Note: Descriptions are shown in the official language in which they were submitted.
CA 02155166 1995-09-25
~-, r
~ ,s ~ ~'
T ITLL? OI~ THE :LNVENT ION
REFRIGFRA'fOR O1. L_, GOMfOSI'1'I0N AND
FLI1ID COMPOSI_TLON FOR REFRI_GERAT'OR
13ACKGBOUNI) OI~ fIIL INVENTION
1 . F f.eld of the lnveni=ion
'This invention relates t:o a refr-i.gerator oil
composition and a fluid composition i'or a rei'rigerator,
and in part=icular to a-~ refrigerator oil composition and
a fluid cornposit;ion containing said oi1_ composition for
I_0 use in a refrigerator, the refrigerator oil_ comprising
a mixed base oil. consisting essentially oi' an a_Lkyl
benzene oil having specific. features and a synthetic
oil and being suited for use in combination with an HFC
refrigerant containing 1,1,1,2-tetraf:Luor~oethane (HFC-
15 134x) and/or pentafluoroethane (HFG-1.25) .
2. Pior Art
Due to the rec~.ent prob:l_ems as to tlhe dest=ruction
of the ozone layer, the use of CfC (ch7_orofluorocarbon)
and HCFC (hydrochlorof:Luorocarbon) which have been
conventionally used as a refrigerant for a refrigerator
is now restricted under a regulation. 'Therefore, as a
replacement of these materials, HFC (hydrofluorocarbon)
has been increasingly employed as a refri~;erant.
Under the circumstances, PAG (p olyalkylene
glycol) and esters which are compatib.l_e w~~th HFC. have
been studied or used as an oil for a r-efrigerator using
an HFC refrigerant. For example, the use of fAG is
CA 02155166 1995-09-25
_ Z_
proposed i.n IJ . S . fal~ent 4 , 7 55 . 316 ; Japanese latent
Unexamined fublicat:ions Nos. I-lei I-198694, I-lei 1-
256594 , He i 7 -2590)3 , Hei 1-259094 , lief J_-25909 5 , Hei.
1-274191, Ile.i. 2-43290, Ilei 2--55797 and Ilei 2-84491. The
use of esters is proposed irr PC'f.l'ubi icat:ion No. fIei 3-
505602 as well as in .Japanese I='at.ent Une~s:amirled
Publicat ions Nos . 1;(e.i 3-88892 , Ile i 2--128991 , Ilei 3-
128992, Hei 3-2(:)0895, Hei 3-227397, liei 4.-20597, Ilei 4-
72390 , Iie i 4-21_8592 and Ilei. 4--249593 .
However, PACa i.s rather hi.g~t~ in hyg;roscopicity
and poor in electric o.harac:terist;ics (volume
resistivity). On the other hand, ester-based oi_Ls are
readily hydrolyzed to generate arr acid thus possibly
giving rise to various probl.erns. Moreover, these oils
are accompanied with s~ ser:ious problem that they are
inferior in lubricity as compared with a mineral
oil/CFC or a mineral oil/HCI~C.
On the other hand, Japanese Patent Unexamined
Publication No. Hei5-157379 describes a refrigerating
2~ system for an HFC-134a refrigerant wherein there is
used a refrigerator oil which is incompatible with a
refrigerant; as an example of such arr i.ncornpatible oi~_,
an alkylbenzene oil. is di_scl.osed t:herei.n. Further,
Japanese Patent; Unexamined Publication No. Hei 5-59386
describes a mixed oil comprising a hydrocarbon compound
and ester or ether, wltick is useful_ ats a refrigs=rator
oil for a refrigerator- using tetrafluoroetlnane.
CA 02155166 2004-07-15
- 3 -
However, it has been found that if an ordinary
alkylbenzene oil is to be used as a refrigerator oil
for HF(;-134a and/or HFC-125, some specific means are
required to be taken on the side of the system, and
that if' an ordinary alkylbenzene oil is used as a
refrigerator oil for HFC-134a and/or HFC-125 without
taking such specific means, the seizure of a
refrigerating compressor used may possibly be caused
after a long period of its operation.
