Note: Descriptions are shown in the official language in which they were submitted.
WO 94/12134 ~ PCT/GB93/û2382
21~9875
WO~ND DRESSINGS
This invention relates to a novel form of wound
dressing and a novel method of treatment of wounds.
The accummulation of wound exudate in secreting
5- ~kin wounds such as decubitus ulcers and surgical
wounds promotes the growth of bacteria and other
organisms which may delay heaIing o~ the wound and in
some casea cause infection of~the wound. It is well
established that wound healing may ~e accele.rated lf
lO- the wound is kept in a 'moist condition' but that
excess exudate must be remQved from the wound.
: ~ :
Polyurethane films have~been used as dressings in
recent years, such films have~a moisture vapour
15`- transmission rate (MVTR) which allows excess exudate
to permeate`whilst keeping a residual amount of
; ; moisture around the;wound~area. More recently
;~ hydxogel;~at~erials have~;been~used, sometimes in~
; conjunction wi~th a polyurethane film dressing to
20- further control the 'moisture'~ content of the wound.
However, the application of a~hydrogel and~ ~
subsequently a~olyurethane dressing proves~wkward ~o
manipuIate. ~A further~disadvantage is that for falrly
`~ ! 25- superficial wounds only relati~ely small amounts of
hydrogei are required~thus a sachet~of hydrogel m!ay
only~be part used~and~the~remainder di~scarded.
Recent~ developments~have sought to overcome this
30O~ problem. ;For~example, European Patent Application
No.42416~describes~ a~reservoir of a hydrogel in a
vacuum;formed well in~a thin~film dressing layer
provided~wlth~an adhesive perimeter portion. The thin
WO~4/12134 PCT/GB93l0~8'
2~ 49 87 . I
film layer is provided with a support layer around the
perimeter of the vacuum formed well. However, such
dressings have only limited stability since the
support layer does nst add to the support of the
hydrogelO In the embodiment-described therein the
dressing layer may be provided with a grid pattern to
permit measurement of wound healing.
European Patent Application No.42~422 discloses a
10- number of hydrogel dressings which include;
(i) a hydrogel mater1al maintained in à cavity
formed in a flexible film membrane; and
(ii) a hydrogel layer maintained in position on a
flexible film by a perimeter defining adhesive coated
lSo foam dam.
However, such dressings although advantageous
~ over the methods of applying hydrogels and dressings: ,
separately, suffer from~a:number of disadvantages.
. 20.
The ~oam dam dressings of the prior art have th
disadvantage that the shouldsr of the dam is aligned.
with the edge of the dressing, the shoulder ea~ily
: catches when a patient moves, thus dislodging the
25- dressing and/or causing discomfort to the patient.
` This is found to be a particular~problem with sacral
: dres~ings. :~Moreover, it is common for the appearance
. of a small:wound~in th ~orm of a pressure ~ore in thP
dermis or epidermis to merely be an i~ndication of a
~ 30 :much larger subcutaneous wound sore. Thus it may be
:: : undesirable to adhere a dre5sing to tissue directly
adja nt to the visible wound.
~ . ~
,
~'094/12134 PCT/GB93l0~82
, .. , 21~987~
Also, because of the need to create a vacuum
ormed well, particularly with the dressings of
European Patent Application No.426422, the dressings
I of the prior art are difficult and expensive to
1 5 manufacture and would tend to re~uire a less
conformable backing layer.
Although the dressings of the prior art are
described as optionally having a grid marked on a
10- backing in order to measura wound healing, this
particular aspec~ of the prior art is disadvantageous
since the grid cannot be easily entered into the
patients records.
: ~ lS. We have now found a form of dressing which
overc4mes or mitigates these problems.
,
According to the invention, we provide an
adhesive dressing comprising a backing layer having a
: 2~- pressure sensiti~e adhesive layer over one surface
thereo~, a hydrogel layer over part of the adAPsive
; surface, a removable pro~ector which covers the
. exposed~adhesi~e layer and the hydrogeI layer; and a
~; continuous con~ormable support~layer which is
~; 25- reversibly attached to the non-adhesive ~urface of the
backing layer and ex~ends ~eyond the backing layer at
l one or more edges.
