Note: Descriptions are shown in the official language in which they were submitted.
215~598
-
REMOVAL OF WATER VAPOR FROM ACIDGAS STREAMS
FIELD OF THE lNv~NllON
This invention relates to the removal of water vapor from gas
streams containing hydrogen sulfide, sulfur dioxide, carbon dioxide
and other acidic gases, and in particular to a process for the
production of elemental sulfur from such gas streams in which water
vapor is removed from the gas to P~h~nçe the production of
elemental sulfur.
BACKGROUND OF THE lNv~NlloN
Acidic gas streams are often encountered in the processing of
natural gas, crude oil, bitumen, heavy oil and coal-derived
liquids, for the removal of the sulfur content. Most often, an
2ls~598
acidic component, for example, hydrogen sulfide, is separated or
formed in the process and it, in turn, becomes a disposal problem.
The sulfur content of such an acidgas is generally converted to
elemental sulfur, and the residual acidgas heCo~es a waste stream.
The removal of sulfur prior to emission of the residual gas streams
to the atmosphere is regulated by laws to minimize the degree of
air pollution.
DESCRIPTION OF THE PRIOR ART
Most commonly, amine absorption is employed in natural gas
processing plants to sweeten sour natural gas through hydrogen
sulfide removal. After regeneration of the amine solution, a gas
stream (acidgas) emerges rich in acidic constituents such as
hydrogen sulfide and carbon dioxide. Generally, a version of the
modified Claus Process is used to recover elemental sulfur from the
acidgas via the following chemical reactions, singly or combined,
H2S + 1/2 02 ~ S + H20 (1) and/or
2H2S + SO2 3S + 2H20 (2)
Reactions (1) and (2) both proceed in a front-end combustion step,
typically in a furnace or burner, resulting in an acid gas
containing a 2:1 stoichiometric ratio (reaction 2) of hydrogen
sulfide to sulfur dioxide. The additional recovery of sulfur is
achieved, based on reaction (2), in staged fixed bed catalytic
reactors operating at successively lower temperatures. An
additional cleanup unit is usually required to achieve the degree
of sulfur removal mandated by emission regulations. The reaction
(2) is exothermic and reversible, and an approach to the limiting
equilibrium conversion of hydrogen sulfide is desirable. In the
21~598
past, this has been achieved by shifting the reaction to the right
by removal of the product, sulfur, by condensation after each
process reaction stage. Also, by successively lowering the stage
temperatures, the equilibrium collveLsion (and sulfur recovery) is
maximized at the outlet of the final stage.
Similarly, the removal of the product water vapor would also
theoretically shift the equilibrium ~ol.ve~ion additionally to the
right. However, the removal of water vapor from modified Claus
process streams by pressure-swing absorption has never been
utilized in practice because the preliminary economic analyses of
such process modification have shown it to be relatively more
capital-intensive and uneconomical. Moreover, the condensation of
water vapor by cooling a modified Claus process stream apparently
has been attempted in the past. Apparently, the attempt using a
stainless steel condensor for water removal, failed due to severe
corrosion. This failure is not surprising, since it is known that
hydrogen sulfide contacting aqueous solutions of sulfur dioxide
forms very corrosive polythionic acids called "Wackenroder's
solution". See tCHEMISTRY OF THE ~T~M~NTS, N. N. Greenwood and A
Earnshaw, Pergamon Press Ltd. 1984, page 849].
It is an object of the present invention to provide a process for
removing water vapor from acidic gas streams.
It is also an object of the present invention to provide a process
for removing water vapor from acidic gas streams in which the water
vapor is removed by dehydration with concentrated sulfuric acid.
It is another object of the invention to provide an improved
process for the production of elemental sulfur from acidgas streams
containing hydrogen sulfide, in which the production of elemental
sulfur is enhanced by removing the product water vapor by
dehydration with concentrated sulfuric acid.
2~5~598
According to the invention, an improved process for the production
of elemental sulfur according to the chemical reactions
H2S + 1/2 02 S + H2O (1), or
H2S + 3/2 O2 ~ SO2 + H2O (2), and
2H2S + SO2 3S + 2H2O (3)
including removing elemental sulfur after each reaction stage by
condensation above the boiling point of water, the improvement
comprising after one or more reaction stages also removing water
vapor by dehydration by direct contact of H2S with concentrated
sulfuric acid of a concentration of about 82 to about 96 weight
percent, is provided.
The sulfuric acid contact may occur following the removal of
elemental sulfur, at one or more stages/locations in the process
depending upon the the amounts of water vapor present at those
stages/locations, and whether the further im~uved sulfur recovery
in a particular reaction stage is economically justifiable.
Even though the modified Claus process preferably involves a series
of stages or reaction steps at successively lower temperatures to
achieve higher equilibrium conversions for chemical reaction (3),
the temperatures used may be less than the dew-point of sulfur
vapor (about 120 C), but not below the dew-point of water (about
100 C).
A further improvement in the production of elemental sulfur may be
achieved if hydrogen sulfide is permitted to react with the
concentrated sulfuric acid, according to the following chemical
2154598
reaction,
H2S + H2SO4 S + SO2 + 2H2O (4)
The extent to which additional elemental sulfur is formed through
reaction (4) is determined by the concentration/strength of the
sulfuric acid and the reaction temperature used in the dehydration
unit. Since SO2 is a by product of this reaction, further
modification of the inlet air : H2S ratios may be required to
accommodate the additional SO2.
Reaction temperatures of ambient to about 120 C have been found to
be appropriate to both enhance the production of elemental sulfur
by both shifting the equilibrium conversion attainable in the
reaction stages as described in equations (1) to (3), and in
promoting additional sulfur recovery from equation (4).
A still further improvement will be achieved if dehydration by
concentrated sulfuric acid contact is combined with the use of
oxygen-enriched air or pure oxygen in a continuous process using
reactions (2) and (3), as will be apparent hereinafter.
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 is a schematic illustration of a straight-through modified
Claus process, as known in the prior art.
Figure 2 is a graph illustrating the conversion of hydrogen sulfide
to sulfur, according to the invention.
Figure 3 is a schematic illustration of an apparatus according to
the invention.
215~598
..
DESCRIPTION OF THE PREFERRED EMBODIMENTS OF THE lN~NllON
The advantages of removing water Yapour are particularly apparent
in terms of impact upon process efficiency. Three different
examples illustrate gains to be achieved by removal of water vapor
from a modified Claus process unit.
Example 1
..
A simple straight-through modified Claus process without tailgas
cleanup is described in figure 1. This process includes a furnace
followed by two stages of catalytic reactors. Specifically, the
process includes a furnace in which about 1/3 of the H2S in the feed
is oxidized at flame temperatures via chemical reaction (2). The
resulting 2:1 ratio of H2S : SO2 is then chemically reacted over
alumina- or titania-based catalysts in two successive reaction
stages according to chemical reaction (3). The inlet temperatures
to the two stages are, for example, about 280C and about 260C,
respectively. After the furnace and after each of the two reactor
stages, sulfur vapor is condensed at about 120C to prevent
condensation of water vapor. Each of these three stages is
adiabatic and attains an H2S conversion such that the effluent gas
composition approaches the equilibrium conversion limit possible
for the given operating conditions.
Curves A to C' in figure 2 represent calculated equilibrium
conversions {for reactions (4) and (2)} as a function of
temperature for four different stream compositions resulting from
a 100% H2S acidgas feed to the furnace. Curve A and the 70~
conversion level define the equilibrium conversion attained in a
stream leaving the furnace and being cooled from 1100C to 227C
(500K). The feed composition to the furnace is given by A in table
1.
215~598
-
Table 1
Feed Composi~ions (wt%)
H2S S02 o2 H20 N2
A 29.58 0.00 14.79 0.00 55.63
B 6.94 3.47 0.00 24.30 65.29
C 2.74 1.37 0.00 31.49 64.40
C' 4.20 2.00 o.00 o.oo 94.00
The sulfur is removed by condensation at about 227C and curve B
shows the equilibrium conversions possible for the resulting stream
composition (composition B in table 1). The diagonal broken line
represents the adiabatic conversion path in the first catalytic
converter. Its intersection with curve B shows that 88% conversion
is reached after one catalytic reaction stage. After cooling to
about 227C, followed by a second stage of adiabatic catalytic
reaction, a final equilibrium conversion of 94% is attained. The
unconverted 6% of sulfur in the feed would necessitate additional
processing for tailgas cleanup as required.
If water vapor were removed after the sulfur condensor, by adding
concentrated sulfuric acid of concentration of about 82 to about 96
wt% after the first catalytic reactor, the composition of stream C
is altered to C'. The equilibrium conversions for the composition
of this dry stream are shown by curve C'. The intersection of the
second adiabatic reactor path with curve C' shows that the second
reactor is now able to achieve a 98.5% conversion.
Removal of water vapor after the furnace, where the bulk of the
water is produced(see composition B), would increase the overall
21~s98
conversion slightly and lower the downstream volumes of total
flowing process gas by more than 25% thus requiring smaller
processing vessels and lowering the capital costs.
Example 2
If the novelty of dehydration of Claus plant acidgaæes is combined
with the novelty of converting H2S directly to elemental sulfur (see
applicant's co-pending U.S. patent application Serial Number
08/198,790, filed 18 February 1994, the disclosure of which is
incorporated herein by reference), their combined application to
the modified Claus process becomes even more advantageous. If a
combined dehydrator/H2S oxidation unit is inserted after the second
catalytic converter of figure 1 as well as a dehydrator after the
first converter, the feed to the dehydrator/oxidation unit
corresponds to that of C' in figure 2. The 4.2% H2S would be
essentially completely converted to elemental sulfur in the
dehydrator/oxidation unit at about 120C and about 96 wt% sulfuric
acid. The resulting tail gas would contain the unreacted 0.46% S02
and 99.54% N2. This would correspond to 99.1% recovery of sulfur.
This recovery could eliminate the need for additional tailgas
cleanup since the low concentration of SO2 in the tailgas mixture
can, for many locations, be discharged directly to the atmosphere.
Example 3
The use of oxygen-enriched air or pure oxygen in place of an air
feed to a modified Claus plant has already been recognized to have
some advantages over a conventional Claus plant. The richer
feedgas(containing no nitrogen for example) increases the
conversions in each reaction stage, and requires a smaller flowing
volume, which in turn reduces equipment size requirements, thus
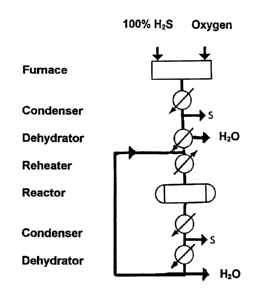
lowering capital costs. Figure 3 shows how an oxygen feed could be
combined with sulfuric acid dehydration in a continuous, re-
21S~5~8
circulating, in which sulfur and water vapor are continuously
removed, which theoretically could achieve a zero emission
operation. The process operates in the same manner as the
aforementioned modified Claus process through reactions (2) and
(3), but is designed to re-circulate the substantially dry and
sulfur-free unreacted H2S/SO2 (2:1) ratio to the catalytic reaction
stage. Perfect control of the 2:1 stoichiometric feed ratio is
essential here, otherwise, the excess gas(H2S or SO2) will build up
in concentration in successive recycle passes, thereby lowering the
conversion and necessitating a purge stream, with its accompanying
recovery problems. If SO2 in excess of the desired 2:1 ratio should
buildup, a pure H2S makeup stream would be required for ratio
control. On the other hand, it may be advantageous to operate the
re-circulation process with an excess of H2S to facilitate a more
complete conversion of SO2 during each pass through the catalytic
converter. The excess H2S could then be re-cycled to the plant feed
(prior to the furnace) since it is nearly pure H2S. If minor
amounts of other gases, e.g. N2 or CO2 are present in the feed, a
purge stream will be necessary irrespective of feed ratio control.
This purge stream could be incinerated since it cannot be recycled.
The details of the above process conditions essentially follow the
established practices employed in the operation of typical modified
Claus process plants, and will be well known to those skilled in
the art.