Note: Descriptions are shown in the official language in which they were submitted.
2148612
16I t.E . P4N-+M T H i; 4Meid$MID
1'E'1CT7R A N S LAT 1 O N
Substihtted benzimidazoles
The invention relates to new substituted benzimidazoles, to a plurality of
processes
for their preparation, and to their use as pesticides.
It has been disclosed that certain phosphoric esters or carbamates, such as,
for
example, the compound O,S-dimethyl-thiolo-phosphoramide or the compound
O-(2-isopropoxypherryl) N-methyl-carbamate, have insecticidal properties (cf.,
for
example, DE 1,210,835 or D:E 1,108,202).
However, the level, or duration, of action of these previously known compounds
is not entirely satisfactory in all fields of application, in particular in
the case of
certain insects or when low concentrations are applied.
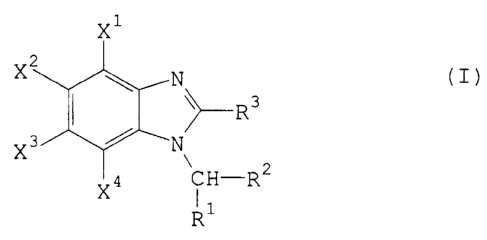
New substituted ben;dmidazoles of the general formula (I)
x
z
X ~ N
3' / ~--R3
X N
4 C H-R2
X I I
R
in which
R' represents hydrogen, alkyl, alkoxy or optionally substituted aryl,
R2 represents hydroxyl, cyano or in each case optionally substituted alkyl,
alkenyl, alkinyl, alkoxy, alkenyloxy, alkinyloxy, alkylthio, amino,
aminoc:arbonyl(-CONH2), alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy,
dialkoxyphosphonyl, (hetero)aryl, (hetero)arylcarbonyl, (hetero)aryloxy-
Le A 29 088-PCT
CA 02148612 2003-11-13
30725-3
carbonyl, (hetero)arylcarbonyloxy or (hetero)aryl-
aminocarbonylaminocarbonyloxy,
R3 represents fluoroalkyl,
Xl, x2, X3 and X4 independently of one another in each case
represent hydrogen, halogen, cyano, nitro, in each case
optionally substituted alkyl, alkoxy, alkylthio,
alkylsulphinyl, alkylsulphonyl or cycloalkyl, optionally
substituted, fused dioxyalkylene, or represent
hydroxycarbonyl, alkylcarbonyl, alkoxycarbonyl or
cycloalkyloxycarbonyl, in each case optionally substituted
amino or aminocarbonyl or in each case optionally
substituted aryl, aryloxy, arylthio, arylsulphinyl,
arylsulphonyl, arylsulphonyloxy, arylcarbonyl,
aryloxycarbonyl, arylazo or arylthiomethylsulphonyl, but
where at least one of the substituents X1, X2, X3 or X4
represents halogenoalkyl, with the exception of the
chloromethyl radical, halogenoalkoxy, halogenoalkylthio,
halogenoalkylsulphinyl, halogenoalkylsulphonyl or
alkylsulphonyl, optionally substituted, fused dioxyalkylene,
or represent hydroxycarbonyl, alkylcarbonyl, alkoxycarbonyl
or cycloalkyloxycarbonyl, in each case optionally
substituted amino or aminocarbonyl, or in each case
optionally substituted aryl, arylthio, arylsulphinyl,
arylsulphonyl, arylsulphonyloxy, arylcarbonyl,
aryloxycarbonyl, arylazo or arylthiomethylsulphonyl,
have been found.
In one aspect, the invention provides a
benzimidazole of the general formula:
- 2 -
CA 02148612 2003-11-13
30725-3
X
XZ
N (I)
I R3
X3 ~ N
X4 C H-RZ
R1
wherein:
Rl is hydrogen or C1-C8-alkyl;
R2 is CN, C1-C8-alkoxy or a substituted amino group
substituted by C1-Ce-alkyl, carbo-C1-C4-alkoxy or a mixture
thereof;
R3 is CF3;
X1, X2, X3 and X4, independently of each other, are hydrogen,
halogen, C1-C6-halogenoalkyl, C1-C6-halogenoalkoxy, or X2 and
X3 together form -O-CFCl-CFCl-O-, provided that X1, X2, X3 or
X4 represents a C1-C6-halogenoalkyl group.
The compounds of the formula (I) may optionally be
present in the form of geometric and/or optical isomers or
regioisomers or their isomer mixtures in varying
composition, depending on the nature and number of the
substituents. The invention claims the pure isomers and the
isomer mixtures.
Furthermore, it has been found that the new
substituted benzimidazoles of the
- 2a -
4~~12
general formula (I)
x
z
X ~ N
3~' ~}-- R3 (1)
~
4 CH-R
X I I
R
in which
R' represents hydrogen, alkyl, alkoxy or optionally substituted aryl,
R2 represents hydroxyl, cy,ano or in each case optionally substituted alkyl,
alkenyl, allcinyl, alkoxy, alkenyloxy, alkinyloxy, alkylthio, amino,
aminocarbonyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy,
dialkoxyphosphonyl, (hetero)aryl, (hetero)arylcarbonyl,
(hetero)aryloxrycarbonyl, (hetero)arylcarbonyloxy or (hetero)arylamino-
carbonylaminocarbonyloxy,
R3 represents fluoroalkyl,
X', X2, X3 and V independently of one another in each case represent hydrogen,
halogen, cyano, nitro, in each case optionally substituted alkyl, alkoxy,
alkylthio, alkylsulphinyl, alkylsulphonyl or cycloalkyl, optionally
substituted, fused dioxyalkylene, or represent hydroxycarbonyl,
alkylcarbonyl, alkoxycarbonyl or cycloalkyloxycarbonyl, in each case
optionally substituted amino or aminocarbonyl or in each case optionally
substituted aryl, aryloxy, arylthio, arylsulphinyl, arylsulphonyl, aryl-
sulphonylox.y, arylcarbonyl, aryloxycarbonyl, arylazo or
arylthiomethy lsulphonyl, but where at least one of the substituents X',
LeA29088 -3-
~148 612
X3 or V represents halogenoallcyl, with the exception of the chloromethyl
radical, halogenoalkoxy, halogenoalkylthio, halogenoallcylsulphinyl,
halogenoalkylsulphonyl or alkylsulphonyl, optionally substituted, fused
dioxyalkylene, or represent hydroxycarbonyl, alkylcarbonyl, alkoxycarbonyl
or cycloalkyloxycarbonyl, in each case optionally substituted amino or
aminocarbonvl, or in each case optionally substituted aryl, arylthio,
arylsulphinyl, arylsulphonyl, arylsulphonyloxy, arylcarbonyl,
aryloxycarbonyl, arylazo or arylthiomethylsulphonyl,
are obtained when
a) 1 H-benzimidazoles of the formula (II),
x
z
is X \ N R 3
s~ I
X ~ N
a H
X
in which
X3 and V have the abovementioned meaning,
are reacted with compounds of the formula (III),
R
A-CH
z
R
in which
L.eA29088 -4-
2148 6 12
A represents a suitable leaving group,
R' has the abovemeritione,d meaning and
R2 has the abovementioned meaning,
if appropriate in the presence of a diluent and, if appropriate, in the
presence of a
reaction auxiliary.
Finally, it has been found that the new substituted benzimidazoles of the
general
formula (I) have a good activity against pests.
Surprisingly, the substituted benzimidazoles of the general formula (I)
according
to the invention show a considerably better insecticidal activity compared
with the.
phosphoric esters or carbamates knovvn- from the prior -art, such as, for
example,
the compound O, S-dimethyl-thiolo-phosphoramide or the compound
0~(2-isopropoxyphenyl) N-methyl-carbamate, which are compounds with a similar
action.
Formula (I) provides a general defnzition of the substituted benzimidazoles
according to the invention. Preferred compounds of the forrnula (I) are those
in
which
R' represents hydrogen, in each case straight-chain or branched alkyl or
alkoxy, each of which has 1 to 8 carbon atoms, or phenyl which is
optionally monosubstituted or polysubstituted by identical or different
substituents, suitable substituents being:
halogen, cyano, nitro, in each case straight-chain or branched alkyl, alkoxy,
alkylthio, alkylsulphinyl or alkylsulphonyl, each of which has 1 to 6 carbon
atoms, in imch case straight-chain or branched halogenoalkyl,
LeA29088 -5-
2148612
halogenoalkoxy, halogenoalkylthio, halogenoalkylsulphinyl or
halogenoalkyl.sulphonyl, each of which has 1 to 6 carbon atoms and 1 to 13
identical or different halogen atoms, in each case straight-chain or branched
alkoxyalkyl, alkoxyalkoxy, alkanoyl, alkoxycarbonyl or alkoximinoalkyl,
each of which has 1 to 6 carbon atoms in the individual alkyl moieties,
divalent dioxyallcylene having 1 to 5 carbon atoms which is optionally
monosubstituied or polysubstituted by identical or different substituents
from the series consisting of halogen and/or straight-chain or branched
alkyl having 1 to 6 carbon atoms and/or straight-chain or branched
halogenoalkyl having 1 to 6 carbon atoms and 1 to 13 identical or different
halogen atoms, or phenyl which is optionally monosubstituted or poly-
substituted by identical or different substituents from the series consisting
of halogen anid/or straight-chain or branched alkyl having 1 to 6 carbon
atoms and/or straight-chain or branched halogenoalkyl having 1 to 6 carbon
.15 atoms and 1 to 13 identical or different halogen atonis,
R2 represents hydroxyl, cyano, or represents alkyl, alkenyl, alkinyl, alkoxy,
alkenyloxy, alkinyloxy, alkylthio, alkylcarbonyl, alkoxycarbonyl, alkyl-
carbonyloxy or dialkoxyphosphonyl, each of which has up to 8 carbon
atoms in the individual alkyl, alkenyl or alkinyl moieties and each of which
is optionally monosubstituted or polysubstituted by identical or different
substituents, suitable substituents in each case being:
halogen, strailht-chain. or branched alkoxy having 1 to 8 carbon atoms, or
aryl having 6 to 10 car-bon atoms or heteroaryl having 2 to 9 carbon atoms
and 1 to 5 hetero atoms - in particular nitrogen, oxygen and/or sulphur -
each of these ary1 or heteroaryl substituents optionally being
monosubstituted or polysubstituted by identical or different substituents,
suitable aryl or heteroaryl substituents being those mentioned in the case of
R',
LeA29088 -6-
2 148612
RZ furthermore represents amino or aminocarbonyl, each of which is optionally
monosubstituted or disubstituted by identical or different substituents,
suitable substituents in each case being:
formyl, strailft-chain or branched alkyl having 1 to 8 carbon atoms,
straight-chain or branched alkenyl having 2 to 8 carbon atonis, straight-
chain or branched alkylsulphonyl having 1 to 8 carbon atoms, carbamoyl,
thiocarbamoy.1 or sulphamoyl, each of which is optionally monosubstituted
or disubstitutfA by identical or different straight-chain or branched alkyl
substituents having 1 to 8 carbon atoni.s, or cycloalkyl, cycloalkylcarbonyl
or cycloalkyloxycarbonyl, each of which has 3 to 8 carbon atoms in the
cycloalkyl r.noiety, alkylcarbonyl, alkenylcarbonyl, alkoxycarbonyl,
alkenyloxycarbonyl, allcylthio-carbonyl, alkoxy-thiocarbonyl or alkylthio-
thiocarbonyl, each of which has 1 to 8 carbon atoms in the individual
straight-chain or branched alkyl moieties, in each case - divalent and
cyclized alkanediylc:arbonyl or alkanediyloxycarbonyl, each of which has
2 to 6 carbon atoms in the alkanediyl moiety, or arylalkyl,
arylalkylcarbcanyl or arylalkyloxycarbonyl, each of which has 6 to 10
carbon atoms in the aryl moiety and 1 to 8 carbon atoms in the straight-
chain or branched alkyl moiety and each of which is optionally
monosubstituted or polysubstituted in the aryl moiety by identical or
different substituents, or aryl, arylcarbonyl or aryloxycarbonyl, each of
which has 6 to 10 carbon atoms in the aryl moiety and each of which is
optionally monosubstituted or polysubstituted in the aryl moiety by
identical or different substituents, suitable aryl substituents in each case
being those rr.ientioned in the case of R',
R2 furthermore represents aryl, arylcarbonyl, aryloxycarbonyl, arylcarbonyloxy
or arylaminocarbonylaminocarbonyloxy, each of which has 6 to 10 carbon
atoms in the aryl moiety and each of which is optionally monosubstituted
or polysubstiltuted by identical or different substituents, suitable aryl
LeA29088 -7-
2148612
substituents in each case being those mentioned in the case of R',
RZ furthermore represents heteroaryl, heteroarylcarbonyl,
heteroaryl.oxycarbonyl, heteroarylcarbonyloxy or
heteroarylaminocarbonylaminocarbonyloxy, each of which has 2 to 9
carbon atoms and 1 to 5 identical or different hetero atoms - in particular
nitrogen, oxygen and/or sulphur - in the heteroaryl moiety and each of
which is optionally monosubstituted or polysubstituted by identical or
different subst ituents, suitable heteroaryl substituents in each case being
the
aryl substituents mentioned in the case of R', and
R3 represents pecfluoroallcyl or partially fluorinated alkyl having 1 to 25 C
atoms and up to 50 F atoms,
X', V, X3 and V iniiependently of orie another in each case represent
hydrogen,
fluorine, chlorine, bromine, iodine, cyano, nitro, in each case straight-chain
or branched alikyl, alkoxy, alkylthio, alkylsulphinyl or alkylsulphonyl, each
of which has 1 to 8 carbon atoms, cycloalkyl having 3 to 8 carbon atoms,
in each case straight-chain or branched halogenoalkyl, halogenoalkoxy,
halogenoalkylthio, halogenoalkylsulphinyl, halogenoalkylsulphonyl, each of
which has 1 to 6 carbon atoms and 1 to 13 identical or different halogen
atoms, or divalent dioxyalkylene having 1 to 5 carbon atoms which is
optionally monosubstituted or polysubstituted by identical or different
substituents fi=om the series consisting of halogen and/or straight-chain or
branched alkyl having 1 to 4 carbon atoms and/or straight-chain or
branched halogenoalkyl having 1 to 4 carbon atoms and 1 to 9 identical or
different halogen atoms, furthermore represent hydroxycarbonyl, in each
case straight-chain or branched alkylcarbonyl or alkoxycarbonyl, each of
which has 1 to 6 carbon atoms in the alkyl moiety, cycloalkyloxycarbonyl
having 3 to 8 carbon atoms in the cycloalkyl moiety, or amino or
aminocarbonyl, each of which is optionally monosubstituted or
LeA29088 -8-
'2148612
polysubstituted by identical or different substituents, suitable amino substi-
tuents in each case being:
in each case straight-chain or branched alkyl having 1 to 6 carbon atoms,
halogenoalkyl having I to 6 carbon atoms and 1 to 13 halogen atoms,
alkoxyalkyl or alkylcarbonyl, each of which has 1 to 6 carbon atoms in the
individual allo/1 moieties, or arylcarbonyl, arylsulphonyl, arylaminocarbonyl
or arylmethylsulphonyl, each of which has 6 to 10 carbon atoms in the aryl
moiety and each of which is optionally monosubstituted or polysubstituted
in the aryl moiety by identical or different substituents, suitable aryl
substituents iri each case being those mentioned in the case of R';
furthermore represent aryl, aryloxy, arylthio, arylsulphinyl, arylsulphonyl,
arylsulphonyloxy, arylcarbonyl, aryloxycarbonyl, arylthiomethylsulphonyl
'15 or arylazo, ea;,h of -which has 6 to 10 cazbon atoms in the aryl 'moiety
and
each of which is optionally monosubstituted or polysubstituted in the aryl
moiety by identical or different substituents, suitable suitable aryl
substituents iri each case being those mentioned in the case of R',
where at least: one of the substituents X', V, X3 or V represents in each
case straight-chain or branched halogenoalkyl (with the exception of the
chloromethyl radical'), halogenoalkoxy, halogenoalkylthio, halogeno-
allcylsulphinyl, or halogenoalkylsulphonyl, each of which has 1 to 6 carbon
atoms and 1 to 13 identical or different halogen atoms, straight-chain or
branched alkylsulphonyl having 1 to 6 carbon atoms or divalent
dioxyalkylene having 1 to 5 carbon atoms which is optionally mono-
substituted or polysubstituted by identical or different substituents from the
series consisting of halogen and/or straight-chain or branched alkyl having
1 to 4 carbon atoms and/or straight-chain or branched halogenoalkyl having
1 to 4 carbon atoms and 1 to 9 identical or different halogen atoms,
furthermore represents hydroxycarbonyl, in each case straight-chain or
LeA29088 -9-
2148 6 12
branched alkylcarbonyl or alkoxycarbonyl, each of which has 1 to 6 carbon
atoms in the alkyl moiety, cycloalkyloxycarbonyl having 3 to 8 carbon
atoms in the cycloalkyl moiety, or amino or aminocarbonyl, each of which
is optionally monosubstituted or polysubstituted by identical or different
substituents, suitable amino substituents in each case being:
in each case slraight-chain or branched alkyl having 1 to 6 carbon atoms,
halogenoalkyl having 1 to 6 carbon atoms and 1 to 13 halogen atoms,
alkoxyalkyl or alkylcarbonyl, each of which has 1 to 6 carbon atoms in the
individual alkyl moieties, or arylcarbonyl, arylsulphonyl, arylaminocarbonyl
or arylmethylsulphonyl, each of which has 6 to 10 carbon atoms in the aryl
moiety and ea;.h of which is optionally monosubstituted or polysubstituted
in the aryl moiety by identical or different substituents, suitable aryl
substituents in each case being those mentioned in the case of R';
furthermore represents aryl, arylthio, arylsulphinyl, arylsulphonyl,
arylsulphonyloxy, arylcarbonyl, aryloxycarbonyl, arylthiomethylsulphonyl
or arylazo, each of which has 6 to 10 carbon atoms in the aryl moiety and
each of' which is optionally monosubstituted or polysubstituted in the aryl
moiety by identical or different substituents, suitable aryl substituents in
each case beir.ig those mentioned in the case of R'.
Particularly prefen-ed compounds of the formula (I) are those in which
R' represents hydrogen, in each case straight-chain or branched alkyl or
alkoxy, each of which has 1 to 6 carbon atoms, or phenyl which is
optionally monosubstituted to trisubstituted by identical or different
substituents, suitable substituents being:
halogen, cyano, nitro, in each case straight-chain or branched alkyl, alkoxy,
alkylthio, alkylsulphinyl or alkylsulphonyl, each of which has 1 to 4 carbon
LeA29088 - 10-
8 6 12
atoms, in each case straight-chain or branched halogenoalkyl,
halogenoalkoxy, halogenoalkylthio, halogenoalkylsulphinyl or
halogenoalkylsulphonyl, each of which has 1 to 4 carbon atoms and 1 to 9
identical or diiYerent halogen atoms, in each case straight-chain or branched
alkoxyalkyl, a.lkoxyalkoxy, alkanoyl, alkoxycarbonyl or alkoximinoalkyl,
each of which has 1 to 4 carbon atoms in the individual alkyl moieties,
divalent dioxyalkylene having 1 to 4 carbon atoms which is optionally
monosubstituted to hexasubstituted by identical or different substituents
from the series consisting of halogen and/or straight-chain or branched
alkyl having 1 to 4 carbon atoms and/or straight-chain or branched
halogenoalkyl having 1 to 4 carbon atoms and 1 to 9 identical or different
halogen atoms, or phenyl which is optionally monosubstituted to penta-
substituted by identical or different substituents from the series consisting
of halogen and/or. straight-chain or branched alkyl having 1 to 4 carbon
atoms and/or straight-chain or branched halogenoalkyl having 1-to 4 carbon
atoms and 1 to 9 identical or different halogen atoms,
RZ represents hydroxyl or cyano, or alkyl, alkenyl, alkinyl, alkoxy,
alkenyloxy,
alkinyloxy, aTkylthio, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy or
dialkoxyphosphonyl, each of which has up to 6 carbon atoms in the
individual AM, alkenyl or alkinyl moieties and each of which is optionally
monosubstituted to pentasubstituted by identical or different halogen
substituents, or alkyl, alkenyl, alkinyl, alkoxy, alkenyloxy, alkinyloxy,
alkylthio, allcylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy or dialkoxy-
phosphonyl, each of which has up to 6 carbon atoms in the individual
alkyl, alkenyl or alkinyl moieties and each of which is optionally monosub-
stituted to trisubstituted by identical or different substituents, suitable
substituents in each case being:
straight-chain or branched alkoxy having 1 to 6 carbon atoms, or aryl
having 6 or 10 carbon atoms or heteroaryl having 2 to 9 carbon atoms and
LeA29088 -11-
2148 512
1 to 4 hetero atoms - in particular nitrogen, oxygen and/or sulphur - each
of which is optionally monosubstituted to trisubstituted by identical or dif-
ferent substituents, suitable aryl or heteroaryl substituents being those
mentioned in ihe case of R',
R2 furthermore represents amino or aminocarbonyl, each of which is optionally
monosubstitutiA or disubstituted by identical or different substituents,
suitable substituents in each case being:
formyl, straight-chain or branched alkyl having 1 to 6 carbon atoms,
straight-chain or branched alkenyl having 2 to 6 carbon atoms, straight-
chain or branched alkylsulphonyl having 1 to 6 carbon atoms, carbamoyl,
thiocarbamoyl or sulphamoyl, each of which is optionally monosubstituted
or disubstituted by identical. or different straight-chain or branched alkyl
substituents having a to 6 carbon atoms, or cycloalkyl, cycloallcylcarbonyl
or cycloalkyloxycarbonyl, each of which has 3 to 7 carbon atoms in the
cycloalkyl moiety, alkylcarbonyl, alkenylcarbonyl, alkoxycarbonyl,
alkenyloxycarbonyl, alkylthio-carbonyl, alkoxy-thiocarbonyl or alkylthio-
thiocarbonyl, each of which has 1 to 6 carbon atoms in the individual
straight-chain or branched alkyl moieties, in each case divalent and
cyclized alkanediylcarbonyl or alkanediyloxycarbonyl, each of which has
2 to 5 cai bon atoms in the alkanediyl moiety, or arylalkyl,
arylalkylcarbonyl or arylallcyloxycarbonyl, each of which has 6 or 10
carbon atoms in the aryl moiety and I to 6 carbon atoms in the straight-
chain or branched alkyl moiety and each of which is optionally
monosubstituted to trisubstituted in the aryl moiety by identical or different
substituents, or aryl, arylcarbonyl or aryloxycarbonyl, each of which has 6
or 10 carbon atoms in the aryl moiety and each of which is optionally
monosubstituted to trisubstituted in the aryl moiety by identical or different
substituents, suitable aryl substituents in each case being those mentioned
in the case of R',
LeA29088 - 12-
2 148612
R2 furthermore represents aryl, arylcarbonyl, aryloxycarbonyl, arylcarbonyloxy
or arylaminocarbonylarninocarbonyloxy, each of which has 6 or 10 carbon
atoms in the aryl moiety and each of which is optionally monosubstituted
to pentasubstituted by identical or different substituents, suitable aryl
substituents in each case being those mentioned in the case of R',
RZ furthermore represents heteroaryl, heteroarylcarbonyl,
heteroaryloxycarbonyl, heteroarylcarbonyloxy or
heteroarylaminocarbonylaminocarbonyloxy, each of which has 2 to 9
carbon atoms and 1 to 4 identical or different hetero atoms - in particular
nitrogen, oxygen and/or sulphur - in the heteroaryl moiety and each of
which is optionally monosubstituted to pentasubstituted by identical or
different substituents, suitable heteroaryl substituents in each case being
the
aryl substituer-ts mentioned in the case of R', and
R3 represent,s CF:;, C',2Fs or C-iF,s,
X', V, X3 and V independently of one another in each case represent hydrogen,
fluorine, chlorine, bromine, cyano, nitro, in each case straight-chain or
branched alkyl, alkoxy, alkylthio, alkylsulphinyl or alkylsulphonyl, each of
which has 1 to 6 carbon atoms, cycloalkyl having 3 to 7 carbon atoms, in
each case straight-chain or branched halogenoalkyl, halogenoalkoxy,
halogenoalkylthio, halogenoalkylsulphinyl, halogenoalkylsulphonyl, each of
which has 1 t:o 4 carbon atoms and 1 to 9 identical or different halogen
atoms, or divalent dioxyalkylene having 1 to 4 carbon atoms which is
optionally monosubstituted to hexasubstituted by identical or different
substituents fi-om the series consisting of halogen and/or straight-chain or
branched allcyl having 1 to 4 carbon atoms and/or straight-chain or
branched halogenoalkyl having 1 to 4 carbon atoms and 1 to 9 identical or
different halogen atoms, furthermore represent hydroxycarbonyl, in each
case straight-chain or branched alkylcarbonyl or alkoxycarbonyl, each of
LeA29088 - 13-
2148 612
which has 1 tc- 4 carbon atoms in the alkyl moiety, cycloalkyloxycarbonyl
having 3 to 7 carbon atoms in the cycloalkyl moiety, or amino or
aminocarbonyl., each of which is optionally monosubstituted or
disubstituted by identical or different substituents, suitable amino sub-
stituents in each case being:
in each case sitraight-chain or branched alkyl having 1 to 4 carbon atoms,
halogenoalkyl having 1 to 4 carbon atoms and 1 to 9 halogen atoms,
alkoxyalkyl or alkylcarbonyl, each of which has 1 to 4 carbon atoms in the
individual alkyl moieties, or arylcarbonyl, arylsulphonyl, arylaminocarbonyl
or arylmethylsulphonyl, each of which has 6 or 10 carbon atom.s in the aryl
moiety and each of which is optionally monosubstituted to pentasubstituted
in the aryl rrioiety by identical or different substituents, suitable aryl
substituents in each case being those mentioned in the case of R'; .