: The amount which the support layer ex~ends beyond
;~ 30 the outer~edge of the backing layer may vary according
to the size and nature of t~e wound to be treatedO
: ~ However, in:general we prefer the support layer to
~: : extend beyond the backing layer by approximately 1 - 5
~, ,
`
W09~/12134 ~ ~ $ 7 PCT/GB93/0238~
cm, more preferably by l - 4 cm and most preferably by
l - 3 cm. The support layer may extend over one edge,
two edges or all four edges of the backing 'ayer.
~' The pressure sensitive adhesive may be a
¦ continuous adhesive layer or may be non-continuous.
In a preferred em~odiement of the present invention
the pressure sensitive adhesive layer is
non-conti~uous, eg. a~pattern spread adhe~ive. In
lO- particularly the portion of the b cking layer around
; the periphery of hydrogel layer may be adhesive free.
Such adhesive free re~ions may be manu~acturPd by
coating the bar.king layer with an adhesive in the
presenc~ of a template. The adhesive f-ee region of
l;~ the backing layer may be from O.l to 5.0mm wide,
preferably from 0.1 to 20mm wide, more preferably from
: 1 to lOmmi wide~
,
The:hydrogel layer may optionally be provided
j 20~ with a support member. The support member may be
I in~igral to the hydr~el layer:or may be adjacent:the
periphery of the hydrogel 1ayer:such peripheral
support members may surround aIl:or par~ o~ the
:~ hydrvgelO ~Preferably, a:peripheral sup~ort member iS
a foam dam member which preferably surrounds the whole
~, oflthç~hydrogel layer.
The dam~member may~surround the whole of the
hydrogel lay:er~: Tha~exposed surface of the dam member
: 3~ may also optiona11y be coated with a pressure
: sensitive adhesi~e layer,~but preferably, the wound
:~ facing side of the dam member is adhesi~e ~ree.
: . :
WO 94/12134 PCTIGB93/02382
~ 1 ~L 9 ~ 7 ~
The dam member may def ine any con~entional shape,
eg. circular, square or rectangular. Alternatively,
the dam member may def ine an internal well ~hich is
dif f erent in shape ~o that def ined by it ' s external
walls. Thus the d m member may be rectangular snaped
on it ' s external walls whilst def ining a circular
shaped well. When the outer walls of the dam member
define a square or rectangular shape the corners of
the dam member and/ or the backing layer may be rounded
10. in order to further alleviate the problem o~ the
dressing, when applied, catching and causing
discomf ort to the patient . In addition, the shoulder
of the dam mem~er adjacent to the backing layer may be
. ~evelled in order to alleviate 'dressing lift'. Such
15- 'dressing lift' may also be alleviated by profilirlg
the dam member such that it ' s thickness at it ' s outer
: edge may be less than tha~ at its inner edge. In a
: similar fashion 'dressing }ift' may be alle~riated in: the support free dressing by prof iling the hydrogel
:; 2 ~ layer . Thus, when ~h~ hydrogel layer is a square or
recta~gular slab, the corners of the sla~ may be of
less thickness ~han the remainder. Alternatively, the
; : whole of the outer edge o~ the slab may be o~ less
~` :~ thickne s than the remainder. Such edge profiling
2;- wouId of cource be applicable to all shapes of
hydrogel layer.
::~ ~
When the hydrogel layer is provided with an
:: : integral support member, the support member may he a 30 reticulated ~member~ or may :comprise a scrim or gauze
of material~. ~ The lntegral suppcrt layer may be a
sc:rim, gauze or net of mat~rial. The integral support
member ma~r comprise a conf ormable sheet, eg . a
- ~
I ~'094/12134 PCT/GB9310~8'
9 8 ~
! - 6 -
plastics sheet provided with a plurali~y of apertures
or, for example, a polyurethane net, eg. a ~EYPOL net.
Further, the integral support member preferably
comprises material whic~ absorbs wound exudate, eg~
cotton, although non-absorbent materials may also be
used. The integral support member will be within th~
hydrogel layer but may be adjacent either the wound
facing or the backing layer facing surface~ It is
preferable`however that the integral support will lie
10- substantially in the middle, measured by depth, of the
hydrogel layer, that it is, substantially in equal
proximity to the wound facing and the backing layer
. facing surface. The integral support preferably
extends substantially to the edges:of the hydrogel
: 1~- layer, althou~h provided the hydrogel layer is
:~ su~ficiently stable this is not essen~ial.
~;
Suitably the backing layer may comprise any of
: ~ : those materials which are conventionally employed ~o
20- form thin film surgical dressing. Suitable ma~erials
include those described in~UK Patent No. 1280631.
