Note: Descriptions are shown in the official language in which they were submitted.
E499
~~~ 19/10
- 1 -
DESCRIPTION
1 (Technical Field]
The present invention relates to use of
nonsteroidal anti-estrogen compounds (hereinafter
referred to as nonsteroidal anti-estrogens) such as
toremifene, expected as a remedy for autoimmune
diseases.
The autoimmune diseases include collagen
diseases and the like. In light of affected parts by
the diseases, there are mentioned, for example,
degenerative diseases of supporting tissues and connec-
tive tissues; autoimmune degenerative diseases of
salivary glands, particularly Sjbgren~s disease;
autoimmune degenerative diseases of kidneys, particular-
ly systemic lupus erythematodes and glomerulonephritis;
autoimmune degenerative diseases of joints, particularly
rheumatoid arthritis; and sutoimmune degenerative
diseases of blood vessels such as generalized
necrotizing angitis and granulomatous angitis; and
multiple sclerosis.
[Background Art]
Immunosuppressants, nucleic acid antagonists,
antimetabolites, etc., are used in the medicinal treat-
ment of autoimmune diseases today. Anti-inflammatory
agents, anticoagulants, etc., are also used in the
- 2 -
1 symptomatic therapies of the diseases. The effects of
these agents are, however, not yet sufficient.
It is known that the immunosuppressants have
side effects of provoking diabetes, renal disorders,
infectious diseases, etc. Also the use of the nucleic
acid antagonist or antimetabolite is frequently
accompanied by side effects such as hepatic disorders
and medullary disorders. Thus the medicinal treatment
of autoimmune diseases is so far very insufficient.
It has been demanded to develop a remedy for
autoimmune diseases which acts on the immune system and
which has a function mechanism different fxom that of
conventional drugs for the diseases and less serious
side effects.
[Disclosure of Invention]
After intensive investigations made for the
purpose of finding the above-described remedy, the
present inventors have found that nonsteroidal anti-
estrogens have an excellent therapeutic effect on the
autoimmune diseases and thus, based on this finding,
completed the present invention.
The present invention relates to a remedy for
autoimmune diseases which comprises as active ingredient
a nonsteroidal anti-estrogen or a pharmaceutically
acceptable salt thereof.
2~.4~fi~~~~
- 3 -
1 [Brief Description of Drawings]
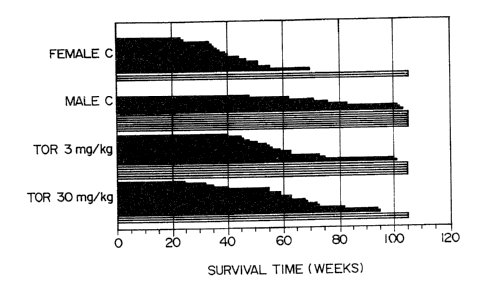
Fig. 1 shows survival times of animals
(NZBxNZW F1 mice: B/W F1 mice) which accepted different
doses of toremifene.
[Best Mode for Carrying Out the Invention]
The nonsteroidal anti-estrogen compounds
usable in the present invention are those having a
triphenyl CZ - C5 alkene or triphenyl C2 - C5 alkane
skeleton. Preferably, they are CZ - C5 alkenes or CZ - C5
alkanes having three phenyl substituents at the 1- .
position and 2-position, wherein any of the phenyl
groups may have a substituent such as a mono- or di-
lower alkyl ( C1 - C3 ) amino lower alkoxy ( G~ - C3 ) group,
or a hydroxyl group, or the alkyl group in the above
alkenes or alkanes may have a substituent such as a
halogen.
Examples of these compounds include toremifene
(JP-B-4 19973), tamoxifen (JP-B-59 21861), 4-hydroxy- ,
tamoxifen (JP-A-54 44644), 3-hydroxytamoxifen (JP-A-57
122049) and N-demethyltoremifene or 4-hydroxytoremifene
(JP-A-3 163015). Toremifene is particularly preferred.
,Ia is well-known that these compounds have an,anti~
neoplastic effect (see Cancer Chemotherapy and
Pharmacology, 17, 109-113 (1986) and the above-mentioned
patent publications).
