Note: Descriptions are shown in the official language in which they were submitted.
~m9z~2
-1-
TONER FOR DEVELOPING ELECTROSTATIC IMAGE,
IMAGE FORMING METHOD AND PROCESS CARTRIDGE
FIELD OF THE INVENTION AND RELATED ART
The present invention relates to a toner,
particularly a negatively chargeable toner, for
developing electrostatic images in image forming
methods, such as electrophotography, and electrostatic
printing. The present invention also relates to an
image forming method and a process cartridge using the
toner.
Hitherto, a large number of electro-
photographic processes have been known, as disclosed
in U.S. Patent Nos. 2,297,691; 3,666,363; 4,071,361
and others. In these processes, an electric latent
image is formed on a photosensitive member comprising
a photoconductive material by various means, then the
latent image is developed and visualized with a toner,
and the resultant toner image is, after transferred
onto a transfer-receiving material, such as paper, as
desired, fixed by heating, pressing, heating and
pressing, etc., to obtain a copy or a print. In the
case of including the step of transferring a toner
image, a step of removing a residual toner remaining
on the photosensitive member is ordinarily also
included.
Known developing methods for visualizing
~~~~z72
-2-
electrical latent images with a toner may include,
e.g., the magnetic brush method described in U.S.
Patent No. 2,874,063, the cascade developing method
disclosed in U.S. Patent No. 2,618,552, the powder
cloud method disclosed U.S. Patent No. 2,221,776, and
a method using an electroconductive magnetic toner
disclosed in U.S. Patent No. 3,909,258.
As for the step of fixing the toner image
onto a sheet material such as paper which is the final
step in the above process, various methods and
apparatus have been developed, of which the most
popular one is a heating and pressing fixation system
using hot rollers.
In the heating and pressing system, a sheet
carrying a toner image to be fixed (hereinafter called
"fixation sheet") is passed through hot rollers, while
a surface of a hot roller having a releasability with
the toner is caused to contact the toner image surface
of the fixation sheet under pressure, to fix the toner
image. In this method, as the hot roller surface and
the toner image on the fixation sheet contact each
other under a pressure, a very good heat efficiency is
attained for melt-fixing the toner image onto the
fixation sheet to afford quick fixation.
Recently, in place of hot rollers, there has
been commercialized a fixing apparatus comprising a
heating member and a pressing member which is disposed
~1492~2
-3-
opposite to the heating member and presses a recording
medium (such as paper) to contact the heating member_
via a film.
On the other hand, in recent years, there
have been also desired high-quality copy or print
images in accordance with the use of digitalized
copying machines and fine toner particles.
More specifically, it has been desired to
obtain a photographic image accompanied with
characters, so that the character images are clear
while the photographic image is excellent in density
gradation faithful to the original. Generally, in a
copy of a photographic image accompanied with
characters, if the line density is increased so as to
provide clear character images, not only the density
gradation characteristic of the photograph image is
impaired, but also the halftone part thereof are
roughened.
Further, resolution failure (collapsion) of
line images and scattering are liable to be caused at
the time of fixation as described above, so that the
image qualities of the resultant copy images are
rather liable to be deteriorated.
Further, in case where the line image density
is increased, because of an increased toner coverage,
a thick toner image is pushed against a photosensitive
member to be attached to the photosensitive member in
2149212
-4-
the toner transfer step, so that a so-called transfer
failure (or a hollow image), i.e., a partial lack
toner image (line images in this case), in the
transferred image, is liable to be caused, thereby
providing poor quality of copy images. On the other
hand, in case where the gradation characteristic of a
photographic image is intended to be improved, the
density of characters or line images is liable to be
lowered, thus providing unclear images.
In recent years, there has been obtained some
improvement in density gradation characteristic by a
system including image density readout and digital
conversion. However, a further improvement has been
desired.
Regarding density gradation characteristic,
it is impossible to obtain a linear relationship
between a developing potential (difference between a
photosensitive member potential and a developer-
carrying member potential) and a resultant (copy)
image density. In a halftone region, a slight change
in developing potential leads to a remarkable change
in image density. This provides a complexity in
obtaining a satisfactory density gradation
characteristic.
Generally, copied images appear clearer
because of an edge effect of attracting an increased
amount of toner so that clear line images can be
t
2149212
-5-
retained in case where a maximum density of ca. 1.30
is attained at a solid image part which is less _
affected by the edge effect.
In case of a photographic image, however, the
maximum density of a photograph appears less at a
glance because of its surface gloss but actually
amounts to a very high level of 1.90 - 2.00.
Accordingly, in a copy of a photographic image, even
if the surface gloss is suppressed, a solid part image
density of ca. 1.4 - 1.5 is required since a density
increase due to the edge effect cannot be expected
because of a large image area.
Accordingly, in providing a copy of a
photographic image accompanied with characters, it
becomes very important to obtain a developing
potential-image density relationship which is close to
the first order (linear} one and also a maximum image
density of 1.4 - 1.5.
Further, the density gradation
characteristic is liable to be remarkably affected by
the saturation charge and the charging speed of a
developer used. In case where the saturation charge
is appropriate for the developing conditions, a
developer showing a slow charging speed provides a low
maximum image density, thus generally thin and blurred
images in the initial stage of copying. In this case,
however, non-problematic images can be obtained if the
-6-
maximum image density is ca. 1.3, as described above,
thus being able to obviate an adverse effect of the
slow changeability. Even in case of the slow charging
speed, the initial copy image density is increased if
the saturation charge is increased. However, on
continuation of copying, the charge of the developer
is gradually increased to finally exceed an
appropriate charge for development, thereby resulting
in a lower copy image density. Also in this case, no
problem occurs in line images if the maximum image
density is ca. 1.3
From the above, it is understood that a
photographic image is more remarkably affected by the
saturation charge and the charging speed of a
developer than a line image.
In case where a smaller particle size toner
is used, the dispersion state of a charge control
agent and a colorant remarkably affects the
changeability of the toner.
A toner for developing electrostatic images
may generally contain a dye called a charge control
agent for controlling the changeability of the toner.
In order to provide a toner with a negative
changeability, chromium complex compounds have been
principally used.
Japanese Laid-Open Patent Application (JP-A)
60-170864, describes that, among such chromiun complex
2149212
compounds, those having a good mutual solubility with
a binder resin show a uniform negative chargeability
and provide clear copy images but are liable to be
accompanies with difficulties, such remaining of a
toner residue on a photosensitive member due to
cleaning failure and filming, and those being
insoluble within a binder resin (particularly in a
polyester resin) show good chargeability and also good
anti-filming characteristic.
However, a metal complex salt compound
insoluble or incompatible with a binder resin shows a
poor dispersibility. Accordingly, when a toner
containing such a metal complex salt compound is
formulated into fine particles, the toner is liable to
be charged excessively particularly in a low-humidity
environment, thus leading to fog or a density
lowering. This is because a fine particle size
fraction and a coarse particle size fraction formed
through a pulverization step of toner production are
caused to have remarkably different contents (weight
ratios) of the charge control agent (i.e.,so-called
localization of a charge control agent), so that toner
particles are caused to have different
chargeabilities.
In case where a fine powder fraction and a
coarse powder fraction recovered from the classifying
step are re-utilized as a material for toner
A
~14927~
_8_
production, the above-mentioned liability of
localization of a charge control agent is further
promoted to cause difficulties, such as a lowering
in image density and fog due to a toner
electrification insufficiency under a low-humidity
condition. For this reason, it has been hitherto
difficult to reutilize both fine powder and coarse
powder by-produced in the classification step for
toner production, and coarse powder alone has been
reutilized as proposed in JP-A 3-209266. JP-A 61-
155464 and JP-A 62-177561 have proposed an azo-type
iron complex as a charge control agent showing good
dispersibility within a binder resin. A toner
containing the azo-type iron complex is, however,
accompanied with difficulties, such as a slow rate of
electrification and a lowering in image density after
a long period of standing or in a high humidity
environment. In recent years, a smaller particle size
(at most 9 um in terms of a weight-average particle
size (diameter)) is recommended for providing high-
quality images. A small particle size toner is liable
to have a remarkably high charge under a low-humidity
condition and cause difficulties, such as thinning of
line images, a lowering in image density and
occurrence of reversal potential fog caused by a toner
charged to an opposite polarity due to charging
failure on a developer-carrying member, such as a
21492'2
-9-
developing sleeve, due to the copresence of the
excessively charged toner.
In order to improve the chargeability of a
toner containing such an azo-type iron complex, JP-A
1-306862 has proposed a silicone resin-coated carrier
which has a high chargeability-imparting effect, and
JP-A 2-153362 has proposed a developing apparatus
including an improved toner layer thickness-regulating
member and an improved toner replenishment-assisting
member. In these proposals, the developing
performance of the toner is retained by charge-
imparting or -assisting members and it is difficult to
retain good image quality for a long period due to
deterioration or soiling of the charge-imparting or
-assisting member.
SUMMARY OF THE INVENTION
An object of the present invention is to
provide a toner for developing electrostatic images
having solved the above-mentioned problems and capable
of retaining a high-quality image forming performance
for a long period.
An object of the present invention is to
provide a toner having a good dispersibility of a
charge control agent and a uniform chargeability,
capable of retaining a high image density for a long
period and capable of providing images free from fog
214922
-10-
and with a high resolution.
Another object of the present invention is to
provide a toner which can be quickly charged and can
provide good toner images similarly as before standing
even after standing for a long period or in a high-
humidity environment.
Another object of the present invention is to
provide a toner which can provide high-quality images
without using a charge-assisting member.
Another object of the present invention is to
provide a fine particle size toner which can provide
satisfactory developed images for a long period under
various environmental conditions even in case of
providing high-resolution developed images.
Another object of the present invention is to
provide a toner which allows re-utilization of fine
powder and coarse powder by-produced in the
classification step in toner production.
Another object of the present invention is to
provide a toner highly suitably adapted to an
electrophotographic process not adversely affecting a
photosensitive member or a developer-carrying member.
A further object of the present invention is
to provide an image forming method and a process
cartridge using such a toner as described above.
According to the present invention, there is
provided a toner for developing electrostatic images,
2149272
-11-
comprising:
(a) a binder resin,
(b) a long-chain alkyl compound represented
by the following formula (1), (2) or (3):
CH3--~CH2-jx CH20H ( 1 ) ,
wherein x denotes an average value in the range of 35
- 150,
CH3-~ CH2-j X -O~- j HCH2 -O~H ( 2 ) ,
R
wherein x denotes an average value in the range of 35
- 150; z denotes an average value in the range of 1 -
5, and R denotes H or an alkyl group having 1 - 10
carbon atoms,
CH3-~CH2~ CH2COOH (3),
wherein y denotes an average value in the range of 35
- 150 ; and
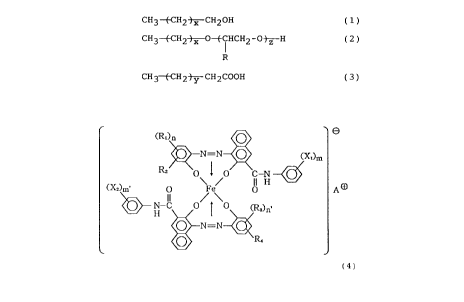
(c) an azo-type iron complex compound
represented by the following formula (4);
~R )n O
N-N O . <X~)m
1 ~ ~-N -
CX~m' O Fe/ p H
I)
N-C O ~ O (~)n.
H
2 5 0 N=N
R.
(4)
214922
-12-
wherein X1 and X2 independently denote hydrogen atom,
lower alkyl group, lower alkoxy group, nitro group or
halogen atom; m and m' denote an integer of 1 - 3; Rl
and R3 independently denote hydrogen atom, Cl-18 alkyl
or alkenyl, sufonamide, mesyl, sulfonic acid group,
carboxy ester group, hydroxy, Cl-18 alkoxy,
acetylamino, benzoylamino or halogen atom; n and n'
denote an integer of 1 - 3; R2 and R4 denote hydrogen
atom or nitro group; and A+ denotes a cation including
75 - 98 mol. s of ammonium ion and another ion
selected from the group consisting of hydrogen ion,
sodium ion, potassium iron and mixtures thereof.