European Patents Nos. 51935j 91800 and ~78740.
:~ Particularly apt materials are polyurethanes, for
example polyester or polyether polyurethanes known as
:, ~5 Estanes (Trade Mark)~ Other apt materials are
elastomeric pdlyether polyesters~ or e~ample tholse
known as ~ytrels (Trade Mark) and polyether
polyamides, for example tho:se known as Pebaxes ~Trade
Mark)O Other favoured materials include ~ydrophilic
~ 30^ ~ po~l~mers ~such as~hy~drophilic polyure~hanes including
:~ : : those~described in~UK Patent ~o. 2093190B, esp~cially
the polyurethane d scribed in Example 2 therein. Such
materials~will typically take up from 5 to 95~ by
`:`: :
: :
: :
WO ~4/12134 PCT/GB93/02382
,. . , ,21,~987a
- 7
~ .
weight of water.
The materials employed in the dressings of the
inven~ion may be moisture vapour permeable. The
5 moisture vapour transmission rate of the materials
employed in the present invention may be measured by a
procedure known as the Payne Cup m~thod, which method
is described in European Patent Application No.
360458.: The method:uses a cup 1.5cm deep with a
lO flanged top. The inner diameter of ~ the flange is such
to provide an area for moi ture vapour transmission of
. 10cm2. In this method lOml of chilled water is added
to the cup and:a sample o~ the materia1 under test,
large enough to completely co~e~ the flange, is
}5 clamped o~er the cup. The comp1ete~assembly is then
weighed and placed in a~cabinet where the temperature
and relati~e humidity ~re maintained at 37C and 10%
~ r~5pectîvely. After 17 hours the cup is~removed from
:;, the ca~inet and allowed to coo1 at room temper ture.
20- After re-weighing, the mass of water lost by vapour
;~ transmission is ca1cula~ed and the resul~ expressed as
in gm 2 24hrs 1 at ~7C at~100~ to lOS;relative:
humidity:dif~erence. H~reinafter the:units for : ~
: moisture~vapour transmission will be abbreviated ~o~ -
25 . gll~ 2 24hrs l. ~ : ~ ~
i
ore importantly, the~overa11 moiskure vapour:
tran mi~ssion rate (MVTR)~of the~dress~ing should eguate
: to 500~- 7000~gm ~24h based on composite
;30'~ ;properties:, ie in heavl1y;exudin~ wounds the gel may
act~as~a~'sink' and~enab1e ~he~moisture~ vapour
permea~le film~;to~" lash off"~excess fluid~ In
: lightly~-xudlng wounds the MVTR shouId be sufficleh~
W094/12134 PCTIGB93/0~82
2~,498~S
to maintain a moist environment and prevent the wound
drying out.
The backing layer may comprise a polyurethane
film or alternatively the backing layer may comprise a
HYTREL (TM). The backing layer may have a thickness
of from 15 to lOO~m, preferably 20 to 80~m and more
preferably 25 to 50~m, for example 27.5~, 30~m, 35~m,
40~m.
! 10
The pressure sensitive adhesive layer may b~
formed from an adhesive which is conventionally used
for contact with the skin. Suitable adhe~ives include
: polyvinyl alkyl ether adhesiva and acrylate ester
15 copolymer a~hesives. Suitable adhesives are described
in ~K Patent No. 1280631 and Europe.n Pat~nts Nos.