The pharmaceutically acceptable salts thereof
include, for example, hydrochlorides, sulfates,
- 4 -
1 citrates, tartrates and phosphates.
Drugs usable in combination with the
nonsteroidal anti-estrogens in the medicinal treatment
of autoimmune diseases include glucocorticoids (e. g.
prednisoione, prednisone, cortisol). Prednisolone is
preferred.
The glucocorticoids themselves have an effect
of treating the autoimmune diseases. The nonsteroidal
anti-estrogens or a pharmaceutically acceptable salt
thereof according to the present invention concomitant
with the glucocorticoids synergistically improve the
effect of treating.
The remedy of the present invention
particularly exhibits an excellent remedial effect on
systemic lupus erythematodes.
Therefore the present invention relates to the
following:
(i) a remedy for autoimmune diseases which
comprises as active ingredient a nonsteroidal anti-
estrogen or a pharmaceutically acceptable salt thereof;
(ii) a remedy recited in (i), wherein the
nonsteroidal anti-estrogen is a compound having a
triphenyl Cz-C~ alkene or triphenyl C2-C5 alkane skeleton;
(iii) a remedy recited in (i) or (ii), wherein the
active ingredient is toremifene or a pharmaceutically
acceptable salt thereof;
(iv) a remedy recited in (i) or (ii), wherein the
autoimmune diseases are collagen diseases, autoimmune
21~~(~0
1 degenerative diseases of kidneys such as nephritis,
particularly glomerulonephritis, and autoimmune
degenerative diseases of blood vessels, salivary glands
and joints;
5 (v) a remedy recited in (i) or (ii), wherein the
autoimmune diseases are systemic lupus erythematodes;
and
(vi) a remedy recited in (i) or (ii) for
concomitant use with a glucocorticoid.
The pharmaceutical composition of the present
invention is administered orally, parenterally or
intravenously.
Usually, a pharmaceutically effective amount
of the active ingredient is used in combination with a
suitable medicinal carrier or other auxiliaries. The
term "pharmaceutically effective amount" herein means an
j
amount capable of exhibiting the intended pharmacolog-
i
ical activity without causing unfavorable side effects.
The accurate amount varies in each case depending on
various factors such as administration methods,
individual natures of the patients and situations in
which the.patient accepts the remedy and, as a matter of
course, structures of derivatives to be administered.
Dose of the active ingredient for adult is
usually 10 to 1000 mg/day, preferably 20 to 500 mg/day,
more preferably 30 to 300 mg/day.
In the case of the concomitant use, dose of
the glucocorticoid for adult is 1 to 100 mg/day,
2~.48~~
_ 6 _
1 preferably 2 to 60 mg, and that of the nonsteroidal
anti-estrogen or the pharmaceutically acceptable salt
thereof for adult is 10 to 700 mg/day, preferably 20 to
500 mg/day, more preferably 30 to 300 mg/day.
The medicinal carrier or other auxiliaries
generally usable in combination with the active
ingredient according to the present invention may be any
of solid and liquid ones and usually selected in
consideration of an administration route. Examples of
the solid carrier include lactose, sucrose, gelatin and
agar, and those of the liquid carrier include water,
syrup, peanut oil and olive oil. Other suitable
carriers and auxiliaries known by those skilled in the
art are also usable. The active ingredient according to
the present invention can be combined with the carrier
or other auxiliaries to form any of various acceptable
preparations such as tablets, capsules, suppositories,
liquid, emulsion and powder.
In the preparations of the remedy of the
present invention, the amount of the nonsteroidal anti-
estrogen or the pharmaceutically acceptable salt thereof
can widely vary depending on the preparation, etc.
Usually, the amount is 0.01 .., 100 by weight, preferably
0.1 ,..70~ by weight, and the balance contains the
medicinal carrier or other auxiliaries.
MRL/Mp-lpr/lpr mice spontaneously develop a
lethal glomerulonephritis, angitis, sialadenitis,
polyarthritis, etc., concurrently with the deposition of
2~~~~~
- 7 -
1 an immune complex with age. Therefore, they are widely
used as experimental models for human systemic lupus
erythematodes, Sjogren's disease, rheumatoid arthritis
and autoimmune angitis such as multiple arteritis.
