Note: Descriptions are shown in the official language in which they were submitted.
DENTIF~ICE COMPOSITION CONTAINING
STABI~IZED SODIUM PE~CARBONATE
BACKGROUND OF THE INVENTION
Periodontal disease affects a majority of
the world's population. The basic cause of the
disease is microbial in nature.
It is well-established that hydrogen
peroxide and other peroxygen-containing agents are
effective in curative and prophylactic treatments
10- with respect to dental plaque, calculus, gingivitis,
mouth odor, tooth stains, mucosal infections, and
the like.
Most peroxy compounds such as hydrogen
peroxide in oral care products tend to be unstable
in storage, mainly due to an inherent chemical
instability, and also to interaction with other
ingredients in the composition. The peroxy compounds
decompose within a relat~vely short period, with a
concomitant premature release of active oxygen.
65284-77
2 ~
Peroxy compounds are difficult to formulate into
toothpastes or gels wh;ch have an acceptable shelf-
life and are capable of liberating active oxygen
when applied to an oral cavity.
Many oral care products have been
formulated which include a peroxy compound, and more
recently oral care products have been developed
which include a peroxy compound having improved
stability. United States patents which describe
peroxy-containing toothpastes, mouthwashes, tablets,
chewing gums, and other forms of oral care products
include 2,275,979; 3,577,521; 3,657,413; 3,885,028;
3,886,265; 4,226,851; 4,302,441; 4,405,599;
4,426,108; 4,431,631; 4,521,403; 4,522,805;
4,528,180; 4,567,036; 4,592,487; 4,592,488;
4,592,489; 4,687,663; 4,812,308; 4,837,008;
4,839,157; 4,849,213; 4,867,988; 4,891,211;
4,897,z58; 4,92S,655; 4,971,782; 4,980,152;
4,988,450; 5,000,941; 5,041,280; 5,085,853;
5,256,402; and the like.
Both organic and inorganic peroxy
compounds have been proposed for use in oral care
products, and typically the peroxy compounds exhibit
one or more disadvantages which limit their
effectiveness in oral hygiene applications.
i~ A 65284-77
'~.
''- 2150~'~
~, .~
.,"
Sodium perborate and potassium chlorate do
not release significant levels of hydrogen peroxide
in water. Sodium perborate also is of questionable
safety because it contains boron which can undergo
systemic absorption.
Sodium percarbonate has a high active
oxygen content (15.28% theoretical) and high water
solubility. It is produced from low cost starting
materials, and it is an environmentally safe
chemical. Sodium percarbonate is potentially a
superior reagent as a hydrogen peroxide-releasing
ingredient in oral care products, except that it is
less stable than sodium perborate.
Stabilizers such as magnesium sulfate are
suitable for stabilizing sodium perborate, but
provide only limited protection with sodium
percarbonate. Various methods for stabilization of
sodium percarbonate have been proposed.
U.S. 2,380,620 discloses that sodium
silicate, magnesium sulphate or gum arabic are
unsatisfactory stabilizers when incorporated in
sodium percarbonate, but diphenylguanidine lessens
the decomposition in the presence of the
conventional stabilizers.
U.S. 3,951,838 discloses that prior
attempts at chemical stabilization of sodium
percarbonate, primarily by magnesium silicate, are
:'
. .
_ -i 21S0~26
generally ineffective in promoting long term
stability, particularly in a humid atmosphere. The
patent proposes coating of the particles with an
aqueous silica sol and drying to accomplish
stabilization.
U.S. 4,075,116 describes cocrystallizing
of sodium percarbonate with other salts known to
form perhydrates such as sodium sulfate, sodium
pyrophosphate, sodium glucoheptonate, sodium
metaborate, and the like.
U.S. 4,171,280 discloses that a non-caking
bleach composition may be formed containing up to 6%
active oxygen by spraying only sufficient hydrogen
peroxide onto sodium carbonate particles to convert
a part of the sodium carbonate to sodium
percarbonate. U.S. 4,260,408 teaches the addition
of sodium phosphate to the composition as a
stabilizer. Both patents demonstrate that an assay
of less than 6% active oxygen (less than 40% sodium
percarbonate) is necessary to obtain satisfactory
stability.
There is continuing research and
development effort to produce sodium percarbonate in
a form which exhibits long term stability under
storage conditions, and when incorporated as a
peroxygen ingredient in commercial products.
.
'i
:
2150426-
,.,~.
,~_
Accordingly, it is an object of this
invention to provide sodium percarbonate in a form
which is stable under ambient temperature and
moisture conditions.
It is a further object of this invention
to provide a dentifrice composition which contains
stabilized sodium percarbonate, and which releases
active oxygen under oral hygiene utilization
conditions.
