Note: Descriptions are shown in the official language in which they were submitted.
A- 19969/A
215064~
Butyne-linked or hexadiyne-linked hindered amines as stabilisers
The present invention relates to novel compounds in which two sterically hindered amine
units are terminally linked through the nitrogen atoms to a 2-butyne or 2,4-hexadiyne unit,
to the use thereof for stabilising organic material against oxidative, thermal or actinic
degradation, to corresponding compositions, to a process for stabilising said material, and
to a process for the preparation of the novel compounds.
A number of hindered amines of the 2,2,6,6-tetramethylpiperidine type are already known
as additives for synthetic polymers, in particular as light stabilisers, including also
derivatives of the l-propargyl-2,2,6,6-tetramethylpiperidine which are substituted in
4-position. The following publications are cited by way of exemplification:
US-A-4 472 547; US-A-4 268 593; US-A-4 344 877. US-A-4 386 127 also discloses
1 -butinyl-2,2,6,6-tetramethyl-4-hydroxypiperidine.
It is also know to use some compounds of the 2,6-diarylpiperidin- l-yl-substituted 2-butene
type as stabilisers (US-A-5 204 474).
A number of scientific publications describe l-butinyl-2,2,6,6-tetramethylpiperidines in
which the piperidine ring is unsubstituted in 4-position, as well as the potential use thereof
as medicament, for example M. E. Zuhair et al., Eur. J. Med. Chem. 27, 93 (1992); J.M.A.
Al-Rawi et al. in Org. Magn. Reson. 19, 91 (1982) and Org. Magn. Reson. 15, 285 (1981);
B. Karlen et al., J. Med. Chem. 13, 651 (1970).
US-A-3 755 586, W. B. Lutz et al., J. Org. Chem. 27, 1695 (1962), and US-A-3 085 093
disclose 1,6-bis(2,2,6,6-tetramethylpiperidin-1-yl)hexadiyne in which the piperidine rings
are unsubstituted in 4-position, as a pharmaceutically active compound.
It has now been found that specific compounds of the 1,4-diamino-2-butyne or
1,6-diamino-2,4-hexadiyne type are surprisingly suitable for use as stabilisers for organic
material.
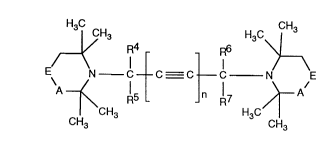
Accordingly, in one of its aspects the invention relates to novel compounds of formula I
215064~
H3C CH3
~CH3 H3C>L,
E~ N--C--C C--C N E (I)
CH3 CH3
wherein n is 1 or 2, A is -CH2- or -CO-;
if A is methylene, E is R1,C~R2 and,
if A is carbonyl, E is, N--R3
Rl is hydrogen;
R2 is -OH; -o-R8; -S-R8; -OCO-R8; -NHCO-R8 or -N(Rl3)Rl4; or
Rl and R2, taken together, are a substituent =O or, together with the linking carbon atom,
O R15
form a five- or six-membered ring of one of the formulae /C\ ~C~ 6 ~
R19 R17 R15 IR17
~C~ ~C ~\C/ \C=O' ~C~ ~R18~ ~C l ~C/ --C_O
H2) k (CH2) k O O
wherein k is 2 or 3;
R3 is C1-CI8alkyl; C3-Cl8alkenyl; C5-C8cycloalkyl; phenyl; naphthyl; C7-C9phenylalkyl;
or Cll-Cl4naphthylalkyl;
R4, R5, R6 and R7 are each independently of one another hydrogen or methyl;
R8 is C1-Cs0aLkyl, C2-Cl8aLkenyl or glycidyl; or C2-C50alkyl which is interrupted by -O-,
-S-, -NR19- and/or Cs-C8cycloalkylene; or R8 is C5-CI2cycloalkyl which is unsubstituted
or substituted by 1 to 4 substituents -R12; C6-C10aryl which is unsubstituted or substituted
by 1 to 4 substituents -Rl2 or -ORl2; or C7-Cs0aralkyl which is unsubstituted or substituted
by Cs-C8cycloalkyl and/or interrupted in the aliphatic moiety by C5-C8cycloalkylene
and/or by oxygen or sulfur, and/or in the aromatic moiety by 1 to 4 substituents -R12 or
215064~
_oR12;
Rl2 is Cl-Cl8alkyl; C2-Cl8alkenyl; Cs-C8cycloaLIcyl; phenyl or benzyl;
Rl3 and Rl4, each independently of the other, have one of the meanings given for R8; or
Rl3 and Rl4, together with the linking nitrogen atom, are cyclic imide of for[nula
o
N/ \Rl 8
\C/
o
whose ring structure contains 4 to 6 carbon atoms;
Rls and Rl6 are each independently of the other H; or Cl-Cl2aLkyl or, taken together, are
straight-chain oc,~linked C4-Cl3alkylene;
Rl7 is H or has one of the meanings of R3;
Rl8 is C2-Cl8alkylene or C2-C6alkenylene; and
Rl9 has one of the meanings of R3.
The inventive compounds may be used with advantage for stabilising organic material
against the harmful action of light, oxygen and/or heat. Furthermore, compounds of
formula I, wherein A is methylene and E is -CH(OH)-, are also useful starting materials
for the preparation of further stabilisers and are, accordingly, also a preferred object of the
invention.
A is preferably methylene and E is preferably R1~C~
Compounds of formula I, wherein n = l, are particularly preferred.
If several identically named radicals occur within a formula, then these radicals may be
identical or different and have each independently of one another one of the given
R7
meanings. Rl and R2, for example, embrace in their shared meaning ~C' C ~17 as
215064~
H C12H25 H
wellas~C~c N-Cl2H25 andalso~C~c N-C12H25 and~ `C N-H-
O O O
Preferred compounds are those of formula I, wherein R4 and R6 are hydrogen. All four
substit~lent.~ R4, R5, R6 and Ri are most preferably hydrogen.
R8, Rl3 or Rl4 defined as aryl are an aromatic hydrocarbon radical such as phenyl or
naphthyl. Aralkyl is aL~yl which is substituted by an aromatic hydrocarbon radical e.g. a
C6-ClOhydrocarbon radical. Illustrative examples thereof are, inter alia, benzyl or
a-methylbenzyl. The given carbon numbers (indices) indicate the total number of carbon
atoms in the cited radical.
R8, Rl3 and Rl4 typically have the following me~ning.c-
methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl, isobutyl, t-butyl, 2-ethylbutyl,
n-pentyl, isopentyl, 1-methylpentyl, 1,3-dimethylbutyl, n-hexyl, 1-methylhexyl, n-heptyl,
isoheptyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, 3-methylheptyl, n-octyl, 2-ethylhexyl,
1,1,3-trimethylhexyl, 1,1,3,3-tetramethylpentyl, nonyl, decyl, undecyl, 1-methylundecyl,
dodecyl, 1,1,3,3,5,5-hexamethylhexyl, tridecyl, tetradecyl, pentadecyl, hexadecyl,
heptadecyl, octadecyl, eicosyl, docosyl, pentacosyl, triacosyl, tetracontyl;
-C2H4-O-C2H4-O-Cl2H2s.-(C2H4-0)4-C4Hg,-(C2H4-)6-c4H9~-(C2H4-0)4-C2Hs.
-(C2H4-0)6-C2Hs;
cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, 2- or 4-methylcyclohexyl, dimethyl-
cyclohexyl, trimethylcyclohexyl, t-butylcyclohexyl;
C6-ClOaryl, such as phenyl or naphthyl; Cl-C4aL~yl-substituted phenyl;
arylaL~yl or substituted arylalkyl, such as benzyl, phenethyl, 3-phenylpropyl,
a-methylbenzyl, a,~-dimethylbenzyl. -C2H4-O-C2H4-O-Cl2H24-C6Hs.
-(C2H4-0)4-CH2-C6Hs, -(C2H4-0)6-C4H8-C6Hs~ -CH2-C6H4-C(CH3)2-C6Hs.
R3, Rl7 and Rl9 are preferably Cl-Cl8aL~yl; C3-CsaL~enyl; cyclohexyl; benzyl anda-methylbenzyl. A further preferred meaning of Rl7 is hydrogen.
If the radicals R8, Rl3 or Rl4 contain aL~yl which is interrupted by -O-, -S- or -NRl9-, then
said aL~yl contains at least 2, preferably at least 4, carbon atoms and is preferably
interrupted by 1 to 6 groups -O-, -S- or -NR19-, more particularly by 1 to 6 groups -O-.
21~06A4
The hetero atoms are preferably attached to carbon atoms and not to other hetero atoms;
structures of the -O-O- or -NRl9-NRl9- type do not occur. Said radicals are mostpreferably polyoxyethylene chains whose ends are saturated by Cl-C8aL~yl.
R8, Rl3 and Rl4 are particularly preferably long-chain and/or buL~y radicals, typically
C6-C36aL~yl; C6-C36aL~cyl which is interrupted by -O-; Cs-CgcycloaLkyl; phenyl;
C7-C36phenylaL~yl; or C7-C36phenylaLkyl which is in~llu~led in the aliphatic moiety by
-O-. C6-Cl8AL~yl or C7-C9phenylaLkyl is particularly preferred.
R3, R8, Rl2, Rl3, Rl4, Rl7 and Rl9 defined as aL~enyl are, within the scope of their given
meanings, typically ethenyl, 1- or 2-propenyl, butenyl, pentenyl, hexenyl, heptenyl,
octenyl, nonenyl, decenyl, undecenyl, dodecenyl, tridecenyl, tetradecenyl, pentadecenyl,
hexadecenyl, heptadecenyl or oct~lecenyl. R3, Rl7 and Rl9 defined as aL~enyl arepreferably allyl; and R8, Rl2, Rl3 and Rl4 defined as aL~enyl are preferably vinyl,
a-methylvinyl or allyl.
R3, Rl2, Rl7 and Rl9 defined as aL~yl are typically methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, tert-butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl,
tetradecyl, pentadecyl, hexadecyl, heptadecyl or octadecyl; and defined as cycloaL~yl are
typically cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, preferably cyclohexyl.
Halogen denotes an atom of the series F, Cl, Br, I and is usually Cl or Br, moreparticularly Cl.