3S399 and 51935. Preferably the adhesi~e is a
~ polyvinyl ether adhesive or an:acrylate ester
; ~ copolymer adhesive formed b~ the copolymerisation of
20- 2-ethylhexyl acrylate,: butyl acrylate and acrylic
acid~
: The adhesive layer may be from 15 to 65~m thick,
for example 20 to 40~m thick and is applied at a
. ~J- weight per un}t area of 10 to 75gsm, more suitably 15
to~65gsm and preferably 25 to 40gsm. I ~;
: The rémovable protector is preferably a 5il icone
:~ Goatsd relea~e paper.: Sui~ably ~he removable
~: 30 ~ protector may have a weight per unit area of 1~0 ~o
: ~140gsm,~and preferably 110 tjo 130gsm, for example
` ~120gsm. The remova~le protector may be divided in~o
tWQ ~r more pieces. Preferably at least one of the
WO94/12 W 1 ~9 8 73 PCT/GB93/0238
. _ 9 _
:
,
! protector pieces is significantly 1arger than the
¦ other or others and covers a major proportion of the
adhesive layer. It is desirable that the stripping
1Oad of the support layer from the bac~ing 1ayer is
great~r than:that of the protector from the adhesive
layer otherwise there is a risk that the support layer
would peel from the backing layer before the protector
can be remov2d.:
10 The hydrogel layer may be any polymer which is
characterised by it's hydrophilicity and insoIubi1ity
;: ~ in water. Such polymers~ preferab1y:comprise a cross: ~ linked macromolecular n~twork. Such hydrophilic
po1ymers may~ be amphoteric,~eg:. contai~ing anionic and
15- cationic~monomers; anionic,~eg. containing
~: : carboxy1ate,~su1phonate, phosphonate group~; cationic,
: eg~ containing quaternary ammonium ions; zwitterionic,
: eg. conta~ining monomers with:a cationic and ~anionicgroup;~or non-ionic,~eg.~containing ~mide, hydroxyl,
: lactam,~polyether, polyhydroxyethy1methacrylate or
~: ~ ; polyvinyl~pyrrolidone~(PVP) groups.
;Preferred hydrogel materials are those containing~
: ~ polyethylene oxide or PVP.~ Such hydroge~s~pr~ferab1y
: ~ contain ~rom~5:to 30% w/w of~the hydrophilir pol ~ er,~
praferab:ly~rom 5 to 20% w/w, most pre~erab1y 5 to 15
; w/w, ~eg.~ lO~ V/W. ;~
:;T~he~hydrogel~ material may be~ an aqueous;or:~a~
sa1ine~:~so1ution~in a gel-like phase and~may comprise
from about~5% to~about 3~0% by weight of~a polyhydrlc
alcohol selected from~the group:consisting of
polypropylene g1ycol~, polye~hylene g1yco1 and
WO94/12l34 PCT/G~93/0~&2
Zl 498~
, -- 10 --
glycerine.
The hydrogel may also contain from about 8% to
about 14% by wei~ht of an isophorone disocyanate
. ;^ terminated prepolymer/ and, when saline, up to about
1 1% by weight of a salt such as sodium chloride, and
I ~he balance water. ~ :
¦ The hydrogel comprisas a water-insoluble,
10 water-swellable cross-linked cellulose derivative,
,
water and a polyol component and the cellulose
~erivative compri~es less:~han 10% by weight of the
gel. `
:
::~ 15- In particular, hydrogels which may he mentioned
: include those described in~patent applica~ion no. WQ
92tl6245~ When the dressing~according to the
invention includes a support member,:the hydrogel may
be relatively more mobile than other pre~rred
~:~ ~ 20- hydrogels althoug~ the less mobile hydrogels may al~o
: : bè used.
:`; : It is~a:further feature:of this invention to
provide~an a~hesive dressing as hereinbefore de~cribed
25- wherein the removable protector ~xtends beyond the
backing layer at one or more~edges, preferably at both
edges and comprises first and second parts, the first
part having a:portion~:~ext~nding away:from the adhesive
surface and ben~ ~ack to :form a v shap~ an~ the second
~ part;having a portion~extending:away ~rom the adhesi~e
: and overlying the v-shaped firs~ part.
According to~a further feature o~ the invention
~ the conformable support layer may be provlded with a
: :
: :
i
WO94/12134 9 PCT/GB93/0~82
^` ~1~987S
~.........
grid mar~ing in order to enable wound healing to be
observed. The extensions of the conformable support
layer beyond one or more edges of the backing layer
are preferably non-adhesive and thus facilitate the
5- removal of the conformable suppo~t layer from the
backing layer.: ~
In another aspect therefore the present invention
provides a method of treatiDg a wound which omprises
lO- applying thereto an adhesive dressing as hereinbefore
described by removing the removable protector,
;~ app~lying ~he hydrogel layer to the wound and the
exposed adhesive:layer to the skin and then removing
~` : . the con~inuous conformable support layer.