According to another aspect of the present
invention, there is provided an image forming method,
comprising:
a charging step of supplying a voltage to a
charging means in contact with a member to be charged
to charge the member to be charged,
a step of forming an electrostatic image on
the charged member to be charged,
a developing step of developing the
electrostatic image with a toner as described above to
form a toner image on the member to be charged,
a transfer step of transferring the toner
image to a transfer-receiving material directly or via
an intermediate transfer member, and
a fixing step of fixing the toner image onto
214922
-13-
the transfer-receiving material.
According to a further aspect of the present.
invention, there is provided a process-cartridge,
comprising at least a developing means and a
photosensitive member,
the developing means and the photosensitive
member being integrated into a cartridge which is
detachably mountable to a main body of an image
forming apparatus,
wherein the developing means contains a
toner as described above.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic illustration of an
image forming apparatus used in Examples of the
present invention.
Figure 2 is an exploded perspective view of
essential parts of a fixing apparatus. used in Examples
of the invention.
Figure 3 is an enlarged sectional view of a
fixing apparatus including a film in a non-driven
state used in Examples of the present invention.
Figure 4 is a partial illustration of a
checker pattern for evaluating the developing
performance of a toner.
Figure 5 is a schematic illustration of an
embodiment of the process-cartridge according to the
214922
-14-
present invention.
DETAILED DESCRIPTION OF THE INVENTION
As a result of our study, it has been found
possible to provide a toner capable of forming stable
images while retaining a high development performance
and without being affected by an environmental change.
An azo-type iron complex, when used as a
charge control agent for an electrophotographic toner,
provides a toner which shows an insufficient charging
speed under a high-humidity condition and fails to
provide a sufficient image density at an initial stage
or a long period of standing under a high-humidity
condition. Under a low-humidity condition, in a long
period of continual use, the toner is liable to cause
an accumulation of an excessive triboelectric charge
(charge-up), thus resulting in images with a low image
density and noticeable fog.
In contrast thereto, a chromium or aluminum
complex compound insoluble in a binder resin
alleviates the above-mentioned problems and has been
therefore widely used. A toner using such a chromium
or aluminum complex compound is accompanied with a
problem that classified fine powder and classified
coarse powder thereof cannot be readily re-utilized.
This is because the chromium or aluminum complex
compound is contained in different weight ratios in
~1492~2
-15-
the classified fine powder, classified medium powder
(used as a toner) and classified coarse powder, so
that a toner produced by re-utilization of the
classified fine powder and the classified coarse
powder is liable to cause a lowering in image density
and fog during a long period of continual use in a
low-humidity environment.
We have noted in combination that an azo-type
iron complex compound shows little localization in
classified powders and that a binder containing an
azo-type chromium complex compound insoluble in a
binder resin shows a good developing performance,
whereby we have succeeded in improving the charge
controllability of an azo-type iron complex compound
while retaining the non-localizability of the azo-type
iron complex compound, by forming micro-domains
(aggregations) of the azo-type iron complex compound
in toner particles.
The formation of an azo-type iron complex
compound is accomplished by the presence of a long-
chain alkyl compound in toner particles. This is
considered because the OH groups or carboxyl groups in
the long-chain alkyl compound respectively form an
associated state and, under the influence of the
associations, the azo-type iron complex compound forms
microdomain. As a result, the azo-type iron complex
compound can be provided with an improved charge
~1492~2
-16-
controllability while maintaining the non-
localizability.
The localization of an azo-type metal complex
in classified fine powder, classified medium powder
(used as a toner) and classified coarse powder
resultant after a classification step in a toner
production process using the azo-type metal complex is
evaluated in the following manner. Each powder
fraction is weighed in a prescribed amount within a
range of 1.0 - 3.0 g and is dispersed in 200 ml of
ethyl alcohol under stirring for 48 hours, followed by
filtration to recover a filtrate. Then, the
absorption spectrum in the visible range of the
filtrate is obtained and a relative absorbance at a
wavelength showing an absorption, e.g., T = 480 nm,
attributable to the metal complex is measured. The
localization characteristic of the metal complex is
evaluated by factors (ratios):
ODF/ODM and ODC/ODM,
wherein ODF denotes an absorbance of a filtrate
obtained from classified fine powder, ODM denotes an
absorbance of a filtrate obtained from classified
medium powder and ODD denotes an absorbance of a
filtrate obtained from classified coarse powder.
An azo-type iron complex compound represented
by the above-mentioned formula (4) wherein A+
comprises 75 - 98 mol. o of ammonium ions, has been
~14~2'~2
-17-
found to exhibit a preferred performance in forming
stable toner images. An azo-type iron complex
compound having cations consisting solely of ammonium
ions tends to provide a toner showing an image density
which slowly increases after standing in a high-
humidity environment. On the other hand, an azo-type
iron complex compound having cations consisting only
of protons or alkali metal ions tends to provide a
toner showing a low image density in a high-humidity
environment.
As a result of our study, the use of cations
including both ammonium ions and alkali metal ions
and/or protons provides a compound giving a toner
showing a good performance after a long period of
standing. The inclusion of ammonium ions at 75 - 98
mol. % provides particularly good results regarding
image density increasing speed and image density level
after the increase.
When the ammonium ion content is below 75 %,
the image density is lowered and, above 98 %, the
image density tends to increase slowly.
As a result of further study of ours, the
azo-type iron complex compound used in the toner
according to the present invention may preferably have
a solubility in methanol of 0.1 - 8 g/100 ml, more
preferably 0.3 - 4 g/100 ml, further preferably 0.4 -
2 g/100 ml.
21492'72
-18-
In case where the solubility is below 0.1
g/100 ml, the charge control agent (azo-type iron
complex compound} shows a low dispersibility in the
toner even if the long-chain alkyl compound is used in
combination, thus providing a toner which has an
unstable triboelectric chargeability and is liable to
cause image fog and scattering.
On the other hand, in case where the
solubility exceeds 8 g/100 ml, the toner performances
are liable to be affected by the temperature and
humidity during a long period of standing in a high
temperature - high humidity environment, so that the
toner chargeability is impaired and it becomes
difficult to obtain a sufficient image density.
The charge control agent may preferably be
used in a proportion of 0.2 - 5 wt. parts per 100 wt.
parts of the binder resin.
The solubility of the charge control agent
may be measured in the following manner.
<Solubility measurement of charge control agent>
2 g of a charge control agent is weighed and
placed in a 300 ml Erlenmeyer flask to which 100 ml of
methanol is added. The system is heated to 50 °C
under stirring and the stirring is further continued
for 1 hour (when all the charge control agent is
dissolved, the charge control agent is further added
successively at an increment of 2 g each under
z~4~z~~
-19-
continued stirring). Then, the system is cooled to
room temperature and the insoluble charge control
agent is removed by a 0.1 um-filter to measure the
absorbance (A) of the solution at a maximum absorption
wavelength by using a spectrophotometer.
On the other hand, a standard solution of the
charge control agent (at a concentration Co (= 0.02
g/1 (= 20 ppm)) is prepared, and the absorbance (Ao)
thereof is measured. From these data, the solubility
of the charge control agent (C (g/1)) is calculated by
A/Ao = C/Co, based on the Lambert-Beeis low
represented by the following formula:
A = loge (IO/I) - ~OCd,
wherein I denotes a transmitted light intensity
through a solution, IO denotes a transmitted light
intensity through a solvent (= methanol), EO denotes
an absorption coefficient, C denotes the concentration
of the charge control agent, and d denotes the
thickness of the solution for the absorbance
measurement.
The azo-type iron complex compound used in
the present invention has a structure represented by
the following general formula (4):
214~~'~~
-20-
a
(R,) n O
N-N O (XOm
O ~ O C-N -
(XZ)m' O Fe/ p
N-C O/ \O CR,)n
1
Q N=N
0
(4)
wherein X1 and X2 independently denote hydrogen atom,
lower alkyl group, lower alkoxy group, nitro group or
halogen atom; m and m' denote an integer of 1 - 3; R1
and R3 independently denote hydrogen atom, C1-18 alkyl
or alkenyl, sufonamide, mesyl, sulfonic acid group,
carboxy ester group, hydroxy, Cl-18 alkoxy,
acetylamino, benzoylamino or halogen atom; n and n'
denote an integer of 1 - 3; R2 and R4 denote hydrogen
atom or nitro group; and A+ denotes a cation including
75 - 98 mol. o of ammonium ion and another ion
selected from the group consisting of hydrogen ion,
sodium ion, potassium iron and mixtures thereof.
The above azo-type iron complex which is
suitably used as a negative charge control agent may
be synthesized according to a known process.
The negative charge control agent may be used
singly or in combination of two or more species or in
combination with another negative charge control
agent.
21492'2
-21-
Representative examples of the azo-type iron
complex represented by the above formula may include
those having structures as shown below wherein A~
denotes the same meaning as defined above:
10
20
~~4~2'~2
-22-
Iron Complex (1)
0
Ci O
O N=N O
O ~ O C-N
\ / II HH
O F a O A~
H li / \
~N-C O ~ O
O N=N O
O CI
Iron Complex (2)
Ci O
O N=N O
C I O ~ O C-N
\ / II H
O Fe O A
H II /
OQ --- N-C O O C I
N-N O
Q cl
25
-23-
Iron Complex (3)
C1 O o
Q N=N O NOZ
O CONH O
\ /
O Fe
A
II / \
O N-C O ~ O
H
NOz Q N=N
O C1
Iron Complex (4)
C 1 O o
O N = N O NOz
02N O ~ O C-N- O
\ / II H
/ F e\ O A~
O N-C O ~ O
NOZ
NOZ
O N=N O
O cl
2192'72
-24-
Iron Complex (5)
OZN O o
Cl ~ N=N
O CH3
O ~ O C-N O
\F e/ II H
O
N-C O/ \O
H
CH3 ~ N=N
C1
NOZ
Iron Complex (6)
0
~ N=N 0
O ~ O ~C-N 0
/ II H
O
O A+
H (I /F e\ CH3
0 N-C O ~ O
CH3 0 N=N
In the toner for developing the electrostatic
images the azo-type ion complex may preferably be used
in an amount of 0.1 - 10 wt. parts, more preferably
0.1 - 5 wt. parts, per 100 wt. parts of the binder
resin_
2142'72
-25-
The long-chain alkyl compound used in the
present invention may be represented by the following
formula (1), (2) or (3).
CH3-~-CH2~CH20H ( 1 ) ,
CH3-f-CH2~O~C) HCH2 -O~H ( 2 ) ,
R
wherein x denotes an average value in the range of 35
- 150; z denotes an average value in the range of 1 -
5, and R denotes H or an alkyl group having 1 - 10
carbon atoms.
The long-chain alkyl compound of the above
formulae may fob example be produced as follows.
Ethylene is polymerized in the presence of a Ziegler
catalyst and, after the polymerization, oxidized to
provide an alkoxide of the catalyst metal and
polyethylene, which is then hydrolyzed to provide an
objective long-chain alkyl compound of formula (1).
By reacting the long-chain alkyl alcohol of formula
(1) with an epoxy group-containing substance, it is
possible to obtain a long-chain alkoxy alcohol of
formula (2). The thus prepared long-chain alkyl
alcohols have little branching and a sharp molecular
weight distribution and are suitably used in the
present invention.
CH3-f-CH2~ CH2COOH ( 3 ) ,
wherein y denotes an integer of 35 - 150.
The long-chain alkyl compound of formula (3)
~~492~2
-26-
may be obtained by oxidizing the long-chain alkyl
compound of formula (1).
For the compound represented by the above
formula (1), (2) or (3), x and y may preferably be 35
- 150. If x and y are below 35, the resultant toner
is liable to cause melt-sticking onto the
photosensitive member or a lower storage stability.
If x and y are larger than 150, the above-mentioned
contribution to toner chargeability (i.e., promoting
the formation of microdomains of the azo-type iron
complex) is lowered, thus being unsuitable for
accomplishing the object of the present invention. z
is preferably at most 5. If z is larger than 5, the
resultant toner is liable to cause melt-sticking onto
the photosensitive member. For similar reasons, it is
preferred that R is H or a C1 - C10 alkyl group.