Among the compounds of formula I, those compounds are of particular interest wherein A
is methylene, E is R1,C~R2, and
R2 is -OH; -o-R8; -OCO-R8; -NHCO-R~ or -N(Rl3~Rl4; or
Rl and R2, taken together, are a substituent =O or, together with the linking carbon atom,
O R15
are a five- or six-membered ring of one of the formulae /C\ ~C\
215064~
R19 R17 R15 R~17
N R15 ~ N \C~\R18 ~ --C--R16 ~ ~N--C = O
~C~ ~C~ ~C~ ~C--O / \o/ / \C_N--R ~ ~_N--R17
wherein k is 2 or 3;
R4, R5, R6 and R7 are hydrogen,
R8 is Cl-CsOaL~yl, CrCl8alkenyl or glyeidyl; or CrCsOalkyl whieh is interrupted by -O-,
-S-, -NRl9- and/or C5-C8eyeloalkylene; or R8 is Cs-Cl2cycloalkyl which is unsubstituted
or substituted by 1 to 4 substituents -Rl2; phenyl which is unsubstituted or substituted by 1
to 4 substituents -Rl2 or -ORl2; or C7-Cl8aralkyl whieh is unsubstituted or substituted in
the aromatie moiety by 1 to 4 substituents -Rl2 or -ORl2;
Rl2 is Cl-Cl8alkyl; C2-Cl8alkenyl; Cs-C8eyeloalkyl; phenyl or benzyl;
Rl3 and Rl4, eaeh independently of the other, have one of the meanings given for R8; or
Rl3 and Rl4, together with the linking nitrogen atom, are cyclic imide of formula
1C
N/ \R18
o
whose ring structure eontains 4 to 6 earbon atoms; and
Rl8 is C2-Cl8aLkylene or C2-C6aLkenylene.
Among these compounds, those compounds of formula I are preferred wherein A is
methylene, E is R1,C~R2, and
R2 is -OH; -o-R8; -OCO-R8; -NHCO-R8 or -N(Rl3)Rl4; or
Rl and R2, together with the linking carbon atom, are a five- or six-membered ring of
215064~
R17
formula /C\ /R or ~C 1 17;
o
R4, Rs, R6 and R7 are hydrogen,
R8 is Cl-C36alkyl, C2-C8alkenyl or glycidyl; or C2-C36alkyl which is inle~ ed by -O-,
-NRl9- and/or Cs-C8cycloalkylene; or R8 is Cs-Cl2cycloalkyl; phenyl which is
unsubstituted or substituted by Rl2 or -ORl2; or C7-Cgaralkyl which is unsubstituted or
substituted in the aromatic moiety by -Rl2;
Rl2 is Cl-C8alkyl; C2-C8alkenyl; cyclohexyl; phenyl or benzyl;
Rl3 and Rl4, each independently of the other, have one of the meanings given for R8; or
Rl3 and Rl4, together with the linking nitrogen atom, are cyclic imide of formula
C
\ /
C
whose ring structure contains 4 to 6 carbon atoms; and
Rl8 is C2-C3alkylene or C2-C3alkenylene;
more particularly those compounds, wherein A is methylene, E is F~,C~R2, and
R2 is -OH; -o-R8; -OCO-R8; -NHCO-R8 or -N(Rl3)Rl4; or
Rl and R2, together with the linking carbon atom, are a five- or six-membered ring of
R17
formula /C\ /R18 or \C I 17;
R4, Rs, R6 and R7 are hydrogen,
215064Q
R8 is Cl-ClgaL~yl, C2-C8alkenyl or glycidyl; or R8 is Cs-Cl2cycloaL~yl; phenyl which is
unsubstituted or substituted by -Rl2; or C7-CgaraLkyl;
Rl2 is Cl-C8aLkyl; vinyl; a-methylvinyl or allyl;
Rl3 and Rl4, each independently of the other, have one of the me~nin~ given for R8.
Of particular interest are those compounds of formula I, wherein n is l or 2, A is
methylene, E is F~C~ and
R2 is -OH; Cl-C~8aL~oxy; allyloxy; C7-Cgaralkoxy; glycidyloxy; C2-Cl8aL~anoyloxy;
C3-C4aL~enoyloxy; benzoyloxy; formamidyl; C2-Cl8alkanoylamido; benzoylamido; or
-N(Rl3)Rl4; or
Rl and R2, together with the linking carbon atom, are a five- or six-membered ring of
R17
formula /C\ /R18 or ~C 1 17;
lco
R4, R5, R6 and R7 are hydrogen;
Rl3 and Rl4 are each independently of the other Cl-Cl8aL~yl; and Rl8 is C2-C3aL~ylene or
C2aL~enylene;
more particularly those compounds, wherein A is methylene, E is R~,C~R2, and
R2 is -OH; C6-Cl8aLkoxy; allyloxy; benzyloxy; glycidyloxy; C2-Cl8aLkanoyloxy; orC3-C4aLkenoyloxy; or
Rl and R2, together with the linking carbon atom, are a five-membered ring of formula
R117
Xo--CH2 ~ \C--N--R17
R4, R5, R6 and R7 are hydrogen.
Of particular importance among these compounds is a compound of formula I, wherein A
is methylene and E is cH, typically the compound
215~64~
H3C CH H3C 3
HO ~N--CH2--C--C--CH2--N~ OH .
~~ CH3 H3C ~~
H3C CH3
The novel compounds of formula I can be obtained in general accordance with the known
processes described, inter alia, in US-A-3 085 093; J. Org. Chem. 27, 1695 (1962); Eur. J.
Med. Chem. 27, 93 (1992); US-A-4 386 127; or J. Med. Chem. 13, 651 (1970); and in the
literature cited therein. Accordingly, 2,2,6,6-tetramethylpiperidine having free l-position
can be reacted with butinyl halides or butinyl dih~ es, or first with plopargyl halide. The
halides used are often chlorides and, preferably, bromides. l-Propargyl-2,2,6,6-tetra-
methylpiperidines obtained may be reacted with formaldehyde and further 2,2,6,6-tetra-
methylpiperidine to the disubstituted butynes, or may be dimerised with a catalyst to the
corresponding hexadiyne.
However, the compounds of formula I are preferably prepared by the following novel
process.
In this process, compounds of formula I are obtained by reacting an ester of an aromatic
sulfonic acid of formula II
O R4 F~6 o
X3 5 --C--C--C C--o S )~3, (II),
O R5 n R7 O
wherein X3 and X3 are each independently of the other phenyl or phenyl which is
substituted by Cl-C8alkyl, Cl-C8aLkoxy or halogen,
with a compound of formula III
H3C>~
HN E (III).
H3CCH
This process also constitutes an object of the invention.
215064~
- 10-
X3 and X3 are preferably phenyl, tolyl, chlorophenyl or methoxyphenyl; more particularly
phenyl or tolyl. X3 and X3 are preferably identical. The educt of formula II in the novel
process is particularly preferably 2-butyne-1,4-diol-ditosylate or 2,4-hexadiyne-1,6-diol-
ditosylate, more particularly 2-butyne-1,4-diol-ditosylate.
Usually about 2 equivalents of the compound of formula III are used per equivalent of the
compound of formula II. It is also advantageous to use the compound of formula III in
excess, typically in an amount of 1.5-4, preferably of 2-3, equivalents of the compound of
formula III per sulfonyloxy group in the compound of formula II. In this case it is possible
to separate the non-reacted educt from the product and to use it again in the reaction.
The reaction is conveniently carried out in an inert solvent. The solvents used are usually
polar organic solvents, such as alcohols, halogenated hydrocarbons, esters, ethers, ketones,
amides, nitriles, tert-amines or sulfoxides. Suitable solvents are, for example, methyl ethyl
ketone, tert-butanol, dimethyl form~mide, tetrahydrofuran, dioxane, chloroform, pyridine,
dibutyl ether, dimethyl sulfoxide, acetonitrile. Acetonitrile is particularly preferred.
During the reaction, the temperature may be in the range from 0 to 200C, conveniently
from 20-160C and, preferably, from 40-140C.
The temperature of the reaction mixture can be kept for the duration of the reaction in the
boiling range (reflux). The procedure consists in heating a reaction mixture containing a
solvent to boiling point, usually under normal pressure, then condensing the evaporated
solvent with a suitable condenser and recycling it to the reaction mixture.
The reaction may be carried out under exclusion of oxygen, typically by flushing with an
inert gas such as argon. However, oxygen does not interfere in every case and the reaction
may accordingly also be carried out without taking the above measure.
When the reaction is complete, working up can be carried out by standard methods. It is
convenient to first dilute the mixture with water, typically by adding the reaction mixture
to the 1- to 4-fold volume of ice water, whereupon the product may be separated direct or
extracted, suitably with e.g. acetate or chloroform. If extraction is carried out, the product
is isolated in customary manner by removing the solvent, conveniently after drying the
organic phase. It is also possible to carry out further purifying steps, such as washing with
an aqueous solution of NaCl, dispersing of activated carbon, filtration, recryst~ ion
-
215064~
_
11
and/or distillation.
Some of the compounds of formula II are known and can be prepared, for example,
according to Angew. Chem. 104, 1652-1654 (1992) or in general accordance with the
process described therein. Compounds of formula III are commonly known and some are
commercially available.
The compounds obtained according to the process described above may be used per se, but
they may also be further derivatised in general accordance with known methods ordescribed processes. As just one example there may be mentioned here the further reaction
of a diol of formula I, wherein E is the group -CH(OH)-, which diol may be converted by
standard methods into the corresponding ethers, esters or, if oxygen is replaced by
nitrogen, into corresponding amines.
The compounds of formula I are suitable for stabilising organic materials against thermal,
oxidative and actinic degradation.
Illustrative examples of such materials are:
1. Polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene,
polybut- l-ene, poly-4-methylpent-1-ene, polyisoprene or polybutadiene, as well as poly-
mers of cycloolefins, for instance of cyclopentene or norbornene, polyethylene (which
optionally can be crosslinked), for example high density polyethylene (HDPE), low
density polyethylene (LDPE), linear low density polyethylene (LLDPE), branched low
density polyethylene (BLDPE).