The present invention will be explained
referring to examples on suppression of lymphadenopathy
glomerulonephritis, angitis, sialadenitis and arthritis
of MRL/Mp-lpr/lpr mice with the nonsteroidal anti-
estrogen compound according to the present invention.
The nonsteroidal anti-estrogen such as
toremifene and the pharmaceutically acceptable salt
thereof according to the present invention exhibit an
excellent remedial effect on degenerative diseases such
as autoimmune diseases, for example, systemic lupus
erythematodes.
Example 1
Treatment of spontaneous autoimmune diseases of MRL/Mp-
lpr/lpr mice by administration of 2f4-(Z)-4-chloro-1,2-
diphenvl-1-butenvl,]phenol-N,N-dimethvlethvlamine
citrate (toremifene citrate)
Eight-week old female MRL/Mp-lpr/lpr mice
(Clea Japan, Inc.) were used in this examination.
Toremifene citrate (JP-B-4 19973) was suspended in
carboxymethylcellulose to prepare a 0.5~ suspension.
This compound (100 mg/kg) was orally administered to
each mouse once a day for 13 weeks. ..
21~18~~
_8_
1 (A) Tnhibition of swelling of spleen and lymph
node of MRL/Mp-lpr/lpr mice with toremifene citrate
Repeated oral administration of 100 mg/kg of
toremifene citrate once a day for 13 weeks inhibited the
swelling of the spleen and lymph node of each mouse (see
Table 1).
The spleen and lymph nodes of the MRL/Mp-
lpr/lpr mice are seriously swollen with age due to the
presence of the lymphoproliferation gene (lpr). The lpr
codes for the Fas antigen in each mouse. However, in
the MRL/Mp-lpr/lpr mice, an abnormality of the genes
disturbs the expression of the Fas antigen. As a
result, autoreactive T-cells are not subjected to
negative selection through the Fas antigen in the thymus
and appear in the peripheral tissues to cause the
swelling of the lymphoid organs and autoimmune symptoms.
The presence of the autoreactive T-cells was confirmed
also in the autoimmune diseases of human beings, such as
rheumatoid arthritis.
The results of this study indicated that the
nonsteroidal anti-estrogen compounds such as toremifene
citrate are capable of inhibiting the appearance of the
,autoreactive T-cells, thereby suppressing the swel~.ing ,
of spleen and lymph node to treat the autoimmune
diseases.
y
- 9 -
Table 1: Effect of toremifene citratel~ on swelling
of spleen and lymph node MRL/Mp-lpr/lpr
mice
Number Spleen weight Lymph node
of 4~ 5~
Group animals Body weight weight
Body weight
Control 11 2.340.74 3~ 6.771.70
2~
Toremifene
citrate 12 1.381.06 3.111.43
treatment
1 1) Toremifene citrate (100 mg/kg) was orally
administered to 8-week old mice once a day for 13 weeks.
2) Only 0.5~ carboxymethylcellulose was given to the
mice of the control group.
3) Standard deviation
Weight of spleen X 100
4) Spleen weight/body weight = Body weight of
mouse
5) Lymph node weight/body = Weight of lymph node x 100
weight Body weight of mouse
(B) Suppression of renal disorder of MRL/Mp-
lpr/lpr mouse with toremifene citrate
An autopsy was performed on the mice of the
control group and the toremifene citrate treated group
after the completion of the administration to examine
their kidneys pathohistologically. The blood urea
nitrogen (BUN) of the serum in each group was examined
to confirm changes in the renal function. As shown in
21~~~~
- to -
1 Table 2, toremifene citrate ameliorated the
glomerulonephritis and healed the renal function in the
MRL/Mp-lpr/lpr mice.
The glomerulonephritis of the MRL/Mp-Ipr/lpr
mice is caused by the deposition of immunocomplexes.
Also in the case of the autoimmune diseases such as
systemic lupus erythematodes (SLE) of human, the
patients suffer from glomerulonephritis concurrent with
the deposition of the immunocomplex. The results
indicated that the nonsteroidal anti-estrogen compounds
such as toremifene citrate are effective remedies for
the degenerative diseases of the kidney, such as the SLE
with renal syndrome and glomerulonephritis.