The long-chain alkyl compound used in the
present invention may suitably be a mixture of
compounds having different molecular weights and can
further contain at most 30 wt. o, preferably at most
wt. o of hydrocarbon compounds free from functional
groups such as hydroxyl and carboxyl group as by-
produced through the above-mentioned production
processes of the compounds of the formulae (1) - (3).
25 The long-chain alkyl compound may preferably have a
number-average molecular weight (Mn) of 150 - 2500, a
weight-average molecular weight (Mw) of 250 - 5000,
214922
-27-
and an Mw/Mn ratio of at most 3.
In case where Mn is below 150 or Mw is below
250, the toner is liable to cause melt-sticking onto
the photosensitive member or a lower storage
stability. In case where Mn exceeds 2500 or Mw
exceeds 5000, the contribution to toner chargeability
is lowered, thus being liable to cause problems, such
as fog.
The long-chain alkyl compound of formula (1)
or (2) used in the present invention may preferably
have an OH value of 2 - 150 mgKOH/g, more preferably
10 - 120 mgKOH/g. If the long-chain alkyl compound
has an OH value below 2 mgKOH/g, the dispersibility
thereof in the binder resin is lowered to result in
ununiform toner chargeability leading to a density
decrease, fog, and inferior image quality in copy
images. In case where the long-chain alkyl compound
has an OH value exceeding 150 mgKOH/g, the
localization of the OH group charge density is
increased to exceed the charge density localization of
the OH groups in the binder resin, so that copy images
in the initial state of image formation are liable to
have a low density and a poor image quality.
Alternatively, even if the initial density is high,
the density is liable to be lowered gradually on
continuation of copying. Further, in case where the
OH value exceeds 150 mgKOH/g, the long-chain alkyl
zm~z7z
-28-
compound is caused to contain a large amount of low-
molecular weight molecules so that the resultant toner
is liable to cause a melt-sticking onto the
photosensitive member and lower the storage stability.
The long-chain alkyl compound of formula (3)
used in the present invention may preferably have an
acid value of 2 - 150 mgKOH/g, more preferably 5 - 120
mgKOH/g. If the long-chain alkyl compound has an acid
value below 2 mgKOH/g, the dispersion thereof in the
binder resin becomes worse, thereby resulting in
inferior image qualities of copy images. Further, as
the carboxyl groups do not sufficiently associate each
other, the environmental characteristic is liable to
be impaired. Further, the resultant toner is liable
to show a low charging velocity, to result in a lower
density at the initial stage of copying. In case
where the acid value of the long-chain alkyl compound
exceeds 150 mgKOH/g, it contains a large amount of
low-molecular weight molecules, the resultant toner is
liable to cause melt-sticking onto the photosensitive
member and lower the storage stability.
The long-chain alkyl compounds, when used
singly, may preferably be contained in an amount of
0.1 - 30 wt. parts, particularly 0.5 - 20 wt. parts,
per 100 wt. parts of the binder resin.
In case where the long-chain alkyl compounds
are used in combination, the total amount thereof may
zl~~z~z
-29-
preferably be 0.1 - 30 wt. parts, more preferably 0.5
- 20 wt. parts, per 100 wt. parts of the binder resin.
It is preferred for the toner according to
the present invention to contain 3 - 90 % by number of
toner particles having a particle size of 5 ~~m or
smaller. Hitherto, it has been considered difficult to
control the charge imparted to toner particles of 5 um
or smaller. Further, such fine toner particles are
considered to impair the fluidity of the toner, soil
the carrier and developing sleeve, cause cleaning
failure and filming onto the drum and scatter to soil
the interior of an image forming apparatus. Thus, it
has been considered necessary to remove or decrease
toner particles of 5 um or smaller.
As a result of our study, however, in case of
a toner containing a specific long-chain alkyl
compound and an azo-type iron complex of the above-
mentioned formula, it has been found that toner
particles of 5 um or smaller are very effective for
providing images of a fine definition and a high
resolution.
In the toner used in the present invention,
it is also preferred that toner particles of 6.35 -
10.08 um constitute 1 - 80 o by number and the toner
has a weight-average particle size of 4.0 - 10 um,
more preferably 4.5 - 9.0 um.
Toner particles of 5 dam or smaller are able
214272
-30-
to strictly cover and faithfully reproduce an
electrostatic image, but an electrostatic image per se
has a higher electric field intensity at the
peripheral edge than the middle or central portion.
As a result, toner particles are attached to the
central portion in a smaller thickness than to the
peripheral part, so that the inner part is liable to
be thin in density. We have found that this problem
can be solved to provide a clear image by using toner
particles of 6.35 - 10.08 um in a proportion of 1 - 80
o by number. This may be attributable to a fact that
toner particles of 6.35 - 10.08 um are supplied to an
inner part having a smaller intensity than the edge of
a latent image presumably because they have a
moderately controlled charge relative to toner
particles of 5 um or smaller, thereby to compensate
for the less coverage of toner particles and result in
a uniform developed image. As a result, a sharp image
having a high density and excellent in resolution and
gradation characteristic can be attained.
Further, it is most preferred that the
contents of the toner particles of 5 pm or smaller in
terms of o by number (N s) and o by volume (V %)
satisfy the relationship of N/V = -0.05N+k, wherein 3
< k < 12, and 5 < N < 90. The toner having a particle
size distribution satisfying the relationship in
combination with the other characteristic features
2.~4~2~2
-31-
according to the present invention accomplishes a
better developing performance with respect to a
digital latent image composed of minute spots.
We have found a certain state of presence of
fine powder accomplishing the intended performance
satisfying the above formula during our study on the
particle size distribution with respect to particles
of 5 um or smaller. For a certain value of N, a large
N/V value is understood to mean that a large
proportion of particles smaller than 5 um are present
with a broad particle size distribution, and a small
N/V value is understood to mean that particles having
a particle size in the neighborhood of 5 pm is present
in a large proportion and particles smaller than that
are present in a small proportion. A further better
thin-line reproducibility and high resolution in a
large quantity of copying or printing are accomplished
when the N/V is in the range of 1.0 - 7.45, N is in
the range of 5 - 90 and the above formula relationship
is satisfied.
Toner particles of 12.7 um or larger are
suppressed to be not more than 2.0 o by volume. The
fewer, the better.
The particle size distribution of the toner
used in the present invention is described more
specifically below.
Toner particles of 5 um or smaller may be
~1492~2
-32-
contained in a proportion of 5 - 90 o by number,
further preferably 9 - 75 ~ by number, of the total _
number of particles. If the content of the toner
particles of 5 um or smaller is below 5 o by number, a
portion of the toner particles effective for providing
a high image quality is few and particularly, as the
toner is consumed during a continuation of copying or
printing-out, the effective component is
preferentially consumed to result in an awkward
particle size distribution of the toner and gradually
deteriorates the image quality. If the content is
above 90 o by number, mutual agglomeration of the
toner particles and charge-up are liable to occur,
thus leading to difficulties, such as cleaning
failure, a low image density, and a large difference
in density between the contour and interior of an
image to provide a somewhat hollow image.
It is preferred that the content of the
particles in the range of 6.35 - 10.08 um is 1 - 80 0
by number, further preferably 5 - 70 o by number.
Above 80 o by number, the image quality becomes worse,
and excess of toner coverage is liable to occur, thus
resulting in a lower thin-line reproducibility and an
increased toner consumption. Below 5 o by number, it
becomes difficult to obtain a high image density in
some cases.
For similar reasons as N, V may preferably be
214922
-33-
0.5 - 70 o by volume.
The k value may preferably be 3 - 12, more
preferably 4 - 10.
If k < 3.0, toner particles of 5.0 um or
below are insufficient, and the resultant image
density, resolution and sharpness decrease. When fine
toner particles in a toner, which have conventionally
been considered useless, are present in an appropriate
amount, they are effective for achieving closest
packing of toner in development and contribute to the
formation of a uniform image. Particularly, these
particles fill thin-line portions and contour portions
of an image, thereby to visually improve the sharpness
thereof. On the other hand, if k > 12, an excess of
fine powder is present, whereby the balance of
particle size distribution can be disturbed during
successive copying or print-out, thus leading to
difficulties such as a somewhat lower image density
and filming.
The amount of toner particles having a
particle size of 12.7 um or larger should be 2.0 o by
volume or smaller, preferably 1.0 o by volume or
smaller, more preferably 0.5 % by volume or smaller.
If the above amount is larger than 2.0 o by volume,
these particles are liable to impair thin-line
reproducibility.
The toner used in the present invention may
z~~s272
-34-
have a weight-average particle size of 4 - 10 um, more
preferably 4.5 - 9 um. This value cannot be
considered separately from the above-mentioned
factors. If the weight-average particle size is below
4 um, the toner is liable to cause soiling of the
interior of an apparatus with scattered toner, a
lowering in image density in a low-humidity
environment and cleaning failure of the photosensitive
member. If the weight-average particle size exceeds 9
um, a minute spot of 100 um or smaller cannot be
developed with a sufficient resolution and noticeable
scattering to non-image part is observed, thus being
liable to provide inferior images.
Examples of the binder resin used in the
toner of the present invention may include polyester
resins, vinyl resins and epoxy resins. Among these,
polyester resins or vinyl resins may preferably be
used in view of charging characteristic and fixing
characteristic.
A polyester resin preferably used in the
present invention may have a composition that it
comprises 45 - 55 mol. o of alcohol component and 55 -
45 mol. % of acid component.
Examples of the alcohol component may
include: diols, such as ethylene glycol, propylene
glycol, 1,3-butanediol, 1,4-butanediol, 2,3-
butanediol, diethylene glycol, triethylene glycol,
2149~'~2
-35-
1,5-pentanediol, 1,6-hexanediol, neopentyl glycol, 2-
ethyl-1,3-hexanediol, hydrogenated bisphenol A,
bisphenols and derivatives represented by the
following formula (A):
IH3
H -~OR~O-O-C-~O-f RO~ H ( A ) ,
CH3
wherein R denotes an ethylene or propylene group, x
and y are independently a positive integer of at least
1 with the proviso that the average of x+y is in the
range of 2 - 10; diols represented by the following
formula (B):
H-fOR'-~O-~O-~-R' -O~H (B) ,
i H3 i H3
wherein R' denotes -CH2CH2-, -CH2-CH- or -CH2-C- ,
CH3
x' and y' are a positive integer of at least 1 with
the proviso that the average of x'+y' is in the range
of 1 - 10.
Examples of the dibasic acid constituting at
least 50 mol. % of the total acid may include
benzenedicarboxylic acids, such as phthalic acid,
terephthalic acid and isophthalic acid, and their
anhydrides; alkyldicarboxylic acids, such as succinic
acid, adipic acid, sebacic acid and azelaic acid, and
their anhydrides; C6 - C18 alkyl or alkenyl-
~1492~2
-36-
substituted succinic acids, and their anhydrides; and
unsaturated dicarboxylic acids, such as fumaric acid,-
malefic acid, citraconic acid and itaconic acid, and
their anhydrides.
An especially preferred class of alcohol
components constituting the polyester resin is a
bisphenol derivative represented by the above formula
(A), and preferred examples of acid components may
include dicarboxylic acids inclusive of phthalic acid,
terephthalic acid, isophthalic acid and their
anhydrides; succinic acid, n-dodecenylsuccinic acid,
and their anhydrides, fumaric acid, malefic acid, and
malefic anhydride.
The polyester resin may preferably have a
glass transition temperature of 40 - 90 °C,
particularly 45 - 85 °C, a number-average molecular
weight (Mn) of 1,000 - 50,000, particularly 1,500 -
20,000, and a weight-average molecular weight (Mw) of
3x103 - 5x106, particularly 4x103 - 1.5x106.