Polyolefins, i.e. the polymers of monoolefins exemplified in the preceding paragraph,
preferably polyethylene and polypropylene, can be prepared by different, and especially
by the following, methods:
a) radical polymerisation (normally under high pressure and at elevated
temperature).
b) catalytic polymerisation using a catalyst that normally contains one or more
than one metal of groups IVb, Vb, VIb or VIII of the Periodic Table. These
metals usually have one or more than one ligand, typically oxides, halides,
215061 l
- 12-
alcoholates, esters, ethers, amines, aLkyls, alkenyls and/or aryls that may be
either 1l- or ~-coordinated. These metal complexes may be in the free form or
fixed on substrates, typically on activated m~gn~.sium chloride, ti~nillm(III)
chloride, alumina or silicon oxide. These catalysts may be soluble or insoluble
in the polymerisation medium. The catalysts can be used by themselves in the
polymerisation or further activators may be used, typically metal aL~yls, metal
hydrides, metal aL~yl h~ es, metal alkyl oxides or metal alkyloxanes, said
metals being elements of groups Ia, IIa and/or Ina of the Periodic Table. The
activators may be modified conveniently with further ester, ether, amine or silyl
ether groups. These catalyst systems are usually termed Phillips, Standard Oil
Indiana, Ziegler (-Natta), TNZ (DuPont), metallocene or single site catalysts
(SSC).
2. Mixtures of the polymers mentioned under 1), for example mixtures of polypropylene
with polyisobutylene, polypropylene with polyethylene (for example PP/HDPE,
PP/LDPE) and mixtures of different types of polyethylene (for example LDPE/HDPE).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl mono-
mers, for example ethylene/propylene copolymers, linear low density polyethylene(LLDPE) and mixtures thereof with low density polyethylene (LDPE), propylene/but-
l-ene copolymers, propylene/isobutylene copolymers, ethylene/but- l-ene copolymers,
ethylene/hexene copolymers, ethylene/methylpentene copolymers, ethylene/heptene
copolymers, ethylene/octene copolymers, propylene/butadiene copolymers, isobutylene/-
isoprene copolymers, ethylene/alkyl acrylate copolymers, ethylene/alkyl methacrylate
copolymers, ethylene/vinyl acetate copolymers and their copolymers with carbon mon-
oxide or ethylene/acrylic acid copolymers and their salts (ionomers) as well as terpoly-
mers of ethylene with propylene and a diene such as hexadiene, dicyclopentadiene or ethy-
lidene-norbornene; and mixtures of such copolymers with one another and with polymers
mentioned in 1) above, for example polypropylene/ethylene-propylene copolymers,
LDPE/ethylene-vinyl acetate copolymers (EVA), LDPE/ethylene-acrylic acid copolymers
(EAA), LLDPE/EVA, LLDPE/EAA and altern~ing or random polyalkylene/carbon mon-
oxide copolymers and mixtures thereof with other polymers, for example polyamides.
4. Hydrocarbon resins (for example C5-Cg) including hydrogenated modifications thereof
(e.g. tackifiers) and mixtures of polyalkylenes and starch.
2150641
- 13-
5. Polystyrene, poly(p-methylstyrene), poly(a-methylstyrene).
6. Copolymers of styrene or a-methylstyrene with dienes or acrylic derivatives, for
example styrene/butadiene, styrene/acrylonitrile, styrene/alkyl meth~crylate, styrene/buta-
diene/aLkyl acrylate, styrene/but~ien(~/alkyl methacrylate, styrene/maleic anhydride,
styrene/acrylonitrile/methyl acrylate; mixtures of high impact strength of styrene copoly-
mers and another polymer, for example a polyacrylate, a diene polymer or an ethylene/-
propylene/diene terpolymer; and block copolymers of styrene such as styrene/butadiene/-
styrene, styrene/isoprene/styrene, styrene/ethylene/butylene/styrene or styrene/ethylene/-
propylene/ styrene.
7. Graft copolymers of styrene or a-methylstyrene, for example styrene on polybut~ ne,
styrene on polybutadiene-styrene or polybutadiene-acrylonitrile copolymers; styrene and
acrylonitrile (or methacrylonitrile) on polybutadiene; styrene, acrylonitrile and methyl
methacrylate on polybutadiene; styrene and maleic anhydride on polybutadiene; styrene,
acrylonitrile and maleic anhydride or maleimide on polybutadiene; styrene and maleimide
on polybutadiene; styrene and alkyl acrylates or methacrylates on polybutadiene; styrene
and acrylonitrile on ethylene/propylene/diene terpolymers; styrene and acrylonitrile on
polyalkyl acrylates or polyalkyl methacrylates, styrene and acrylonitrile on acrylate/buta-
diene copolymers, as well as mixtures thereof with the copolymers listed under 6), for
example the copolymer mixtures known as ABS, MBS, ASA or AES polymers.
8. Halogen-containing polymers such as polychloroprene, chlorinated rubbers, chlorinated
or sulfochlorinated polyethylene, copolymers of ethylene and chlorinated ethylene, epi-
chlorohydrin homo- and copolymers, especially polymers of halogen-containing vinyl
compounds, for example polyvinyl chloride, polyvinylidene chloride, polyvinyl fluoride,
polyvinylidene fluoride, as well as copolymers thereof such as vinyl chloride/vinylidene
chloride, vinyl chloride/vinyl acetate or vinylidene chloride/vinyl acetate copolymers.
9. Polymers derived from a,~-unsaturated acids and derivatives thereof such as polyacry-
lates and polymethacrylates; polymethyl methacrylates, polyacrylamides and polyacrylo-
nitriles, impact-modified with butyl acrylate.
10. Copolymers of the monomers mentioned under 9) with each other or with other
unsaturated monomers, for example acrylonitrile/ butadiene copolymers, acrylonitrile/-
alkyl acrylate copolymers, acrylonitrile/aLkoxyalkyl acrylate or acrylonitrile/vinyl halide
215064~
- 14-
copolymers or acrylonitrile/ aLkyl methacrylate/butadiene terpolymers.
11. Polymers derived from unsaturated alcohols and amines or the acyl derivatives or
acetals thereof, for example polyvinyl alcohol, polyvinyl acetate, polyvinyl stearate, poly-
vinyl benzoate, polyvinyl maleate, polyvinyl butyral, polyallyl phthalate or polyallyl
melamine; as well as their copolymers with olefins mentioned in 1) above.
12. Homopolymers and copolymers of cyclic ethers such as polyaL~ylene glycols, poly-
ethylene oxide, polypropylene oxide or copolymers thereof with bisglycidyl ethers.
13. Polyacetals such as polyoxymethylene and those polyoxymethylenes which contain
ethylene oxide as a comonomer; polyacetals modified with thermoplastic polyurethanes,
acrylates or MBS.
14. Polyphenylene oxides and sulfides, and mixtures of polyphenylene oxides with sty-
rene polymers or polyamides.
15. Polyurethanes derived from hydroxyl-terminated polyethers, polyesters or polybuta-
dienes on the one hand and aliphatic or aromatic polyisocyanates on the other, as well as
precursors thereof.
16. Polyamides and copolyamides derived from ~ mines and dicarboxylic acids and/or
from aminocarboxylic acids or the corresponding lactams, for example polyamide 4, poly-
amide 6, polyamide 6/6, 6/10, 6/9, 6/12, 4/6, 12/12, polyamide 11, polyamide 12, aromatic
polyamides starting from m-xylene (li~mine and adipic acid; polyamides prepared from
hexamethylenediAmine and isophthalic or/and terephthalic acid and with or without an
elastomer as modifier, for example poly-2,4,4,-trimethylhexamethylene terephth~l~mide
or poly-m-phenylene isoph~h;~l~mide; and also block copolymers of the aforementioned
polyamides with polyolefins, olefin copolymers, ionomers or chemically bonded or graf-
ted elastomers; or with polyethers, e.g. with polyethylene glycol, polypropylene glycol or
polytetramethylene glycol; as well as polyamides or copolyamides modified with EPDM
or ABS; and polyamides condensed during processing (RIM polyamide systems).
17. Polyureas, polyimides, polyamide-imides and polybenzimidazoles.
18. Polyesters derived from dicarboxylic acids and diols and/or from hydroxycarboxylic
21506~
- 15-
acids or the corresponding lactones, for example polyethylene terephth~ te, polybutylene
terephthalate, poly-1,4-dimethylolcyclohexane terephth~ e and polyhydroxybenzoates,
as well as block copolyether esters derived from hydroxyl-termin~ted polyethers; and also
polyesters modified with polycarbonates or MBS.
19. Polycarbonates and polyester carbonates.
20. Polysulfones, polyether sulfones and polyether ketones.
21. Cro.s~link~d polymers derived from aldehydes on the one hand and phenols, ureas and
melamines on the other hand, such as phenoVform~l~ehyde resins, urealformaldehyde
resins and melamine/formaldehyde resins.
22. Drying and non-drying aLkyd resins.
23. Unsaturated polyester resins derived from copolyesters of saturated and unsaturated
dicarboxylic acids with polyhydric alcohols and vinyl compounds as cro.~slinking agents,
and also halogen-containing modifications thereof of low fl~mm~bility.
24. Cro.~.clink~ble acrylic resins derived from substituted acrylates, for example epoxy
acrylates, urethane acrylates or polyester acrylates.
25. Alkyd resins, polyester resins and acrylate resins crosslinked with melamine resins,
urea resins, polyisocyanates or epoxy resins.
26. Crosslinked epoxy resins derived from polyepoxides, for example from bisglycidyl
ethers or from cyclo~liph~tic diepoxides.
27. Natural polymers such as cellulose, rubber, gelatin and chemically m~dified homolo-
gous derivatives thereof, for example cellulose acetates, cellulose propionates and cellu-
lose butyrates, or the cellulose ethers such as methyl cellulose; as well as rosins and their
derivatives.
28. Blends of the aforementioned polymers (polyblends), for example PP/EPDM, Poly-
amide/EPDM or ABS, PVC/EVA, PVC/ABS, PVC/MBS, PC/ABS, PBTP/ABS,
PC/ASA, PC/PBT, PVC/CPE, PVC/acrylates, POM/thermoplastic PUR, PC/thermoplastic
2150644
- 16-
PUR, POM/acrylate, POM/MBS, PPO/HIPS, PPO/PA 6.6 and copolymers, PA/HDPE,
PA/PP, PA/PPO.
Accordingly, the invention further relates to compositions comprising, (a) an organic
material susceptible to oxidative, thermal and/or actinic degradation and, (b) at least one
compound of formula I, as well as to the use of compounds of formula I for stabilising
organic m~t~ l against oxidative, thermal or actinic degradation.
The invention also relates to a process for stabilising organic m~ter1~l against therm~l,
oxidative and/or actinic degradation, which comprises adding to said m~tçri~l at least one
compound of formula I.
The use of compounds of formula I as stabilisers in synthetic organic polymers is of
particular interest, as are corresponding compositions.
The organic materials to be protected are preferably natural, semi-synthetic or, preferably,
synthetic organic materials. Particularly preferred are synthetic organic polymers or
mixtures of such polymers, in particular thermoplastic polymers such as polyolefins, more
particularly polyethylene and polypropylene (PP). Organic materials which are also
particularly preferred are coating compositions. Photographic materials will be taken to
mean, in particular, those materials which are described in Research Disclosure 1990,
31429 (pages 474-480) for photographic and other techniques of reproduction. Coating
m:~teri~l.c which are usefully stabilised according to this invention are described, inter alia,
in Ullmann's Encyclopedia of Industrial Chemistry, 5. ed., Vol. A18, pp. 359-464, VCH
Verlagsgesellschaft, Weinheim 1991.
The compounds of formula I are usually added to the material to be stabilised in amounts
of 0.01 to 10%, preferably of 0.01 to 5% and, most preferably, of 0.01 to 2%, based on the
total weight of the stabilised composition. It is particularly preferred to add the novel
compounds in amounts of 0.05 to 1.5%, preferably of 0.1 to 0.5%.
Incorporation in the organic materials may be effected, for example, by blending them
with, or by applying thereto, the compounds of formula I and further optional additives by
methods which are commonly used in the art. If the organic materials are polymers,
especially synthetic polymers, the incorporation may be effected before or during the
fabrication of shaped articles or by applying the dissolved or dispersed compound to the
polymer, with or without subsequent evaporation of the solvent. In the case of elastomers,
21506~4
-
these may also be stabilised as latices. A further means of blending the compounds of
formula I into polymers consists in adding said compounds before, during or directly after
the polymerisation of the corresponding monomers or before cross-linking The
compounds of formula I may be added per se, but also in encapsulated form (e.g. in
waxes, oils or polymers). If the cornpounds of formula I are added before or during
polymeri.~tion, they can also act as regulators for the chain length of the poly mers (chain
terminators).
The compounds of formula I may also be added in the form of a masterbatch which
contains these compounds to the plastics to be stabilised, typically in a concentration of
2.5 to 25% by weight.
The compounds of formula I may conveniently be incorporated by the following
techniques:
- as emulsion or dispersion (e.g. to latices or emulsion polymers),
- as dry mixture while blending additional components or polymer mixtures,
- by direct addition to the processing apparatus (e.g. extruder, internal mixer, and the
like), and
- as solution or melt.
Novel polymer compositions may be used in a wide range of forms and processed todifferent products, typically including sheets, filaments, ribbons, moulded articles,
profiles, or as binders for paint systems, adhesives or putties.
In addition to cont~ining the compounds of formula I, the compositions of the invention
may contain as additional component (c) one or more than one conventional additive such
as the ones listed below.
The standard additives are conveniently used in amounts of 0.1-10% by weight, typically
of 0.2-5% by weight, based on the polymer to be stabilised.
1. Antioxidants
1.1. Alkylated monophenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-tert-butyl-
4,6-dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-butylphenol,
2,6-di-tert-butyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol, 2-(o~-methylcyclo-
21~064~
_
- 18-
hexyl)-4,6-dimethylphenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tricyclohexylphenol,
2,6-di-tert-butyl-4-methoxymethylphenol, 2,6-dinonyl-4-methylphenol, 2,4-dimethyl-6-
( 1 '-methylundec- 1 '-yl)phenol, 2,4-dimethyl-6-( 1 '-methylheptadec- 1 '-yl)phenol, 2,4-di-
methyl-6-(1'-methyltridec-1'-yl)phenol and mixtures thereof.
1.2. Alkylthiomethylphenols, for example 2,4-dioctylthiomethyl-6-tert-butylphenol,
2,4-dioctylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-dido-
decylthiomethyl-4-nonylphenol.
1.3. Hydroquinones and aL~ylated hvdroql~inon~s, for example 2,6-di-tert-butyl-4-
methoxyphenol, 2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-di-
phenyl-4-octadecyloxyphenol, 2,6-di-tert-butylhydroquinone, 2,5-di-tert-butyl-4-hydroxy-
anisole, 3,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyphenyl stearate,
bis(3,5-di-tert-butyl-4-hydroxyphenyl) adipate.
1.4. Tocopherols, for example a-tocopherol"3-tocopherol, ~-tocopherol, ~tocopherol and
mixtures thereof (Vitamin E).
1.5. Hydroxylated thiodiphenyl ethers, for example 2,2'-thiobis(6-tert-butyl-4-methyl-
phenol), 2,2'-thiobis~4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol), 4,4'-thio-
bis(6-tert-butyl-2-methylphenol), 4,4'-thiobis(3,6-di-sec-amylphenol), 4,4'-bis(2,6-dim-
ethyl-4-hydroxyphenyl) disulfide.
1.6. Alkylidenebisphenols, for example 2,2'-methylenebis(6-tert-butyl-4-methylphenol),
2,2'-methylenebis(6-tert-butyl-4-ethylphenol), 2,2'-methylenebis[4-methyl-6-(a-methyl-
cyclohexyl)phenol], 2,2'-methylenebis(4-methyl-6-cyclohexylphenol), 2,2'-methylene-
bis(6-nonyl-4-methylphenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), 2,2'-ethylidene-
bis(4,6-di-tert-butylphenol), 2,2'-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2'-methy-
lenebis[6-(a-methylbenzyl)-4-nonylphenol], 2,2'-methylenebis[6-(a,oc-dimethylbenzyl)-
4-nonylphenol], 4,4'-methylenebis(2,6-di-tert-butylphenol), 4,4'-methylenebis(6-tert-
butyl-2-methylphenol), 1,1-bis(5-tert-butyl-4-hydroxy-2-methylphenyl)butane, 2,6-bis(3-
tert-butyl-5-methyl-2-hydroxybenzyl)-4-methylphenol, 1,1,3-tris(S-tert-butyl-4-hydroxy-
2-methylphenyl)butane, 1,1-bis(S-tert-butyl-4-hydroxy-2-methyl-phenyl)-3-n-dodecylmer-
captobutane, ethylene glycol bis[3,3-bis(3'-tert-butyl-4'-hydroxyphenyl)butyrate], bis(3-
tert-butyl-4-hydroxy-S-methyl-phenyl)dicyclopentadiene, bis[2-(3'-tert-butyl-2'-hydroxy-
S'-methylbenzyl)-6-tert-butyl-4-methylphenyl]terephthalate, 1,1-bis(3,5-dimethyl-2-
21SD6~
- 19-
hydroxyphenyl)butane, 2,2-bis(3,5-di-tert-butyl-4-hydroxyphenyl)propane, 2,2-bis(5-tert-
butyl-4-hydroxy-2-methylphenyl)-4-n-dodecylmercaptobutane, 1,1,5,5-tetra(5-tert-butyl-
4-hydroxy-2-methylphenyl)pentane.
1.7. O-, N- and S-benzyl compounds, for e~r~mplP 3,5,3',5'-tetra-tert-butyl-4,4'-dihydroxy-
dibenzyl ether, octadecyl-4-hydroxy-3,5-dimethylbenzylmercaptoacet~te, tris(3,5-di-tert-
butyl-4-hydroxybenzyl)amine, bis(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)dithio-
terephth~l~te, bis(3,5-di-tert-butyl-4-hydroxybenzyl)sulfide, isooctyl-3,5-di-tert-butyl-4-
hydroxybenzylmercaptoacetate.
1.8. Hydroxybenzylated malonates, for example dioctadecyl-2,2-bis(3,5-di-tert-butyl-2-
hydroxybenzyl)malonate, dioctadecyl-2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)malo-
nate, didodecylmercaptoethyl-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate, bis[4-
(1, 1 ,3,3-tetramethylbutyl)phenyl]-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate.
1.9. Aromatic hydroxybenzyl compounds, for example 1,3,5-tris(3,5-di-tert-butyl-4-hy-
droxybenzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-2,3,5,6-
tetramethylbenzene, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)phenol.
1.10. Triazine compounds, for example 2,4-bis(octylmercapto)-6-(3,5-di-tert-butyl-4-
hydroxyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxy-
anilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-
1,3,5-triazine, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris-
(3,5-di-tert-butyl-4-hydroxybenzyl)isocyanurate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-di-
methylbenzyl)isocyanurate, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenylethyl)-1,3,5-tri-
azine, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hexahydro-1,3,5-triazine,
1,3 ,5-tris(3 ,5-dicyclohexyl-4-hydroxybenzyl)isocyanurate.
1.11. Benzylphosphonates, for example dimethyl-2,5-di-tert-butyl-4-hydroxybenzylphos-
phonate, diethyl-3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl-3,5-di-tert-
butyl-4-hydroxybenzylphosphonate, dioctadecyl-5-tert-butyl-4-hydroxy-3-methylbenzyl-
phosphonate, the calcium salt of the monoethyl ester of 3,5-di-tert-butyl-4-hydroxybenzyl-
phosphonic acid.
1.12. Acylaminophenols, for example 4-hydroxylauranilide, 4-hydroxystearanilide, octyl
N-(3 ,5-di-tert-butyl-4-hydroxyphenyl)carbamate.
