Note: Descriptions are shown in the official language in which they were submitted.
BASF Aktiengesellschaft 940188 O.Z. OOSO/44957
2151770
The preparation of polymers based on 1-vinylimidazoles
The present invention relates to a novel process for preparing
5 polymers ~ased on l-vinylimidazole by precipitation polymeriza-
tion in the presence of compounds which form free radicals.
1-Vinylimidazoles are normally polymerized in aqueous or ethan-
olic solution. To prepare higher molecular weight or crosslinked
lO polymers or special copolymers it is frequently more favorable to
use the precipitation polymerization method.
EP-A 162 388 discloses the preparation of 1-vinylimidazole
copolymers by precipitation polymerization in benzene.
Furthermore, A. Chapiro et al., Eur. Polym. J., 24 (1988), 1019
describe the precipitation polymerization of 1-vinylimidazole in
benzene, toluene or tetrachloromethane. The preparation of
copolymers of 1-vinylimidazole and 4-aminostyrene by precipita-
20 tion polymerization in benzene is described by R.F.C. Bay et al.,Polymer, 32 (1991), 2456.
The polyvinylimidazoles prepared in tetrachloromethane have, how-
ever, only low molecular weights, while the polymers obtained in
25 benzene or toluene result as crosslinked gels which are difficult
to work up.
An additional disadvantage of this known process is that the
solvents used are toxicologically very objectionable.
It is an object of the present invention to find a process which
allows the use of toxicologically less objectionable reaction
media.
35 We have found that this object is achieved by a process
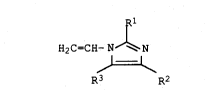
(a)-10-100% by weight of a compound of the general formula I
IRl
H2C=CH-N ~ N
,~
R3 R2
BASF AXtiengesellschaft 940188 O.Z. 0050/44957
~ -- 21~1770
where Rl, R2 and R3 are identical or different and each is
hydrogen, Cl-Cl8-alkyl or phenyl,
(b) 0-90% by weight of another monoethylenically unsaturated
monomer capable of free-radical copolymerization, and
(c) 0-20% by weight of a monomer which contains at least two non-
conjugated ethylenic double bonds,
10 in the presence of compounds which form free radicals, wherein
the polymerization is carried out in an organic solvent or
solvent mixture which contains no aromatic groups and, apart from
oxygen, no heteroatom and in which the resulting polymer is of
low solubility or insoluble.
Suitable monomers (a) are vinylimidazoles of the formula I
Rl
20 H2C=C~-N ~ N
,1=1~
R3 R2
25 where Rl, R2 and R3 are identical or different and are each hydro-
gen, Cl-Cl8-alkyl or phenyl, for example 1-vinylimidazole, 2-meth-
yl-1-vinylimidazole, 2-ethyl-1-vinylimidazole, 2-propyl-1-vinyl-
imidazole, 2-butyl-1-vinylimidazole, 2,4-dimethyl-1-vinylimid-
azole, 2,5-dimethyl-1-vinylimidazole, 2-ethyl-4-methyl-1-vinyl-
30 imidazole, 2-ethyl-5-methyl-1-vinylimidazole, 2,4,5-trimethyl-
l-vinylimidazole, 4,5-diethyl-2-methyl-1-vinylimidazole,
4-methyl-1-vinylimidazole, 4-ethyl-1-vinylimidazole, 4,5-dime-
thyl-l-vinylimidazole, 5-methyl-1-vinylimidazole, 2,4,5-tri-
ethyl-l-vinylimidazole, 2-phenyl-1-vinylimidazole, 2-undecyl-1-
35 vinylimidazole or 2-stearyl-1-vinylimidazole. It is also possible
to use mixtures of said monomers in any desired ratios. Monomers
of group ~a) which are preferably used are l-vinylimidazole,
2-methyl-1-vinylimidazole, 4(5)-methyl-1-vinylimidazole,
2-ethyl-1-vinylimidazole and 2-ethyl-4(5)-methyl-1-vinylimida-
40 zole. 1-Vinylimidazole and 2-methyl-1-vinylimidazole are very
particularly preferred. The monomers are used in amounts of
10-100% by weight, preferably 25-100% by weight.
Suitable monomers (b) are further monoethylenically unsaturated
45 monomers capable of free-radical copolymerization, or mixtures
thereof, for example N-vinyllactams such as N-vinylpyrrolidone
and N-vinylcaprolactam, N-vinyloxazolidinone, N-vinyltriazole,
BASF Aktiengesellschaft 940188 O.Z. 0050/44957
-- 21~1770
N-vinyl-N-methylacetamide, (meth)acrylic esters such as methyl,
ethyl, hydroxyethyl, propyl, hydroxypropyl, butyl, ethylhexyl,
decyl, lauryl, i-bornyl, cetyl, palmityl, phenoxyethyl or stearyl
acrylate or the corresponding methacrylates, (meth)acrylamides
5 such as acrylamide, N-methylolacrylamide, N,N-dimethylamino-
propylacrylamide, N-tert-butylacrylamide, N-tert-octylacrylamide,
N-undecylacrylamide or the corresponding methacrylamides, vinyl
esters with 2-30, in particular 2-14, carbon atoms in the mole-
cule such as vinyl acetate, vinyl propionate, vinyl laurate,
10 vinyl neooctanoate, vinyl neononanoate, vinyl neodecanoate, sty-
rene, vinyltoluene, ~-methylstyrene, unsaturated carboxylic acids
such as acrylic acid, methacrylic acid, crotonic acid, maleic
acid, fumaric acid, itaconic acid or their anhydrides, 2-acryl-
amido-2-methylpropanesulfonic acid, acrylic esters having a basic
15 nitrogen atom such as diethylaminoethyl acrylate, dimethylamino-
ethyl acrylate, dimethylaminopropyl acrylate or the corresponding
methacrylates, 2-vinylpyridine, 4-vinylpyridine. Particularly
preferred are N-vinylpyrrolidone, N-vinylcaprolactam, alkyl
(meth)acrylates, vinyl acetate, styrene, acrylic acid, meth-
20 acrylic acid, maleic acid and monomers which have a basic nitro-
gen atom. N-Vinylpyrrolidone is very particularly preferred. The
monomers are used in amounts of 0-90% by weight, preferably 0-75%
by weight.
25 Suitable monomers (c) are those compounds which are capable of
free-radical copolymerization and which contain at least two non-
conjugated ethylenic double bonds in the molecule. Examples of
suitable monomers (c) are diacrylates or dimethacrylates or at
least dihydric saturated alcohols, eg. ethylene glycol diacry-
30 late, ethylene glycol dimethacrylate, 1,2-propylene glycol
diacrylate, 1,2-propylene glycol dimethacrylate, 1,4-butanediol
diacrylate, 1,4-butanediol dimethacrylate, hexanediol diacrylate,
hexanediol dimethacrylate, neopentyl glycol diacrylate, neopentyl
glycol dimethacrylate, 3-methylpentanediol diacrylate and
35 3-methylpentanediol dimethacrylate. The acrylates and meth-
acrylates of alcohols with more than 2 OH groups can also be used
as monomers (c), eg. trimethylolpropane triacrylate or tri-
methylolpropane trimethacrylate. Also suitable are diacrylates or
dimethacrylates of polyethylene glycols or polypropylene glycols
40 with molecular weights of, in each case, 100-9000. Polyethylene
glycols and polypropylene glycols used to prepare the diacrylates
or dimethacrylates preferably have a molecular weight of, in each
case, 200-2000. Apart from the homopolymers of ethylene oxide and
propylene oxide it is also possible to use block copolymers of
45 ethylene oxide and propylene oxide or copolymers of ethylene
oxide and propylene oxide which contain the ethylene oxide and
propylene oxide units in random distribution. The oligomers of
BASF Aktiengesellschaft 940188 O.Z. 0050/44957
4 21S177~
ethylene oxide and propylene oxide are also suitable for pre-
paring the crosslinkers, eg. diethylene glycol diacrylate,
diethylene glycol dimethacrylate, triethylene glycol diacrylate,
triethylene glycol dimethacrylate, tetraethylene glycol diacry-
5 late and/or tetraethylene glycol dimethacrylate. Also suitable ascrosslinkers are vinyl esters of ethylenically unsaturated
C3-C6-carboxylic acids, eg. vinyl acetate, vinyl methacrylate or
vinyl itaconate. Also suitable as crosslinkers are vinyl esters
of saturated carboxylic acids containing at least 2 carboxyl
10 groups, and di- and polyvinyl ethers of at least dihydric
alcohols, eg. divinyl adipate, butanediol divinyl ether and
trimethylolpropane trivinyl ether as well as acrylamides or
methacrylamides of at least difunctional saturated amines such as
methylenebis(acrylamide) or ethylenebis(methacrylamide). Further
15 suitable monomers (c) are allyl esters of ethylenically
unsaturated carboxylic acids, eg. allyl acrylate and allyl meth-
acrylate, allyl ethers of polyhydric alcohols, eg. pentaery-
thritol triallyl ether, triallylsucrose and pentaallylsucrose.