Examples of a vinyl monomer for providing the
vinyl resin may include: styrene; styrene derivatives,
such as o-methylstyrene, m-methylstyrene, p-
methylstyrene, p-methoxystyrene, p-phenylstyrene, p-
chlorostyrene, 3,4-dichlorostyrene, p-ethylstyrene,
2,4-dimethylstyrene, p-n-butylstyrene, p-tert-
butylstyrene, p-n-hexylstyrene, p-n-octylstyrene, p-n-
nonylstyrene, p-n-decylstyrene, and p-n-
2149272
-37-
dodecylstyrene; ethylenically unsaturated monoolefins,
such as ethylene, propylene, butylene, and
isobutylene; unsaturated polyenes, such as butadiene;
halogenated vinyls, such as vinyl chloride, vinylidene
chloride, vinyl bromide, and vinyl fluoride; vinyl
esters, such as vinyl acetate, vinyl propionate, and
vinyl benzoate; methacrylates, such as methyl
methacrylate, ethyl methacrylate, propyl methacrylate,
n-butyl methacrylate, isobutyl methacrylate, n-octyl
methacrylate, dodecyl methacrylate, 2-ethylhexyl
methacrylate, stearyl methacrylate, phenyl
methacrylate, dimethylaminoethyl methacrylate, and
diethylaminoethyl methacrylate; acrylates, such as
methyl acrylate, ethyl acrylate, n-butyl acrylate,
isobutyl acrylate, propyl acrylate, n-octyl acrylate,
dodecyl acrylate, 2-ethylhexyl acrylate, stearyl
acrylate, 2-chloroethyl acrylate, and phenyl acrylate,
vinyl ethers, such as vinyl methyl ether, vinyl ethyl
ether, and vinyl isobutyl ether; vinyl ketones, such
as vinyl methyl ketone, vinyl hexyl ketone, and methyl
isopropenyl ketone; N-vinyl compounds, such as N-
vinylpyrrole, N-vinylcarbazole, N-vinylindole, and N-
vinyl pyrrolidone; vinylnaphthalenes; acrylic acid
derivatives or methacrylic acid derivatives, such as
acrylonitrile, methacryronitrile, and acrylamide; the
esters of the above-mentioned a,a-unsaturated acids
and the diesters of the above-mentioned dibasic acids.
2I4~~~2
-38-
Examples of a carboxy group-containing vinyl
monomer may include: unsaturated dibasic acids, such
as malefic acid, citraconic acid, itaconic acid,
alkenylsuccinic acid, fumaric acid, and mesaconic
acid; unsaturated dibasic acid anhydrides, such as
malefic anhydride, citraconic anhydride, itaconic
anhydride, and alkenylsuccinic anhydride; unsaturated
dibasic acid half esters, such as mono-methyl maleate,
mono-ethyl maleate, mono-butyl maleate, mono-methyl
citraconate, mono-ethyl citraconate, mono-butyl
citraconate, mono-methyl itaconate, mono-methyl
alkenylsuccinate, monomethyl fumarate, and mono-methyl
mesaconate; unsaturated dibasic acid esters, such as
dimethyl maleate and dimethyl fumarate; a,a-
unsaturated acids, such as acrylic acid, methacrylic
acid, crotonic acid, and cinnamic acid; a,a-
unsaturated acid anhydrides, such as crotonic
anhydride, and cinnamic anhydride; anhydrides between
such an a,a-unsaturated acid and a lower aliphatic
acid; alkenylmalonic acid, alkenylglutaric acid,
alkenyladipic acid, and anhydrides and monoesters of
these acids.
It is also possible to use a hydroxyl group-
containing vinyl monomer: inclusive of acrylic or
methacrylic acid esters, such as 2-hydroxyethyl
acrylate, and 2-hydroxyethyl methacrylate; 4-(1-
hydroxy-1-methylbutyl)styrene, and 4-(1-hydroxy-1-
2149272
-39-
methylhexyl)styrene.
The vinyl resin may have a glass transition-
point of 45 - 80 °C, preferably 55 - 70 oC, a number-
average molecular weight (Mn) of 2.5x103 - 5x104, and
a weight-average molecular weight (Mw) of 1x104 -
1.5x106.
In the present invention, it is also possible
to use a mixture binder resin including a vinyl
homopolymer or copolymer, a polyester, polyester,
epoxy resin, polyvinyl butyral, rosin, modified rosin,
terpene resin, phenolic resin, aliphatic or alicyclic-
hydrocarbon resin or aromatic petroleum resin, in
addition to the above-mentioned binder resin.
In case of using a mixture binder resin
including two or more resins of the same or different
types, the two or more resins may preferably have
different molecular weights and may be mixed with each
other in appropriate ratios.
The toner according to the present invention
may be either a magnetic toner or a non-magnetic
toner. In order to constitute a magnetic toner, it is
preferred to use a magnetic material as described
below.
Examples of the magnetic material contained
in the insulating magnetic toner used in the present
invention may include: iron oxides, such as magnetite,
hematite, and ferrite; iron oxides containing another
,/r~
2149272
-40-
metal oxide; metals, such as Fe, Co and Ni, and alloys
of these metals with other metals, such as A1, Co, Cu,
Pb, Mg, Ni, Sn, Zn, Sb, He, Hi, Cd, Ca, Mn, Se, Ti, W
and V; and mixtures of the above.
Specific examples of the magnetic material
may include: triiron tetroxide (Fe304), diiron
trioxide (Y-Fe203), zinc iron oxide (ZnFe204), yttrium
iron oxide (Y3Fe5012), cadmium iron oxide (CdFe204),
gadolinium iron oxide (Gd3Fe5012), copper iron oxide
(CuFe204), lead iron oxide (PbFe12019), nickel iron
oxide (NiFe204), neodymium iron oxide (NdFe203),
barium iron oxide (BaFe12019), magnesium iron oxide
(MgFe204), manganese iron oxide (MnFe204), lanthanum
iron oxide (LaFe03), powdery iron (Fe), powdery cobalt
(Co), and powdery nickel (Ni). The above magnetic
materials may be used singly or in mixture of two or
more species. Particularly suitable magnetic material
for the present invention is fine powder of triiron
tetroxide or Y-diiron trioxide.
The magnetic material may have an average
particle size (Dav.) of 0.1 - 2 um, preferably 0.1 -
0.3 um. The magnetic material may preferably show
magnetic properties when measured by application of 10
kilo-Oersted, inclusive of: a coercive force (Hc) of
20 - 150 Oersted, a saturation magnetization (us) of
50 - 200 emu/g, particularly 50 - 100 emu/g, and a
residual magnetization (ar) of 2 - 20 emu/g.
2149272
-41-
The magnetic material may be contained in the
toner in a proportion of 10 - 200 wt. parts, _
preferably 20 - 150 wt. parts, per 100 wt. parts of
the binder resin.
The toner according to the present invention
may optionally contain a colorant, inclusive of
arbitrary pigments or dyes.
Examples of the pigment may include: carbon
black, aniline black, acetylene black, Naphthol
Yellow, Hansa Yellow, Rhodamine Lake, Alizarine Lake,
red iron oxide, Phthalocyanine Blue, and Indanthrene
Blue. It is preferred to use 0.1 - 20 wt. parts,
particularly 1 - 10 wt. parts, of a pigment per 100
wt. parts of the binder resin. For similar purpose,
there may also be used dyes, such as azo dyes,
anthraquinone dyes, xanthene dyes, and methine dyes,
which may preferably be used in an amount of 0.1 - 20
wt. parts, particularly 0.3 - 10 wt. parts, per 100
wt. parts of the resin.
In the present invention, it is also
possible
to incorporate one or two or more species of release
agent, as desired, within a toner.
Examples of the release agent may include:
aliphatic hydrocarbon waxes, such as low-molecular
weight polyethylene, low-molecular weight
polypropylene, microcrystalline wax, and paraffin wax,
oxidation products of aliphatic hydrocarbon waxes,
2149272
-42-
such as oxidized polyethylene wax, and block
copolymers of these; waxes containing aliphatic esters
as principal constituents, such as carnauba wax,
montanic acid ester wax, and partially or totally
deacidified aliphatic esters, such as deacidified
carnauba wax. Further examples of the release agent
may include: saturated linear aliphatic acids, such as
palmitic acid, stearic acid, and montanic acid;
unsaturated aliphatic acids, such as brassidic acid,
eleostearic acid and parinaric acid; saturated
alcohols, such as stearyl alcohol, behenyl alcohol,
ceryl alcohol, and melissyl alcohol; polyhydric
alcohols, such as sorbitol; aliphatic acid amides,
such as linoleylamide, oleylamide, and laurylamide;
saturated aliphatic acid bisamides, methylene-
bisstearylamide, ethylene-biscaprylamide, and
ethylene-biscaprylamide; unsaturated aliphatic acid
amides, such as ethylene-bisolerylamide,
hexamethylene-bisoleylamide, N,N'-dioleyladipoylamide,
and N,N'-dioleylsebacoylamide, aromatic bisamides,
such as m-xylene-bisstearoylamide, and N,N'-
distearylisophthalylamide; aliphatic acid metal salts
(generally called metallic soap), such as calcium
stearate, calcium laurate, zinc stearate, and
magnesium stearate; grafted waxes obtained by grafting
aliphatic hydrocarbon waxes with vinyl monomers, such
as styrene and acrylic acid; partially esterified
2149272
-43-
products between aliphatic acids and polyhydric
alcohols, such as behenic acid monoglyceride; and
methyl ester compounds having hydroxyl group as
obtained by hydrogenating vegetable fat and oil.
The particularly preferred class of release
agent in the present invention may include aliphatic
hydrocarbon waxes because of good dispersibility
within the binder resin (preferably one having an acid
value of 5 - 50), thus providing not only a good
fixability of the resultant toner but also a minimum
abrasion of an organic photoconductor when used in
combination with the toner according to the present
invention.
Specific examples of the release agent
preferably used in the present invention may include
e.g., a low-molecular weight alkylene polymer obtained
through polymerization of an alkylene by radical
polymerization under a high pressure or in the
presence of a Ziegler catalyst under a low pressure;
an alkylene polymer obtained by thermal decomposition
of an alkylene polymer of a high molecular weight; and
a hydrocarbon wax obtained by subjecting a mixture gas
containing carbon monoxide and hydrogen to the Arge
process to form a hydrocarbon mixture and distilling
the hydrocarbon mixture to recover a residue.
Fractionation of wax may preferably be performed by
the press sweating method, the solvent method, vacuum
~1~~272
-44-
distillation or fractionating crystallization. As the
source of the hydrocarbon wax, it is preferred to use
hydrocarbons having up to several hundred carbon atoms
as obtained through synthesis from a mixture of carbon
monoxide and hydrogen in the presence of a metal oxide
catalyst (generally a composite of two or more
species), e.g., by the Synthol process, the Hydrocol
process (using a fluidized catalyst bed), and the Arge
process (using a fixed catalyst bed) providing a
product rich in waxy hydrocarbon, and hydrocarbons
obtained by polymerizing an alkylene, such as
ethylene, in the presence of a Ziegler catalyst, as
they are rich in saturated long-chain linear
hydrocarbons and accompanied with few branches. It is
further preferred to use hydrocarbon waxes synthesized
without polymerization because of their structure and
molecular weight distribution suitable for easy
fractionation.
As for the molecular weight distribution of
the wax, it is preferred that the wax shows a peak in
a molecular weight region of 400 - 2400, further 450 -
2000, particularly 500 - 1600. Hy satisfying such
molecular weight distribution, the resultant toner is
provided with preferable thermal characteristics.
The release agent may preferably be used in
an amount of 0.1 - 20 wt. parts, particularly 0.5 - 10
wt. parts, per 100 wt. parts of the binder resin.
2149272
-45-
The release agent may be uniformly dispersed
in the binder resin by a method of mixing the release-
agent in a solution of the resin at an elevated
temperature under stirring or melt-kneading the binder
resin together with the release agent.
A flowability-improving agent may be
optionally blended with the toner to improve the
flowability of the toner. Examples thereof may
include: powder of fluorine-containing resin, such as
polyvinylidene fluoride fine powder and
polytetrafluoroethylene fine powder; titanium oxide
fine powder, hydrophobic titanium oxide fine powder;
fine powdery silica such as wet-process silica and
dry-process silica, and treated silica obtained by
surface-treating such fine powdery silica with silane
coupling agent, titanium coupling agent, silicone oil,
etc.
A preferred class of the flowability-
improving agent includes dry process silica or fumed
silica obtained by vapor-phase oxidation of a silicon
halide. For example, silica powder can be produced
according to the method utilizing pyrolytic oxidation
of gaseous silicon tetrachloride in oxygen-hydrogen
flame, and the basic reaction scheme may be
represented as follows:
SiCl4 + 2H2 + OZ -~ SiOZ + 4HC1.