;~ ~ ~ 15. - ` : : :
~: :We ~urthe~ provide~a method of manufacturing an
a~hesive dressing as;hereinbefore described. This
; I method comprises taking a;backing layer provided with
a support layer on a fixst side. The support layer
: 20- preferably be~lng:provided with a grid pattern, a
~: ~ second side of the backing ~layer is coat d~with an
~` adhesive layer and then a protector is applied to ~he
. ~ adhesive using conventional methods knnwn ~ se. In
; an automate~ pro~ess, the dressing may optionally be
~, ~ 25~ wound-:~nto a reel~ Subseguently, the reel~is
unrolled~ the protector is remsved from the adhesive
;~ ~ layer~,~ the~hydrogel layer, which: may be in;the form of
;~: ~ a 'sl~b' is po~itioned onto the adhesive.:~ ~he
protector~is,then~reapplied and~the~:dressings may be
30- cu~ t~;siæe.
When;~the~ hydrogel l:ayer comprises an integral
support me~ber~the process~of manufacture will be as
~ ~ : : ` : ` : :
WO94/12134 PCTIGB93/Q238~
9~,~15
.- 12 -
hereinbefore described. When the dressing according
to the invention comprises a dam member, the dam
member may be applied after the hydrogel la~er or the
hydroge~ layer may be supported in the dam me~ber
prior to applying the supported hydrogel layer to the
backing layer. ~:Preferably however, the dam member is
applied to~the backing layer;and then the hydrogel
. layer is appl~ied.
10. The dressing may be plac~d in a bacteria-proof
pack, sealed and sterilised by conventional methods,
: including, for example,:using ethylene oxide or
~:; . irradiation.
: :;
: : 15. Preferred embodiments~of adhesive dressings of
:: the present~invantion will now be:described by way of
:~ ~ exampIe only and w1th reference to~the accompanying
:. drawings, in which
,
, I :: .
~ ~ 20- Figure 1 is a cross section through an ambodiment
:~: ~ ~ of a:dressing according to the invention,
~;`
~ Figure 2~is an expanded perspective view of the~
:~ ~: embodiment of Figure~l.~ ` ::
2~.
~Figure 3 is a cross section of an~embodlmen~ of
tAe invention:provided~w1th~a~peripheral dam member,
Figure 4 is~a cross section of a embodiment of
30: ::the invention~provided with an integral support
: : member.
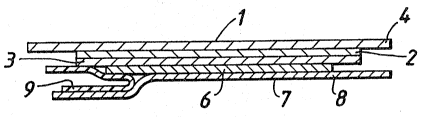
;Figures l and 2`shows~an adh sive dressing ~1
: :
.'O~./12134 PCT/GB93/02382
. 21~987,~ ,
¦ which comprises a backing layer (2) formed from a fil~
of polyether polyurethane. ThP backing layex (2) has
on one surface a pressure sensitive adhe~ive layer t3)
formed from polyacrylate ester adhesive. On the
non-adhesive surface of the backing layer (2) is a
support layer (4). The support layer (4) may compxise
a silicone or polyethylene coated papex or a
: transparent film of polyethylene ox polypropylene.
The support layer (4,) is marked with a ~rid (5) to
l0- enable wound haaling to be measured. A hydrogel (6)
is attached to the adhesi~e layex ~3)~ The adhesive
:; layer (3) and the hydrogel~(6~ are provided with a
protector (7) made from a~silicone coatPd:release
~~~ paper.
~ 15. ~ :
! The protec~or (7) comprises two components. A
large protector (8~ is essentially:flat and overlap.s
the smaller protector (9) which smaller pr~tector (9)
~ is folded into:a v-shape.:
:~ ~ : 20. ~ :
~ ~ :
~: ~ The support layer (4). is provided with edges (lO~
which;extend beyo~d the edge~(11) of t~he backing layer
(2)~ and adhesive layer~(3).~
, ~ l In Figure 3 the d~essin~ is provided with a;dam
. . :member (12) around the periphery of:the:hydro~el layer
In: Figure~ 4 the hydrogel: layer (6) is providPd:
; 30wlth~an lntegral :support member ~(13j being a scrim.
In use~the larger~plecé (8) of the;protector is
removed:first and: the dressing held by the overlylng
::
~'094/12134 PCT/GB93/023B?
.
2~98~S
- 14 -
portion of piece (9) and edge of the support layer
(4). In such a case the larger area of the dressing
! is adhered to the skin, then the smaller piece (9) and
the support layer (4) may be removed. Alternatively,
5- the smaller protector piece (9~ may be removed before
application and the dressing handled asectically by
the edges of the support layer (4) which project
beyond the adhesive (3) and film (2) layers.
: 10.
lS.
:
:~ :
20.
:
.: '
25.
~ ~ I
.
30.
,
;
~ .
: