NMR ;CDCl3, ~) : 0.96 (3H, t, J=7.2Hz), 1.13 ~3H, t,
J=7.5Hz), 1.34-1.49 (2H, m), 1.62-1.76 (2H, m),
2.24-2.39 (2H, m), 4.17-4.22 (2H, m), 4.23 (2H,
s), 6.95 (lH, t, J=7.7Hz)
(2) (Z1-2-(3-Methylbutyloxycarbonyl)-2-pentenylbromide
I~ (Neat) : 2910, 1700, 1630 cm 1
NMR (CDCl3, ~) : 0.95 (6H, d, J=6.4Hz), 1.13 (3H, t,
J=7.5Hz), 1.55-1.81 (3H, m), 2.27-2.36 (2H, m),
4.19-4.26 (4H, m), 6.95 (lH, t, J=7.6Hz)
(3) (Z)-2-(3-Methylbutyloxycarbonyl)-2-butenylbromide
IR (Neat) : 2920, 1700, 1635 cm
NMR (CDCl3, 3) : 0.94 (6H, d, J=6.4Hz), 1.54-1.81
(3H, m), 1.92 (3H, d, J=7.3Hz), 4.19-4.26 (4H,
m), 7.06 (lH, q, J=7.3Hz)
Preparation 14
To 3,3-dimethylbutyryl chloride (24.225 g) was added
dropwise paraldehyde ~7.928 g). To the mixture was added
zinc chloride (25 mg). The mixture was stirred at 90C
for 5 hours. The reaction mixture was distilled to give
1-chloroethyl 3,3-dimethylbutyrate (15.49 g) (55-57C/14
mmHg~.
NMR (CDCl3, ~) : 1.05 ~9H, s), 1.79 (3H, d, J=6Hz),
2.24 (2H, s), 6.55 (lH, q, J=6Hz)
Preparation 15
To a solution of 3-ethynylpyridine (30.94 g) in
WO94/10177 PCT/JP93/0150~
~4~6~9 - 38 -
benzene (300 mi) was 2,2'-azobisisobutylnitrile (493 mg)
under reflux and to the m~xture was added dropwise
tributyltin hydride (87~`32 g) over 50 minutes under
nitrogen. The mixtùre was refluxed for 1 hour and
evaporated in vacuo. The residue was chromatographed on
silica gel to give 3-~(E)-2-tributylstannylvinyl~pyridine
(55.3i g) as an oil.
IR (Film) : 2900, 1730, 1580 cm 1
NMR (CDCl3, ~) : 0.8-1.6 (27H, m), 6.85 (lH, d,
J=19.6Hz), 6.99 (lH, d, J=19.6Hz), 7.2-7.3 (lH,
m~, 7.7-7.8 (lH, m), 8.4-8.5 (lH, m), 8.6-8.65
~lH, m)
Preparation 16
To a cooled solution of diphenylmethyl
7~-formamido-3-methylsulfonyloxy-3-cephem-4-carboxylate
(69.29 g) in N,N-dimethylformamide (690 ml) was added
3-[(E)-2-tributylstannylvinyl)pyridine ~55.19 g) and
lithium bromide (24.67 g) at 5C. The mixture was
2~ degassed by nitrogen for 5 minutes. To the mixture was
added bis(acetnitrile)palladium(II) chloride (737 mg) and
the mixture was stirred at 5C for 30 minutes and at room
temperature for 20 hours. The mixture was poured into
ice-water (4.5 Q) and the precipitates were collected by
~ ration and washed with cold water. The precipitates
were dissolved in tetrahydrofuran and to the solution was
added a mixture of ethyl acetate and water. The separated
organic layer was washed with brine, dried over magnesium
sulfate and evaporated in vacuo. The residue was
dissolved in acetnitrile (300 ml) and the solution was
washed with hexane (300 ml) twice. The acetnitrile layer
was evaporated in vacuo and the residue was
chromatographed on silica gel to give diphenylmethyl
7~-formamido-3-l(E)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylate (54.89 g).
21 9 7 6 0 9 PCT/JP93/01505
WO94/10177
- 3~ - -
IR (Nujol) : 3200, 1760, 1670 cm
NMR (DMSO-d6, ~) : 3.79 and 4.10 (2H, ABq,
J=17.6Hz), 5.28 (lH, t, J=4.9Hz), 5.89 llH, dd,
J=8.9 and 4.9Hz), 7.07 llH, s), 7.16 (lH, d,
J=16.3Hz), 7.3-7.5 (13H, m), 8.19 (lH, s),
8.45-8.55 (2H, m), 9.19 (lH, d, J=8.9Hz)
Preparation 17
To a solution of diphenylmethyl 7~-formamido-3-r(E)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (54.8 g) in
methanol (550 ml) was added dropwise conc. hydrochloric
acid (91.7 ml) at 5C and the mixture was stirred at room
temperature for 4 hours. The mixture was poured into a
mixture of ethyl acetate (2 Q) and ice water (1 Q) and
adjusted to pH 7 by addition of 5N aqueous sodium
hydroxide solution. The separated organic layer was
washed with water and brine, dried over magnesium sulfate
and evaporated in vacuo. To the residue was added ethyl
acetate and the crystalline solid was collected by
filtration, washed with ethyl acetate and diisopropyl
ether and dried in vacuo to give diphenylmethyl
7~-amino-3-[(E)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylate (22.56 g).
IR (Nujol) : 1750, 1700 cm
NMR (DMSO-d6, ~) : 2.43 (2H, br s), 3.71 and 4.04
(2H, ABq, J=17.7Hz), 4.88 (lH, d, J=5.1Hzj, 5.12
(lH, d, J=5.1Hz), 7.01 (lH, d, J=15.6Hz), 7.17
(iH, d, J=15.6Hz), 7.05 (lH, s), 7.2-7.6 (12H,
m), 8.4-8.5 (2H, m)
3~
Preparation 18
To a suspension of diphenylmethyl 7~-amino-3-~(E)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (2.35 g) and
anisole (2.4 ml) in dichloromethane (12 ml) was added
trifluoroacetic acid l4.8 ml) at 5C. The mixture was
WO94/10177 PCT/JP93/0150~
~,~4~6~9 - ~o
stirred at 5C for 1.5 hou~s;x The reaction mixture was
poured into diisopropyl~her and the precipitates were
collected by filtrat ~ , washed with diisopropyl ether,
and dried in vacuo to give 7~-amino-3-~(E)-2-(pyridin-
3-yl)vinyl]-3-cephem-4-carboxylic acid
bis(trifluoroacetate) (2.79 g).
NMR (DMSO-d6, ~) : 3.87 and 4.11 (2H, ABq, J=17Hz),
5.24 (lH, d, J=5Hz), 5.32 (lH, d, J=5Hz), 7.19
(lH, d, J=16Hz), 7.63 (lH, t, J=7Hz), 7.67 (lH,
d, J=16Hz), 8.15 (lH, dd-like), 8.61 (lH, dd,
J=1.5Hz, 5Hz), 8.78 (lH, d, J=1.5Hz)
Preparation 19
The following compounds were obtained according to a
similar manner to that of Preparation 1.
(1) Diphenylmethyl 7~-~ert-butoxyc~r~onylamino-3-[(Z)-2-
(6-methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate
IR (Nujol) : 1765, 1705, 1670 cm
NMR (DMSO-d6, ~) : 1.41 (9H, s), 2.43 (3H, s), 3.30
and 3.53 (2H, ABq, J=17.7Hz), 5.21 (lH, d,
J=4.8Hz), 5.24 (lH, dd, J=8.9Hz, 4.8Hz), 6.44
(lH, d, J=12.5Hz), 6.51 (lH, d, J=12.5Hz), 6.83
(lH, s), 7.20-7.49 (12H, m), 8.08 (lH, d,
J=8.9Hz), 8.26 ~lH, s)
~2) Diphenylmethy 7~-tert-butoxycarbonyl~m; no-3-[(z)-2-
(2-methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate
IR (Nujol) : 1760, 1695, 1660 cm
NMR (DMSO-d6, ~) : 1.39 (9H, s), 2.40 (3H, s), 3.10
and 3.29 (2H, ABq! J=17.3Hz), 5.12 (lH, d,
J=4.7Hz), 5.50 (lH, dd, J=8.3Hz), 6.61 (lH, d,
J=12.1Hz), 6.71 (lH, d, J=12.1Hz), 6.83 (lH, s),
7.14-7.49 (12H, m), 8.02 (lH, d, J=8.8Hz), 8.35
(lH, m)
W094/10177 2 1 4 7 6 0 9 PCT/JP93/01505
- 41 -
Preparation 20
The following compounds were obtained according to a
similar manner to that of Preparation 3.
(1) 7~-Amino-3-[(Z)-2-(6-methylpyridin-3-yl)vinyl]-3-
cephem-4-carboxylic acid dihydrochloride
IR (Nujol) : 3320, 1760, 1690 cm 1
NMR (DMSO-d6, ~) : 2.75 (3H, s), 3.52 and 3.65 (2H,
ABq, J=17.3Hz), 5.18 (lH, d, J=5.1Hz), 5.34 (lH,
d, J=5.1Hz), 6.75 (2H, s), 7.81 (lH, d,
J=8.4Hz), 8.38 (lH, d, J=8.4Hz), 8.67 (lH, s)
(2) 7~-Amino-3-[(Z)-2-(2-methylpyridin-3-yl)vinyl]-3-
cephem-4-carboxylic acid dihydrochloride
IR (Nujol) : 3320, 1750, 1680 cm 1
NMR (DMSO-d6, ~) : 2.71 (3H, s), 3.50 and 3.60 (2H,
AB~, J=17.2Hz), 5.14 (lH, d, J=5.0Hz), 5.26 (lH,
d, J=5.0Hz), 6.74 (lH, d, J=12.OHz), 6.83 (lH,
d, J=12.0Hz), 7.76 (lH, dd, J=7.8Hz, 5.8Hz),
8.21 (lH, d, J=7.8Hz), 8.64 (lH, d, J=5.8Hz)
Preparation 21
The following compounds were obtained according to a
similar manner to that of Preparation 6.
(1) l-Chloroethyl l-propylbutyl carbonate
IR (Neat) : 1769, 663 cm 1
NNR ~CDCl~, ~) : 0.92 (6H, t, J=7Hz), 1.2-1.7 (8H,
m), 1.83 (3H, d, J=6Hz), 4.80 (lH, m), 6.43 (lH,
q, J=6Hz)
(2) l-Chloroethyl cyclobutylmethyl carbonate
IR (Neat) : 1767, 663 cm 1
NMR (CDC13, ~) : 1.7-2.2 (6H, m), 1.84 (3H, d,
J=6Hz), 2.68 (lH, m), 4.18 (2H, d, J=7Hz),
W094/10177 2 1 4~ 6 0 9- PCT/JP93/01505
- 42 -
5.43 (lH, q, J=6Hz)
Preparation 22
The following compo~n~s were obtained according to a
i similar manner to that of Preparation 8.
(1~ 1-Bromoethyl 1-propylbutyl carbonate
IR (Neat) : 1763, 590 cm 1
NMR (CDCl3, ~) : 0.92 (3H, t, J=7Hz), 1.2-1.7 (8H,
m), 2.03 (3H, d, J=6Hz), 4.8-4.9 (lH, m), 6.61
(lH, q, J=6Hz)
!2) 1-Bromoethyl cyclobutylmethyl carbonate
IR (Neat) : 1764, 590 cm 1
NMR (CDCl3, ~) : 1.7-2.2 (6H, m), 2.03 (3H, d,
J=6Hz), 2.68 (1~, m), 4.19 (2H, d, J=7Hz), 6.61
(lH, ~, J=6Hz)
(3) 1-Bromoethyl cyclohexyl carbonate
IR INeat) : 1750 cm 1
NMR (CDCl3, ~) : 1.2-2.0 (lOH, m), 2.03 (3H, d,
J=6Hz), 4.6-4.8 (lH, m), 6.62 (lH, q, J=6Hz)
Preparation 23
To a solution of diphenylmethyl
7~-(t-butoxycarbonylamino)-3-chloromethyl-3-cephem-4-carb-
oxylate (1 kg) in acetone ~2~ Q) was added sodium iodide
(291 g) and then triphenylphosphine (611 g) portionwise at
ambient temperature. The reaction mixture was stirred for
a day. The precipitate was filtered and washed twice with
acetone (2 Q) to give diphenylmethyl 7~-~t-
butoxycarbonylamino)-3-[(triphenylphosphonio)methyl-3-
cephem-4-carboxylate iodide. To a suspension of
diphenylmethyl 7~-(t-butoxycarbony~amino)-3-
3~ [(triphenylphosphonio)methyl]-3-cephem-4-carboxylate
WO94/10177 21 4 7 6 0 9
~ - 43 -
iodide oDtained above in ethyl acetate (17 ~) and water
(8.5 ~) was added potassium carbonate (1.1 kg) in water (4
Q) and pH was adjusted to 10-10.5. After stirring for 30
minutes at ambient temperature, the aqueous layer was
S separated and extracted twice with ethyl acetate (2 Q x
2). The combined organic layer was washed with saturated
sodium chloride solution (S Q) and evaporated under
reduced pressure. To the residue in acetonitrile (8 ~)
and water (1 Q) was added nicotinaldehyde (202 ml)
dropwise under ice cooling. The mixture was stirred for
2.5 days at 3C and then poured into ethyl acetate (15 ~)
and 5% sodium hydrogensulfite. The organic layer was
separated, washed with 5% sodium hydrogensulfite solution
(3 ~) and saturated sodium chloride solution (5 Q), dried
over magnesium sulfate and evaporated under reduced
pressure. The residue was cGlumn chromatographed on
silica gel (5 kg) (dichloromethane:acetone = 19:1 (V/V))
and the fractions cont~; n ing the object compound was
evaporated. Acetone (1 ~) was added to the residue and
the mixture was stirred ~or 6 hours and allowed to stand
for 14 hours. The precipitate was filtered and dried
under reduced pressure to give diphenylmethyl
7~-(t-butoxycarbonylamino)-3-[(Z)-2-(pyridin-3-yl)vinyl]-
3-cephem-4-carboxylate (300.7 g). The filtrate was
evaporated and the residue was recrystallized in the same
manner above to give the object compound (76 g).
IR (Nujol) : 1765, 1700 cm
NMR (DMSO-d6, ~) : 1.41 (9H, s), 3.30 and 3.56 (2H,
ABq, J=17.5Hz), 5.21 (lH, d, J=4.8Hz), 5.56 (lH,
dd, J=8.3Hz, 4.8Hz), 6.49 (lH, d, J=12.2Hz),
6.55 (lH, d, J=12.2Hz), 6.79 (lH, s), 7.27-7.65
(12H, m), 8.08 ilH, d, J=8.3Hz), 8.38 (lH, m),
8.43 (lH, m)
wo 94/10l721 ~ 6 0 9 44 _ PCT/JP93iO150C
Preparation 24
'~o a suspension of 7~-~mino-3-~(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-car~oxylic acid (303 mg) in acetone
(3 ml) and N,N-dimethyl~f~rmamide (2 ml) was added dropwise
~, 1,8-diazabicyclo~5.4.0]undec-7-ene (DBU) (152 mg) in
acetone (1 ml) at 5C. After stirring for 30 minutes,
i-bromoethyl-1-ethylpropylcarbonate (266 mg) was added at
the same temperature. The reaction mixture was stirred
for an hour, then was poured into a mixture of ethyl
1~ acetate and water. The organic layex was separated,
washed with water twice and brine, dried over magnesium
~ulfate. After addition of oxalic acid (90 mg), the
solution was stirred for an hour. The precipitate was
filtered and dried under reduced pressure to give
1-(1-ethylpropyloxycarbonyloxy)ethyl
7~-amino-3-[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylate oxalate (195 mg).
IR (KBr) : 3429, 1790, 1755, 1628 cm 1
NMR ~DMSO-d6, ~ : 0.7-0.9 (6H, m), 1.34 (1.5H, d,
J=5.4Hz), 1.45 (1.5H, d, J=5.4Hz), 1.3-1.6 (4H,
m), 3.25 (0.5H, d, J=17.9Hz), 3.30 (0.5H, d,
J=17.9Hz), 3.62 (lH, d, J=17.8Hz), 4.40-4.60
(lH, m), 4.35-5.00 (lH, m), 5.16 (lH, d,
J=5.1Hz), 6.4-6.75 (2H, m), 7.3-7.4 (lH, m),
7.6-7.7 (lH, m), 8.4-8.6 (2H, m)
Preparation 25
The following compound was obtained according to a
similar manner to that of Preparation 24.
l-Cyclohexyloxycarbonyloxyethyl 7~-amino-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate oxalate
IR (KBr) : 3429, 1790, 1755 cm 1
NMR (DMSO-d6, ~) : 1.0-2.0 (lOH, m), 1.33 (1.5H, d,
J=5.4Hz), 1.43 (1.5H, d, J=5.4Hz), 3.25 (0.5H,
WO94/10177 21 4 7 6 0 9 PCT/JP93/01505
_ - ~5 -
d, J=18.0~z), 3.29 (0.5H, d! J=18.0Hz), 3.62
~lH, d, J=18.0Hz), 4.4-4.6 ~lH, m), 4.9-5.0 ~lH,
m), 5.1-5.2 ~lH, m), 6.4-6.8 (2H, m), 7.3-7.4
(lH, m), 7.6-7.7 ~iH, m), 8.48 (2H, br s)
Example 1
To a solution of 7~-amino-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylic acid dihydrochloride (12 g)
and N-~trimethylsilyl)acetamide ~49.9 g) in methylene
chloride (240 ml) was added 2-(2-aminothiazol-4-yl)-2-
acetoxyiminoacetyl chloride hydrochloride (syn isomer)
(10.9 g) under ice-cooling. After being stirred for 4
hours at the same temperature, the mixture was added
dropwise to diisopropyl ether (1.5 Q). The precipitate
containing 7~-~2-(2-aminothiazol-4-yl)-2-acetoxyimino-
acetamido]-3-~(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylic acid (syn iscmer) was collected by filtration,
and dried in vacuo. The precipitate was dissolved in 10%
methanol aqueous solution ~280 ml), and thereto was
ammonium chloride (5.12 g) and the mixture was adjusted to
pH 8 with a sodium carbonate aqueous solution. The
solution was stirred at room temperature for 30 minutes
maintaining pH 8 with a sodium carbonate aqueous solution.
The solution was adjusted to pH 6 with lN hydrochloric
acid, and evaporated in vacuo to remove methanol. The
solution was subjected to column chromatography on HP-20
(Trademark : Mitsubishi Kasei Corporation) and eluted
with 1~% isopropyl alcohol aqueous solution. The
fractions cont~ining the object compound were collected
~,0 and lyophilized to give crude product, which was purified
by preparative HPLC utilizing a C18 ~ Bondapak resin
(Trademark : Waters Associates, Inc.) to afford 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylic acid (syn
isomer) (0.36 g).
WO94/10177 PCT/JP93/0150
2 1 ~? 60 9 - ~6 -
IR (Nujol) : 1750, 1650 cm 1
NMR (DMSO-d6, ~) : 3.19 and 3.54 (2H, ABq,
- J=17.7Hz), 5.24 t:IH, d, J=4.8Hz), 5.81 (lH, d,
J=8.2Hz and 4.8Hz), 6.54 (lH, d, J=12.2Hz), 6.61
i (lH, d, J=12.2Hz), 6.65 (lH, s), 7.12 (2H, br
s~, 7.34 (lH, m), 7.63 (lH, m), 8.43 (2H, m),
9.51 (lH, d, J=8.2Hz), 11.29 (lH, s)
Example 2
The following compounds were obtained according to a
similar manner to that of Example 1.