. . : . .
furthermore represent aryl, aryloxy, arylthio, arylsulphinyl, arylsulphonyl,
arylsulphonyloxy, arylcarbonyl, aryloxycarbonyl, arylthiomethylsulphonyl
or arylazo, each of which has 6 or 10 carbon atoms in the aryl moiety and
each of which is optionally monosubstituted to pentasubstituted in the aryl
moiety by identical or different substituents, suitable suitable aryl
substituents in each case being those mentioned in the case of R';
where at least one of the substituents X', V, X3 or V represents in each
case straight-chain or branched halogenoalkyl (with the exception of the
chloromethyl radical), halogenoalkoxy, halogenoalkylthio,
halogenoalkyl sulphinyl or halogenoalkylsulphonyl, each of which has 1 to
4 carbon atorris and 1 to 9 identical or different halogen atoms, straight-
chain or branched alkylsulphonyl having 1 to 4 carbon atoms or divalent
dioxyalkylene having 1 to 4 carbon atoms which is optionally monosubsti-
tuted to hexasubstituted by identical or different substituents from the
series
consisting of halogen and/or straight-chain or branched alkyl having 1 to 4
LeA29088 - 14-
2 148 612
carbon atoms and/or straight-chain or branched halogenoalkyl having 1 to
4 carbon atoms and 1 to 9 identical or different halogen atoms, furthermore
represents hyiiroxyc:arbonyl, in each case straight-chain or branched
alkylcarbonyl or alkoxycarbonyl, each of which has 1 to 4 carbon atoms in
the alkyl moiety, cycloalkyloxycarbonyl having 3 to 7 carbon atoms in the
cycloalkyl moiety, or amino or aminocarbonyl, each of which is optionally
monosubstitutfxl or disubstituted by identical or different substituents,
suitable amino substituents in each case being:
in each case siraight-chain or branched alkyl having 1 to 4 carbon atoms,
halogenoalkyl having 1 to 4 carbon atoms and 1 to 9 halogen atoms,
alkoxyalkyl or alkylcarbonyl, each of which has 1 to 4 carbon atoms in the
individual alkyl moieties, or arylcarbonyl, arylsulphonyl, arylaminocarbonyl
or arylmethylsulphonyl, each of which has 6 or 10 carbon atoms in the aryl
moiety and each of which is optionally -monosubstiituted to pentasubstituted
in the aryl rrioiety by identical or different substituents, suitable aryl
substituents in each case being those mentioned in the case of R';
fiuthermore ;represents aryl, arylthio, arylsulphinyl, arylsulphonyl,
arylsulphonyloxy, arylcarbonyl, aryloxycaibonyl, arylthiomethylsulphonyl
or arylazo, each of which has 6 or 10 carbon atoms in the aryl moiety,
such as phenyl or naphthyl, and each of which is optionally
monosubstitutEA to pentasubstituted in the aryl moiety by identical or
different substituents, suitable suitable aryl substituents in each case being
those mentioned in t:he case of R'.
Very particularly pref'erred compounds of the formula (I) are those in which
R' represents hycirogen, in each case straiight-chaiin or branched alkyl or
alkoxy, each of which has 1 to 4 carbon atoms, or phenyl which is
optionally monosubstituted or disubstituted by identical or different
LeA29088 - 15-
~.1486 12
substituents, suitable substituents being:
halogen, cyano, nitro, in each case straight-chain or branched alkyl, alkoxy,
alkylthio, allcylsulphinyl or allcylsulphonyl, each of which has 1 to 3 carbon
atoms, in i:ach case straight-chain or branched halogenoalkyl,
halogenoalkoxy, halogenoalkylthio, halogenoalkylsulphinyl or
halogenoalkylsulphonyl, each of which has 1 to 3 carbon atoms and 1 to 7
identical or different halogen atoms, in each case straight-chain or branched
alkoxyalkyl, alkoxyalkoxy, alkanoyl, alkoxycarbonyl or alkoximinoalkyl,
each of which has 1 to 3 carbon atoms in the individual alkyl moieties,
divalent dioxyalkylene having 1 to 3 carbon atoms which is optionally
monosubstituted to tetrasubstituted by identical or different substituents
from the series consisting of halogen and/or straight-chain or branched
alkyl having 1 to 3 carbon atoms and/or straight-chain or branched
halogenoalkyl having 1 to 3. carbon atoms and 1* to 7 identical or different
halogen atoms, or phenyl which is optionally monosubstituted to tri-
substituted by identical or different substituents from the series consisting
of halogen and/or straight-chain or branched alkyl having 1 to 3 carbon
atoms and/or :rtrraight-chain or branched halogenoalkyl having 1 to 3 carbon
atoms and 1 to 7 identical or different halogen atoms,
Rz represents hyclroxyl or cyano, or alkyl, alkenyl, alkinyl, alkoxy,
alkenyloxy,
alkinyloxy, alkylthio, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy or
dialkoxyphosphonyl, each of which has up to 4 carbon atoms in the
individual alkyl, alkenyl or alkinyl moieties and each of which is optionally
monosubstituted to trisubstituted by identical or different halogen
substituents - in particular fluorine, chlorine and/or bromine substituents -
or alkyl, alk:enyl, alkinyl, alkoxy, alkenyloxy, alkinyloxy, alkylthio,
alkylcarbonyl,, alkoxycarbonyl, alkylcarbonyloxy or dialkoxyphosphoryl,
each of which has up to 4 carbon atoms in the individual alkyl, alkenyl or
alkinyl moieties and each of which is optionally monosubstituted or di-
LeA29088 - 16-
2148612
substituted by identical or different substituents, suitable substituents in
each case being:
straight-chain or branched alkoxy having 1 to 3 carbon atoms or phenyl
which is optionally monosubstituted or disubstituted by identical or
different substituents, suitable phenyl substituents being those mentioned in
the case of R'.
RZ furthermore represents amino or aminocarbonyl, each of which is optionally
monosubstitutcxi or disubstituted by identical or different substituents,
suitable substiituents in each case being:
formyl, straight-chain or branched alkyl having 1 to 4 carbon atoms,
strai.ght-chain or branched alkenyl haviing 2 to 4 carbon atoms, straiight-
chain or branched alkylsulphonyl having *1 to 4 carbon atoms, carbamoyl,
thiocarbamoyl or sulphamoyl, each of which is optionally monosubstituted
or disubstituted by identical or different straight-chain or branched alkyl
substituents having I to 4 carbon atoms, or cycloalkyl, cycloalkylcarbonyl
or cycloalkylaxycarbonyl, each of which has 3 to 6 carbon atoms in the
cycloalkyl rrioiety, alkylcarbonyl, alkenylcarbonyl, alkoxycarbonyl,
alkenyloxycarbonyl, alkylthio-carbonyl, alkoxy-thiocarbonyl or alkylthio-
thiocarbonyl, each of which has 1 to 4 carbon atoms in the individual
straight-chain or branched alkyl moieties, in each case divalent and
cyclized alkanediylcarbonyl or alkanediyloxycarbonyl, each of which has
2 to 4 carbon atoms in the alkanediyl moiety, phenylalkyl,
phenylalkylcarbonyl or phenylalkyloxycarbonyl, each of which has 1 to 4
carbon atoms in the straight-chain or branched alkyl moiety and each of
which is monosubstituted or disubstituted in the phenyl moiety by identical
or different substituents, or phenyl, phenylcarbonyl or phenyloxycarbonyl,
each of which is optionally monosubstituted or disubstituted in the phenyl
moiety by ideiitical or different substituents, suitable phenyl substituents
in
LeA29088 - 17-
2148612
each case being those mentioned in the case of R',
Rz furthermore represents phenyl, phenylcarbonyl, phenyloxycarbonyl,
phenylcarborniloxy oi- phenylaminocarbonylaminocarbonyloxy, each of
which is optionally monosubstituted to trisubstituted by identical or
different substituents, suitable phenyl substituents in each case being those
mentioned in the case of R',
R2 furthermore represents heteroaryl, heteroarylcarbonyl,
heteroaryl.oxycarbonyl, heteroarylcarbonyloxy, or
heteroarylam'vnocarbonylaminocarbonyloxy, each of which has 2 to 9
carbon atoms and 1 tc- 3 identical or different hetero atoms - in particular
nitrogen, oxygen and/or sulphur - in the heteroaryl moiety and each of
which. is optionally monosubstituted to trisubstituted by identical or
different substituents, suitable heter6aryl substituents in each case being
the
phenyl substiluents mentioned in the case of R'. Heteroaryl radicals which
may be mentioned are pyridyl, furanyl, thiophenyl, piperidinyl or pyrrolyl,
and
R3 represents CF3,
X', V, X3 and X4 independently of one another in each case represent hydrogen,
chlorine, broinine, cyano, nitro, in each case straight-chain or branched
alkyl, alkoxy, alkylthio, alkylsulphinyl or alkylsulphonyl, each of which has
1 to 4 carbor- atoms, cycloalkyl having 3, 5 or 6 carbon atoms, in each
case straight-chain or branched halogenoalkyl, halogenoalkoxy,
halogenoalkyl.thio, halogenoalkylsulphinyl, halogenoalkylsulphonyl, each of
which has 1 to 3 carbon atoms and I to 7 identical or different halogen
atoms, or divalent dioxyalkylene having 1 to 3 carbon atoms which is
optionally monosubstituted to tetrasubstituted by identical or different
substituents from the series consisting of halogen and/or straight-chain or
LeA29088 - 18-
~148 612
branched alkyl having 1 to 3 carbon atoms and/or straight-chain or
branched halo;;enoalkyl having 1 to 3 carbon atoms and 1 to 7 identical or
different halogen atoms, furthermore represent hydroxycarbonyl, in each
case straight-chain or branched alkylcarbonyl or alkoxycarbonyl, each of
which has 1 to 3 carbon atoms in the alkyl moiety, cycloalkyloxycarbonyl
having 3, 5 or 6 carbon atoms in the cycloalkyl moiety, or amino or
aminocarbony:l, each of which is optionally monosubstituted or
disubstituted by identical or different substituents, suitable amino sub-
stituents in each case being:
in each case straight-chain or branched alkyl having 1 to 3 carbon atoms,
halogenoalkyl having 1 to 3 carbon atoms and 1 to 7 halogen atoms,
alkoxyalkyl or alkylcarbonyl, each of which has 1 to 3 carbon atoms in the
individual alkyl moieties, or phenylcarbonyl, phenylsulphonyl,
phenylaminocarbonyl or phenylmethylsulphonyl, each of which 'is
optionally monosubstituted to trisubstituted in the phenyl moiety by
identical or di:fferent substituents, suitable phenyl substituents in each
case
being those mentioned in the case of R';
furthermore represent phenyl, phenyloxy, phenylthio, phenylsulphinyl,
phenylsulphor.iyl, phenylsulphonyloxy, phenylcarbonyl, phenyloxycarbonyl,
phenylthiomethylsulphonyl or phenylazo, each of which is optionally
monosubstitutA to trisubstituted in the phenyl moiety by identical or
different substituents, suitable suitable phenyl substituents in each case
being those mentioned in the case of R',
where at least one of the substituents X', V, X3 or V represents in each
case straight-chain or branched halogenoalkyl (with the exception of the
chloromethyl radical), halogenoalkoxy, halogenoalkylthio,
halogenoalkyl,sulphinyl or halogenoalkylsulphonyl, each of which has 1 to
3 carbon atonis and 1 to 7 identical or different halogen atoms, straight-
LeA29088 - 19-
2148 6 12
chain or branched alkylsulphonyl having 1 to 3 carbon atoms or divalent
dioxyalkylene having 1 to 3 carbon atoms which is optionally monosubsti-
tuted to tetrasiubstituted by identical or different substituents from the
series
consisting of halogen and/or straight-chain or branched alkyl having 1 to 3
carbon atoms and/or straight-chain or branched halogenoalkyl having 1 to
3 carbon atom.s and 1 to 7 identical or different halogen atoms, furthermore
represents hydroxycarbonyl, in each case straight-chain or branched
alkylcarbonyl or alkoxycarbonyl, each of which has 1 to 3 carbon atoms in
the alkyl moi,-4, cycloalkyloxycarbonyl having 3, 5 or 6 carbon atoms in
the cycloalkyl moiety, or amino or aminocarbonyl, each of which is
optionally monosubstituted or disubstituted by identical or different
substituents, suitable amino substituents in each case being:
in each case :-traight-chain or branched alkyl having 1 to 3 carbon atoms,
1*5 halogenoallcyl. having l to - 3 carbon atoni.s and.1 to *7 halogen atoms,
alkoxyalkyl or alkylcarbonyl, each of which has 1 to 3 carbon atoms in the
individual alkyl moieties, or phenylcarbonyl, phenylsulphonyl,
phenylaminocarbonyl or phenylmethylsulphonyl, each of which is
optionally monosubstituted to trisubstituted in the phenyl moiety by
identical or different substituents, suitable phenyl substituents being in
each
case those mentioned in the case of R';
fiuthermore represent phenyl, phenylthio, phenylsulphinyl, phenylsulphonyl,
phenylsulphoriyloxy, phenylcarbonyl, phenyloxycarbonyl, phenylthiomethyl-
sulphonyl or phenylazo, each of which is optionally monosubstituted to
trisubstituted in the phenyl moiety by identical or different substituents,
suitable suitable phenyl substituents in each case being those mentioned in
the case of R'.
The following substituted benzimidizoles of the general formula (I) may be
mentioned individually in addition to the compounds mentioned in the
preparation
LeA29088 -20-
2148612
examples:
x
z
X. N
~
3 ~ / ~}--R3 (~)
X N
4 CH-RZ
X 11
R
LeA29088 -21-
2148 6 12
X1 X2 _ X3 X4 Ri R2
Br H CI-CH2-S02- H H i ZHs
COOCzHs
Br H C6H5-S-CH2-SO2- H H CzHs
COOC~Hs
H p-CH3-C6H4-S02-0- p-CH3-C6H4-S02-O- H H CzHs
COOCzHs
Br H C6H5-O-CO- H H CZHS
COOCzIis
Br H 0, H H CiHs
COOCzHs
Br H n-C6H13-0-CO- H H C2H5
COOCzHs
Br. H H. H H q.,HS
COOC.~HS
Br H F3C-S- H H C7HS
COOCzHs
Ci H F3C-S- H H qHs
COO(;Hs
COO-C6H5 H F3C-O- H H -O-C2H5
Le A 29 088 - 22 -
2143512
XI X2 X3 X4 R1 . R2
O, ci H CF3 H H qH,
O N
COOCHS
0,c/ H F3C-O- H H C~HS
O N
COOCzHs
Br H CIFCH-CF2-S- H H CzHs
N
/ \COOCzHs
Br H CIFCH-CF2-S- H H -O-C2H5
Br H F3C-CHF-CF2-S- H H CzHs
N
/ \COOC~HS
Br H F3C-CHF-CF2-S- H H -O-CZHS
CF3 H CF3 H H C7HS
N
/ \COOCZHS
CF3 H CF3 H H -0-C2H5
COO-n-C3H7 H CF3. ' H H. C.2H~
N
/ \COOCzHs
COO-i-C3H7 H CF3 H H C~HS
\COOCzHs
COO-n-CqHq H CF3 H H CiH,
/N
\COOqHS
L e A 29 088 - 23 -
214S612
Xi X2 X3 X4 R1 R2
H
COO-s-C4H9 H CF3 H H j'' s
,"- N \COOqHs
H
'
COO-C6H5 H CF3 H H I
/
\CooqHs
COO-s-C4H9 F3C-0- H H -O-C2H5
COO-n-C3H7 H CF3 H H -O-C2H5
COO-i-C3H7 H CF3 H H -O-C2H5
COO-n-C4Hq H CF3 H H -O-C2H5
COO-s-C4H9 H CF3 H H -O-CZHS
COO-C6H5 H CF3 H H -0-C2H5
COO-n-C3H7 H F3C-O- H H /N
COO-i-C3H7 H F3C-O- H H
N
~'~OOCzHs
H
COO n-C4H9 H' F3C-0- H H s
/N
\COpCHs
COO-s-C4H9 H F3C-O- H H
/
\coOqHs
H
COO-C6H5 H F3C-O- H H j~ s
\CooqHs
N
COO-n-C3H7 H F3C-0- H H -O-C2H5
C00-i-C3H7 H F3C-0- H H -O-C2H5
COO-n-C4H9 H F3C-O- H H -O-C2H5
-24-
LeA2
CA 02148612 2003-11-13
30725-3
The 1H-benzimidazoles of the formula (II)
mentioned in the preparation of the substituted
benzimidazoles of the formula (I) can also be employed as
pesticides, just like the compounds of the formula (I).
1H-Benzimidazoles of the formula (II) which are preferably
mentioned are those in which the substituents have the
preferred and particularly preferred meanings mentioned in
the case of the compounds of the formula (I). The following
compounds of the formula (II) are mentioned individually:
1
X
~-C F 3
Xx:) N
N (II)
X
X4 4 H
x 1 x 2 x 3 x 4
H CF3 Br H
H _C_C\0 H
CF3 CH2-CF3
H -OCF3 C1 H
H -OCF3 Br H
H -O-CFC1-CFC1-O H
In one aspect, the invention provides a
substituted 1H-benzimidazole of the general formula:
XZ
~-CF3
X3 N
H
- 25 -
CA 02148612 2003-11-13
30725-3
wherein X2 to X3 represent the following combinations:
x 2 x 3
CF3 Br
-OCF3 Cl
-OCF3 Br
If, for example, 5(6)-phenyl-2-trifluoromethyl-
benzimidazole and chloromethyl ethyl ether are used as
starting compounds, the course of the reaction of the
process according to the invention can be represented by the
following equation:
- 25a -
2148612
2 CN ~-CF3 + 2 C!-CH2 O-CZHs
N
~~--CF3
a-(:'~:N
- HI;i ~
C H2 O-C2H5
---- +
base
N
\>--CF3
N
CHZ O--C2H5
Formula (II) provide:3 a general definition of the III benzimidazoles required
as
starting substances for carrying out the process according to the invention.
In this
fortnula (II), R3, X', :)(z, X3 and X4 preferably represent those radicals
which have
already been mentioned in connection with the description of the compounds of
the formula (I) accor(iing to the invention as being preferred for these
substituents.
The 1H-benzimidazoles of the formula (II) are known or can be obtained in
analogy to known processes (cf., for example, J. Amer. Chem. Soc. 71, 1292
[1953]; US 3,576,818).
Formula (III) provides a general defniition of the compounds furkhermore
required
as starting materials :For carrying out the process according to the
invention.
In this formula (III), R' and RZ preferably represent those radicals which
have
already been mentioned in connection with the description of the substances of
the
LeA29088 -26-
2148612
formula (1) according to the invention as being preferred for these
substituents.
A preferably represents a leaving radical customary in alkylating agents,
preferably
halogen, in particular chlorine, bromine or iodine, or in each case optionally
substituted allcylsulphonyloxy, alkoxysulphonyloxy or arylsulphonyloxy, such
as,
in particular, niethariesulphonyloxy, trifluoromethanesulphonyloxy,
methoxysulphonyloxy, ethoxysulphonyloxy or p-toluenesulphonyloxy.
A furthermore also represent.s an alcohol, alkanoyloxy or alkoxy group, such
as,
for example, a hydroxyl, acetoxy or methoxy group.
The compounds of the forrnula (III) are known or can be obtained in analogy to
known processes (cf.,, for example, DE 2,040,175; DE 2,119,518; Synthesis
1973,
703).
. . .. . . .
Suitable diluents for canying out the process according to the invention are
inert
organic solvents. These include, in particular, aliphatic, alicyclic or
aromatic,
optionally halogenated hydrocarbons, such as, for example, benzine, benzene,
toluene, xylene, cl dorobenz~ene, dichlorobenzene, petroleum ether, hexane,
cyclohexane, dichloromethane, chloroform or carbon tetrachloride; ethers, such
as
diethyl ether, diisopropyl ether, dioxane, tetrahydrofuran or ethylene glycol
dimethyl ether or ethylene glycol diethyl ether; ketones, such as acetone,
butanone
or methyl isobutyl ketone; nitriles, such as acetonitrile, propionitrile or
benzoni-
trile; amides, such as N,N-dimethylformamide, N,N-dimethylacetamide,
N-methylformanilide, N-methylpyrrolidone or hexamethylphosphoric triamide;
esters, such as medryl acetate or ethyl acetate, or bases, such as pyridine,
or
organic acids, such as formic acid or acetic acid.
The process according to the invention is preferably carried out in the
presence of
a suitable reaction auxiliary. Suitable reaction auxiliaries are all customary
inorganic or organic bases. These include, for exarnple, the hydrides,
hydroxides,
LeA29088 -27-
2148 6 12
amides, alcoholates, acetates, carbonates or hydrogencarbonates of alkaline
earth
metals or alkali met<<ls, such as, for example, sodium hydride, sodium amide,
lithium diethylamide, sodium methylate, sodium ethylate, potassium tert.-
butylate,
sodium hydroxide, potassium hydroxide, ammonium hydroxide, sodium acetate,
potassium acetate, c:alcium acetate, ammonium acetate, sodium carbonate,
potassium carbonate, potassium hydrogencarbonate, sodium hydrogencarbonate or
ammonium carbonate, organolithium compounds, such as n-butyllithium, and also
tertiary amines, such as trimethylamine, triethylamine, tributylamine, di-
isopro-
pylethylamine, tetraniethylguanidine, N,N-dimethylaniline, pyridine,
piperidine,
N-methylpiperidine, N,N-dimethylaminopyridine, diazabicyclooctane (DABCO),
diazabicyclononene (I)BN) or diazabicycloundecene (DBU).
In those cases where A in formula (III) represents an alcohol, alkanoyloxy or
alkoxy group, other suitable reaction auxiliaries are organic or inorganic
acids,
' such as, .for example, suJphurric acid, hydrochloric acid, p-
toluenesulphonic acid,
perfluorobutanesulphonic acid or strongly acidic ion exchangers.
If appropriate, the process according to the invention can also be carried out
in a
two-phase system, such as, for example, water/toluene or
water/dichloromethane,
if appropriate in the presence of a suitable phase-transfer catalyst. Examples
of
such catalysts which may be mentioned are: tetrabutylammonium iodide,
tetrabutylammonium bromide, tetrabutylammonium chloride, tributyl-methyl-
phosphonium bromicle, trimethyl-C13/C15-alkylammonium chloride, trimethyl-
C13/C15-alkylammonium bromide, dibenzyl-dimethyl-ammonium methylsulphate,
dimethyl-C12/C.14alkylbenzylammonium chloride, dimethyl-C12/C14-alkyl-benzyl-
ammonium bromide, tetrabutyl ammonium hydroxide, triethylbenzylammonium
chloride, methyltrioctylammonium chloride, trimethylbenzylammonium chloride,
15-crown-5, 18-crown-6 or tris-[2-(2-methoxyethoxy)-ethyl]-amine.