In the above preparation step, it is also
2149272
-46-
possible to obtain complex fine powder of silica and
other metal oxides by using other metal halide
compounds such as aluminum chloride or titanium
chloride together with silicon halide compounds. Such
is also included in the fine silica powder to be used
in the present invention.
It is preferred to use fine silica powder
having an average primary particle size of 0.001 - 2
pm, particularly 0.002 - 0.2 pm.
Commercially available fine silica powder
formed by vapor phase oxidation of a silicon halide to
be used in the present invention include those sold
under the trade names as shown below.
AEROSIL 130
(Nippon Aerosil Co.) 200
300
380
TT 600
MOX 170
MOX 80
COK 84
Cab-O-Sil M-5
(Cabot Co.) MS-7
MS-75
HS-5
EH-5
Wacker HDK N 20
2149272
-47-
(WALKER-CHEMIE GMHH) V 15
N 20E
T 30
T 40
D-C Fine Silica
(Dow Corning Co.)
Fransol
(Fransil Co.)
It is further preferred to use treated silica
fine powder obtained by subjecting the silica fine
powder formed by vapor-phase oxidation of a silicon
halide to a hydrophobicity-imparting treatment. It is
particularly preferred to use treated silica fine
powder having a hydrophobicity of 30 - 80 as measured
by the methanol titration test.
Silica fine powder may be imparted with a
hydrophobicity by chemically treating the powder with
an organosilicone compound, etc., reactive with or
physically adsorbed by the silica fine powder.
Example of such an organosilicone compound
may include: hexamethyldisilazane, trimethylsilane,
trimethylchlorosilane, trimethylethoxysilane,
dimethyldichlorosilane, methyltrichlorosilane,
allyldimethylchlorosilane, allylphenyldichlorosilane,
benzyldimethylcholrosilane, bromomethyl-
dimethylchlorosilane, a-chloroethyltrichlorosilane,
a-chloroethyltrichlorosilane, chloromethyldimethyl-
-48-
chlorosilane, triorganosilylmercaptans such as
trimethylsilylmercaptan, triorganosilyl acrylates,
vinyldimethylacetoxysilane, dimethylethoxysilane,
dimethyldimethoxysilane, diphenyldiethoxysilane,
hexamethyldisiloxane, 1,3-divinyltetramethyldi-
siloxane, 1,3-diphenyltetramethyldisiloxane, and
dimethylpolysiloxane having 2 to 12 siloxane units per
molecule and containing each one hydroxyl group bonded
to Si at the terminal units. These may be used alone
or as a mixture of two or more compounds.
The flowability-improving agent used in the
present invention may have a specific surface area of
at least 30 m2/g, preferably 50 m2/g, as measured by
the BET method according to nitrogen adsorption. The
flowability-improving agent may be used in an amount
of 0.01 - 8 wt. parts, preferably 0.1 - 4 wt. parts,
per 100 wt. parts of the toner.
In case where the toner according to the
present invention is used for constituting a two-
component type developer, the toner is blended with a
carrier. Examples of the carrier used in the present
invention may include: surface-oxidized or -unoxidized
powder of metals, such as iron, nickel, copper, zinc,
cobalt, manganese, chromium and rare earth metals,
particles of alloys of these metal, oxide particles,
and ferrite particles.
A coated carrier obtained by coating the
214972
-49-
above carrier particles with a resin may preferably
be used particularly in a developing method wherein a
developing bias is supplied with an AC bias voltage.
The coating may be performed according to known
methods inclusive of a method applying a coating
liquid obtained by dissolving or suspending a coating
material such as a resin into a solvent onto the
surface of carrier core particles, and a method of
powder blending carrier core particles and a coating
material.
Examples of the coating material firmly
applied onto the core particles may include:
polytetrafluoroethylene, monochlorotrifluoroethylene
polymer, polyvinylidene fluoride, silicone resin,
polyester resin, styrene resin, acrylic resin,
polyamide, polyvinyl butyral, aminoacrylate resin,
basic dyes and lakes thereof, silica fine powder and
alumina fine powder. These coating materials may be
used singly or in combination of plural species.
The coating material may be applied onto the
core particles in a proportion of 0.1 - 30 wt. %,
preferably 0.5 - 20 wt. o, based on the carrier
core particles. The carrier may preferably have an
average particle size of 10 - 100 um, more preferably
20 - 70 }un.
A particularly preferred type of carrier may
comprise particles of a magnetic ferrite such as Cu-
z~~~~~2
-50-
Zn-Fe ternary ferrite surface-coated with a fluorine-
containing resin or a styrene-based resin. Preferred
coating materials may include mixtures of a fluorine
containing resin and a styrene copolymer, such as a
mixture of polyvinylidene fluoride and styrene-methyl
methacrylate resin, and a mixture of
polytetraluforoethylene and styrene-methyl
methacrylate resin. The fluorine-containing resin may
also be a copolymer, such as vinylidene
fluoride/tetrafluoroethylene (10/90 - 90/10)
copolymer. Other examples of the styrene-based resin
may include styrene/2-ethylhexyl acrylate (20/80 -
80/20) copolymer and styrene/2-ethylhexyl
acrylate/methyl methacrylate (20 - 60/5 - 30/10 - 50)
copolymer. The fluorine-containing resin and the
styrene-based resin may be blended in a weight ratio
of 90:10 - 20:80, preferably 70:30 - 30:70. The
coating amount may be 0.01 - 5 wt. o, preferably 0.1 -
1 wt. o of the carrier core.
The coated magnetic ferrite carrier may
preferably include at least 70 wt. o of particles
of 250 mesh-pass and 400 mesh-on, and have an average
particle size of 10 - 100 um, more preferably 20 -
70 um. A sharp particle size distribution is
preferred.
The characteristic values of a binder resin
and a long-chain alkyl compound and the particle size
2~492~2
-51-
distribution of a toner referred to herein may be
measured according to the following methods.
(1) Glass transition temperature Tg
Measurement may be performed in the following
manner by using a differential scanning calorimeter
("DSC-7", available from Perkin-Elmer Corp.).
A sample in an amount of 5 - 20 mg,
preferably about 10 mg, is accurately weighed.
The sample is placed on an aluminum pan and
subjected to measurement in a temperature range of
30 - 200 °C at a temperature-raising rate of 10 °C/min
in a normal temperature - normal humidity environment
in parallel with a blank aluminum pan as a reference.
In the course of temperature increase, a main
absorption peak appears in the temperature region of
40 - 100 °C.
In this instance, the glass transition
temperature is determined as a temperature of an
intersection between a DSC curve and an intermediate
line pressing between the base lines obtained before
and after the appearance of the absorption peak.
(2) Molecular weiqht distribution (for binder resin)
The molecular weight (distribution) of a
binder resin may be measured based on a chromatogram
obtained by GPC (gel permeation chromatography).
In the GPC apparatus, a column is stabilized
in a heat chamber at 40 °C, tetrahydrofuran (THF)
z~4~z~2
-52-
solvent is caused to flow through the column at that
temperature at a rate of 1 ml/min., and 50 - 200 ul of
a GPC sample solution adjusted at a concentration of
0.05 - 0.6 wt. o is injected. The identification of
sample molecular weight and its molecular weight
distribution is performed based on a calibration curve
obtained by using several monodisperse polystyrene
samples and having a logarithmic scale of molecular
weight versus count number. The standard polystyrene
samples for preparation of a calibration curve may be
available from, e.g., Pressure Chemical Co. or Toso
K.K. It is appropriate to use at least 10 standard
polystyrene samples inclusive of those having
molecular weights of, e.g., 6x102, 2.1x103, 4x103,
1.75x104, 5.1x104, 1.1x105, 3.9x105, 8.6x105, 2x106
and 4.48x106. The detector may be an RI (refractive
index) detector. For accurate measurement, it is
appropriate to constitute the column as a combination
of several commercially available polystyrene gel
columns in order to effect accurate measurement in the
molecular weight range of 103 - 2x106. A preferred
example thereof may be a combination of p-styragel
500, 103, 104 and 105 available from Waters Co.; a
combination of Shodex KF-801, 802, 803, 804, 805, 806
and 807 available from Showa Denko K.K.
(3) Molecular weight distribution (for lon chain
alkyl compound)
2149272
-53-
The molecular weight (distribution) of a
long-chain alkyl compound may be measured by GPC under
the following conditions:
Apparatus: "GPC-150C" (available from Waters Co.)
Column: "GMH-HT" 30 cm-binary (available from
Toso K.K.)
Temperature: 135 °C
Solvent: o-dichlorobenzene containing 0.1 g of
ionol.
Flow rate: 1.0 ml/min.
Sample: 0.4 ml of a 0.15 ~-sample.
Based on the above GPC measurement, the
molecular weight distribution of a sample is obtained
once based on a calibration curve prepared by
monodisperse polystyrene standard samples, and re-
calculated into a distribution corresponding to that
of polyethylene using a conversion formula based on
the Mark-Houwink viscosity formula.
(4) Measurement of acid values and OH values
1) re: Acid value
A sample material is accurately weighed and
dissolved in a mixture solvent, and water is added
thereto. The resultant liquid is titrated with O.1N-
NaOH by potentiometric titration using glass
electrodes (according to JIS K1557-1970).
2) re: Hydroxyl value (OH value)
A sample is accurately weighed into a 100 ml-
2149272
-54-
volumetric flask, and 5 ml of an acetylating agent is
accurately added thereto. Then, the system is heated
by dipping into a bath of 100 °C ~ 5 °C. After 1 - 2
hours, the flask is taken out of the bath and allowed
to cool by standing, and water is added thereto,
followed by shaking to decompose acetic anhydride. In
order to complete the decomposition, the flask is
again heated for more than 10 min. by dipping into the
bath. After cooling, the flask wall is sufficiently
washed with an organic solvent. The resultant liquid
is titrated with a N/2-potassium hydroxide solution in
ethyl alcohol by potentiometric titration using glass
electrodes (according to JIS K0070-1966).
(5) Particle size distribution measurement
Coulter Multisizer II (available from Coulter
Electronics Inc.) is used as an instrument for
measurement, to which an interface (available from
Nikkaki K.K.) for providing a number-basis
distribution, and a volume-basis distribution and a
personal computer PC 9801 (available from NEC K.K.)
are connected.
For measurement, a 1 $-NaCl aqueous solution
as an electrolytic solution is prepared by using a
reagent-grade sodium chloride. Into 100 to 150 ml of
the electrolytic solution, 0.1 to 5 ml of a
surfactant (preferably an alkylbenzenesulfonic acid
salt) is added as a dispersant, and 2 to 20 mg of a
~A
X149212
-55-
sample is added thereto. The resultant dispersion of
the sample in the electrolytic liquid is subjected to
a dispersion treatment for about 1 - 3 minutes by
means of an ultrasonic disperser, and then subjected
to measurement of particle size distribution in the
range of 2 - 40 }un by using the above-mentioned
Coulter Multisizer II with a 100 micron-aperture to
obtain a volume-basis distribution and a number-basis
distribution. Form the results of the volume-basis
distribution and number-basis distribution in the
range of 2 - 40 um, a weight-average particle size
(D4) is calculated with a central value of each
channel taken as a representative value of the
channel.
Next, an embodiment of the image forming
method according to the present invention will be
described with reference to Figures 1 - 3. Figure 1
shows an electrophotographic apparatus usable as an
example of a copying machine or a printer for
practicing the image forming method according to the
present invention. The apparatus includes a
developing means 1 containing a toner 13 according to
the present invention. The toner may be a magnetic
toner or a non-magnetic toner. In an image forming
apparatus other than the one shown in Figure 1, it is
possible to use a developing means including a two-
component type developer comprising a toner and a
z~~sz~z
-56-
carrier.
