Note: Descriptions are shown in the official language in which they were submitted.
WO ~4/05~744 21~ ~ g ~ 6 PCI`/GB93/02269
CAPSULE CAP ~ -
1, .`.'`'`
TECHNICAL FIELD t--
The present invention relates to a cap ~or fitting to
the body of a capsule for containing a pharmaceutically ~;
active material. In particular, though not exclusively,
the cap i5 for use with a controlled release capsule
construction which comprises a male plug engaged within a
neck of a female body; the male plu~ being formed of a
water-swellable material which swells 50 as to disengage
the female boày upon ex~osure to an aaueous medium. -
,.
BACKGROUND ~-
International Patent Specification WO 90/0~168
discloses a capsule of this type which comprises a water
swella~le male plug engaged withln a female body. A
pharmaceu~ically active material is~contained ~ithin t.~_
device. When the capsule is exposed to water, the male
hydrogel plug swells and eventually disengages itself from
the remale body, thereby allowing the pharmaceutically
active material contained within the capsule to be
released. It has been found that the tlme taken to ! :
release the pharmaceutical materi_ is predictable and
reproducible, so that the device may be used to release
pharmaceutically active material within the body of a
patient after a predeter~._ned time interval. .:~is may, , '
for example, be useful in the treatment of medical
,
';
W094/09744 PCT/GB93/0226~ ~
2 ~ i !,-- ~ ~ ' -
-
conditions where it is desirable to administer a
pharmaceutically active material to the patient sometime
through the night while the patient is asleep, so as to 1~5 . ';'
provide a desired level~of the drug in the patient in
accordance with his needs, for Pxamp e during the night or
when he awa~es. It may also be useful to allow dosing of ~ `-
materials at a predetermined point as the capsule passes
through the gastro-intestinal tract, for example in the
colon.
Conventional hard gelatin capsules are produced and
filled in large numbers using high speed automatic ;~
machinery. Such capsules comprise a body and a cap.
Normally the cap is pre-fitted to the capsule body during
manufacture of the capsule. During filling, the rilling
machine removes the cap, fills the capsule with
pha~maceutical material, and then replaces the cap - often
in a manner such that the cap is locked onto the capsule
body. Patent Specification U~S. 3,399,803 discloses a
self-loc~ing medicament capsule wherein the body has a
.
groove near its open end, and the cap has a corresponding
ridge which snaps into the groove so as to lock the cap
and body together. U.S. 4,442,g41 discloses a
bayonet-type arrangement whereby a rais~d portion on the . -
cap is engaged into a groove on the body.
European Patent Specification 246804 also discloses a >
capsule body having a groove near its mouth for the
purposes of pre~enting the capsule distorting from its
'`~,'
~ 3
cylindrical form, which may cause difficulty in fitting -~
the cap onto the capsule body. ~-
U.S. patent specification 4,4~7,327 discloses ~ `~
number of capsule configurations wherein the cap is
intended to be Iocked onto the body in a tamper-proof
manner. Whilst not addr2ssing the same problem as the
present invention, some of the cap shapes have a mouth
which is narrower than the hemispherical end of the cap.
However, in the type of controlled release capsule
construction disclosed in WO 90/09168 it may be
inconvenient to employ pre-locked capJbody assemblies,
since in a filling machine a numb~r of steps are required :~
to be carried out between the disengagement of the -
pre-locked assemblies and their reassembly. It would
therefore he convenlent to provide the caps and the bodies
separately within the filling machine for subsequent
assembly as part of the filling process. However,
conventional caps have a tendency to nest and jam one
inside another during storage, and more particularly as
the caps are directed sequentially down a chute in a
~illing machine. One reason for this is that the caps are
not truly cylindrical but taper slightly (i.e. are
frustro-conical) from a wider open end to a narrower ---
hemispherical end. The degree of taper is slight and is
intended to asslst removal of the cap from its mould ~
during production. Typically the taper is about 0.012 ~ :
inch (304 microns), that is to say =he radius at the upper
AMEN~ S~
i`` ` . .. ... ~ .