When canying out the process according to the invention, the reaction
temperatures can be varied within a substantial range. In general, the process
is
LeA29088 -28-
2148 6 12
carried out at temperatures between -70 C and +200 C, preferably at
temperatures
between 0 C and 130 C.
The process according to the invention is conventionally carried out unde:r
atmospheric pressure. However, it can also be carried out under increased or
reduced pressure.
To carry out the proa~ss according to the invention, 1.0 to 5.0 mol,
preferably 1.0
to 2.5 mol, of compoi.znd of' the formula (III) and, if appropriate, 0.01 to
5.0 mol,
preferably 1.0 to 3.0 rnol, of reaction auxiliary are generrally employed per
mole of
1 H-benzimidazole of the formula (H).
In a particular embodiment, it is also possible to first silylate the
1H-benzimidazoles of the formula (II) in a preceding reaction step with the
aid of
conventional silylatic-n processes, for example -using hexamethyldisilazane or
trimethylsilyl chloride, if appropriate in the presence of a suitable
catalyst, such as,
for example, sulphuric acid, trifluoroacetic acid, ammonium sulphate,
imidazole or
saccharin, at temperatures between -20 C and +50 C, and to react the 1-
trimethyl-
silylbenzimidazoles thus obtainable in a subsequent second step with
alkylating
agents of the formula (II) using the process according to the invention. In
this
case, it is advantageous to add tin tetrachloride to the alkylation reaction
to act as
a catalyst (cf., for emunple, Chem. Heterocycl. Comp. USSR 24, 514 [1988])
The reaction is carried out and the reaction product is worked up and isolated
by
known processes (cf in this context also the preparation examples).
The end products of the fonnula (I) are purified with the aid of conventional
processes, for example by column chromatography or by recrystallization.
They are characterized with the aid of the melting point or, in the case of
compounds which da not form crystals -in particular in the case of regioisomer
LeA29088 -29-
~1.4 3 6 12
mixtures -, with the ziid of proton nuclear resonance spectroscopy ('H-NMR).
The active compounds are suitable for combating animal pests, preferably
arthropods and nematodes, in particular insects and arachnids encountered in
agriculture, in forests, in the protection of stored products and materials,
and in the
hygiene sector. They are active against normally sensitive and resistant
species and
against all or some stages of development.
The abovementioned pests include:
From the order of the Isopoda, for example, Oniscus asellus, Armadillidium
vulgare and Porcellio scaber;
from the order of the Diplopoda, for example, Blaniulus guttulatus;
from the order of ihe Chilopoda, for example, Geophilus carpophagus and
Scutigera spec;
from the order of the Symphyla, for example, Scutigerella immaculata;
from the order of the Thysanura, for example, Lepisma saccharina;
from the order of the Collembola, for example, Onychiurus armatus;
from the order of the Orthoptera, for example, Blatta orientalis, Periplaneta
americana, Leucophaea maderae, Blattella germanica, Acheta domesticus,
Gryllotalpa spp., Locusta migratoria migratorioides, Melanoplus differentialis
and
Schistocerca gregaria;
from the order of the Dermaptera, for example, Forficula auricularia;
from the order of the Isoptera, for example, Reticulitermes spp.;
from the order of the Anoplura, for example, Phylloxera vastatrix, Pemphigus
spp.,
Pediculus humanus corporis, l:-laematopinus spp. and Linognathus spp.;
from the order of the Mallophaga, for example, Trichodectes spp. and Damalinea
spp=;
from the order of the Thysanoptera, for example, Hercinothrips femoralis and
Thrips tabaci;
from the order of the Heteroptera, for example, Eurigaster spp., Dysdercus
LeA29088 -30-
2148612
intermedius, Piesma (juadrata, Cimex lectularius, Rhodnius prolixus and
Triatoma
spp.;
from the order of the Homoptera, for example, Aleurodes brassicae, Bemisia
tabaci, Trialeurodes vaporariorum, Aphis gossypii, Brevicoryne brassicae,
Cryptomyzus ribis, Doralis fabae, Doralis pomi, Eriosoma lanigenun,
Hyalopterus
muidinis, Macrosiphum avenae, Myzus spp., Phorodon humuli, Rhopalosiphum
padi, Empoasca spp., Euscelis bilobatus, Nephotettix cincticeps, L.ecaiium
comi,
Saissetia oleae, Laodelphax striatellus, Nilaparvata lugens, Aonidiella
aurantii,
Aspidiotus hederae, Pseudococcus spp. and Psylla spp.;
from the order of the Lepidoptera, for example, Pectinophora gossypiella,
Bupalus
piniarius, Chei.matobia brumata, Lithocolletis blancardella, Hyponomeuta
padella,
Plutella maculipennis, Malacosoma neustria, Euproctis chrysorrhoea, Lymantria
spp., Bucculatrix thurberiella, Phyllocnistis citrella, Agrotis spp., Euxoa
spp., Feltia
spp., Earias insulana, Heliothis spp., Laphygrna exigua, Mamestra brassicae,
Panolis flammea, Prodenia litura, Spodoptera spp., Trichoplusia ni; Carpocapsa
pomonella, Pieris spp., Chilo spp., Pyrausta nubilalis, Ephestia kuehniella,
Galleria
mellonella, Tineola bisselliella, Tinea pellionella, Hofinannophila
pseudospretella,
Cacoecia podana, Capua reticulana, Choristoneura fiuniferana, Clysia
ambiguella,
Homona magnani:ma and Torlrix viridana;
from the order of the Coleoptera, for example, Anobium punctatum, Rhizopertha
dominica, Bruchidiu> obtectus, Acanthoscelides obtectus, Hylotrupes bajulus,
Agelastica alni, Leptinotarsa decemlineata, Phaedon cochleariae, Diabrotica
spp.,
Psylliodes chrysocephala, Epilachna varivestis, Atomaria spp., Oryzaephilus
surinamensis, Anthonomus spp., Sitophilus spp., Otiorrhynchus sulcatus,
Cosmopolites sordidus, Ceuthorrhynchus assimilis, Hypera postica, Dermestes
spp.,
Trogoderma spp., Ani:hrenus spp., Attagenus spp., Lyctus spp., Meligethes
aeneus,
Ptinus spp., Niptus hololeucus, Gibbium psylloides, Tribolium spp., Tenebrio
molitor, Agriotes spp., Conodenas spp., Melolontha melolontha, Amphimallon
solstitialis and Costelytra zealandica;
from the order of the Hymenoptera, for example, Diprion spp., Hoplocampa spp.,
Lasius spp., Monomorium pharaonis and Vespa spp.;
LeA29088 -31 -
2148612
from the order of the: Diptera, for example, Aedes spp., Anopheles spp., Culex
spp., Drosophila melanogaster, Musca spp., Fannia spp., Calliphora
erythrocephala,
Lucilia spp., Chrysomyia spp., Cuterebra spp., Gastrophilus spp., Hyppobosca
spp.,
Stomoxys spp., Oestrus spp., Hypode:rma spp., Tabanus spp., Tannia spp., Bibio
hortulanus, Oscinella frit, Phorbia spp., Pegomyia hyoscyami, Ceratitis
capitata,
Dacus oleae and Tipula paludosa;
from the order of the Siphonaptera, for example, Xenopsylla cheopis.
Ceratophyllus spp.;
from the order of the Arachnida, for example, Scorpio maurus and Latrodectus
mactans;
from the order of the .Acarina, for example, Acanas siro, Argas spp.,
Omithodoros
spp., Deimanyssus gallinae, Eriophyes ribis, Phyllocoptnrta oleivora,
Boophilus
spp., Rhipicephalus spp., Amblyomma spp., Hyalomma spp., Ixodes spp.,
Psoroptes spp., Choirioptes spp., Sarcoptes spp., Tarsonemus spp., Bryobia
15. ~ praetiosa, Panonychus 'spp. and Tetranychus. spp.
The plant-parasitic nematodes include Pratylenchus spp., Radopholus similis,
Ditylenchus dipsaci, 'Tylenchulus semipenetrans, Heterodera spp., Meloidogyne
spp., Aphelenchoides spp., Longidonts spp., Xiphinema spp., Trichodorus spp..
:20
The active compound:, according to the invention are not only active against
plant,
hygiene and stored product pests, but also, in the veterinary medicine sector,
against animal parasites (ectoparasites and endoparasites) such as scaly
ticks,
Argasidae, scab mites, Trombidae, flies (stinging and sucking), parasitic fly
larvae,
:25 lice, hair lice, bird lio.-, fleas and endoparasitic worms.
They are active against norrnally-sensitive and resistant species and strains
and
against all parasitic and non-parasitic development stages of the ecto- and
endoparasites.
:30
The active compounds according to the invention are distinguished by a
powe:rful
LeA29088 -32-
2148612
insecticidal activity.
They can be emplo;yed particularly successfully for combating plant-injurious
insects, such as, for example, against the larvae of the mustard beetle
(Phaedon
cochleariae) or against the caterpillars of the cabbage moth (Plutella
maculipennis)
or against other Plutella species, such as, for example, Plutella xylostella,
or
against the tobacco bud worm (Heliothis virescens) and for combating plant-
injurious mites, suchi as, f'or example, against the greenhouse red spider
mite
(Tetranychus urticae), or for combating plant-injurious nematodes, such as,
for
example, against the nematode species Globodera rostochiensis.
In addition, the active compounds according to the invention can also be
employed
for combating hygiene and stored product pests, such as, for example, against
the
house fly (Musca donnestica) or against the grain weevil (Sitophilus
granarius) or
against cockrokh'species, such as, for example, Blattella germanica or
Periplaneta
americana.
Moreover, the active compounds according to the invention can be employed
particularly successfully for combating parasitic pests of warm-blooded
species,
:20 such as, for example, against scab mites (Psoroptes ovis).
In addition, the active compounds according to the invention also have a
powerful
fungicidal activity and can be employed in practice for combating undesired
microorganisms. The active compounds are also suitable for use as fungicides.
Fungicidal agents in plant protection are employed for combating
Plasmodiophoromycetes, Oomycetes, Chytridiomycetes, Zygomycetes,
Ascomycetes, Basidiornycetes and Deuteromycetes.
Some causative organi-sms of fungal diseases which come under the generic
names
listed above may be mentioned as examples, but not by way of limitation:
LeA29088 -33-
~1486 12
Pythium species, such as, for example, Pythium ultimum;
Phytophthora species, such as, for exarnple,
Phytophthora infestans;
Pseudoperonospora species, such as, for example, Pseudoperonospora humuli or
Pseudoperonospora cubense;
Plasmopara species, such as, fbr example, Plasmopara viticola;
Peronospora species, such as, for example, Peronospora pisi or Peronospora
brassicae;
Erysiphe species, such as, for example, Erysiphe graminis;
Sphaerotheca species, such as, for example, Sphaerotheca fuliginea;
Podosphaera species, such as, for example, Podosphaera leucotricha;
Venturia species, sucht as, for example, Venturia inaequalis;
Pyrenophora species, such as, for example, Pyrenophora teres or Pyrenophora
graminea (conidia fonn: Drechslera, synonym: Helminthosporium); .
15Cochliobolus species, such as,' for example, Cochliobolus sativus (conidia
forin:
Drechslera, synonym: Helminthosporium);
Uromyces species, such as, for example, Uromyces appendiculatus;
Puccinia species, such as, for example, Puccinia recondita;
Tilletia species, such as, for example, Tilletia caries;
Ustilago species, such as, for example, Ustilago nuda or Ustilago avenae;
Pellicularia species, such as, for example, Pellicularia sasakii;
Pyricularia species, such as, for example, Pyricularia oryzae;
Fusarium species, such as, for example, Fusarium culmotwn;
Botrytis species, such as, for example, Botrytis cinerea;
Septoria species, such as, for example, Septoria nodorum;
Leptosphaeria species, such as, for example, I.eptosphaeria nodorum;
Cercospora species, suich as, for example, Cercospora canescens;
Altemaria species, such as, for example, Altemaria brassicae and
Pseudocercosporella species, such as, for example, Pseudocercosporella
herpotrichoides.
Le A 29 088 - 34 -
'2148 612
The good toleration, by plants, of the active compounds, at the concentrations
required for combating plant diseases, perniits treatment of above-ground
parts of
plants, of vegetative propagation stock and seeds, and of the soil.
In this context, the acitive compounds according to the invention can be
employed
with particular success for conibating cereal diseases such as, for example,
against
the causative organisrn of powdety mildew of cereals (Erysiphe graminis) or
for
combating diseases in fruit and vegetable growing such as, for example,
against
the causative organism of tomato blight (Phytophthora infestans) or against
the
causative organism oi' downy mildew of grapevine (Plasmopara viticola), or for
combating rice diseases such as, for example, against the causative organism
of
rice blast disease (Pyricularia oryzae).
Moreover, if used at appropriate application rates, the active compounds
according
to the invention can bE; used as defoliants, desiccants, agents for destroying
broad-
leaved plants and, especially, as weed-killers. By weeds, in the broadest
sense,
there are to be understood all plants which grow in locations where they are
undesired. Whether tl7e substances according to the invention act as total or
selective herbicides depends essentially on the amount used.
The active compound3 according to the invention can be used, for example, in
connection with the fcdlowing plants:
DigUledon weeds of ~:hegenera: Sinapis, Lepidium, Galium, Stellaria,
Matricaria,
Anthemis, Galinsoga, Chenopodium, Urtica, Senecio, Amaranthus, Portulaca,
Xanthium, Convolvulus, Ipomoea, Polygonum, Sesbania, Ambrosia, Cirsium,
Carduus, Sonchus, Solanum, Rorippa, Rotala, Lindemia, L.amium, Veronica,
Abutilon, Emex, Datwa, Viola, Galeopsis, Papaver and Centaurea.
I?icotvledon cultures of the genera: Gossypium, Glycine, Beta, Daucus,
Phaseolus,
Pisum, Solanum, Linum, lpomoea, Vicia, Nicotiana, Lycopersicon, Arachis,
LeA29088 -35-
2148612
Brassica, Lactuca, Cucumis and Cucurbita.
Monocotvledon weeds of the genera: Echinochloa, Setaria, Panicum, Digitaria,
Phleum, Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena, Cypeivs,
Sorghum, Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria, Eleocharis,
Scirpus, Paspalum, Ischaemum, Sphenoclea, Dactyloctenium, Agrostis, Alopecunas
and Apera.
onoco edon cultures of the genera: Oryza, Zea, Triticum, Hordeum, Avena,
Secale, Sorghum, Panicum, Sacchatvni, Ananas, Asparagus and Allium.
However, the use of the active compounds according to the invention is in no
way
restricted to these genera, but also extends in the same manner to other
plants.
The compounds are suitable, depending on the concentration, for the total
combating of weeds, for example on industrial tenain and rail tracks, and on
paths
and squares with or without tree plantings. Equally, the compounds can be
employed for combating weeds in perennial cultures, for example
afforestations,
decorative tree plantinigs, orchards, vineyards, citrus groves, nut orchards,
banana
plantations, coffee plantations, tea plantations, rubber plantations, oil palm
plantations, cocoa plantations, soft fruit plantings and hopfields, and for
the
selective combating of weeds in annual cultures.
The active compounds according to the invention can be employed with
particular
:25 success here for combating monocotyledon and dicotyledon weeds in
monocotyledon and dicotyledon cultures, such as, for example, maize, wheat or
soya.
Depending on their particular physical andlor chemical properties, the active
compounds can be converted into the customary formulations, such as solutions,
emulsions, suspensioris, powders, foams, pastes, granules, aerosols, natural
and
LeA29088 -36-
2148612
synthetic materials iinpregnated with active compound, very fine capsules in
polymeric substance;; and in coating compositions for seed, furthermore in
formulations used with buniing equipment, such as fi.unigating cartridges,
fumigating cans and futnigating coils and the like, as well as ULV cold- and
warm-mist formulaticins.
These formulations are produced in a known maruier, for example by mixing the
active compounds with extenders, that is liquid solvents, liquefied gases
under
pressure and/or solid carriers, optionally with the use of surface-active
agents, that
is emulsifying agents and/or dispersing agents and/or foam fonming agents. In
the
case of the use of water as an extender, organic solvents can, for example,
also be
used as auxiliary solvents. As liquid solvents, there are suitable in the
main:
aromatics, such as xylene, toluene or alkylnaphthalenes, chlorinated aromatics
or
chlorinated aliphatic hydrocarbons, such as chlorobenzenes, chloroethylene,s
or
methylene chloride,.aliphatic hydrocarbons, such as cyclohexane or paraffins,
for
example petroleum firactions, alcohols, such as butanol or glycol as well as
their
ethers and esters, ket:ones, such as acetone, methyl ethyl ketone, methyl
isobutyl
ketone or cyclohexan.one, strongly polar solvents, such as dimethylformamide
and
dimethyl sulphoxide, as well as water; by liquefied gaseous extenders or
carriers
there are meant liquids which are gaseous at normal temperature and under
atmos-
pheric pressure, for example aerosol propellants, such as halogenohydrocarbons
as
well as butane, propane, nitrogen and carbon dioxide; as solid carriers there
are
suitable: for example ground natural minerals, such as kaolins, clays, talc,
chalk,
quartz, attapulgite, nlontmorillonite or diatomaceous earth, and ground
synthetic
minerals, such as higNy disperse silica, alumina and silicates; as solid
carriers for
granules there are suitable, for example crushed and fra.ctionated natural
rocks such
as calcite, marble, ptnnice, sepiolite and dolomite, as well as synthetic
granules of
inorganic and organic meals, and granules of organic material such as sawdust,
coconut shells, maize cobs and tobacco stalks; as emulsifying and/or foam-
forming
agents there are suitable: for example non-ionic and anionic emulsifiers, such
as
polyoxyethylene fatty acid esters, polyoxyethylene fatty alcohol ethers, for
example
LeA29088 -37-
_
2~.48 6 12
alkylaryl polyglycol ethers, alkylsulphonates, alkyl sulphates,
arylsulphonates as
well as albumen hydrolysis products; as dispersing agents there are suitable:
for
example lignin-sulphite waste liquors and methylcellulose.
Adhesives such as carboxymethylcellulose and natural and synthetic polymers in
the form of powders, granules or latexes, such as gum arabic, polyvinyl
alcohol
and polyvinyl acetate;, as well as natural phospholipids, such as cephalins
and
lecithins, and synthetic phospholipids, can be used in the formulations.
Further
additives can be mineral and vegetable oils.
It is possible to use caAorants such as inorganic pigments, for example iron
oxide,
titanium oxide and Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs,
azo dyestuffs and melml phthalocyanine dyestuffs, and trace nutrients such as
salts
of iron, manganese, boron, copper, cobalt, molybdenum and tin.
The formulations in general contain between 0.1 and 95 per cent by weight of
active compound, preferably between 0.5 and 90%.
The active compowlds according to the invention can be present in their
commercially available formulations and the use forms prepared with these
formulations as a rr.iixture with other active compounds, such as
insecticides,
attractants, sterilants, acaricides, nematicides, fungicides, growth-
regulating
substances or herbicides. The insecticides include, for example, phosphates,
carbamates, carboxyl ates; chlorinated hydrocarbons, phenylureas and
substances
produced by microorganisms, inter alia.
The active compounds according to the invention can furthermore be present in
their commercially available formulations and in the use forms, prepared from
these formulations, as a mixture with synergistic agents. Synergistic agents
are
compounds which ir.icrease the action of the active compounds, without it
being
necessary for the syriergistic agent added to be active itself.
LeA29088 -38-
j14 36 12
The active compound content of the use forms prepared from the commercially
available formulations can vary within wide limits. The active compound
concentration of the use forms can be from 0.0000001 to 95 per cent by weight
of
active compound, preferably between 0.0001 and 1 per cent by weight.
The compounds are employed in a customary manner appropriate for the use
forms.
When used against hygiene pests and pests of stored products, the active
compounds are distinguished by an excellent residual action on wood and clay
as
well as a good stability to alkali on limed substrates.
The active compourids which can be used according to the invention are also
suitable for combatir-g insects, mites, ticks etc. in the sector of animal
keeping and
cattle breeding, betteT results, for example. higher milk production, greater
weight,
more attractive animal pelt, longer life etc., can be achieved by combating
the
pests.
The application of the active compounds which can be used according to the
invention occurs in this sector in a known fashion, for example, by oral
application
in the form of, tablets, capsules, potions or granules, by means of dermal or
external application in the form of, for example, dipping, spraying, pouring-
on,
spotting-on and dusting, as well as by means of parenteral application in the
form
of, for example, inje;.tion, and, furthermore, by means of the feed-through
process.
In addition, application as moulded articles (collar, ear tag) is also
possible.
When used as fungicides, the active compounds according to the invention can
also be present in the formulations as a mixture with other known active
compounds, such as fungicides, insecticides, acaricides and herbicides, and in
mixtures with fertilizers and growth regulators.
LeA29088 -39-
21~3 16 12
When used as fungicides, the active compounds can be used as such, in the form
of their formulations, or the use forms prepared therefrom, such as ready-to-
use
solutions, suspension,, wettable powders, pastes, soluble powders, dusts and
granules. They are used in the customary manner, for example by watering,
spraying, atomizing, scattering, dusting, foaming, brushing on and the like.
It is
furthermore possible to apply the active compounds by the ultra-low volume
method or to inject the active compound formulation or the active compound
itself
into the soil. The seeci of the plants can also be treated.
When used as fungicides in the treatment of parts of plants, the active
compound
concentrations in the use forms can be varied within a substantial range. They
are,
in general, between 1 and 0.0001% by weight, preferably between 0.5 and 0.001%
by weight.
When used as fungicides in the treatment of seed, amounts of active corrqwund
of
0.001 to 50 g per kilogram of seed, preferably 0.01 to 10 g, are generally
required.
When used as fungicides in the treatment of soil, active compound
concentrations
of 0.00001 to 0.1% by weight, preferably 0.0001 to 0.02% by weight, are
required
at the place of action.
When used as herbiciiies, the active compounds according to the invention, as
such
or in the form of their fonnulations, can also be used as a mixture with known
herbicides for combating weeds, fuiished formulations or tank mixes being
possible. Suitable herbicides for the mixtures are known herbicides, for
example
anilides such as, for example, diflufenican and propanil; arylcarboxylic acids
such
as, for example, dichloropicolinic acid, dicamba or picloram; aryloxyalkanoic
acids
such as, for examplo-1, 2,4-D, 2,4-DB, 2,4-DP, fluroxypyr, MCPA, MCPP and
triclopyr; aryloxy-phenoxy-alkanoic esters such as, for example, diclofop-
methyl,
fenoxaprop-ethyl, fluazifop-butyl, haloxyfop-methyl and quizalofop-ethyl;
azinones
such as, for example, chloridazon and norflurazon; carbamates such as, for
LeA29088 -40-
2143 512
example, chlorpropham, desmedipham, phenmedipham and propham; chloro-
acetanilides such as, for example, alachlor, acetochlor, butachlor,
metazachlor,
metolachlor, pretilacliilor and propachlor; dinitroanilines such as, for
example,
oryzalin, pendimethalin and trifluralin; diphenyl ethers such as, for example,
acifluorfen, bifenox, fluoroglycofen, fomesafen, halosafen, lactofen and
oxyfluorfen; ureas such as, for example, chlortoluron, diuron, fluometuron,
isoproturon, linuron and methabenzthiazuron; hydroxylamines such as, for
example, alloxydim, clethodim, cycloxydim, sethoxydim and tralkoxydim;
imidazolinones such as, for example, imazethapyr, imazamethabenz, imazapyr and
imazaquin; nitriles such as, for example, bromoxynil, dichlobenil and ioxynil;
oxyacetamides such as, for example, mefenacet; sulphonylureas such as, for
example, amidosulfiiron, 'bensulfiuron-methyl, chlorimuron-ethyl,
chlorsulfuron,
cinosulfuron, metsu].furon-methyl, nicosulfiaron, primisulfuron,
pyrazosulfuron-
ethyl, thifensulfuron-methyl, triasulfuron and tribenuron-methyl;
thiocarbamates
such as, for example, butylate, cycloate, di-allate, EPTC,- esprocarb,
molinate,
prosulfocarb, thiobeiicarb and tri-allate; triazines such as, for example,
atrazine,
cyanazine, simazine, simetxyn, terbutryn and terbutylazine; triazinones such
as, for
example, hexazinone, metaniitron and metribuzin; others such as, for example
aminotriazole, benfuresate, bentazone, cinmethylin, clomazone, clopyralid,
difenzoquat, dithiopyr, ethofiunesate, fluorochloridone, glufosinate,
glyphosate,
isoxaben, pyridate, quinchlorac, quinmerac, sulphosate and tridiphane.