(1) 7~-12-(2-Aminothiazol-4-yl)-2-hydroxyiminoacetamido]-
3-[(E)-2-(pyridin-4-yl)vinyl]-3-cephem-4-carboxylic
acid (syn isomer)
IR (Nujol) : 1760, 1550 cm 1
NMR (DMSO-d6, ~) : 3.71 and 4.04 (2H, ABq,
J=17.7Hz), 5.25 (lH, d, J=4.9Hz), 5.85 (lH, dd,
J=4.9Hz, 8.2Hz), 6.68 (lH, s), 6.98 (lH, d,
J=16.3Hz), 7.15 ~2H, s), 7.39 (2H, d, J=5.8Hz),
7.61 (lH, d, J=16.3Hz), 8.54 (2H, d, J=5.8Hz),
9.53 (lH, d, J=8.2Hz), 11.33 (lH, s)
(2) 7~-l2-(2-Aminothiazol-4-yl)-2-hydroxyiminoacetamido]-
3-1(E)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylic
acid (syn isomer)
IR (Nujol) : 3200, 1755, 1650 cm 1
NMR (DMSO-d6, ~) : 3.70 and 4.00 (2H, ABq,
J=17.5Hz), 5.23 (lH, d, J=4.6Hz), 5.83 (lH, d,
'0 J=8.1Hz), 6.69 (lH, s), 7.02 (lH, d, J=16.4Hz),
7.14 (2H, s), 7.39 (lH, s), 7.87 (lH, d,
J=16.4Hz), 7.87 (lH, m), 8.45 (lH, m), 8.63 (lH,
s), 9.52 !lH, d, J=8.lHz), 11.34 (lH, br s)
W094/10177 214 7 6 0 9 PCT/JP93/01505
- 47 -
Example 3
Triethylamine (1.50 ml, 10.6 mmol) was added to a
suspension of 7~-amino-3-[(Z)-2-(pyridin-3-yl)vinyl]-3-
cephem-4-carboxylic acid dihydrochloride (syn isomer)
(1.00 g, 2.66 mmol) in tetrahydrofuran (20 ml).
Trimethylsilyl chloride (0.84 ml, 6.65 mmol) was added
dropwise to the cold mixture (5C) during a period of 5
minutes. The mixture was stirred for 30 minutes at room
temperature and cooled again to ca. 5C. To the mixture
was added a solution of 1-~2-(2-aminothiazol-4-yl)-2-
trityloxyiminoacetyl]benzotriazole-3-oxide (syn isomer)
(1.45 g, 2.66 mmol) in N,N-dimethylformamide (15 ml) over
a period of 10 minutes. The mixture was stirred overnight
at room temperature and concentrated under reduced
pressure to remove most of the tetrahydrofuran. The
concentrate was poured into ice water (100 ml) with
vigorous stirring to give 7~-~2-(2-aminothiazol-4-yl)-2-
trityloxyiminoacetamido]-3-~(Z)-2-(pyridin-3-yl)vinyl]-3-
ceph~m-4-carboxylic acid (syn isomer) (955 mg).
IR (Nujol) : 1725, 1650 cm 1
NMR (DMSO-d6, ~) : 2.95 and 3.34 (2H, ABq,
J=17.OHz), 5.21 (lH, d, J=4.9Hz), 5.82 (lH, dd,
J=8.2 and 4.9Hz), 6.43 (lH, d, J=12.2Hz), 6.5~
(lH, s), 6.89 (lH, d, J=12.2Hz), 7.25-7.30 (18H,
m), 7.65 (lH, d, J=8.3Hz), 8.42-8.46 (2H, m),
9.90 (lH, d, J=8.2Hz)
Example 4
To a cooled solution of 7~-~2-(2-aminothiazol-4-yl)-
2-trityloxyiminoacetamido]-3-~(Z)-2-(pyridin-3-yl)vinyl~-
3-cephem-4-carboxylic acid (syn isomer) (1.39 g, 1.94
mmol) and potassium carbonate (147 mg, 1.07 mmol) in
N,N-dimethylformamide (14 ml) was added iodomethyl
pivalate (515 mg, 2.13 mmol) at 0C. The mixture was
?,5 stirred at about 5C for 30 minutes. The reaction mixture
W094/10177 PCT/JP93iO150C
~4~609 - 48 -
~as poured into a mixture of ice water (90 ml) and ethyl
acetate (120 ml). The organic layer was separated, washed
with water and with brine,~`d`~ied over magnesium sulfate,
and concentrated under reduced pressure. The residue was
triturated with diisopropyl ether. The precipitates were
collected ~y filtration to give pivaloyloxymethyl
7~-[2-(2-aminotniazol-4-ylj-2-trityloxyiminoacetamido]-
3-[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer) (283.7 mg).
10NMR (~MSO-d6, ~) : 1.14 (9H, s), 3.32 and 3.65 (2H,
ABq, J=18.OHz), 5.35 (lH, d, J=4.9Hz), 5.64 and
5.80 (2H, A~q, J=5.9Hz), 6.01 (lH, dd, J=8.2Hz
and 4.9Hz), 6.51 (lH, d, J=12.lHz), 6.59 (lH,
s), 6.66 (lH, d, J=12.1Hz), 7.23-7.39 (18H, m),
157.64-7.68 (lH, m), 8.46-8.47 (2H, m), 9.95 (lH,
J=8.2Hz)
Example 5
Pivaloyloxymethyl 7~-[2-(2-aminothiazol-4-yl)-2-
trityloxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)vinyl]-3-
cephem-4-carboxylate ~syn isomer) (185 mg, 0.223 mmol) was
stirred in 90% formic acid (0.69 ml) for 40 minutes at
room temperature. The reaction mixture was filtered, and
the filtrate was poured into a mixture of ice water (10
ml) and ethyl acetate (10 ml). The aqueous layer was
adjusted to pH 5 with sodium hydrogencarbonate. The
organic layer was separated, washed with water and with
brine, dried over magnesium suifate, and concentrated
under reduced pressure. The residue was triturated with
~0 diisopropyl ether. The precipitates were collected by
filtration to give pivaloyloxymethyl 7~-[2-(2-amino-
thiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-
3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer) (72.9 mg).
IR (Nujol) : 1740, 1655 cm 1
NMR (DMSO-d6, ~) : 1.12 (9H, s), 3.29 and 3.65 (2H,
WO94/10177 2 1 4 7 6 0 9 PCT/JP93/01505
- 49 -
~Bq, J=ï7.~Hz), 5.28 (lH, d, J=4.8Hz), 5.60 and
~.76 (2H, ABq, J=5.9Hz), 5.86 (lH, dd, J=8.1Hz
and 4.8Hz), 6.48 (lH, d, J=12.1Hz), 6.59 (lH, d,
J=12.1Hz), 6.66 (lH, s), 7.17 (2H, br s), 7.35
(lH, dd, J=4.8Hz and 8.0Hz), 7.65 (lH, d,
J=8.0Hz), 8.45-8.47 (2H, m), 9.53 (lH, d,
J=8.1Hz), 11.32 ~lH, s)
Example 6
To a solution of 7~-amino-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylic acid dihydrochloride (2 g)
and N-(trimethylsilyl)acetamide (10.5 g) in methylene
chloride (40 ml) was added 2-(2-aminothiazol-4-yl)-2-
methoxyiminoacetyl chloride hydrochloride (syn isomer)
1~ (2.01 g) at -10 ~ -20C. After being stirred for 1 hour
under ice-cooling, the mixture was poured into a mixture
of water (230 ml) and me'hylene chloride (150 ml) and
adjusted to pH 6 with an a~ueous sodium hydrogencarbonate
solution. The separated aqueous layer was adjusted to pH
5 with lN hydrochloric acid, and evaporated to remove
metnylene chloride. The solution was adjusted to pH 5
with lN hydrochloric acid, subjected to column
chromatography on HP-20, and eluted with 15% isopropyl
alcohol aqueous solution. The fractions containing the
object compound was collected and lyophilized to give
crude product, which was purified by preparative HPLC
utilizing a C18 ~ ~ondapac resin (Trademark : Waters
Associates, Inc.) to afford 7~-[2-(2-aminothiazol-4-
yl)-2-methoxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)-
0 vinyl~-3-cephem-4-carboxylic acid (syn isomer) (1.0 g).
IR (Nujol) : 3200, 1750, 1650 cm 1
NMR (DMSO-d6, ~) : 3.17 and 3.54 (2H, ABq,
J=17.6Hz), 3.83 ~3H, s), ~.23 (lH, d, J=4.8Hz),
~.79 (lH, dd, J=8.1Hz, 4.8Hz), 6.52 (lH, d,
3~ J=12.2Hz), 6.64 (lH, d, J=12.2Hz), 6.73 (lH, s),
~ 4~ 6 PCT/JP93/0150~
7.22 (2H, br s), 7.3~ (lH, m) 7.65 (lH, m),
8.45 (2H, m), 9.~ ~lH, d, J=8.2Hz)
Example 7
The following compounds were obtained according to
similar manners to those of Examples 1, 3 and 6.
(1) 7~-l2-(2-Aminothiazol-4-yl)-2-methoxyiminoacetamido]-
3-[(E)-2-(pyridin-4-yl)vinyl]-3-cephem-4-carboxylic
acid (syn isomer)
IR (Nujol) : 3250, 1780, 1660 cm 1
NMR tDMSO-d6, ~) : 3.72 and 4.06 (2H, ABq,
J=17.7Hz), 3.86 (3H, s), 5.25 ~lH, d, J=4.9Hz),
5.84 (lH, dd, J=8.1Hz, 4.9Hz), 6.77 (lH, s),
7.00 (lH, d, J=16.3Hz), 7.24 (2H, br s), 7.40
(2H, d, J=5.9Hz), 7.61 (lH, d, J=16.3Hz), 8.55
(2H, d, J=5.9Hz), 9.68 (lH, d, J=8.lHz)
(2) 7~-~2-(2-Aminothiazol-4-yl)-2-methoxyiminoacetamido]-
3-~(E)-2-(pyridin-3-yl)vinyl~-3-cephem-4-carboxylic
acid (syn isomer)
IR (Nujol) : 3250, 1750, 1650 cm
NMR (DMSO-d6, ~) : 3.72 and 4.02 (2H, ABq,
J=17.5Hz), 3.86 (3H, s), 5.23 (lH, d, J=4.8Hz),
5.80 (lH, dd, J=8.1Hz, 4.8Hz), 6.77 (lH, s),
7.02 (lH, d, J=16.4Hz), 7.24 (2H, s), 7.39 (lH,
m), 7.54 (lH, d, J=16.4Hz), 7.87 (lH, m), 8.46
(lH, m), 8.63 (lH, s), 9.67 (lH, d, J=8.1Hz)
Example 8
To a stirred solution of 7~-[2-(2-aminothiazoi-4-yl)-
2-methoxyiminoacetamido]-3-[(E)-2-(pyridin-3-yl)vinyl]-3-
cephem-4-carboxylic acid (syn isomer) (500 mg, 1.03 mmol)
in N,N-dimethylformamide (10 ml) was added potassium
carbonate (71.2 mg, 0.515 mmol) at 5C. The mixture was
WO94/10177 2 1 4 7 6 0 9 PCT/JP93/01505
stir~ed at 5C for 30 minutes. To the solution was added
iodomethyl pivalate (249 mg, 1.03 mmol). The mixture was
stirred at 5C for 30 minutes. The reaction mixture was
poured into a mixture of ethyl acetate and water. The
organic layer was separated, washed with water and with
brine, dried over magnesium sulfate, and concentrated
under reduced pressure. The residue was triturated with
diisopropyl ether. The precipitates were collected by
filtration to give pivaloyloxymethyl 7~-[2-(2-amino-
thiazol-4-yl)-2-methoxyiminoacetamido]-3-[(E)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
(368 mg, 0.613 mmol).
IR (Nujol) : 1765, 1740, 1665 cm
NMR (DMSO-d6, ~) : 1.16 (9H, s), 3.77 and 4.14 (2H,
ABq, J=17.9Hz), 3.85 (3H, s), 5.29 (lH, d,
J=4.8Hz), 5.89 and 5.98 (2H, ABq, J=5.9Hz), 5.91
(lH, dd, J=4.8Hz and 8.1Hz), 6.78 (lH, s), 7.16
(lH, d, J=16.2Hz), 7.25 (2H, br s), 7.42 (lH,
m), 7.42 (lH, d, J=16.2Hz), 7.92 (lH, m), 8.49
(lH, m), 8.68 (lH, m), 9.69 (lH, d, J=8.1Hz)
Example 9
The following compounds were obtained according to
similar manners to those of Examples 4 and 8.
2~
(1) 1-Acetoxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
methoxyiminoacetamido~-3-[(E)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1760, 1665 cm 1
NMR (DMSO-d6, ~) : 1.50 and 1.51 (total 3H, two d,
J=5.4Hz), 2.06 and 2.08 (total 3H, two s), 3.75
and 4.13 (2H, ABq, J=17.8Hz), 3.86 (3H, s), 5.28
and 5.29 (total lH, two d, J=4.8Hz), 5.83-5.92
(lH, m), 6.76 and 6.77 (total lH, two s), 6.99
and 7.06 (total lH, two d, J=5.4Hz), 7.13 (lH,
WO94/10177 9 - 52 - PCT/JP93/01505
d, J=16.5Hz), 7.24 (2H, br s), 7.36 (lH, d,
J=16.5Hz), 7.40-7.47 (lH, m), 7.87-7.91 tlH, m),
8.48-8.50 (lH, m), 8.67 (lH, br s), 9.69 (lH, d,
J=8.lHz) ;~-
, .
(2) 1-(Isopropyloxyczrbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-methoxyiminoacetamido]-3-[(E)-2-
(pyridin-4-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1740, 1655 cm 1
NMR (DMSO-d6, ~) : 1.23-1.26 (6H, m), 1.53 and 1.54
(total 3H, two d, J=5.4Hz), 3.75 and 4.12 (2H,
~B~, J=17.0Hz), 3.85 (3H, s), 4.76-4.83 (lH, m),
5.28 and 5.31 (total lH, two d, J=4.9Hz),
5.85-5.93 (lH, m), 6.75 (lH, s), 6.91 and 6.96
(total lH, two d, J=5.4H~), 7.08 (lH, d,
J=16.2Hz), 7.24 (2H, br s), 7.43-7.44 (2H, m),
7.47 (lH, d, J=16.2Hz), 8.57 (2H, d, J=5.9Hz),
9.69 (lH, d, J=8.1Hz)
~0
Example 10
To a solution of 2-(2-tritylaminothiazol-4-yl)-2-
trityloxyiminoacetic acid (syn isomer) (20.2 g) in
dichloromethane (150 ml) were added triethylamine (5.02
ml) and phosphorus pentachloride (6.69 g) at 5C. The
mixture was stirred at 5C for 1 hour. After a solution
of 7~-amino-3-~(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylic acid (10.9 g) in dichloromethane (218 ml) and
bis(trimethylsilyl)acetamide (26.7 ml) was stirred at room
temperature for 45 minutes, to the solution was added the
~ctivated solution obtained above. The mixture was
stirred at 5C for 2 hours, and then at room temperature
for 15 hours. The reaction mixture was poured into a
mixture of ethyl acetate (600 ml), tetrahydrofuran (600
~5 ml) and water (1.2 R). The organic layer was separated,
21~ 7 6 0 9 PCT/JP93/01505
WO94/10177
- ~3 -
~ashed with water and with brine, dried over magnesium
sulfate, and evaporated in vacuo. The residue was
triturated with diisopropyl ether. The precipitates were
collected by filtration to give 7~-~2-~2-tritylaminothiazol-
4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylic acid ~syn isomer) (21.1 g).
IR (Nujol) : i770, i670 cm 1
NMR (DMSO-d6, ~) : 3.21 and 3.50 (2H, ABq, J=18Hz),
5.28 (lH, d, J=5Hz), 5.88 (lH, dd, J=5Hz, 8Hz),
6.59 tlH, s), 6.61 (2H, s), 7.0-7.4 (31H, m),
7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 8.77 (lH, s),
3.94 (lH, d, J=8Hz)
Example 11
The following compounds were obtained according to
similar manners to those of Examples 1, 3, 6 and 10.
(1) 7~-[2-(2-Tritylaminothiazol-4-yl)-2-difluoromethoxy-
iminoacetamido]-3-[(Z)-2-(pyridin-3-yl)vinyl]-3-
,0 cephem-4-carboxylic acid (syn isomer)
NMR (DMSO-d6, ~) : 3.33 and 3.69 (2H, ABq,
J=17.9Hz), 5.28 (lH, d, J=4.8Hz), 5.73 (lH, dd,
J=7.6Hz, 4.9Hz), 6.68 (2H, s), 6.97 (lH, s),
7.28 (2H, ~r s), 7.32-7.34 (15H, m), 7.81-7.88
(lH, m), 8.23-8.28 (lH, m), 8.72-8.78 (2H, m),
9.32 (lH, d, J=7.6Hz)
(2) 7~-[2-(2-Amino-4-thiazolyl)-2-trityloxyimino-
acetamido]-3-[(Z)-2-(2-methylpyridin-3-yl)vinyl]-3-
cephem-4-carboxylic acid (syn isomer)
IR (Nujol) : 1755, 1640 cm 1
NMR (DMSO-d6, ~) : 2.44 (3H, s), 2.72 and 3.17 (2H,
ABq, J=16.9Hz), 5.15 (lH, d, J=4.9Hz), 5.79 (lH,
dd, J=8.2Hz, 4.9Hz), 6.49 (lH, d, J=12.1Hz),
~5 8.33 (lH, m), 9.85 (lH, d, J=8.2Hz)
WO94/10177 ` PCT/JP93/015
41 609 - 5~ -
3) 7~-~2-~2-Amino-4-thiazolyl)-2-trityloxyimino-
acetamido]-3-[(Z)-2-(6-methylpyridin-3-yl)vinyl]-3-
cephem-4-carboxylic acid (syn isomer)
IR (Nujol) : 1750, 1740, 1650 cm 1
NMR (DMSO-d~ 2.44 (3H, s), 3.01 and 3.35 (2H,
ABq, J=16.7Hz), 5.21 (lH, d, J=4.8Hz), 5.83 (lH,
dd, J=8.4Hz, 4.8Hz), 6.42 (lH, d, J=12.lHz),
6.59 (lH, s), 6.79 (lH, d, J=12.1Hz), 7.17-7.30
~18H, m), 7.51-7.56 (lH, m), 8.34 (lH, m), 9.91
(lH, d, J=8.4Hz)
(4) 7~-[2-~2-Aminothiazol-4-yl)-2-trityloxyimino-
acetamido]-3-[(E)-2-(pyridin-3-yl)vinyl~-3-cephem-4-
carboxylic acid (syn isomer)
IR (Nujol) : 1730, 1650 cm 1
NMR (DMSO-d6, ~) : 3 61 znd 3.81 (2H, ABq, J=17Hz),
5.17 (lH, d, J=5Hz), 5.82 (lH, dd, J=5Hz, 8Hz),
6.62 (lH, s), 6.70 (lH, d, J=16Hz), 7.1-7.4
(18H, m), 7.77 (lH, d, J=16Hz), 7.8 (lH, m),
8.33 (lH, d, J=5Hz), 8.62 (lH, d-like), 9.91
(lH, d, J=8Hz)
(5) 7~-[2-(2-Amino-4-thiazolyl)-2-hydroxyimino-
acetamido~-3-~(Z)-2-(6-methylpyridin-3-yl)vinyl]-3-
cephem-4-carboxylic acid (syn isomer)
IR (Nujol) : 3260, 1740, 165S cm 1
NMR (DMSO-d6, ~) : 2.45 (3H, s), 3.19 and 3.51 (2H,
ABq, J=17.7Hz), 5.23 (lH, d, J=4.8Hz), 5.81 (lH,
dd, J=8.2Hz, 4.8Hz), 6.49 (lH, d, J=12.2Hz),
6.57 (lH, d, J=12.2Hz), 6.65 (lH, s), 7.11 (2H,
br s), 7.20 (lH, d, J=8.1Hz), 7.54 (lH, d,
J=8.1Hz), 8.33 (lH, s), 9.50 (lH, d, J=8.2Hz),
11.28 (lH, s)
(6) 7~-[2-(~-Amino-4-thiazolyl)-2-hydroxyiminoacetamido]-
WO94/10177 2 1`4 7 6 0 9 PCT/JP93/01505
- ',5 -
3-~(Z)-2-(2-methylpyridin-3-yl)vinyl]-3-cephem-4-
carboxylic acid (syn isomer)
IR (Nujol) : 1750, 1660 cm 1
NMR (DMSO-d6, ~) : 2.43 ~3H, s), 2.88 and 3.25 (2H,
ABq, J=17.1Hz), 5.10 (lH, d, J=4.8Hz), 5.69 (lH,
dd, J=8.2Hz, 4.8Hz), 6.54 (lH, d, J=12.1Hz),
6.62 (lH, s), 6.76 (lH, d, J=12.1Hz), 7.12 (2H,
br s), 7.16 (lH, m), 7.45 (lH, d, J=6.5Hz), 8.34
~lH, d, J=4.8Hz), 9.42 (lH, d, J=8.2Hz), 11.32
(lH, s)
Example 12
~o a solution of 7~-~2-(2-tritylaminothiazol-4-yl)-
2-trityloxyiminoacetamido]-3-~(Z)-2-(pyridin-3-yl)vinyl]-
3-cephem-4-carboxylic acid (syn isomer) (1.5 g) in
N,N-dimethyl~ormamide (15 ml) was added cesium carbonate
(255 mg) at 5C. The mixture was stirred at 5C for 15
minutes. To the mixture was added l-bromoethyl pivalate
(328 mg). The mixture was stirred at 5C for 7 hours.