- 4
en~ of the frustro-conical section adjacent the ~:
. .
hemispherical end is 0.012 inch less than the radius at ~.
the open end of the cap. However, this taper may b~e -.
sufficient to allo~ the hemispherical end of one cap to
get jammed inside the open end of an adjacent cap.
It is an object of the present invention to mitigate ~
this problem by providing a cap construc~ion wherein .~
jamming together of the caps is minimised. ~
SUMMARY OF THE INVENTION ~-
,.
Thus, the present invention provides a cap for '-
fitting to a ~ody of a capsule, the cap having a tubular
open end portion for engaging the capsule body and a domed
closed end portion, the domed closed end portion having an
external diameter greater than the internal diametPr of .
the mouth of the tubular open end portion and wherein the
domed closed end portion is ~lattened, such as to be ,~:
non-hemispherical, and have a major radius extending .
substantially transversely to a longitudinal axis of the
tubular open end~portion, and a minor radius extending ~`
:;
substantially along said longitudinal axis,.such as to `:
substantially avoid interlocking of nested caps.
The domed closed end portion has an external diameter '
greater than the internal diameter of the tubular open end
portion. Generally speaklng, this may be achieved in one .`
of a number o~ ways; or some or all of them in
combination. Firstly, the taper of the cap can be reduced ~:
compared to conventional caps. Secondlyl the wall
`. ,'',.
~E~ D S~fET
. ,',~.
- 5
thickness of the cap can be increased. Thirdly, an
inwardly extending protrusion or protrusions may be
provided adjacent the mouth of the open end portion. `-
According to the first provision the taper may be
reduced. Conventional substantially cylindrical caps
generally have a taper such that the radial width of the ;~
cap reduces slightly from the open end to the closed end.
This taper assists removal of the cap from its moulding
pin during production of the cap. Usually, this taper is
of the order of 300 microns. In an embodiment of the
invention, the taper is reduced to no more than 250
microns. This has the effect of increasing the external
radius of the domed portion relative to the internal ;~
radius of the tubular end portion. The taper is the ` ;
difference between the radius of the domed end portion and ;-
the radius of the mouth of the tubular portion.
Secondly, the wall thicXness may be increased. In
order to increase the difference between the internal `-
diameter of the mouth of the tubular open end portion and l-~
the external diameter of the domed portion, it is ^
preferred to increase the wall thic~ness (over that
conventionally used) such that ~he wall thickness is at
least 200 microns, (e.g. 220 to 280 microns). A
conventional hard gelatin cap has a wall thickness of
approximately 0.005" (127 microns).
According to a third provision, an inwardly extPnding
protrusion or protrusions may be provided adjacent the
~M~N~D ~HEET
-
6` .
.; ., .
- 5a -
:
mouth of the open end portion, such as to further minimise ~
any tendency to nesting and jamming. The protruslons may ~;
be individual protrusions or the protrusions may together
form a continuous inwardly extending ridge.
In one embodiment the cap is wider at its domed end `-~
than its open end; the transverse external diameter of the -;
domed end portion exceeding the transverse external ~ .
diameter o~ the open end portion, usually by up to 5% of -
the diameter.
Advantageously, the domed end of the cap may be
flattened relative to conventional hemispherical caps so ~`
as to increase the angle between a tangent at a position `~
' .
,." ~
'1"`'
~,
. .
, . .
, ' , ,', .. "
! . .
, ;~.
' ..':'
~MEN~ SHEE7 ~,
'' "`-"
':'~''':
W094/0~744 2 ~ PCT/GB93/02269 ;,~-;
.;`, 1-,
, ,,,. 1.