When used as herbicides, a mixture with other known active compounds, such as
fungicides, insecticicles, acaricides, nematocides, bird repellants, plant
nutrients and
agents which improve soil structure, is also possible.
The active compounds can be used as such, in the form of their formulations or
in
the use forms prepared therefrom by further dilution, such as ready-to-use
solutions, suspensions, emulsions, powders, pastes and granules. They are used
in
the customary manner, for example by watering, spraying, atomizing or
scattering.
LeA29088 -41 -
CA 02148612 2003-11-13
30725-3
When used as herbicides, the active compounds
according to the invention can be applied either before or
after emergence of the plants. They can also be
incorporated into the soil before sowing.
When used as herbicides, the amount of active
compound used can vary within a substantial range. It
depends essentially on the nature of the desired effect. In
general, the amounts used are between 0.001 and 10 kg of
active compound per hectare of soil surface, preferably
between 0.005 and 5 kg per hectare.
Accordingly, the invention also provides:
compositions comprising the compounds of the invention;
methods of preparing the compositions and methods of
combating pests and parasites using the compounds and
compositions of the invention; and uses of the compounds and
compositions of the invention for combating pests and
parasites.
The preparation and use of the active compounds
according to the invention can be seen from the following
Examples.
- 42 -
2148612
PiepFUation Exama:
~
~
--N
~--CF3 ~ N
~
'
N I \C F3
CHZ O-CZHs "~ N
CHZ O-C2H5
7.9 g (0.03 mol) of 5(6)-phenyl-2-trifluoromethyl-lH-benzimidazole and 8.2 g
(0.06 mol) of pulveri:,-,ed potassium carbonate are refluxed for 15 minutes in
70 ml
of ethyl acetate, and the mixture is subsequently treated with 3.9 g (0.04
mol) of
(chloromethyt ethyl ether in :20 ml of ethyl acetate and refluxed for a
further 4
hours, with stirring. For working up, the cooled reaction mixture is washed
twice
using in each case 40 ml of water, dried over sodium sulphate, and
concentrated
in vacuo, and the m;idue is purified by chromatography over silica gel
(eluent:
dichloromethane).
6.9 g (71% of iheory;) of 1-ethoxymethyl-5(6}phenyl-2-trifluoromethyl-
benzimidazole are obtained as a regioisomer mixture in a ratio of 1:1.
'H-NMR (DMSadb/tetramethylsilane): d = 5.84 (s, 2H); 5.89 (s, 2H) ppm [in each
case N-CH -U-].
The following substituted benzimidazoles of the general formula (I) are
obtained
in a corresponding nianner and following the general preparation instructions:
LeA29088 -43-
2148612
x
2
N
\ ~ \ ~~-CF3 {I)
X3~ ~ N
2
X CH-R
II
R
Ex. Xl :(2 X3 X4 Rl R2 Physical
No. Properties
2 Br H CF3 H H CH M.P. 90-90 C
-N
C-OCH3
//
0
3 Br H CF3 H H -N,qHs m.p.70-74 C
C-OCHI
//
0
4 Br H CF3 H H ,n-qH7 m.p.75-79 C
-N
C-OCH3
//
0
Br H CF3 H H -CH=CH2 m.p.53-56 C
(H) (CF3) (H) (Br) (82:18)
6 Br H CF3 H H -CO-C(CH3)3 m.p.120-123 C
7 Br H CF3 H H -CH2-C6H5 m.p.80-84 C
8 Br H CF3 H H -CO-C6H5 m.p.163-166 C
LeA29088 -44-
~148612
Ex. X' XZ X3 X4 Rl R2 Physical
No. Properties
9 Br H( CF3 H H -CH=CH-CH3 m.p.80-83 C
(H) (CF'3) (H) (Br) (93:7)
Br F.[ CF3 H H C1 m.p.60-63 C
v,,CH =C
C H (E/Z=64:36)
3
11 H (CH3)=,N-CO- H H H -O-C2H5 1 H-NIvR*):
(13) ((CH3)2N-CO-) 5.59; 5.60;
7.54-8.62
12 H F2CH-CF2-0- H H H -O-C2H5
(1i) (F2CH-CF2-O-)
13 Br H CF3 H H -O-i-C3H7 1H-NMR
(H) (CF'3) (H) (Br) 5.94; 6.00
(63:37)
14 Br H: CF3 H H -O-n-C3H7 M.P. 70 73 C
(H) (CF'3) (1D (Br) (76:24)
Br H: CF3 H H -0-(CH2)3-C6H5 1H-NMR*):
(H) (CF'3) . ~) (Br) 5.94; 6.00.
(64:36)
16 Br H: CF3 H H-O-CH2-C=-CH m.p.71-73 C
17 Br H: CF3 H H m.p.195-200 C
0 _
-O-C-NH-c-NH ~ , C!
18 Br H: CF3 H H-O-CO-C(CH3)3 m.p.98-101 C
19 Br H CF3 H H C 1H-NMR#):
(H) (CF 3) (H) (Br) -O-CH2 0 6.08; .14
(70:30)
LeA29088 -45-
2148612
Ex. xl X2 X3 X4 Rl R2 Physical
No. Properties
20 Br H CF3 H H -O-C2H5 m.p.82-85 C
(H) (CF3) (H) (Br) (87:13)
21 Br H CF3 H H CH a m.p.128-130 C
-N
C-OCsHs
//
0
22 Br H CF3 H H CN m.p.147-151 C
23 H C6B5-CO- H H H -O-C2H5 1H-NMR*):
(H) (C6H5-CO-) 5.89
(1:1)
24 H C6H5-CO- H H H _N ci~ m,p,105-109 C
(Ei) (C6H5-CO-) ~C-ocH, (1:1)
o
25 H C6H5-CO- H H H CN m,p.102-105 C
Ci) (C6H5-CO-) (1:1)
26 H Ce~115.' H H If -N H_NMR*).
0-1) (C6H5) 'C-OCH66,02; 5,98
o/ (40:60)
27 Br H CF3 H H -0-CH2-CH2-O- cH3 1H-1VMRR):
(H) (C1'3) (H) (Br) 5.94; 6.03
28 Br H CF3 H H -N qHs m.p.103-106 C
C-0CzHs
//
0
LeA29088 -46-
~14 8 6 12
Ex. X1 ;K2 X3 X4 R1 R2 Physical
No. Properties
29 Br H CF3 H H in"CH, m.p.92-94 C
-N
C-0 CHS
//
0
30 Br l~ CF3 H H / icA m.p.70-73 C
-N
C-OC=HS
//
0
31 Br H CF3 H H /CH2 'CHs nyp.70-74 C
-N
// C-OCzHs
0
32 Br F[ CF3 H H /rHs m.p.70-73 C
-N
.=
C-03C,H9 .
0
33 H -()-(CH2)3-0- H H -O-C2H5 m.p.70-74 C
34 Br H CF3 H H -N(CH3)2
(x HCI)
35 H -()-(CH2)3-0- H H -N CH3 m.p.105-108 C
C-OCH3
//
0
LeA29088 -47-
'z14861?
Ex X1 X2 X3 X4 R1 R2
No. Physical
Properties
36 Br H CF3 H H /CC~Hii m.p.80-83 C
-N
C-OC2H5
/
0
37 Br H CF3 H H c6Hs m.p,135-136 C
-N
C-OCiHs
S
38 Br 1I CF3 H H CH2 CH=CH,
m.p.76-78 C
C-OC=HS
0
39 Br H CF3 H H ~aHs m.p,174-176 C
-N
// C--OC2Hs
S
40 Br H CF3 H H ,CH2 CH CHj
-N
C-OCm.p.109-112 C
iHs
//
0
41 Br CF3 H H -S-CH3 m.p.56-60 C
(H) (CF'3) (H) (Br) (1:1)
42 H CF3 CI H H -COOC2H5
(CI) (CF3)
LeA29088 -48-
Ex. X1 =(2 X3 X4 RI R2
No. Physical
Properties
43 H CF3 Cl H H ci
/-\
44 H C1 CF3 H H cl
/-\
45 H CF3 Cl H H Cl
~ D C
46 H (:l CF3 H H Cl
\-/ C
47 H CF3 Ct H H
((;1) (CF3) &HO=
48 H ClF3 Cl H H O
11
(C:1) (CF3) -P-OC=HS
OqHS
49 H CF3 Cl H H Cg3
(C:l) (CF3) \-/
50 H CF3 CI H -O-C2H5 -O-C2H5 Ii1.p.90-92 C
(C1) (CF3)
51 H CF3 Cl H H -CO-C(CH3)3
(Cl) (CF3)
52 H CI CF3 H H CN
53 H CF3 CI H H CN
54 H CF3 CI H H -CO-NH2
(CI) (CF3)
LeA29088 -49-
Ex. X1 X2 X3 X4 R1 R2 Physical
No. Properties
55 H CF3 Cl H H -CO-C6H5
(Cl) (CF3)
56 H CF3 Cl H H -OCH(CH3)2 1H-NMR
(CI) (CF3) A: 5.66; 7.83;
8.23
B: 5.71; 8.00;
8.06
57 H CF3 Cl H H 0 _ 1H-NMIIt*):
(1~1) (CF3) -C ~ i F A: 5.67; 7.43;
8.33 '
B: 5.73; 7.63;
8.10
58 H CF3 Cl H H O _ 1H-NMR*):
(CI) (CF3) -C ~ i CaHs A: 5.75; 7.45;
8.30
B : 5.78; 7.75;
7:97
59 -H CF3 CI H H p Cxs 1H-NMe):
(C1) (CF3) C A: 5.60; 7.41;
CH 8.28
' B: 5.63; 7.63;
8.06
60 H CF'3 Cl H H O _ 1H-NNIIt:
(Cl) (CF3) -C ~ i C A: 5.71; 7.42;
Cl 8.28
B: 5.75; 7.66;
8.06
IeA29088 -50-
4148612
Ex. Xi )'2 X3 X4 Rl R2 Physical
No. Properties
61 H CF3 CI H H 0 C 1H_NMR*):
((;1) (CF3) -C 0 A: 5.67; 7.39;
C 8.29
B: 5.73, 7.60 and
8.05
62 H CF3 CI H H 0 _ 1H-NMR*):
(Cl) (CF3) -C ~ i B A: 5.83; 7.68;
8.25
B: 5.90; 7.75;
8.03
63 H CF3 CI H H O _ 1H-NMR*):
(CI) (CF3) -C ~ / C A: 5.60; 7.38;
H3C 8.26
B: 5.64, 7.62 and
8.04
64 H CF3 Cl H H 0 Cl C 1H-NMR*):
(CI) (CF3) 5.30;'7.54;
8:22
B: 5.35; 7.75;.
8.02
65 H CF:; C1 H H 0 Cl 1H-NMR*):
(Cl) (CF3) -CCH2 ~:_\ C A 5.11; 7.15;
8.23
B: 5.15; 7.40;
8.02
IeA29088 -51 -
2148f12
Ex. Xi X2, X3 X4 RI R2 Physical
No. Properties
66 H CF 3 Cl H CH3 CN 1H-NMR}):
(Cl) (CF3) A: 5.60; 7.80;
8.34
B: 5.65; 7.96;
8.13
s
67 H CF3 Cl H H ,~Hs IH-NMR :
(C!) (CF3) -N
C-OCHA: 5.86; 7.98;
3
8.33
0 B: 5.90; 8.03;
8.21
68 H CF3 Cl H H ,~~H9 1H-N1~IIt}):
(CI) (CF3) -N A 5.85; 7.99;
/C-0C1H5 8.32
0 B: 5.90; 8.02;
8.22
69 H CF3 Cl H H CH 3 1H-NMR*):
(Cl.)(CF3) -N A:5.87;'7.98;
C-OCH3
i~ 8.34
0 B: 5.91; 8.05;
8.22
70 H -C,-CF2-CF2-O- H H -O-CH(CH3)2 1H-NMR*):
5.61; 7.45; 7.65
71 H -Cl-CF2-CF2-O- H H -CO-C6H5
m.p.141-143 C
72 H -G-CF2-CF2-0- H H CN mp.132-134 C
73 H -O-CF2-O- H H -0-CH(CH3)2 m.p.76-78 C
LeA29088 -52-
~148 612
Ex.
No. Xl ,i{2 X3 X4 Rl R2 Physical
Properties
74 H -O-CF2-O- H H -CO-C6H5 m.p,188-189 C
75 H -O-CF2-O- H H CN mp,145-147 C
76 H CF3 Br H H -O-CH(CH3)2 1H_NMR'):
(13r) (CF3) A: 5.65; 8.03;
8.23
B: 5.69; 8.05;
8.20
77 H CF3 Br H H Cl lH-N1VR.):
(Br) (CF3) ~~C A: 5.56; 7.59;
8.29
B: 5.59; 7.61;
8.26
78 H CF3 H H H -O-C2H5 1H-NMF:*):
(Fi) (CF3) A: 5.38; 7.18-
7.94;.
B: 5.40
79 H CF3 H H H OH 1H-N1vIR
(F~) (CF3) 2.2; 7.76;
8.1
80 H CF'3 Br H H -O-C2H5 IH-NMP, ).
(B(CF3) A: 5.64; 8.03;
8.21
B: 5.72; 8.06;
8.18
81 H CF3 Br H H -O-C2H5 mp.66 C
82 H Br CF3 H H -O-C2H5 1H-NMR*):
B: 5.72; 8.05;
8.17
Le A 29 088 - 53 -
214Sl 6 12
Ex. X1 X2 X3 X4 Rl R2 Physical
No. Properties
83 H CF3 Br H H -O-n-C3H7 1H-NMR*):
(Br) (CF3) A: 6.67; 8.08;
8.27
B: 5.69; 8.11;
8.25
84 H CF3 Br H H-O-CH2-C=-CH 1H-NM.R*):
(l3r) (CF3) A: 5.51; 7.89;
8.17
B: 5.71; 7.93;
8.21
85 H CF3 Cl H H -O-C2H5 1H-NMR*):
(Cl) (CF3) A: 5.69; 7.82;
8.23
B: 5.71; 8.00;
8.03
86 H C.F3 CI H H -O-C2H5 mp.73 C
87 H (11 CF3 H H -O-C2H5 1H-NMR*):
B: 5.71; 8.00;
8.03
88 H C]?3 Cl H H-O-CH(CH2F)2 1H-NMR*):
(C:1) (CF3) A: 5.83; 7.78;
8.03
B: 5.89; 8.01;
8.26
89 H CF3 Cl H H -O-n-C3H7 1H-NMR*):
(Cl) (CF3) . A: 5.70; 7.80;
8.06
B: 5.73; 7.99;
8.21
LeA29088 -54-
2148612
Ex. X1 X2 X3 X4 Rl R2 Physical
No. Properues
90 H CF3 Cl H H-O-CH2-C. CH 1H-NMR*):
;CI) (CF3) A: 5.73; 7.81;
8.04
B : 5.77; 8.00;
8.02
91 H (:F3 Cl H H CH3 1H-NMR*):
(Cl) (CF3) !NN A: 5.90; 8.00;
C-OC=HS
i/ 8.21
0 B: 5.93; 8.03;
8.31 =
92 H CF3 Cl H H ,C2Hs 1H-NMR*):
(Cl) (CF3) ~N A: 5.89; 8.00;
C-OC2Hs 8.21
0 B: 5.95; 8.03;
8.33
93 H CF3 Cl H H. /n-qH7 1H-NMTt*):
(C1) (CF3) = -N A: 5.89; 8:00;
C-OrHs
i/ 8.22
0 B: 5.91; 8.04;
8.32
94 H CF3 Cl H H -CO-OC2H5 mp.73 C
((:1) (CF3)
95 H CF3 Cl H CH3 -CO-OC2H5 1H-NMR*):
((:1) (CF3) A: 1.91; 5.34;
7.57; 8.12
96 H -O-CF2-O- H H -O-C2H5 m.p.92 C
LeA29088 -55-
2143612
Ex. X1 X2 X3 X4 Rl R2 Physical
No. Properties
97 H -O-CF2-O- H H-O-CH(CH2F)2 mp,.64 C
98 H -O-C:F2-O- H H -O-n-C3H7 m.p, 41 C
99 H -O-CF2-O- H H-O-CH2-C CH mp.87 C
100 H -O-CF2-O- H H -N CI43 mp.93 C
C-OCZHS
//
0
101 H -O-CF2-O- H H -N ,C7HS mp.67 C
C-OCzHs
//
0
102 H -O-CF2-O- H H /rl-qH., 1H-NIvIIZ*~-
____N 5.89; 7.51
C-OC=HS
//
0
103 H -,O-CF2-CF2-O- H H -O-C2H5 1H-NIvSR*):
5.63; 7.52; 7.63
104 H -1:)-CF2-CF2-O- H H -0-CH(CH2F)2 1H-NMR*):
5.82; 7.42; 7.68
105 H -O-CF2-CF2-O- H H ,~3 m.p.118 C
-N
C-OCH5
//
0
LeA29088 -56-
148 612
Eh. X1 X2 X3 X4 Rl R2 Physical
No. Properties
106 H -O-CF2-CF2-O- H H SHs m.p.85 C
-N
C-OCZH
//
0
107 H -O-CF2-CF2-O- H H / n-CsHT n1.p.103 C
-N
C-OC=Hs
//
0
108 H -O-CF2-CF2-O- H H -O-n-C3H7 1H-NMR*):
5.75; 7.48; 7.54
109 H -O-CF2-CF2-O- H H-O-CH2-C=CH 1H-NNIIt*):
5.81; 7.49; 7.68
110 H -O-CF2-CF2-0- H H -CO-OC2H5 m.p.90 C
111 H -0-CF2-CF2-O- H CH3 -CO-OC2H5 1H-1VMR*):
5.84; 5:34; 7.65 .'
112 H ==O-CF2-CHF-O- H H -O-C2H5 1H-N1v1R*):
(=-o-CHF-CF2-o-) 5.84; 7.64; 7.71
113 H =-O-CF2-CHF-O- H H-O-CH(CH2F)2 IH-IVMR*):
(-o-CI-iP-CF2-O-) 5.81; 6.01;
7.35; 7.61
114 H -0-CF2-CHF-O- H H -O-n-C3H7 1H-NMR*):
(-O-CHF-CF2-O-) 5.70; 6.03;
7.50; 7.60
115 H -O-CF2-CHF-O- H H-O-CH2-C CH 1H-NMR*):
(-o-CHF-CF2-o-) 5.56; 6.00;
7.46; 7.54
LeA29088 -57-
Ex. X1 X2 X3 X4 Rl R2 Physical
No. Properties
116 H -0-CF2-CHF-0- H H CH 3 IH-NMR*):
(-O-CHF-CF2-O-) -N. 5.78; 6.01;
C-OC=HS
i~ 7.43; 7.57
0
117 H -O-CF2-CHF-0- H H ,q2Hs IH-NMR#):
(-O-CHF-CF2-O-) -N 5.80; 6.00;
C-OC2H3
7.45; 7.48
0
118 H -0-CF2-CHF-O- H H ,n-qH, IH-NMR*}:
(-O-CHF-CF2-O-) -N 5.85; 6.05;
C-OC=HS
ii 7.53-7.68
0
119 H -=0-CF2-CHF-O- H H -CO-OC2H5 1H-NMR*):
(=-O-CHF-CF2-O-) 4.98; 6.03;
7.09; 7.63
120 H -0-CF2-CHF-O- H CH3 -CO-OC2H5 IH-NMR ):
(-O-CCHF-CF2-O-) 1.86; 6.01;
7.19; 7.62
121 H -O-CF2-CClF-O- H H. -O-C2H5 IH-NMR*):
(-O-CCIF-CF2-O-) 2.35; 7.15-7.98
122 H -'0-CF2-CC1F-0- H H -O-n-C3H7
(-,D-CCIF-CF2-O-)
LeA29088 -58-
z148612
Ex. XI '(Z X3 X4 Rl R2 Physical
No. Properties
123 H -0-CF2-CCIF-O- H H -O-CH2-C=CH
(-,O-CCIF-CF2-O-)
124 H -O\ c zO H H -O-C2H5 1H-NNIIt
F3C' "CR,fF3 5.62; 7.28; 7.32
125 H -O\ C~' O- H H CH 3 1H-NMR*):
F3C' \CH2 -CFs C-OCHS A: 5.78; 7.32;
7.44
0 B: 5.80; 7.32;
7.44
126 H --O\, O- H H ,C2HS 1H-NMR s.
-
]3C' CH2 ~F7 -N L-Q"3HS A: 5.76; 7.30;
~+
~i 7.42
0 B: 5.78; 7.30;
7.42
127 H --O\C'0~-- H H iN ~sK, 1H-i~iMR ):
Frs~ ~1:H2 CF3 A: 5.76; 7.32;
C--OqHs
7.42
0 B: 5.78; 7.32;
7.42
128 H CF-{O H H H -O-C2H5
(H) (CF30)
129 H CF-;O CF3O H H -O-C2H5 1H-NMR*):
5.50; 7.78; 7.82
LeA29088 -59-
1214 ~ 6 1~
Ex. X1 ):2 X3 X4 Ri R2 Physical
No. Properties
130 H CF3O CF3O H H -O-n-C3H7 IH-NMR*):
5.51; 7.75; 7.79
131 H CF'30 CF30 H H-O-CH2-CCH 1H-NMR#):
5.48; 7.76; 7.80
132 H CF'30 CF3O H H-O-CH(CH2F)2 1H-NMIIt*):
5.80; 7.78; 7.84
133 H C113-S02- H CF3 H -O-C2H5 1H-NMR*):
(CF3) (H) (CH3-S02-) (H) 5.80; 8.25; 8.56
134 H CF3 (CH3O) H H-O-C2H5 1H-NMR*):
(CH3O) CF3 A: 5.49; 7.05;
7.70
B: 5.50; 7.10;
7.73
135 H (C2H5)2N-CO- H H H-O-C2H5 1H-NMIt*):
(H) ((C2H5)2N-CO-) 5.73; 5.74;
7.29-8.63
136 H C21150-CO- H H H-O-C2H5 1H-NMR
(H) (C2H50-CO-) 5.72; 5.74;
7.65-8.59
137 H C6H,;-CO-NH- H H H -O-C2H5 1H-NNIIt*):
(H) (C6H5-CO-NH-) 5.70; 7.21-8.48;
7.98
138 H CH30-CO- H H H-O-C2H5 1H-NMR*):
(H) (CH3O-CO-) 5.72; 5.74;
7.68-8.59
LeA29088 -60-
~14~~12
The following subsi:ituted benzimidazoles of the general formula (Ia) are
additionally obtained in a corresponding manner:
a
X. ~ N
~ C ~}-CF3 (la)
X~ ~ N
CH2-O-C2H5
Ex. No. X2 X3 Physical
Properties
139 ~Iiz-OC2Hs H 1H-NMR*):
Cl cl )-CH= SiJz-N-
C11; 5.69; 5.70;
ct ~, cH- so= N 7.03-8.05
p
*):
140 \ ~>-Clij SOZ-NH- H IH-NMR
5.65; 5.67;
(H) 6.71-8:03 .