Also suitable as crosslinkers are methylenebismethacrylamide,
20 divinylethyleneurea, divinylpropyleneurea, divinylbenzene, divi-
nyldioxane, tetraallylsilane, tetravinylsilane, 1,7-octadiene,
diallyl phthalate, trivinylcyclohexane, 1,9-decadiene or
triallyltriazinetrione. Divinylethyleneurea, allyl methacrylate
and diacrylates and dimethacrylates of at least dihydric alcohols
25 are particularly preferred.
In the case where copolymerization of the crosslinking monomers
(c) is desired, they are used in amounts of 0.01-20% by weight,
preferably 0.02-15% by weight, particularly preferably 0.1-8% by
30 weight.
The polymerization is carried out as precipitation polymerization
in a solvent in which the monomers are soluble and the resulting
polymers-are of low solubility or insoluble. The solvents used
35 according to the invention are organic solvents which have no
aromatic groups and, apart from oxygen, contain no heteroatom.
Suitable and preferred solvents are those selected from the group
consisting of saturated hydrocarbons with 5-12 carbon atoms,
dialkyl ethers with 2-12 carbon atoms, the C3-Cl2-ketones and
40 C1-C22-alkyl esters of Cl-C22-carboxylic acids.
Examples of suitable hydrocarbons are pentane, cyclopentane, hex-
ane, cyclohexane, methylcyclohexane, heptane, octane or iso-
octane. Examples of suitable ethers are dimethyl ether, diethyl
45 ether, diamyl ether, tert-butyl methyl ether or dibutyl ether.
Suitable ketones are dialkyl ketones such as acetone, methyl
ethyl ketone, diethyl ketone or methyl amyl ketone. The reaction
BASF Aktiengesellschaft 940188 O.Z. 0050/44957
215i770
can also be carried out in alcohols such as n-butanol, 2-meth-
yl-2-butanol, isoamyl alcohol, hexanol, cyclohexanol, octanol or
decanol. Examples of suitable alkyl carboxylates are ethyl for-
mate, methyl acetate, ethyl acetate, isopropyl acetate, isobutyl
5 acetate, stearyl acetate, 2-ethylhexyl 2-ethylhexanoate, methyl
stearate, isopropyl myristate or isopropyl palmitate. It is also
possible to use mixtures of said solvents.
Preferred solvents are pentane, hexane, heptane, cyclohexane,
10 methylcyclohexane, tert-butyl methyl ether, acetone, methyl ethyl
ketone, n-butanol, methyl acetate, ethyl acetate, isopropyl ace-
tate or isobutyl acetate. Heptane, cyclohexane or ethyl acetate
are very particularly preferred. The amount of solvent is prefer-
ably chosen so that the reaction mixture can be stirred during
15 the polymerization. The solids content of the reaction mixture is
preferably in the range from 10 to 40% by weight.
It is also possible to add to the mixture small amounts of up to
10% by weight, preferably up to 4% by weight, particularly pre-
20 ferably up to 2% by weight, based on the monomers used, of water,methanol, ethanol, isopropanol, protective colloids or emulsi-
fiers in order to exert a beneficial influence on the morphology
of the products or the viscosity of the reaction mixture. It is
also possible in this way to have a beneficial influence on other
25 properties of the copolymers, eg. the residual contents of
monomers or solvents during or after a workup step. Examples of
suitable protective colloids are polyvinylpyrrolidones, partially
hydrolyzed polyvinyl acetates, cellulose ethers or copolymers of
N-vinylpyrrolidone and vinyl acetate. The amounts of water and/or
30 emulsifiers present during the precipitation polymerization, if
used, are only such that the mixture of all the components still
appears homogeneous before the polymerization starts.
