Note: Descriptions are shown in the official language in which they were submitted.
094/1~8g 214 ~ PCT~S9311~79
PROCESS FOR PROTECTING A S~RFACE ~SING SI~ICATE COiMPOUNDS
Field of the Invention
The present invention relates to a method for forming a
a protective layer on a rigid surface of an article. The
protective layer is formed by applying a layer of a silicate-
containing solution to the rigid surface, drying the layer,
and applying an acidic solution to the dried layer in order to
form the protective layer.
Backaround of the In~ention
10The de truction of metallic and wood articles, such as
~; through abrasion and corrosion, has a substantial economic
impact on many industries. Inhibiting such destructive forces
is therefore of substantial commercial and practical value.
One method for inhibiting abrasion and corrosion is the use of
a protective barrier or coating over an article's exposed
-surfaces~
- ~ .
Various types of protective barriers have been used. For
example,~ organic ~compositions, ~such as paints, varnishes,
- -lac~quers~ and~ the like can~be applied directly over the
20~ surfacè~`~of~;the~article.~ In order to act as a protective
-barrier~, the~organic composition must be compatible with the
treatèd~ surface. -Also, with ~ome organic compositions the
surface~;must be pre~-treated before application so that proper
bonding~ and~adhesion oc~curs to the surface. When applying
paints~to~an;aluminum~or aluminum alloy surface, for example,
the surface~must be thoroughly cleaned and "roughed-upll or
"pickled"~ so that~the;paint adheres to the~surface. When
relativèly thin paints are used, however, the "roughed-up~ or
;-"pick}ed" underlying surface may be seen through the paint
layer, which may~ ble Inde,sirla~le~. ;The protective barrier
-;~ formed by some organic compositions may also be relatively
soft and~not resiseant~to abrasions or corrosion.
Another type of protective barrier uses silicate
~-~P~compounds which are chemically bonded to various metallic
~35~ surfa¢es~ `It is widely known that various silicate compounds
; can~be-used to form hard, smooth surfaces that are resistant
to abrasion and corrosion. U.S. Patent No. 3,658,662 to
,~
W094/1~8g ~ PCT~S93111279 ~1
214~19 ~
--2--
Casson, et al. discloses lithographic plates made of aluminum
or aluminum alloy material that are silicated to provide a
hard, smooth barrier between the plate's surface and the
corrosive diazonium salts and other photosensitive coatings
used in the lithographic process. Another advantage of
silicate compounds is their heat and fire resistant
properties. U.S. Patent No. 4,Bl0,741 to Kim, for example,
discloses an elaborate process for producing a fire-resistant,
non-combustible material containing silicate compounds.
However, such silicated materials still allow unacceptably
high levels of corrosion, and are prone to be dissolved by
-solvents.
- One type of coating which uses silicate compounds is
applied using electrolytic processes, such as that disclosed
in U.S. Patent ~o. 3i658,662 to Casson, et al. This process
involves the use of a basic electrolyte solution of sodium
; silicate or;other salts and a piece of aluminum which acts as
an~anode.~ Electricity i9 supplied between the aluminum anode
and a csthode in order to cause an aluminum silicate barrier
; 20 to form on the surface of the aluminum anode. This process,
however, cannot be u~ed on surfaces which do~not conduct
electricity,~ such as wood.
Some~prior art protective coatings, particularly coatings
for metals~, a1so contain toxic substances, such~as chromates
~and cadmium. Such substances are both harmful to the
environmént~and expensive to dispose of. The processes for
~ -- creating s-uch coatings, such as the widely used chromate
S - conversion coating, also create other waste disposal problems.
The coatings created by such proces~es, moreover, lack great
resistance ~o abrasion.i There is thereifore a great need for
an improved protective coating for metals and other materials
~- that~ does not use substances that are toxic or otherwise
harmful to the environment and that confers greater resistance
.,
`~ to abrasion.
, ~ ~
~ 35 - Summary
x~ ~ In contrast to prior art protective coatings, the
-~ protective layer formed by the method of the present invention
., ,
,`', ~ ~
; ~,
, " -
;,,,
WO94/1~89 ~1 4 9 9 1 3 PCT~593/1~79
provides a coating for a relatively rigid surface of an
article that is highly resistant to abrasion, corrosion,
solvents, fire, and other destructive forces. This protective
layer is formed from non-toxic materials by a process which
s itself does not generate further hazardous waste. The present
invention therefore represents a significant improvement in
the art of protective layers for coatings for rigid surfaces.
In one embodiment, a method for treating a rigid surface
of an article in order to form a protective layer on that
rigid surface is disclosed which comprises the steps of:
(a) forming a hydrophilic surface on the rigid
surface of the article;
(b) applying a silicate-containing solution to the
hydrophilic surface, thereby forming a continuous layer
of the silicate-containing solution on the hydrophilic
surface;
tc) drying the layer of silicate-containing
solution on the hydrophilic surface to produce a dried
layer of silicate material on the rigid surface of the
article; and
-~ (d) exposing the dried laye~ of silicate material
to an acid, thereby forming the protective layer on the
- ; ~ rigid surface.
- ~ ~ In this method, the hydrophilic surface can be created by any
-~25 of ~arious means, including chemical means and mechanical
~,,
~-~ means, such as sanding the surface. The hydrophilic surface
can also be rinsed before it is exposed to the silicate-
containing solution.
In this method,ilt~hejsil~cate-containing solution contains
between 104 and 100% (by volume) of a silicate compound.
~ Preferably, the silicate-containing solution contains between
`~ 20% and 40% (by ~olume) of the silicate compound. The
silicate-containing solution is also preferably a sodium
silicate solution, although the silicate-containing solution
-~ 35 can as well be a potassium silicate solution. When the
hydrophilic surface is exposed to tne silicate-containing
~; solution, it can be exposed for between l and 60 seconds in
..:
,: :
,-: ., ,. ,, . , , , . ,, , , . ,.. ~ .... ... .
W094/1æ89 PCT~S93111279 ~
21499i9
; -4-
order to form the layer of silicate-containing solution.
. Preferably, however, the hydrophilic surface is exposed to the
silicate-containing solution for 20 seconds or less
The drying step of the present method preferably
comprises heating the layer of silicate-containing solution on
~:; the hydrophilic surface. The layer of silicate-containing
solution should be heated to at least 302F. In this method,
the heating step can comprise exposing the layer of silicate-
containing solution on the hydrophilic ~urface to an
environment having an initial temperature of between about
68F and 480F and then raising the temperature of the
environment at a rate of between about 30F and 60F per
, minute until a temperature of between 302F and 480F is
reached. Preferably, this step comprises exposing the layer
of silicate-containing solution on the hydrophilic surface to
the environment having an initial temperature of between 120F
; and~150F~and~then raising the temperature of the environment
at~ a-~rate-of between about 30F and 60F per minute until a
- temperature~of~between 305F and 350F is reached. The entire
2~0 drying~step~lasts~for between about 2 minutes and 50 minutes,
and preferably for between 6 minutes a~d 15 minutes.
The acid used in the present method can be an acidic
solution~which contains between 1~ and 99~ by volume of an
acidj~al~t~hough the acidic solution preferably contains between
~- 25 lO~ and~30%~by volume of the acid. The acid can be phosphoric
~- ~ acid, which is inexpensive and readily available. The dried
layer of silicate material is exposed to the acidic solution
for between about 5 seconds and 120 seconds, and preferably
~; ~ for,between 20 seconds and 50,seconds. ! In this embodiment,acidic solution can be between 68F and 180~F when the dried
layer of silicate material is exposed to the acidic solution.