~- / Ci= 8p2-UH
141 CE;H5-SO2-NH- H
(H) (C6H5-SO2-NH-)
142 Ci H IH-NMR*):
C Cl 5.32; 5.63;
7.15-8.46
~ / 90=-NH-
LeA29088 -61 -
21_496 12
Ex. X2 X3 Physical
No. Properties
143 611 CF, HI H-NMR*):
~3 , 5.18; 5.63;
6.95-8.40
~) 9J2-NH-
144 0 H 1H-NMR
I I
(CH3)3C-CH1 O-C- Q 5.81; 5.82;
II 7.65-8.62
(H) )iC-CFLi O-C
145 Cl 0 H 1H-NMR
Cl-(~ ~NH-C-NH- 62(Clo) ~ ~ NHC-NH- 6.78-8.15
146 F'~H o o H IH-NMR
IL 5.53;=6.45-8.07
Fc
NIi-C-NH- F~~
FO O
II
(H) 0 < NH-c-NH-
147 ci j 0 H 1H-NIv~t
CiHsO-(CH=)=40--(~-)-NH-C-NH- CI O 5.51; 5.54;
'
11
(~ c,H,o{cti,h ~NH-C-NH) 6.71-8.01
LeA29088 -62-
2148M
Ex. X2 X3 Physical
No. Properties
148 ci
- H
-C,H,o{~; i NH-C-NH- Cl
0
(H) II
(-c,R7o.<CN)r~NH-C-NH-
149 0 H 1 H-NMR
C.iHsO{CHqk \~fNH-C-NH- 5.54; 5.58;
0 6.72-8.08
(H) II
CaI-IsO-(CHrv \ / NH-C-NH)-
150 H 1H-NIVIIZ*):
H'o~(cx'~ \ / Nx-c-Nx- 5.49; 5.53;
0
(H) i-- H,o{CH,)i \ / NH-C-NH- 6.61-8.11
The 'H-NNIR. spectra were recorded in deuterochloroform (CDC13) or
hexadeutero-d.imeth,yl sulphoxide (DMSO4) with tetramethylsilane (TMS)
as the internal standard. The data given are the chemical shift as 8 value in
ppm.
Le A 29 088 - 63 -
2148612
Prepmation of the slbwrting comnound:
Example II-1:
-N
~-CF3
H ~---CF3
cr N
N
H
18.4 g(0.092 mol) of 3,4-diaminobiphenyl and 150 ml of trifluoroacetic acid
are
refluxed for 5 hours. Excess trifluoroacetic acid is subsequently distilled
off, the
residue is partitionect between 200 ml of ethyl acetate and 70 ml of water,
the
organic phase is separated off, washed with in each case 70 ml of saturated
aqueous sodium hydrogen carbonate solution and water, dried over sodium
sulphate and concent3ated in vacuo, and the residue is purified by
chromatography
over silica gel (eluent: cyclohexanelethyl acetate 2:1).
18.3 g (76% of theory) of 5(6)-phenyl-2-trifluoromethyl-1H benzimidazole as a
1:1
regioisomer mixture of melting point 177-182 C are obtained.
NH
2
NH2
88 g (0.4 mol) of 4-=ino-3-nitro-biphenyl (92 per cent) are hydrogenated with
molecular hydrogen in 30(X) ml of inethanol in the presence of 10 g of Raney
nickel at 60 C and a pressure of 5 bar. For working up, the Raney nickel is
filtered off and the filtrate is concentrated in vacuo.
LeA29088 -64-
___
8 6 12
69.2 g (86% of theory) of 3,4-diaminobiphenyl of melting point 96-99 C are
obtained (purity according to HPLC 92%).
NHZ
NO2
43 g(0.15 mol) of 4-acetamido-3-nitro-biphenyl (90 per cent) and 1.6 g (0.03
mol)
of sodium methylate are refluxed for 2 hours in 500 ml of inethanol. For
working
up, the cooled reaction mixture is poured into 1300 ml of ice-water and stin-
ed for
10 minutes, and the precipitate which has separated out is then filtered off
with
suction and dried.
33 g (94% of theory) of 4-amino-3-nitro-biphenyl of melting point 163-165 C
are
obtained (purity according to HPLC 92%).
O
( ?-- NH-C\
~--' C H3
NO2
A mixture of 50.4 ml (1.2 mol) of 98 per cent strength nitric acid and 60 ml
of
glacial acetic acid is added dropwise with stimng at 70 C to a suspension of
84.4 g (0.4 mol) of 4acetamido-biphenyl (compare, for example, Beilstein
Volume
12, 4th Supplement, p. 3248) in 340 ml of glacial acetic acid, and, when the
addition has ended, the mixture is stiured for a further hour at 70 C. For
working
up, the cooled reaction mixture is poured into 1.00 ml of ice-water and
stiired for
10 minutes, and the precipitate which has separated out is filtered off with
suction,
washed with 200 ml of water and dried.
L e A 29 088 - 65 -
100 g (88% of theory;, of 4-acetamido-3-nitro-biphenyl of melting point 128-
131 C
are obtained (purity according to HPLC 90%).
The following 1 H-benzimidazoles of the formula
~
X
z
N
3~ I ~--CF3
X N
H
X
are obtained in a corresponding manner.
LeA29088 -66-
2t 48 G 12
Ex. X1 X2 X3 X4 Physical
No.
Properties
II-2 Br H CF3 H mp.149-151 C
(H) (CF3) p (Br)
11-3 H H C6H5-CO- H mp.120-122 C
((,'6H5-CO-) (H)
II-4 H CH3-CO- H H mp.145-149 C
(H) (CH3-CO-)
II-5 H C1-CH2-S02- H H mp.197-200 C
(H) (CI-CH2-SO2-)
11-6 H -O-CH2-CH2-CH2-O- H mp. >230 C
11-7 H H3C-SO2- H H
(M (Ii3C-SO2-)
11-8 Br H C1-CH2-S02- H mp.180-187 C
(H) (C1-CH2-S02-) (H) (Br)
11-9 H CF3 Br H mp.209 C
(Br) (CF3)
11-10 H -O-CF2-O- H ni.p.242 C
II-11 H -O-CF2-CF2-0- H m.p.235-237 C
11-12 H -O-CF2-CHF-O- H mp.217 C
(-O-CHF-CF2-O-)
11-13 H -O-CFCI-CFCI-O- H m.p.185 C
11-14 H CF30 CI H mp.144 C
(Cl) (CF30)
A2948S -67-
2148 512
Ex.
No. X1 X2 X3 X4 Physical
Properties
II-15 H ~~~c "lo+ H mp.209 C
F,C' 'C'H2-CF3
11-16 H CF3O H H m,p,168 C
(H) (CF30)
11-17 H CF3O CF3O H m.p.158 C
11-18 H CH3-S02- H CF3 mp.105 C
(CF3) (H) (CH3-SO2-) (H)
11-19 H CF3 CH3O H m.p.60 C-
(CH30) (CF3)
11-20 H (C2H5)N-CO- H H rrLp, 125 C
(H) ((C2H5)N-CO-)
II-21 H C2H50-CO- H H mp.140 C
(H) (C2H50-CO-)
11-22 H C6H5-CO-NH- H H mp.*202 C
(H) (C6H5-CO-NH-)
11-23 H CH3O-CO- H H m.p.157 C
(H) (CH3O-CO-)
11-24 H (CH3)2N-CO- H H m.p, 226-227 C
(H) ((CH3)2N-CO-)
11-25 H F2CH-CF2-0- H H mp.181 C
(H) (F2CH-CF2-O-)
11-26 H C6H5-SO2-NH- H H mp, 70 C
(H) (C6H5-SO2-NH-)
LeA29088 -68-
M86 12
Ex. XI X2 X3 X4 Physical
No.
Properties
II-27 H Cl H H
~-9o,-NH- Cl m.p.67 C
(H) ~ / 9J2-Nx
II-28 H CF3 H H r3lp, 79 C
-902-NH- CF3
(H) C 902-NH-
II-29 H 0
11 H H m.p.214-215 C
(3i3)3C-CH= O-C-
O
(H) tl
(CHAC-CHj-O-C-)
II-30 H - ct 0 H H m.p.254-255 C
ct-~ / NH-c-NH-
(H) EdHLH-
-69-
Le
~14~~12
Ex. X1 X2 X3 X4 Physical
NO= Properties
11-31 H F'C4;No 0 H H mp.103 C
FsC--~ II
O } NH C-NH-
\ /
F,C-CH,
O
II
(H) O\-/ 1VH-C-IVH-
II-32 H c[ _ H H m.p. 186 C
C1H,()(CF~)i-G \ / NH-C-NH-
cl O
(r-1-40.(CNk-G\ / MH-c11-NH)-
11-33 H cl H H mp.144 C
nc,E[,accx, \ / rrH-C-rht-
ct p
l
"'qH~C~ \ / ~-Cl-IiH-
11-34 H = _ 0 H H mP= 207 C
~H:~=(c~h \ / rrH-C-r[x-
0
-1
~ (r-,,,0{cx,h O taH-C-rnt)-
LeA29088 -70-
~143612
Ex. X1 X2 X3 X4 Physical
No.
Properties
11-35 H o
~-c,:",o{Clt,~ ~ / NH-c-NH- H H m.p.201 C
0
n
iC- H7O(CH2), ~ / A1H-C-NH-
11-36 H C, "~IOCH' H H m.p.80 C
CI- C/ (M1 S0= N
~N -or,
CI ~ / CIii 9~1
~
11-37 H (CF3)2N- H H
(H) ((CF3)2N-)
II-38 H H m.p.68 C
(H) ~ ~ CEis 11-39 H CF3S H H m.p.174 C
(H) (CF3S)
I1-40 H FC1CH-CF2-O- H H m.p.57 C
(H) (FCICH-CF2-O-)
LeA29088 -71-
8612
Ex. X1 }(2 X3 X4 Physical
No. Properties
11-41 H Cl= o H H m.p.176 C
11
Cl- ~NH-C-NH- Cl 0
11
(1-~ C ~ ~ (1NHNH)
11-42 H CH, so,-NH--O so,-rn-i- H H
01) ( II-43 H p H H m.p.190 C
~N1H-C-NH
I I
~~ (i a NH-OC-NH- 11-44 H _ 0 H H m.p.208 C
cH,O{CHA NH-c-NH-
0
11
-NH-
Of) cH,O-,CNh ~NH-c
LeA29088 -72-
__
2148612
Ex. X1 X2 X3 X4 Physical
No. Properties
II-45 H yCH3)3C-O-CO- H H m.p.162 C
(H) ((CH3)3C-O-CO-)
11-46 H COOCH3 H H .p.70 C
SOz-NH- COOCH,
(H) QH H
i:O
, NH-C-NH Cl O
\ II
~ , NH-C-NH-
(M
I1-48 H ~--~ 0 H H m.p.61 C
02N--(, . >--C-NH- . 0 ~: II = .
~ 02N O C-NH- .
II-49 H QCNH H H m. p.76 C
- 0
C:L I I
C-NH-
(R) C1
LeA29088 -73-
2 t 4 3 6 12
ER= X1 X2 X3 X4
No Physical
.
Properties
11-50 H (-'H,O-CP-N-9Jz-NH- H H
~H3
Ei3O=CO-N-87Z NH-
~7
11-51 H COOH H H m.p.250 C
(H) (COOH)
11-52 H (CH3)3(:-NH-CO- H H m.p.79 C.
(H) ((CH3)3C-NH-CO-)
II-53 H ~H ~ H H m.p.39 C
F',C-C-=NH-C-
I
CH3 CH3 0-
11
F3C-C-NH-C
(H) ~H,
11-54 H A(C-CH2- H H
(H) (NC-CH2-)
11-55 H NH2 H H
(H) (NH2)
II-56 H HOOC-CH2- H H
(H) (HOOC-CH2-)
II-57 H F3C-S02- H H
(H) (F3C-SO2-)
The 'H-NMR. spectra were recorded in deuterochloroform (CDC13) or
hexadeuterodimethyl sulphoxide (DMSO-d6) with tetramethylsilane (TMS)
as the internal standard. The data given are the chemical shift as b value in
ppm.
Chloro-(2-halogeno-1-fluoromethyl-ethoxy)-methanes of the formula
Le A 29 088 - 74 -
2148612
/C H2X
Ci-CH2-O-C\H
CH2F
in which
X represents fluorine or chlorine
[Specifically, these are chloro-(2-fluoro-l-fluoromethyl-ethoxy)-methane
(formula
(I), X = fluorine) and chloro-(2-chloro-l-fluoromethyl-ethoxy)-methane
(formula
(I), X = chlorine).)
can be obtained by rEmcting halogenated isopropanols of the formula
.. . . . . .
CH2X
HO-C\H
C H2F
in which
X represents fluorine or chlorine,
with formaldehyde and hydrogen chloride at -20 to +20 C.
They can be used for the preparation of substituted benzimidazoles of the
forrnula
Le A 29 088 - 75 -
2148612
;K
2
X N
X3 Y' N~--C F3 C H2X
~
4 CH2-O-C\H
CH2F
in which
X represent fluoi:-ine or chlorine and
X', V, X3 and V independeritly of one another in each case represent hydrogen,
halogen, cyano, nitro, in each case optionally substituted alkyl, alkoxy,
alkylthio, al]icylsulphinyl, alkylsulphonyl or cycloalkyl, optionally
substituted, fused dioxyalkylene; or hydroxycarbonyl, alkylcarbonyl;
alkoxycarbonyl, cycloEakyloxycarbonyl, in each case optionally substituted
ami.no or arninocarbcinyl or in each case optionally substituted aryl,
aryloxy, arylthio, arylsulphinyl, arylsulphonyl, arylsulphonyloxy,
arylcarbonyl, aryloxyc:arbonyl, arylazo or arylthiomethylsulphonyl, but
where at least one of the substituents X', V, X3 or V represents halogeno-
alkyl wi.th the exception of the chloromethyl radical, halogenoalkoxy,
halogenoalkylthio, halogenoalkylsulphinyl, halogenoalkylsulphonyl,
alkylsulphonyl, optionally substituted, fused dioxyalkylene, or
hydroxycarbonyl, alkylcarbonyl, alkoxycarbonyl, cycloalkyloxycarbonyl, in
each case optionally substituted amino or aminocarbonyl, or in each case
optionally substituted aryl, arylthio, arylsulphinyl, arylsulphonyl,
arylsulphonyloxy, arylcarbonyl, aryloxycarbonyl, arylazo or arylthio-
methylsulphoriyl,
from benzimidazoles of the formula
LeA29088 -76-
21~~~12
x
2
x N
3 ~--C F3
x N
Example X H
192 g of 1,3-difluoro-2-propanol were treated with 66 g of paraformaldehyde
(finely powdered). At -10 C, a vigorous stream of hydrogen chloride gas was
then
passed in, with stirring, until a clear 2-phase mixture had formed. The
organic
phase was subsequenitly separated off, dried using calcium chloride and
subjected
to fiactional distillation in vacuo. With a boiling point of 50 to 54 C at 20
mbar,
183 g(60% of theory) of chl~oro-(2-fluoro-1-fluoromethyl-ethoxy)-methane were
obtained. The characteristic absorptions in the NMR spectra were as follows:
'H-NMR: 5.6 ppm and 4.55 ppm.
19F-NMR: -233 ppm.
Fluorinated 1,3-benz&-dioxoles of the forrrmula
LeA29088 -77-
R
Ft 2 4 3
1 O 2 CF3
R3 6 4 O CHXCF3
R
in which
X represents hydrogen, iluorine, chlorine or broniine and
R' and R4 can be identical or different from each other and in each case
denote
hydrogen, halogen, C,-C6-alkyl, C,-C6-alkoxy, halogeno-C,-C6-alkyl,
C6-C,o-aryl, COOI1, CN, NCO, COO-C,-C6-alkyl, NH-C,-C6-alkyl or
N(C,-C6-alkyl)2, and
RZ and R3 represent NO2 or NHZ,
can be obtained by reacting 1,2-dihydroxybenzenes
R
R2 ~ OH
3 ~ ~
R 4 OH
R
in which
R' to R4 have the abovementioned meaning,
with hexafluorobutene of the formula
Le A 29 088 - 78 -
~14 86 12
CF3 X2
,
X C F3
cis-trans
in which
X' represents hydrogen or halogen and
~ represents halogen,
in the presence of a base and of a diluent at -20 to +200 C,
or by first reacting 1,2-dihydroxybenzenes which have a protective group, of
the
formula
R
R2 pRs
1
R 3 4 OH
R
in which
R' to R4 have the ab)vementiioned meaning and
RS represents a protective group or
RS together with R' represents a-C(CH3)2-0- radical
LeA29088 -79-
2148612
with a hexafluorobutene of tlle formula
CF3 X2
X CF3
cis-trans
in which
X' represents hydrogen or halogen and
V represents hallogen,
thus resulting in an intermediate of the formula
R
R? OR5
3 CF3
R O-C =C
R' I X ~
CF3
in which
R' to R4, RS and X' lqave the abovementioned meaning,
eliminating the protective group RS from the intermediate of the above
formula,
and
reacting the resulting OH compound with a base, thus obtaining 1,3-benzo-
dioxoles
of the above formula.
Le A 29 088 - 80 -
1,3-Benzo-dioxoles which have two adjacent amino groups can be converted with
trifluoroacetic acid tc- give the corresponding benzimidazole, for example of
the
following formula
R
N C 0 C F3
F=3C--~ I K
Ii O CHXCF3
R4
~
F
in which
R', R4 and X have the abovernentioned meaning.
By alkylation, benzirnidazole. derivatives can be'obtained from these which
are
substituted on the niti ogen atom by a
9
R
-CH radical.
Le A 29 088 - 81 -
21.4--8612
Examples
E-x=le 1 a
2-(2,2,2-Trifluoroethyl)-2-trifluoromethyl-1,3benzodioxole
11 g of pyrocatechol were (fissolved in 200 ml of dimethylformamide and the
solution was treatecl with 1.8 g of 45% strength by weight aqueous sodium
hydroxide solution. The mixture was treated dropwise at 75 C with 20 g of
2-chloro-1,1,1,4,4,4-hexafluoro-2-butene. Stirring was continued for 30
minutes at
75 C. The batch was subsequently poured into 500 ml of ice-water and extracted
using diethyl ether. The organic phase was washed with water, dried with
magnesium sulphate and concentrated. Finally, the product was distilled under
a
high vacuum. The yield was 15 g(=56 ,/o), and the boiling point 60 C at 10
mbar.
The NMRspectra- showed the following characteristic ab sorptions: 19F-1VNIR: -
59.0
and -84.6 ppm. 'H-NMR 3.02 ppm.
Ex=le 2a
2-(1-Chloro-2,2,2-triiluoroethyl)-2-trifluoromethyl-1,3-benzodioxole
110 g of pyrocatechol were dissolved in 1500 ml of acetonitrile, and the
solution
was treated with 200 g of trielhylamine. The mixture was treated dropwise at
75 C
with 235 g of 2,3-dichloro- 1,11,1,4,4,4-hexafluoro-2-butene. Stirring was
continued
for 2 hours at 75 C.1200 ml of the solvent were subsequently distilled off in
vacuo, and the residue was taken up i.n 1500 ml of water. The product was
extracted using diethyl ether, and the organic phase was washed twice using
10%
strength by weight aclueous sodium hydroxide solution and once with water.
After
drying with magnesium sulphate, the organic phase was concentrated and
subjected
to fractional distillation in vacuo. The yield was 258 g (= 84% of theory).
The
boiling point was 6:3 C at 12 mbar. The NMR spectra showed the following
L.eA29088 -82-
~14-8 6 1 w
characteristic absorptions: '9F-NMR: -66.8 and -79.7 ppm. 'H-NMR: 4.71 ppm.
Examples 3a
2-(1,1,1,4,4,4-Hexafluoro-2-butenoxy)-methoxybenzene
260 g of 2-methoxyphenol were dissolved in 1 1 of dimethylformamide (technical
grade) and the solution was treated with 220 g of 45% strength sodium
hydroxide
solution. 400 g of 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene were then added
dropwise with stirring at 22 C:. Stirring was continued for 2 hours up to 22
C. The
mixture was then txezted with 1.5 1 of ice-water and extracted with methylene
chloride.
The combined organic phases were washed twice using 10% strength sodium
hydroxide solution and once using saturated NaCI solution, dried using MgSO4
and
distilled. The yield was 329 g (58% of theory), and the boiling point was 68-
70 C
at 12 mbar. The NMR spectra showed the following characteristic
absorptions:19F-
NMR -57.6 and -67.9 ppm. 'H-NMR: 5.92 ppm.
Bxamnle 4a
2-(1,1,1,4,4,4-Hexafluoro-2-butenoxy)-phenol
286.1 g of 2-(1,1,1,4,4,4-hexa.fluoro-2-butenoxy)-methoxybenzene of Example 3a
were dissolved in a rnixture of 500 ml of glacial acetic acid and 500 ml of
48%
strength hydrobromi.c acid, and the mixture was treated with 5 g of
triethylbenzylammonium chloride. The mixture was stirred at a bath
temper=ature
of 150 C until a gas-chroniatographic check showed that the reaction was
complete. The mixture was then allowed to cool and treated with 2 kg of ice-
water. The aqueous phase was extracted thoroughly using CH2C12. After drying
with MgSO4, the solvent was stripped off and the residue distilled in vacuo.
The
Le A 29 088 - 83 -
yield was 200 g(50 'o of theory), and the boiling point was 80 C at 16 mbar.
The
NMR spectra showed the following characteristic absorptions: 14F-NMR: -59.6
and
-69.6 ppm. 'H-NMR: 6.1 ppm.
Exa nr ple 5a
2-(2,2,2-Trifluoroethyl)-2-trifl.uoromethyl-1,3-benzodioxole
200 g of 2-(1,1,1,4.,4,4-Hexafluoro-2-butenoxy)-phenol of Example 4a were
dissolved in 400 ml of acetonitrile and the solution was treated with 5 g of
triethylamine. The mixture mias stirred for 4 h at 70 C. It was then distilled
in
vacuo. The yield was 162 g (81% of theory), and the boiling point was 60 C at
10 mbar. The NMR spectra showed the following characteristic absorptions: 19F-
NMR: -59.0 and -84.6 ppm. 'H-NMR 3.02 ppm.
~Aa=le 6a
2-(2-Chloro- 1,1,1,4,4,,4-hexafluoro-2-butenoxy)-1-benzyloxybenzene
20 g of 2-benzyloxyphenol were dissolved in 100 ml of dimethylformamide and
the solution was treated with 9 g of 45% strength sodium hydroxide solution.
23 g
of 2,3-dichloro-1,1,1,4,4,4-hexafluoro-2-butene were then added dropwise at
room
temperature. After the exothermic reaction had subsided, stining was continued
for
1 hour at room temperature. and the mixture was poured into water and
extracted
using tert.-butyl methyl ether. After drying with MgSO4, the solvent was
stripped
off. The yield was 29 g (74% of theory). The NMR spectra showed the following
characteristic absorptiions: 'vF--NMR -59.5; -60.5; -61.7 and -62.8 ppm.
LeA29088 -84-
Exa=le 7a
2-(2-Chloro- 1,1,1,4,4~,4-hexafluoro-2-butenoxy)-phenol
24.4 g of 2-(2-chloro- 1,1,1,4,4,4-hexafluoro-2-butenoxy)-1-benzyloxybenzene
of
Example 6a were diissolved in 150 ml of tetrahydrofuran and the solution was
treated with 3 bar hydrogen for 4 hours at room temperature in the presence of
2 g
of Pd/C (10% strength). The imixture was subsequently filtered and the
filtrate was
concentrated and distilled in vacuo. The yield was 13.2 g (69% of theory), and
the
boiling point was 56 C at 0.15 mbar.
F&=le 8a
2-(1-Chloro-2,2,2-triiliuoroethyl)-2-trifluoromethyl-1,3-benzodioxole
. , .
11.7 g of 2-(2-chloro-1,1,1,4,4,4-hexafluoro-2-butenoxy)phenol of Example 7a
were dissolved in 40 ml of tert.-butyl methyl ether and the solution was
treated
with 40 ml of 1N sodium hydroxide solution. After the mixture had been stirred
for 30 minutes at room temperature, the organic phase was separated off, dried
using MgSO4 and distilled. The yield was 10 g (88% of theory), and the boiling
point was 63 C at 12 mbar. The NMR spectra showed the following characteristic
absorptions: '9F-NM1Z -66.8 and -79.7 ppm. 'H-NMR: 4.71 ppm.