The reaction mixture was poured into a mixture of ethyl
acetate (100 ml), tetrahydrofuran (100 ml) and ice-water
(200 ml). The organic layer was separated, washed with
water and with brine, dried over magnesium sulfate, and
evaporated in vacuo. The residue was chromatographed on
silica gel to give l-pivaloyloxyethyl
7~-~2-(2-tritylaminothiazol-4-yl)-2-trityloxyimino-
acetamido]-3-[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylate (syn isomer) (665 mg).
IR (Nujol) : 1770, 1730, 1670 cm 1
NMR (DMSO-d6, ~) : 1.04 (9H, s), 1.31 and 1.45
(total 3H, d, J=5Hz), 3.30 and 3.61 (2H, ABq,
J=18Hz), 5.3-5.4 (lH, m), 5.9-6.0 (lH, m),
6.4-6.8 (4H, m), 7.0-7.4 (31H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 8.77 (lH, br s), 9.9-10.0
(lH, m)
W094/10177 PCT/JP93/015
- 56 -
~4~609
Example 13
The following compounds were obtained according to
similar manners to those of Examples 4, 8 and 12.
(1) l-(Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-trityloxyiminoacetamido)-3-
~(E)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
NMR ~DMSO-d6, ~) : 1.1-1.9 (lOH, m), 1.56 (3H, d,
J=5Hz), 3.79 and 4.13 (2H, ABq, J=17Hz), 4.5-4.6
(lH, m), 5.35 and 5.38 ~total lH, d, J=5Hz),
5.76 (lH, s), 6.0-6.1 (lH, m), 6.63 (lH, s),
6.95 (lH, m), 7.15 ~lH, d, J=16Hz), 7.2-7.5
(19H, m), 7.8-8.0 (lH, m), 8.49 (lH, d, J=SHz),
8.68 (lH, m), 9.96 (lH, d, J=8Hz)
(2) ~ivaloyloxymethyl 7~-~2- ! 2-aminothiazol-4-yl)-2-
trityloxyiminoacetamido]-3-~E)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1760, 1730, 1670 cm 1
NMR (DMSO-d6, ~) : 3.80 and 4.15 (2H, ABq, J=18Hz),
5.37 (lH, d, J=5Hz), 5.91 and 6.01 (2H, ABq,
J=6Hz), 6.0-6.1 (lH, m), 6.63 (lH, s), 7.2-7.5
(20H, m), 7.93 (lH, d-like), 8.50 (lH, d,
J=4Hz), 8.69 (lH, br s), 9.96 (lH, d, J=8Hz)
(3) 1-(3-Methylbutyryloxy)ethyl 7~-[2-(2-tritylamino-
thiazol-4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1730, 1670, 1510 cm
NMR (DMSO-d6, ~) : 0.8-0.9 (6H, m), 1.32 and 1.44
(total 3H, d, J=5Hz), 2.0-2.2 (3H, m), 3.3 and
3.6 (2H, m), 5.32 (lH, d, J=5Hz), 5.9-6.0 (lH,
m), 6.4-6.9 (4H, m), 7.1-7.3 (31H, m), 7.4 (lH,
WO94/10177 21 4 7 6 0 9 PCT/JP93/0150~
- 57 -
m~, 8.4-8.5 ~2H. m)! 8.78 (lH, s)
(4) Pivaloyloxymethyl 7~-~2-(2-amino-4-thiazolyl)-2-
trityloxyiminoacetamido]-3-~(Z)-2-(2-methylpyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 3340, 1780, 1745, 1675, 1610 cm 1
NMR ~DMSO-d6, ~) : 1.16 (9H, s), 2.44 (3H, s), 3.21
and 3.48 (2H, ABq, J=17.7Hz), 5.28 (lH, d,
J=4.9Hz), 5.61 and 5.79 (2H, ABq, J=5.9Hz), 5.96
(lH, dd, J=8.2Hz, 4.9Hz), 6.57 (lH, d,
J=11.9Hz), 6.57 (lH, s), 6.75 (lH, d, J=11.9Hz),
7.15-7.29 (18P., m), 7.46 (lH, m), 8.36 (lH, m),
9.88 (lH, d, J=8.2HZ)
(5) Pivaloyloxymethyl 7~-~2-(2-amino-4-thiazolyl)-2-
trityloxyiminoacetamido}-3-~(Z)-2-(6-methylpyridin-
3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1770, 1750, 1665 cm 1
NMR (DMSO-d6, ~) : 1.14 (9H, s), 2.44 (3H, s), 3.22
and 3.51 (2H, ABq, J=17.5Hz), 5.35 (lH, d,
J=4.9Hz), 5.66 and 5.81 (2H, ABq, J=5.9Hz), 6.01
(lH, dd, J=8.3Hz), 6.46 (lH, d, J=12.0Hz), 6.60
(lH, s), 6.62 (lH, d, J=12.0Hz), 7.19-7.30 (18H,
m), 7.52-7.56 (lH, m), 8.34-8.35 (lH, m), 9.95
(lH, d, J=8.3Hz)
(6) 1-(3-Methylbutoxycarbonyloxy)ethyl 7~-~2-(2-trityl-
aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-~(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1760, 1680 cm 1
NMR (DMS~-d6, ~) : 0.80 and 0.87 (total 6H, d,
J=7Hz), 1.3-1.7 (6H, m), 3.26 and 3.60 (2H, ABq,
J=18Hz), 4.1-4.2 (2H, m), 5.32 and 5.33 (total
lH, d, J=5Hz), 5.9-6.0 (lH, m), 6.3-6.8 (4H, m),
WO94/10177 PCT/JP93/015r~
~ 609 - 58 -
7.l-7.3 (31H, m)! 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 8.76 (lH, s), ~.94 (lH, d, J=8Hz)
'7) l-(Isobutyloxycarbonyloxy)ethyl 73-[2-(2-
tritylaminothiazol-4-yl)-2-trityloxyiminoacetamido}-
3-~(Z)-2-(3-pyridyl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR (Nujol) : 1770, 1740, 1670, 1580 cm 1
NMR (DMSO-d6, ~) : 0.8-0.9 (6H, m), 1.3-1.4 (3H, m),
1.8-2.0 (lH, m), 3.29 and 3.61 (2H, ABq,
J=17.6Hz), 4.24 (2H, d, J=7.0Hz), 5.32 (lH, d,
J=4.9Hz), 5.91 (lH, dd, J=8.2Hz, 4.7Hz), 6.5-6.9
(4H, m), 7.2-7.4 (31H, m), 7.6-7.7 (lH, m),
8.4-8.5 (2H, m), 8.78 (lH, br s), 9.93 (lH, d,
J=8.2Hz)
(3,3-Dimethylbutyryloxy)ethyl 7B-[2-(2-tritylamino-
thiazol-4-yl)-2-trityloxyiminoacetamido~-3-[(Z)-2-
(3-pyridyl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1770, 1740, 1670, 1580 cm
NMR (DMSO-d6, ~) : 0.92 (9H, s), 0.98 (2H, s), 1.45
(3H, d, J=5.4Hz), 3.41 and 3.60 (2H, ~3q,
J=18.1Hz), 5.32 (lH, d, J=4.2Hz), 5.9-6.0 (lH,
m), 6.4-6.9 (4H, m), 7.1-7.4 (31H, m), 7.6-7.7
(lH, m), 8.4-8.5 (2H, m), 8.78 (lH, s), 9.9-10.0
(lH, m)
(9) 1-(Hexyloxycarbonyloxy)ethyl 7~-[2-(2-
tritylaminothiazol-4-yl)-2-trityloxyiminoacetamido~-
3-[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR (Nujol) : 1770, 1750, 1670 cm
NMR (DMSO-d6, ~) : 0.8-1.6 (14H, m), 3.30 and 3.60
(2H, ABq, J=18Hz), 4.0-4.1 (2H, m), 5.32 and
5.33 (total lH, d, J=5Hz), 5.9-6.0 (lH, m),
WO94/10177 21 4 7 6 0 9 PCT/JP93/OlS0~
- 59 -
6.5-8.8 (4H, m), 7.1-7.4 (31H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 8.78 (lH, s), 9.94 and 9.95
(total lH, d, J=8Hz)
(10) 1-Isobutyryloxyethyl 7~-~2-(2-tritylaminothiazol-4-
yl)-2-trityloxyiminoacetamido]-3-r(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1770, 1740, 1720, 1670, 1510 cm 1
NMR (DMSO-d6, ~) : 1.0-1.1 ~6H, m), 1.32 and 1.44
(total 3H, d, J=5Hz), 3.1-4.7 (2H, m), 5.32 and
5.33 (total lH, d, J=5Hz), 5.9-6.0 (lH, m),
6.5-6.8 (4H, m), 7.2-7.3 (31H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 8.78 (lH, s), 9.93 and 9.95
(total lH, d, J=8Hz)
(11) 1-(4-Methylpentoxycarbcnyloxy)ethyl 7~-[2-(2-
tritylaminothiazol-4-yl)-2-trityloxyiminoacetamido]-
3-~(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR (Nujol) : 1770, 1740, 1670 cm 1
NMR (DMSO-d6, ~) : 0.8-0.9 (6H, m), 1.1-1.3 (2H, m),
1.36 and 1.48 (total 3H, d, J=5Hz), 1.4-1.6 (3H,
m), 3.29 and 3.60 (2H, ABq, J=18Hz), 4.0-4.1
(2H, m), 5.32 and 5.33 (total lH, d, J=5Hz),
5.9-6.0 (lH, m), 6.5-6.8 (4H, m), 7.1-7.3 (31H,
m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 8.78 (lH,
s), 9.93 and 9.94 (total lH, d, J=8Hz)
(12) 1-(1-Ethylpropoxycarbonyloxy)ethyl 7~-~2-(2-
tritylaminothiazol-4-yl)-2-trityloxyiminoacetamido]-
3-[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR (Nujol) : 1770, 1730, 1670 cm 1
NMR (DMSO-d6, ~) : 0.7-0.9 (6H, m), 1.36 and 1.49
(total 3H, d J=5Hz), 1.4-1.6 (4H, m), 3.2-3.7
WO94/10177 PCT/JP93/01C
~4~609 - 60 -
~2H, m), 4.50 (l-H, m), 5.32 (lH, d, J=5Hz),
5.9-6.0 (lH, m~, 6.4-6.8 (4H, m), 7.1-7.3 (31H,
m), 7.1-7.8 ~lH, m), 8.5 (2H, m), 8.77 (lH, s),
9.95 (lH, d, J=7Hz)
(13) Cycloheptylcarbonyloxymethyl 7~- E 2-~2-aminothiazol-
4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
NMR (DMSO-d6, ~) : 1.3-1.9 (13H, m), 3.33 and 3.65
(2H, ABq, J=18Hz), 5.36 (lH, d, J=5Hz), 5.65 and
5.76 (2H, ABq, J=6Hz), 6.00 (lH, dd, J=5Hz,
8Hz), 6.51 (lH, d, J=12Hz), 6.59 (lH, s), 6.65
(lH, d, J=12Hz), 7.1-7.3 (18H, m), 7.6-7.7 (lH,
m), 8.3-8.5 (2H, m), 9.95 (lH, d, J=8Hz)
(14) 1-~(lR,2S,5R)-2-Isopropyl-5-methylcyclohexyloxy-
carbonyloxy]ethyl 7~-[2-(2-aminothiazol-4-yl)-2-
trityloxyiminoacetamido)-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1770, 1740 cm 1
NMR (DMSO-d6, ~) : 0.7-2.0 (18H, m), 1.37 and 1.49
(total 3H, d, J=5Hz), 3.28 and 3.62 (2H, ABq,
J=18Hz), 4.45 (lH, m), 5.36 (lH, d, J=5Hz),
5.9-6.1 (lH, m), 6.5-6.8 (4H, m), 7.1-7.4 (18H,
m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 9.94 and
3.96 (total lH, d, J=8Hz)
(15) l-Methylcyclohexylcarbonyloxymethyl 7~-[2-(2-amino-
thiazol-4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1740 cm 1
NMR (DMSO-d6, ~) : 1.10 (3H, s), 1.2-1.5 (8H, m),
1.9-2.0 (2H, m), 3.30 and 3.62 (2H, ABq,
J=18Hz), 5.36 (lH, d, J=5Hz), 5.69 and 5.81 (2H,
WO94/10177 21 ~ 7 6 0 9 PCT/JP93/0150~
- 61 -
ABq, J=6Hz), 6.01 (lH, dd, J=5Hz, 8Hz), 6.53
(lH, d, J=12Hz), 6.59 (lH, s), 6.67 (lH, d,
J=12Hz), 7.1-7.9 (18H, m), 7.66 (lH, m), 8.47
(2H, m), 9.95 (lH, d, J=8Hz)
(16) (E)-2-(Isobutoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-
4-thiazolyl)-2-trityloxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1765, 1700, 1670 cm 1
NMR (DMSO-d6, ~) : 0.88 (6H, d, J=6.7Hz), 1.00 (3H,
t, J=7.4Hz), 1.87 (lH, m), 2.31 (2H, m), 3.26
and 3.61 (2H, ABq, J=18.0Hz), 3.87 (2H, d,
J=6.5Hz), 4.84 and 4.92 (2H, ABq, J=11.9Hz),
5.33 (lH, d, J=4.9Hz), 5.96 (lH, dd, J=8.3Hz,
4.9Hz), 6.47 (lH, d, J=12.1Hz), 6.58 (lH, s),
6.59 (lH, d, J=12.1Hz), 6.98 (lH, t, J=7.8Hz),
7.30 (16H, m), 7.64 (lH, m), 8.44 (2H, m), 9.95
(lH, d, J=4.9Hz)
(17) (E)-2-(Isobutoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-
4-thiazolyl)-2-trityloxyiminoacetamido]-3-[(Z)-2-(2-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3400, 1770, 1695, 1670 cm 1
NMR (DMSO-d6, ~) : 0.89 (6H, d, J=6.7Hz), 1.03 (3H,
t, J=7.4Hz), 1.91 (lH, m), 2.26 (3H, m), 2.44
(3H, s), 3.10 and 3.42 (2H, ABq, J=17.5Hz), 3.89
(2H, d, J=6.4Hz), 4.85 and 4.96 (2H, ABq,
J=11.8Hz), 5.25 (lH, d, J=4.9Hz), 5.91 (lH, dd,
J=8.3Hz, 4.9Hz), 6.56 (lH, s), 6.57 (lH, d,
J=12.0Hz), 6.70 (lH, d, J=12.0Hz), 7.01 (lH, t,
J=7.7Hz), 7.12-7.28 (17H, m), 7.44 (lH, m), 8.35
(lH, m), 9.88 (lH, d, J=8.3Hz)
3~
WO94/10177 PCT/JP93/OlS~
2 ~ 4~ 609 - 62 -
-
118) (E)-2-(Isobutoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-
4-thiazolyl)-2-trit~loxyiminoacetamido]-3-[(z)-2-(6-
methylpyridin-3~ vinyl~-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1765, 1700, 1680, 1665 cm
NMR ~DMSO-d6, ~) : 0.92 (6H, d, J=7.0Hz), 1.00 (3H,
t, J=7.6Hz), 1.85-1.92 (lH, m), 2.26-2.44 (2H,
m), 2.44 (3H, s), 3.25 and 3.58 (2H, ABq,
J=17.6Hz), 3.87 (2H, d, J=7.0Hz), 4.85 and 4.93
(2H, ABq, J=12.0Hz), 5.33 (lH, d, J=4.9Hz), 5.96
(lH, dd, J=8.3Hz, 4.9Hz), 6.31 (lH, d,
J=12.0Hz), 6.54 (lH, d, J=12.0Hz), 6.55 (lH, s),
6.98 (lH, t, J=7.6Hz), 7.05-7.29 (18H, m),
7.51-7.56 (lH, m), 8.32 (lH, m), 9.94 (lH, d,
J=8.3Hz)
(19) (E)-2-(n-Hexyloxycarbonyl)-2-pentenyl 7~-[2-(2-amino-
4-thiazolyl)-2-trityloxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1705, 1670, 1605 cm
NMR (DMSO-d6, ~) : 0.78-0.83 (3H, m), 0.99 (3H, t,
J=7.4Hz), 1.24 (6H, m), 1.49-1.57 (2H, m),
2.21-2.36 (2H, m), 3.26 and 3.60 (2H, ABq,
J=17.7Hz), 4.07 (2H, t, J=6.6Hz), 4.82 and 4.92
(2H, ABq, J=11.9Hz), 5.33 (lH, d, J=4.9Hz), 5.98
(lH, dd, J=8.3Hz, 4.9Hz), 6.47 (lH, d,
J=12.1Hz), 6.58 (lH, s), 6.59 (lH, d, J=12.1Hz),
6.96 (lH, t, J=7.7Hz), 7.23-7.37 (18H, m),
7.63-7.67 (lH, m), 8.43-8.46 (2H, m), 9.95 tlH,
d, J=8.3Hz)
(20) (E)-2-(n-Butoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-4-
thiazolyl)-2-trityloxyiminoactamido~-3-[(Z)-2-(pyridin-
3-yl)vinyl]-3-cephem-4-carbox~late (syn isomer)
WO94/10177 2 1 ~ 7 6 0 9 PCT/JP93/01505
-
- 63 -
I~ (Nujol) : 1765, 1710, 1680 cm
NMR (DMSO-d6, ~) : 0.86 (3H, t, J=7.2Hz), 0.99 (3H,
- t, J=7.4Hz), 1.22-1.41 ~2H, m~, 1.49-1.63 (2H,
m), 2.21-2.36 (2H, m), 3.26 and 3.61 (2H, ABq,
J=17.9Hz), 4.08 (2H, t, J=6.4Hz), 4.82 and 4.91
(2H, ABq, J=11.8Hz), 5.33 (lH, d, J=4.9Hz), 5.97
(lH, dd, J=8.4Hz, 4.9Hz), 6.47 (lH, d,
J=12.1Hz), 6.58 (lH, s), 6.59 (lH, d, J=12.1Hz),
6.96 (lH, t, J=7.7Hz), 7.23-7.36 (18H, m),
7.63-7.67 (lH, m), 8.43-8.46 (2H, m), 9.94 (lH,
d, J=8.4Hz)
(21) (E)-2-(Isopentyloxycarbonyl)-2-butenyl 7~-[2-(2-
amino-4-thiazolyl)-2-trityloxyiminoacetamido~-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3410, 1770, 1680 cm 1
NMR (DMSO-d6, ~) : 0.86 (6H, d, J=6.1Hz), 1.42-1.67
(3H, m), 1.88 (3H, d, J=7.2Hz), 3.26 and 3.61
(2H, ABq, J=17.7Hz), 4.11 (2H, t, J=6.7Hz), 4.81
and 4.93 t2H, ABq, J=11.9Hz), 5.33 (lH, d,
J=5.lHz), 5.99 (lH, dd, J=8.4Hz, 5.lHz), 6.47
(lH, d, J=12.1Hz), 6.58 (lH, s), 6.59 (lH, d,
J=12.1Hz), 7.08 (lH, q, J=7.2Hz), 7.23-7.37 (18H, m),
2; 7.63-7.67 (lH, m), 8.43-8.46 (2H, m), 9.94 (lH, d,
J=8.4Hz)
(22) (E)-2-(Ethoxycarbonyl)-2-butenyl 7~-[2-(2-amino-4-
thiazolyl)-2-trityloxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1765, i700, 1665 cm 1
NMR (DMSO-d6, ~) : 1.19 (3H, t, J=7.1Hz), 1.88 (3H,
d, J=7.2Hz), 3.27 and 3.62 (2H, ABq, J=17.7Hz),
4.12 (2H, q, J=7.1Hz), 4.81 and 4.93 (2H, ABq,
WO94/10177 PCT/JP93/01'--
~4~6~9 - 64 -
J=11.9Hz), 5.34 (lH, d, J=4.9Hz), 5.79 (lH, dd,
J=8.3Hz, 4.9Hz), 6.47 (lH, d, J=12.lHz), 6.58
(lH, s), 6.59 (lH, d, J=12.1Hz), 7.08 (lH, q,
J=7.2Hz), 7.23-7.37 (18H, m), 7.64-7.68 (lH, m),
8.43-8.46 (2H, m), 9.94 (lH, d, J=8.3Hz)
(23) (E)-2-~Isobutoxycarbonyl)-2-butenyl 7~-[2-t2-amino-4-
thiazolyl)-2-trityloxyiminoacetamido~-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1775, 1700, 1600 cm
NMR (DMSO-d6, ~) : 0.88 (6H, d, J=6.7Hz), 1.85-1.91
(4H, m), 3.27 and 3.61 ~2H, ABq, J=17.9Hz), 3.87
(2H, d, J=6.7Hz), 4.84 and 4.94 (2H, ABq,
J=11.9Hz), 5.33 (lH, d, J=5.0Hz`, 6.47 (lH, d,
J=12.3Hz), 6.58 (lH, s), 6.59 (lH, d, J=12.3Hz),
7.09 (lH, q, J=7.2Hz), 7.30-7.36 (18H, m),
7.63-7.67 (lH, m), 8.43-8.46 (2H, m), 9.94 (lH, d,
J=8.2Hz)
~0
( 7 4) ~E)-2-(Isopentyloxycarbonyl)-2-pentenyl 7~-[2-(2-
amino-4-thiazolyl)-2-trityloxyiminoacetamido]-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
2S IR (Nujol) : 1765, 1700, 1670, 1600 cm 1
NMR (DMSO-d6, ~) : 0.86 (6H, d, J=6.4Hz), 0.99 (3H,
t, J=7.5Hz), 1.43-1.70 (3H, m), 2.22-2.48 (2H,
m), 3.27 and 3.61 (2H, ABq, J=17.8Hz), 4.11 (2H,
t, J=6.7Hz), 4.82 and 4.92 (2H, ABq, J=11.8Hz),
5.34 (lH, d, J=4.9Hz), 5.98 (lH, dd, J=8.4Hz,
4.9Hz), 6.47 (lH, d, J=12.1Hz), 6.58 (lH, s),
6.59 (lH, d, J=12.1Hz), 6.96 (lH, t, J=7.7Hz),
7.23-7.37 (18H, m), 7.63-7.67 (lH, m), 8.43-8.46
(2H, m), 9.95 (lH, d, J=8.4Hz)
WO94/10177 21 ~ 7 6 0 9 PCT/JP93/01~05
- 65 -
~25, (E)-2-(Ethoxycarbonyl)-2-penten~l 7~-[2-(2-amino-4-
thiazolyl)-2-trityloxyiminoacetamido~-3-~(Z)-2-
- (pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1765, 1700, 1665 cm 1
NMR ~DMSO-d6, ~) : 0.99 (3H, t, J=7.1Hz), 1.20 (3H,
t, J=6.8Hz), 2.23-2.33 (2H, m), 3.27 and 3.61
t2H, ABq, J=17.8Hz), 4.12 (2H, q, J=7.1Hz), 4.81
and 4.91 (2H, ABq, J=11.8Hz), 5.33 (lH, d,
J=4.8Hz), 5.98 (lH, dd, J=8.4Hz, 4.8Hz), 6.47
(lH, d, J=12.0Hz), 6.58 (lH, s), 6.58 (lH, d,
J=12.0Hz), 6.96 (lH, t, J=7.6Hz), 7.26-7.36
(18H, m), 7.63-7.67 (lH, m), 8.43-8.46 (2H, m),
9.94 (lH, d, J=8.4Hz)
(26) (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 7~-~2-t2-
aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-
(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR (Nujol) : 1800, 1770, 1720 cm
NMR (DMSO-d6, ~) : 2.13 (3H, s), 3.37 and 3.70 (2H,
ABq, J=18Hz), 4.89 and 5.04 (2H, ABq, J=14Hz),
5.36 (lH, d, J=5Hz), 6.02 (lH, dd, J=5Hz, 8Hz),
6.48 (lH, d, J=12Hz), 6.59 (lH, s), 6.63 (lH, d,
J=12Hz), 7.2-7.4 (18H, m), 7.6-7.7 (lH, m),
8.4-8.5 (2H, m), 9.93 (lH, d, J=8Hz)
(27) 1-[3,3-Dimethylbutoxycarbonyloxy]ethyl 7~-[2-(2-
tritylamino-4-thiazolyl)-2-trityloxyiminoacetamido]-
3-~(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR (Nujol) : 1750, 1670 cm 1
NMR (DMSO-d6, ~) : 0.82 and 0.89 (9H, two s, 9H),
1.33-1.56 (5H, m), 3.26 and 3.60 (2H, ABq,
3~ J=17.6Hz), 4.11-4.18 (2H, m), 5.31 and 5.32 tlH,
W094/10177 - PCT/JP93/015
- 66 -
~,4~609
two d, J=4.9Hz), 5.88-5.98 (lH, m), 6.44-6.77
(4H, m), 7.14-7.29-(31H, m), 7.63-7.67 (lH, m),
8.44-8.46 (2H. m), 8.78 (lH, m), 9.94 and 9.95
(lH, two d, ~=8.2Hz)
(28) 1-(n-PentyloxycarbonyloXy)ethyl 7~-~2-(2-tritylamino-
4-thiazolyl)-2-trityloxyiminoacetamido~-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol): 1755, 1740, 1640 cm
NMR (DMSO-d5, ~) : 0.80-0.86 (3H, m), 1.21-1.58 (9H,
m), 3.26 and 3.65 (2H, ABq, J=17.7Hz), 4.05-4.12
(2H, m), 5.31 and 5.32 (lH, two d, J=4.9Hz),
5.89-5.96 (lH, m), 6.48-6.76 (4H, m), 7.14-7.29
(31H, m), 7.63 (lH, m), 8.44-8.47 (2H, m), 8.78
(lH, m), 9.94 and 9.95 (lH, two d, J=8.3Hz)
(29) 1-(1-Ethylpropoxycarbonyloxy)ethyl 7~-[2-(2-amino-4-
thiazolyl)-2-trityloxyiminoacetamido)-3-[(Z)-2-(2-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol): 1770, 1740, 1660 cm
NMR (DMSO-d6, ~) : 0.73-0.89 (6H, m), 1.02-1.57 (7H,
m), 2.44 (3H, m), 3.08 and 3.49 (2H, ABq,
J=17.6Hz), 4.49-4.58 (lH, m), 5.28 (lH, d,
J=4.9Hz), 5.92-6.02 (lH, m), 6.54-6.82 (4H, m),
7.13-7.31 (18H, m), 7.41-7.49 (lH, m), 8.36-8.37
(lH, m), 9.86 and 9.87 (lH, two d, J=8.3Hz)
(30) 1-(Cyclohexyloxycarbonyloxy)ethyl 7B- E 2-(2-amino-4-
thiazolyl)-2-trityloxyiminoacetamido~-3-[(Z)-2-(2-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol): 1770, 1740, 1670 cm
NMR (DMSO-d6, o) : 1.17-1.99 (lOH, m), 1.38 and 1.52
W094J10177 21 1 7 6 Q 9 PCT/JP93/01505
- ~7 -
(3H, two d, J=5.4Hz), 2.44 (3H, s), 3.09 and
3.29 (2H, ABq, J=17.7Hz), 4.59 (lH, m), 5.28 and
5.29 (lH, two d, J=4.9Hz), 5.98 (lH, m), 6.57
(lH,-s), 6.58 (lH, d, J=12.1Hz), 6.71 (lH, d,
J=12.1Hz), 6.76 (lH, m), 7.13-7.31 (18H, m),
7.45 (lH, m), 8.35 (lH, m), 9.88 and 9.89 (lH,
each d, J=8.lHz)
(31) l-(Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-amino-4-
thiazolyl)-2-trityloxyiminoacetamido]-3-[(Z)-2-(6-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1750, 1660 cm
NMR (DMSO-d6, ~) : 1.09-1.94 (lOH, m), 1.36 and 1.49
(3H, two d, J=5.4Hz), 2.44 (3H, s), 3.26 and
3.61 (2H, ABq, J=17.4Hz), 4.53 (lH, m), 5.34 and
5.35 (lH, two d, J=4.9Hz), 6.02 and 6.03 (lH,
two dd, J=8.4Hz, 4.9Hz), 6.43-6.78 (3H, m), 6.60
(lH, s), 7.19-7.30 (18H, m), 7.53-7.57 (lH, m),
8.35 (lH, m), 9.95 and 9.96 ~lH, each d,
J=8.4Hz)
(32) l-(Ethoxycarbonyloxy)ethyl 7~-[2-(2-aminothiazol-4-
yl)-2-trityloxyiminoacetamido~-3-~(Z)-2-~pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1740, 1682 cm 1
NMR (DMSO-d6, ~) : 1.19 and 1.21 (total 3H, each t,
J=7.1Hz), 1.36 and 1.49 (total 3H, each d,
J=5.4Hz), 3.29 and 3.65 (2H, ABq, J=18.9Hz),
4.14 (2H, m), 5.36 and 5.37 (total lH, each d,
J=4.9Hz), 5.98-6.08 (lH, m), 6.48-6.77 (3H, m),
6.59 (lH, s), 7.26-7.30 (18H, m), 7.63-7.70 (lH,
m), 8.45-8.47 (2H, m), 9.94 and 9.95 (total lH,
two d, J=8.4Hz)
~5
WO94/10177 609 - 68 - PCT/JP93/01Cr~
t..) l-Acetoxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
trityloxyiminoaceta~ido]-3-~(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-A~carboxylate (syn isomer)
IR (Nujol) : 1735, 1660 cm
S NMR (DMSO-d6, ~) : 1.33 and 1.44 (total 3H, each d,
J=5.4Hz), 2.03 and 2.04 ttotal 3H, each s), 3.29
and 3.67 (2H, ABq, J=17.0Hz), 5.34-5.39 (lH, m),
5.97-6.09 (lH, m), 6.43-6.87 (3H, m), 6.59 (lH,
s), 7.20-7.37 (18H, m), 7.65-7.69 (lH, m),
8.45-8.48 (2H, m), 9.92-9.96 (lH, m)
t34) l-(Cyclopentyloxycarbonyloxy)ethyl 7~-[2-t2-
aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-
[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR (Nujol) : 1745, 1665 cm
NMR (DMSO-d6, ~) : 1.35 and 1.48 (total 3H, two d,
J=5.4Hz), 1.40-1.90 t8H, m), 3.28 and 3.64 (2H,
ABq, J=18.0Hz), 4.95-5.06 (lH, m), 5.36 and 5.37
(total lH, two d, J=4.9Hz), 5.98-6.08 (lH, m),
o.48-6.79 (3H, m), 6.59 (lH, s), 7.13-7.39 (18H,
m~, 7.62-7.70 (lH, m), 8.43-8.52 (2H, m),
9.93-9.97 (lH, m)
t35) l-(Isopropyloxycarbonyloxy)ethyl 7~-[2-t2-amino-
thiazol-4-yl)-2-trityloxyiminoacetamido~-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate tsyn
isomer)
IR tNujol) : 1735 cm
NMR (DMSO-d6, ~) : 1.18-1.24 (6H, m), 1.3~ and 1.48
(total 3H, each d, J=5.4Hz), 3.28 and 3.64 (2H,
ABq, J=17.8Hz), 4.76 and 4.78 (total lH, each t,
J=6.2Hz), 5.35 and 5.37 tlH, two d, J=4.9Hz),
5.98-6.08 (lH, m), 6.48-6.76 (3H, m), 6.59 tlH,
s), 7.24-7.29 (18H, m), 7.62-7.70 (lH, m),
wo g4,l0l,7 2 1 4 7 6 0 9 PCT/JP93/01505
- 69 -
~.~5-8.47 ~2H, m), 9.94 and 9.96 (lH, two d,
J=8.5Hz)
.
t36) 1-(Cyclohexyloxycarbonyloxy)ethyl 7~-l2-(2-amino-
thiazol-4-yl)-2-trityloxyiminoacetamido~-3-i(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1740, 1665 cm 1
NMR (DMSO-d6, ~) : 1.09-1.90 (lOH, m), 1.35 and 1.48
(total 3H, each d, J=5.4Hz), 3.28 and 3.64 (2H,
ABq, J=17.3Hz), 4.45-4.59 (lH, m), 5.34-5.37
(lH, m), 5.98-6.04 (lH, m), 6.48-6.78 (3H, m),
6.59 (lH, s), 7.18-7.39 (17H, m), 7.61-7.72 (lH,
m), 8.43-8.~2 (2H, m), ~.92-9.99 (lH, m)
~5
Example 14
To a solution of 7~-[2-difluoromethoxyimino-2-(2-
triphenylmethylaminothiazol-4-yl)acetamido~-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylic acid (syn
isomer) (6.05 g) in methanol-(30 ml) was added conc.
hydrochloric acid (4.5 ml) and the mixture was stirred at
room temperature for 1.5 hours. The reaction mixture was
poured into a mixture of water and ethyl acetate. The
separated aqueous layer was adjusted to pH 5 with lN
2~ sodium hydroxide solution, washed with ethyl acetate twice
and evaporated to remove ethyl acetate. The solution was
adjusted to pH 3 with aqueous sodium hydrogencarbonate
solution, subjected to column chromatography on Diaion
HP-20, and eluted with aqueous 20% isopropyl alcohol
solution. The fractions cont~ining the object compound
was collected and lyophilized to give crude product, which
was purified by preparative HPLC utilizing a C18 ~
Bondepak resin (Trademark : Waters Associates, Inc.) to
afford 7~-~2-(2-aminothiazol-4-yl)-2-difluoromethoxyimino-
3~ acetamido]-3-[(Z)-2-(pyridin-3-yl)vinyl~-3-cephem-4-
WOg4/10177 ` PCT/JP93/OlS~-
~6~9 - 70 -
carboxylic acid (syn isomer) ~748 mg).
iR (Nujol) : 1740 cm
NMR (DMSO-d6, ~) : 3.22 and 3.58 (2H, ABq, J=17.7Hz),
5.28 (lH, d, J=4.8Hz), 5.85 (lH, dd, J=4.8Hz,
8.0Hz), 6.55 (lH, d, J=12.2Hz), 6.62 (lH, d,
J=12.2Hz), 6.99 (lH, s), 7.12 (lH, t, J=71.1Hz),
7.32-7.38 (lH, m), 7.36 (2H, br s), 7.63-7.69
(lH, m), 8.43-8.47 (2H, m), 10.01 (lH, m)
Example 15
A solution of 1-pivaloyloxyethyl 7~-E2-(2-
tritylaminothiazol-4-yl)-2-trityloxyiminoacetamido}-
3-[(Z)-2-(pyridin-3-yl)vinyl~-3-cephem-4-carboxylate
(syn isomer) (640 mg) in 90% formic acid aqueous solution
i5 (4.8 ml) was stirred at room temperature for 4.5 hours.
The insoluble material in the reaction mixture was
filtered off. The filtrate was poured into a mixture of
ethyl acetate and water. The mixture was adjusted to pH
5. The organic layer was separated, washed with water and
with brine, dried over magnesium sulfate, and evaporated
in vacuo. The residue was triturated with diisopropyl
ether. The precipitates were collected by filtration and
dried to give 1-pivaloyloxyethyl 7~-~2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-~pyridin-3-yl)vinyl-
3-cephem-4-carboxylate (syn isomer) (219 mg).
IR (Nujol) : 1760, 1720, 1650, 1590, 1500 cm
NMR (DMSO-d6, ~) : 1.08 and 1.12 (total 9H, s), 1.29
and 1.42 (total 3H, d, J=5Hz), 3.29 and 3.65
(2H, ABq, J=18Hz), 5.28 and 5.30 (total lH, d,
J=5Hz), 5.87 and 5.89 (total lH, dd, J=5Hz,
8Hz), 6.43 (lH, d, J=12Hz), 6.54 (lH, d,
J=12Hz), 6.65 (lH, s), 6.6-6.8 (lH, m), 7.13
(2H, br s), 7.2-7.4 (lH, m), 7.6-7.7 (lH, m),
8.4-8.5 (2H, m), 9.52 and 9.54 (total lH, d,
J=8Hz), 11.3 (lH, s)
WO94/10177 _ 7l _ PCT/JP93/01505
Example 16
The following compounds were obt~;ne~ according to
similar-manners to those of Examples 5 and 15.
(1) l-Methylcyclohexylcarbonyloxymethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z~-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1740, 1660, 1600 cm 1
NMR (DMSO-d6, ~) : 1.08 (3H, s), 1.1-1.6 (8H, m)
1.8-2.0 (2H, m), 3.27 and 3.62 (2H, ABq,
J=18Hz), 5.28 (lH, d, J=5Hz), 5.66 and 5.78 (2H,
ABq, J=6Hz), 5.86 (lH, dd, J=5Hz, 8Hz), 6.49
(lH, d, J=12Hz), 6.65 (lH, s), 6.64 (lH, d,
J=12Hz), 7.14 (2H, br s), 7.3-7.4 (lH, m),
7.6-7.7 (lH, m), 8.45 (2H, m), 9.53 (lH, d,
J=8Hz), 11.3 (lH, s)
(2) l-(Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido)-3-[(E)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1630 cm 1
NMR (DMSO-d6, ~) : 1.1-2.0 (13H, m), 3.73 and 4.09
(2H, ABq, J=17Hz), 4.55 (lH, m), 5.27 and 5.30
Itotal lH, d, J=5Hz), 5.8-6.0 llH, m), 6.67 (lH,
s), 6.92 (lH, m), 7.12 llH, d, J=16Hz), 7.14
(2H, br s), 7.3-7.5 (2H, m), 7.8-8.0 (lH, m),
8.49 (lH, d, J=4Hz), 8.68 (lH, br s), 9.54 (lH,
d, J=8Hz), 11.3 (lH, s)
(3) Pivaloyloxymethyl 7~-[2-(2-aminothiazol-4-yl)-2-
hydroxyiminoacetamido]-3-l(E)-2-(pyridin-3-yl)vinyl]-
3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1770, 1740, 1650 cm 1
W094/10177 PCT/JP93/015
~4~609 - 72 -
NMR (DMSO-d6, ~) : 1.16 ~9H, s), 3.76 and 4.i2 (2H,
ABq, J=18Hz), 5.28 (lH, d, J=5Hz), 5.8-5.9 (lH,
- m), 5.88 and 5.98 (2H, ABq, J=6Hz), 6.69 (lH,
s), 7.14 (lH, s), 7.15 (lH, d, J=16Hz), 7.3-7.5
~3H, m), 7.92 (lH, d, J=8Hz), 8.49 (lH, d,
J=8Hz), 8.68 (lH, br s), 9.54 (lH, d, J=8Hz),
11.3 (lH, br s)
(4) 1-[(lR,2S,5R)-2-Isopropyl-5-methylcyclohexyloxy-
carbonyloxy]ethyl 7~-[2-(2-aminothiazol-4-yl)-2-
hydroxyiminoacetamido~-3-l(Z)-2-(pyridin-3-yl)vinyl]-
3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1730 cm 1
NMR (DMSO-d6, ~) : 0.74 (3H, d, J=7Hz), 0.8-2.1
i5 (15H, m), 1.35 and 1.45 (lH, d, J=5Hz), 3.24 and
3.62 (2H, ABq, J=18Hz), 4.3-4.5 (lH, m),
5.27-5.28 (total lH, d, J=5Hz), 5.87 (lH, m),
6.4-6.7 (4H, m), 7.12 (2H, br s), 7.2-7.4 (lH,
m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, br s), 9.51
and 9.53 (total lH, d, J=8Hz), 11.3 (lH, s)
(5) Cycloheptylcarbonyloxymethyl 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1750, 1650 cm
NMR (DMSO-d6, ~) : 1.3-1.7 (lOH, m), 1.7-2.0 (2H,
m), 2.4-2.5 (lH, m), 3.29 and 3.64 (2H, ABq,
J=18Hz), 5.28 (lH, d, J=5Hz), 5.62 and 5.73 (2H,
ABq, J=6Hz), 5.86 (lH, dd, J=5Hz, 8Hz), 6.48
(lH, d, J=12Hz), 6.63 (lH, d, J=12Hz), 6.76 (lH,
s), 7.12 (2H, br s), 7.34 (lH, dd, J=5Hz, 8Hz),
7.65 (lH, d, J=8Hz), 8.45 (2H, m), 9.52 (lH, d,
J=8Hz), 11.3 (lH, s)
(6) 1-(3-Methylbutoxycarbonyloxy)ethyl 7~-[2-(2-
2147609
WO94/10177 PCT/JP93/01505
- 73 -
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1740, 1640, 1590, 1500 cm 1
, ~MR ~DMSO-d6, ~) : 0.88 (6H, d, J=6Hz), 1.3-1.7 (6H,
m), 3.29 and 3.63 (2H, ABq, J=18Hz), 4.12 and
4.13 (total 2H, t, J=7Hz), 5.28 and 5.29 (total
lH, d, J=5Hz), 5.88 (lH, m), 6.3-6.8 (4H, m),
7.19 (2H, br s), 7.3-7.4 (lH, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 9.52 and 9.53 (total lH, d,
J=8Hz), 11.3 (lH, s)
(7) 1-(3-Methylbutyryloxy)ethyl 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido}-3-l(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer~
IR (Nujol) : 1750, 1660, 1600, 1510 cm 1
NMR (DMSO-d6, ~) : O.87 and 0.88 (total 6H, d,
J=7Hz), 1.30 and 1.41 (total 3H, d, J=5Hz),
1.8-2.0 (lH, m); 2.15 and 2.17 (total 2H, d,
?0 J=7Hz), 3.30 and 3.64 (2H, ABq, J=17Hz), 5.28
and 5.29 (total lH, d, J=5Hz), 5.87 (lH, m),
6.4-6.8 (4H, m), 7.13 (2H, br s), 7.2-7.4 (lH,
m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 9.51 and
9.53 (total lH, d, J=8Hz), 11.3 (lH, s)
(8) l-Isobutyryloxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
hydroxyiminoacetamido~-3-[(Z)-2-(pyridin-3-yl)vinyl]-
3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1770, 1740, 1670, 1520 cm
NMR (DMSO-d6, ~) : 1.0-1.1 (6H, m), 1.30 and 1.41
(total 3H, d, J=5Hz), 3.29 and 3.65 (2H, ABq,
J=18Hz), 5.28 and 5.29 (total lH, d, J=5Hz),
5.8-5.9 (lH, m), 6.4-6.9 (4H, m), 7.13 (2H, br
s), 7.2-7.4 (lH, m), 7.6-7.7 (lH, m), 8.46 (2H,
m), 9.51 and 9.53 (total lH, d, J=8Hz), 11.3
(lH, s)
WO94/10177 PCT/JP93/OI~r
- 7q -
~4~6~9
i9) l-Hexyloxycarbonyloxyethyl 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-l(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1750, 1660, 1600, 1510 cm 1
NMR IDMSO-d6, ~) : 0.8-1.0 (3H, m), 1.1-1.7 (llH,
m), 3.27 and 3.63-(2H, ABq, J=18Hz), 4.0-4.1
12H, m), 5.2-5.3 (lH, m), 5.8-6.0 (lH, m),
6.4-6.8 (4H, m), 7.13 (2H, br s), 7.3-7.4 (lH,
m), 7.6-7.7 (lH, m), 8.46 (2H, m), 9.52 and 9.53
(total lH, d, J=8Hz), 11.3 (lH, s)
(10) l-(l-Ethylpropoxycarbonyloxy)ethyl 7~-[2-(2-amino-
thiazol-4-yl)-2-hydroxyiminoacetamido~-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3300, 1770, 1740, 1650, 1600, 1510 cm
NMR (DMSO-d6, ~) : 0.7-0.9 (6H, m), 1.35 and 1.45
(total 3H, d, J=5Hz), 1.4-1.6 (4H, m), 3.24 and
3.28 (total lH, d, J=18Hz), 3.62 (lH, d,
J=18Hz), 4.51 (lH, m), 5.27 (lH, d, J=5Hz), 5.87
(lH, m), 6.4-6.7 (4H, m), 7.15 (2H, br s),
7.2-7.4 (lH, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 9.52-9.54 (total lH, d, J=8Hz), 11.3 (lH, s)
(11) 1-(3,3-Dimethylbutyryloxy)ethyl 7~- r 2-(2- minothiazol-
4-yl)-2-hydroxyiminoacetamido]-3-l(Z)-2-(3-pyridyl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1775, 1740, 1655, 1600, 1505 cm 1
NMR (DMSO-d6, ~) : 0.9-1.0 ~llH, m), 1.30-1.42
(total 3H, d, J=5.4Hz), 3.25 and 3.64 (2H, ABg,
J=17.8Hz), 5.28-5.29 (total lH, d, J=4.9Hz),
5.8-5.9 (lH, m), 6.5-6.9 (4H, m), 7.22 (2H, br
s), 7.3-7.4 (lH, m), 7.6-7.7 (lH, m), 8.4-8.5
(lH, m), 9.53 and 9.56 (total lH, d, J=8.1Hz),
11.36 (lH, s)
WO94/10177 21~ 7 6 0 9 PCT/JP93/01~05
- 7S -
~12) l-~Isobutyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-~(Z)-
2-(3-pyridyl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 176S, 17S0, 1655, 1600, 1505 cm 1
NMR (DMSO-d6, ~) : 0.87 (6H, d, J=6.7Hz), 1.34 and
1.4S ~3H, d, J=5.4Hz), 1.80-2.0 (lH, m), 3.25
and 3.64 (2H, ABq, J=17.9Hz), 3.89 (2H, d,
J=6.6Hz), 5.27 and 5.28 (total lH, d, J=4.8Hz),
5.8-5.95 (lH, m), 6.4-6.8 (4H, m), 7.20 (2H, br
s), 7.3-7.4 (lH, m), 7.6-7.7 (lH, m), 8.4-8.5
(2H, m), 9.52 and 9.55 (total lH, d, J=8.2Hz),
11.34 (lH, s)
(13) 1-(4-Methylpentoxycarbonyloxy)ethyl 7~-[2-(2-amino-
thiazol-4-yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3300, 1770, 1745, 1660, 1610, 1520 cm 1
NMR (DMSO-d6, ~) : 0.8-0.9 (6H, m), 1.2-1.7 (8H, m),
3.25 and 3.63 (2H, ABq, J=18Hz), 4.0-4.1 (2H,
m), 5.27 and 5.29 (total lH, d, J=5Hz), 5.8-6.0
(lH, m), 6.47 and 6.51 (total lH, d, J=12Hz),
6.6-6.8 (3H, m), 7.13 (2H, br s), 7.3-7.4 (lH,
m), 7.6-7.7 (lH, m), 8.46 (2H, m), 9.52 and 9.S3
(total lH, d, J=8Hz), 11.3 (lH, s)
(14) (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 7~-~2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxy~ate (syn
isomer) -1
IR (Nujol) : 3200, 1800, 1765, 1730, 1660 cm
NMR (DMSO-d6, ~) : 2.11 ~3H, s), 3.34 and 3.68 (2H,
ABq, J=18Hz), 4.86 and S.00 (2H, ABq, J=14Hz),
5.28 (lH, d, J=SHz), S.86 (lH, d, J=5.8Hz), 6.44
WO94/10177 PCT/JP93/015~-
~ 6~9 - ~6 -
and 6.60 (2H, ABq, J=12Hz), 6.65 (lH, s), 7.13
(2H, br s), 7.2-7.3 (lH, m), 7.6-7.7 (lH, m),
8.4 (2H, m), 9.S0 (lH, d, J=8Hz), 11.3 (lH, s)
(15) 1-~(3,3-Dimethylbutoxy)carbonyloxy}ethyl 7~-~2-(2-
amino-4-thiazolyl)-2-hydroxyiminoacetamido)-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1755, 1730, 1650 cm 1
NMR (DMSO-d6, ~) : 0.89 (9H, s), 1.33 and 1.44 (3H,
each d, J=5.5Hz), 1.48-1.57 (2H, m), 3.29 and
3.63 (2H, ABq, J=17.9Hz), 4.10-4.18 (2H, m),
5.27 and 5.28 (lH, each d, J=4.9Hz), 5.86 and
5.88 (lH, each dd, J=8.3Hz, 4.9Hz), 6.45-6.73
(4H, m), 7.13 (2H, br s), 7.32-7.37 (lH, m),
7.63-7.67 (lH, m), 8.46 (2H, m), 9.51 and 9.53
(lH, each d, J=8.3Hz), 11.30 (lH, s)
(16) l-(n-Pentyloxycarbonyloxy)ethyl 7~-(2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido~-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1740, 1640 cm 1
NNR (DMSO-d6, ~) : 0.83-0.89 ~3H, m), 1.26-1.58 (9H,
m), 3.25 and 3.63 (2H, ABq, J=17.7Hz), 4.06-4.11
(2H, m), 5.27 and 5.28 (lH, two d, J=4.8Hz),
5.83-5.92 (lH, m), 6.44-6.73 (4H, m), 7.12 (2H,
br s), 7.31-7.37 (lH, m), 7.63-7.67 (lH, m),
8.46 (2H, m), 9.51 and 9.53 (lH, two d,
J=8.2Hz), 11.30 (lH, s)
(17) l-(l-Ethylpropoxycarbonyloxy)ethyl 7~-~2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido]-3-~(Z)-2-(2-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
W094/10177 ~14 7 6 0 9 PCT/JP93/01~05
- 77 -
IR (Nujol) : 1760, 1750, 1640 cm 1
NMR (DMSO-d6, ~) : 0.80-0.88 (6H, m), 1.37 and 1.48
~3H, two d, J=5.4Hz), 1.46-'.55 (4H, m), 2.44
(3H, m), 3.04 and 3.45 (2H, ABq, J=17.9Hz),
4.49-4.54 (lH, m), 5.20 (lH, d, J=4.9Hz),
5.78-5.87 (lH, m), 6.51-6.80 (4H, m), 7.11 (2H,
s), 7.12-7.28 (lH, m), 7.40-7.47 (lH, m),
8.35-8.37 (lH, m), 9.45-9.47 (lH, each d,
J=8.3Hz), 11.28 (lH, s)
(18) (E)-2-(Isobutoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-
4-thiazolyl)-2-hydroxyiminoacetamido]-3-l(Z)-2-(2-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3250, 1765, 1695, 1655 cm
NMR (DMSO-d6, ~) : 0.8S (6H, d, J=6.7Hz), 1.01 (3H,
t, J=7.7Hz), 1.90 (lH, m), 2.26 (2H, m), 2.38
(3H, s), 3.06 and 3.40 (2H, ABq, J=17.6Hz), 3.88
(2H, d, J=6.4Hz), 4.82 and 4.92 (2H, ABq,
J=11.9Hz), 5.17 (lH, d, J=4.9Hz), 5.76 (lH, dd,
J=8.2Hz, 4.9Hz), 6.53 (lH, d, J=12.OHz), 6.62
(lH, s), 6.68 (lH, d, J=12.0Hz), 6.98 (lH, t,
J=7.7Hz), 7.12-7.16 (3H, m), 7.43 (lH, m), 8.35
~ , m), 3.46 (lH, d, J=8.2Hz), 11.29 (lH, s)
(19) Pivaloyloxymethyl 7~-12-(2-amino-4-thiazolyl)-2-
hydroxyiminoacetamido]-3-l(Z)-2-(2-methylpyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 3270, 1770, 1740, 1655 cm 1
NMR (DMSO-d6, ~) : 1.14 (9H, s), 2.43 (3H, s), 3.18
and 3.49 (2H, ~3q, J=17.8Hz), 5.20 (lH, d,
J=4.8Hz), 5.56 and 5.74 (2H, ABq, J=5.9Hz), 5.81
(lH, d, J=8. Hz, 4.8Hz), 6.53 (lH, d, J=11.9Hz),
6.63 (lH, s), 6.73 (lH, d, J=11.9Hz), 7.13 (2H,
br s), 7.17 (lH, m), 7.44 (lH, m), 8.36 (lH, m),
W094/10177 ` pcT/Jp93/ol~r
- 78 -
6~9
9.46 (lH, d, J=8.2Hz), 11.29 ~lH, s)
(20) l-(-Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido]-3-[(Z)-2-(2-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1775, 17SO, 1660, 1605 cm
NMR (DMSO-d6, ~) : 1.12-1.98 (lOH, m), 1.35 and 1.47
(3H, each d, J=5.4Hz), 2.44 (3H, s), 3.06 and
3.38 (2H, ABq, J=18.2Hz), 4.55 (lH, m), 5.19 and
5.21 (lH, each d, J=4.9Hz), 5.83 (lH, m), 6.55
(lH, d, J=12.2Hz), 6.61 (lH, s), 6.62 (lH, m),
6.77 (lH, d, J=12.2Hz), 7.12 (2H, br s), 7.16
(lH, m), 7.44 (lH, m), 8.36 (lH, m), 9.45 and
9.47 (lH, d, J=8.2Hz), 11.28 (lH, s)
(21) l-(Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido~-3-[(Z)-2-(6-
methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1745, 1650 cm 1
NMR (DMSO-d6, ~) : 1.05-1.96 (lOH, m), 1.35 and
1.45 (3H, each d, J=5.4Hz), 2.45 (3H, s), 3.24
and 3.60 (2H, ABq, J=18.0Hz), 4.52 (lH, m), 5.27
and 5.28 (lH, each d, J=4.9Hz), 5.83-5.92 (lH,
m), 6.39-6.74 (3H, m), 6.64 and 6.65 (lH, two
s), 7.13 (2H, br s), 7.20 (lH, m), 7.50-7.55
(lH, m), 8.33 (lH, s), 9.52 and 9.53 (lH, each
d, J=8.2Hz), 11.30 (lH, s)
(22) Pivaloyloxymethyl 7~-~2-(2-amino-4-thiazolyl)-2-
hydroxyiminoacetamido]-3-[(Z)-2-(6-methylpyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1750, 1650, 1590 cm
NMR (DMSO-d6, ~) : 1.12 (9H, s), 2.45 (3H, s), 3.28
WO94/10177 21 ~ 7 6 0 9 PCT/JP93/01505
- 79 -
and 3.~2 (2H, ABq, J=18.2Hz), 5.27 (lH, d,
J=4.9Hz), 5.62 and 5.77 (2H, ABq, J=5.9Hz), 5.86
(lH, dd, J=8.2Hz, 4.9Hz), 6.42 (lH, d,
J=12.0Hz), 6.60 (lH, d, J=12.0Hz), 6.66 (lH, s),
- 5 7.13 (2H, br s), 7.19-7.23 (lH, m), 7.50-7.56
(lH, m), 8.32-8.33 (lH, m), 9.53 (lH, d,
J=4 9Hz), 11.31 (lH, s)
(23) (E)-2-(Isobutoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-
4-thiazolyl)-2-hydroxyiminoacetamido~-3-~(Z)-2-(6-
methylpyridin-3-yl)vinyl~-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1760, 1700, 1660 cm 1
NMR (DMSO-d6, ~) : 0.87 (6H, d, J=6.7Hz), 0.98 (3H,
t, J=7.5Hz), 1.81-1.95 (lH, ~), 2.19-2.34 (2H,
m), 2.44 (3H, s), 3.23 and 3.58 (2H, ABq,
J=18.0Hz), 3.8S (2H, d, J=6.7Hz), 4.82 and 4.89
(2H, ABq, J=11.8Hz), 5.25 (lH, d, J=4.9Hz), 5.81
(lH, dd, J=8.2Hz, 4.9Hz), 6.37 (lH, d,
J=12.0Hz), 6.53 (lH, d, J=12.0Hz), 6.65 (lH, s),
6.95 (lH, t, J=7.SHz), 7.13 (2H, br s),
7.13-7.16 (lH, m), 7.49-7.54 (lH, m), 8.30-8.31
(lH, m), 9.52 (lH, d, J=8.2Hz), 11.30 (lH, s)
2S (24) (E)-2-(Isobutoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-
4-thiazolyl)-2-hydroxyiminoacetamido~-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1760, 1710, 1690, 1640 cm 1
NMR (DMSO-d6, ~) : 0.88 (6H, d, J=6.7Hz), 0.98 (3H,
t, J=7.6Hz), 1.88 (lH, m), 2.26 (2H, m), 3.22
and 3.60 (2H, ABq, J=17.9Hz), 3.86 (2H, d,
J=6.5Hz), 4.81 and 4.89 (2H, ABq, J=11.9Hz),
5.25 (lH, d, J=4.9Hz), 5.81 (lH, dd, J=8.2Hz,
4.9Hz), 6.43 (lH, d, J=12.1Hz), 6.56 (lH, d,
WO94/10177 PCT/JP93/01~'
- 80 -
6~9
J=12.1Hz), 6.64 (lH, s), 6.95 (lH, t, J=7.7Hz),
7.12 (2H, br s), 7.32`(lH, m), 7.63 (lH, m),
8.44 (2H, m), 9.52 ~lH, d, J=8.2Hz), 11.30 (lH,
s ) ~, ~
(25~ ~E)-2-(n-Hexyloxycarbonyl)-2-pentenyl 7~-[2-i2-amino-
4-thiazolyl)-^-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3280, 1775, 1705, 1645 cm
NMR (DMSO-d6, ~) : 0.82-0.87 (3H, m), 0.94-1.00 (3H,
m), 1.25 (6H, m), 1.49-1.57 (2H, m), 2.18-2.33
(2H, m), 3.21 and 3.59 (2H, ABq, J=17.9Hz), 4.06
(2H, m, J=6.5Hz), 4.79 and 4.88 (2H, AB~,
J=11.8Hz), 5.25 (lH, d, J=4.9Hz), 5.82 (lH, dd,
J=8.2Hz, 4.9Hz), 6.43 (lH, d, J=12.0Hz), 6.56
(lH, d, J=12.0Hz), 6.64 (lH, s), 6.94 (lH, t,
J=7.7Hz), 7.13 (2H, br s), 7.30-7.36 (lH, m),
7.62-7.66 (lH, m), 8.44-8.45 (2H, m), 9.51 (lH,
23 d, J=8.2Hz), 11.30 (lH, s)