Other objects and advantages of the
present invention shall become apparent from the
accompanying description and examples.
,
~ - .
. .
~,
'
'~
, .
~ 2150~26
DESCRIPTION OF THE INVENTION
One or more objects of the present
invention are accomplished by the provision of a
dentifrice composition comprising (1) between about
20-50 weight percent of polyalkylene glycol;
(2) between about 1-15 weight percent of sodium
percarbonate; (3) between about 30-60 weight percent
of sodium bicarbonate; (4) between about
0.1-5 weight percent of colloidal silica;
(5) between about 0.05-0.5 weight percent of
fluoridating ingredient; (6) between about
0.2-2 weight percent of alkali metal C10-C18 alkyl
sulfate anionic surfactant; (7) between about
0.2-2 weight percent of alkali metal C10-C18
acylsarcosinate anionic surfactant;
(8) between about 1-8 weight percent of nonionic
surfactant; and (9) between about 0.2-1 part by
weight of water per part of sodium percarbonate.
An important advantage of a present
invention dentifrice composition is the long term
stability of the sodium percarbonate ingredient,
which releases hydrogen peroxide under dentifrice
utilization conditions. The dentifrice composition
in the form of a toothpaste formation provides
active oxygen in the oral cavity during dental
brushing. The sodium percarbonate content of an
invention toothpaste formulation does not degrade
and generate gas during storage in a capped
toothpaste tube over a period of six months under
ambient conditions.
~ 2150426
::
The polyalkylene glycol ingredient of an
invention dentifrice composition preferably is
selected from oxyalkylated diols which have a
molecular weight in the range between about
400-12,000. Polyethylene glycols are commercially
available under tradenames such as Carbowax 200,
300, 400, 600, 900, 1000, 20000, 4000, 6000 and 8000
(Union Carbide), in which the number values are
approximations of average molecular weight.
Polyethylene-propylene glycols are commercially
available under tradenames such as
Pluracare/Pluronic L-31 and L-35 (BASF).
The polyalkylene glycol ingredient serves
as a hydrophilic vehicle for the other dentifrice
ingredients. It enhances the compatibility of the
ingredients when they are incorporated as
constituents in a dentifrice composition.
The sodium percarbonate ingredient of an
invention dentifrice composition is employed in the
form of a crystalline powder, which typically has an
average particle size between about 1-100 microns,
and preferably the particle size is in the range of
5-40 microns. Methods of manufacturing sodium
percarbonate are described in technical publications
such as U.S. 4,966,76Z and references cited therein.
An essential aspect of a present invention
dentifrice composition is the inclusion of a
controlled quantity of water content. As
;~,
21~0~26
. ,_
demonstrated in Example I, the sodium percarbonate
ingredient in a dentifrice formulation is stabilized
against degradation by the inclusion of between
about 0.2-1 part by weight of water per part of
sodium percarbonate.
The sodium bicarbonate ingredient of an
invention dentifrice composition functions as a soft
abrasive, and additionally it imparts a clean and
fresh feel in the oral cavity when an invention
toothpaste formulation is utilized. The sodium
bicarbonate preferably has an average particle size
between about 5-200 microns.
The colloidal silica ingredient of an
invention dentifrice composition can be selected
from amorphous silica compounds which function as a
thickening agent relative to the polyalkylene glycol
ingredient. Commercial colloidal silica compounds
are available under tradenames such as Sylodent 15
and Sylodent 2 (W.R. Grace), Aerosil 200 (Degussa)
and Cabosil fumed silica (Cabot). The colloidal
silica ingredient is compatible with the
polyalkylene glycol ingredient.
Aerosil 200 is a preferred type of
hydrophilic fumed silica having a surface area of
about 200 M2/g, and an average particle size between
about 10-12 nanometers. Aerosil R972 is a
hydrophobic fumed silica having a surface area of
about 100 M2/g, and an average particle size of about
15 nanometers.
2150~26
, .. ~,.
.
The alkali metal C10-Cl8 alkyl sulfate
anionic surfactant ingredient of an invention
dentifrice composition is illustrated by surface-
active compounds such as sodium and potassium salts
of sulfate esters of n-decanol, n-tridecanol, lauryl
alcohol, myristyl alcohol, cetyl alcohol, and
stearyl alcohol.
The alkali metal C10-C18 acylsarcosinate
anionic surfactant ingredient is illustrated by
surface-active compounds such as sodium and
potassium salts of sarcosine aminoacid, which are
N-substituted with acyl groups derived from fatty
acids which include capric acid, lauric acid,
myristic acid, palmitic acid, stearic acid,
oleic acid, and the like.