~-~ ; In one embodiment, steps (b) to (d) above are repeated a
plurality of~ times in -order to provide improved corrosion
resistance and other characteristics. In this and other
~35 embodimen-s, the protective layer is rinsed to remove excess
~ :
-~r 2 1 4 ~ ~ 1 9
WO94/12289 - PCT~S93/1~79
Another embodiment of the present in~ention comprises an
article having a rigid surface on which a protective layer has
been formed, the protective layer being formed by:
- ~a) forming a hydrophilic surface on the rigid
surface of the article;
~b) applying a silicate-containing solution to the
hydrophilic surface, thereby forming a continuous layer
of the silicate-containing solution on the hydrophilic
surface;
~c) drying the layer of silicate-containing
solution on the hydrophilic surface to produce a dried
layer of silicate material on the rigid surface of the
article; and
~d) exposing the dried layer of silicate material
to an acid, thereby forming the protective layer on the
rigid surface.
-: In another, alternative embodiment of the present
invent~ion, a method for treating an article having a rigid
surface~in order to form a protective layer on the rigid
~-~ 20 surface is provided which comprises the steps of:
~ a) applying a uniform l~yer of a silicate-
: containing solution to the rigid surface of the articlei
-~- : (b) drying the uniform layer of the silicate-
~ ~ .
containing solution to produce a dried layer of silicate
material on the rigid surface; and
c) exposing the solid layer of silicate material
to an ac~id, thereby forming a protective layer on the
~: rigid surface.
This method can additionally comprise the step of providing a
hydrophilic lsurface on the rigid surface before applying the
uniform layer of the silicate-containing solution to the rigid
surface.
- In yet another embodiment of the present in~ention, a
protective layer on a rigid surface is disclosed, the
: prot~ctive layer comprising:
an outer layer comprising silicon dioxide, the outer
. layer being non-porous; and
~,~
WO94/1~89 ~ , PCT~593/11279 ~
2149919
an inner layer comprising silicon dioxide and a
metal oxide, the inner layer being water-soluble.
These and other aspects and embodiments of the present
invention will be discussed in further detail below.
Brief Descri~tion of the Drawinqs
Figure l is a cross-~ectional view of a metallic article
having a protective layer formed thereon comprising one inner
layer of-silicate salt and an outer bi-layer.
Figure 2 is a cross-sectional view of a metallic article
having a protective layer formed thereon comprising two inner
layers of silicate salt and an outer bi-layer.
Figure 3 is a cross-sectional view of a metallic article
ha~ing a protective layer formed thereon comprising two bi-
layers.
Figure 4 is a cross-sectional view ~f a wood article
having a protective layer formed thereon comprising two inner
layers of silicate salt and an outer bi-layer.
-~ Figure 5 is `a cross-sectional view of a wood article
;~ having a protective layer formed thereon comprising an inner
layer of silicate salt and an outer bi-layer.
~ Figure 6 is a graphic illustrat~on of one aspect of a
-~ preferred embodiment of the present method for treating rigid
~ articles in order to form a protective layer thereon.
; ~ Detailed Descri~tion of the In~ention -
~ 25 The present in~ention comprises an improved method of
-~ treating a rigid surface of an article in order to create a
~- - protective layer on that surface. It is believed that the
surface of any relatively rigid material, such as a metal, can
be treated according,tolthe,pjresent method in order toiprotect
the surface of that material. The protective layer created by
the present method is belie~ed to contain substantial amounts
of silicon dioxide, and has been found to be substantially
resistant to most chemical sol~ents.
The protective layer created by the inventive method can
- 35 be used to facilitate paint adhesion to a surface, as well as
to impart a glossy finish to such a surface. The protective
layer, however, has been found to be especially useful in
,-, ~
~ WOg4/~28g 214-9 919 PCT~S93/11279
.. ...
--7--
protecting sur~aces from environmental damage such as
corrosion and abrasion. In particular, the protective layer
created by the present method has been found to be of great
use in protecting metal surfaces from corrosion. For example,
aluminum and steel surfaces treated according to the present
method have been substantially protected from corrosion.
Treated aluminum and steel surfaces were subjected ~o ASTM-
B117 Accelerated Salt Spray Corrosion Tests and to paint
adhesion tests per Mil. Specification C-5541, with various
exposure durations. Aluminum alloys were subjected to such
tests for 168 and 336 hours, and were found to meet and exceed
the standards specified for aluminum alloys subjected to these
~ tests. Steel and zinc-coated steel alloys were also subjected
to such tests for 1.5, 2, 4, 12, 24, 48, and 96 hours, and
were likwise found to meet and exceed the standards set for
such alloys.
}n one embodiment, the process disclosed herein forms a
;p~rotecti~e;layer comprising an outer layer of silicon dioxide
over~one or~m~re inner layers of silicate salt. To form such
~-` 20 a~protecti~e~1ayer, the process begins by creating a fixed
surface~on an~article, such as metallic or wood article. The
fixed surface is created by first washing the surface with
detergent and hot water. With metallic articles, the surface
is then exposed to a fixing acid solution which etches or
25~ chemical~ reacts with the surface to form a thin film layer
thereon. The film layer contains binding substances, such as
mètallic oxide~material, which enables the layer of silicate
salt formed in the next step to bind to the surface. In one
~- aspect of this embodiment, the fixing acid is phosphoric acid,
`~ ~ 30 which forms a` thinlfilm layer comprising metallic oxide and
metallic ortho-phosphate material. After exposing the surface
on the metallic article to the acid, excess acid, oxides, or
other impurities not tightly bonded to the surface are then
forcibly removed by wiping the surface with a cloth or by
~35 washing the surface with pressurized hot water. With wood
articles, no further steps are needed to create a fixed
surface.
..,., ~ :
"..
c ~
WO94/1~89 - PCT~S93/1~7s ~
:-;14991g
.:
: -8-
After a fixed surface is created on the article, it is
~then exposed to an alkali or alkaline-earth metal silicate
`~``;tsolution. Although various types of silicate solutions may be
used in the process, in the preferred process the fixed
surface is exposed to 18-33% (v/v) sodium silicate solution.
After exposure, the fixed surface is dried completely,
preferably at a temperature of 302F or more, to form a thin
layer of silicate salt over the fixed surface. During the
drying step, it is postulated that a portion of the silicate- 10 salt is converted into silicon dioxide. With wood surfaces,
it is postula~ed that during this first exposure, the silicate
salt solution is partially absorbed into the wood fibers
located along the surface. The amount of silicate salt
~-solution absorbed is dependent upon the type of wood, the
porosity of the wood, and the length of exposure.
Because cracks and other imperfections can occur in the
first~layer of silicate salt during the process, the entire
~-process may~ be repeated to form a plurality of layers of
-~silicate~sal~t over the lower layers of silicate salt. Each
layer of silicate salt provides greater protection to the
article against abrasion, corrosion, fi~e and heat. After the
~ ~fina~ layer of silicate salt is formed and dried, it is then
5 ~'','~, , ~ exposed to a strong~acid, such as an acidic solution. During
this exposure, the final layer of silicate salt is transformed
into an outer bi-layer structure comprising an inner layer of
silicate salt and an outer layer of silicon dioxide. When the
entire process is completed, the protective layer comprises a
`~-plurality of inner layers of silicate salt at least partially
converted into silicon dioxide and an outer bi-layer
comprising an lnnér layelr of silicate salt and an outer layer
of silicon dioxide.
~- ~-The above stated process may be further modified by
exposing each dried layer of silicate salt to an acid before
- forming a subse~uent layer of silicate salt thereover. This
3~5~ creates a plurality of bi-layers, similar to the outer bi--
layer described àbove, over the fixed surface which improves
~`~the protective layers resistance to abrasion, corrosion, fire
~ '
~ WO94/12~89 214 9 ~13 PCT~S93tl~79
.:
_ g _
and heat. When using this alternative proc~ss, each layer of
silicate salt is transformed into an inner bi-layer comprising
a layer of silicate salt and an outer layer of silicon
dioxide. For most applications, one inner bi-layer is formed
undex the outer bi-layer to provide sufficient protection,
When the entire process is completed, a protective layer is
formed over the metallic or wood article to protect it against
abrasion, corrosion, heat, and fire.