- 6 -
on the domed end where the domed end meets an adjacent
cap, and the inside wall of the adjacent cap when nested
thereto. In particular, the minor radius of the flattened
dome may be from 60 to 80~ of the major transversely
extending radius (i.e. in the direction transverse to the
longitudinal axis of the tubular open end portion). The
flattened dome may be substantially hemi-ovoid in shape or
it may be a slmilar non-geometrically defined shape.
Thus, the terms "radius" and "diameter" as used herein are~;
not used in a strict geometrical sense but in a general
sense.
Another aspect of the invention relates to a capsule
comprising the cap fitted to a capsule body. In a
preferred arrangement, the capsule body has a reduced ~-
diameter neck regior. adjacent the open mouth of the body,
and the cap nas an inwardly extending ridge between the
tubula, open end portion and the domed portion, the ridge ;
being engaged within the -eck region of the body to lock
the cap to the body.
A further aspect of the invention provides a method
of filling the capsule, which comprises,
~i) providing a supply of the caps and a separate
su~ly of capsule bodies;
(ii) fee~ing a body to a filling location and
introducing a unit dose of phar~a~eutically. ~ ,~
actlve material into the body; and
(iii) closing the capsule by fitting a cap over an
open end of the capsule. -
W094/09744 PCT/GB93/022~9 ~ ~
2~ 36 "~
.. i. .. .
- 7 -
The method may rurther comprise the step of -~
introducing a plug of a water swellable material into~a
neck of the l^illed capsule body prior to fitting the cap
thereto. Preferably the capsule body has a flared
outwardly extending open mouth and the cap has inwardly
extending protrusions, and the methcd comprises the
further step of pressing the cap onto the body such that
the inwardly extending protrusions clip over the flared -.
mouth of the body to lock the cap in~place on the body.
The present invention is particularly applicable to a : .
controlled release capsule which c_mprises a male plug
engaged ~ithin a neck portion of a female k-iy; the male ,-.
plug belng substantially cylindrical and formed from a ~-
water-swellable mater.ial which swells so as to disengage .~
the female body upon exposure to an aqueous medium. The l~ :
water swellable material is preferably as disclosed
W090/0916~. :
DETAILED DESCRIPTION OF PREFERRED EM~`DIMENTS
Embodiments of the present inYention will now be j ~-~
described by way of example only with reference to the
drawings wherein;
Figure 1 is a cross sectional elevation oî a firs~ l-
embodiment;
Figure 2 is a schematic elevation of a series of caps
in the chute of a capsule filling machine; ~.
Figure 3 is an elevation of a second cap i ~
construction; and `~:
2,!L(s~ t5~ pCI'/C;1~93/02~6Y ~,
~ - 8 -
Figure 4 is a part cross sectional elevation of a
series of caps according to the second construction in a
filling machine chute.
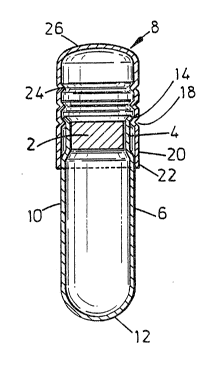
The capsule shown in Figure l comprises a male plug 2
formed of a hydrogel material inserted in neck 4 of female
body 6. The capsule is closed with a cap 8. Typically, a -~
size "0" capsule size is employed.
The body 6 comprises a cylindrical maln portion 10
and closed end 12. The main body narrows to the neck
portio.~ ~ which is substantlally cylindrical so as to
receive the male plug 2 with a close tolerance. The neck
portion then flares out to a flared mouth portion 14 of a
diameter substantially the same as the diameter of the ;~
main body portion 10. ~-
The cap 8 comprises a tubular (substantially
cylindrical) open end portion 20 having a mouth 22, and at
its inner end a ridge 18 or series of detents for locking
the cap onto the body and a stop ring 2~ for locating the
cap on the body. The cap has a domed closed end 26 which
is flattened relative to a hemisphere. In the embodiments
shown, the upstanding radius (along the longitudinal axis
of the -ubular open end portion) is approximately 70% of ' -~
the transverse radial direction. This flattening helps
avoid jamming of th~ nested caps by increasing the angle
between the dome and the mouth of an adjacent cap when ,
nested thereto. The cap wall thickness is substantially
250 microns.