The nonionic surfactant ingredient of an
invention dentifrice composition is selected from
surface-active organic polymers which include
polyethylene-polypropylene block polymers
(Pluronics); C6-C18 alcohols with 1-15 moles of
ethylene oxide per mole of alcohol; C6-C18 alcohols
with 1-10 moles of propylene oxide per mole of
alcohol; C6-C18 alcohols with 1-15 moles of ethylene
oxide and 1-10 moles of propylene oxide per mole of
alcohol; C6-Cl8 alkylphenols with 1-15 moles of
ethylene oxide and/or propylene oxide;
polyoxyethylene monoester of sorbitol with a C10-C18
fatty acid, such as Polysorbate 20 [polyoxyethylene
(20)sorbitan monolaurate]; and the like.
, '
' '- 2150426
~, v
The nonionic surfactant provides
solubilizing, dispersing and emulsifying activities
relative to the other dentifrice composition
ingredients. The two anionic surfactant ingredients
contribute additional surface-active functions, and
the combination of three surfactant ingredients also
provides an effective foaming action during dental
hygiene brushing with an invention toothpaste
formulation.
The fluoridating ingredient of an
invention dentifrice is selected from fluoride-
providing salts which have anti-caries efficacy.
Suitable salts include sodium fluoride; potassium
fluoride; cuprous fluoride; stannous fluoride;
stannous chlorofluoride; sodium fluorosilicate;
ammonium fluorosilicate; sodium monofluorophosphate;
and the like.
The anticalculus e~ficacy of an invention
dentifrice composition can be increased by the
addition of between about 0.5-10 weight percent of
an alkali metal pyrophosphate ingredient. Suitable
alkali metal pyrophosphates include dialkali metal
and tetraalkali metal pyrophosphate and mixtures
thereof in a hydrated or unhydrated form.
Illustrative of pyrophosphate salts are Na2H2P207,
Na4P207 and K4PzO~-
~t
,. .
~' 2150~23~
;....... '~
.
11
A toothpaste formulation can be preparedconveniently by blending each of the ingredients
into the polyalkylene glycol ingredient, which
normally is a viscous liquid at room temperature.
Conventional adjuvants can be included.
Suitable adjuvants include whitening
agents such as titanium dioxide; preservatives;
silicones; chlorophyll compounds; antibacterial
agents such as cetyl pyridinium chloride; flavorants
such as oils of spearmint and peppermint; sweetening
agents such as sucrose, saccharin, aspartame and
sodium cyclamate; colorants such as FDC Red 40,
FDC Green 3, DC Red 19 and D&C Green 5; humectants
such as glycerin; gelling agents such as sodium
carboxymethylcellulose; abrasives such as alpha-
alumina, particulate polyvinyl chloride, and calcium
phosphate; and the like.
A flavorant is employed in a quantity
between about 0.2-2 weight percent. A sweetener
ingredient is employed in a quantity between about
0.1-5 weight percent, and a colorant additive
quantity is between about 0.005-0.3 weight percent.
By the practice of the present invention,
an oral care product can be prepared which has
superior properties for retarding plaque and
calculus formulation, and controlling bad breath and
gingivitis. Important advantages derive from the
.,,'1. '
:
- 2150~2G
presence of stabilized sodium percarbonate as a
component of an oral care product, when the oral
care product is applied to an oral cavity following
a prescribed hygiene regimen.
As an alternative ingredient to sodium
percarbonate, the present invention also
contemplates the use of other stabilized inorganic
peroxyhydrate compounds which yield hydrogen
peroxide when dissolved in an aqueous medium, such
as sodium pyrophosphate peroxyhydrate.
The following examples are further
illustrative of the present invention. The
components and specific ingredients are presented as
being typical, and various modifications can be
derived in view of the foregoing disclosure within
the scope of the invention.
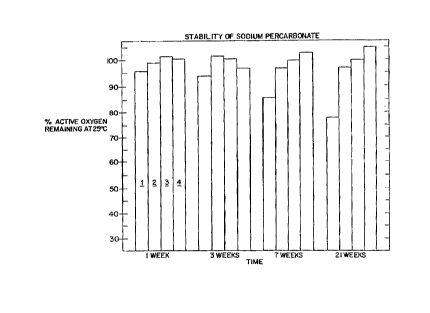
Figures 1-3 are bar graphs which are a
representation of the comparative stability data
corresponding to the 14 formulations as prepared and
tested in Example I.
For test purposes in Example I, the level
of active oxygen content retained in each
formulation under simulated storage conditions is
determined as follows:
,~j
.
!
