Note: Descriptions are shown in the official language in which they were submitted.
` _ 2152357
Specification
Technological Aspect
This invention provides an agent for improving cerebral
metabolism, including glucose ester derivatives (glucose
pyranose derivatives), which is excellent in penetrating
activity across the blood-brain barrier.
Background
Transport from blood to the cerebral tissue of substances
necessary for the maintenance of cerebral function is extremely
impeded by the blood-brain barrier. Glucose, which is a sole
and very important energy source for the cerebral tissue, is
therefore, no exception as reported by Goldstein (Scientific
America, 254:74-83, 1986). The supply of glucose from blood
to inside of brain is controlled by carrier proteins which
are specific for the blood-brain barrier, through their
selective and active transport from the outside of the barrier
to the cerebral tissue. Glucose transported to the cerebral
tissue, is metabolized by hexokinase to glucose-6-phosphate,
which is a very important intermediate in glucose catabolism
system, and then entered into a metabolic pathway where it
is degraded up to an end product of the energy generating
system while generating simultaneously with high energy-
phosphoric compounds such as ATP through its linked
2152357
.. ,
phosphorylation reaction under influence of oxidation,
decarboxylation and other reactions.
Approximately 20 % of the total oxygen consumption in the body
takes place in the brain where a large amount of glucose is
concomitantly consumed. In the cerebral tissue, however, there
is no sugar storage in the form of glycogen, and therefore,
the brain is liable to fall into a state of energy metabolism
dysfunction within a short time when glucose supply is disturbed
due to blood hypoglycemia or to a decrease in the capacity
of the blood-brain barrier transport, the conditions of which
are just similar to those of respiratory or circulation
disorders.
In an acute situation, disorders appear first in an
metabolically active tissue site with the highest sugar
consumption, then to the next site in an order of sugar
consumption degrees. A cerebrum dysfunction starts at a blood
sugar level lower than 60 mg/dl, and hypoglycemic coma may
be caused when its level becomes lower than 20 mg/dl. In this
case, 50 % of human subjects will die unless they get an
improvement in the cerebral energy metabolic disorder within
5 minutes. Even if survived, they may suffer from sequela
such as dementia and a vegetable state depend on the degree
of disorder. Similar to brain hypoxia, an irreversible
neuronal symptom is known to progress in some cases even afer
revival from coma as a result of regressive degeneration due
to delayed cell death following excess release of glutamic
acid and elicitation of a calcium concentration increase in
2152357
neuronal cells, particularly when the coma state prolonged
or spasm occurred repeatedly.
The current treatment that has been considered best for cerebral
disorders resulting from hypoglycemic shock and disturbance
in consciousness associated with diabetic hypoglycemia is oral
or intravenous administration of glucose. This therapy method
has been widely exercised. This treatment can raise a blood
sugar level immediately. However, since glucose per ce cannot
cross the barrier, glucose needs to be captured first by carrier
proteins and then subjected to an active and selective transport
together with its carrier through the blood-brain barrier into
cerebral tissues. Because of this fact, a time-lag of a few
minutes is inevitable between administration of glucose and
attainment of a sufficient concentration of glucose at a site
of the cerebral tissue. This detriment in glucose
administration has remained to be solved, since supply of energy
source at a sufficient level to the cerebral tissue of these
patients must be carried out as urgently as possible.
In the same token as above, a decreased capacity in the active
transport of glucose at the blood-brain barrier due to aging
or cerebral disorders can result in reducing cerebral metabolic
functional competence and causing necrosis of the cerebral
tissue. Thereby, the administration of glucose, as mentioned
above, to the patients with incompetence in active transport
of glucose through the blood-brain barrier would result in
only an increase in blood sugar levels, but hardly in providing
their cerebral tissue with energy source. Thus, this therapy
. ` 2152357
-
cannot be an efficient one for improvement or maintenance of
cerebral metabolism in these patients.
In ventors of this invention studied this problem with use
of 1, 3, 4, 6-tetra-0-acetyl-N-(m-iodobenzoyl)-glucosamine
and 1, 3, 4, 6-tetra-0-pivaloyl-N-(m-iodobenzoyl)-glucosamine,
and reported a high transport rate of glucosamine ester
derivatives across the blood-brain barrier in Journal of
Labelled Compounds and Radiopharmaceuticals, 30:300-303, 1991.