Referring again to Figure 1, the surface of _a
photosensitive member 3 (e. g., an OPC photosensitive
drum, an amorphous silicon photosensitive drum or a
polysilicon photosensitive drum) is charged by a
charging means 11 (e. g., a contact charging means such
as a charging roller as shown, a charging brush or a
charging blade) supplied with a voltage from a bias
voltage application means 34. Then, the charged
surface of the photosensitive member 3 is irradiated
with light 5 (e.g., laser light or light from a
halogen lamp) carrying image data to form an
electrostatic image on the photosensitive member. The
electrostatic image is developed with a magnetic toner
13 (in this embodiment) on a developing sleeve 6
enclosing a magnetic field generating means 15 (e. g.,
a magnet) of the developing means 1 also equipped with
a toner applicator blade 8 (e.g., an elastic blade or
a magnetic blade) for applying the toner 13 onto the
developing sleeve 6. The development is performed by
either the normal development scheme or the reversal
development scheme to form a toner image on the
photosensitive member 3. At the developing station,
the developing sleeve may be supplied, as desired,
with an alternating, a pulse, and/or a DC bias voltage
from a bias voltage application means 12. When the
toner image on the photosensitive member 3 arrives at
2~492~2
-57-
a transfer station to which also a transfer material
is conveyed, the back side (side opposite the
photosensitive member 3) of the transfer member P is
pressed and charged by a transfer means 4 (e.g., a
transfer roller as shown or a transfer belt) to which
a voltage is applied from a bias application means 33,
to electrostatically transfer the toner image on the
photosensitive member 3 onto the transfer material P.
As the case may be, the toner image on the
photosensitive member 3 can be transferred onto an
intermediate transfer member (not shown, such as an
intermediate transfer drum or an intermediate transfer
belt) and then to the transfer material P.
The toner image on the transfer material P
separated from the photosensitive member 3 may be
fixed onto the transfer material P by a heat-and-
pressure application means 35 (e.g., a fixing means as
shown wherein a pressure roller 23 is pressed against
a fixed heat-generating member 21 via a heat-resistant
sheet 22; or a heat-pressure roller fixing means). A
portion, if any, of the toner remaining on the
photosensitive member 3 after the transfer step may be
removed, as desired, from the surface of the
photosensitive member 3 by a cleaning means 7 (e.g., a
cleaning blade as shown, a cleaning roller or a
cleaning brush). The photosensitive member 3 after
the cleaning is again subjected to an image forming
2149272
-58-
cycle as described above starting from the charging
step by the charging means 11.
The photosensitive member 3 as a member to be
charged and also an electrostatic image-bearing member
generally comprises a photosensitive layer and an
electroconductive substrate and is rotated in the
direction of an arrow as indicated. The developing
sleeve 6 comprising a non-magnetic cylinder as a toner
carrying member is rotated in the same direction as
the photosensitive member 3 at the developing station.
Inside the developing sleeve 6, a multi-polar
permanent magnet (magnet roll) 15 as a magnetic field-
generating means is fixedly disposed. The magnetic
toner 13 contained inside the developing means 1 is
applied by the applicator blade 8 onto the surface of
the developing sleeve, and the toner particles
constituting the toner are triboelectrically charged
by friction with the applicator blade 8 and/or the
developing sleeve 6. The toner may be uniformly
applied by the applicator blade 8 in a layer of e.g.,
10 - 300 um on the surface of the developing sleeve 6.
At the developing station, the developing sleeve 6 may
be supplied with an AC bias voltage of f = 200 - 4000
Hz and Vpp = 500 - 3000 V.
At the developing station, toner particles
are transferred onto the electrostatic image on the
photosensitive member due to the electrostatic force
214922
-59-
of the photosensitive member surface and the action of
an AC or pulse bias voltage.
Incidentally, in Examples described
hereinafter, an image forming apparatus having
structure as shown in Figures 1 to 3 was used, of
which the included members are denoted by reference
numerals as shown below.
That is, reference numeral 3 denotes an
electrostatic image-bearing member (photosensitive
drum); 11, a charger (charging roller); 2, a process-
cartridge; 7, a cleaning means; 5, an exposure means;
15, a developer container; 6, a developer-carrying
member (developing sleeve); 15, a magnetic field
generating means; 8, a layer thickness-regulating
elastic member; 4, a transfer means (transfer roller);
20, a stay; 21, a heating member; 21a, a heater
substrate; 21b, a heat-generating member; 21c, a
surface protective layer; 21d, a temperature-detecting
element; 22, a fixing film; 23, a pressing roller; 24,
a coil spring; 25, a film edge-regulating member; 26,
an electricity-supplying connector; 27, an electricity
interrupting member; 28, an inlet guide; and 29, an
outlet guide (separation guide).
Further, Figure 5 is a schematic sectional
view of a process-cartridge detached from a main body
of an image forming apparatus as described above. The
process-cartridge at least includes a developing means
2149272
-60-
and an electrostatic image-bearing member which are
integrated into a cartridge, so as to be detachably
mountable to a main body of an image forming
apparatus, such as a copying machine or a laser beam
printer.
In this embodiment shown in Figure 5, the
process-cartridge integrally includes a developing
means 1, a drum-shaped electrostatic image bearing
member (photosensitive drum) 3, a cleaner including a
cleaning blade 7, and a primary charger (charging
roller) 11.
In this embodiment, the developing means 1
includes a toner layer thickness-regulating member 8
and a toner vessel containing a magnetic toner 13. At
the time of development, a prescribed bias electric
field is applied between the photosensitive drum 3 and
the developing sleeve 6 carrying the magnetic toner 13
to effect a development of an electrostatic image
formed on the photosensitive drum 3.
Hereinbelow, the present invention will be
described based on specific Examples.
Resin Production Example 1
Terephthalic acid 12 mol. o
Fumaric acid 18 mol. $
Adipic acid 10 mol. o
Trimellitic anhydride 12 mol. $
Hisphenol derivatives of the above-
2149272
-61-
described formula (A)
(R = propylene, x + y = 2.2) 15 mol.
(R = ethylene, x + y = 2.2) 33 mol. g
The above ingredients were subjected to poly-
condensation to obtain a polyester (called "Resin A")
having Mn = 5,000, Mw = 57,000, Tg = 60 oC, acid
value = 20, OH value = 20.
Resin Production Example 2
Styrene 87 wt. parts
Hutyl acrylate 13 "
Di-tert-butyl peroxide 3
The above ingredients were added dropwise in
4 hours to 200 wt. parts of xylene heated to the
reflux temperature. Further, the polymerization was
completed under xylene reflux (138 - 144 °C), followed
by heating to 200 °C under a reduced pressure to
remove the xylene. The thus-obtained resin is called
"Resin B".
Styrene 75 wt.part (s)
Butyl acrylate 25 "
2,2-Bis(4,4-di-tert-butyl-
peroxycyclohexyl)propane 0.1 "
Benzoyl peroxide
0.1 "
To a mixture liquid comprising the above
ingredients, 170 wt. parts of water containing 0.12
wt. part of partially saponified polyvinyl alcohol was
added, and the system was vigorously stirred to form a
,.
214 92 72
-62-
suspension liquid. The suspension liquid was added to
a reaction vessel containing 50 wt. parts of water and
aerated with nitrogen, and was subjected to suspension
polymerization at 80 °C for 8 hours. After the
reaction, the product was washed to obtain Resin C.
The above Resin B and Resin C at a weight
ratio of 70:30 were dissolved in xylene and uniformly
mixed, followed by removal of xylene to obtain Resin
D, which showed a molecular weight distribution
providing peaks at molecular weights of 1.2x104 and
8x105, Mn (number-average molecular weight) - 0.7x104
and Mw (weight-average molecular weight) - 2.5x105,
and Tg = 61 °C.
Resin Production Example 3
Styrene 80.0 wt. parts
Butyl acrylate 10.0 "
Monobutyl maleate 10.0 "
Di-tert-butyl peroxide (.0
Resin E was prepared from the above
ingredients otherwise in the same manner as in
production of Resin B in Resin Production Example 2
above.
Resin E 40.0 wt.part(s)
Styrene 45.0 "
Butyl acrylate 15.0 "
Divinylbenzene 0.5 "
Benzoyl peroxide 0.5 "
2149~~2
-63-
A mixture liquid comprising the above
ingredients was subjected to suspension polymerization
in the same manner as in production of Resin C in
Resin Production Example 2 to obtain Resin F, which
showed Tg = 60 °C, Mn = 1x104 and Mw = 1x105.
Example 1
Resin A 100 wt. parts
Magnetic iron oxide 90 wt. parts
(average particle size (Dav.) - 0.15 um,
He = 115 oersted, as = 80 emu/g,
6r = 11 emu/g)
Long-chain alkyl alcohol of
Formula (1) 3 wt. parts
(x = 48 as an average value, Mn = 440,
Mw = 870, Mw/Mn = 1.98, OH value = 66)
Azo-type iron complex (1) 2 wt.%
(A+ - 90 %:NH4, 10 %:Na+ and H+
mixture; SMeOH (solubility in methanol)
- 0.87 g/100 ml)
The above ingredients were pre-mixed by a
Henschel mixer and melt-kneaded through a twin screw
extruder at 130 °C. After cooling, the melt-kneaded
product was coarsely crushed by a cutter mill,
pulverized by a jet stream pulverizer, and classified
by a pneumatic classifier to obtain a magnetic toner
(1) having a weight-average particle size (D4) of 6.6
um, content of s 5 um particles: 49.3 % (N, % by
2149272
-64-
number), 9.6 $ (V, $ by volume). The characterizing
data of the toner are summarized in Table 1. _
The localization factors of the azo-type iron
complex in the fine and coarse power fractions were
ODF/ODM = 1.012 and OD~/ODM = 0.998.
100 wt. parts of the magnetic toner (1) and
1.0 wt. part of hydrophobic silica surface-treated
with hexamethyldisilazane were blended in a Henschel
mixer to obtain Developer No. 1.
The thus-obtained Developer No. 1 was charged
in a commercially available digital copying machine
("GP-55", available from Canon K.K.) and subjected to
image formation of 5x104 sheets under normal
temperature/low humidity (N/L = 23.5 °C/5 $RH)
conditions and further 3x104 sheets under high
temperature/high humidity (H/H = 32.5 oC/80 $RH)
conditions. Further, Developer No. 1 was also charged
in a commercially available analog copying machine
("NP-9800", available from Canon K.K.) and subjected
to image formation of 2x105 sheets under the normal
temperature/low humidity (N/L) conditions and further
1x105 sheets under the high temperature/ high humidity
(H/H) conditions. The results of the image formation
tests are shown in Tables 3 and 4.
In Tables 3 and 4, the evaluation results are
indicated by symbols respectively indicating the
following performances.
lL
2149272
-65-
~: Very good
o: Good
oa: Practically of no problem
d: Slightly problematic
x: Practically unacceptable
Further, a commercially available laser beam
printer ("LBP-SX", available from Canon K.K.) was
remodeled as shown in Figure 1 (schematic view). More
specifically, the process cartridge 2 was equipped
with a urethane rubber-made elastic blade 8 and a
charging roller 9. Further, the main body was
equipped with a charging roller 4 and the heat-fixing
apparatus was remodeled into an apparatus 35 shown in
Figure 1, Figure 2 (exploded perspective view) and
Figure 3 (sectional view). Image formation was
performed by using Developer No. 1 under the following
conditions.
An OPC photosensitive member 3 was primarily
charged at a potential of -600 volts and exposed to
form an electrostatic latent image thereon having a
light part potential VL of -150 volts. At the
developing station, the photosensitive drum 3 and the
developing sleeve 6 (enclosing a magnet 15) were
disposed with a gap of 300 um so that the developer
layer on the sleeve 6 did not contact the
photosensitive member 3, and an AC bias (f = 1800 Hz,
Vpp = 1500 V and a DC bias (VD = -400 V) were applied
A
214~2~2
-66-
in superposition from a bias application means 12 to
the developing sleeve 6, thereby developing the _
electrostatic latent image by a reversal development
scheme to form a toner image on the OPC photosensitive
member 3. The thus-formed toner image was transferred
onto plain paper by applying a positive transfer
potential and the plain paper carrying the toner image
was applied through the heat fixing apparatus 35 to
fix the toner image onto the plain paper. In the
heat-fixing apparatus, the surface temperature
detected by a sensor element 21d of a heating member
21 was set to 130 °C, and a total pressure of 6 kg was
applied between the heating member 21 and a pressing
roller 23 with a nip of 3 mm between the pressing
roller 23 and a fixing film 22. The fixing film 22
comprised a 50 um-thick heat-resistant polyimide film
coated, on its side contacting the transfer material
P, with a low-resistivity release layer comprising
polytetrafluoroethylene with an electroconductive
substance dispersed therein.