''~ 21S042G
.,, ,.. ~
13
A 1-2 gram sample of a formulation is
weighted accurately, and transferred into a 250 ml
Erlenmeyer flask. A 75-100 ml aliquot of 3 M
sulfuric acid is added dropwise while the flask
contents are swirled qently. The acidified aqueous
medium then is titrated with 0.1 N KMnO4 solution
until a permanent pink color is evident.
active oxygen = V x N x 0.8
Sample weight (grams)
where V is ml of KMnO4 solution consumed; and N is
normality of KMnO~ solution.
~ 2150426
'~_
14
EXAMPLE I
This Example illustrates the stability of
hydrogen peroxide-releasing formulations in
accordance with the present invention.
A series of gel formulations are prepared
by blending the ingredients listed in the Table.
The effect of water content on the
stability of sodium percarbonate is tested for the
14 formulations in the Table. Figs. 1-3 are bar
graphs which summarize the comparative stability
data at temperatures of 25~C and 37.5~C over
extended periods of time.
The comparative data demonstrate that the
highest sodium percarbonate stability is exhibited
by gel formulations which have a water content
between about 0.2-1 part by weight per part of
sodium percarbonate.
',
;'''
21~0~26
. '.~,,,_ 15
. ~ U)
O ~O ~1 0
...
~ O
.
U~
CO ~o ~,, o
In
, ,, o ~~ ~ o
"
. .
O N ~~ O
," ,1 ~
~ o~D ~ O
a u~
U~~O~, O
Ul
~ ~ ~
~ .
O
I p
O
m ~ o~D ~1 0
~: -
a ~ 0
~. ~ ~ ~ _I o
~ r7 ~~o ~ O
. . --
~1 ~ O~D ~1 0
. ~
~ ~,
. '
o
o
...
... .
~ ~ X s~
o ~ r s
U
~n
o e
o
V ~
a
~ o
~ , e ~~
1l ) C
C ~ o
o ~ o ~v ~)
H P~ ~:UJ .¢ 4
2150~6
..,~
,_
EXAMPLE II
This Example illustrates the preparation
of a stable dentifrice composition in accordance
with the present invention.
A pre-blend is prepared with the following
ingredients:
Parts By Weight
Polyethylene glycol (M.W.400) 34.45
Polyethylene glycol (M.W. 8000)0 . 9
10 Sodium percarbonate 6.0
Aerosil 200 (Degussa) 1.0
Tetrasodium pyrophosphate 2.0
Water 2.0
The pre-blend is admixed with additional
inqredients to form a composition with a toothpaste
consistency:
Weiqht Percent
Polyethylene glycol (M.W. 400)34.45
Polyethylene glycol (M.W. 8000)o.g
20 Sodium percarbonate 6.0
. Aerosil 200 (Degussa) 1.0
Tetrasodium pyrophosphate 2.0
Water 2.0
Sodium bicarbonate 51.0
25 Flavor 0.75
Saccharin 0.9
Sodium lauryl sulfate 1.0
'~ 2i50426
r
17
The invention toothpaste is more stable
than a control toothpaste which has a zero weight
percent water content, when tested at 37.5~C for
21 days. The invention toothpaste has an active
oxygen loss of about 4%, and the control toothpaste
has an active oxygen loss in the range of about
10-14 percent.
It is advantageous to minimize the
presence of any polyvalent metal ions such as
copper, iron, manganese, nickel, and the like, in
the formulations, since this type of impurity tends
to catalyze the decomposition of peroxygen
compounds.
.~
'''
~ .
..
:-;
''- 2I~426
.. .
18
EXAMPLE III
This Example illustrates the preparation
of a stable toothpaste formulation in accordance
with the present invention.
A formulation with a toothpaste
consistency is prepared by blending the following
ingredients:
;' Parts By Weiqht
PEG-8(1)34.50
10 Sodium fluoride 0.25
Sodium saccharin 1.10
Sodium lauryl sulfate 0.50
Sodium lauroyl sarcosinate 0.50
Flavorant 279194~2) 1.20
Sodium bicarbonate(3) 50.32
Pluronic F-127 (BASF) (4) 5.00
Aerosil 200 (Degussa) 2.50
Sodium percarbonate~5) 3.00
Water 1.20
Polyethylene glycol (M.W. 400).
Haarmann & Reimer.
(3) Grade 3; Church & Dwight Co.
(4) Polyoxyethylene-polyoxypropylene block polymer.
(5) 5-40 Micron particle size range.
.
'-~ 2150g26'
The final homogeneous formulation is
deaerated and filled into standard capped toothpaste
tubes. After storage at 25~C for 21 weeks, the
capped tubes do not show any evidence of tube
distortion from qas generation within the dentifrice
contents.
:
,, ,~ , .
. . .
' .