In one embodiment, the present method can be used to
protect metallic and wood surfaces. As used herein, the term
"metallic" refers to surfaces containing metal and metal
alloys, including steel, aluminum, and associated alloys.
~ Also as used herein, the term "wood" refers to all types of
woods or wood products. It is anticipated that this
embodiment of the present method may also be used on articles
- made of other materials.
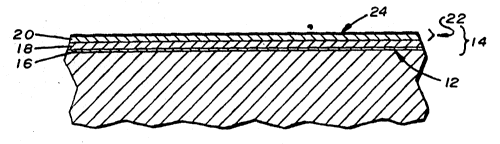
As shown in Figure 1, the method disclosed herein can be
used to form a protecti~e layer 14 on a fixed surface 12 of an
article 10, such as a metallic article. The protective layer
14 ~is thought to comprise an outer bi-layer structure 22
covering~an inner layer of silicate salt material 18. When
treating metallic articles according to this embodiment of the
present method, a fixed surface 12 is created on the article
10. However, the creation of a fixed surface in the present
method is optional. To create a fixed sùrface, the surface to
be treated is first c}eaned and dried. Although no particular
method of cleaning and drying i8 preferred, warm water and a
~detergent have proven to be sati~factory. Next, the surface
is expoQed to a fixing acid solution which etches or
chemically reacts wïth the surface to form a rhin film layèr
16 thereon. The thin film layer 16 preferably contains a
binding substance, such as a metallic oxide material, which
enables the layer of silicate salt formed later to bind to the
surface. In one aspect of this embodiment, the fixing acid- ~ 5 solution is 20~ to 25% phosphoric acid and is left on the
surface for 3 to 10 minutes. When phosphoric acid is used as
the fixing acid solution, it is postulated that it etches and
wO94/1~8s PCT~S93111~79 ~ t ~ ;
2149919
--10-- ~- .
chemically reacts with the surface to form a thin film layer
16 comprising metal ortho-phosphate and a metal oxide salt.
When acids stronger than phosphoric acid, such as hydrochloric
acid, are used on metallic surfaces, excessive oxidation of
the surface may occur.
Although any method may be used to expose the surface to
be treated to a fixing acid solution, one method is to
completely submerge the article lO in an acid bath comprising -~
the fixing acid solution. Preferably, the acid bath is heated
to l20F-l40F to speed up the reaction and to generate a msre
uniform appearance on the surface. For higher or lower
concentrations of acid solution, however, the reaction times
~ and temperatures may be decreased or increased accordingly.
After the film layer 16 is formed on the metallic
surface, the surface is then wiped with a cloth or washed in
warm water to forcibly remo~e any excess acid or any
impurities. The impurities, generally referred to as smut
material, comprise various oxides and phosphate salts
(typically copper and magnesium salts) which form on the
sur~ace. This washing step, called desmutting, is conducted
for appearance value only, since the smut material does not ~`
appear to hinder the creation of or the functioning of the
protective layer. In the preferred process, desmutting is
conducted by washing the film layer 16 with warm or hot water,
120F-140F. Pressure and scrubbing action as well as other
desmutting methods may also be used. The smut material is
~-~ apparent by a dark grey to black color on aluminum alloys.
After cleaning and rinsing, the film layer 16 is then dried.
Next, the film layer 16 is exposed to an alkali-metal or
alkaline-earth metal`'silicate solution to form the innèr layer
of silicate salt over the fixed surface 12. The alkali-metal
silicate solution can be selected from the group consisting of
potassium silicate, sodium silicate, and lithium silicate.
The alkaline-earth metal silicate solution can be selected -~
35 ~ from the group consisting of beryllium silicate, magnesium j~
silicate, and calcium silicate. In a preferred embodiment,
the film layer 16 is exposed to 18-33% (v/~) sodium silicate
'
~ W094/1~89 2 1 4 9 9 19 PCT~S93/1~79
,,,"~
solution by immersion or spraying. When used on metallic
articles lO, it is postulated that during the exposure to the
sodium silicate solution, the metallic ortho-phosphate or
metallic oxide material found in the film layer 16 chemically
bonds with the sodium silicate in the solution to form the
inner layer of silicate salt 18. The actual exposure time to
the silicate solution can vary from about 1 to 10 minutes,
depending upon the type of article and surface being treated.
With smooth surfaces, for example, shorter exposure time is
required than with rough or pitted surfaces. Also, some
materials require longer exposure times than others. For
example, surfaces made of aluminum orlaluminum alloys require
~ a shorter exposure time than magnesium or magnesium alloy
surfaces.
After exposing the film layer 16 to the solution of
sodium silicate,~the layer of silicate salt 18 is then allowed
to dry completely~at a sufficiently high temperature. It is
important~that~the film layer 16~be dried completely ~efore
proceeding to the next step in the process. In a preferred
-embodimént, the drying step is carried out in an oven at 302F
or more. At this temperature, complete drying takes between
-~ ~about ~2 and 30 minutes. It has been discovered that when
~- sodium silicate is dried at 302F or more, a portion of it is
converted in~o silicon dioxide, which appears to increase the
~;-25~ ~resistance of the inner layer }8 to abrasion and corrosion.
By increa~sing~ or decreasing the temperature or the drying
time, the amoun~ of conversi~n of sodium silicate into silicon
dioxide can be increased or decrea~ed, respectively, to create
layers having different abrasion and corrosion-resistant
properties~
;~ ~ After the inner layer 18 has dried completely, it is then
pre~ferably exposed again to the 18-33~ (v/v) sodium silicate
solu~ion for about one to three minutes and then dried in the
same manner as above to form a second layer of silicate salt
20 over~the first inner }ayer 18. Unlike the first exposure
- to~the~sodium silicate solution used to create the inner layer
18, the length of exposure to the sodium silicate solution to
,,:,- ~ .
WOg4/L~89 . ~ ?~; - PCT~Sg3/11279 ~
2149919 :
-12-
form the second layer 20 is not as critical. Again, by
controlling the drying temperature and the drying time, the
amount of sodium silicate converted into silicon dioxide may
be selectively controlled.
After the second layer 20 has dried completely, it is
then preferably exposed to an acid, such as an acidic solution
selected from the group consisting of acetic acid, boric acid,
carbonic acid, hydrochloric acid, nitric acid, phosphoric
acid, and sulfuric acid. During this exposure, the second
layer 20 is converted into a uniform bi-layer 22. Bi-layer 22
comprises the lower portion of the second layer 20 and an
outer layer of silicon dioxide 24. During the exposure to the
acid solution, the acid chemically reacts with the silicate
material in the second layer 20 to form the outer layer of
silicon dioxide 24. In the preferred process, the second
layer 20 is exposed to 20-25~ (v/v) phosphoric acid heated
between 120F-140F. When the acid is heated to this
- temperature, the exposure time is between about 15 and 30
seconds. For aluminum, if the temperature of the acid is room
temperature (about 68F to 140F), the exposure time should be
between about ewo and seven minutes. Fo~lowing this exposure,
the~out~er-layer of silicon dioxide 24 will be hard and smooth
and~have-a uniform appearance. Because it is harder than
sodium s~licate, the protective layer 14 formed with the bi-
25~ layer structure 22 is more resistant to abrasion and corrosion
than the single layers of sodium silicate.
;, - : ~ , ,
As shown in Figure 2, instead of converting the second
~layer of silicate salt 20 into a bi-layer structure 22, a
third layer of silicate salt 26 can be formed over the second
~; 30 layer of silicate salt'2d to form a second protective layer 25
which provides greater protection than protective layer 14.
In order to form the second protective layer 25, the third
layer 26 is formed using the steps cited above used to form
the second layer 20. Rather than exposing the second layer 20
to phosphoric acid after drying completely, it is instead
exposed to the 18-33~ sodium silicate solution. After drying,
~; the third layer of silicate salt 26 is then formed which is
~ :
~ W094/~289 2 ~ q 9 ~ 1 9 ~CT~S9~ 79 `;;
-13-
then exposed to 20-25~ phosphoric acid to form an outer bi-
layer 28. Like bi-layer 22, bi-layer 28 comprises the lower
portion of ~he third layer 26 made of sodium silicate and an
outer layer of silicon dioxide 29.