Ex le
2,2-Dimethyl-4-(1,1,11,4,4,4-hexafluoro-2-butenoxy)-1,3-benzodioxole (Formula
V,
RS together with R' == -C(CH-3)2-0- radical)
46 g of 2,2-dimethyl-4-hydroxy-l,3-benzodioxole (Formula IV, RS together with
R3 =-C(CH3)2-0- radical) were dissolved in 200 ml of N-methylpyrrolidone and
the solution was treated widt 31 g of 40% strength by weight aqueous sodium
LeA29088 - 85 -
21412
hydroxide solution. 54.8 g of 2-chloro- 1, 1, 1,4,4,4-hexafluoro-2-butene were
subsequently added dropwise at room temperature with stin-ing. Stirring was
continued for 1 hour, and the batch was then poured into water and extracted
using
tert.-butyl methyl ether. The organic phase was washed using 10% strength by
weight aqueous sodilun hydroxide solution and dried using magnesium sulphate,
and the readily volatile components were removed on a rotary evaporator. This
gave 73.8 g (= 80% of theory) of a product whose purity was 95% according to
gas chromatography. The characteristic absorptions in the NMR spectra were:
19F-
NMR: -58.1 and -68.5 ppm. 'H-NMR: 6.73, 6.55, 6.03 and 1.70 ppm.
Exanmle l0a
1,2-Dihydroxy-3-(1,1,1,4,4,4-hexafluoro-2-butenoxy)-benz.ene
65 g of the product of Example 9a and 200 nil -of concentrated aqueous
hydrochloric acid were. refluxed for 4 hours with stimng. The batch was
subsequently diluted with 300 ml of water and extracted using methylene
chloride.
After drying with rriagnesiutn sulphate, the solvent was stripped off from the
organic phase, giving 54 g of a product of 90% purity. Recrystallization from
cyclohexane gave o:)lourless crystals having a melting point of 105 C. The
characteristic absorptions in the NMR spectra were as follows: 'gF-NMR -57.7
and
-67.7 ppm. 'H-NMR: 6.77, 6.50, 6.21 and 5.42 ppm.
Exam in e 11 a
2-(2,2,2-Trifluoroeth)/l)-2-(trifluoromethyl)-4-hydroxy-1,3-benz,odioxole
(Formula
(I), R' =OH, X=H., A=CH, RZ andR3=H)
43.5 g of the product of Example l0a were dissolved in 300 ml of acetonitrile,
and
1.5 g of triethylamine were added at room temperature. After the mixture had
been
stirred for 2 hours at room temperature, the solvent was stripped off and the
Le A 29 088 - 86 -
2 ~ 0~~2
residue was distilled in vacuo. The yield was 17 g (= 39% of theory), the
boiling
point was 85 C at 0.15 mbar, and the melting point was 65 C. The
characteristic
absorptions in the NlvIR spectra were as follows: 19F-NMR -59.0 and -84.5 ppm.
'H-NMR: 6.80, 6.55, 6.2 and 3.01 ppm.
Examnle 12a
2,2-Dimethyl-4-(3-chloro-1,1,1,4,4,4-hexafluoro-2-butenoxy)-1,3-benzodioxole
(Formula (V), R' and RS together are -C(CH3)2-0-, X' = Cl, R2 + R3 = H, A =
CH)
33.2 g of 2,2-dimeth.yl-4-hydroxy-1,3-benzodioxole were reacted analogously to
Example 9a with 47 g of 2,3-dichloro- 1, 1, 1,4,4,4-hexafluoro-2-butene. The
product
obtained was distilled in vaci.io, and a 1:1 molar mixture of cis/transisomers
was
obtained. The yield was 51 g70%0 of theory), and the boiling point was 70 C
at 0.15 mbar. The characteristic absorptions in the NMR *spectra were as
follows:
19F-NIVIR: -60.0, -61.6, -62.2 and 63.4 ppm. 'H-NMR: 6.79, 6.65 to 6.48 and
1.7 ppm
Exam in e 13a
1,2-Dihydroxy-3-(3-chloro-1, ]1,1,4,4,4-hexafluoro-2-butenoxy)-benz.ene
(Formula
(V),R'=OH,RZ+R3=H,A=CH,RS=H,X'=Cl)
18 g of the product of Example 12a were reacted analogously to Example 1 Oa
with
50 ml of concentrated hydrochloric acid. 15.7 g of a product with a purity of
97%
were obtained. The product was a 1:1 molar mixture of the cis/trans isomers.
The
characteristic absorptions in the NMR spectra were as follows: 19F-NMR: -60.2,
-61.3, -62.2 and -63.3 ppm. '1:-1-NNIR: 6.80, 6.45 and 6.25 ppm.
Le A 29 088 - 87 -
Example 14a
2-(1-Chloro-2,2,2-trifluoroethyl)-2-trifluoromethyl-4-hydroxy-1,3-benzodioxole
15 g of the product of Examp;le 13a were dissolved in 50 ml of acetonitrile
and the
solution was treated with 1. rni of triethylamine. The mixture was stiirred
for 15
niinutes, the solvent was then. stripped off, and the residue was distilled in
vacuo.
For purification, the product was taken up in diethyl ether and filtered
through
silicon dioxide. After the diethyl ether had been stripped off, 10.5 g of the
product
(= 70% of theory) remained. The melting point was 139 to 141 C. The
characteristic absorptiions in tl:ie NMR spectra were as follows: 19F-NMR: -
66.6 and
-79.3 ppm. 'H-NMR 8.4, 6.76, 6.60, 6.50 and 4.70 ppm.
Exain~le 15a
.15 .
5-Nitro-2-(2,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-benzodioxole
A solution of 54.4 g of 2-(2,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-
benzodioxole
in 75 ml of methylene chloride was added dropwise at 10 C to a mixture of 40
ml
of 65% strength by weight nitric acid and 40 ml of concentrated sulphuric
acid.
Stirring was contiinufA for 1 hour at room temperature and the batch was then
poured into ice-water, the organic phase was separated off, and the aqueous
phase
was extracted using methylene chloride. The combined organic phases were
washed with water, dried and freed from volatile components. 95 g of the
product
(= 86% of theory), which hacl a melting point of 87 to 88 C, remained.
The NMR spectra showed the following characteristic absorptions: 19F-NIVIR -
59.0
and -69.4 ppm. 'H-NMR: 3.10 ppm.
LeA29088 -88-
21.48612
Examnle 16a
5-Nitro-2-(1-chloro-2,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-benzodioxole
613 g of 2-(1-chloro-2,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-benz.odioxole
of
Example 2a were dissolved in 1.2 1 of methylene chloride and the solution was
added dropwise at 0 to 10 C to a mixture of 400 ml of 65% strength nitric acid
and 400 ml of conceritraterl sWphuric acid. Stirring was continued for 2 hours
at
room temperature. Then, the rr,iixture was poured carefully into 2 1 of ice-
water and
extracted using methylene chloride. The combined organic phases were washed
twice using water, dried and c:oncentrateri. The yield was 652 g (93% of
theory).
The NMR spectra showexi the following characteristic absorptions: 19F-NMR -
66.4
and -79.2 ppm. 'H-NTvIR 4.81. ppm.
Eyle 17a
5,6-Dinitro-2-(2,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-benzodioxole
317 g of the product of Example 15a were introduced, and a mixture of 250 ml
of
100% strength by weight nitric acid and 350 ml of concentrated sulphuric acid
were added dropwise, with stirring. The mixture was stirred for 2 hours at 55
C.
The batch was then allowed to cool and poured into ice-water. The product was
extracted using methylene chloride, and the methylene chloride phase was
washed
until neutral using sodium hiydrogencarbonate solution, dried and freed from
readily volatile components on a rotary evaporator. The yield was 339 g (= 94%
of theory), and the melting point was 101 to 103 C.
"Ihe NMR spectra showed the following characteristic absorptions: 'gF-NNIR: -
60.9
and -86.5 ppm. 1H-Nl'dR 3.18 ppm.
L e A 29 088 - 89 -
22
Exam lp e 18a
5,6-Dinitro-2-(1-chloro-2,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-
benzodioxole
352 g of 5-nitro-2-(1-chloro-2.,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-
benzod.ioxole
of Example 16a were introduced and treated with a mixture of 250 ml of 100%
strength by weight nitric aciLd and 350 ml of concentrated sulphuric acid. The
mixture was sti;rred iFor 2 hoiurs at 60 C. After cooling, the mixture was
poured
into ice-water and extracted using methylene chloride. The methylene chloride
phase was washed with sodium hydrogencarbonate solution and dried and then
evaporated on a rotary evapoirator. The yield was 392 g (91% of theory), and
the
melting point was 125 C. Th.e NMR spectra showed the following characteristic
absorptions: 19F-NMR -68.5 and -81.0 ppm. 'H-NMR: 4.86 ppm.
.. ~'E&=le 19a
5-Amino-2-(2,2,2-trifluoroethyl)-2-trifluoromethyl-1, 3-benzodioxole
57.4 g of the product of ExanTle 15a were dissolved in 400 ml of
tetrahydrofuran
and hydrogenated wirh hydrogen for 5 hours at 30 C at 50 bar in the presence
of
4 g of catalyst (palla(hum on charcoal, 10% strength by weight). The mixture
was
then subjected to filtration, the solvent was removed and the residue was
distilled
under a high vacuurn. Thi:s gave 37 g of product (= 63% of theory) having a
boiling point of 83 C at 0.07 mbar. '9F-NMR: -59.0 and -84.6 ppm. 'H-NMR:
2.98 ppm.
Example 20a
5-Amino-2-(1-chloro-2,2,2-trifluoroethyl)-2-trifluoromethyl-1, 3-benzodioxole
72 gof5-nitro-2-(1-crdoro-2,2,2-trifluoroethyl)-2-trifluoromethyl-1,3-
benzodioxole
LeA29088 -90-
~ 14-8 6 .t 2
of Example 16a were dissolved in 500 ml of tetrahydrofuran and hydrogenated
for
hours at room temperature with 15 to 20 bar hydrogen using 5 g of palladium on
charcoal (5% strength). The mixture was subsequently filtered and the solvent
was
stripped off in vacuo. The yield was 60 g (93% of theory), and the boiling
point
5 was 80 to 82 C at 0.1 mbar. Zhe NMR spectra showed the following
characteristic
absorptions: 19F-NMR -66.5 and -79.4 ppm. 'H-NMR: 4.68 ppm.
Exarnple 21 a
5,6-Diamino-2-(2,2,2.-trifluoroethyl)-2-trifluoromethyl-1,3-benzodioxole
339 g of the product of Example 17a were dissolved in 2000 ml of
tetrahydrofuran
and treated with 20 g of catalyst (palladium on charcoal, 5% strength by
weight).
The mixture was hydrogenated with hydrogen for 13 hours at 25 to 30 bar and at
room temperaturE. The batch was then filtered and the solvent was stripped off
iii
vacuo. A solid remained. The yield was 274 g (= 96% of theory). 19F-NMR -61.2
and -86.6 ppm. 'H-NMR: 3.02 ppm.
Ex mple 22a
2-(2,2,2-Trifluoroeth)il)-2-trifluoromethyl-1,3-benzodioxole
306.5 g of 2-(1-chloro-2,2,2-irifluoroethyl)-2-trifluoromethyl-1,3-
benzodioxole of
Example 2a were dissolved in 500 ml of THF and the solution was treated with
101 g of triethylamine and 30 g of palladium on charcoal (5% strength by
weight).
The mixture was then hydrogenated for 48 hours at 110 C under 100 bar
hydrogen. The mixtw-e was subsequently filtered, the solvent was stripped off,
and
the residue was subjected to f actionation in vacuo. The yield was 126 g (46%
of
theory), and the boil'vig point was 60 C at 10 mbar. The NMR spectra showed
the
following characteristic absorptions: 19F-NMR: -59.0 and -84.6 ppm. 'H-NMR:
3.02 ppm.
LeA29088 -91 -
Fluoroalkyl(ene) group-containing o-phenylenediamines of the formula
I
R 3
NHR
R2 NH2
X
in which
R' represents CF_,, OCF3, SCF3, S02-C,-C6-alkyl, which can be straight-chain
or branched and fully or partially substituted by fluorine, N(CF3)2, a phenyl
or phenoxy radical with CF3 or CN in the 4-position and, if appropriate,
other substituents, 1,1õ2,3,3,3-hexafluoropropoxy, 1,1,2-trifluoro-2-chloro-
ethoxy, 1,1,2,2-tetrafluoroethoxy, 1,1,2-trifluoro-2-chloro-ethylthio or
1,1,2,3,3,3-hexafluoropropylthio,
R2 independently of R' represents F, Cl, Br, CN, CH3, OCF3, SC~-Cl-C6-alkyl,
which can bestraight-c:hain or branched and fully or partially substituted by
fluorine, Ci:)O-C,-C16-alkyl, COOC65, 1,1,2,2-tetrafluoroethoxy,
1,1,2,3,3,3-hexafluoropropoxy or 1,1,2-trifluoro-2-chloro-ethoxy and
R3 represents hydrogen, CCCH3 or COCF3,
it being possible for R' and R? together to represent a-4-CFCl-CFCI-O-radical,
with the exception of the compounds described in EP-A 251,013 and
EP-A 487,286, can bel obtained by dinitrating a benzene derivative of the
formula
D
Ds
LeA29088 -92-
214-3612
in which
D' represents CF30, CF3S, CHF2CF2O, CHFCI-CF2O, CF3CHFCF2O, CF3CFZO,
CF3CF2CF2O, CF3CF2S or CF3CHFCF2O and
D2 represents CF3O, ClF3S, CHF2CF2O, CHFCI-CF2O, CF3CHF-CF2O,
CF3CF2O, CF3CF2CF2O, CF3CF2S, CF3CHFCF2O, fluorine, chlorine,
bromine, C,-C6-alkyl or C,-C6-alkoxy,
subsequently reducing the nitro groups and thus obtaining compounds in which
R'
and R2 are in the 4- and 5-position relative to the amino groups and have the
meanings of D' and U.
If it is desired to prepare compounds in which R' has the abovementioned
meaning
and is in the 4-position relative to the amino groups and R2 represents Cl or
Br in
the 5-position relative to the aulvno groups, it is possible, for example, to
react a
nitrobenzene derivative of the formula
I
R 20 Cl or in which
R' has the abovernentioned meaning and
Hal represents fluorine, chlorine or bromine,
with ammonia to exchange the Hal group for an amino group, and to reduce the
resulting nitraniline.
LeA29088 -93-
4 Z 14 86?2
If it is desired to prepare compounds in which R' has the abovementioned
meaning
and is in the 4-position relatiive to the amino groups, R2 represents chlorine
or
bromine in the 6-position relative to the amino groups and R3 denotes
hydrogen,
it is possible, for example, to react a nitraniline of the formula
R NOZ
~
NH2
in which
R' has the aboveinentionexi meaniing.
with a chloriinating or brominatiing agent thus introducing a chlorine or
bromine
atom into the meta-position relative to the nitro group, and subsequently to
reduce
the nitro group.
If it is desired to prepare compounds in which R' denotes a donor group in the
4-position relative to the two amino groups, R2 denotes an acceptor group, for
example COO-C,-C6-alkyl, CN, CF3 or SC2-C,-C6-a.lkyl and R3 is other than
hydrogen, it is possible, for example, to mononitrate a benzene derivative of
the
formula
D
A
in which
LeA29088 -94-
8 6 12
D' has the abovementioned meaning and
A represents CF, S02-Ci-C6-alkyl, which can be straight-chain or branched
and fully or partially substituted by fluorine, or represents COO-C,-C6-alkyl
or CN,
(the NO2 group enters in the para-position relative to D'), to reduce the NO2
group
to the NH2 group, to acylate the NH2 group, for example with acetic acid or
trifluoroacetic acid, to cany out another mononitration reaction (this NO2
group
enters in the ortho-position relative to the NHCOR group in which R is, for
example, CH3 or CF3), to reduce this NO2 group to the NH2 group and, if
appropriate, if it is desired to prepare a compound of the above formula where
R3
is hydrogen, to elimiriate the acyl group by hydrolysis.
The fluoroalkyl(ene)-group-comtairiing o-phenylenediamines in which R3 denotes
hydrogen can be initially reacted with trifluoroacetic acid to give
2-trifluoromethylbenzimidazoles of the formula
R
N
~ ~--C F3
N
R H
and these can then be reacted further with compounds of the formula
5
R
/
A-CH
R4
in which R' and R2 assume the scope of the above meanings,
Le A 29 088 - 95 -
~143612
R4 represents hydrogen, alkyl, alkoxy or optionally substituted aryl,
RS represents hydroxyl, cyano or in each case optionally substituted alkyl,
alkenyl, alkinyl, alkoxy, alkenyloxy, alkinyloxy, alkylthio, amino,
aminocarbonyl, alk~ylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy,
dialkoxyphosphonyl, (hetero)aryl, (hetero)arylcarbonyl,
(hetero)aryloxycarbonyl, (hetero)arylc.arbonyloxy or (hetero)arylamino-
carbonylaminocarbonyloxy and
A denotes a suitable leaving group.
Leaving groups are known to a person skilled in the art, examples being
halogen,
alkyl(alkoxy, aryl)sulphonyloxy, hydroxyl or alkoxy.
Em=les
Exmples lb to 6b (Dinitration and reduction)
Exam. ln e 1 b
320 g of 1,2-bis-(2-cl-Aoro- 1,1,2-trifluoroethoxy)-benz,ene were added
dropwise to
500 g of a mixed acici containing 33% strength by weight HNO3 and 67% strength
by weight H2SO4. After one hour at 40 C, 250 ml of 20% strength by weight
oleum were added dropwise. 1he mixture was subsequently heated at 80 C, and
stining was continuol for 15 hours. A further 120 ml of 20% strength by weight
oleum and 250 g of the abovementioned mixed acid were then added dropwise.
After 6 hours at 80 to 82 C, the mixture was cooled and poured onto ice. The
organic phase was separated off and washed with water. After azeotropic drying
using 1,2-dichloroetliane, 350 g of 96% by weight pure 1,2-dinitro-4,5-bis-
(2-chloro-1,1,2-trifluoroethoxi~)-benzene were obtained (oil, np : 1.4832, GC
99.1%)
LeA29088 -96-
~14312
350 g of this dinitro compoiund were added dropwise to a mixture of 1.5 1 of
ethanol, 50 ml of water, 30 ml of concentrated aqueous hydrochloric acid and
470 g of iron filings, and the mixture was refluxed for a total of 15 hours.
When
cold, the solution was filtered and concentrated and the residue was
recrystallized
from cyclohexane. 216 g of 1,2-diamino-4,5-bis-(2-chloro-1,1,2-
trifluoroethoxy)-
benzene having a melting point of 58 to 60 C were obtained.
E&=le 2b
Analogously to Exarnple 1, :1,2-bis-(1,1,2,3,3,3-hexafluoropropoxy)-benzene
was
used to prepare the oarresponding 4,5-dinitro compound (oil, nD : 1.4852) and
the
corresponding 4,5-diamino compound (oil, 87% by weight pure).
Ex~le 3b
Analogously to Example 1, 1-(1,1,2-trifluoro-2-chloroethoxy)-2-chlorobenzene
was
used to prepare the corresponcling 4,5-dinitro compound (melting point 56 to
57 C)
and the corresponding 4,5-diamino compound (melting point 67 to 68 C).
x 4b
Analogously to Example 1, 1-trifluoromethoxy-2-bromobenzene was used to
prepare the corresponding 4,5-dinitro compound (melting point 73 to 75 C) and
the
corresponding 4,5-diamino compound (oil, purity 98% by weight, nD : 1.5485).
EAa=1e 5b
Analogously to Example 1, 1-trifluoromethoxy-2-chlorobenzene was used to
prepare the corresponding 4,5-dinitro compound (melting point 55 to 56 C) and
the
corresponding 4,5-diamino compound (melting point 56-57 C).
LeA29088 -97-
2148 6 12
Ex=le 6b
1-(1,1,2,3,3,3-Hexafluoroprop)xy)-2-chloro-benzene was used to prepare the
corresponding 4,5-dinitro compound (oil) and the corresponding 4,5-diamino
compound (oil).
Examnles 7b to 12b
Treatment with ammonia under pressure and reduction
Examnle 7b
260 g of 3-nitro-2,5-dichlorobenzotrifluoride, 130 ml of water and 10 g of
tetraethylammonium chloride were introduced into an autoclave, and 120 ml of
liquid ammonia were injected.. The mixture was then heated to 130 C and
stirred
for 10 hours at this temperature. After cooling, the batch was filtered and
the
precipitate which had been separated off was washed with water and dried 194 g
of 2-amino-3-nitro-5-chloro-bmzotrifluoride with a melting point of 67 C were
obtained.
134 g of the nitranil'uie obtained as described above were dissolved in 800 ml
of
ethanol, and 20 ml of water, 10 ml of concentrated aqueous hydrochloric acid
and
160 g of iron filings were then added. The mixture was refluxed for 15 hours
and
then cooled, and subjected to filtration with suction, the filter residue was
washed
with dichloromethane, and the organic phases were subsequently freed from the
solvent under reduced pressure. 171 g of 5-chloro-3-trifluoromethyl-
1,2-diaminobenzene with a melting point of 53 C were obtained.
Exam le
Analogously to Example 7, 3-nitro-4,6-dichloro-difluorochloromethoxybenzene
was
LeA29088 -98-
~~~~6-IL 2
used to obtain fust 3-niilro-4amino-6-chloro-difluorochloromethoxybenz.ene
(melting point 73 C) and therefrom 3,4-diamino-6-chloro-difluorochloromethoxy-
benzene (oil).
F~=le 9b
Analogously to Exarnple 7, 3-bromo-5-nitro-6-chlorobenzotrifluoride was used
to
prepare fust 3-bromo-5-riitro-i5-amino-benzotrifluoride (melting point 80 to
82 C)
and therefrom 3-bronio-5,6-diamino-benzotrifluoride (melting point 52 to 54
C).
E&=le lOb
Analogously to Exarr.iple 7, 3--cyano-4-chloro-5-riitro-benzotrifluoride was
used to
prepare first 3-cyano-4-amino-S-riitro-benzotrifluoride (melting point 99 to
100 C)
and therefrom 3-cyano-4,5-diamino-benzotrifluoride.
Em=le llb
Analogously to Example 7, 3,6-dichloro-5-nitro-benzotrifluoride was used to
prepare first 3-chloro-5-riitro-6-amino-benzotrifluoride (melting point 53 to
54 C)
and therefrom 3-chloro-5,6-diamino-benzotrifluoride.
Exam in e 12b
2-Bromo-4-fluoro-5-ndtro-(1,1,2-trifluoro-2-chloro)-ethoxybenzene was used to
prepare fust 2-brorno-4-amiino-5-nitro-(1,1,2-trifluoro-2-chloro-ethoxy)-
benzene
(melting point 90 C) and thereirom 2-bromo-4, 5-diamino-(1,1,2-trifluoro-2-
chloro)-
ethoxybenzene.
LeA29088 -99-
1436 12
Exam
(Halogenation of a niltianiline and reduction)
24 g of finely pulverulent 2-nitro-4-trifluoromethylmercaptoaniline were
dissolved
in 50 ml of tri-fluoroacetic acid, and 18 g of bromine were metered in at 20
C.
Stirring was then continued for 3 hours at 20 C and for a finther 30 minutes
at
40 C. The mixture poiured into water and the product taken up in
dichloromethane.
After the solvent had been removed, 31 g of 6-bromo-2-nitro-4-trifluoromethyl-
mercapto-aniline were: obtained.
155 g of the nitranilvle thus prepared were refluxed for 15 hours with 15 ml
of
water, 10 ml of conce:ntrated aqueous hydrochloric acid and 70 g of iron
filings in
700 ml of ethanol, the mixture was then filtered, the filtrate was freed from
the
solvent under reduced pressure and the solid crude product was recrystallized
from
cyclohexane. 112 g of 6-bromo-4-trifluoromethyl-mercapto-1;2-diaminobenzene
with a melting point of 60 to 61 C were obtained.
mple 14b
Ex
Analogously to Example 13, 27 g of 2-nitro-4-trifluoromethyl-sulphonylaniline
in
100 ml of acetic acid were brominated with 18 g of bromine.