215064~
- 20 -
1.13. Esters of ~B-(3,5-di-tert-butyl4-hydroxyphenyl)propionic acid with mono- or poly-
hydric alcohols, e.g. with methanol, ethanol, oct~-lec~nol, 1,6-hexanediol, l,9-nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene
glycol, triethylene glycol, pentaerythritol, tris(hyd~ ye~lyl) isocyanurate, N,N'-bis(hy-
droxyethyl)oxamide, 3-thi~n~eç~nol~ 3-thiapent~dec~nol, trimethylhP~r~nPdiol, trimethyl-
olpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.14. Esters of ,3-(5-tert-butyl4-hydroxy-3-methylphenyl)propionic acid with mono- or
polyhydric alcohols, e.g. with methanol, ethanol, oct~dec~nol, 1,6-hexanediol, l,9-nonane-
diol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene
glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl) isocyanurate, N,N'-bis-
(hydroxyethyl)oxamide, 3-thi~lln~ec~nol~ 3-thiapent~-lec~nol, trimethylhexanediol, tri-
methylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.15. Esters of ,~-(3,5-dicyclohexyl-4-hydroxyphenyl)propionic acid with mono- or poly-
hydric alcohols, e.g. with methanol, ethanol, oct~ec~nol, 1,6-hexanediol, l,9-nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene
glycol, triethylene glycol, pentaery~hritol, tris(hydroxyethyl) isocyanurate, N,N'-bis(hy-
droxyethyl)oxamide, 3-thiaundecanol, 3-thiapent~ec~nol, trimethylhexanediol, trimethyl-
olpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.16. Esters of 3,5-di-tert-butyl-4-hydroxyphenyl acetic acid with mono- or polyhydric
alcohols, e.g. with methanol, ethanol, oct~ec~nol, 1,6-hexanediol, l,9-nonanediol, ethy-
lene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl) isocyanurate, N,N~-bis(hydroxy-
ethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpro-
pane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.17. Amides of ,B-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid e.g. N,N'-bis(3,5-di-
tert-butyl-4-hydroxyphenylpropionyl)hexamethylene~ minP, N,N'-bis(3,5-di-tert-butyl-
4-hydroxyphenylpropionyl)trimethylenedi~mine, N,N'-bis(3,5-di-tert-butyl-4-hydroxy-
phenylpropionyl)hydrazine .
2. UV absorbers and li~ht stabilisers
21506~
2.1. 2-(2'-Hydroxyphenyl)benzotriazoles, for example 2-(2'-hydroxy-5'-methylphenyl)-
benzotriazole, 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)benzotriazole, 2-(5'-tert-butyl-2'-
hydroxyphenyl)benzotriazole, 2-(2'-hydroxy-5'-(1,1,3,3-tetramethylbutyl)phenyl)benzo-
triazole, 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-
2'-hydroxy-5'-methylphenyl)-5-chloro-benzotriazole, 2-(3'-sec-butyl-5'-tert-butyl-2'-
hydroxyphenyl)benzotriazole, 2-(2'~hydroxy-4'-octyloxyphenyl)benzotriazole, 2-(3',5'-
di-tert-amyl-2'-hydroxyphenyl)benzotriazole, 2-(3',5'-bis-(a,a-dimethylbenzyl)-2'-
hydroxyphenyl)benzotriazole, mixture of 2-(3'-tert-butyl-2'-hydroxy-5'-(2-octyloxycar-
bonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-5'-[2-(2-ethylhexyloxy)-car-
bonylethyl]-2'-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-5'-(2-
methoxycarbonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-5'-(2-
methoxycarbonylethyl)phenyl)benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-5'-(2-octyl-
oxycarbonylethyl)phenyl)benzotriazole, 2-(3'-tert-butyl-5'-[2-(2-ethylhexyloxy)carbonyl-
ethyl]-2'-hydroxyphenyl)benzotriazole, 2-(3'-dodecyl-2'-hydroxy-5'-methylphenyl)benzo-
triazole, and 2-(3'-tert-butyl-2'-hydroxy-5'-(2-isooctyloxycarbonylethyl)phenylbenzotri-
azole, 2,2'-methylene-bis[4-(1,1,3,3-tetramethylbutyl)-6-benzotriazole-2-ylphenol]; the
transesterification product of 2-[3'-tert-butyl-5'-(2-methoxycarbonylethyl)-2'-hydroxy-
phenyl]-2H-benzotriazole with polyethylene glycol 300; [R-CH2CH2-COO(CH2)3~,
where R = 3'-tert-butyl-4'-hydroxy-5'-2H-benzotriazol-2-ylphenyl.
2.2. 2-Hydroxybenzophenones, for example the 4-hydroxy, 4-methoxy, 4-octyloxy, 4-de-
cyloxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-dimethoxy
derivatives.
2.3. Esters of substituted and unsubstituted benzoic acids, as for example 4-tertbutyl-
phenyl salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoyl resorcinol, bis(4-
tert-butylbenzoyl) resorcinolj benzoyl resorcinol, 2,4-di-tertbutylphenyl 3,5-di-tert-butyl-
4-hydroxybenzoate, hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, octadecyl 3,5-di-tert-
butyl-4-hydroxybenzoate, 2-methyl-4,6-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxy-
benzoate.
2.4. Acrylates, for example ethyl a-cyano-~,p-diphenylacrylate, isooctyl a-cyano-,B,~-di-
phenylacrylate, methyl a-carbomethoxycinn~m~te, methyl a-cyano-,B-methyl-p-methoxy-
cinn~m~te, butyl a-cyano-,3-methyl-p-methoxy-cinn~m~tf~, methyl oc-carbomethoxy-p-
methoxycinn~m~te and N-(,3-carbomethoxy-~-cyanovinyl)-2-methylindoline.
215064~
2.5. Nickel compounds, for example nickel complexes of 2,2'-thio-bis[4-(1,1,3,3-tetra-
methylbutyl)phenol], such as the 1:1 or 1:2 complex, with or without additional ligands
such as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel dibutyldi-
thiocarbamate, nickel salts of the monoalkyl esters, e.g. the methyl or ethyl ester, of 4-
hydroxy-3,5-di-tert-butylbenzylphosphonic acid, nickel complexes of ketoximes, e.g. of
2-hydroxy-4-methylphenyl undecylketoxime, nickel complexes of 1-phenyl-4-lauroyl-5-
hydroxypyrazole, with or without additional ligands.
2.6. Sterically hindered ~mines, for example bis(2,2,6,6-tetramethyl-piperidyl)sebacate,
bis(2,2,6,6-tetramethyl-piperidyl)succinate, bis(l,2,2,6,6-pentamethylpiperidyl)sebacate,
bis(l,2,2,6,6-pentamethylpiperidyl) n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate,
the condensate of 1-(2-hydroxyethyl)-2,2,6,6-tetramethyl-4-hydroxypiperidine and succi-
nic acid, the conden~e of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenedi-
amine and 4-tert-octylamino-2,6-dichloro-1,3,5-triazine, tris(2,2,6,6-tetramethyl-4-piperi-
dyl) nitrilotriacetate, tetrakis(2,2,6,6-tetramethyl-4- piperidyl)-1,2,3,4-butane-tetracar-
boxylate, 1,1'-(1,2-ethanediyl)bis(3,3,5,5-tetramethylpiperazinone), 4-benzoyl-2,2,6,6-
tetramethylpiperidine, 4-stearyloxy-2,2,6,6-tetramethylpiperidine, bis(l,2,2,6,6-penta-
methylpiperidyl)-2-n-butyl-2-(2-hydroxy-3,5-di-tert-butylbenzyl)malonate, 3-n-octyl-
7,7 ,9,9-tetramethyl- 1 ,3,8-triazasprio[4.5]decane-2,4-dione, bis( 1 -octyloxy-2,2,6,6-tetra-
methylpiperidyl)sebacate, bis(l-octyloxy-2,2,6,6-tetramethylpiperidyl)succinate, the
condensate of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylene~ mine and 4-mor-
pholino-2,6-dichloro- 1,3,5-triazine, the condensate of 2-chloro-4,6-bis(4-n-butylamino-
2,2,6,6-tetramethylpiperidyl )-1,3,5 triazine and 1,2-bis(3-aminopropylamino)ethane, the
condensate of 2-chloro-4,6-di(4-n-butylamino-1,2,2,6,6-pentamethylpiperidyl)-
1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane, 8-acetyl-3-dodecyl-7,7,9,9-tetra-
methyl- 1 ,3,8-triazaspiro[4.5]decane-2,4-dione, 3-dodecyl- 1 -(2,2,6,6-tetramethyl-4-piperi-
dyl)pyrrolidine-2,5-dione, 3-dodecyl-1-(1,2,2,6,6-pentamethyl-4-piperidyl)pyrrolidine-
2,5-dione.
2.7. Oxamides, for example 4,4'-dioctyloxyoxanilide, 2,2'-dioctyloxy-5,5'-di-tert-butox-
anilide, 2,2'-didodecyloxy-5,5'-di-tert-butoxanilide, 2-ethoxy-2'-ethyloxanilide, N,N'-
bis(3-dimethylaminopropyl)oxamide, 2-ethoxy-5-tert-butyl-2'-ethoxanilide and its mix-
ture with 2-ethoxy-2'-ethyl-5,4'-di-tert-butoxanilide and mixtures of ortho- and para-
methoxy-disubstituted oxanilides and mixtures of o- and p-ethoxy-disubstituted oxani-
lides.
21506~
2.8. 2-(2-Hydroxypheny~ 3~s-tri~7ines~ for example 2,4,6-tris(2-hydroxy-4-octyloxy-
phenyl)-1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-
1,3,5-~ri~7inP, 2-(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine,
2,4-bis(2-hydroxy-4-propyloxyphenyl)-6-(2,4-dimethylphenyl)-1,3,5-tri~7in~., 2-(2-hy-
droxy-4-octyloxyphenyl)-4,6-bis(4-methylphenyl)-1,3,5-tri~7inç, 2-(2-hydroxy-4-dodecyl-
oxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-tri~7in~, 2-[2-hydroxy-4-(2-hydroxy-
3-butyloxy-propoxy)phenyl]-4,6-bis(2,4-dimethyl)-1,3,5-triazine, 2-[2-hydroxy-4-(2-
hydroxy-3-octyloxy-propyloxy)phenyl]-4,6-bis(2,4-dimethyl)- 1,3,5-tri~7in~
3. Metal deactivators, for example N,N'-diphenyloxamide, N-salicylal-N'-salicyloyl
hydrazine, N,N'-bis(salicyloyl) hydrazine, N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenyl-
propionyl) hydrazine, 3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalyl di-
hydrazide, oxanilide, isophthaloyl dihydrazide, sebacoyl bisphenylhydrazide, N,N'-di-
acetyladipoyl dihydrazide, N,N'-bis(salicyloyl)oxalyl dihydrazide, N,N'-bis(salicyloyl)-
thiopropionyl dihydrazide.