W094tO9744 ~1 4 ~ PCT~GB93/02269 ',`!,'",
9 _ '
In the embodiments shown, the domed portion extends `~
outwardly slightly beyond the tubular open end portion.
I'he male plug 2 is formed of a hydrogel material
(such as disclosed ~n WO 9C/09168) and is usually inserted
so that the upper end of the plug is level with or below
the upper end of the capsule body.
The cap is ~ormed of a water soluble ma~erial, such
as gelatin. The capsule body is formed of a water i~-
insoluble material, which may be a water insoluble ~;
plastics material or may be ~elatin coated with a f
water-impermeable coating.
The capsule body i5 formed in conventional manner by
dipping a mould pin into a gelatin solution and allowing
to dry. The gelatin is then coated with a ~-
water-impermeable coati-.g (e.g. by dip-coating) after the
capsule body is stripped from ~he mould pin and trimmed ~o j~
size. ~l_ernatively, the water-impermeable coa-~g may ^- ~-
applie~ by spray coatlng or vapour deposit~_n o~.-o the ;,
capsule body. ;~
The walls of the female body may be formed from a
wide varietv of materials. They may be of homogenous 3 ;.
construc~ions or they may be laminated. Examples of
mater~ls suitable for use in the construction of the body
include polyethylene, polypropylene,
poly(methylmethacrylate), polyvinyl chloride, polystyrene,
polyurethanes, polytetrafluoroethylene, nylons,
polyformaldehydes, polyesters, oellulose aceiate ~nd nitro
cellulose.
W094/097~ PCT/GB93/0226~ ~
Z ~ 5 ~; - 10
However, a ~-eferred construction uses an impermeable
coating to cover the exterior of a body which has been
formed fxom a water soluble material. The coating may
conveniently be formed by dippin~ the body in a solution ---
of a material which forms a layer which is impermeable to
water. Alternatively, the hody might be spray-coated. A ;~
preferred class of capsule bodies are conventional hard
gelatin or starch capsule bodies coated with a solution of
polyvinyl chlorlde or a polyvinyl acetate copolymer or an
ethyl cellulose solution.
Figure , shows three nested caps 8 travelling down a
delivery chute 30 in a filling machine, prior to being
fitted onto the capsule body. During assembly, the
capsule body is first filled with pharmaceutically active
material. Then, the hydrogel plug is positioned in the
neck c~ the body and inserted into the body 50 as to be -;~
locat~ correctly (usually either flush with the top or
the bcdy or slightly recessed). Finally, the cap is
fitted over the mouth of the capsule body to fo~m the
assembled capsule as shown in Figure 1.
Figure 3 shows a second embodiment which is generally
similar to the first embodiment, analo~ous parts being
labelled with the same reference numerals, but with the
addition of a further ring 28 adjacent the open mouth 22
of the open ended portion 20 of the cap. As can be seen
in Figure 4, this help~ further assist prevention of
ja~-ng together of the nested caps in the filling chute.
'` '
W094/09744 ~ PCT/GB93/02269 ,~
- 11 - i
The ring 28 locates at the lower end of the neck 4 (see
Figure 1) when the cap is fitted onto the body.
Depending on the intended application, the cap may be
enteric coated to prevent dissolution in the stomach. In ;~
the higher pH of the intestine the enteric coating
dissolves exposing the water soluble cap, which in turn ;
dissolves in the aqueous medium. The enteric coating may
be any coating material known in the art, such as those
disclosed in WO 90/09168.
As used herein, the term enteric coating includes all
coatings (whether pH dependent or not) which are able to
pass through the stomach and dissolve in the intestine.
This includes coating materials, such as fats, which
dissolve preferentially under the enzymatic regime
prevailing in the intestine.
';',
i
`~:
. .
,,