When the entire process is completed, relati~ely smooth,
hard protective layers 14 and 25 may be formed on the surface
of the article 10, comprising one or two inner layers of
silicate salt 18 and 20, covered by outer bi-layers 22 or 28,
respectively. The protective layers 14 and 25 are very
resistant to abrasion and corrosion caused by acidic,
alkaline, and salt water action, and are glass-like in
appearance. These layers can be covered with organic
compositions, such as paints, varnishes, and the like.
It has been discovered that cracks or pores may be formed
on the top surface of each inner layer of sodium silicate 18,
20 by using the foregoing method. This may affect the overall
functioning of the protective layers 14 and 25. Such cracks
or pores may be in part due to the thermodynamic properties of
the underlying materials being treated. One way of overcoming
thiC problem is discussed below with regard to drying a
silicate-containing solution at different initial and final
temperatures and for differing amounts~of time. Another way
~ of dealing with this problem is to expose each lower layer of
-~ silicate salt 18 or 20 to an acid before forming a subsequent
2~ layer of silicate salt thereover. In this way a plurality of
bi-layers may be formed over the fixed surface 12.
Figure 3 shows a third protective layer 50 formed on the
exposed surface of article 10 comprising two bi-layers 52 and
55 formed over the fixed surface 12 of article 10. Using the
steps cited above to form protective layers 14 and 25, the two
bi-layers 52 and 55 are manufactured over the fixed surface
12. Film layer 16 is first formed over the fixed surface 12.
~ An inner bi-layer 52 comprising a first layer of silicate salt
: 18 and first layer of silicon dioxide 54 is then formed over
the fixed surface 12. After the inner bi-layer 52 is formed,
~ , ,
an outer bi-layer 55 is formed thereover comprising a second
layer of silicate salt 20 and an outer layer of silicon
,:.
'
,~ ,
WO94/12289 ' ; PCT~S93t11279 ~
2149~19
-14-
dioxide 57. For some applica~ions, however, where additional
protection is needed, an additional bi-layer ~not shown) may
be formed over the fixed surface 12.
To form protective layer So over the ar~icle 10, the
fixed surface 1~, film layer ~.6 and first layer of silicate
salt 18 are first formed on the article 10 using the process
cited above. Af~er the first layer 18 has dried completely
and partially been converted into silicon dioxide, it is then
immersed in phosphoric acid heated to between about 120F and
140F for about 15 to 30 seconds in order to form an inner
layer of silicon dioxide 54. The inner layer 54 is similar to
the outer layer 24 created when forming the protective layer
~ 14. After cooling, the inner bi-layer 52 i5 then exposed to
an 18-33% (v/v) sodium silicate solution for about one to
three minutes and dried to form a second layer of sodium
silicate 20 thereover. The se~_ond layer 20 is then immersed
in hot phosphoric acid as above for about 15 to 30 seconds to
form the oùter layer of silicon dioxide 57 thereover. When
the process is com~21eted, the inner and outer bi-layers 52 and
55, respectively, are formed o~er the article 10.
This e~bodiment of the preseint method can also be used on
various wood surfaces to provide protection against abrasion,
corrosion, heat and fire. As seen in Figure 4, in an
alternative eimbodiment, a protective layer 34 can be formed on
the exposed surfaces of an article 30 made of wood. As with
m~tallic articles, in this embodiment a fixed surface 32
should first be formed on the article 30 so that the
protecti~e layer 34 will properly adhere to the ~rticle 30.
The fixed surface 32 is formed by wa~hing and rinsing it with
hot or warm wateir~a~d aiidet!ergent !`fior ~e~eral mihutesl to
remo~e all dirt particles or foreign substances from the wood
pores. Since wood is more porous than most metallic
substances, washing the wood `surface and re~oving foreign
substances is~ more important than~with metallic surfaces.
Unlike the proceqs us~d on metallic surfaces, no acid is u ed
to`create the fixed surface 32.
~`
:
~ W094tl~89 214 9 919 PCT~S93/tl279
.. .
- 1 S -
After the fixed surface 32 has been prepared, it is then ~
- exposed to a silicate-containing solution as described above. ~-
In a preferxed embodiment, the fixed surface 32 is exposed to
an 18-33% (v/v) solution of sodium silicate for between about
S one and three minutes. During this step, the sodium silicate
solution is allowed to soak into the wood pores and surface
cracks to form a first layer of silicate salt 36. After
exposure, the first layer of sodium silicate 36 is then dried
at or near room temperaeure. Higher temperatures may be used,
as tolerated by the wood article. It is postulated that
during the exposure to the sodium silicate solution, this
solution i8 absorbed into the wood fibers, and that as the
~ silicate matexial dries it hardens and forms crystal
structures between the wood fibers. It is also postulated
that a small portion of the sodium silicate is converted into
silicon dioxide during drying. As a result, the first layer
of silica~te~salt~36 is~relatively hard and tightly bound to
the~ fixed urface~ 32. After the first layer 36 has been
formed,`~;subsequent second and third layers of silicate salt 38
~and~40,~ respèctively, are formed over the first layer 36. As
when~;treating~metallic articles according to this embodiment
of~the~present~method, it is important that the second and
third~làyers~of silicate salt 38 and 40 be dried sufficiently,
in~oxder to form a suitable amount of silicon dioxide, before
25 ~ proceedin~g;with the~next~step.~ ;
After the third layer of silicate s~alt 40 has dried, it
is-~then~exposed~to~an acid~, as in the treatment of the article
10. In~a~preferred embodiment, 20~ to 25% (v/v) phosphoric
~- acid is used at a temperature of 120F-180F for about 3
min:utes;.' ~It'is a~iso~lpos~ulated that the outer layèr 40i is i`
converted into a bi-layer 41 comprising an outer layer made of
s~il1con~dioxide 42 and the inner layer made of inner layer 40.
- After the outer layer of silicon dioxide 42 has dried, a hard,
smooth protective layer 34 is formed over the fixed surface 32
- 35 ~ of~article 30 comprising two inner layers silicate salt 36,
38, covered by bi-layer`41.
". ~ , ~ ,
?'.' ~ ~ ~
''~'~,' "
' ~
I, ~
',~',~
WOg4/1~89 ' ~ - PCT~593/11279 ~ ¦ ~
2149919
-16-
As shown in Figure 5, for some applications it may be
necessary to form an alternative protective layer 45
comprising one inner layer of silicate material 36 and one
outer bi-layer 4l.
The following examples are used to illustrate the methods
described above.
EXAMPLE 1
A method of treating an aluminum or aluminum alloy
surface using the foregoing embodiment of the present
invention is described as follows:
Two samples of aluminum panels were treated - sample l
comprised 2024 T-3 stock while a sample 2 comprised 6061 T-6
stock. Both panels measured 3 inches (W) x lO inches (L) and
.039 inches thick. Samples l and 2 were first cleaned with a
detergent and water and then immersed in a 25~ solution of
ortho-phosphoric acid at 140F for three minutes. The acid
solution was heated to speed up the reaction and to provide a
~more uniform appearance. S~amples l and 2 were then removed
~- ~from~the~acid ~solution with sample l having a dark black
~20 smutty film~and sample 2 having a light grey smutty film. The
smutty films on both samples were des~utted by wiping each
sample with a sponge and hot water. Once desmutted, the
surface of samples 1 and 2 have a distinct silvery-white
color. It is believed that this color is due to the formation
of aluminum ortho-phosphate on the surface of each sample.
~ Samples l and 2 were then cooled by placing them in a 60F
; water bath for one minute. While wet, thé samples were then
placed in a 16~ sodium silicate solution for one minute.