4. Phosphites and phosphonites~ for example triphenyl phosphite, diphenyl aL~yl phos-
phites, phenyl dialkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite, triocta-
decyl phosphite, distearyl pentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl) phos-
phite, diisodecyl pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl) pentaerythritol
diphosphite, bis(2,6-di-tert-butyl-4-methylphenyl)pentaerythritol diphosphite, bis(isode-
cyloxy)pentaerythritol diphosphite, bis(2,4-di-tert-butyl-6-methylphenyl)pentaerythritol
diphosphite, bis(2,4,6-tris(tert-butylphenyl)pentaerythritol diphosphite, tristearyl sorbitol
triphosphite, tetrakis(2,4-di-tert-butylphenyl) 4,4'-biphenylene diphosphonite, 6-isooctyl-
oxy-2,4,8,10-tetra-tert-butyl-12H-dibenz[d,g]-1,3,2-dioxaphosphocin, 6-fluoro-2,4,8,10-
tetra-tert-butyl- 1 2-methyl-dibenz[d,g] -1 ,3,2-dioxaphosphocin, bis(2,4-di-tert-butyl-6-
methylphenyl)methylphosphite, bis(2,4-di-tert-butyl-6-methylphenyl)ethylphosphite.
5. Peroxide scavengers, for example esters of ,B-thiodipropionic acid, for example the
lauryl, stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the zinc salt of
2-mercaptobenzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide, penta-
erythritol tetrakis(,B-dodecylmercapto)propionate.
6. Polyamide stabilisers, for example, copper salts in combination with iodides and/or
phosphorus compounds and salts of divalent manganese.
21S0644
- 24-
7. Basic co-stabilisers, for example, mel~minP, polyvinylpyrrolidone, dicyandiamide, tri-
allyl cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides, polyure-
thanes, aLkali metal salts and ~lk~line earth metal salts of higher fatty acids for example
calcium stearate, zinc stearate, m~gn~osium behenate, m~gn~osillm stearate, sodium rici-
noleate and potassium p~lmit~te, antimony pyrocatecholate or tin pyrocatecholate.
8. Nucleating agents, for example, 4-tert-butylbenzoic acid, adipic acid, diphenylacetic
acid.
9. Fillers and reinforcing agents, for example, calcium carbonate, silicates, glass fibres,
talc, kaolin, mica, barium sulfate, metal oxides and hydroxides, carbon black, graphite.
10. Other additives, for example, plasticisers, lubricants, emulsif1er.s, pigments, optical
brighteners, flameproofing agents, antistatic agents and blowing agents.
11. Benzofuranones and indolinones, for example those disclosed in US-A-4 325 863,
US-A-4 338 244 or US-A-5 175 312, or 3-[4-(2-acetoxyethoxy)phenyl]-5,7-di-tert-butyl-
benzofuran-2-one, 5,7-di-tert-butyl-3-[4-(2-stearoyloxyethoxy)phenyl]benzofuran-2-one,
3,3'-bis[5,7-di-tert-butyl-3-(4-[2-hydroxyethoxy]phenyl)benzofuran-2-one], 5,7-di-tert-bu-
tyl-3-(4-ethoxyphenyl)benzofuran-2-one, 3-(4-acetoxy-3,5-dimethylphenyl)-5,7-di-tert-
butyl-benzofuran-2-one, 3-(3,5-dimethyl-4-pivaloyloxyphenyl)-5,7-di-tert-butyl-benzofu-
ran-2-one.
The following Examples further illustrate the invention. All parts or percentages, in the
Examples as well as throughout the rest of the description and in the claims, are by
weight, unless otherwise indicated. The following abbreviations are used in the Examples:
GC: gas chromatography;
HPLC: high pressure liquid chromatography;
DSC: differential scanning cal()rimeter;
H-NMR: nuclear magnetic resonance of the nuclide lH.
A) Working Examples
Al) 2-Butyne-1,4-diol-ditosylate (compound l) is prepared in accordance with Angew.
Chem. 104, 1652-1654 (1992).
215064~
- 25 -
A2) Preparation of 1,4-bis(4-hexyloxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
A2a) P'l~p~dtion of 4-hexyloxy-2,2,6,6-tetramethylpiperidine
A 2.5 litre sulfonation flask, equipped with glass stirrer, condenser and 500 ml dropping
funnel, is charged, under nitrogen, with 314.6 g (2 mol) of 4-hydro~y-2,2,6,6-tetra-
methylpiperidine, 100 g (0.1 mol) of polyethylene glycol 1000, 1.4 1 of toluene and 561 g
(10 mol) of KOH in powder form. The suspension is heated to 80C and to the yellow
solution are then added dropwise 363.2 g (2.2 mol) of l-bromohexane over 45 minutes.
The batch is stirred for S hours at 80C, whereupon KBr precipitates. After cooling, the
reaction mixture is diluted with water and toluene and the organic phase is separated,
washed until neutral and dried over Na2SO4. The solvent is removed by evaporation on a
rotary evaporator.
The residue is distilled at 76C/0.08 torr, affording 205 g (43%) of the title product
(compound 2a), having a purity of >99% (GC analysis).
Microanalysis calculated found
%C 74.63 74.82
%H 12.94 13.04
%N 5.80 5.66
H-NMR (CDCl3)
0.68 ppm (1 H, s): NH
0.87-0.91 ppm (3 H, t) : CH3 (hexyl)
0.96-1.04 ppm (2 H, t) : CH2 (piperidine)
1.14 and 1.19 ppm (12 H, s) : CH3 (piperidine)
1.31-1.4 ppm (6 H, m) : -(CH2)3- (hexyl)
1.53-1.63 ppm (2 H, m) : -O-C-CH2 (hexyl)
1.93-1.98 ppm (2 H, m) : CH2 (piperidine)
3.44-3.49 ppm (2 H, t) : -O-CH2-
\ /
3.61-3.68 ppm (1 H, m) C
A2b) 1,4-Bis(4-hexyloxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
A round-bottomed flask, equipped with magnetic stirrer and condenser, is charged, under
argon, with 193.1 g (0.8 mol) of compound 2a), 63.1 g (0.16 mol) of compound 1) and
21506~4
- 26 -
500 ml of acetonitrile.
The mixture is refluxed overnight and then poured onto 700 g of ice and extracted with
500 ml of acetate. The organic phase is then dried and concentrated by evaporation. The
residue is taken up in hexane/acetate = 2: 1 and filtered over silica gel. The solvent is
distilled off, affording 49.5 g (58%) of the title product (compound 2) as a clear viscous
liquid.
Microanalysis calculated found
%C 76.63 76.62
%H 12.11 12.04
%N 5.26 5.32
H-NMR (CDCl3)
0.86-0.91 ppm (6 H, t) : CH3 (hexyl)
1.08 and 1.21 ppm (24 H, s) : CH3 (piperidine)
1.29-1.43 ppm (16 H, m) : CH2 (piperidine), -(CH2)3- (hexyl)
1.50-1.57 ppm (4 H, m) : O-C-CH2- (hexyl)
1.78-1.83 ppm (4 H, m) : CH2 (piperidine)
3.32 ppm (4 H, s): N-CH2-C_C
3.40-3.45 ppm (4 H, t): -O-CH2-
\ /
3.53-3.58 ppm (2H, m) C
H
A3) Preparation of 1,4-bis(4-benzyloxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
A3a) Preparation of 4-benzyloxy-2,2,6,6-tetramethylpiperidine
In general accordance with Example A2a), 314.6 g (2 mol) of 4-hydroxy-2,2,6,6-tetra-
methylpiperidine are reacted with 376.2 g (2.2 mol) of benzyl bromide.
The crude product is distilled at 84C/0.006 torr, affording 280 g (57%) of a liquid which
gradually crystallises. Melting point: 30C
Microanalysis calculated found
%C 77.68 77.66
%H 10.19 10.29
%N 5.66 5.60
21506~
-
- 27 -
H-NMR (CDC13~
0.71 ppm (1 H, s) : NH
l.OS and 1.13 ppm (2 H, t) : CH2 (piperidine)
1.15-1.17 ppm (12 H, s) CH3
1.99-2.05 ppm (2 H, m) : CH2 (piperidine)
\
3.76-3.86ppm (1 H,m) :
H
4.58 ppm (2 H, s) : -O-CH2-
7.26-7.35 ppm (5 H, m) : aromatic ring
A3b) 1,4-Bis(4-benzyloxy-2,2,6,6-tetramethylpiperidine)but-2-yne
In general accordance with Example A2b), 202 g (0.84 mol) of the compound of Example
A3a) are reacted with 41.4 g (0.17 mol) of 2-butyne-1,4-diol-ditosylate (compound 1) in
S00 ml of acetonitrile.
The crude product is recrystallised from methanol, affording 38.2 g (41%) of the title
product as a solid which melts at 80.5C.
Microanalysis calculated found
%C 79.36 79.35
%H 9.62 9.81
%N 5.14 5.06
H-NMR (CDC13)
1.06 and 1.23 ppm (24 H, s) CH3
1.42-1.50 ppm (4 H, t) : CH2 (pipeAdine)
1.84-1.89 ppm (4 H, m) : CH2 (piperidine)
3.33 ppm (4 H, s) : N-CH2-C_C
\ /
3.64-3.74 ppm (2 H, m) /C~
H
4.54 ppm (4 H, s) : -O-CH2-
7.24-7.42 ppm (10 H, m) : aromatic ring
21506491
- 28 -
A4) Preparation of 1,4-bis(4-methoxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
A4a) Preparation of 4-methoxy-2,2,6,6-tetramethylpiperidine
In a 2.5 1 sulfonation flask, equipped with glass stirrer, thermometer and condenser, 236 g
(1.5 mol) of 4-hydroxy-2,2,6,6-tetramethylpiperidine, 4 g (15 mmol) of 18-crown-6, 420 g
(7.5 mol) of powdered KOH and 30 g (0.21 mol) of sodium sulfate are added to 1.5 1 of
tetrahydrofuran (THF). The mixture is then heated to 35C and 212 g (1.5 mol) of methyl
iodide, dissolved in 150 ml of THF, are then added dropwise over 4 hours. The batch is
then poured onto ice/acetate and the organic phase is separated, dried and cautiously
concentrated by evaporation on a rotary evaporator.