1H The present inventors took notice of an
alkylbenzene oil (alkylbenzenes) which is free from
hydrolysis and hygroscopicity and made an extensive
study to finally find out that if a mixture comprising
an alkylbenzene oil having a specific property and a
15 specific synthetic oil is used as a refrigerator oil
for IIFC-134a and/or HFC-1.2 5 , the seizure of the
refrigerating compressor can be avoided, thus
indicating an excellent lubricity of the alkylbenzene
oil, and that the alkylbenzene oil is capable of
maintaining a high reliability for a long period of
time. This invention has thus been accomplished :in one
aspect.
It has further been found out by the present
inventors that when a phosphorus compound is added in
25 a specific ratio to the above mixed base oil compr:is:ing
an alkylbenzene oil having a specific property and a
synthetic oil and the resulting mixture is used as a
CA 02155166 1995-09-25
~ ~ ~~ ~3 ~. ~
- 4- -
refrigerator oil composition in a re-1'rigerator, the
wear resistance and .Load resistance c>f the refrigerai;or
can be improved. ~I'hi.s invention has thus been
accomplished in another aspect.
Summary oi' Lhe Invention
The object of the present; invention is to
provide <i refrigerator- oil composition to be used with
an HFC refri geraznt containing HFC-134a and/or HFC-125 ,
which enables a refrigerati..ng compressor to be
prevented from .its seizure, i_s excel.:l-ent in .lubricity
and retains high reliability for a long period of time.
Another object of the present: invention is t:o
provide a fluid composition for use in a refrigerator,
which comprises the above refrigerator o.il composition
and an HFC refrigerant containing HFC-139.a and/or HFC-
125.
In a first aspect of this invention, there is
provided a refrigerator oil for use with an I-IFC
refrigerant- containing HFC-1.34a and/or HfC-125, which
comprises
(A) 70 to 99% by weight of an alky:lbenzene oil
containing at Least 60~ by weight: (b<-rsed on the total
weight of the component (A)) of al.ky~lbenzenes having a
molecular weight of 200 to 350 and
(B) 30 to 1 ~ by weight of a synthetic oil
containing oxygen.
CA 02155166 2004-07-15
_ 5 -
In <i second aspect of this invention, there is
provided an oil composition for use with an HFC
refrigerant containing HFC-134a and/or HFC-125, which
comprises:
a mixed base oil comprising:
(A) 70 to 99% by weight of an alkylbenzene oil
containing at least 60% by weight (based on the total
weight of the component (A)) of alkylbenzenes having a
molecular weight of 200 to 350 and
(B) 30 to 1% by weight of a synthetic oil
containing oxygen, and
(C) 0.005 to 5.0 parts by weight (based on 100
parts by weight of the mixed base oil) of a p]losphOrus
compound.
1.5 In a third aspect of this invention, there is
provided a fluid composition for use in a refrigerator
which comprises:
[I] an HFC refrigerant containing HFC-134a
and/or HFC-125 and
[II] a refrigerator oil comprising:
(A) 70 to 99% by weight of an alkylbenzene oil
containing at least 60% by weight (based on the total
weight of the component (A)) of alkylbenzenes having a
molecular weight of 200 to 350, and
(B) 30 to 1% by weight of a synthetic oil
containing oxygen.
CA 02155166 2004-07-15
p
In a fourth aspect of this invention, there is
provided a fluid composition for use in a refriger-at-or
which comprises:
[IJ an IIFC refrigerant containing HFC-134a
and/or HFC-125 and
[II] an oil composition which comprises a mixed
base oil comprising
(A) 70 to 99~ by weight of an alkylbenzene oil
containing at least 60~ by weight (based on the total
weight of the component (A)) of alkylbenzenes having a
molecular weight of 200 to 350 and
(B) 30 to 1% by weight of a synthetic oil
containing oxygen, and
(C) 0.005 to 5.0 parts by weight (based on 100
Parts by weight of the mixed base oil) of a phosphorus
compound.
This invention will be further exp.l.ained in
detail with reference to the following preferred
embodiments.
The component (A) of the refrigerator oil or oil
composition (these expressions wi7_1 hereinafter bc;
collectively referred to simply as a refrigerator oi:l
composition) proposed by this invention comprises an
alkylbenzene oil containing at least 60o by weight
(based on total weight of the component (A)) of
alkylbenzenes having a molecular weight of 200 to 350.