However, anticipated has been the appearance of glucose
derivatives such as those that have a high transport rate at
the blood-brain barrier, regardless of the active transport
regulatory condition, and can reach the cerebral tissues
quickly so as to be converted to glucose-6-phosphate, therefore
act very effectively in improvement of the cerebral metabolism
in the patients who suffered blood hypoglycemic shock or
diabetic hypoglycemic coma. Thus, this invention is intended
to explore this type of contemplation.
Disclosure of The Invention
These inventors gave rise to the discoveries described below,
leading to the invention presented herein. The discoveries
are as follws: 1) glucose ester derivatives, having a transport
mechanism different from that for glucose, can reach the
cerebral tissue after crossing the blood-brain barrier without
any substantial time lag that often occurs in case of glucose
transport; 2) in the cerebral tissues, glucose ester derivatives
can be converted to the aforementioned catabolic intermediate
metabolite, glucose-6-phosphate, at much more accelerated rate
--4--
2152357
than the catabolic rate of these derivatives present in somatic
tissues outside the blood-brain barrier. These findings led
us to this invention described herein. That is, in this
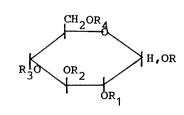
invention, a glucose ester derivative of the formula below.
CH20R
~ ~ H,OR
R30\0R2 ~/ ( 1 )
R1
~The anormeric substitute in the formula is eitherOL or~ ;
R - R4, are identlcal or different each other, and each
represents H atom, a straight or branched acyl group having
2 to 8 carbons, or acyl group containing a ring structure,
and in addition, at least 1 of said R - R4 is the acyl group
is used as an active ingredient of the present cerebral
metabolism improving agent.]
The Best Modes for The Practice of The Invention
Any of the glucose ester derivatives, shown as the general
formula above, can be converted quickly into glucose-6-phosphate
following administration. Because of this characteristic,
these derivatives are most effective in improvement of cerebral
metabolism in such patients who suffered from blood hypoglycemic
shock, and diabetic hypoglycemic coma that require urgently
the supply of glucose energy source, and also in the patients
who suffer from dementia or motor function disorder resulted
2I 52357
from lack of glucose energy source due to the transport
disfunction at the blood-brain barrier.
The most preferable glucose ester derivatives in this invention
are as follows: 1, 3, 4, 6,-tetra-0-acetyl-D-glucose; 1, 2,
3, 4, 6,-penta-0-acetyl-D-glucose; 1, 2-di-0-acetyl-3,
4, 6-tri-0-(2-methylbutyryl)-D-glucose; 1, 3,-di-0-acetyl-
6-0-butyryl-D-glucose; 1, 3, 4,-tri-0-acetyl-6-0-
nicotinoyl-D--glucose; 1, 2-di-0-ber.zoyl-D-glucose; 1-0-
cinnamoyl-D-glucose, etc. In particular, 1, 2-di-0-
acetyl-D-glucose, 3, 4, 6-tri-0-acetyl-D-glucose, 1, 3, 4,
6-tetra-0-acetyl-D-glucose, 1, 2, 3, 4, 6-penta-0-acetyl-
D-glucose are very effective and preferred.
Glucose ester derivatives of this invention can be administered
orally or non-orally in formulas of tablets, capsules, granules,
syrup, troche, elixir, injection, and suspension that are
manufactured in general pharmacological preparation methods
with use of fillers, disintegrant, binders, lubricants,
sweetening agents, alcohol, solubilizer, buffering agents,
water-soluble bases, emulsifying agents, suspending agents.
The effects of the invention shall be minutely stated in
connection with the following ~eference Examples and Examples,
and however, these should not be taken as being limitative
to the present invention and the working effects thereof.