The residue is distilled at 67C/16 mm, affording 190 g (74%) of a clear liquid.GC Purity: 95%
Microanalysis calculated found
%C 70.12 70.31
%H 12.36 12.39
%N 8.18 8.04
H-NMR (CDCl3~
0.71 ppm (1 H, s) : NH
0.97-1.03 ppm (2 H, t) CH2
1.15 and 1.19 ppm (12 H, s): CH3 (piperidine)
1.95-2.00 ppm (2 H, m) CH2
3.37ppm (3 H,s): O-CH3
\ /
3.52-3.62ppm (1 H,m) C
A4b) 1,4-Bis(4-methoxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
In general accordance with Example A2b), 188.4 g (1.1 mol) of the compound of Example
A4a) are reacted with 53.1 g (0.22 mol) of 2-butyne-1,4-diol-ditosylate (compound 1) in
650 ml of acetonitrile.
The crude product is distilled at 170C/0.08 torr and then crystallised.
Melting point: 62C, yield: 40 g (46%).
Microanalysis calculated found
%C 73.42 73.17
%H 11.30 11.43
215069~
- 29 -
%N 7.13 6.91
H-NMR (CDCl3)
1.08 and 1.23 ppm (24 H, s): CH3 (piperidine)
1.29-1.37 ppm (4 H, t) : CH2 (piperidine)
1.80-1.86 ppm (4 H, m) : CH2 (piperidine)
3.33 ppm (10 H, s): N-CH2-C--C and -O-CH3
\ /o
3.42-3.52 ppm (2 H, m) /C~
H
A5) Preparation of 1,4-bis(4-hydroxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
l,CH3 H3C 3
HO ~N--CH2--C--C--CH2--N~ OH
H3C CH3
In general accordance with Example A2b), 833 g (5.3 mol) of 4-hydroxy-2,2,6,6-tetra-
methylpiperidine are reacted with 418 g (1.05 mol) of 2-butyne-1,4-diol-ditosylate
(compound 1) in 3.6 1 of acetonitrile.
The crude product is recrystallised from ethanol, affording 295 g (76%) of the title product
as white crystals. Melting point: 203.4C.
Microanalysis calculated found
%C 72.48 72.52
%H 11.06 11.12
%N 7.68 7.75
H-NMR (DMSO)
1.01 and 1.11 ppm (24 H, s) CH3
1.11-1.19 ppm (4 H, t) : CH2 (piperidine)
1.60-1.66 ppm (4 H, m) : CH2 (piperidine)
3.34 ppm (4 H, s) : N-CH2-C-C
\ /o
3.67-3.76 ppm (2 H, m) /C\
H
4.37-4.39 ppm (2 H, d) : -OH
21506~
- 30 -
A6) Preparation of 1,4-bis(4-rn-octylcarbonyloxyl-2,2,6,6-tetramethylpiperidinyl)
but-2-yne
Into a 2 1 round-bottomed flask, equipped with m~gn~.tic stirrer, are put 72.9 g (0.2 mol) of
1,4-bis(4-hydroxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne (compound 5), 86.2 g
(0.5 mol) of methyl pelargonate, 8 g of dibutyltin oxide and 800 ml of xylene. A Vigreux
~lictill~tion unit is mounted on the round-bottomed flask. The flask is heated to 150C and
the methanol is allowed to distill off first. The temperature is then raised slightly, so that
the xylene also gradually distills off.
The residue is distilled at 60C/0.008 torr, affording 99 g (77%) of a viscous liquid.
Microanalysis calculated found
%C 74.48 74.25
%H 11.25 11.23
%N 4.34 4.24
H-NMR (CDCl3)
0.86-0.9 ppm (6 H, t) : CH3 (pelargonic acid)
1.13 and 1.22 ppm (24 H, s) : CH3 (piperidine)
1.20-1.4 ppm (20 H, m) : -(CH2)s- (pelargonic acid)
1.45-1.57 ppm (4 H, t) : CH2 (piperidine)
1.56-1.62 ppm (4 H, m) : OCC-C-CH2-
1.76-1.82 ppm (4 H, m) : CH2 (piperidine)
2.23-2.32 ppm (4 H, t) : OOC-CH2-
3.32 ppm (4 H, s): N-CH2-C_C
\ /
5.03-5.11 ppm (2 H, m) C
H
A7) Preparation of 1,4-bis(4-ln-heptadecyl-carbonyloxyl-2~2~6~6-tetramethylpiperidinyl)
but-2-yne
In general accordance with Example A6), 48.1 g (132 mmol) of compound 5 are reacted
with 100 g (0.33 mol) of methyl stearate.
The crude product is then recrystallised from ethanol, affording 88.6 g (75%) of white
crystals. Melting point: 68C.
Microanalysis calculated found
%C 77.62 77.53
215~
- 31 -
%H 12.13 12.19
%N 3.12 3.14
H-NMR (CDCl3~
0.86-0.9 ppm (6 H, t) : CH3 (stearic acid)
1.13 and 1.22 ppm (24 H, s) : CH3 (piperidine)
1.20-1.35 ppm (56 H, m) : -(CH2)l4-
1.46-1.49 ppm (4 H, t) : CH2 (piperidine)
1.48-1.62 ppm (4 H, m) : OOC-C-CH2-
1.76-1.81 ppm (4 H, m) : CH2 (piperidine)
2.23-2.28 ppm (4 H, t) : OOC-CH2-
3.32 ppm (4 H, s) : N-CH2-C_C
5.02-5.10 ppm (2 H, m) ~C~H
A8) Preparation of 1,4-bis(4-~n-pentadecylcarbonyloxyl-2~2~6~6-tetramethylpiperidinyl)
but-2-yne
In general accordance with Example A6), 72.9 g (0.2 mol) of 1,4-bis(4-hydroxy-2,2,6,6-
tetramethylpiperidinyl)but-2-yne are reacted with 135.2 g (0.5 mol) of methyl p~lmit~te.
The crude product is recrystallised from ethanol, affording 121 g (72%) of white crystals.
Melting point: 61C.
Microanalysis calculated found
%C 77.08 76.93
%H l l.g8 11.99
%N 3.33 3.32
H-NMR (CDC13~
0.86-0.9 ppm (6 H, t) : CH3 (palmitic acid)
1.12 and 1.22 ppm (24 H, s) : CH3 (piperidine)
I.20-1.40 ppm (48 H, m) : -(CH2)12-
1.45-1.54 ppm (4 H, t) : CH2 (piperidine)
1.53-1.68 ppm (4 H, m) oOC-C-CH2-
1.76-1.82 ppm (4 H, m) : CH2 (piperidine)
2.23-2.30 ppm (4 H, t) : OOC-CH2-
3.32 ppm (4 H, s) : N-CH2-C_C
2150~
- 32 -
5.02-5.10 ppm (2 H, ln) c
A9) Preparation of 1,4-bis(4-rethylcarbonyloxyl-2~2~6~6-tetramethylpiperidinyl)but-2-yne
Into a 1.5 1 sulfonation flask, equipped with glass stirrer, thermometer, condenser and
dropping funnel, are put 100 g (274 mmol) of the compound of Example AS), 83.1 g(823 mmol) of triethylamine and 1 l of THF. Then 76.1 g (823 mmol) of propionyl
chloride, dissolved in 100 ml of THF, are added dropwise at 20C. The batch is stirred
overnight at 25C, insoluble salt is then removed, and the solvent is removed byevaporation.
The residue is recrystallised from methanol, affording 52 g (39%) of a substance which
melts at 78C.
Microanalysis calculated found
%C 70.55 70.44
%H 10.15 10.12
%N 5.88 5.68
H-NMR (CDC13~
1.09-1.15 ppm (18 H, s,t) : CH3 (propionic acid), CH3 (piperidine)
1.22 ppm (12 H, s) : CH3 (piperidine)
1.43-1.53 ppm (4 H, t) : CH2 (piperidine)
1.77-1.82 ppm (4 H, m) : CH2 (piperidine)
2.25-2.33 ppm (4 H, q) : OOC-CH2-
3.32 ppm (4 H, s) : N-CH2-C_C
\ /
5.02-5.12ppm (2H,m) : / ~
H
A10) Preparation of 1,4-bis(4-acetoxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
AlOa) Preparation of 4-acetoxy-2,2,6,6-tetramethylpiperidine
Into a 1.5 1 sulfonation flask, equipped with glass stirrer, thermometer and condenser, are
put 157 g (1 mol) of 4-hydroxy-2,2,6,6-tetranethylpiperidine, 60 g (1 mol) of acetic acid,
300 g (3 mol) of acetic acid anhydride and 5 drops of concentrated sulfuric acid. The batch
is stirred overnight at 60C under nitrogen and cooled to 25C, and then ice-cold sodium
21506~
hydroxide is poured in until the pH is 10.
After extraction with diethyl ether, the organic phase is dried, filtered and concentrated by
evaporation.
The residue is distilled at 102C/l9 torr, affording 150 g (75%) of a clear liquid.
GC purity: c. 98%.
Microanalysis calculated found
%C 66.29 66.03
%H 10.62 10.76
%N 7.03 6.93
H-NMR (CDCl3)
0.97 ppm (1 H, s) : NH
1.07-1.18 ppm (8 H, s,t) : CH3 and CH2 (piperidine)
1.23 ppm (6 H, s) : CH3 (piperidine)
1.89-1.98 ppm (2 H, m) CH2
2.11 ppm (3 H, s) : OOC-CH3
5.3-5.23 ppm (1 H, m) : ~(
AlOb) 1,4-Bis(4-acetoxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
In general accordance with Example A2b), 39.5 g (0.1 mol) of 2-butyne- 1,4-diol-ditosylate are reacted with 59.8 g (0.3 mol) of 4-acetoxy-2,2,6,6-tetramethylpiperidine
(compound lOa) in 360 ml of acetonitrile.
The crude product is recrystallised from acetonitrile, affording 20.3 g (45%) of a
colourless solid which melts at 113C.