Samples l and 2 were then removed from the sodium silicate
solution and placed in an o~en heated to 300 to 315F for
approximately thirty minutes to dry. After ten minutes,
- ~ samples l and 2 were removed from the o~en and allowed to cool
to room temperature. It was noted that both samples l and 2
. 1
~ ~ ha~e a smooth, glassy surface. Once cooled, samples l and 2
-- 35 were returned to the 18~ sodium silicate solution for three
minutes. Samples l and 2 were then placed back into the
heated over for thirty minutes, then removed and allowed to
~,, ' .
~wo 94,l228g 2 1 ~ g ~ 1 9 PCT~sg3lll2?9 -
-17-
cool to room temperature. When cooled, samples 1 and 2 were
again placed in the 25~ ortho-phosphoric acid for three
minutes.
The surface of samples 1 and 2 appear hard, smooth, and
glassy. The anti-corrosiYe properti~s of sample 1 and 2 were
tested using ASTM B-117 or Mil. Specifications, C-5541. The
paint adhesion property of samples 1 and 2 were also tested to
Mil. Specifications, C-5541. Both samples 1 and 2 passed,
according to the specifications for each test.
The heat and fire resistant property of each sample was
tested by comparing the burning of an untreated piece of
similar aluminum with the treated samples. The~treated and
untreated samples were exposed to an acetylene flame which
burns between 2,000F~and 2,500F. The untreated samples
decomposed to ash in approximately 30 seconds. No pooling
residue was noted. The treated aluminum samples~l and 2, on
~the other hand~,~bent after a few seconds of exposure. After
approximàtèly 2 minutes, 8 seconds, the aluminum material
began~ slowly running out from between the two ~ides of the
~-20~ coating~làyer.~ At~that point the test was then stopped. No
alumin~m~ash residue~was found. ~ -;
EXAMP~E 2
- A method for treating an article made of a steel or steel
alloy surface using the foregoing embodiment of~the present
2~5 ~ invention;~is as~follows:
Three~samples of;steel panels 1-3 made of 4130 steel were
treated,~ all me;asuring 4 i:nches (W) and 6 inches ~L) and 0.041
inches thick. Samples 1-3 were first cleaned with a detergent
~ and hot water to remove grease and oil and then immersed in
;~ 30 25% (by ~ol~me) solution of ortho-phosphoric acid maintained
at 120F. This step pro~ides iron ortho-phosphate on the
surfaces of the panels to which sodium silicate may bond.
Samples 1-3 were then rinsed~with cool water and immersed in
a 33~ ~by ~olume) sodium silicate solution maintained at 40F.
The samples 1-3 were then dried at 305F for 30 minutes to
form a first layer of sodium silicate partially converted into
~;~ silicon dioxide on the exposed surfaces. Samples 1-3 were
,: :
i
WOg4/1~89 ` PCT~S93/11279 ~ !
2149~ 18-
allowed to cool to room temperature and then immersed in a 25~
(v/v) solution of ortho-phosphoric acid at 120F for
approximately 15 seconds. This step forms a hard, insoluble
layer of silicon dioxide over the first layer of sodium
silicate, thereby creating a bi-layer structure over the
surface. Samples 1-3 were then rinsed and cooled and re-
immersed in the 33% of sodium silicate solution to form a
second layer of sodium silicate over the exposed surfaces.
Samples 1-3 were then dried at 305F for approximately 30
minutes and allowed to cool to room temperature. Samples 1-3
were then immersed in a 25~ (v/v) ortho-phosphoric acid
solution at 120F for approximately 15 seconds to~form a bi-
layer structure of sodium silicate and silicon dioxide over
the samples. Samples 1-3 were then rinsed with cool water and
dried t 305F for 5 minutes. Samples 1-3 were tested in
accordance with ASTM-B117 salt spray test and passed
successful1y the 1/3 hour, l hour, 2 hour, and 24 hours tests,
which ~indicates that the protective layer formed in the
process~acts as a cqrrosion inhibitor under standard testing
conditions.
EXAMPLE 3
A~method for treating copper or copper alloy surface
using the~foregoing embodiment of the present invention is as
;follows:~
2~5~ A sample of copper tubing was treated. The tubing
meaoured~12 inches (L) x 1/2 inches ~I/D) x 1/8 inches side
thic~ness. The sample was first cleaned with a detergent and
hot water to remove grease and oil and then immersed in the
25~ (v/v) solution of ortho-phosphoric acid at 120F. This
~30 step provldes a~filml'layer of copper ortho-phosphate on the
exposed surface of the sample to which, it is postulated,
sodium silicate can bond. The sample was then rinsed with
~,
cool water and immersed in 33% (by ~olume) sodium silicate
so1utLon ma1nta}ned at 40F. The sample was then dried at
305F for~approximately 30 minutes to form a first layer of
sodium silicate and silicon dioxide over the film layer. The
~ sample was then allowed to cool to room temperature and then
,, ~
~: ;
,-",' :
~ WO94/L~89 ~14 ~ ~19 PCT~S93/1~79
-19-
immersed .in a 25~ (v/v) ortho-phosphoric acid solution at
120F for 15 seconds. The sample was then rinsed with cool
water and re-immersed in a 33~ (by volume) sodium silicate
solution to form a second layer of sodium silicate over the
surface. The sample was then dried at 305F for approximately
30 minutes and allowed to cool to room temperature. The
sample was then immersed in the 25% (v/v) ortho-phosphoric
acid solution at 120F for approximately 15 seconds to form an
outer layer of silicon dioxide over the second layer of sodium
silicate. The sample was then rinsed with cool water and
dried at 305F for~5 minutes. The sample was tested in
accordance with ASTM-B117 salt spray test and passed
~ successfully the 48 hour test, indicating that the protective
- layer formed in the process acts as a corrosion inhibitor
~15 under standard testing conditions.
- EXAMPLE~4
A~method of treating~a wood surface using the foregoing
embodiment~of~the present invention is described~as follows:
A~wood~ sample made of pine is~ first cut measuring 2
~ 20 inches~ (W)~ x~4 inc~hes (L) x 6 inches (H). A 20~ solution of
- sodium silicate~is then pored into a container measuring 18
-~ inches ~(H)~ x 6 inches diameter. The container has an air
valve ~(a~lso~-~known as a "Shrader" valve) and a sealing lid so
that pressure could be held inside the container when closed.
-25~ The wood~sample is then~placed into the container filled with
~-the~sodium~silicate~so}ution. The container was then closed
and sealeditlghe. An air hose was connected to the air valve
and air~was forced into the container to create an internal
pressure of approximately 70 psi. The wood sample remained in
the solution under~ip~essure'for tèn minutes and then removed
a~d-allowed to dry for two hours at 90F. When the wood
~sample was dry, it was returned to the sodium silicate
solution and placed under pressure as described above. The
wood sample was then dried for two hours at 90 degrees
~35 Fahrenheit. Once dry, the wood sample was placed into a 25%
~jv~) ortho-phosphoric acid solution at 140F for 5 minutes.
-~The wood sample was then removed from the acid solution and
,~,
.
,, ,
WO 94/12289 ` ~ PCTN593111279 ~ ~ ~
2149~19
-20- ! ;
rinsed with cool tap water to wash off the excess acid. The
wood was then left to dry for 2 more hours. Once dry, the
sample was slightly darker in appearance. No o~her surface
changes were visible.
The heat and flame resistance of the wood sample was
tested as follows: The treated wood sample and untreated
sample were exposed to an acetylene flame which burns at a
temperature between 2,000F - 2,500CF. The treated and
untreated samples were exposed to the flame for one minute.
Within seconds of being exposed, the untreated sample burned
with a visible flame and smoke. Burning continued for
approximately ten minutes. With the treated sample,
combustion occurred within seconds after being exposed to the
flame. After removing the flame, however, combustion stopped
within three seconds, and after 15 seconds, no smoke was
detected. After 30 seconds, the surface of the treated sample
was slightly warm to the touch.