The molecular weight of the copolymers can, if desired, be
35 reduced by adding regulators to the polymerizing mixture, for
example halogen compounds such as tetrachloromethane, chloroform,
bromotrichloromethane, allyl compounds such as allyl alcohol or
2,5-diphenyl-1-hexene, aldehydes, formic acid or formic esters.
Polymerization regulators which contain sulfur in bound form are
40 preferably used. Examples of compounds of this type are inorganic
bisulfites, sulfites, disulfites and dithionites or organic sul-
fides, disulfides, polysulfides, sulfoxides, sulfones and mer-
capto compounds. Compounds which are particularly preferably used
are mercapto alcohols, mercapto carboxylic acids and mercapto-
45 alkanes with from two to 30 carbon atoms in the molecule, forexample 2-mercaptoethanol, 3-mercaptopropanol, 3-mercapto-1,2-
propanediol, 4-mercaptobutanol, cysteine, mercaptoacetic acid,
BASF AXtiengesellschaft 940188 O.Z. 0050/44957
. ~_
6 2 1 S 1 7 7 0
3-mercaptopropionic acid, mercaptosuccinic acid, n-butyl mercap-
tan, n-hexyl mercaptan, n-dodecyl mercaptan or tert-dodecyl mer-
captan. If polymerization regulators are used they are employed
in amounts of 0.1-15, preferably 0.1-5, % of the weight of the
5 monomers present in the polymerization.
The monomers are subjected to free-radical polymerization, ie.
compounds which form free radicals under the polymerization
conditions are needed to initiate the homo- or copolymerization.
10 Initiators which form free radicals are all conventional peroxy
and azo compounds, for example peroxides, hydroperoxides and per-
oxy esters such as hydrogen peroxide, dibenzoyl peroxide,
di-tert-butyl peroxide, tert-butyl hydroperoxide, diacyl perox-
ides such as dilauroyl peroxide, didecanoyl peroxide and diocta-
lS noyl peroxide or peresters such as tert-butyl peroctanoate, tert-
butyl perpivalate, tert-amyl perpivalate or tert-butyl perneode-
canoate, as well as azo compounds such as 2,2'-azobis(2-amidino-
propane) dihydrochloride, 2,2'-azobis[2-(2-imidazolinyl)propane]
dihydrochloride, 4,4'-azobis(4-cyanovaleric acid), 2,2'-azo-
20 bis(2,4-dimethylvaleronitrile), 2,2'-azobisisobutyronitrile,
2,2'-azobis(2-methylbutyronitrile), dimethyl 2,2'-azobis(isobuty-
rate), 2,2'-azobis(4-methoxy-2,4-dimethylvaleronitrile),
1,1'-azobis(1-cyclohexanecarbonitrile), 2,2'-azobis(2,4,4-trime-
thylpentane) or 2-(carbamoylazo)isobutyronitrile. It is also, of
25 course, possible to use mixtures of initiators or the known redox
initiators. Examples of redox initiators are combinations of at
least one peroxo compound such as potassium, sodium or ammonium
persulfate, sodium hypochlorite, sodium perborate, sodium percar-
bonate, hydrogen peroxide, tert-butyl hydroperoxide or di-tert-
30 butyl peroxide and at least one reducing agent such as ascorbicacid, lactic acid, citric acid, sodium sulfite or sodium bisul-
fite, acetone sulfite, sodium dithionite, sodium N-hydroxymethyl-
sulfinate or a tertiary amine such as dimethylaniline. The initi-
ators are employed in conventional amounts, eg. 0.1-6% of the
35 weight of the monomers to be polymerized.