Table 2: Improvement of renal function and amelioration
of glomerulonephritis of MRL/Mp-lpr/lpr mice
with toremifene citrate
Number Glomerulonephritis BUN
1~
Group of (mg/dl
)2~
animals
Control 11 2.4 0.7 3~ 43.123.9
Toremifene
citrate 12 1.2 0.7 24.614.9
treatment
1) The kidney was fixed in 10~ buffered formalin, and
then paraffin sections thereof were prepared by an
ordinary method to prepare HE and PAS stained
specimens. The extent of the disorder of the renal
~1~~QQ~
_ 11 _
1 glomeruli was scored and classified into the
following groupsn
0 (no disorder),
1 (slight disorder),
2 (medium disorder), and
3 (heavy disorder).
Twenty-five renal glomeruli were observed for each
mouse and the average thereof was calculated.
2) The BUi~ was determined with a Fuji Dry Chem
Analyzer.
3) Standard deviation.
(C) Inhibition by toremifene citrate of
sialadenitis an~c~itis and arthritis of MRL/Mp-lpr/lpr :.:.
mice
The salivary gland, renal blood vessel and knee
joint of each mouse in the control group and the
toremifene citrate treated group were histopathological-
ly examined.
As shown in Table 3, toremifene citrate
prevented the mice from being attacked by sialadenitis,
angitis and arthritis.
These results indicated that the nonsteroidal
,anti-estrogen compounds such as toremifene citrate and
tamoxifen citrate can be used as the remedy for
autoimmune sialadenitis (SjSgren's disease), autoimmune
arthritis (chronic articular rheumatism) and autoimmune
angitis (necrotizing angitis and granulomatous angitis).
21~~~0~~
- 12 -
Table 3: Effect of toremifene citrate for preventing
MR~/Mp-lpr/lpr mice from being attacked by
sialadenitis, angitis and arthritis
Number Sialadenitis Angitis Arthritis
Group of 1) 1) 1)
animals
Control 11 2.20.6 Z~ 2.10.7 1.60.9
Toremifene
citrate 12 0.90.8 0.90.8 0.40.5
treatment
1 1) The salivary gland, kidney and knee joint were
fixed in 10~ buffered formalin, and then paraffin
sections thereof were prepared by an ordinary method to
prepare HE and PAS stained specimens. The extent of the
disorder was scored and classified into the following
groups:
0 (no disorder),
1 (slight disorder),
2 (medium disorder), and
3 (heavy disorder).
2) Standard deviation.
~~~g~~.
- 13 -
1 Example 2
Effect of concomitant use of toremifene citrate with
alucocorticoid on MRL/Mp-lpr/lpr mice
Twelve-week old female MRL/Mp-lpr/lpr mice were
used in the examination. Thirty miligrams per kg or 15
mg/kg of toremifene citrate (TOR) was orally -,:
administered to each mouse twice a day for 9 weeks from
the 12th week to the 21st week. A glucocorticoid
(prednisolone), 8, 4 and 2 mg/kg/day, were subcutaneous-
ly administered to mice once a day as a positive control
drug. The concomitant use of tremifene with the
glucocorticoid was also carried out according to the
same regimen as above. The kidney was taken out from
each mouse the day after the completion of the whole
administration period and fixed in a PLP fixative.
Frozen sections were made from the fixed kidney and used
for an immunostaining with an anti-Mac-2 monoclonal
antibody (Hybritec Inc., San Diego, USA). The number of
Mac-2 positive cells (activated macrophages) invading
each of 10 to 20 glomeruli of the kidney, which is
hereinafter referred to as Mac 2 number, was counted
under a microscopy to determine an average Mac 2 number
per glomerulus. The degree of severeness of
glomerulonephritis was estimated in terms of the average
Mac 2 number (n = 13 for each group). Table 4 shows the
results.