EXAMPLE 5
A method of treating a magnesium alloy surface using the
~20 ~foregoing~em~odiment of the present invention is described as
follQws~
Three sampIes of magnesium panels were treated - all
samples were a magnesium-nickel alloy measuring 4 inches (W)
x 6 inches (L) x 0.041 inches thick. Samples 1-3 were first
~ cleaned~with a detergent and hot water to remove grease and
oil and then immersed in a 25% (v/v) solution of ortho-
phosphoric acid at 120F. This _tep provides magnesium ortho-
"~ ,
phosphate to which it is poqtulated the sodium silicate may
bond,. The sampleqj!were tjhen,rinsed,with cool water and
immer~ed in a 33% (v/v) 40F sodium silicate solution for 5
minutes. The samples were then dried at 305~F for 10 minutes
~:- to form a first layer of sodium silicate. The panels were ~i
allowed to cool to room temperature and then immersed in a 25%
; (v/v) solution of ortho-phosphoric acid at 120~ for 15
~-35 seconds (this step can vary from 15 to 45 seconds). A
;~ uniform, outer layer of silicon dioxide was thereby formed on
- the surface exposed to the acid solution. The samples were
,~,' ~ ~
~ W094/lZ~g 214 9 ~19 PCT~S93/1~7g
. , ~
-21-
then rinsed with cool water and re-immersed in the 33~ (v/~)
sodium silicate solution. The samples were then dried at
305F for 30 minutes and allowed to cool to room temperature.
The samples were then immersed in a 25~ (v/~) ortho-phosphoric
acid solution at 120F for 15 seconds, forming a final layer
of silicon dioxide. The samples were then rinsed with cool
water and dried at 305F`for 5 minutes. -
EXAMPLE 6
A method for treating nickel or nickel ~lloy surface
using the foregoing embodiment of the present invention is
described as follows:
A sample of nickel plated tubing was treated - the sample
was nickel plated measuring 12 inches (1) c 1/2 inch (O.D.)
and 1/8 inch side wall; thickness. The sample was first
cleaned with a detergent and hot water to remove grease and
oil~ and then immersed in a 25~ ~(v/v) solution of ortho~
ph~sphoric~acid~àt~120 degr~ees~Fahrenheit. This step provides
ickel~ortho~-phosphate to which the sodium~ silicate may bond.
The~sample~was,~then~rlnsed with cool water and immersed in a
33~(v/v)~ 40 degree~Fàhrenheit sodium silicate solution for 5
, minutes~. The sample was~then dried at~305 degrees Fahrenheit
for 10 minutes to form a first layer of sodium silicate.
During,the drying~process, a portion of the sodium silicate
was~partially con~erted Lnto silicon dioxide. The sample was
25~ allowed to ~cool to room temperature and immersed in a 25%
(Y/V~ -solutlon ~of ortho-phosphoric acid at 120 degrees
Fahrenheit~for~15 seconds (this step can ~ary from 15 to 45
seconds). A uniform outer layer of silicon dioxide is thereby
formed on the surface expo~ed to the acid solution. The
sample was~then rinsed litX cool'!water and re-immerqed in t'he
: . .
, ~ 33~sodium silicate solution. The sample was then dried at
305~degrees Fahrenheit for 10 minutes and allowed to cool to
room ~temperature. The`sample was then immersed,in a ~5%
ortho-phosphoric acid solution at ?o degrees Fahrenheit for 5
35 ~ minutes forming a final layer of silicon dioxide. The sample
~ wa~s then rinsed with cool water and dried at 305~ for 5
"',,~ minutes to dry.
G: '
~-': ' :
_"~
W094/1~9 ' '~ ' PCT~593/11~7~
214g~19 . , "
-22-
EXAMPLE 7
A method for treating a ,silver or silver'alloy surface
using the foregoing embodiment of the present invention is
described as follows:
A sample of silver plated tubing was treated. The
sample measured 12 inches ~L) 1/2 inch (O.D.) and had 1/8 inch
side wall thickness. The sample was first c}eaned with a
detergent and hot water to remove grease and oil and then
immersed in a 25% (v/v) solution of ortho-phosphoric acid at
120F. This step provides silver nitrate on the surface of
the silver, to which it i8 postulated the sodium silicate
bonds. The sample was then rinsed with cool water and
immersed in a 33~ (v/v) 40F sodium silicate solution for 5
minutes. The sample was then dried at 305F for 10 minutes,to
form a first layer of sodium silicate. During the drying
process, a portion of the sodium silicate was partially
converted~into~silicon dioxide. The~sample was allowed to
cool to~room temperature~ and then immer~ed in a 25% (v/v)
solution~o ortho-phosphoric acid at 70F for 5 minuees. A
~ ~ uniform~;outer layer of silicon dioxide is thereby formed on
the surf~ace exposed to the acid solutio~. The sample was then
rinsed~witb cool water and re-immersed in the 33~ (v/v) sodium
silicate~solution. The sample was then dried at 305F for 10
minutes and allowed to cool to room temperature. The sample
was then;~immersed in a 25~ (v/v) ortho-phosphoric acid
solution at 70F for 5 minutes, forming a final layer of
silicon~dioxide. The sample was then rinsed with cool water
' and dried at 305F for 5 minutes.
EXAMPLE 8
A meth`od for treàting titanium or titanium alloy surface
~ using the foregoing embodiment of the present invention is
-,,`'~ described as follows:
A~sample of titanium plate was treated - the sample
measured 3 inches (L) x 3 inches (W) and 0.02 inches thick.
r
The sample was first cleaned with a detergent and hot water to
remove grease and oil and then immersed in a 10~ (v/v)
,~ ................................................................................ .
~- solution of ortho-phosphoric acid at 120F. This step
". ~ .
~ WO94/1~89 21~9~19 rcT~s93/l~79 i ~
provides titanium ortho-phosphate and titanium oxide on the
surface of the titanium with which it is postulated the sodium
silicate may bond. The sample was then rinsed with cool water
and immersed in a 33~ (v/v) 40F codium silicate solution for
5 minutes. The sample was then dried at 30SF for lO minutes
to form a first layer of sodium silicate. During the drying
process, a portion of the sodium silicate was partially
converted into silicon dioxide. The sample was allowed to
cool to room temperature and immersed in a 25~ (v/v~ solution
of ortho-phosphoric acid at 70F for 5 minutes. A uniform
outer layer of silicon dioxide is thereby formed on the
surface exposed to the acid solution. The sample was then
rinsed with cool water and re-immersed in the 33~ (by volume)
sodium silicate solution. The sample was then dried at 305F
for lO mlnutes and allowed to cool to room temperature. Then,
the sample was immersed in a 25% ~v/v) ortho-phosphoric acid
solue~ion~ at 70~F for 5 minutes, f orming a final layer of
silicon~dloxide.~ The sample was then rinsed with cool water
and dried at 305'F for 5 minutes.
~In a~preferred embodiment of the present invention, the
present ~method can be used to treat any relatively rigid
surf~ace in order to protect the surface of that material.
Metals, such as~ aluminum, steel, zinc, and magnesium, have
b~een found to be~particularly suited for treament according to
~this embodiment. Those of skill in the art will be able to
-~~determine, through routine experimentation, those materials or
surfa~es which cannot be treated according to this embodiment
of the invention to plr,od!uce ~a pr~tectiv~ellayer on the surfaces
of such materials. However, it is noted that materials which
will deyrade due to the drying temperatures employed in this
-~embodiment of the present method or due to adverse chemical
reactions with any of the chemicals or reagents, such as the
,.~
silicate solution, used in this embodiment will probably not
~35 be practical to use as surfaces on which to form the
~- ~ protective layer according to this embodiment of the present
i~ invention.