The precipitation polymerization is normally carried out under
inert gas atmosphere. The polymerization can be carried out, for
example, by introducing all the components present during the
40 polymerization into a polymerization vessel, starting the reac-
tion and, if necessary, cooling the reaction mixture in order to
control the temperature. However, it is also possible to start
the polymerization with only a few or a portion of the components
and to meter the remainder of the components in continuously or
45 batchwise, singly or together, over periods which may differ
according to circumstances, depending on the progress of the
polymerization. However, it is also possible for initially only
BASF Aktiengesellschaft 940188 O.Z. 0050/44957
7 21517~0
the diluent to be present and for the monomers and the polymer-
ization initiators to be introduced separately, batchwise or con-
tinuously.
5 The temperature during the polymerization is generally from 40 to
160, preferably from 50 to 120 C. It can be controlled variously
during the reaction by a program. The polymerization is prefer-
ably carried out under atmospheric pressure but can also be car-
ried out under reduced or elevated pressure. If the polymeriza-
10 tion temperature is above the boiling point of the solvent, thepolymerization is carried out in pressure-tight equipment under
pressures up to 8 bar.
The polymerization process is preferably controlled so that the
15 resulting copolymer is in the form of a fine-particle powder. The
average particle size of the polymer powder is 0.01-500, prefer-
ably 0.5-200, ~m. After the polymerization, the crosslinked copo-
lymer is separated from the other components of the reaction mix-
ture, for example by filtration, decantation or centrifugation.
20 The resulting powder can be subjected, if necessary, to further
suitable separating, washing, drying or m; 11; ng processes.
Those polymers obtained by the process according to the invention
which are soluble in water or another suitable solvent preferably
25 have K values (determined by the method of H. Fikentscher, Cellu-
lose-Chemie, 13 (1932) 58-64 and 71-74 in aqueous solution at 25 C
and a polymer concentration of 1% by weight) in the range from 10
to 200.
30 After the reaction, the polymers can be converted in the same
medium, or after isolation in another medium, by a suitable
reagent to a quaternized form. Suitable for the quaternization
are, for example, alkyl halides having 1-18 carbon atoms in the
moleculej eg. methyl chloride, ethyl chloride, propyl chloride,
35 hexyl chloride, dodecyl chloride or lauryl chloride, as well as
benzyl halides such as benzyl chloride. The corresponding iodine
or bromine compounds are also, of course, suitable. Further
suitable quaternizing agents are dialkyl sulfates, in particular
dimethyl sulfate and diethyl sulfate. In some cases it is also
40 sufficient to convert the polymers into the salt form by treat-
ment with an acid. The quaternization can take place completely
or partially.
The polymers obtained according to the invention can be used, for
45 example, for binding bile acids in the blood to reduce the cho-
lesterol level or for selective removal of heavy metal ions from
solutions, and as auxiliaries in cosmetic formulations, for
BASF Aktiengesellschaft 940188 O.Z. 0050/44957
_
8 215177~
example to adjust the rheological behavior. The polymers accord-
ing to the invention can also be used as detergent additive to
inhibit transfer of dyes during the washing process.
5 Examples
Example 1
400 g of ethyl acetate, 100 g of N-vinylimidazole, 10 g of divi-
10 nylethyleneurea and 1 g of tert-butyl perpivalate were stirred at
72 C in a 2000 ml flask which was equipped with a stirrer, reflux
condenser, thermometer and an apparatus for working under protec-
tive gas for 2 hours. The resulting product was filtered off on a
suction funnel, washed with 100 g of ethyl acetate and dried in a
15 vacuum oven at 50 C for 8 hours. 111 g of a white powder with an
apparent density of 67 g/100 ml and an average particle size of
124 ~m were obtained.
Example 2
600 g of cyclohexane, 100 g of N-vinylimidazole, 10 g of
divinylethyleneurea and 1 g of azobisisobutyronitrile were
stirred at 77 C in a 2000 ml flask which was equipped with a
stirrer, reflux condenser, thermometer and an apparatus for
25 working under protective gas for 4.5 hours. The resulting product
was filtered off on a suction funnel, washed with 200 g of cyclo-
hexane and dried in a vacuum oven at 50 C for 8 hours. 109 g of a
white powder with an apparent density of 6 g/100 ml and an
average particle size of 30 ~m were obtained.