Microanalysis calculated found
%C 69.61 69.65
%H 9.89 9.83
%N 6.24 6.25
H-NMR (CDCl3)
1.12 and 1.23 ppm (24 H, s) : CH3 (piperidine)
1.43-1.53 ppm (4 H, t) : CH2 (piperidine)
1.77-1.82 ppm (4 H, m) : CH2 (piperidine)
2150fil~
- 34-
2.02 ppm (6 H, s) : OOC-CH3
3.32 ppm (4 H, s) : N-CH2-CeC
\
5.00-5.11 ppm (2 H, m) C
Al l) Preparation of 1,4-bis(4-acetamido-2,2,6,6-tetramethyl piperidinyl)but-2-yne
Al la) Preparation of 4-ace~mido-2,2,6,6-tetramethylpiperidine
In a 10 l reactor, equipped with glass stirrer, thermometer and dropping funnel, 625 g
(4 mol) of 4-amino-2,2,6,6-tetrame~hylpipelidine are dissolved in 2.8 1 of toluene. To this
solution is added dropwise a solution of 450 g (4.4 mol) of acetic acid anhydride in 400 ml
of toluene so that the temperature remains below 25C, and the batch is stirred for about
one hour after this addition. Then 4 1 of a 2N solution of sodium hydroxide are poured into
the reaction solution. After thorough mixing, the organic phase is separated, dried and
concentrated by evaporation. The residue is recrystallised from acetonitrile, affording
687 g (80%) of the title product in a crystalline modification which contains water of
cryst~ tion. Melting point: 125C.
Microanalysis calculated found
%C 61.06 61.09
%H 11.18 11.30
%N 12.98 13.00
H-NMR (CD30D)
1.00-1.08 ppm (2 H, t) CH2
1.13 and 1.23 ppm (12 H, s) : CH3 (piperidine)
1.73-1.78 ppm (2 H, m) CH2
1.91 ppm (3 H, s~ : OC-CH3
4.11-4.18 ppm (1 H, m) : ><
Allb) 1,4-Bis(4-acetamido-2,2,6,6-tetramethylpiperidinyl)but-2-yne
In general accordance with Example A2b), 79 g (0.2 mol) of 2-butyne-1,4-diol-ditosylate
are reacted with 159 g (0.8 mol) of 4-acetamide-2,2,6,6-tetramethylpiperidine from
Example Al la) in 700 ml of acetonitrile.
~ 21 5 06~ 4
The crude product is recrystallised from dioxane, affording 42 g (47%) of the title product
as colourless substance which decomposes at 251 C.
Microanalysis calculated found
%C 69.91 69.70
%H 10.38 10.50
%N 12.54 12.47
H-NMR (CD30D)
1.15 and 1.21 ppm (24 H, s) : CH3 (pipeAdine)
1.23-1.35 ppm (4 H, t) : CH2 (piperidine)
1.62-1.67 ppm (4 H, m) : CH2 (pipeAdine)
1.91 ppm (6 H, s) : OC-CH3
3.37 ppm (4 H, s) : N-CH2-C_C
4.04-4.13 ppm (2 H, m~ : ><
A12) 1,4-Bis(4-allyloxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
A12a) 4-Allyloxy-2,2,6,6-tetramethylpiperidine
In general accordance with Example A2a), 156 g (1 mol) of 4-hydroxy-2,2,6,6-tetra-
methylpiperAdine are reacted with 145 g (1.2 mol) of allyl bromide.
The crude product is distilled, affording 159 g (81%) of a liquid having a boiling point of
40C/3 mbar. GC puAty: > 98%.
Microanalysis calculated found
%C 73.04 72.94
%H 11.75 11.73
%N 7.10 7.08
A12b) 1,4-Bis(4-allyloxy-2,2,6,6-tetramethylpiperidinyl)but-2-yne
In general accordance with Example A2b), 148 g (750 mmol) of 4-allyloxy-2,2,6,6-tetra-
methylpiperidine are reacted with 59 g (150 mmol) of 2-butyne-1,4-diol-ditosylate in
500 ml of acetonitrile.
The crude product is distilled off at 170C/0.08 torr, affording 50.2 g (75%) of the title
product (compound 12). Melting point: 53C.
Microanalysis calculated found
%C 75.63 75.53
2150641
- 36 -
%H 10.88 11.08
%N 6.30 ~ 6.34
H-NMR (CDCl3)
1.07 and 1.22 ppm (24 H, s) CH3
1.28-1.43 ppm (4 H, t) : CH2 (piperidine)
1.79-1.84 ppm (4 H, m) : CH2 (piperidine)
3.32 ppm (4 H, s) : N-CH2-C-C
3.57-3.67 ppm (2 H, m) : >~
3.99-4.02 ppm (4 H, d) -O-CH2-
5.12-5.30 ppm (4H, q) : =CH2
5.86-5.99 ppm (2 H, m) : -CH=
A13) 1,4-Bis(triacetonamine ethylene ketal)but-2-yne
>~Ha ~O,ccH2
A mixture of 199 g (1 mol) of triacetonamine ethylene ketal and 79 g (0.2 mol) of
compound 1 in 700 ml of acetonitrile is kept at reflux temperature for 5 hours. The crude
product obtained after cooling is recrystallised from methanol, affording 80 g (89%) of the
title product. Melting point: 123C.
Microanalysis calculated found
%C 69.61 69.84
%H 9.89 10.04
%N 6.24 6.34
H-NMR (CDC132
1.20 ppm (24 H, s) CH3
1.68 ppm (8 H, s) : CH2 (piperidine)
3.37 ppm (4 H, s) : N-CH2-
3.91 ppm (8 H, s) -o-cH2-cH
21~064~
A14) 1,4-Bis(7-dodecyl-2,2,10,10-tetramethyl-1,5,7-triazaspiror4~sldecane-6~8-dione
yl)but-2-yne
O - C N~N ~H2--C----C -C~ c N--C12H25
O H3C CH3 0
In general accordance with Example A2b), 39.4 g (0.1 mol) of 3-dodecyl-7,7,9,9-tetra-
methyl- 1,3,8-triazaspiro[4,5]decane-2,4-dione are reacted with 7.9 g (20 mmol) of
2-butyne- 1,4-diol-ditosylate in 420 ml of acetonitrile. The crude product is first
recryst~llised from 300 ml of n-hexane and then from ethanol until the constant melting
point of 162C is reached.
Microanalysis calculatedfound
%C 71.73 71.81
%H 10.59 10.62
%N 10.04 10.09
A15) 2,4-Hexadiyne-1,6-diol-ditosylate
Into a 1.5 1 slllf~ting flask, equipped with thermometer, magnetic stirrer, dropping funnel
and condenser, are put 54.6 g (0.5 mol) of 2,4-hexadiyne-1,6-diol, 220 g (1.16 mol) of
toluene-4-sulfonyl chloride and 750 ml of acetonitrile. While cooling with an ice bath.
56 g (1 mol) of a solution of KOH in 95 ml of water are added dropwise over 30 minutes.
The reaction mixture is stirred at 20-25C for 24 h and then 700 ml of water and 1 1 of
diethyl ether are added. The organic phase is separated, washed with a solution of sodium
bicarbonate and water, dried with sodium sulfate and concentrated by evaporation. The
product is recrystallised twice from methanol, affording 32 g of the title product. Melting
point: 95C (compound 15).
Microanalysis calculatedfound
%C 57.40 57.38
%H 4.34 4.36
%N 15.32 15.03
21S064~
- 38 -
A16) 1,6-Bis(4-hydroxy-2,2,6,6-tetramethylpiperidin-1-yl)-2,4-hexadiyne
H3C CH H C CH3
HO ~N ~CH2--(C--C)2-CH2--N/~ OH
CH3 H3C ~--
H3C CH3
In general accordance with Example A2b), 2.5 g (6 mmol) of compound 15 are reacted
with 4.7 g (30 mmol) of 4-hydroxy-2,2,6,6-tetramethylpiperidine in 30 ml of acetonitrile.
The crude product is recrystallised from toluene, affording 2 g (83%) of the title product
as white crystals. Decomposition point: 234C.
Microanalysis calculated found
%C 74.18 74.28
%H 10.38 10.38
%N 7.21 7.16
A17) 1,6-Bis(4-benzyloxy-2,2,6,6-tetramethylpiperidine)-2,4-hexadiyne
- ~ -CH2--(C--C)2CH2--N~ O CH
H3C CH3
In general accordance with Example A2b), 15 g (36 mmol) of compound 15 are reacted
with 44.5 g (180 mmol) of the compound of Example A3a) in 150 ml of acetonitrile. The
crude product is recrystallised from equal parts of each of toluene, acetonitrile and ethyl
acetate. Each end product of the above formula has a melting point of 171C.
Microanalysis calculated found
%C 80.24 80.13
%H 9.21 9.31
%N 4.92 4.92
2150~q~
- 39 -
B) Use Examples
Bl) Stabilising polypropylene sheets against light
1 g each of the novel stabilisers described in the above Examples are mixed with 1000 g of
polypropylene having a melt flow index of 4.0 g/10 min. (230C; 2.16 kg), supplied by
Statoil, St~th~lle7 Norway, and the following additives:
0.5 g of pentae,ylhlilyl tetrakis(3-[3',5'-di-tert-butyl-4'-hydroxyphenyl]propionate);
1.0 g of tris(2,4-di-tert-butyl-phenyl)phosphite;
1.0 g of FILOFIN g) Blue G (pigment; Ciba Geigy S.p.A., Origgio, Italy);
1.0 g of calcium stearate.
A mixture without any novel stabiliser is prepared for comparison purposes.
The mixture is extruded at 200-230C and the granules so obtained are processed by the
injection moulding process at 200-220C to sheets of 2 mm.
The sheets are exposed according to ASTM D 2565-85 in a Weather-O-Meter(~ 65 WR
(Atlas Electric Devices, Chicago, USA) at a black standard temperature of 63 + 3C. The
samples are examined at regular intervals and the onset of chalking is determined. The
exposure time until the onset of chalking is listed in the following Table 1.
Tab. 1: Exposure time until the onset of chalking
Stabiliser of Example Exposure time (h)
none 620
A2 3420
A3 3800
A4 4130
A12 3800
The samples stabilised according to the invention have excellent fastness to light.