(26) iE)-2-(n-Butoxycarbonyl)-2-pentenyl 7~-[2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3300, 1780, 1710, 1650 cm 1
NMR (DMSO-d6, ~) : 0.87 (3H, t, J=7.2Hz), 0.98 (3H,
t, J=7.5Hz), 1.23-1.41 (2H, m), 1.49-1.63 (2H,
m), 2.18-2.33 (2H, m), 3.23 and 3.66 (2H, ABq,
~0 J=17.9Hz), 4.07 (2H, t, J=6.4Hz), 4.79 and 4.88
(2H, ABq, J=11.8Hz), 5.26 (lH, d, J=4.9Hz), 5.82
(lH, dd, J=~.2Hz, 4.9Hz), 6.43 (lH, d,
J=12.1Hz), 6.56 (lH, d, J=12.1-~z), 6.64 (lH, s),
6.94 (lH, t, J=7.7Hz), 7.12 i2H, br s),
7.30-7.36 (lH, m), 7.62-7.66 (lH, m), 8.43-8.45
WO94/10177 21 ~ 76og PCT/JP93/01505
~2H, m), 9.51 (lH, d, J=8.2Hz), 11.30 (lH, s)
- (27) (E3-2-(3-Methylbutoxycarbonyl)-2-butenyl 7~-[2-(2-
amino-4-thiazolyl)-2-hydroxyiminoacet~mi~o]-3-[(Z)-2-
(pyridin-3-yl)vinyl~-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3310, 1780, 1710, 1655, 1610 cm 1
NMR (DMSO-d6, ~) : 0.87 (6H, d, J=6.5Hz), 1.42-1.52
(2H, m), 1.58-1.69 (lH, m), 1.85 (3H, d,
J=7.2Hz), 3.23 and 3.60 (2H, ABq, J=17.8Hz),
4.09 (2H, t, J=6.6Hz), 4.79 and 4.88 (2H, ABq,
J=11.8Hz), 5.25 (lH, d, J=4.9Hz), 5.83 (lH, dd,
J=8.2Hz, 4.9Hz), 6.43 (lH, d, J=12.lHz), 6.57
(lH, d, J=12.1Hz), 6.64 (lH, s), 6.64-6.99 (3H,
m), 6.99-7.13 (lH, m), 7.62-7.66 (lH, m), 8.45
(2H, m), 9.51 (lH, d, J=8.2Hz), 11.30 (lH, m)
(28) (E)-2-(Ethoxycarbonyl)-2-butenyl 7~-[2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl}-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1700, 1650 cm 1
NMR (DMSO-d6, ~) : 1.20 (3H, t, J=7.1Hz), 1.85 (3H,
d, J=7.2Hz), 3.24 and 3.61 (2H, ABq, J=17.9Hz),
4.11 (2H, q, J=7.1Hz), 4.78 and 4.89 (2H, ABq,
J=11.8Hz), 5.26 (lH, d, J=4.9Hz), 5.84 (lH, dd,
J=8.2Hz, 4.9Hz), 6.43 (lH, d, J=12.1Hz), 6.57
(lH, d, J=12.1Hz), 6.65 (lH, s), 7.04 (lH, q,
J=7.2Hz), 7.13 (2H, s), 7.30-7.36 (lH, s),
7.62-7.66 (lH, m), 8.45 (2H, m), 9.51 (lH, d,
J=8.2Hz), 11.31 (lH, s)
(29) (E)-2-(Isobutoxycarbonyl)-2-butenyl 7~-[2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-
3-yl)vinyl]-3-cephem-4-carboxylate ~syn isomer)
W094/10177 PCT/JP93/015r
- 82 -
~4~6~9
IR (Nujol) : 3280, 1765, 1700, 1650 cm 1
NMR (DMSO-d6, ~) : 0.88 (6H, d, J=6.7Hz), 1.84-1.91
(4H, m), 3.23 and 3.60 (2H, ABq, J=18.0Hz), 3.85
(2H, d, J=6.7Hz), 4.81 and 4.90 (2H, ABq,
J=11.9Hz), 5.25 ~lH, d, J=4.8Hz), 5.82 (lH, dd,
J=8.lHz, 4.8Hz),`~6.43 (lH, d, J=12.lHz), 6.S6
llH, d, J=12.1Hz), 6.64 (lH, s), 7.07 (lH, q,
J=7.2Hz), 7.12 (2H, br s), 7.30-7.36 (lH, m),
7.62-7.66 (lH, m), 8.43-8.45 (2H, m), 9.52 (lH,
d, J=8.1Hz), 11.32 (lH, s)
(30) (E)-2-(3-Methylbutoxycarbonyl)-2-pentenyl 7~-[2-(2-
amino-4-thiazolyl)-2-hydroxyiminoacetamido]-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3280, 1770, 1700, 1640 cm 1
NMR (DMSO-d6, ~) : 0.87 (6H, d, J=6.5Hz), 0.97 (3H,
t, J=7.5Hz), 1.43-1.71 (3H, m), 2.18-2.33 (2H,
m), 3.23 ~nd 3.60 (2H, ABq, J=17.9Hz), 4.10 (2H,
t, J=6.7Hz), 4.79 and 4.87 (2H, ABq, J=11.8Hz),
5.26 (lH, d, J=4.9Hz), 5.82 (lH, dd, J=8.2Hz,
4.9Hz), 6.43 (lH, d, J=12.lHz), 6.56 (lH, d,
J=12.1Hz), 6.64 (lH, s), 6.93 (lH, t, J=7.7Hz),
7.13 (2H, s), 7.30-7.36 (lH, m), 7.62-7.66 (lH,
m), 8.43-8.45 (2H, m), 9.52 (lH, d, J=8.2Hz),
11.30 (lH, s)
(31) (E)-2-(Ethoxycar~onyl)-2-pentenyl 7~-[2-(2-amino-4-
thiazolyl)-2-hydroxyiminoacetamido]-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 3280, 1770, 1705, 1640 cm
NMR (DNSO-d6, ~) : 0.97 (3H, t, J=7.5Hz), 1.20 (3H,
t, J=7.1Hz), 2.17-2.32 (2H, m), 3.23 and 3.61
(2H, ABq, J=17.7Hz), 4.11 (2H, q, J=7.OHz), 4.77
WO94/10177 21 4 7 6 0 ~ PCT/JP93/01505
- 83 -
and 4.87 (2H, ABq, J=11.8Hz), 5.26 (lH, d,
J=4.8Hz), 5.82 (lH, dd, J=8.2Hz, 4.8Hz), 6.43
(lH, d, J=12.lHz), 6.57 (lH, d, J=12.lHz), 6.65
(lH, s), 6.93 (lH, t, J=7.7Hz), 7.12 (2H, br s),
7.29-7.36 (lH, m), 7.62-7.66 (lH, m), 8.46 (2H,
m), 9.51 (lH, d, J=8.2Hz), 11.30 (lH, s)
(32) l-(Ethoxycarbonyloxy)ethyl 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1740, 1650 cm 1
NMR (DMSO-d6, ~) : 1.16 and 1.22 (total 3H, each t,
J=7.1Hz), 1.33 and 1.44 (total 3H, each d,
J=5.4Hz), 3.25 and 3.64 (2H, ABq, J=17.8Hz),
4.13 and 4.14 (total 2H, each q, J=7.1Hz), 5.28
and 5.29 (total lH, each d, J=4.8Hz), 5.83-5.92
(lH, m), 6.45-6.74 (3H, m), 6.65 (lH, s), 7.12
(2H, br s), 7.31-7.37 (lH, m), 7.64-7.67 (lH,
m), 8.44-8.52 (2H, m), 9.51 and 9.53 (total lH,
each d, J=8.2Hz), 11.30 (lH, s)
(33) l-Acetoxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)vinyl~-
3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 174C, 1650 cm
NMR (DMSO-d6, ~) : 1.30 and 1.40 (total 3H, each d,
J=5.5Hz), 2.01 and 2.02 (total 3H, each s), 3.27
and 3.64 (2H, ABq, J=16.9Hz), 5.27 and 5.28
(total lH, each d, J=8.6Hz), 5.83-5.92 (lH, m),
6.42-6.84 (3H, m), 6.65 (lH, s), 7.12 (2H, br
s), 7.31-7.38 (lH, m), 7.64-7.68 (lH, m),
8.45-8.46 (2H, m), 9.51 and 9.52 (total lH, each
d, J=8.2Hz), 11.30 (lH, s)
(34) 1-(Cyclopentylo~carbonyloxy)ethyl 7~-~2-(2-
W094/10177 PCT/JP93/OlS'
9 - 84 -
6~ -
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer) ~
IR (Nujol) : 1740, 1660 cm
NMR (DMSO-d6, ~) : 1.33 and 1.44 (total 3H, each d,
J=5.4Hz), 1.50-1.80 (lOH, m), 3.25 and 3.63 (2H,
ABq, J=18.0Hz), 4.97-5.03 (lH, m), 5.27 and 5.29
~total lH, each d, J=4.9Hz), 5.83-5.92 (lH, m),
6.45-6.73 (3H, m), 6.64 (lH, s), 7.13 (2H, br
s), 7.36-7.38 (lH, m), 7.64-7.67 (lH, m),
8.44-8.50 (2H, m), 9.52 and 9.54 (total lH, two
d, J=8.2Hz), 11.31 (lH, s)
(35) l-(Isopropyloxycarbonyloxy)ethyl 7~-~2-(2-amino-
thiazol-4-yl)-2-hydroxyiminoacetamido]-3-[tZ)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1740, 1660 cm
NMR (DMSO-d6, ~) : 1.21-1.26 (6H, m), 1.33 and 1.44
(total 3H, two d, J=5.4Hz), 3.25 and 3.63 (2H,
ABq, J=18.0Hz), 4.72-4.81 (lH, m), 5.27 and 5.29
(total lH, two d, J=4.9Hz), 5.83-5.92 (lH, m),
6.45-6.S7 (3H, m), 6.65 ~lH, s), 7.12 (2H, br
s), 7.31-7.37 (lH, m), 7.63-7.66 (lH, m),
8.43-8.50 (2H, m), 9.51 and 9.53 (total lH, two
d, J=8.2Hz), 11.30 (lH, s)
(36) l-(Cyclohexyloxycarbonyloxy)ethyl 7~-~2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
~pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1740, 1655 cm 1
NMR (DMSO-d6, ~) : 1.14-1.87 (lOH, m), 1.34 and 1.45
(total 3H, each d, J=5.2Hz), 3.25 and 3.63 (2H,
AB~, J=18.lHz), 4.48-4.59 (lH, m), 5.24-5.30
2147609
WO94/10177 PCT/JP93/0150~
-
- 85 -
(lHr m), 5.83-5.92 (lH, m), 6.48-6.74 (3H, m),
6.64 (lH, s), 7.12 (2H, br s), 7.29-7.38 (lH,
m), 7.63-7.67 (lH, m), 8.42-8.48 (2H, m), 9.51
and 9.53 (total lH, each d, J=8.2Hz), 11.30 (lH,
- 5 s)
Example 17
The following compounds were obtained according to
similar manners to those of Examples 4, 8 and 12.
(1) 1-(2-Ethylbutyryloxy)ethyl 7~-[2-(2-aminothiazol-4-
yl)-2-trityloxyiminoacetamido]-3-l(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1792, 1757, 1697 cm 1
NMR (DMSO-d6, ~) : 0.7-0.9 (6H, m), 1.3-1.6 (7H, m),
2.1-2.2 (lH, m), 3.40 and 3.65 (2H, ABq,
J=18Hz), 5.36 (lH, d, J=5Hz), 6.0-6.1 (lH, m),
6.4-6.9 (4H, m), 7.1-7.4 (18H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 9.9-10.0 (lH, m)
(2) l-Cyclopentylcarbonyloxyethyl 7~-12-(2-tritYlamino-
thiazol-4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl~-3-cephem-4-carboxylate (syn
isomer)
IR (Nujol) : 1770, 1725, 1665 cm 1
NMR (DMSO-d6, ~) : 1.32 and 1.44 (total 3H, d,
J=5Hz), 1.4-1.9 (8H, m), 2.7-2.8 (lH, m), 3.30
and 3.61 (2H, ABq, J=18Hz), 5.32 (lH, d, J=5Hz),
5.8-5.9 (lH, m), 6.5-6.9 (4H, m), 7.1-7.3 (31H,
m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 8.78 (lH,
br s), 9.9-10.0 (lH, m)
(3) l-Cyclohexylcarbonyloxyethyl 7~-12-(2-aminothiazol-4-
yl)-2-trityloxyiminoacetamido~-3-l(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
W094/10177 PCT/JP93iO15f
86 -
IR ~KBr) : 1790, 1751, 1683, 1620 cm 1
NMR (DMSO-d6, ~) : 1.1-1.9 (lOH, m), 1.32 and 1.44
(total 3H, d, J=5Hz), 2.1-2.3 (lH, m), 3.2-3.4
(lH, m~, 3.6-3.8 (lH,~ m), 5.36 and 5.37 (total
lH, d, J=5Hz), 6.0-6.1 (lH, m), 6.4-6.9 (4H, m),
7.1-7.4 (18H, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 9.94 and 9.96 (total lH, d, J=8Hz)
(4) l-Propionyloxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
trityloxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1788, 1755, 1682, 1618 cm 1
NMR (DMSO-d6, ~) : 0.9-1.1 (3H, m), 1.32 and 1.44
(total 3H, d, J=5Hz), 3.2-3.3 (lH, m), 3.6-3.7
(lH, m), 5.3-5.4 (lH, m), 6.0-6.1 (lH, m),
6.5-6.9 (4H, m), 7.1-7.3 (18H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 9.9-10.0 (lH, m)
(5) l-Valeryloxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
trytyloxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (~3r) : 1790, 1755, 1684, 1618 cm 1
NMR (DMSO-d6, ~) : 0.8-0.9 (3H, m), 1.2-1.6 (7H, m),
2.2-2.3 (2H, m), 3.2-3.4 (lH, m), 3.66 (lH, d,
J=18Hz), 5.3-5.4 (lH, m), 6.0-6.1 (lH, m),
6.4-6.9 (4H, m), 7.1-7.4 (18H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 9.9-10.0 (lH, m)
(6) l-(l-Propylbutoxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
NMR (DMSO-d6, ~) : 0.7-0.9 (6~, m), 1.1-1.6 (llH,
m), 3.2-3.4 (lH, m), 3.5-3.7 (lH, m), 4.65 (lH,
m), 5.36 (lH, d, J=5Hz), 6.0-6.1 (lH, m),
WO94/10177 PCT/JP93/01505
-- 21478609
6.5-6.7 ~4H, m), 7.2-7.4 (18H, m), 7.6-7.7 ~lH,
m), 8.4-8.5 (2H, m), 9.94 and 9.96 (total lH, d,
J=8Hz)
- 5 (7) l-Butyloxycarbonyloxyethyl 7~-~2-(2-aminothiazol-4-
yl)-2-trityloxyiminoacetamido~-3-[(Z)-2-(pyridin-3-
yl)vinyl~-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1788, 1765, 1684, 1620 cm
NMR (DMSO-d6, ~) : 0.82 and 0.88 (total 3H, t,
J=7Hz), 1.2-1.7 (4H, m), 1.36 and 1.49 (total
3H, d, J=5Hz), 3.2-3.4 (lH, m), 3.65 (lH, d,
J=18Hz), 4.0-4.1 (2H, m), 5.35 and 5.37 (total
lH, d, J=5Hz), 6.0-6.1 (lH, m), 6.5-6.8 (4H, m),
7.1-7.4 (18H, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 9.94 and 9.95 (total lH, d, J=8Hz)
(8) 1-(2,2-Dimethylpropoxycarbonyloxy)ethyl 7~-12-(2-
aminothiazol-4-yl-2-trityloxyiminoacetamido]-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (KBr) : 1790, 1763, 1684, 1618 cm
NMR (DMSO-d~, ~) : 0.87 and 0.90 (total 9H, s), 1.38
and 1.50 (total 3H, d, J=5Hz), 3.2-3.4 (lH, m),
3.65 (lH, d, J=18Hz), 3.82 (2H, s), 5.35 (lH, d,
J=5Hz), 6.0-6.1 ~lH, m), 6.4-6.8 (4H, m),
7.1-7.3 (18H, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 9.94 and 9.95 (total lH, d, J=8Hz)
(9) l-(l-Methylbutoxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (KBr) : 1790, 1759, 1684, 1618 cm
NMR (DMSO-d6, ~) : 0.7-1.5 (13H, m), 3.2-3.3 (lH, m),
3.64 (lH, d, J=18Hz), 4.6-4.8 (lH, m), 5.36 (lH,
~4~ PCr/JP93/015~
- 88 -
d, J=5Hz), 6.0-6.1 ~lH, m), 6.4-6.8 (4H, m),
7.2-7.4 (18H, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 9.9-10.0 (lH, m)
(10~ 1-(4-Methylpentancyloxy)ethyl 7~-[2-(2-aminothiazol-
4-yl)-2-trityloxyiminoacetamido]-3-[(Z)-2-(pyridin-
3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1791, 1751, 1683, 1618 cm
NMR (DMSO-d6, ~) : 0.7-0.9 (6H, m), 1.2-1.6 (6H, m),
2.2-2.4 (2H, m), 3.2-3.4 (lH, m), 3.65 (lH, d,
J=18.1Hz), 5.2-5.4 (lH, m), 5.9-6.1 (lH, m),
6.4-5.9 (4H, m), 7.1-7.4 (18H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 9.8-10.0 (lH, m)
(11) l-(Butyryloxy)ethyl 7~-r2-(2-aminothiazol-4-yl)-2-
trityloxyiminoacetamido]-3-[(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1787, 1758, 1681, 1619 cm
NMR ~DMSO-d6, ~) : 0.7-0.9 (3H, m), 1.32 and 1.44
(3H, d, J=5.4Hz), 1.4-1.6 (2H, m), 2.2-2.3 (2H
n), 3.2-3.4 (2H m), 3.66 (lH, d, J=18.5Hz),
5.3-5.4 (lH, m), 5.9-6.1 (lH, m), 6.4-6.8 (4H,
m), 7.1-7.4 (18H, m), 7.6-7.7 (lH, m), 8.4-8.5
(2H, m), 9.9-10.1 (lH, m)
~12) 1-(2-Ethylbutoxycarbonyloxy)ethyl 73-[2-(2-
aminothiazol-4-yl)-2-trityloxyiminoacetamido~-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
lR (KBr) : 1789, 1764, 1681, 1619 cm
NMR (DMSO-d6, ~) : 0.7-0.9 (6H, m), 1.1-1.6 (8H, m),
3~2-3.4 (lH, m), 3.65 (lH, d, J=18.1Hz), 3.9-4.1
(2H, m), 5.2-5.4 (lH, m), 5.9-6.1 (lH, m),
6.4-6.8 (4H, m), 7.1-7.4 (18H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 9.9-10.1 (lH, m)
WO94/10177 21 ~ 7 6 0 9 PCT/JP93/01505
-
- 89 -
~13~ l-(Propoxycarbonyloxy)ethyl 7~-[2-(2-aminothiazol-4-
yl)-2-trityloxyiminoacetamido]-3-~5Z)-2-(pyridin-3-
yl-)vinyl}-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1791, 1762, 183, 1616, 1540 cm
NMR (DMSO-d6, ~) : 0.83, 0.88 (3H, t, J=7.4Hz),
1.36, 1.49 (3H, d, J=5.4Hz), 3.2-3.4 (lH, m),
3.65 (lH. d, J=17.8Hz), 4.05 (2H, m) ! 5.34-5.38
(lH, m~, 5.95-6.10 (lH, m), 6.40-6.80 (4H, m),
7.1-7.4 (18H, m), 7.6-7.7 (lH, m), 8.4-8.5 52H,
m), 9.85-10.0 (lH, m)
(14) l-lCyclobutylmethyloxycarbonyloxy)ethyl 7~-~2-(2-
aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-
~(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR ~KBr) : 1786, 1763, 1684, 1618 cm
NMR 5DMSO-d6, ~) : 1.36 and 1.49 5total 3H, d,
J=5Hz), 1.6-2.1 56H, m), 2.5-2.6 (lH, m),
3.2-3.4 ~lH, m), 3.65 51H, d, J=18Hz), 5.36 and
5.37 (total lH, d, J=5Hz), 6.0-6.1 51H, m),
6.5-6.8 (4H, m), 7.2-7.4 (18H, m), 7.6-7.7 (lH,
m), 8.4-8.5 (2H, m), 9.95 51H, d, J=8Hz)
515) 1-51-Ethylpropoxycarbonyloxy)ethyl 7~-[2-
acetoxyimino-2-(2-aminothiazol-4-yl)acetamido~-3-
[~Z)-2-pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
(syn isomer)
IR 5KBr) : 1766.5, 1762.6, 1718.6, 1683.6 cm 1
NMR (DMSO-d6, o) : O.83 (6H, t, J=7.4Hz), 1.34 and
1.46 ~3H, two d, J=5.4Hz), 1.45-1.56 54H, m),
2.14 (3H, s), 3.27 and 3.68 (2H, ABq, J=18.3Hz),
4.49 (lH, m), 5.32 and 5.33 (lH, two d,
J=4.9Hz), 5.90 and 5.91 51H, two dd, J=8.1Hz,
4.9Hz), 6.45-6.72 53H, m), 7.04 and 7.07 (lH,
two s), 7.32-7.36 (3H, m), 7.63-7.72 (lH, m),
W094/10177 PCT/JP93iO15~-
~ 6~9 - go -
8.45-8.47 (2H, m), 9.93 and 9.95 (lH, two d,
J=8.lHz)
Example 18
i The following compounds were obtained according to
similar manners to those of Examples 5 and 15.