After working up, 32 g of 2-nitro-6-bromo-4-trifluoro-methylsulphonyl-anialine
were obtained. Melting point 147 C.
32 g of the nitramine thus prepared were reduced with iron filings in alcohol
and
aqueous hydrochloric acid. 24 g of 3-bromo-5-trifluoromethylsulphonyl-
phenylene-
1,2-diamine were obtained, melting point 155-157 C.
LeA29088 - 100-
214~~~2
Examnle 15b
Analogously to Example 14, 27 g of 2-nitro-4-trifluoromethylsulphonyl-aniline
in
100 ml of acetic acid were chlorinated with 10 g of chlorine. 29 g of
2-nitro-4-trifluoromethylsulphonyl-6-chloro-aniline were obtained, melting
point:
138-139 C.
13 g of 3-chloro-5-triifluoromethylsulphonyl-1,2-phenylenediamine (melting
point:
143-145 C) were obtained by reduction.
Ex=les 16 to 20
(Nitration and reduction in 2 steps)
Agij?n1e 16
263 g of 4-(2,6-dichloro-4-tri:fluoromethyl)-phenoxy-acetanilide were
dissolved in
1100 ml of dichloromethane and introduced at 10 C. 88 g of 98% strength by
weight nitric acid were theni added dropwise at this temperature. Stirring was
continued for 1 hour at 10 C and for 2 more hours at 30 C. After 300 ml of
water
had been added, the phases were separated and the organic phase was freed from
dichloromethane under reduced pressure. 253 g of 2-nitro-4-(2,6-dichloro-
4-trifluoromethylphenoxy)-aaetanilide with a melting point of 138-140 C
remained.
91 g of the acetanilid.e thus pi=epared were dissolved in 800 ml of dioxane,
10 g of
Raney nickel were added, and the mixture was hydrogenated at 25 to 45 C in a
hydrogenation apparatus with a maximum hydrogen pressure of 50 bar. After
releasing the pressure and filtration, the dioxane was distilled off under a
slight
vacuum. 65 g of 2-,amino-4-=(2,6-dichloro-4-trifluoromethyl-phenoxy)-
acetanilide
with a melting point of 222-223 C remained.
LeA29088 - 101 -
2148612
E}cample 17
Analogously to Exarrple 16, 3-trifluoromethyl-4-methoxy-acetanilide was used
to
prepare first 3-trifluoromelhyl-4-methoxy-6-nitro-acetanilide (melting point
143-144 C) and therefrom 3-trifluoromethyl-4-methoxy-6-amino-acetanilide
(melting point 164-165 C).
Example 18
Analogously to Example 16, 3-trifluoromethyl-4-fluoro-
trifluoromethylacetanilide
was used to prepare first 3-trifluoromethyl-4-fluoro-6-nitro-
trifluoromethylacetani-
lide (melting point 78 C) and therefrom 3-trifluoromethyl-4-fluoro-6-amino-
trifluoromethylacetanilide (me;lting point 92-93 C).
Exa=1e.19.
Analogously to Exarr.ple 16, 3-trifluoromethyl-4-bromo-
trifluoromethylacetanilide
was used to prepare first 3-trifluoromethyl-4-bromo-6-nitro-trifluoromethyl-
acetanilide (melting point 110 to 112 C) and therefrom 3-trifluoromethyl-4-
bromo-
6-amino-trifluorometlrylacetardlide (melting point 63-65 C).
Fy
,a=le 20
Analogously to Example 16, 3-trifluoromethylthio-4-chloro-tri-
fluoromethylacetanilide was used to prepare first 3-trifluoromethylthio-4-
chloro-
6-nitro-trifluoromethylacetanillide (melting point 99-100 C) and therefrom
3-trifluoromethylthio--4-chloro-6-amino-trifluoromethylacetanilide (melting
point
88-90 C).
Le A 29 088 - 102 -
2148612
Example 21
0.2 mol of 3-brorno-5-trif.luoromethyl-phenylene-diamine and 150 ml of
trifluoroacetic acid were refluxed for 3 hours. For working up, excess
trifluoroacetic acid was distilled off and the residue was partitioned between
100 ml of water and :300 ml of ethyl acetate. The organic phase was separated
off,
washed in succession with in each case 100 ml of aqueous sodium
hydrogencarbonate solution and water, dried over sodium sulphate and
concentrated in vacuo. The residue was purified by chromatography on silica
gel
(eluent: cyclohexanelethyl acetate 1:1).
This gave 4-bromo-6-trifli,ioromethyl-2-trifluoromethyl-1H benzimidazole of
melting point 149-151 C.
le 22
0.03 mol of 4-bromo-6-trifluoromethyl-2-trifluoromethyl-1H benzimidazole and
0.06 mol of pulverulient pot2issium carbonate were refluxed for 15 minutes in
70 ml of ethyl acetate, 3.9 g(0.04 mol) of chloromethyl methyl thioether in 20
ml
of ethyl acetate were; then acided, and the mixture was refluxed for a
fiirther 4
hours, with stirring. For working up, the cooled reaction mixture was washed
twice
using in each case 40 ml of water, dried over sodium sulphate and concentrated
in
vacuo, and the residue was purified by chromatography on silica gel (eluent:
dichloromethane).
This gave 1-methylthiorriethyl-4-bromo-6-trifluoromethyl-2-trifluoromethyl-
benzimidazole of ine;lting point 56-60 C.
Le A 29 088 - 103 -
214 86~2
Use Examdes:
In the Use Examples 4fich follow, the compounds listed below were employed as
comparison substances:
0
ii
~ O-C -NH-C H3
! (A)
~
0-1-C3H7
O-(2-Isopropoxyphenyl) NI-methyl-carbamate (compare, for example,
DE 1,108,202)
0
I I
CHI~O-P=NH2 (B)
I
SCH3
O,S-Dimethyl-thiolo-phosphoramide (compare, for example, DE 1,210,835)
LeA29088 -104-
2148612
Example A
Ptaedon larvae test
Solvent: 7 parts by weilot of dimethylfornnanide
Emulsifier: 1 part by weiglit of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent and the stated amount of
emulsifier, and the ccmcentrate is diluted with water to the desired
concentration.
Cabbage leaves (Brassica oleracea) are treated by being dipped into the
preparation
of the active compowld of the desired concentration and are infested with
mustard
beetle larvae (Phaedon cochleariae), while the leaves are still moist.
After the specified period of time, the destruction in per cent is detemiined.
100%
means that all the beetle laniae have been killed; 0% means that none of the
beetle larvae have been killed.
In this test, a superioir activity compared with the prior art is shown, for
example,
by the following corripounds of the Preparation Examples: 13, 14, 16, 18, 20,
22,
28, 29, 30, 56, 76, 80, 84, 85, 86, 89, 103 and 109.
Le A 29 088 - 105 -
Table A
Phaedon Iarvae Test
Active componnds Active compound Degree of de.s-
concenhabion in % tniction in %
after 3 days
0 (A) 0.1 100
11
ao O-C -NH-CH3 0.01 70
0,001 0
-iCH,
(known)
F,C -N (85) 0.1 100
0_01 100
Cl -~N
0.001 100
CHz -OCHs
C1 --N
~-CF,
--N
3C
CH: -()CzHs
F,C -N (86) 0,1 100
~}--CF, 0.01 100
C1 ~N\ 0.001 100
CH2 -OCZHS
LeA29088 - 106-
2:t43612
Table A: ( ntinuati"
Phaedon Imvae Tesit
Ac6ve compoimds Active compoimd Degree of des-
concenhation in % huction in %
after 3 days
F3C N(80) 0.1 100
Q-CF, 0,01 100
Br
CH2--OC2HS 0,001 100
+
Br N
I ~>--CF3
~C
/
3C N'
CH2-OCZHS
F2C1~0 -. N (103) 0,1 100
1 I
--CF3 0.01 100
FzCO N
0.001 100
CHz-OCzHs
F CO ~ -N (109) 0.1 100
Z I ~ \~----CF, 0.01 100
F2C~O / ~N
0,001 100
CH2 -O-CHz -C-CH
LeA29088 -107-
2148612
Table A: (Conti.n=W
Phaedon Ia><vae Test
Active compoiuids Active con>Ipound Degree of de.s-
concentration in % tniction in %
after 3 days
F,C .N (89) 0.1 100
>--CF, 0.01 100
Cl
N\ 0.001 100
CHZ-O-n-qH7
C1 N
>___<~F
3
3C N~
CH2-O-ri-C3H7
F,C N (90) 0.1 100
,> -CF3
0.01 100
Cl N 0.001 100
CH=-O-CH2 -C=CH
+ =
C1
CF3
3C N
C'H2-O-CHZ-C-CH
LeA2908~ - 108-
Table A: (ContinuatiQu)
Phaedon Larvae Test
Active compotuxls Active conipowid Degtee of des-
concenhation in % tniction in %
after 3 days
F3C 11 (84) 0.1 100
~ ~--=CF3 0.01 100
Br 14 \ 0.001 100
CH2-O-CH2-C=CH
+
sr rirr
~ ~~--CF,
3C
CH2-0-0l2-C=CH
F3C -N (56) 0.1 100
~-CFs 0,01 100
Cl =N
0.001 100
CHZ-O-C:H(CH3)2
+
C .\ N
I >--'CF3
/
3C N\
CH2-O-C:H(CH3h
I.eA29088 -109-
~~~8 6 12
Table A: Gontinuat;iM)
Phaedon I.arvae Test
Active compouxb Active compound Degree of des-
concentra6on in % huction in %
after 3 days
F,C -N (76) 0
,1 100
>--CF; 0.01 100
0.001 100
CH2-O-(--H(CH3)=
+
Br N
\
I >--CF,
/
3C N\
CH2-0a:H(CH;~
Br (13) 0.1 100
\ 'N 0,01 100
~ \>--CF, 0.001 100
/
F,C *N\
+ CH2-O-(:H(CHA
F3C .N
~}-CF3
N
Br CH2-O-CH(CH3)2
LeA29088 - 110-
'Z1UM
Table A: (Continuati"n
Phaedon Lvvae Tes't
Active compounds Active compoiuid Degme of des-
concentration in % hucfion in %
after 3 days
Br (14) 0,1 100
~= ~N\ 0.01 100
~--CF' 0.001 100
~ ~N
F3C
+ CH2-C)-n-C3H.,
F'C N
--CF3
~ .. .
Br CH2-O=-n-C3H7 ='
Br (16) 0.1 100
N\ 0.01 100
>--CFs 0.001 100
~ :Iq~
F3C CH2-O-CH2-C-CH
Br (18) 0.1 100
~~ 0.01 100
CF3 0.001 100
F3Ci
CH2-O-CO-C(CH3)3
L.eA29Q88 -111-
~~~~~12
1Ta1 e A-(Continuati,~,1
Phaedon Laivae Test
Ac6ve compounds Aclive compound Degree of des-
concenhation in % tntction in %
after 3 days
Br (20) 0.1 100
~ N 0.01 100
~'F3 0.001 100
F3C N~
+ CHz-Q-CzHs
F3C _-N
}--C:F3
--N
~ = Br CHZ-C--CHS .
Br (22) 0.1 '100
N 0.01 100
~ '>-'CF, 0.001 100
~
F,C N\
CH2--C13
Br (28) 0.1 100
N 0.01 100
'>--CF, 0.001 100
F3C N ~~ zHs
CHz-N
COC)C2H5
LeA29088 -112-
2143612
Table A: (Contino?t
Phaedon Lmvae Tesit
Active compounds Active conipouuid Degree of de.s-
concenhafion in % tniction in %
after 3 days
Br (29) 0.1 100
\ ~N 0.01 100
~ ~CF3 0,001 100
F3C /
- N CH=-Nfi-C3Hl
COOC2Hs
Br (30) 0,1 100
-N 0,01 100
X>-CF 0.001 i00
,N'
FC
CHZ-N
COOCzHs
L.eA29088 - 113-
Z143612
x le
Plutella test
Solvent: 7 parts by weigbt of dimethylformamide
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparal:ion of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent and the stated amount of
emulsifier, and the concentrate is diluted with water to the desired
concentration.
Cabbage leaves (Brassica oleracea) are treated by being dipped into the
preparation
of active compound of the desired concentration and are infested with
caterpillars
of the diamond-back inoth (Plutella maculipennis) while the leaves are still
moist.
After the specified period of time, the destruction in per cent is determined.
100%
means that all the caterpillai-s have been killed; 0% means that none of the
caterpillars have been killed.
In this test, a superior activity compared with the prior art is shown, for
example,
by the following compounds af the Preparation Examples: 2, 3, 13, 14, 18, 20,
21,
28, 29, 30, 52, 56, 76, 80, 84, 85, 86, 103, 109 and 131.
LeA29088 - 114-
~~.48612
Table R
Plutella Test
Active compournls Active compo><nxi Degnee of des-
concentration in % tnction in %
after 3 days
O (A) 0.1 100
11
O-C-NH-CHj 0,01 100
0.001 10
CI-i-C3H7
(known)
F,C -N~ (85) 0.1 100
CF3 0,01 100
C1 ~N
0,001 100
CH2 -OC=HS
+
C1 N
~CF'3
3C I-N, CHz-OCZHS
F,C
\ ~-N~ (86) 0.1 100
CF, 0.01 100
C1
'N 0.001 100
CH2-OC,HS
Le A 29 098 - 115 -
~143 6 12
Table $= (C'ontinuati"n
Plutella Test
Active compowids Active con>Ipo ond Deglee of des-
concenhaaion in % tcuction in %
after 3 days
(84) 0.1 100
F'C 0.01 100
~ --CF, 0.001 100
Br N
CH=-O-CFiZ-C=CH
+
Br N
:~C\
'
(
/}~.CF 3
,C N
CHz-O-C:H-C=CH:
F,C CCN N (80) 0,1 100
0,01 100
Br 0.001 100
CH2 -C-C:2HS
+
Br
\ iN
~--C:F3
~ ~N
3C \
CH,-OC,Hs
F1C"O \ N (103) 0.1 100
I -
CF, 0_01 100
FZ C~O /
]~N~
0.001 100
CHz --C1C2H5
Le A 29 088 - 116 -
~14 8 6 12
Table B: (('Qn 'n 1 tup-u)
Plutella Test
Active compounds Active compound Degree of de.s-
concentra6on in % tn><ction in %
after 3 days
F C \ 'v\ (109) 0, i 100
2 I 0,0: 100
~
F2C" 0 /
h\ 0.001 100
CHz-O~Hz-C=CH
CF3-0 \ ~,h (131) 0.1 100
1 ~~--CF, 0.01 10C
/
CF,-0 A 0.001 100
.CH2-O-CH2-C=CH
CI ::C J JJ~~~ (52) 0,1 100
~ ~---CF, 0,01 lOC
CF, N' 0,001 100
-CN
CH2
F'C ,N~ (56) 0,1 100
, CF, 0,01 100
C1
N
+ 0,001 100
CH2-O~'H(CH3)z
N
C ~N\-CF3
3c
CHz-O-CH(CH3h
LeA29088 - 117-
2148 612
Table B: (Continuati9W
Plutella Test
Active comipounds Active compoiuid Degnre of de.s-
concenhation in % trnction in %
after 3 days
(76) 0,1 100
0.01 lOC
Br / ,N.
CH2-O-CH(CH,j 0.001 100
+
Br N
~=--CF,
jC N
CH2-0-C:H(CH3)_ Br " =
(2) 0.1 100
\ 0,01 100
~ 0.001 100
CFs / N% CF
CHz-ItiT
CbOCIi,
Br
(3) . 0.1 100
~ lv 0.01 loo
0,00 i 100
CF, 14% /=zHs
CH2-N
COOCH3
LeA29088 - 118-
12
Tahle B: (Continuati,m)
Plutella Test
Active compowxls Active compound Degree of des-
concenhation in % tniction in %
after 3 days
Br (13) 0.1 100
\ 0.01 100
I ~CF' 0.001 100
/
F,C~ -N
+ CH,-O4ZH(CH3n
FjC
---CF
3
/ .N
Br CHz-0-CH(CHi)a
Br (14) 0,1 100
\ N 0,01 1 CO
I \~F' 0.001 100
/
F3C -N
+ CH2-O-~1-CH;
F3C N
C ~_CF'
'N
Br CHz-O-n-C3H;
Br (18) 0.1 100
N 0.01 100
I ,}-CF, 0.001 100
F3C N~
CHz -O-CO-C(CH3)3
TeA29088 -119-
2148 6~2
Table B: (Continuatim)
Plutella Test
Active conipotuxk Active conipotmd Degtee of des-
concenhation in % truction in %
after 3 days
Br (20) 0,1 100
N 0.01 100
0.001 100
~- N
F3C
+ CH3-O-CzHs
F;C ~\ N
I ~ ~--CF3
N
Br CH; -O-CHS ,
Br (21) 0.1 100
~ 0.01 100
I >--C:F, 0.001 100
~
CF3 N% CH3
CH2-N
COOC2H5
Br (28) 0.1 100
IIINCH N 0.01 100
~--CF, 0.001 100
F3Cs
CH 2-N
COOCzHs
LeA29088 - 120-
~148 6 12
Table B: Continuati ~
Plutella Test
Active compoimds Active compound Degtee of des-
concentra6on in % huction in %
after 3 days
Br (29) 0.1 100
0.01 100
F 0,001 100
3 C
CHz-N
COOC2H,
Br (30) 0.1 100
N 0,01 100
I ~~~F3 0.001 100
N
FjC % ,i.CjHr
CH2-N
COOC21=5
I,eA29088 - 121 -
2148612
EAample C:
Heliothis viresceris Test
Solvent: 7 parts by weight of dimethylformainide
Emulsifier: 1 part by weight of allcylaryl polyglycol
ether
To produce a suitable preparatiion of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent and the stated amount of
emulsifier, and the concentrate is diluted with water to the desired
concentration.
Soya shoots (Glycine max) are treated by being dipped into the preparation of
115 active compound of the desired concentration and are infested with the
tobacco
budworm (Heliothis virescens) while the leaves are still moist.
After the specified period of tirne, the destruction in per cent is determined
100%
means that all the wonns have been killed; 0% means that none of the worms
have
been killed.
In this test, a superior activity compared with the prior art is shown, for
example,
by the following compounds of the Preparation Examples: 3, 13, 16, 18, 20, 52,
53, 56, 76, 80, 84, 85, 86, 89, 90, 103, 109 and 131.
Le A 29 088 - 122 -
2~4 86 12
Table C:
Heliothis vi>rescens T'est
Active compouncb Active con>Ipoumd Degree of des-
concenhation in % ttuction in %
after 3 days
O (A) 0.1 10
11
.1O-C-IVH-CH3
~
( known )
F3C . N (85) 0.1 100
, ~ ~>---CF3
C1 ~
CH2-OC2H5
CI
:)c ~>---CF,
~ N
3C ,
CHz-OCzHs
F,C N (86) 0.1 100
~}--CF3
Cl ~ N
CH1-OC2H5
Le A 29 088 - 123 -
21480612
Table C: Continuai:i r
Heliothis virescens Test
Active compounds Active compound Degme of des-
concentration in % tniction in %
after 3 days
F,C (84) 0.1 100
I ~ ~>--CF3
Br N \
CHi-O-CHz-C=CH
Br N
~>--CFs
N
3C: ~
CHz-O-CH,-C=CH
(80) 0.1 100
FsC D-N ~
)--CF,
I Br N\
C:H2 -OC, Hs
Br
N
~)--CF,
:~:N
,C
C:Hz-OCzHs
F C~ (103) 0.1 100
2I II ~CF3
FzC~O / N
CH2-OC2Hs
LeA29088 - 124-
2148612
Table C. (Continua&u)
Heliothis virescens 'Test
Active compounds Active compowid Degree of de.s-
concentration in % ttuction in %
after 3 days
F C"O (109) 0.1 100
2 1 ~> -CF;
F'C~O
CH=-0.CH2-C-CH
CF3-0 ~N (131) 0.1 100
~ ~~---CF3
CFj-0 / N
CH2-O-CHz-C_CH
F,C N (89) 0.1 100
~}-CF,
"
C! N
C13x-Oi--C3H,
-~-
C1
'
~/}_'CF,
3 N
C'Hz-O-n-qH,
Le A 29 088 - 125 -
2148612
Table C: Continua.tQW
Heliothis vi>-cscens 'Test
Active compoun& Active conltpoi,md Degicee of de.s-
concentration in % tnilction in %
after 3 days
F3C \ ~N (90) 0.1 10C
~ ; CF3
---
C1 /
CH2-0=CH2-C=CH
+
_-N
~ ~>--CF3
~~
~
CHz-C)-CHz-C=CH
Cl N (52) 0.1 100
~~>--Cr3
CF3
C H2-CN
CF, :::~:NN\ (5
3) 0.1 100
~--CF,
C1II ~ CH1-CN
LeA29088 - 126-
214 8 612
Table C: ( ntinuatim)
Heliothis vimcens ']Cest
Active compom& Ac6ve conipouid Degme of de.s-
concenttation in % huc6on in %
after 3 days
F3C ~ N (56) 0.1 100
Cl N
\CH2-O-CH(CH,),
+
3C
CH, -O-CH(CH3)~
F3C N (76) 0.1 100
~}-CF3
Br
CHz-O-CH(CH3)2
+
Br ~~
~}--CF3
3C N\
CHz-O-CH(CH3h
Br (3) 0.1 100
CF3 GHS
CH2-N
COOCH3
Le A 29 088 - 127 -
2148612
Table C: (ContinuatiLQW
Heliottiis vinscens Test
Active compounds Active compound Degiee of de.s-
concentration in % tniction in %
after 3 days
Bi (13) 0.1 100
N
F3C
+ CHõ-O-CH(CH,h
F3C N
Eh. CH; O CH(GH3}s
Br (16) 0.1 100'
N
>-CF,
F,C
CHZ--O-CH=-C-CH
Br (18) 0.1 100
~~-CF3
I:N N
F,C ~ \
CHZ--O-CO-C(CH,)3
LeA29088 - 128-
_._.._
~148 6) 12
Table C:(Contiinuati,~i
Heliothis vi>->escens 'Cest
Active compo>In>ds Active compound Degree of de.s-
concentration in % truction in %
after 3 days
F;r (20) 0.1 100
~ CN I ~> -CF3
/
F,C N %
-F Cli,-O-(,HS
F3C \ N
I \>__CF3
/ N
13r C1H,-0-CHs .
LeA29088 129-
2148612
Exam. 1:
Tetranychus test (OP resistant)
Solvent: 7 parts by weigbt of dimethylformamide
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent and the stated amount of
emulsifier, and the concentrate is diluted with water containing emulsifier to
the
desired concentration:c.
Bean plants (Phaseolus vulgaris) which are heavily infested with all
development
stages of the greenhouse red spider mite (Tetranychus urticae) are dipped into
a
preparation of active -;,ompound of the desired concentration.
After the specified period of time, the destruction in per cent is determined
100%
means that all the spicier mites have been killed; 0% means that none of the
spider
mites have been killed.
In this test, a superior activity compared with the prior art is shown, for
example,
by the following compounds of the Preparation Examples: 2, 3, 4, 16, 20, 83
and
84.
LeA29088 - 130-
Table D:
Tehanychus Test (IJP resistant)
Active conipounds Aefive conipound Degree of
concenha6on in % dlestmiction in %
after 7 days
O (B) 0.01 60
11
CH,O- i -NH2 0,001 0
SCH3
(known)
N (84) 0,01 100
0.001 95
F,C C"'CW%
Br CH2=~MH2-C=CH
+ =
Br N
~>--.CF',
3C N \
CH2--O-CH2-C=CH
Br (2) 0.01 100
:CN N 0.001 45
~-- -CF3
CFl \ CH3
CH2-N
\COOCH;
LeA29088 - 131 -
2148612
Table D: (Continuaticiu)
Tehanychus Test (QP-resistant)
Active compoun& Active compoumd Degree of
o
concenhation in % destfion in %
after 7 days
Br (3) 0.01 100
N 0,001 60
>--CF3
CF3 N, C2Hs
CH,-N
,
COOCH3
(4) 0.01 100
Br 0,001 60
x5\>_CF3
CF/n-C,H7
CH2-N
\
COOCH3
Br (16) 0.01 100
N 0,001 60
>-CF,
F,C N \
CH,--O-CHZ-C-CH
LeA29088 - 132-
Table D: (Continuatior
Tetianychus Test (C-P resistaint)
Active conipounds Active compotmd Degme of
concentration in % destnxhon in %
after 7 days
Br (20) 0.01 98
N 0.001 80
~}-CF,
F3C N
-- C:HZ-O-CHS
F3C N
I >-CF3
Br CH2-O-CzHs
CF3\ N (83) 0.01 100
~~F3 0.001 80
Br / N
CH:2-O-n-qH1
Br N
CFs CHf2-O-n-CA
Le A 29 088 - 133 -
2148612
xam le E:
Plutella Test
Solvent: 31 patls by we:ight of acetone
Emulsifier: 1 part by weigllt of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent and the stated amount of
emulsifier, and the concentrate is diluted with water containing emulsifier to
the
desired concentration.