'~
WO94/1~89 PCT~S93/11279 ~, ~
21~9~19
-24-
The protecti~e layers formed by this embodiment of the
present method are somewhat flexible, and exhibit strong
adhesion to the surfaces on which they are forme~. The "ri~id
surfaces" which can be treated according to this embodiment of
the present method, therefore, need only be sufficiently rigid
to allow the formation of the present protective layer
thereon. Surfaces made from wood, aluminum, steel, zinc, and
magnesium have-been found to be sufficiently rigid for these
purposes. Other materials, however, can also be used. For
example, a material which by itself lacks sufficient rigidity
to have a protective layer formed thereon can be attached to
a sufficiently rigid material and thereafter be treated
~ according to the present method in order to form a protective
layer on the surface of that material. Surfaces made rigid in
this way are explicitly included in the definition of a
"rigid" surface. Through routine experimentat~on, one of
skill in the art can determine whether a particular material
is sufficiently rigid by itself to undergo the present
- treatmene or whether such a material must be supported. Such
experimentation can, for example, consist of subjecting a
surface~made from a particular material to the present method
to determine whether a protective layer can be formed thereon.
The protective layer formed according to this embodiment
of the present invention is most effective when the silicate-
containing solution u9ed to form the protective layer can be
applied as a uniform layer 63 to a rigid surface 61 on which
it is desired to form the protective layer. In order to form
such a uniform layer 63 on the rigid surface 61 in this
embodiment, -it is advantageous to prepare the surface 61, by
either chemical or'mechanlcallmleans, to~provide a "wettable"
or hydrophilic surface 62 on the rigid surface 61. The term
'hydrophilic" as used herein describes a surface on which
w er and/or other liquids will form a uniform, continuous wet
film or layer. A hydrophilic surface is one which will act to
~;~3~ carry a liquid, such as water or an aqueous solution, so that
when the }iquid is applied to such a surface the liquid
spreads evenly over the entire surface in a uniform,
.
' ' `
~ WO94/1~89 214 9 9 19 PCT~S93111279
-25-
continuous wet film or layer. As an illustration, a waxed
surface on a car on which water "beads" is not a hydrophilic
surface.
Surfaces can be rendered hydrophilic by any means known
to the art. The removal of excess oxides, oils, and other
contaminants on a surface, such as the surface of a metal, is
often sufficient to render that surface hydrophilic. Methods
such as sanding, sandblasting, and using various chemical
cleaners can be used to remove such oxides, oils, and other
contaminants. Where the rigid surface 61 is a metal surface,
the hydrophilic surface 62 can be formed by exposing that
surface to an acid, preferably an acidic solution such as a
solution of 20% to 25~ (by volume) phosphoric acid. The
phosphoric acid acts as a "chemical sandpaper~' to render the
surface hydrophilic. The particular method to use in order to
render a rigid surface hydrophilic will depend on the
characteristics of the particular surface to be treated,
including its shape and the material it is made from, as will
be obvious to one of skill in the art. The methods discussed
above for preparing a "fixed surface" can also be employed to
produce a hydrophilic surface accordin~ to this e~bodiment of
the present invention.
By providing such a hydrophilic ~urface 62, a uniform,
- continuous film or layer 63 of a silicate-containing solution
can be applied to the rigid surface 61 to be treated. It is
believed, however, that methods of providing a uniform layer
of the silicate-containing solution on the surface being
treated other than applying the silicate solution to a
hydrophilic surface can a1soj be used;to perfor~m the present
method. For example, a gel or emulsion containing a silicate
material could instead be applied to a surface such that a
continuous, relatively uniform layer of the gel is spread over
the surface. Alternatively, the silicate solution can be
sprayed on the rigid surface. The surface would then be
treated as in the remaining steps of the present method.
Following the preparation of the hydrophilic surface 62
on the material to be treated, the surface 62 is preferably
W09411~89 PCT~S93/1~79 ~ , ~
2149919 :~'
-26-
rinsed in order to remove impurities and~or chemical cleaning
residues from the surface. Such rinsing can be accomplished
by applying water, preferably at a temperature of between
approximately 68~F and 140F, to the hydrophilic surface 62.
The surface 62 can then be dried.
A silicate-containing solution is next applied to the
cleaned, hydrophilic surface 62 so as to form a continuous,
thin layer 63 of the solution on the hydrophilic surface 62.
In a preferred embodiment, the silicate-containing solution is
a sodium silicate solution, comprising silicon dioxide,
silicic acid (H2Si205), sodium oxide, and water (available from
the PQ Corporation, Tacoma, WA as Liquid N or Liquid 0~.
However, silicate-containing solutions made up of other
silicate compounds can also be used. Solutions of potassi~um
silicate, metallo-silicates (including aluminum silicate,
magnesium silicate, iron silicate, copper silicate, zinc
siIicate, and manganese silicate), and organo-silicates can be
used,~where potassium, other metals, or organic compounds
~ replace the sodium in a sodium silicate solution. For
examp'e, KASIL #l, a potassium silicate solution available
from the PQ Corporation, can be used~in place of a sodium
~silicate solution. The silicate-containing solution is made
up of between approximately lO~ and lO0~ by volume of a
silicate compound, such as sodium silicate or potassium
~25 -~silicate. Preferably, the solution contains between 20~ and
40~ -(by volumej of the silicate compound. The solution is
also preferably applied when at a temperature of between
approximately 68F and 140F.
The hydrophilic surface 62 is exposed to the silicate-
containing 'solution for between approximately l and 60seconds, and preferably between l and 20 Qeconds. In one
embodiment, such exposure is accomplished by immersing the
surface of the material being treated. Such exposure of the
~ .
hydrophilic surface 62 to the silicate-containing material,35 however, should be minimized in order to provide the treated
urface with enhanced corrosion resistance.
~` '
''~
~ wog4/~e89 214 9 ~19 rCT~S93/1~79 ~ ~
-27-
Once a uniform, continuous film or layer 63 of the
silicate-containing solution is formed on the hydrophilic
surface 62, the solution is dried. In a preferred
embodiment, such drying can be accomplished by exposing the
surface of the treated material to an environment having an
initial temperature of between 68F and 4B0F, and preferably
having an initial temperature of between 120F and 150F. The
temperature of the environment surrounding the treated
hydrophilic surface is then raised by between approximately
30F per minute and 60F per minute until a final temperature
of between about 302F and 480F, and preferably between 305F
and 350F, is reached. In this way, the temperature of the
silicate-containing solution and the surface underlying it is
raised from a lower initial temperature to a higher
1~ temperature. In some cases, for example when a material of
low~thermal conducti~ity is used in the surface being treated,
the~;environement surrounding the surface càn be~at a higher
initiàl~temperature, since the surface~ will itself riae in
temperature~slow}y due to its low thermal conductivity. It is
lmportant,~ howe~er, that the silicate-containing
solution/dried silicate material reaeh a temperature of at
leas~t 302F during this drying step.
The drying of the layer of silicate-containing solution
63~ can~be accomplished, for~example, by placing the treated
~25 ~surface in a drying oven. O~her methods known to those of~ ~ skill in~the art are, of;course, a}so possible. For example,
infrared radiation from a heat lamp can also be used to dry
the layer of the silicate-containing solution 63 and raise it
to a temperature~ ~ovle 30l2F. In addition, agents can be
added to the silicate-containing solution to aid in the drying
and hardening of the layer 63. Zinc oxides, when present in
such a solution in an amount of`up to 7% (by volume) of the
solution,~ will assist in the hardening of the solution.
The drying ti~e for a film or layer 63 of the silicate-
~ containing solution on the hydrophilic surface 61 will dependin part on the thickness of the film or layer 63, the form or
shape of the surface and article being treated, and the
,:
,~ '
9 PCT~593/11279
-28- -
composition of the material being treated. For example, a
material with a high thermal conductivity, such as aluminum,
can be first exposed to a temperature at the lower end of the
above-specified range of drying temperatures, after which the
ambient temperature surrounding the aluminum surface can be
raised. On the other hand, the present method will work best
on a material with a lower thermal conductivity when that
material is first exposed to a higher temperature. In most
cases, an appropriate drying time will be between
approximately 2 and 50 minutes, and preferably between 6 and
15 minutes.