(1) 1-(2-Ethylbutyryloxy)ethyl 7~-~2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido}-3-~(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1786, 1776, 1749, 1670 cm
NMR (DMSO-d6, ~) : 0.7-0.9 (6H, m), 1.3-1.7 (7H, m),
2.15 (lH, m), 3.2-3.7 (2H, m), 5.29 (lH, d,
J=5Hz), 5.8-5.9 (lH, m), 6.4-6.9 (4H, m), 7.12
(2H, s), 7.3-7.4 (lH, m), 7.6-7.7 (lH, m),
8.4-8.5 (2H, m), 9.S2 and 9.54 (total lH, d,
J=8Hz), 11.3 (lH, s)
(2) l-Cyclopentylcarbonyloxyethyl 7~-[2-(2-aminothiazol-
4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1784, 1753, 1678, 1620 cm
NMR (DMSO-d6, ~) : 1.30 and 1.41 (total 3H, d,
J=5Hz), 1.4-1.9 (8H, m), 2.6-2.8 (lH, m), 3.28
and 3.65 i2H, ABq, J=18Hz), 5.28 and 5.29 (total
lH, d, J=5Hz), 5.8-5.9 (lH, m), 6.4-6.9 (4H, m),
7.13 (2H, br s), 7.2-7.3 (lH, m), 7.6-7.7 (lH,
m), 8.3-8.4 (2H, m), 9.51 ana 9.53 (total lH, d,
J=8Hz), 11.3 (lH, s)
~0
(3) l-Cyclohexylcarbonyloxyethyl 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
I~ (KBr) : 1782, 1751, 1674, 1616 cm 1
NMR (DMSO-d6, ~) : 1.1-1.9 (lOH, m), 1.30 and 1.40
WO94/10177 21 ~ 7 6 0 9 PCT/JP93/01505
-
- 31 -
(total 3H, d, J=5Hz), 2.1-2.3 (lH, m), 3.24 and
3.65 (2H, ABq, J=18Hz), 5.28 and 5.29 (total lH,
d, J=5Hz), 5.8-6.0 (lH, m), 6.4-6.8 (4H, m),
7.13 (2H, br s), 7.3-7.4 (lH, m), 7.6-7.7 (lH,
m), 8.4-8.5 (lH, m), 9.52 and 9.53 (total lH, d,
J=8Hz), 11.3 (lH, s)
(4) l-Propionyloxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
hydroxyiminoacetamido~-3-~(Z)-2-(pyridin-3-yl)vinyl]-
3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1778, 1757, 1678, 1620 cm
NMR (DMSO-d6, ~) : 1.0-1.1 (3H, m), 1.3 and 1.40
~total 3H, d, J=5Hz), 2.30 (2H, q, J=7Hz),
3.2-3.3 (lH, m), 3.6-3.7 (lH, m), 5.2-5.3 (lH,
m), 5.8-5.9 (lH, m), 6.4-6.9 (4H, m), 7.12 (2H,
br s), 7.3-7.4 (lH, m), 7.6-7.7 (lH, m), 8.4-8.5
(2H, m), 9.51 and 9.52 (total lH, d, J=8Hz),
11.3 (lH, s)
(5) l-Valeryloxyethyl 7~-[2-(2-aminothiazol-4-yl)-2-
hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)vinyl~-
3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1782, 1759, 1670, 1618 cm
NMR (DMSO-d6, ~) : 0.84 (3H, t, J=7Hz), 1.2-1.6 (4H,
m), 1.30 and 1.40 (total 3H, d, J=5Hz), 2.28
(2H, t, J=6Hz), 3.2-3.3 (lH, m), 3.64 (lH, d,
J=18Hz), 5.28 and S.29 (total lH, d, J=5Hz),
5.8-5.9 (lH, m), 6.4-6.9 (4H, m), 7.13 (2H, br
s), 7.3-7.4 (lH, m), 7.6-7.7 (lH, m), 8.4-8.5
(2H, m), 9.51 and 9.53 (total lH, d, J=8Hz),
11.3 (lH, s)
! 6) l-(l-Propylbutyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido~-3-[(Z)-
2-(pyridin-3-yl)vinyl~-3-cephem-4-carboxylate (syn
WO94/10177 PCT/JP93/015~-
- 92 -
6~9
isomer)
IR ~KBr) : 1784, 1759, 1674, 1618 cm
NMR (DMSO-d6, ~) : 0.8-0.9 (6H, m), 1.2-1.6 (llH,
m), 3.2-3.3 (lH, m), 3.6-3.7 (lH, m), 4.6-4.7
(lH, m), 5.27 (lH, d, J=5Hz), 5.8-5.9 (lH, m),
6.4-6.7 (4H, m), 7.14 (2H, br s), 7.3-7.4 (lH,
m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 9.51 and
9.53 (total lH, d, J=8Hz), 11.3 (lH, s)
(7) l-Butyloxycarbonyloxyethyl 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido~-3-[~Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1767, 1678, 1620 cm
NMR (DMSO-d6, ~) : O.87 (3H, t, J=7Hz), 1.2-1.7 (7H,
m), 3.2-3.4 (lH, m), 3.64 (lH, d, J=18Hz), 4.09
and 4.10 (total 2H, t, J=6Hz), 5.27 and 5.29
(total lH, d, J=5Hz), 5.8-5.9 (lH, m), 6.4-6.8
(4H, m), 7.13 (2H, br s), 7.2-7.4 (lH, m),
7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 9.51 and 9.53
(total lH, d, J=8Hz), 11.3 (lH, s)
(8) 1-(2,2-Dimethylpropoxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (KBr) : 1767, 1674, 1616 cm 1
NMR (DMSO-d6, ~) : 0.89 (9H, s), 1.36 and 1.46
(total 3H, d, J=5Hz), 3.2-3.3 (lH, m), 3.63 (lH,
d, J=18Hz), 3.81 and 3.82 (total 2H, s), 5.27
and 5.29 (total lH, d, J=5Hz), 5.8-5.9 (lH, m),
6.4-6.8 (4H, m), 7.12 (2H, br s), 7.3-7.4 (lH,
m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 9.51 and
9.52 (total lP., d, J=8Hz), 11.3 (lH, s)
(9) l-(l-Methylbutoxycarbonyloxy)ethyl 7~-[2-(2-
wo g4,l0l77 2 1 4 7 6 0 9 PCT/JP93/OlSOS
-
- 93 -
aminot~iazol-4-yl)-2-hydroxyiminoacetamido]-3- r ( z ) -2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (KBr) : 1780, 1759, 1673, 1616 cm
S NMR (DMSO-d6, ~) : 0.8-0.9 (3H, m), 1.1-1.6 (lOH,
mj, 3.2-3.3 (lH, d, J=18Hz), 4.6-4.8 (lH, m),
5.2-5.3 (lH, m), 5.8-5.9 (lH, m), 6.4-6.8 (4H,
m), 7.13 (2H, br s), 7.2-7.4 (lH, m), 7.6-7.7
(lH, m), 8.4-8.5 (2H, m), 9.51 and 9.53 (total
lH, d, J=8Hz), 11.3 (lH, s)
(10) 1-(4-Methylpentanoyloxy)ethyl 7~-~2-(2-aminothiazol-
4-yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 3338, 1789, 1751, 1670, 1616 cm 1
NMR (DMSO-d6, ~) : 0.83 (6H, d, J=6.3Hz), 1.3-1.6
(6H, m), 2.28 (2H, t, J=7.6Hz), 3.2-3.4 (lH, m),
3.64 (lH, d, J=17.9Hz), 5.2-5.3 (lH, m), 5.8-6.0
(lH, m), 6.4-6.8 (4H, m), 7.12 (2H, br s),
7.2-7.4 (2H, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 9.4-9.6 (lH, m)
(11) l-(Butyryloxy)ethyl 7~-~2-(2-aminothiazol-4-yl)-2-
hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-3-yl)vinyl]-
3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 3411, 1780, 1758, 1670, 1618 cm
NMR (DMSO-d6, ~) : 0.7-1.0 (3H, m), 1.30 and 1.40
(3H, d, J=5.5Hz), 1.2-1.6 (2H, m), 2.2-2.4 (2H,
m), 3.2-3.4 (lH, m), 3.65 (lH, d, J=18.OHz),
5.2-5.4 (lH, m), 5.8-6.0 (lH, m), 6.4-6.9 (4H,
m~, 7.13 (2H, br s), 7.1-7.4 (2H, m), 7.6-7.7
(lH, m), 8.4-8.6 (2H, m), 9.4-9.6 (lH, m), 11.3
(lH, s)
(12) 1-(2-Ethylbutoxycarbonyloxy)ethyl 7~-[2-(2-
wo 94/10~9 Pcr/JPg3/o15r^
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (KBr) : 1762, 1683, 1616 cm
NMR (DMSO-d6, ~) : 0.84 (6H, t, J=7.4Hz), 1.1-1.6
(8H, m), 3.2-3.4 (lH, m), 3.63 ~lH, d,
J=17.9Hz), 4.02 (2H, d, J=5.6Hz), 5.2-5.3 (lH,
m), 5.8-5.9 (lH, m), 6.4-6.8 (4H, m), 7.12 (2H,
br s), 7.2-7.4 (2H, m), 7.6-7.7 (lH, m), 8.4-8.6
(2H, m), 9.51 and 9.53 (lH, d, J=8.3Hz), 11.29
(lH, s)
i13) l-(Propoxycarbonyloxy)ethyl 7~-~2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido~-3-~(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1762, 1675, 1616, 1533 cm 1
NMR (DMSO-d6, ~) : 0.87 (3H, t, J=7.4Hz), 1.34 and
1.45 (3H, d, J=5.4Hz), 1.4-1.7 (2H, m), 3.2-3.3
(lH, m), 3.64 (lH, d, J=17.9Hz), 4.05 (2H, m),
5.26-5.30 (lH, m), 5.8-6.0 (lH, m), 6.4-6.8 (4H,
m), 7.13 (2H, br s), 7.2-7.4 (lH, m), 7.6-7.7
(lH, m), 8.4-8.5 (2H, m), 9.51 and 9.53 (lH, d,
J=8.3Hz), 11.30 (lH, s)
(14) l-(Cyclobutylmethyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-
2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer)
IR (KBr) : 1762, 1674, 1620 cm
NMR (DMSO-d6, ~) : 1.34 and 1.44 (total 3H, d,
J=5Hz), 1.6-2.1 (6H, m), 2.5-2.7 (lH, m),
3.2-3.7 (2H, m), 4.07 and 4.08 (2H, d, J=7Hz),
5.27 and 5.29 (total lH, d, J=5Hz), 5.8-5.9 (lH,
m), 6.4-6.8 (4H, m), 7.15 (2H, br s), 7.2-7.4
(lH, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H, m), 9.52
WO94/10177 214 7 6 0 9 PCT/JP93/01505
- 95 -
and 9.S3 (total lH, d, J=8Hz), 11.3 (lH, s)
Example 19
To a solution of 7~-amino-3-[(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylic acid dihydrochloride
(2 g) and N-(trimethylsilyl)acetamide (6.98 g) in
dichloromethane (40 ml) was added 2-acetoxyimino-2-(2-
aminothiazol-4-yl)acetylchloride hydrochloride (syn
isomer) (1.81 g) under ice-cooling. After being stirred
for 1 hour at the same temperature,the mixture was poured
into diisopropyl ether. The resulting precipitate was
collected by filtration, dissolved in water, and adjusted
to pH 4 with an aqueous sodium bicarbonate solution. The
solution was subjected to Diaion HP-20, and the desired
compound was eluted with 20% isopropyl alcohol aqueous
solution. The fractions containing the object compound
were collected, evaporated in vacuo to remove isopropyl
alcohol, and lyophilized to give 7~-[2-acetoxyimino-2-
(2-aminothiazol-4-yl)acetamido]-3-[(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylic acid (syn isomer).
IR (~3r) : 1772.3, 1683.6 cm 1
NMR (DMSO-d6, ~) : 2.14 (3H, m), 3.22 and 3.60 (2H,
ABq, J=17.7Hz), 5.29 (lH, d, J=4.8Hz), 5.85 (lH,
dd, J=8.0Hz, 4.8Hz), 6.55 (lH, d, J=11.6Hz),
6.62 (lH, d, J=11.6Hz), 7.07 (lH, s), 7.31-7.35
(3H, m), 7.63-7.72 (lH, m), 8.43-8.46 (2H, m),
9.93 (lH, d, J=8.0Hz)
Example 20
To a solution of l-(l-ethylpropoxycarbonyloxy)ethyl
7~-[2-acetoxyimino-2-(2-aminothiazol-4-yl)acetamido]-3-
[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn
isomer) (100 mg) in methanol (1 ml) was added concentrated
hydrochloric acid (32.8 ~l) at room temperature. After
being stirred for 1 hour at the same temperature. The
WO94/10177 ~ ` PCT/JP93/015~'
~ 6~9 - 96 -
mixture was poured into a mixture of ethyl acetate and
water. The separated organic layer was washed with brine,
dried over magnesium sulfate, and evaporated in vacuo to
give 1-(1-ethylpropoxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
(62.9 mg).
IR tNujol) : 3300, 1770, 1740, 16S0, 1600, 1510 cm
NMR (DMSO-d6, ~) : 0.7-0.9 ~6H, m), 1.35 and 1.45
(total 3H, d, J=5Hz), 1.4-1.6 (4H, m), 3.24 and
3.28 (total lH, d, J=18Hz), 3.62 (lH, d,
J=18Hz), 4.51 (lH, m), 5.27 (lH, d, J=5Hz), 5.87
(lH, m), 6.4-6.7 (4H, m), 7.15 (2H, br s),
7.2-7.4 (lH, m), 7.6-7.7 (lH, m), 8.4-8.5 (2H,
m), 9.52-9.54 (total lH, d, J=8Hz), 11.3 (lH, s)
Example 21
To a solution of 2-(2-aminothiazol-4-yl)-2-
trityloxyiminoacetic acid (syn isomer) (1.14 g) in
N,N-dimethylacetamide (20 ml) was added portionwise
potassium carbonate (367 mg) under ice-cooling. After
stirring for one hour at the same temperature, at 3-10C,
the mixture was added to the solution prepared by adding
dropwise bis(trimethylsilyl)acetamide (4.62 ml) to a
suspension of 7~-amino-3-[(Z)-2-(pyridin-3-yl)vinyl~-3-
cephem-4-carboxylic acid dihydrochloride (1.0 g) in
dichloromethane (30 ml) at ambient temperature, stirring
for 30 minutes and then cooling to 3-5C with ice cooling.
After stirring for 3 hours, the mixture was evaporated and
the residue was added dropwise to water. The precipitate
was filtered, washed with water and solved in
tetrahydrofuran. The solution W2S dried over magnesium
sulfate and evaporated under reduced pressure. The
residue was column chromatographed on silica gel
(dichloromethane:methanol = (4:1 - 3:1)) to give
WO94/10177 21 ~ 7 6 0 9 PCT/JP93/01505
~.
- 97 -
7~-~2-(2-aminothiazol-4-yl)-2-trityloxyiminoacetamido]-
3-~(Z)-2-pyridin-3-yl)vinyl]-3-cephem-4-carboxylic acid
(syn isomer) (12.6 g).
IR (Nujol) : 1725, 1650 cm 1
, NMR (DMSO-d6, ~) : 2.95 and 3.34 (2H, ABq,
J=17.0Hz), 5.21 (lH, d, J=4.9Hz), 5.82 (lH, dd,
J=8.2 and 4.9Hz), 6.43 (lH, d, J=12.2Hz), 6.58
(lH, s), 6.89 (lH, d, J=12.2Hz), 7.25-7.30 (18H,
m), 7.65 (lH, d, J=8.3Hz), 8.42-8.46 (2H, m),
iO 9.90 (lH, d, J=8.2Hz)
Example 22
To a solution of l-(l-ethylpropoxycarbonyloxy)ethyl
7~-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-
[(Z)-2-(pyridin-3-yl)vinyl~-3-cephem-4-carboxylate (syn
isomer) (150 mg) in ethyl acetate (4.5 ml) was added 4N
hydrochloric acid in ethyl acetate (0.119 ml) at 5C. The
mixture was stirred at 5C for 1 hour. The precipitate in
the reaction mixture was collected by filtration, washed
with ethyl acetate and with diisopropyl ether, and dried
in vacuo to give l-(l-ethylpropoxycarbonyloxy)ethyl
7~-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-
[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
dihydrochloride (syn isomer) (110 mg).
IR (KBr) : 1782, 1753, 1672, 1632 cm 1
NMR (DMSO-d6, ~) : 0.7-1.0 (6H, m), 1.3-1.7 (7H, m),
3.41 and 3.77 (2H, ABq, J=18Hz), 4.4-4.6 (lH,
m), 5.3-5.4 (lH, m), 5.8-6.0 (lH, m), 6.6-6.9
(4H, m), 7.31 (lH, s), 7.8-7.9 (lH, m), 8.2-8.3
(lH, m), 8.6-8.8 (2H, m), 9.77 (lH, d, J=8Hz),
12.4 (lH, br s)
Example 23
The following compounds were obtained according to a
similar manner to that of Example 22.