Cabbage leaves (Brassica oleracea) are treated with the preparation of active
compound of the des;ired concentration. A treated leaf is placed into a
plastic dish
and infested with larvae (L2) of the, diamond-back moth (Plutella xylostella).
After
three days, in each case one iuntreated leaf is used to continue the *feeding
of the
larvae.
After the specified pc;riod of time, the destruction in per cent is
determined. 100%
means that all the animals have been killed; 0% means that none of the animals
have been killed.
In this test, a superior activit:y compared with the prior art is shown, for
example,
by the following conipound of the Preparation Examples: 87.
Le A 29 088 - 134 -
2148612
Table E:
Plutella Test
Active conipowxls Active compound Degree of de.s-
concentration in % tniction in %
after 7 days
O (A) 0.01 0
ii
O-C-NH-CH3
O-'i-CH,
( known )
CiN (87) 0.01 100
I ~ '}--CF3
~~ N
CFy ,,
CH2-C2HS
Le A 29 088 - 135 -
Example F
Ptraedon Test
Solvent: 31 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent and the stated amount of
emulsifier, and the concentrate is diluted with water containing emulsifier to
the
desired concentration.
Cabbage leaves (Brassica oleracea) are treated with the preparation of active
compound of the desired concentration. A treated leaf is placed into a plastic
dish
and infested with larvae (L2) of the mustard beetle (Phaedon cochleariae).
After
three days, in each aLse one untreated leaf is used to continue the feeding of
the
larvae.
After the specified period of time, the destruction in per cent is determined.
100%
means that all the animals have been killed; 0% means that none of the animals
have been killed.
In this test, a superior activity compared with the prior art is shown, for
example,
by the following compound of the Preparation Examples: 87.
Le A 29 088 - 136 -
2148612
Table F:
Ptrac,~don Test
Active compounds Active conipouuid Degme of de.s-
concenhation in % tniction in %
after 7 days
O (A) 0.01 0
n
~ ,O-C:-NH-CH3
O-i-CH,
( known. )
Cl ~ N (87) 0.01 100
I
' N
CF3 %
CH2-O-C2H3
Le A 29 088 - 137 -
2 148612
Ex=le G:
Ctiitical concentration tesdnematodes
Test nematode: Globodera rostochiensis
Solvent: 31 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent, the stated amount of
emulsifier is added and the concentrate is diluted with water to the desired
concentration.
The preparation of active compound is intimately mixed with soil which is
heavily
infested with the test nematodes. 'Ihe coricentration of the active compound
in the
preparation is of practically no importance here, only the amount of active
compound per unit volume of soil, which is given in ppm (= mg/1), being
decisive.
The treated soil is fiilled into pots, potatoes are planted therein, and the
pots are
kept at a greenhouse temperature of 20 C.
Affter six weeks, the potato roots are examined for cysts and the degree of
effectiveness of the active compound is determined in %. The degree of
effectiveness is 100% if infestation is avoided completely and 0% if the level
of
infestation is just as Hgh as in the control plants in untreated, but equally
infested,
soil.
In this test, a superioir activity compared with the prior art is shown, for
example,
by the following cornpound of the Preparation Examples: 13, 20 and 85.
LeA29088 - 138-
2148612
Table G:
Nematode Test (Globodera mstochiensis)
Active compoiux>s Degree of destn.iction in % at an
active conipo><md concentration of
5ppm
(A) 0
ii
O--C-NH-CH3
C\"
I
/
O--i-qH7
(known)
CF3 N (85) 100
}-CF3
CI . N
, ~ .
CHZ-O-n-qH7
CI Cr~
~
~CFj
/
CF3 M~
CHz -O'GiHs
LeA29088 - 139-
2148W
Table G: (ContinuatjQn)
Nematode Test (Glabodera rostochiensis)
Active conipounds Degree of de.stnic6on in % at an
active compotuid concenhation of
ppm
Br (13) 100
-N
~ '}--CF3
CF3 ~N
CHr-O-~C3H;
CF3
-N
Br CHx- O-i-C3F~
Br (20) 100
CF3 ~
CH2-O-CZHS
CF3 N
~}--CF;
--N
Br CHz- O-C2Hs
LeA29088 - 140-
2148612
Example H:
Psoivptes ovis Test
Solvent: 35 parls by Nveight of ethylene glycol monomethyl ether
Emulsifier: 35 pails by weight of nonylphenol polyglycol ether
To produce a suitable preparation of active compound, 3 parts by weight of
active
compound are mixed with 7 parts of the solvent emulsified mixture indicated
above, and the emulsion concentrate thus obtained is diluted with water to the
desired concentration.
l ml of this preparation of active compound is pipetted into PP blister
filntis of a
.15 suitable~ size. Approximately 25 mites are then.transferred inlo the
preparation of
active compound.
After 24 hours, the a,.-tivity of the preparation of active compound is
detetmined
in %. 100% means that all mites have been killed, 0% means that no mites have
been killed.
In this test, a superior activity compared with the prior art is shown, for
example,
by the following compounds of the Preparation Examples: 2, 3, 4, 6, 9, 10, 13,
14,
15, 16, 18, 19, 20, 43, 49, 51, 78 and 85.
Le A 29 088 - 141 -
2~48 6~2
Table H
Psoropte.s ovis Test
Active compounds Active compound Degree of des-
concenhation in tiuction in %
ppm of a.i.
Br (2) 10 100
N
'~-CF,
CF3 CH,
CH2-N
~ COOCH3
Br (3) .10 100
\ N
CF3 N~ ,CzHs
CH2- N\COOCH,
(4) 10 100
Br
\ ~.
~~Fs
CF3 /n-C3H7
CH2=N
\COOCH3
LeA29088 - 142-
2148612
Table H (Continuatbn :
Psomptes ovis Test
Active compowxls Active conipotmd Degree of des-
concentration in tniction in %
ppm of a.i.
Br (6) 10 100
~ N
I ~>----CF3
~ h
CF, \ ~O
CH2 -C
C(CH,),
Br (9) . 10 100
I ~}--C:F,
CF, N
CH2-CH=CH-CH3
CF3 ~--C:F,
Br CHZ-- CH~H-CH3
Le A 29 088 - 143 -
1486 1~
Table H (Continuatim):
Psompkes ovis Test
Active conipounds Active compoimd Degtee of des-
concenhation in tniction in %
ppm of a.i.
Br (10) 10 100
N 1 100
CF3 N' /CH3
CH2'v~ACH=C\
C1
Br (13) 10 100
N 1 100
\>CFs
CF3 N \
CH2 --O-i-C3H,
CF3 N
\>CF3
N
Br CHz-- O-i-C3H7
LeA29088 - 144-
2148612
Table H (Continuati<)4:
Psoiopte.s ovis Test
Active comiwtmds Active compouid Degiee of des-
concenhation in tnxtion in %
ppm of ai.
Br (14) 10 100
r! 1 100
( ~~--CF;
CF3 1,I
,
CH2-O-n-C3H,
+
CF3 N
'-"CF3
Br CHz-_ O-n-C3H,
Br (15) 10 IOC
N 1 100
}--CF
\
CF3 N
('-H2-O{CH2)3-C6H5
+
Cr3
\>-CF3
N
CH1- O{CH2)3-C6H5
Br
L,e A 29 088 - 145 -
~148 6 1~
Table H (ContinuatiQn):
Psomptes ovis Test
Active compomds Active conipoimd Degree of des-
concentratiion in tntction in %
ppm of a.i.
Br (16) 10 100
~ N 1 100
CFI
~ N
, \ CH2-0-CH2-C-CH
Br (18) 10 100
~ ,N 1 100
I \CF3
/ N
CF, 11 O
CH2 --C
C(CH3)1
LeA29088 - 146-
~148 6 12
Table H ContinuatiQo:
Psomptes ovis Test
Active compounds Active conipoimd Degiee of des-
concenhation in tnction in %
ppm of a.i.
Br (19) 10 100
N N 1 100
I ~>--CF3 CI
CF3 \
CH2--O-CH2
+ -
CF3 N
I ~}--CF3 Cl
Br
CH2--O-CH2 Br (20) 10 100
N
\\,----CF3
N
CF3 (:Hz-O-CzHs
CF; 11 N
\)---CF3
B: (:Hz-O-CHS
Le A 29 088 - 147 -
IS 6 2
Table H (Continuati~ m):
Psoioptes ovis Test
Active conipoumb Active compoimd Degree of de.s-
concenhation in tniction in %
ppm of a.i.
,N (78) 10 100
\>---CF,
CF3
CH=-O-CzH<
CF3 N
\>---CF3
'"N
CI-12-O-C2Hs
F,C ~ (85) 10 .100
I \-CF3
/
CI N\
CH2-OCzHs
CI
\-CF,
C N
C N
3~
GH2-OC2H5
Le A 29 088 - 148 -
~148 6 12
Table H Continuati~i :
Psoivptes ovis Test
Active compounds Active compound Degme of de.s-
concenhation in trucfion in %
ppm of ai.
Br (80) 10 100
I ~--CF, 1 100
CF3
CHz-0~H.
+
CF3 , L ~,-CF3
/ N
Br
C:Hz-O-CzH5
CF, ~ NN (43) 10 100
I ~~--CF3 Cl
/
C1 \
CH2
LeA29088 - 149-
214 8 612
Table H (Continuati"n:
Psoroptes ovis Test
Active coropouids Active compound Degzee of des-
concenhation in tiuction in %
ppm of a.i.
N (49) 10 10C
~~--CF, CF,
CF, 0--N%
C1 + CH2
CL O,N
~>--CFa CFj CF3
CHZ
CF, ~N (51) 10 100
\> -CFz .
C1 --N
0
i~
+ C H2 C~,
Cl~ C(CH3)3
~~--CF3
CF3 ~h i0
CH2-C~
C(CH3;3
Le A 29 088 - 150 -
148 6 12
E&=Ie I:
Peiiplanefa ametic=i Test:
Solvent: 35 pails by weight of ethylene glycol monomethyl ether
Emulsifier: 35 parls by weight of nonylphenol polyglycol ether
To produce a suitable preparation of active compound, 3 parts by weight of
active
compound are mixecl with 7 parts of the solvent emulsified mixture indicated
above, and the emulsion concentrate thus obtained is diluted with water to the
desired concentration,2 ml of this preparat:ion of active compound are
pipetted onto filter paper discs
(diameter: 9.5 cm) located in suitably sized Petri dishes. After the filter
discs have
dried, five cockroaches (Periplaneta americana) are transferred into the Petri
dishes
and covered.
After 3 days, the activity of the preparation of active compound is determined
in
%. 100% means that all cockroaches have been killed, 0% means that no
cockroaches have been killel.
In this test, a superior activity compared with the prior art is shown, for
example,
by the following con-ipounds of the Preparation Examples: 3, 14, 16, 17, 18,
20,
22, 43, 53, 55, 56, 76, 79, 80, 83, 85, 87, 89, 90, 128, 129 and 131.
LeA29088 -151-
T 1 I:
Periplaneta ameiicami Test
Ac6ve compouxh Ac6ve conipotmd Degree of de.s-
concentrafion in tiuction in %
ppm of a.i.
Br (3) 1000 100
~ 100 100
~ \}~F3
CF; / ~N ,CxHs
CHZ-N
,
COOCH3
Br (14) 1000 100
N
\\?--CF3
N
CF,
+ CIt-O-n-C3H7
CF3. N
Br CH2-Oi--qH7
LeA29088 - 152-
2148612
Table I (Continuatian):
Periptanefa americmra Test
Active compoimds Active compound Degme of de,s-
concenhation in truction in %
ppm of ai.
Br (16) 1000 100
100 100
j ~-CF3
CF;
+ CHz-O-CHz-C=CH
CF3 N
>--CF,
N
Br CH2-O-CH2-C-CH
B' (17) 1000 100
N
~~ Fs
CFs N x O O _
u u
CHs-O~-NH-C-NH ~ ~ 1
Br (18) 1000 100
-N
( ~>--CF,
CFs 'N \ ~O
CH2--C
C(CH,)3
I.e A 29 088 - 153 -
~.~48 6 12
Table I (Continuation):
Peziplaneta ametic.mra Test
Active compotmds Active compound Degree of de.s-
concenttation in truction in %
ppm of a.i.
Br (20) 1000 100
\ ~N 100 100
{ F3
CF~ ~
+ CHZ-OL,H~
CF3~
c =;
Y~r
Cjt.-o-C2H.
B_
Br (22) 1000 100
100 >50
CF3 CH2-CN
LeA29088 - 154-
2148612
Table I (Continuaticm):
Peiiplaneta americana Test
Active compoimds Active compound Degree of des-
concentration in tniction in %
ppm of a.i.
CF3 N (79) 1000 100
~N>--CF3 100 100
~ CH2 OH
~ N'
I ~~--CF3
/
CF, N,
CH2-OH
CF, ~ N (43) 1000 100
I /
CH2CF~
C1 CN~
CF3 Cl'r-N' N (55)1000 100
~Fs
Cl \ i0
+ CHI C
Cl ~\ N' C6Hs
I ~-CF]
~
CF3~ N~ O
CH2-C,, C6H5
LeA29088 - 155-
214-8612
Table I Continuatiou):
Periptaneta anieticarra Test
Ac6ve compourKb Ac6ve compoiuid Degme of des-
concenha6on in tniction in %
ppm of a.i.
CI N (53) 1000 lOC
-~N~-CF3
CFj
CH,-CN
CF,~~~N (56) 1000 100
11 \>--CF3 100 100
Cl + \CIt--C1-i-C3H,
CI N
N
C"'Ll ~-C F3
CF3 \ CH2-O-i-C3H7
CF,. N (76) 1000 100
~ ~>---CF, 100 100
Br
+ N C;Hz O-i-C3H,
Br N
\">--CF;
CF, ~
(--H2-O-iZ3H,
Le A 29 088 - 156 -
Table I (Continuaticn):
Peliplaneta ameticmra Test
Active compovnds Active compound Degree of cie.s-
concenhation in truction in %
ppm of a.i.
F30 (128) 1000 100
~}--CFs 100 >50
+ CH, OC2H5
~ N
~ / C~>-CF;
h
F30 ~
CHz-OCZH5
CF3O ~ ~N (129) 1000 100
( ~~--CF3 100 >50
CF3O/ ~'
(:H2-O-C2HS
CF3O N (131) 1000 100
~}---CF3
~N
CFj
CHZ-O-CH2-C=CH
LeA29088 -157-
2.148 6 12
Table I (Continuati)n):
Peiipianeta americana Test
Active compoiuids Active compoiuid Degree of des-
concenha6on in truction in %
ppm of a.i.
CF3-1 ~ N ~ (85) 1000 100
\\>--CF3 100 100
+ CHZ-OCzHs
C C-- N~
I ~~-CF3
~N
CF3 CH2-OCzHs
CI (87) 1000 100
%-CF3
CF3
CHz-OC2Hs
CF3 N (80) 1000 100
i ~---CF3 N
B
+ CHz-OC2Es
Br -N \
\-CF3
N
CF,
CHZ-OCzHs
LeA29088 - 158-
2148612
Table I (Continuatiot~:
Petiplaneta americana Test
Active compourik Active conipoiuid Degree of des-
concentration in tiuction in %
ppm of ai.
CF, a ,N (89) 1000 100
~---CF3
Cl -N
+ CHZ-O-n-C,H,
\
Ci IN
~}--CF;
CF3 ~N \
- CH2-O-n-C3H,
CF3 O ~ -N (90) 1000 100
II ~--CF, 100 >50
C- 1'
+ CHz-O-CH,-C=CH
\
C N
~>---CF;
CF3O
CHZ-O-CHz-C=CH
LeA29088 - 159-
2.148612
Table I Continuatioa):
Peiiplanefa anieticana Test
Active compounds Active conipoiuid Degree of des-
concentration in tnwtion in %
ppm of a.i.
CF3
N (83) 1000 100
',):
~~-CF3
Br
+ CH,-O-n-CH,
Br ~ ~}---CF3
''N
CF, \
CH.z-O-n-C., H,
LeA29088 - 160-
2,48612
Emmple =
iViusca domestica Tesit
Solvent: 35 parts by weight of ethylene glycol monomethyl ether
Emulsifier: 35 parts by weight of nonylphenol polyglycol ether
To produce a suitable preparation of active compound, 3 parts by weight of
active
compound are mixed with 7 parts of the solvent emulsified mixture indicated
above,
and the emulsion coricentrate thus obtained is diluted with water to the
desired
concentration.
2 ml of this preparatiion of active compound are pipetted onto filter paper
discs
(diameter: 9.5 cm) loc:ated In suitably sized Petri dishes. After the filter
discs have
dried, 25 test animals (Musca domestica; strain WHO [N])) are transferred into
the
Petri dishes and covezed.
After 3 days, the activity of the preparation of active compound is determined
in %.
100% means that all flies have been killed, 0% means that no flies have been
killed
In this test, a superior activity compared with the prior art is shown, for
example, by
the following compotnzds of the Preparation Examples: 16, 17, 18, 20, 56, 76,
80,
83, 87 and 89.
LeA29088 -161-
___.
r11$~~2
Table J
1VLusca domestica Test
Active compow& Active compoimd Degree of des-
concenhation in tniction in %
ppm of a.i.
Br (16) 1000 100
100 100
CF3
+ CH,--O-CH,-C=CH
C-; \ N -
I / I \X-CF3
N
Br CHZ--O-CHZ-C-CH
Br (17) 1000 100
'}-_CF, 100 100
N O O
CF ~
~ CH; -O-C-tJH-C-Mi-(~ ~}- Cl
Br (18) 1000 100
--N
~}--CF3
CF3 O
CHz-C~
C(CHO;
LeA29088 - 162-
Table J: (Continuatioa)
Musca domestica Tat
Active compotzods Active compound Degree of des-
concentration in tniction in %
ppin of ai.
Br (20) 1000 100
N 100 100
a
CF;
+ CHz-O-C_HS
CFj'-, \ h
~ 3
Br CH2-O-C2F5
CF, a ~N1000 100
~>--CF;
L- ---N
\
B + CH2-O-n-C3H,
r
\iN-
-CF,
CF
a
CHZ-O-n-CjH,
I.e A 29 088 - 163 -
1, 4 8 6 12
Table J: (Continuatic m)
Musca doniestica Test
Active compowKis Active compound Degiee of des-
concentration in trix6on in %
ppm of a.i.
CF3 ~ N (89) 1000 100
~ ~>---CFj
C1 / --N
+ %
Cl CH2-O-n-C3H, --N
~ ---CFj
CF; ~
CHZ-O-n-Cj H,
Ci N (87) 1000 100
\>CFa
N
CF3 CHz-OCZHS
CF,-, \ N (80) 1000 100
I CN\-CF3
~
Br
+ CHZ-C-C2H5
Br CN\-CF3
CF3 N~
C:H2-OC,H5
LeA29088 - 164-
2148612
Table J: (Continua.ticl)
Musca domestica TL-st
Active compowxis Active compound Degree of des-
concenhation in tcuction in %
ppm of a.i.
Cr' N (56) 1000 100
~ ~h}-CFs
CI
+ CH2 O-i-C3H,
C \
I L ~}--CF,
CFj N\
CHZ-O-i.qH,
CF, N (76) 1000 100
c ~>---CFj
Br N
\
Br + CHz O-i-C3H,
~ ~~=--CF;
CF3 N\
CH2-O-i-C,H,
Le A 29 088 - 165 -
2 liL48
xam le K:
Phyt:ophthora Test (to:mato)/protective
Solvent: 4.7 parts by weight of acetone
Emulsifier: 0.3 part by weight of alkyl-aryl polyglycol ether
To produce a suitable preparation of active compound, one part by weight of
active
compound is mixed with the stated amounts of solvent and emulsifier, and the
concentrate is diluted with water to the desired concentration.
To test for protective activity, young plants are sprayed with the preparation
of active
compound until dripping wet. After the spray coating has dried on, the plants
are
inoculated with an aqueous spore suspension of Phytophthora infestans.
The plants are then placed in an incubation cabin at 20 C and about 100%
relative
atmospheric humidity.
The test is evaluated 3 days after inoculation.
In this test, a clearly superior activity compared with the prior art is
shown, for
example, by the compound of Preparation Example 48.
LeA29088 - 166-
~14 8 6 12
Table K:
Phytophthora Test (toimato)/pivtective
Active compound Degcee of effectiveness
in %of the unheated
contml at an active
con-poimd concentration
of 10 ppm
CF3 (48) 57
~---CF3
C1 N 0
CH2-P-OCZHS
+ I
OCZHs
CI :~N \CF3
p,j 0
CF3 õ
CHZ-P-OC2H5
OCZHs
LeA29088 - 167-
Exam e L:
Plasmopaia Test (vir)es)/pivtective
Solvent: 4.7 paits by weight of acetone
Emulsifier: 0.3 pait by weight of allcyl-aryl polyglycol ether
To produce a suitablle preparation of active compound, one part by weight of
active compound is nzixed with the stated amounts of solvent and emulsifier,
and
the concentrate is dih.rted with water to the desired concentration.
To test for protective activity, young plants are sprayed with the preparation
of
active compound until dripping wet. After the spray coating has dried on, the
plants are inoculated with an aqueous spore suspension of Plasmopara viticola
and
then remain in a humidity chamber at- 20 C to 22 C and 100% relative
atmospheric humidity for one day. The plants are then placed in a greenhouse
at
21 C and 90% atmospheric humidity for 5 days. The plants are then moistened
and
placed in a humidity chamber for one day.
The test is evaluated 6 days after the inoculation.
In this test, a clearly superior activity compared with the prior art is
shown, for
example, by the conipounds of the following Preparation Examples: 48, 53 and
130.
L.e A 29 088 - 168 -
Table L:
Plasmopara Test (vines)/protective
Active compound Degiee of effectiveness
in % of the imtreated
conttol at an active
compound concenttation
of10ppm
Cl N (48) 60
1 c ~___cF
3
1=T O
CF3 I I
+ CH2-P-OC:Hg
CF3 \ /N OC=Hj
\>CF3
Cl L-,N O
CHz-P-OCZHj
OCzHs
CF3 (53) 73
~}--CF;
CI N
\
CH2-CN
CF3O ~ N (130 74
\>CF, )
CF30'
CH2-O-n-C3H7
LeA29088 - 169-
MS612
xam le M:
Post-emergence Test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, one part by weight of
active compound is rn ixed with the stated amount of solvent, the stated
amount of
emulsifier is added and the concentrate is diluted with water to the desired
concentration.
Test plants which have a height of 5 - 15 cm are sprayed with the preparation
of
active compound in such a way as to apply the particular amounts of active
compound desired per unit area. After three weeks, the degree of damage to the
plants is rated in % damage in comparison to the development of the untreated
control.
The figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, a clearly superior activity compared with the prior art combined
with
a comparable crop plant selectivity is shown, for example, by the compound of
Preparation Example 79.
LeA29088 - 170-
Table M:
Post-emergence test (giEenhouse)
Active compoiuid Application VNieat Alopacum Lolim Setana Anwurthus Galiutn
pblygornnn Veronira Vlola
rAe
in g/ha
CF3 N (79) 2000 10 95 99 100 100 95 100 100 100
~-CF,
Cl
N
-' + CH2-OH
C N
+ ~--CF,
~
CF3
CHz-OH
Z\z
}-.+
C>O
~-~
y.-..,.