Under the above set conditions, an image
formation test (a printing test) was performed
continuously for 7000 A4-sheets at a rate of 8 A4-
sheets/min. while replenishing the developer as
required under normal temperature/normal humidity (N/N
- 25 °C/60 oRH) conditions.
Similar image formation tests were performed
214922
-67-
under high temperature/high humidity (H/H = 32.5 °C/90
oRH) conditions and low temperature/low humidity (L/L
- 10 °C/15 %RH) conditions. In the high temperature -
high humidity environment, after a 6500 sheets image
formation test, the apparatus and developer were left
standing for 5 days in the same environment and then
further subjected to a 500 sheet image formation test.
The results are shown in Tables 5 and 6.
Exam les 2 - 21 and Comparative Examples 1 8
Toners having particle size distributions
respectively shown in Table 1 were prepared in the
same manner as in Example 1 except that prescriptions
also shown in Table 1 were used. (In Table 1, values
x, y and z are average values.) The localization
factors of the metal complex compounds (inclusive of
azo-type iron complex compound used in Examples) for
the respective toners are shown in Table 2. From
these toners, Developers Nos. 2 - 21 and Comparative
Developers Nos. 1 - 8 were prepared in the same manner
as in Example 1.
The resultant developers were respectively
evaluated by the same image formation as in Example 1.
The results are summarized in Tables 3 - 6.
The evaluation items listed in Tables 3 - 6
are supplemented hereinbelow.
<Evaluation by Digital copier GP-55 and Analog copier
NP-9800 (Tables 3 and 4)>
-68-
The image resolution was evaluated as
follows. An original image was prepared so as to
comprise 12 types of resolution images including
different number of thin lines per mm, i.e., 2.8, 3.2,
3.6, 4.0, 4.5, 5.0, 5.6, 6.3, 7.1, 8.0, 9.0 and 10.0
lines/mm, respectively, each type including 5 thin
lines spaced regularly so as to have a line width and
a spacing which were equal to each other. A copy
image was prepared by reproducing the original image
under the respective image forming conditions and
observed through a magnifying glass, whereby the
largest number of lines/mm at which the adjacent lines
could be observed clearly separately was taken as a
resolution.
Higher number means a higher resolution.
<Evaluation by Laser beam printer LBP-SX (Tables 5 and
6)>
The evaluation was performed in the following
manners for the respective items.
(1) Image density
The density of an image formed on an ordinary
plain paper for copying machine (75 g/m2) after
printing 7000 sheets was evaluated by a MacBeth
Reflection Densitometer (available from MacBeth Co.)
as a relative density against a density of 0.00
allotted to a printed white background portion.
(2) Fog
214922
-69-
Image fog (o) was evaluated as a difference
between the whiteness of a white background portion of
a printed image and the whiteness of an original
transfer paper by measurement with "Reflectometer"
(available from Tokyo Denshoku K.K.). A fog value
exceeding 4 % is practically problematic.
(3) Image quality
A checker pattern shown in Figure 4 was
printed out and the dot reproducibility was evaluated
by counting the number of lacked dots. The results
were evaluated according to the following standards:
~ (very good): lack of 2 dots or less/100 dots
o (good): lack of 3 - 5 dots/100 dots
D (fair): lack of 6 - 10 dots/100 dots
x (poor): lack of 11 dots or more/100 dots
(4) Fixability
A fixed image was rubbed with a soft tissue
paper under a load of 50 g/cm2, and the fixability was
evaluated by a lowering ($) in image density after the
rubbing. The results were evaluated according to the
following standards.
~ (excellent): 5 0 or below
o (good): at least 5 o and below 10 0
4 (fair): at least 10 o and below 20 0
x (poor): at least 20 o
(5) Anti-offset characteristic
A sample image having an image percentage
2149272
-~0-
of about 5 ~ was printed out, and the anti-offset
characteristic was evaluated by the degree of
soiling on the image after printing of 3000 sheets.
The results were evaluated by the following
standards.
~: Very good (non-observable)
o: Good (substantially non-observable)
Q: Fair
x: Poor
(6) Sleeve soiling
After the printing test, the state of
residual toner sticking onto the developing sleeve
surface and the influence thereof on the printed
images were evaluated by observation with eyes. The
results were evaluated according to the following
standards.
Q: Very good (not observable)
o: Good (substantially non-observable)
Q: Fair (sticking was observed but did not affect
the images)
x: Poor (much sticking was observed and resultant
in image irregularity)
(7) Film soiling
After the printing test, the state of
residual toner sticking onto the surface of the fixing
film was evaluated by observation with eyes. The
results are evaluated according to the following
~I49~'~2
-71-
standards.
~: Very good (not observable)
o: Good (substantially non-observable)
D: Fair
x: Poor
15
25
2149272
-72-
\ U1 O tf1 N G
-Z N N c''7 r1
N ~
,' 01 pp
~
. N
N
,dj~ ~ M ~ N Lf1
O
M
N
t0 ~ N O
L1 o ~ r r
y
o 4' 0 0
~ ~n o cn o m o m
do do
~ N ~
CT
+
I~ ~
~G
00
r dP N dP O I~
0 dP t
'
O O O
"I ' ~ ~ ~
r rC II N ~ x N ~ ~ N ~ ~ II N
~ Op II II ..
~
r-I r +' ~, +. ..~ ,~
-1 x ~. x +~+. +~
t z ~ z x ~
~ ~ ~ ~ ~
~ ~
~ , ~ ~ ~ x N
~ ~
a~
~ a, ~
O U1
~l'
.~ C
b f-1 II 00
1D
r ~ ~
II
y
8 ~ 1'~ .7
11 M
~
Y ,~ 00 CO
II
r ~8~ II ~~o
x
~ .a~ x
0 8
a ~ .x
. ~ O
U
O
v ~ U ~ o r1 ,.1 .~-I
w
o .- ~ ,'
v o o~
~ '-
II a
a
a
ox
b b
a~
+~ g
0 +
b b ro
O ~ N r,.~ v'
2149212
-73-
ryj M N
8
p .
~n , , r
N
r "' o r
co
N M ~ ~ 10
N
N O ~ r
N
r r r
r
0 0 0
0 0
~n ~ ° ~n
v
aAa N l0 ~ O u1 ~ p l0
dP l0 dP
O v 001 +~ O ~ ~ ~ O ,~ - y,1
II N ~ ~ x If N ~ ~ II N
z ~~ ~ ~ z ~~ ~ ~ z ~~
=I o '~ .=i o
~ O N 0~0
-.i ro ~ v ~ ~ II '- ~ II
_ ~ M
'tJ m O N ~ M (0 ~ ~ N
O r .-I
II II M fl
x ~~o w x~~o
_ ~ ro
b
it
8
'n '° r co
~3 ~ ~ ~i
21492'72
-74-
N
'- C
N N ~ ~
N
1~
t~
U1 I~ H
r ~, r
~O
O O
M
M ~ O ~ O
M V' M
'
C
O W fl
1D ~ ~ ~
C)
x
0
o u~ o v7 r- . m ~ o
' _ o
M ~
II ~ pr ~7 r1 N . ~ M
l0 O 1D
_
~ il ~ II
O N II v ~ ~, ~ - O N ~
O V~ (V p N '~ ~
N
(d ~ O O b ~ "d' M M ~ M
II ~ M II ~
ri C' M r-I Op ~ ~ v II ,ro
r-I a- ,~ ~ O ~ O 01
II C' tf1 c~ 11
x '
II II II II ~ II II
x~~o ~ II II
~~o
w ~ xrx~ ~o c x~~~ 1
x
.~,
0
b b
>~
+g +g ~ o
-
u
b
-~ ..
+~
0
2149272
-75-
01 ( W N 1D V~ O
f7
e- N e- N N N M yJ
r-
C~0 '- ~f7 O ~
M
O C ,p ~
M O N t!1 O O
01 O O~ O lf1 GMp OJ
~f7 M r7 M N
OO M fV tf1 O I~ N
U1 r ~p
41
,
ri
y,
O rtS
b
ro~
0 0
b
~ b b b '~
.~
a~
0
w
.~
~
G~, ~ ~ ~w
. . .~,
.~,
~n
m
o o o ~ o~ao o~ro o~,
O o 0 o W O U
o
o W o W U
o
GL W
W ~ FC G7 w Ga f
w .~
8
0
N N
2149272
-76-
r ~ ~ _ _
N N N N N !~
8
N OD tf1 r
. ~,.~ O
O OD N
r- N 01 N
O O f~ O ~.,~ O
O
O c7
M ~ N ~ ~
ZT
W N 0p ~p ~, M
1 O
~O r t0 ,
~O O
II
ro x
N * ~ N U7
~
Y.
N '~ N
N (3,
U7
U ~ _
U ~ m
g
*
U ~ ~ ~ o r~
0 0
x '- ~ O
p
II W
~
M II
* '~ _ ~ N ~ ~~ x
v N
II ~ ~ ~ ~ _
M
ro O ~p N N
II ~
M II .-I
r~ x~~o
i
b
.
a~
8
IT N
N
tJ7 UI U1 tn
U
* ~ ~ ~
* .-I
~N ~ O
O ~ ~ ~
~ ~ ~
~
O O O M
W W
~'. ~'. * * *
C CL
r
G
N M ~
'-
v
8 8 8
8 3
i
~A
Image
2149272
_78_
Table 2
Localization factors of azo-type iron
complex compounds (and related compounds)
Ex. or To classified To classified
Comp. Ex. fine powder coarse powder
ODF/ODI"j ODD /ODI"1
Ex. 1 1.012 O.ggg
2 1.015 0.990
3 1.014 0.991
4 1.014 0.992
5 1.016 0.988
6 1.020 0.982
7 1.013 0.995
8 1.010 O.ggg
9 1.009 0.997
10 1.025 0.980
11 1.015 0.990
12 1.010 0.997
13 1.014 0.992
14 1.023 0.981
15 1.020 0.980
16 1.010 O.gg7
17 1.025 O.ggg
18 1.013 0.988
19 1.010 O.ggg
...cont.
~,'
2~492~2
-79-
Table 2 (continued)
20 1.018 p,9g7
21 1.013 p,ggg
Comp.
Ex. 1 1.013 p,gg7
2* 1.120 0.870
3* 1.085 0.902
1.012 0.996
5* 1.210 0.860
6* 1.113 0.885
7 1.019 0.996
8 1.012 p,ggg
* The contents of aluminum or chromium in
classified fine powder, coarse powder and medium
powder (toner) were respectively measured by the
atomic absorption spectrometry and the ratios among
these values were obtained.
25
~149~'~2
-80-
8
U
,
~ O O O O O O O O O O
O O
O o O O O O .- o
m H o~ o~oo ao0o coao o~~ co
* ~\ \ \ \ \ \ \ \ \ \ \
N 0 0 0 0 0 0 0 0 0 0
~ ~7
N . 0 0 0o aom ooao o~ao o~
~ vl
0
o ~ ~~ ~ ~ O O O O O ~ ~ O
M
~ ~ O O O O O O Q O
\
00 l0O ~-O O N COl0 N
~1' ~t'V' ~t'~' ~'~t''d'~' V'
H ~ .-r- ~ ~ -
~ c c-~ .-
O O
O O O O O O O O
O O
U7 r- r-00 0000 00op 0100 01
v E-~ \ \ \ \ \ \ \ \ \ \
O O O O O O O O O O
r-I O O al O\61 0101 O 01 O1
r-
~ ~ ~ ~
' I
~ + ~ ~ O O O O O ~ ~ O
o O 7
C7 c
S
n t
rti u
O O O O O ~ ~ O
CW n
N O CO I~~ 0000 O CO O .+J O O
f~ M (~ In Ul~' '~'~' ~'~' tf7~' I~
N
rd ~ (n
,. H .- ~ ~- c-r- c-~ c-~
\
'-
z ~ o o ~ ~ n
'~
~ 0 0 0 0 0 . o o . b c
-,
r M U7 ~ ~ ~ 6101 0101 ~ 01 01 U7
-I
ACS * ~ H \ \ \ \ \ \ \ \ \ \ ~ N O
a o 0 0 0 0 o 0 0 0 0 ~
l
r o o o~ o~ov o~o~ o a~ o~
M ~ ~ _O
O ~Dr~ OO~ ~ ~ ~ ~ O ~ ~
_ O
H H ~
+
~ O O O O O Q O O ~ O U
~ _ _~
N O CO f~00 a001 O O O ~ N ~ ~ In
* CW n u~~r ~r~r ~ m n um n H O ~ -a-~
H ~ .-r--c-~ ~ c- c- ~
~
H
O .. .... ..