- 14 ._2~~~~~~
Table 4: Suppression of glomerulonephritis of MRL/Mp-
lpr/lpr mice by concomitant use of toremifene
citxate with glucocorticoid
Group Mac 2 number
Control 7.5 ~ 1.5
Toremifene citrate (TOR) 30 mg/kg 6.2 ~ 1.0
15 mg/kg 6.5 ~ 1.2
Prednisolone (P) 8 mg/kg 5.8 ~ 0.8
4 mg/kg 7.9 ~ 0.7
2 mg/kg 9.4 ~ 1.0
Control 11.3 ~ 1.2
Prednisolone (P) 4 mg/kg 9.1 ~ 1.4
2 mg/kg 7.7 ~ 1.0
P 4 mg/kg & TOR 30 mg/kg (concomitant use) 4.1 ~ 0.5*
P 4 mg/kg & TOR 15 mg/kg (concomitant use) 4.3 t 0.5*
P 4 mg/kg & TOR 7.5 mg/kg (concomitant use) 3.5 * 0.5*
P 2 mg/kg & TOR 30 mg/kg (concomitant use) 3.6 ~ 0.7*
P 2 mg/kg & TOR 15 mg/kg (concomitant use) 2.8 ~ 0.5*
P 2 mg/kg & TOR 7.5 mg/kg (concomitant use) 4.3 ~ 0.6*
* P < 0.01 (t-test)
1 All the groups treated by concomitant use of
toremifene citrate (TOR) with prednisolone (P) exhibited
significant decrease in Mac 2 number as compared with
the control and the prednisolone treated group. On the
2~.~8~~J~v ,
- 15 -
1 other hand, the prednisolone treated group and the
toremifene citrate treated group did not exhibit any
significant decrease in Mac 2 number as compared with
the control. The results of these tests indicates that
the concomitant use of the both drugs synergistically
suppresses the glomerulonephritis.
Example 3
Comparison of survival time
NZB x NZW mice (B/W F1 mice) were used as a
pathological model of autoimmune diseases (systemic
lupus erythematodes). Effect of toremifene citrate on
the survival time of the animals was investigated.
Experimental animals:
F1-hybrids of NZB (female) and NZW (male) mice (B/W F1
mice): Imported from,BOmholtgaard, Denmark at the age.of
five weeks.
Test aroups and doses:
Control (male): administration polyethyleneglycol (peg)
3 times a week per os
Control (female): administration peg 3 times a week per
os
Toremifene citrate 30 mg/kg/day: administration 70
mg/kg in polyethylene glycol solution
3 times a week per os to female
NZB x NZW F1 mice
Toremifene citrate 3 mg/kg/day: administration 7 mg/kg
in polyethylene glycol solution 3 times
2~.4~f~~
- 16 -
1 a week per os to female NZB x NZW F1
mice
The survival time of the animals in different
test groups is presented in Fig. 1. All but two female
control animals have died during the almost two years'
follow-up time. Fifty percents of the animals in this
group died before/at the age of 40 weeks, and 20~ (4/20)
were alive after one year.
In the male control group, five animals died
during the first 24 Weeks (not shown in Fig. 1) due to
aggressive behaviour and thereby acquired infection.
These five were excluded from the results. Forty-seven
percents of the male control mice are still alive after
almost two years' time.
In both toremifene treatment groups the life
span of the animals has lengthened clearly when compared
to the female control animals. In the 3 mg/kg
toremifene treatment group only one (1120) animal had
died at/before the age of 40 weeks and three (3/20)
animals in the 30 mg/kg toremifene group.
After one year 80~ and 85$ of the animals were
alive in the 3 mg/kg and 30 mg/kg toremifene treated
,groups, respectively, which is nearer the percentage of,
the male control animals (~ 90~) than that of the female
control group (20~).
Moreover, 25~ (5/20) and 10~ (2120) of the
animals are still alive after almost two years' time in
the lower and higher toremifene dosage group, respec-
- 1~ ..
1 tively.
The follow-up data of 60 female and 15 male F1-
hybrids of NZB x NZW F1 mice (B/W F1 mice) show that
toremifene treatment has clearly extended the life span
of female mice.
Example 4
Examples of preparations comprising the
nonsteroidal anti-estrogen or the pharmacologically
acceptable salt thereof as active ingredient will be
given below, which by no means limit the preparations of
the present invention.
Preparation Example 1
Formulation of prepared 200 mg tablet.
Toremifene citrate 20 mg
Starch 85 mg
Lactose 90 mg
Magnesium stearate 5 mg
Preparation Example 2
Formulation of prepared 200 mg tablet.
Tamoxifen citrate 20 mg
Starch 85 mg
Lactose 90 mg
Magnesium stearate 5 mg