Example 3
400 g of methyl ethyl ketone, 100 g of N-vinylimidazole, 10 g of
allyl methacrylate and 1 g of 2,2'-azobis(2-methylisobutyroni-
35 trile) were stirred at 74 C in a 2000 ml flask which was equippedwith a stirrer, reflux condenser, thermometer and an apparatus
for working under protective gas for 2.5 hours. The resulting
product was filtered off on a suction funnel, washed with 100 g
of methyl ethyl ketone and dried in a vacuum oven at 50 C for
40 8 hours. 106 g of a white powder with an apparent density of
10 g/100 ml and an average particle size of 11 ~m were obtained.
Example 4
45 400 g of cyclohexane, 50 g of N-vinylimidazole, 50 g of N-vinyl-
pyrrolidone and 2 g of divinylethyleneurea were heated to 77 C in
a 2000 ml flask which was equipped with a stirrer, reflux
. BASF Aktiengesellschaft 940188 O.Z. 0050/44957
- 9 21~1770
condenser, thermometer and an apparatus for working under protec-
tive gas. As soon as this temperature was reached, 0.5 g of dime-
thyl 2,2'-azobisisobutyrate was added dropwise over the course of
2 hours. The mixture was then stirred at this temperature for a
5 further 4 hours. The resulting product was filtered off on a suc-
tion funnel, washed with 200 g of cyclohexane and dried in a vac-
uum oven at 50 C for 8 hours. 101 g of a white powder with an
apparent density of 32 g/100 ml and an average particle size of
12 ~m were obtained.
Example 5
400 g of cyclohexane were heated to 77 C in a 1000 ml flask which
was equipped with a stirrer, reflux condenser, thermometer and an
15 apparatus for working under protective gas. As soon as this tem-
perature was reached, 70 g of 1-methyl-2-vinylimidazole and 0.7 g
of mercaptoethanol were added dropwise over the course of 1 hour
and, in parallel to this, 0.4 g of azobisisobutyronitrile in
20 ml of cyclohexane was added dropwise over the course of
20 2 hours. The mixture was then stirred at this temperature for a
further 4 hours. The resulting product was filtered off on a suc-
tion funnel, washed with 200 g of cyclohexane and dried in a vac-
uum oven at 50 C for 8 hours. 68 g of a white powder were ob-
tained. A solution of the polymer in water was clear and color-
25 less and had a K value of 26.2 (determined by the method ofH. Fikentscher, Cellulose-Chemie 13 (1932) 58-64 and 71-74 at 25 C
and a polymer concentration of 1% by weight).
Example 6
400 g of ethyl acetate, 100 g of N-vinylimidazole and 17.3 g of
stearyl acrylate were heated to 70 C in a 2000 ml flask which was
equipped with a stirrer, reflux condenser, thermometer and an
apparatus for working under protective gas. As soon as this
35 temperature was reached, 1 g of tert-butyl perpivalate in 20 ml
of cyclohexane was added dropwise over the course of 2 hours. The
mixture was then stirred at this temperature for a further
4 hours. The resulting product was filtered off on a suction
funnel, washed with 200 g of cyclohexane and dried in a vacuum
40 oven at 50 C for 8 hours. 116 g of a white powder were obtained. A
solution of the polymer in ethanol had a K value of 31 (deter-
mined by the method of H. Fikentscher, Cellulose-Chemie 13 (1932)
58-64 and 71-74 at 25 C and a polymer concentration of 1% by
weight).
BASF Aktiengesellschaft 940188 O.Z. 0050/44957
~` 215177~
Example 7
15 g of the polymer powder from Example 1 were dispersed in 200 g
of ethanol and, at 40 C, 30 g of dimethyl sulfate were added over
5 the course of 30 minutes. The mixture was subsequently heated to
60 C and stirred at this temperature for a further 3 hours. The
product was then filtered off on a suction funnel, washed with
100 ml of water, stirred twice in 400 ml of a 10~ strength
aqueous NaCl solution for 15 minutes, again filtered off with
10 suction, washed with twice 100 ml of water and dried in a vacuum
oven at 50 C for 8 hours. 21 g of a polymer powder with an
exchange capacity of 5.8 meq/g were obtained.