M ~ tf1
- N M d'Ln l0f~ 0001 O z * * -K -k
c--
2149272
-81 -
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 o Q
0 0 ~ ~ 0 0 o r-0 0 0 ~o
00 01t~ I~00 01a1 1~01 CO00 u1 M u1 tl1~ ~ N M
\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ r-~r-i\ \
O O O O O O O O O O O l0 l0M ~O~ -~pp ~p
00 O O0 CO01 0101 0001 00d1 Ln M l0 t11(x~w N M
c-
o ~ o ~ 0 0 0 ~ ~ o o x x x Q x x x x
o ~ 0 0 0 0 0 ~ ~ o o Q x Q Q x x x Q
O1 n O O l0 opO l0l~ N Wit'~ N O O u'1O N O
M ~'~' cNd "d'Wit'~1'~' ~"d' O O ~ M a1 O O O
c-~ r ~ r c- r ~ ~ c- r r r
O
O ' O ~ O O O O O O O ~p ~pO ~p~p M
~
Ol 00 l~01 0100 OO61 CO~1 Lfl to00 tntIllflLC7tll
\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \
O O O O O O O O O O O M M O M l0 M ~O M
01 O 01 0061 O 01 O1O Q101 l0 l0o0 l0LC1l0t1~l0
o ~ 0 0 0 ~ o C ~ o p x x x a x x x x
o ~ 0 0 0 ~ o ~ ~p o ~ Q Q d o Q d Q x
O N CO a0O N N N N t~O O O O t!1I~ O O ~
d'~1 tIl~I'~'~f1d'tI1N M M M N ~-N ~-
c-r r r r r r ~~ ~ r r r c- r r r r ~
O O O O O O O
O O O O O M M ~ M N
O r
O O O O O f~ f~ j
Ol O1 O O1O 00 00lD lDLn l0
c- ~ Ol r-c- r-~ c- \ \
\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \
O O O O O O O O O O O ~ ~ O O r- W p ~
O O 01 01O O O O O O O I~ t~p1 OpI~ n
c- s-'- c-c- a-r-
o ~ ~ o ~ ~ ~ ~ ~ O ~ x x d o x x x x
o ~ p o ~ ~ ~ ~ ~ ~ ~ 0 0 0 o Q 4 o d
O N OO 00O N N N N O N O W O O O M ~ O
~ Ul ~CWf1 ~ u~ ult11M 1 ~ ~ M M N M U
M
r ~ ~ ~ ~ ~ ~ r-c- r c- r r r r r r ~ c-
S U
M
N M ~Y'~ lD(~ OpQ1 O ~ c- N M ~'Ul ~OI~ 00 *.
c-c- a- r-~ ~ c- N N
H
249272
-82-
,x
0
U
U
O O O O O O O O O O O
N
* td
O O O O O O O O O O O
O O O O O O O O O
r-
x
~I N O l0 f~l0 l~l~ O ~- 00t17
\ ~ ~f''
x
~ M M M M M ~ ~ M M
H ~ ~ ~- ~ ~ c-c- - - -
r r c ~-
O
* ~ O O O O ~ ~-c- O r- O r-
U1 \
U N u1 0~ o~oo m (~ t~t~ N ~ a~t~
N N \ \ \ \ \ \ \ \ \ \ \
H
0 0 0 0 0 0 0 0 0 0 o s
; ; o m
N ~ ~ a a o0 00ao a;o0 0;ao
~
o c ,r,
~
'r
x x
x M ~ ~ O O O O O O OQ O O
N
~f1N a1 O 01 O O N M N 00
U
~l'V'M ~f'M V'~Y'Wit'ct'V~M
H r- c-c- r-~ ~- -
~ c ~ .-~
x
O O '-
~ O O O O O
O O O O
~J-~ O O
N Ul r- c-N 00CO 0000 0101 O1O1
N O \ \ \ \ \ \ \ \ \ \ \
E-t
0 0 0 0 0 0 0 0 0 0 0
N o o a, o,m ao00 0 0, o,o
,v,
~ ~ ~ ~ ~ x
x~ -~
' N ~ ~ ~ ~ ~ ~ O ~ ~ O O ~a ~
M .
-
!a tI7M O O O N ~- ~ M M O
~ ~r~r ~ ~r ~ ~ ~r~ ~r~
rI U
~n H ~- .-r- ~-.- ~ ~- .-.-
H
M O O O O O O O O O O O
-
U \ O O O O O O r-
Hl U7 ~ ~ ~ ~ \ ~ ~ \ \ \ \
\ \ \ \ \ \
* O O O O O O O O O O O
N
N U1 O O 01 O~01 ~ 01 O O O O
-~
\
~ ~ ~ ~ ~ O ~ O ~ ~ ~ ~ tI1U7
~
N
O N ~ ~ N ~'N ~ N
* r
Ca
~ ~ ~r ~ ~ rr~ ~ ~r ~r~ N * ~ v
H .- .-.- - -
~ c c ~ r r- r c- O
~1 M U]
.
N
O c- N M Wit'tlll0(~ 00O~ O
r-
2149272
-83-
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 o Q
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 ~ o ~ ~ ~ ~ x x d d x x x x
N t!1~p O O tI1M N O O O O t11O O O h tf)
V' M M d'd' M ~' ~' ~'d' O 00 N r-h d101 01
r r r r r ~ ~ r r r ~ O r r O O O O
O O O O O ~ O O O O O M ~p
00 00Op 0000 h 01 00 d000 d' l0 M
\ \ \ \ \ \ \ \ \ \ \ * \ \
O O O O O O O O O O O * M ~D*
a1 00~ O161 0041 61 0001 tll l0 M
~ o ~ p o O p ~ t~ d x ~ d x x x x
'TJ
'ct'0000 c-N O tf1~' ~Y'M N O O Lf1ll1h h O
~' M M ~'~' ~!'~' ~' V~~' N O M ~ a1 61~
r ~-r ~c-r r ~- r ~ ~ 0 0 r- s-
,
O O O O
O O O ~ O O lD M a- l0l0 l0l0 N
O O O O
00 O~.- h ~ c- Ql01
\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \
O O O O O O O O O O lD ~ c- M l0 l0l0 N
O O101 O O 00O O 01O tn h h ~ptn ~f~M M
r-c- c- c-
~ o ~ ~ ~ ~ d 4 Q Q d Q x x
N
r1
~f1O O V'M h O ~ ~ M N ~ tf1O tnO N rl
ct'~'ct'~'V' M ~' ~' ~'~' ~ O M N r- ~ - -
c- c-r- c-~ c-~ r- ~ c- r- c- c- ~ r r a a
c- r
O O O O O O O
O O O ~ ~ O O l0 M M ~p
O O O O O O O
\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \
O O O O O O O O O O ~ r- O O M M M M
O 0101 O O 01O O O O h (~ 01
r c-~- ~ -
c ~
-
l
r W
'~ ~ O ~ ~ O ~ ~ ~ ~ O O O O O O Q Q ~ O
u7 '-M tn~' ~'N ~I1~ M O h O h O O O Op
d' ~'~' Wit'Wit''~'d" ~N ~f'~' M M ~ M N M M
N O O
c- ~ ~ ~ r r r ~ ~ r r r c- r
*
V' ~ M ~' UllO (~00 al O c- * * * *
r- - -
v ~ a c ~ ~ ~ N N r- N M V'Lfll0h 00 *
H
21492'2
-84-
~n
' o
U
~ ~ 0 0 0 0 0 ~ o o Q o o Q ~
x d
'
~
o
v
-
o
~
b
a
a ~ o0 00 ~ ~ N M d101 O ~ c-~ O M 00~ 00
m
~ r' ~- r-N N N r-~ N N M N N M c-- -
~
a s
4-1
0
r-1
N
IfS fW f1 M Wit''ct'M M ~l''cTM 01N M 01 C'tf1V'
W
~' 'V'V' ~'~' ~'Wit'd'~' M ~' 'c1'M 'ct'~!'~'
W ~ r ~ r c-~ ~-r- r
(I~
r ~ r r r r r- r
I
Zs
~ N M N M M N N M M N OpN N I~ M N N
Z~
cr d'Wit'M d' ~'M ~'V' Wit'
r r ~ r r r r
x r r r r r r r ~ r c-
S~
mW f1 V' Lf7ct'LI1~' d''d'cHM O M cf'c- l0LC1U1
~Y'd' Wit'V' ~'~' ~'Wit'V'd' C ~f'V'd' ~'' '
~1 ~
H r r r r r r r r c-c-
r ~ ~ r r a- r
b'~
N
a c~ t~ m o ~o ~ou, ~n.o u~~r,u~~s u,~r m o ~o
~
w~
lIS l0 tf1t!1U1 U71~ tn~' Lnd' ~'~' InM l0t~ l0
~
~t'V'~t'~'~t'Wit'~T Wit'V' C V' Wit'~t'~'
W r r r r ~ ~ r r r r r r- r r r r- ~
U~
-r-I 1~ l0 Lnl0 tI1Lf7l0L(1~O~CltllLn lfld' l0l0 l0
Wit'C Wit'~' ~'~' ~'Wit'Wit'~' ~'~' ~'~' '
V
~, r- ~ r r--r-r r r r r r
r r r r
H '-
O c-N M V' tt1l0 I~
O c- N M cf'tol0 l~00 01~ ~ r- c- -
~ r
zm~z~z
-85-
p ~ ~ ~ x x Q x x x x x
l~ 00 0000 O I~M ~p00 O1M O
r r r r M V~N ~'d' d'M lf1
LflIn M M f~ ~ Op OpM c-t!1N
d' ~" cY'~' N N N N N N N N
~ a- ~ ~ ~ N c- c-~ c-~
~' N N N tf1 ~ 1~ 00~ N V'
' 'cf'Wit'N N N N N N N N
c- r r r- r ~ ~ e-~ r r-
ut u1 ~'d' 00 u100 O u7 ~ lO
'd'd' ~'~' N N N M N N N N
~ r ~ c- c- r ~ r ~ r-r c-
l~ I~ f~l0 ~- O O N M s-O 00
V' ~' d'Wit'M M M M M M M N
r c- ~ ~ ~ r c- ~ c- r r r
l0 ~O tfWf1 M O M ~ N N 01
V' V' cf'Q' M M M M M M M N
r a- ~ ~ r r r r ~ ~ c-
l0 CW D lO ~ N M UW11 M ~!'1N
~'~t'M M M ~ M M M M
~ r- c- ~ ~ c- ~ c-
tf1 00 01 O ~-
N N '- N M ~ ~ ~pr pp
H
2149272
-86-
Table 6
Evaluation of LBP-SX
Fixability Anti- Sleeve Film
offset soil soil
Ex. 1 Q ~
Ex. 2 ~ ~
Ex. 3 ~ ~ o Q
Ex. 4 ~ ~ o Q
Ex . 5 ~ ~ o
Ex. 6 ~ ~ o
Ex. 7 ~ ~ o
Ex. 8 ~o
Ex. 9 ~ o o
Ex. 10 0
Ex. 11 p Q o 0
Ex. 12 ~ ~ o
Ex. 13 0
Ex. 14 ~ A 4 0
Ex . 15 ~ ~ ~
Ex. 16 ~
Ex . 17 /~ o
Ex. 18 ~ ~ ~
Ex. 19
Ex. 20 ~ ~ o
...cont.
2149272
Table 6 (continued)
Ex. 21 ~ ~ o
Comp.
Ex . 1 0 o x p,
Comp.
Ex . 2 o p, o
Comp.
Ex . 3 d, x x 4
Comp.
Ex. 4 x A o 0
Comp.
Ex. 5 0 0 A
Comp.
Ex. 6 4 x x q
Comp.
Ex . 7 o La x
Comp .
Ex. 8 4 x x D
25