[Reference Example 1]
The compound, 1, 3, 4, 6-tetra-0-acetyl-2-18F-D-glucose
~ . 2152357
._
(18F-AFDE) labelled with an electrophilic reagent, 18F, was
prepared according to the method of Shiue et al (Journal of
Nuclear Medicine, 23:889-903, 1982); 18F acetate, obtained
by passing 18.2 mg 18F through a column filled with sodium
acetate, was subjected to the reaction at 0 C with 3, 4, 6-
tri-O-acetyl-glucal in solvent of freon-11, resulting in a
yield of 60 mg 18F-AFDG.
tReference Exa~ple 2]
The control compound, 2-18F-D-glucose (18F-FDG) was obtained
in an amount of 10 mg by addition of 5 ml of 1 N solution of
hydrochloric acid to 30 mg of 18F-AFDG of the above Reference
Example 1 followed by heating the mixture at 130 C for 15
minutes.
tExample 1 ]
Normal male mice of the ddy strain at 6 weeks of age were
injected intravenously through tail vein with 0.05 ml of mM
18F in DMSO solution. Measurement of its blood concentrations
and radioactivities in the cerebral tissue of the mice revealed
that the radioactivity could be detected in the cerebral tissue
immediately after administration, and levels of the
radioactivity increased up to 30 minutes, whereas the blood
radioactivity levels decreased consistently following
administration, and at 10 minutes of administration, and
thereafter the radioactivity was found to be higher in the
cerebral tissue than in the blood (Figure 1).
`~ . 2152357
tExample 2]
Two tenth ml of 18F-FDG or 18F-AFDG in 50 % DMSO solution was
injected into the carotid artery of three different groups
of rats: the first group of rats injected without addition
of glucose; the second group with 20 mM glucose; the third
group with 80 mM glucose, and then the radioactivity of the
respective labelled compound transported into the cerebral
tissue was measured. The transport rate of 18F-FDG to the
cerebral tissue was decreased inversely as the concentration
of glucose was increased. On the other hand, 18F-FDG, an ester
derivative, could reach the cerebral tissue at transport rates
higher than 90 % with no competition even to the maximum
concentration of glucose, 80 mM (Figure 2). The finding that
the transport of the glucose ester derivative to the cerebral
tissue was not influenced by varying concentratioins of glucose
indicates that there is a transport mechanism across the
blood-brain barrier for the glucose ester derivative different
from the mechanism for glucose transport.
tExample 3]
Mice were given an intravenous injection of 0.05 ml solution
of 0.5 mM 18F-AFD in DMSO. The cerebral tissue harvested at
0.5, 2, 5, 60 and 180 minutes were homogenized for one minute
in ethanol, and then centrifuged at 700 x g for 10 minutes.
The supernatant was collected for individual measurements of
F-AFDG, its intra-cerebral metabolites, 18F-FD and 18F-FDG-
6-phosphate.
It was noted that approximately 40 % of 18F-AFDG reached the
~ 2152357
cerebral tissue, and also its intra-cerebral metaboletes,
F-FD and 18F-FDG-6-phosphate emerged within 0.5 minute
following administration. Thereafter, 18F-AFDG was metabolized
into 18F-FDG-6-phosphate, and the concentration of the latter
was raised markedly (Figure 3).
When intra-cerebral concentrations of metabolites were compared
with those in the blood at 5, 60 and 180 minutes following
administration of 18F-AFDG, 18F-FDG-6-phosphate was found at
the highest proportion in the cerebral tissue at the respective
time points of measurement, and at 180 minutes the entire
18F-AFDG was found to be metabolized into 18F-FDG-6-phosphate,
demonstrating a very rapid metabolic rate of the glucose ester
derivative to glucose-6-phosphate, especially in the cerebral
tissue (Figures 4 and 5).
In Figures 4 and 5, peaks at 0, 0.25, and 0.75 on axis represent
peaks of 18F-FDG-6-phosphate, 18F-FDG and 18F-AFDG,
respectively, where the highest peak measured is designated
100 %, and other two peaks are expressed as relative ratios
of the highest.
Lgend of Drawings
[Figure 1]
Kinetic changes in radioactivities in the blood and the cerebral
tissue of the mice injected with 18F-AFDG.
tFigure 2]
Transport rates to the cerebral tissue of 18F-AFDG and 8F-
2152357
FDG administered to rats with varying concentrations of glucose.
tFigure 3]Kinetic changes of 18F-AFDG, and its metabolites in the cerebral
tissue of the mice administered with this labelled compound.
tFigure 41
Kinetic changes in the ratio of intra-cerebral concentrations
of 18F-AFDG and its metabolites in the mice administered this
labelled compound.
lFigure 5]
Kinetic changes in the ratio of blood concentrations of18F-
AFDG and its metabolites in mice administered this labelled
compound.
-1 0 -