After the silicate-containing solution has dried on the
surface of the material being treated, as described above, the
dried silicate layer 65 is exposed to an acid, such as an
acidic solution, in order to form the protective layer
,
according to the present invention. ~An acidic solution used
in this~embodiment can comprise between l~ and 99% (by volume)
of~an~acid~,~ and preferably ~etween l0~ and 30~ (by volume) of
~ su-ch~an~acid. In a preferred embodiment, the acidic solution
- 20 ~ used~is~a~phosphoric acid solution, because phosphoric acid is
-~- cheap, relatively safe, and widely avai~able. However, other
~- ac~ids can~also be used. For example, gaseous carbon dioxide,
which is a weak acid, can be passed over or through the
surface~being treated. A~gaseous acid such as carbon dioxide
25~ ~ is~particularly useful when the surface being ~reated is
relatlvely porous, such as a wood surface.
Whén an acidic~solution is used, the acidic solution is
preferably at~a temperature of between approximately 68F and
180F when applied to the dried layer of silicate material 65.
Any method1of applying the acidic solution 'to thè dried
silicate layer 65 known to those of skill in the art can be
used~. For example, if the surface to which the silicate-
containing solution was applied encompasses the entire surface
of an article or even just the entire surface of one member of3-5 an~article, the article or member can be immersed or "dipped~
in the acidic solution. Alternatively, the acidic solution
;can be~applied to a surface that comprises only part of an
.~.. ,, :
;, ~
2 ~ 1 9
WO94112289 PCT~S93/1~7
-29-
article or a member of an article by spraying the acidic
solution onto the article, or by any other means. The acidic
solution is preferably exposed to the layer of silicate
material for between about 5 seconds and 120 seconds. More
preferably, the acidic solution is exposed to the layer for
between 20 seconds and 50 seconds.
During the application of the acidic solution, the
protective layer is formed on the surface being treated. The
protective layer comprises an outer layer 67 exposed to the
acid, made up of mostly silicon dioxide, and an inner layer
made up of the dried s~licate material 65. The dried silicate
material Ç5 comprises both silicon dioxide and other oxides.
For example, when the silicate-containing solution is sodium
silicate, the dried silicate material will be made up of
silicon dioxide and sodium oxide. By exposing the surface of
the silicate material 64 to an acid, the oxides and other
soluble contaminants are remo~ed from the surface of the outer
layer 67, leaving a non-porous outer layer 67 comprising
mainly silicon dioxide. It is this outer layer 67 of silicon
dioxi:de that is believed to impart to the protective layer the
increased resistance to corrosion, abra~ion, and other sources
of damage to a surface. By contrast, the inner layer 65 is
relatively more soluble water and other solvents.
Following the application of the acidic solution to the
-25 dried silicate material 65, the surface being treated is can
-~ be rinsed to remove excess acidic solution. Such rinsing can
be accomplished by applying water, preferably at a temperature
of between approximately 68F and 140F, to the treated
surface. W;ater can, be applied to the surfa,ce either by
spraying or by immersion in water or by any other means. The
surface is then allowed to dry.
If desired, the foregoing ~teps of applying a silicate-
containing solution, drying the solution on the surface of a
- material, and then exposing the dried silicate material to an
acid can be repeated a plurality of times. In this aspect of
the present embodiment, each protective layer should be rinsed
after the formation of the protective layer in order to remove
WO94/1Z~9 PCT~59311~79 ~ j ~
214~919
-30-
excess acid before next applying a new layer of the silicate-
containing solution. By repeating these steps, a plurality of
protective layers can be formed on a surface. By forming a
plurality of protective layers, the protective properties of
the protective layer can be enhanced. For example, the
corrosion resistance of a piece of metal on which the
protective layer of the present invention has been formed can
be increased by increasing the number of protective layers on
that piece of metal.
- EXAMPLE 9
A Method of Treatin~ an Aluminum Surface
Two samples of aluminum panels are treated - sample 1
comprises 2024 T-3 stock while sample 2 comprises 6061 T-6
stock. Both panels measure 3 inches (W) x 10 inches ~L) and
.039 inches thick. The surfaces of Samples 1 and 2 are
provlded with hydrophilic surfaces by wet sanding the surfaces
of the panels with an e~lectric hand sander for 5 minutes.
These hydrophilic surfaces are then rinsed with a detergent
and water. While wet, the samples are immersed in a 16% (by
volume) sodium silicate solution for 20 seconds at
~;~ approxi~ately room temperature in ordqr to coat the panels with a uniform layer of the sodium silicate solution. Samples
1 and 2 are then removed from the sodium silicate solution and
placed in~ an oven heated initially to about 130F. The
~; 25 temperature of the oven is then raised by about 40F per
minute until ~ temperature of 310F is reached. The coated
panels remain in the oven for about 12 minutes altogether.
After 12 minutes, samples 1 and 2 are removed from the oven
and allowed to cool to about l00F. Once cooled, samples 1
and 2 are immer~ed in a 25% (by volume) ortho-phosphoric acid
solution for 40 seconds. Following this, the surfaces of
samples 1 and 2 are rinsed to remove excess ortho-phosphoric
acid solution.
The surface of samples 1 and 2 appear hard, smooth, and
. .
glassy. The anti-corrosive properties of sample 1 and 2 are
tested using ASTM B-117 and Mil. Specifications, C-5541.
These samples are subjected to such tests for 168 and 336
~ WO94/1~89 21~ 9 ~ ~ 3 PCT~S93/1~79
1'
-31-
hours, and are found to exceed the standards specified by
these tests. The paint adhesion properties of samples 1 and
2 are also tested to Mil. Specifications, C-5541. Both
samples 1 and 2 pass, according to the specifications for each
test.
The heat and fire resistant properties of each sample are
tested by comparing the burning of an untreated piece of
similar aluminum with the treated samples. The treated and
untreated samples are exposed to an acetylene flame which
burns between 2,000 and 2,500 degrees Fahrenheit. The
untreated samples decompose to ash in approximately 30
seconds. The treated aluminum samples 1 and 2, on the other
hand, bend after a few seconds of exposure. After
approximately 2 minutes, the aluminum material begins slowly
running out from between the two sides of the coating Iayer.
EXAMPLE 10
A Method for Treatina a Steel Surface
Three samples of steel panels 1-3 made of 4130 steel are
tréated, all measuring 4 inches ~W) and 6 inches (L) and 0.041
. ~
inches thick. The surfaces of SampIes 1-3 are provided with
hydrophilic surfaces by wet sanding the~surfaces of the panels
with an~electric hand sander for 5 minutes each. These
hydrophilic surfaces are then rinsed with a detergent and
~; water. While wet, the samples are immersed in a 16~ (by
volume) sodium silicate solution for 20 seconds at
approximately room temperature in order to coat the panels
with~a uniform layer of the sodium silicate solution. Samples
3 were then placed in an oven at 310F for 12 minutes.
After 12 minutes, samples 1-3 are removed from the oven and
allowed to cool to àbout 100F. Once cooled, samples 1-3 are
immersed in a 25% (by volume) phosphoric acid solùtion for 40
~ seconds. Following this, the surfaces of sa~ples 1-3 are
- rinse~ to remove excess phosphoric acid solution.
-~ Samples 1-3 were tested in accordance with ASTM-B117 salt
~35 spray test and passed successfully the 1/3 hour, 1 hour, 2,
24, 48, and 96 hours tests, which indicates that the
J:
, , .
."
W094/12289 PCT~S93/11279 ~
21 ~9 9 19
32~
protective layer formed in the process acts as a corrosion ¦ :
inhibitor under standard testing conditions.
Although the present invention has been described in
terms of certain preferred embodiments, other embodiments and t
alternatives known to those of skill in the art are also
hereby included. Therefore, the embodiments described herein ~:-
are merely examples of the present invention and are not meant :~
to limit its scope.
.
.
: ~ _ .
.
'~
', ``
:~