WO94/10177 PCT/JP93/015~F
- 98 -
G~9
(1) (E)-2-(n-Butyloxycarbonyl)-2~-pentenyl 7~-~2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-~Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
dihydrochloride (syn isomer)
IR (KBr) : 1781.9, 1716.3, 1700.9, 1683.6 cm 1
NMR (DMSO-d6, ~) : 0.89 ~3H, t, J=7.1Hz), 0.98 (3H,
t, J=7.6Hz), 1.28-1.39 (2H, m), 1.54-1.62 ~2H,
m), 2.22-2.29 (2H, m), 3.36 and 3.75 (2H, ABq,
J=17.8Hz), 4.09 ~2H, t, J=6.1Hz), 4.77 and 4.84
(2H, ABq, J=11.6Hz), 5.34 ~lH, d, J=4.4Hz), 5.85
~lH, dd, J=7.7Hz, 4.4Hz), 6.59 (lH, d,
J=11.8Hz), 6.67 (lH, d, J=11.8Hz), 6.82 (lH, s),
6.95 (lH, t, J=7.6Hz), 7.7S-7.82 (lH, m),
8.15-8.19 (lH, m), 8.69-8.74 (2H, m), 9.74 (lH,
d, J=7.7Hz), 12.32 (lH, s)
(2) l-(Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
dihydrochloride (syn isomer)
IR (KBr) : 1782, 1757, 1670, 1632 cm 1
NMR (DMSO-d6, ~) : 1.1-2.0 (13H, m), 3.40 and 3.78
(2H, ABq, J=18Hz), 5.36 and 5.40 (total lH, d,
J=5Hz), 5.8-5.9 (lH, m), 6.8-6.9 (4H, m),
7.8-7.9 (iH, m), 8.2-8.3 (lH, m), 8.7-8.8 (2H,
m), 9.76 (lH, d, J=8Hz), 12.4 (lH, s)
(3) 1-(2-Ethylbutyryloxy)ethyl 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate dihydrochloride (syn
isomer)
IR (KBr) : 1782, 1749, 1670, 1630 cm 1
NMR (DMSO-d6, ~) : 0.7-0.9 (6H, m), 1.3-1.6 (7H, m),
2.0-2.2 (lH, m), 3.41 and 3.79 (2H, ABq,
J=18Hz), 5.38 (lH, d, J=5Hz), 5.8-5.9 (lH, m),
WO94/1017721 4 7 ~ O 9 PCT/JP93/01505
_ 99 _
6.7-6.8 (3H, m), 6.84 (lH, s), 7.8-7.9 (lH, m),
8.2-8.3 tlH, m), 8.7-8.9 (2H, m), 9.77 (lH, d,
J=8Hz), 12.41 (lH, s)
) 1-(3-Methylbutyryloxy)ethyl 7~-~2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-~pyridin-3-yl)-
viny~]-3-cephem-4-carboxylate dihydrochioride (syn
isomer)
IR (KBr) : 1784, 1753, 1676, 1632 cm 1
10NMR (DMSO-d6, ~) : 0.89 (6H, m), 1.32 and 1.40
~total 3H, d, J=5Hz), 1.8-2.1 (lH, m), 2.17 and
2.20 (total 2H, d, J=6Hz), 3.41 and 3.80 (2H,
ABq, J=18Hz), 5.37 and 5.39 (lH, m), 6.5-6.9
(4H, m), 7.8-7.9 (lH, m), 8.2-8.3 (lH, m),
8.7-8.8 (2H, m), 9.77 (lH, d, J=8Hz), 12.4 (lH,
s)
Example 24
~(RS)-(1-Ethylpropoxycarbonyloxy)ethyl] 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
(590 mg) was dissolved in acetonitrile:pH 3 phosphate
buffer (37:63) (120 ml). The mixture was subjected to
preparative HPLC utilizing a C18 ~ Bondapac resin
~5 (Trademark : Waters Associates, Inc.) and eluted with a
solvent system comprised acetonitrile:pH 3 phosphate
buffer (37:63) at a flow rate of 80 ml/minute using LC-8A
pump (Trademark: Shi m~ 7.U Corporation). The fractions
containing the more polar compound at retention time of
27.3 minutes were combined and evaporated in vacuo to
remove acetonitrile. The residue was adjusted to pH 7
with sodium bicarbonate aqueous solution, and extracted
with ethyl acetate. The extract was dried over magnesium
sulfate, and evaporated in vacuo to give the more polar
one (~ompound A) (134 mg) of two diastereomers ~i.e.,
WO94/10177 PCT/JP93/015~
- 100 -
6~g
- [(R)-l-(l-ethylpropoxycarbonyloxy)ethyl 7~-~2-(2-
aminothiazol-4-yl-)-2-hydroxyiminoacetamido]-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
and [(S)-1-(l-ethylprop~xycarbonyloxy)ethyl] 7~-~2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)].
The less polar one (Compound B) (121 mg) of the above
diastereomers at retention time of 31.2 minutes was
obtained according to a similar manner to that as
described for Compound A.
Compound A :
IR (KBr) : 1783.8, 1758.8, 1675.8, 1618.0 cm 1
NMR (DMSO-d6, ~) : 0.83 (6H, t, J=7.5Hz), 1.35 (3H,
d, J=5.4Hz), 1.52-1.60 (4H, m), 3.27 and 3.62
(2H, ABq, J=18.1Hz), 4.48-4.54 (lH, m), 5.27
(lH, d, J=4.9Hz), 5.85 (lH, dd, J=8.3Hz, 4.9Hz),
6.46 (lH, d, J=12.lHz), 6.59 (lH, d, J=12.lHz),
6.63 (lH, s), 6.64-6.72 (lH, m), 7.12 (2H, br
s), 7.32-7.38 (lH, m), 7.63-7.68 (lH, m), 8.46
(2H, m), 9.51 (lH, d, J=8.3Hz), 11.3 (lH, s)
~a]D : -436.2 (C=0.5, CHCl3)
Compound B :
IR (KBr) : 1783.8, 1758.8, 1675.8, 1654.6, 1618 cm 1
NMR (DMSO-d6, ~) : 0.83 (6H, t, J=7.3Hz), 1.45 (3H,
d, J=5.4Hz), 1.47-1.59 (4H, m), 3.23 and 3.61
(2H, ABq, J=18.0Hz), 4.45-4.52 (lH, m), 5.27
(lH, d, J=4.9Hz), 5.88 (lH, dd, J=8.2Hz, 4.9Hz),
6.52 (lH, d, J=12.1Hz), 6.63-6.68 (lH, m), 6.64
(lH, m), 6.67 (lH, d, J=12.1Hz), 7.11 (2H, s),
7.31-7.37 (lH, m), 7.61-7.65 (lH, m), 8.44-8.46
(2H, m), 9.52 (lH, d, J=8.2Hz), 11.3 (lH, s)
~a]23 : -546.6 (C=0.5, CHCl3)
WO94/10177 214 7 6 0 9 PCT/JP93/01505
- 101 -
Example 25
Two diastereomers [i.e.,
[(R)-l-(isovaleryloxy)ethyl] 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-l(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
and
[(S)-l-tisovaleryloxy)ethyl] 7~-[2-(2-aminothiazol-4-
yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)] were obtained
according to a similar manner to that of Example 24.
The more polar one (Compound C) of the above
diastereomers
IR (KRr) : 1789.6, 1751.0, 1733.7, 1683.6 cm 1
NMR (DMSO-d6, ~) : 0.88 (6H, d, J=6.6Hz), 1.30 (3H,
d, J=5.5Hz), 1.89-2.02 (lH, m), 2.17 (2H, d,
J=6.6Hz), 3.30 and 3.65 (2H, ABq, J=17.9Hz),
5.28 (lH, d, J=4.9Hz), 5.86 (lH, dd, J=8.2Hz,
4.9Hz), 6.43 (lH, d, J=12.1Hz), 7.14 (2H, s),
7.32-7.38 (lH, s), 7.64-7.68 (lH, m), 8.45-8.47
(2H, m), 9.42 (lH, d, J=8.2Hz), 11.3 (lH, s)
[a]D3 : ~457 0 (C=0.5, CHC13)
The less polar one (Compound D) of the above
diastereomers
IR (KBr) : 1781.9, 1749.1, 1683.6, 1670.1 cm
NMR (DMSO-d6, ~) : 0.87 (6H, d, J=7.1Hz), 1.41 (3H,
d, J=5.3Hz), 1.89-1.96 (lH, m), 2.16 (2H, d,
J=6.9Hz), 3.27 and 3.65 (2H, ABq, J=17.8Hz),
5.29 (lH, d, J=4.8Hz), 5.90 (lH, dd, J=8.3Hz,
4.8Hz), 6.49 (lH, d, J-12.1Hz), 6.65 (lH, s),
6.65 (lH, d, J=12.1Hz), 6.76-6.80 (lH, m), 7.13
(2H, s), 7.31-7.38 (lH, m), 7.63-7.67 (lH, m),
8.46 (2H, m), 9.53 (lH, d, J=8.3Hz), 11.3 (lH,
s)
WO94/10177 ; PCT/JP93/015~-
~4~6~9 - 102 -
,.
23
[a]D : ~545 4 (C=0.5, CHC13)
Example 26
Two diastereomers [i.e.
[(R)-1-(2-Ethylbutyryloxy)ethyl] 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
and
[(S)-1-(2-Ethylbutyryloxy)ethyl] 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-~(~)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
were obtained according to a similar manner to that of
Example 24.
The more polar one of the above diastereomers
IR (KBr) : 1786, 1749, 1676, 1620 cm 1
NMR (DMSO-d6, ~) : 0.80 (6H, t, J=7Hz), 1.30 (3H, d,
J=5Hz), 1.4-1.6 (4H, m), 2.15 (lH, m), 3.31 and
3.65 (2H, ABq, J=18Hz), 5.29 (lH, d, J=5Hz),
5.86 (lH, dd, J=5Hz, 8Hz), 6.43 (lH, d, J=12Hz),
6.60 (lH, d, J=12Hz), 6.65 (lH, s), 6.76 (lH, q,
J=5Hz), 7.16 (2H, br s), 7.36 (lH, dd, J=5Hz,
8Hz), 7.67 (lH, d, J=8Hz), 8.4-8.5 (2H, m), 9.53
(lH, d, J=8Hz), 11.3 (lH, s)
The less polar one of the above diastereomers
IR (KBr) : 1782, 1749, 1676, 1616 cm 1
NMR (DMSO-d6, ~) : 0.79 (6H, t, J=7Hz), 1.42 (3H, d,
J=5Hz), 2.15 (lH, m), 3.26 and 3.64 (2H, ABq,
J=18Hz), 5.29 (lH, d, J=5Hz), 5.87 (lH, dd,
J=5Hz, 8Hz), 6.50 (lH, d, J=12Hz), 6.65 (lH, s),
6.65 (lH, d, J=12Hz), 6.79 (lH, q, J=5Hz), 7.13
(2H, br s), 7.35 ~lH, dd, J=5Hz, 8Hz), 7.65 (lH,
d, J=8Hz), 8.4-8.5 (2H, m), 9.54 (lH, d, J=8Hz),
11.3 (lH, s)
WO94/10177 2 1 ~ 7 6 0 9 PCT/JP93/01505
- 103 -
Example 27
The hydrochloric acid salts of the compounds A and B
obtained in Example 24 were obtained according to a
similar manner to that of Example 22.
Bis (hydrochloric acid) salt of the compound A.
IR (KBr) : 1781.9, 1772.3, 1756.8, 1683.6,
1670.1 cm 1
Bis (hydrochloric acid) salt of the compound B.
IR (KBr) : 1789.6, 1772.3, 1751.0, 1683.6,
1670.1 cm~
Example 28
The hydrochloric acid salts of the compounds C and D
obtained in Example 25 were obtained according to a
similar manner to that of Example 22.
Bi~ (hydrochloric acid) salt of the compound C
IR (KBr) : 1781.9, 1772.3, 1756.8, 1683.6,
1670.1 cm~1
Bis (hydrochloric acid) salt of the compound D
IR (~3r) : 1789.6, 1772.3, 1751.0, 1683.6,
1670.1 cm 1
Example 29
Two diastereomers ~i.e.,
I(R)-1-Cyclohexyloxycarbonyloxyethyl] 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-~(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
and
[(S)-1-Cyclohexyloxycarbonyloxyethyl] 7~-~2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-l(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)]
W094/10177 PCT/JP93/015~- -
~ 6~9 - 104 -
were obtained according to a similar manner to that of
Example 24.
.
The more polar one (Compound E) of the above
diastereomers
IR ~KBr) : 1782, 1759, 1674, 1618 cm 1
NMR (DMSO-d6, ~) : 1.1-1.9 (lOH, m), 1.33 (3H, d,
J=5Hz), 3.28 and 3.63 (2H, ABq, J=18Hz), 4.54
(lH, m), 5.27 (lH, d, J=5Hz), 5.86 (lH, dd,
J=5Hz, 8Hz), 6.48 (lH, d, J=12Hz), 6.60 (lH, d,
J=12Hz), 6.73 (lH, q, J=5Hz) 7.13 (2H, br s),
7.35 (lH, dd, J=5Hz, 8Hz), 7.66 (lH, d, J=8Hz),
8.47 (2H, m), 9.52 (lH, d, J=8Hz), 11.3 (lH, s)
The less polar one (Compound F) of the above
diastereomers
IR (KBr) : 1782, 1757, 1674, 1618 cm 1
NMR (DMSO-d6, ~) : 1.1-1.9 (lOH, m), 1.44 (3H, d,
J=5Hz), 3.25 and 3.63 (2H, ABq, J=18Hz), 4.51
(lH, m), 5.29 (lH, d, J=5Hz), 5.89 (lH, dd,
J=5Hz, 8Hz), 6.51 (lH, d, J=12Hz), 6.65 (lH, s),
6.6-6.7 (2H, m), 7.13 (2H, br s), 7.3-7.4 (lH,
m), 7.64 (lH, d, J=8Hz), 8.3-8.6 (2H, m), 9.53
(lH, d, J=8Hz), 11.3 (lH, s)
2i
Example 30
The hydrochloric acid salts of the compounds E and F
obtained in Example 29 were obtained according to a
similar manner to that of Example 22.
Bis (hydrochloric acid) salt of the compound E
IR (KBr) : 1782, 1757, 1674, 1632 cm
NMR (DMSO-d6, ~) : 1.1-1.9 (lOH, m), 1.35 (3H, d,
J=5Hz), 3.38 and 3.74 (2H, ABq, J=18Hz), 4.55
(lH, m), 5.34 (lH, d, J=5Hz), 5.86 (lH, dd,
W094/10177 21 ~ 7 6 0 9 PCT/JP93/01505
- 1~5 -
J=5.8Hz~, 6.6-6.7 (3H. m),6.82 (lH, s), 7.77
~lH, dd, J=5Hz, 8Hz), 8.13 (lH, d, J=8Hz), 8.72
(2H, m), 9.74 (lH, d, J=8Hz), 12.3 (lH, s)
. Bis (hydrochloric acid) salt of the compound F
IR (KBr) : 1782, 1757, 1668, 1633 cm 1
NMR IDMSO-d6, ~) : 1.1-1.9 (lOH, m), 1.42 (3H, d,
J=5Hz), 3.39 and 3.78 (2H, ABq, J=18Hz), 4.4-4.6
(lH, m), 5.38 (lH, d, J=5Hz), 5.90 (lH, dd,
J=5Hz, 8Hz), 6.62 (lH, q, J=5Hz), 6.67 (lH, d,
J=12Hz), 6.76 (lH, d, J=12Hz), 6.83 (lH, s),
7.84 (lH, m), 8.19 (lH, d, J=8Hz), 8.6-8.8 (2~,
m), 9.76 (lH, d, J=8Hz), 12.3 (lH, s)
Example 31
To a suspension of l-(l-ethylpropyloxycarbonyloxy)-
ethyl 7~-amino-3-l(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylate oxalate (250 mg) in dichloromethane (20 ml)
was added dropwise bis(trimethylsilyl)acetamide (558 ml)
at an ambient temperature. The reaction mixture was
stirred for 30 minutes and cooled to 5C with ice-water
bath. 2-acetoxyimino-2-(2-aminothiazol-4-yl)acetyl
chloride hydrochloride (syn isomer) (141 mg) was added
portionwise to the solution at the same temperature.
After stirring for 30 minutes, the mixture was poured into
a mixture of water and ethyl acetate. The aqueous layer
was extracted with ethyl acetate. The combined organic
layer was washed with water and brine, dried over
magnesium sulfate and evaporated under reduced pressure to
give 1-~l-ethylpropyloxycarbonyloxy)ethyl 7~-[2-
acetoxyimino-2-(2-aminothiazol-4-yl)acetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl)-3-cephem-4-carboxylate (syn isomer)
(187 mg).
IR (KBr) : 1766.5, 1762.6, 1718.6, 1683.6 cm 1
NMR (DMSO-d6, ~) : 0.83 (6H, t, ~=7.4Hz), 1.34 and
W094/10177 PCT/JP93/01~r-
- 106 -
,6~9
1.46 (3H, two d, J=5.4Hz), 1.45-1.56 (4H, m),
2.14 (3H, s), 3.27 and 3.68 (2H, ABq, J=18.3Hz),
- 4.49 (lH, m), 5.32 and 5.33 (lH, two d,
J=4.9Hz), 5.90 and 5.91 (lH, two dd, J=8.1Hz,
4.9Hz), 6.45-6.72 (3H, m), 7.04 and 7.07 (lH,
two s), 7.32-7.36 (3H, m), 7.63-7.72 (lH, m),
8.45-8.47 (2H, m), 9.93 and 9.95 (lH, two d,
J=8.lHz)
Example 32
The following compound was obtained according to a
similar manner to that of Example 31.
l-Cyclohexyloxycarbonyloxyethyl 7~-[2-acetoxyimino-
2-(2-aminothiazol-4-yl)acetamide]-3-~(Z)-2-(pyridin-3-yl)-
vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (KBr) : 1759, 1670, 1619, 1537 cm
NMR (DMSO-d6, ~) : 1.0-2.2 (13H, m), 3.0-3.8 (2H,
m), 4.4-4.65 (lH, m), 5.2-5.4 (lH, m), 5.9-6.0
(lH, m), 6.4-6.8 (3H, m), 7.06 (lH, d,
J=4.17Hz), 7.2-7.6 (3H, m), 7.6-7.8 (lH, m),
8.47 (2H, br s), 9.9-10.0 (lH, m)
Example 33
The following compound was obtained according to a
similar manner to that of Example 20.
1-(Cyclohexyloxycarbonyloxy~ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido~-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
IR (Nujol) : 1740, 1655 cm 1
NMR (DMSO-d6, ~) : 1.14-1.87 (lOH, m), 1.34 and 1.45
(total 3H, each d, J=5.2Hz), 3.25 and 3.63 ~2H,
ABq, J=18.1Hz), 4.48-4.59 (lH, m), 5.24-5.30
(lH, m), 5.83-5.92 (lH, m), 6.48-6.74 (3H, m),
WO94/10177 2147609 PCT/JP93/01505
-
- 107 -
6.64 (lH, s), 7.12 (2H, br s), 7.29-7.38 (lH,
m), 7.63-7.67 (lH, m), 8.42-8.48 (2H, m), 9.51
- and 9.53 (total lH, each d, J=8.2Hz), 11.30 (lH,
s)
The following compounds can be obtained according to
similar manners to those of Examples mentioned above.
(1) Cyclohexyloxycarbonyloxymethyl 7~-[2-(2-aminothiazol-
4-yl)-2-hydroxyiminoacetamido~-3-[(Z)-2-(pyridin-3-
yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
(2) 1-(4-pentenyloxycarbonyloxy)ethyl
7~-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-
3-[(Z)-2-(pyridin-3-yl)vinyl]-3-cephem-4-
carboxylate (syn isomer)
i5 (3) 1-(2-propylvaleryloxy)ethyl 7~-[2-(2-aminothiazol-
4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-(pyridin-
3-yl)vinyl]-3-cephem-4-carboxylate (syn isomer)
(4) 1-(1-Ethylpropoxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate sulfate
(syn isomer)
(5) 1-(1-Ethylpropoxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
p-toluenesulfonate (syn isomer)
(6) 1-(Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate sulfate
(syn isomer)
(7) 1-(Cyclohexyloxycarbonyloxy)ethyl 7~-[2-(2-
aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-[(Z)-2-
(pyridin-3-yl)vinyl]-3-cephem-4-carboxylate
p-toluenesulfonate (syn isomer)