CA 02155166 2004-07-15
To be more specific, it is required for the
al.kylbenzene oil to contain, based on total weight of
the component: (A) , at: least 60 ~ by weight, preferably
at least 65o by weight, more preferably at least 70% by
weight, still more preferably at least 80% by weight,
most preferably 100 by weight of alkylbenzenes having
a molecular weight of 200 to 350. If there is employed
an alkylbenzene oil which does not meet the above
requirements to the seizure of a refrigerating compressor
used may possibly be caused after a Long period of its
operation, thus undesirably affecting the reliability
of the refrigerator oil.
Further, in view of improving the property for
preventing the generation of seizure of a refrigerating
compressor during a long period of its operation, the
alkylbenzene oil. may desirably be selected from those
containing, based on total weight of the component (A),
at least 30% by weight, more preferably at least 35% by
weight, most preferably at least 40% by weight of
alkylbenzenes having a molecular weight of 200 to 300.
As for the alkylbenzene oil const.itut.ing the
component (A) of the refrigerator oil composition of
this invention, there is no restriction with respect to
the molecular structure of the component alkylbenzenes
as far as the molecular weight thereof falls within the
range of from 200 to 350. However, in view of
improving a long-term rel.iabi.lity of a refrigerating
CA 02155166 1995-09-25
system, iL i_s preferable to select <rtt alkyi_benzene oi:l
(a) composed of a:l.kya_benzenes ltavi_n~r 1 to 4 alkyl
groups , each group c:orttaining 1 t=o l ;) carbon atoms and
the Lota_l. amount; of carbon atoms i.n the ~ulky.l. group
being 9 to 19, and more preferably 1~u sel.ect
alkylbenzenes having 1 t;o 4 a_Lkyl groups, each group
containing 1 Lo 15 carbon moms and t:he total amount oi.'
carbon atoms in the alkyl group being ,j t.o 1. i .
Examples of alkyl groups cont;ainin.g 1 to 19
carbon atoms are methyl , ethy 1. , propyl ( incl.t.tding all
isomers), butyl (including all isomers), pentyl
(including all isomers), hexy~_ (.including all isomers),
heptyl (including all isomers), octyl. (including all
isomers) , nonyl (including all. isomers) , decy=1
(including all i.somers), undecyl (inc:luding all
isomers), dodecyl (including all isomers), tridecyl
( including all isomers ) , tet:radecy:l_ ( including all
isomers), pentadecyl (including a:L1 isomers), hexadecyl
(including all isomers), heptadecyl_ (:including all
isomers), octadecyl (incvluding~ all isomers) and
nonadecyl (including all isomers).
'These alkyl groups may be st;raig~ht-chain or
branched-chain ones. However, i.n view oi' the stability
and viscosity oi' the rzlky:Lbenzenes, branched-c:haa:in
2~ a:il{yl groups are preferable, arnd t:he branched--chain
alkyl groups drat are derived 1'rorn ol..igorners oi' o7_efins
CA 02155166 2004-07-15
- 9 -
such as propylene, butene and isobutylene in view of
availability.
The number of alkyl groups in the a_lkylbenzenes
defined in the above (a) is confined to 1 i;o 4.
However, in view of the stability and availability of
the alkylbenzenes, it is the most preferable to select.
alkylbenzenes having one or two alkyl groups, i.e.,
a monoalkylbenzene, a dialkylbenzene or a mixture of
them.
It is also possible to employ not only the
alkylbenzenes defined in the above (a) which have the
same molecular structure, but also those having
different molecular structures as long as there are
satisfied the conditions that they contain 1 to 4 alkyl
1.5 groups, each group containing 1 to 19 carbon atoms and
the total amount of carbon atoms in the alkyl group
being 9 to 19.
It is permissible for the alkylbenzene oil
constituting the component (A) to contain less than 40%
by weight, preferably less than 35% by weight, or more
preferably less than 30% by weight, of alkylbenzenes
having a molecular weight of less than 200 or more than
350. However, it i.s preferable that the molecular
weight of such alkylbenzenes be confined to a range of
more than 350 to 450, more preferably more than 350 to
430 in view of retaining reliability during a long
period of operation of a compressor used.
CA 02155166 1995-09-25
~~.~_)~.~'
__ i o -
Wi t:h respec:i: t:o t;he alkyl.benze.nes having; a
rnol.ecular- wo.ight ranging from more: t;ltrm .'.350 i_.o 450,
there are n<~ restri_cL,ions _Ltnposec.l on tlrc~ molecular
structure' thereof as L'ar' as the molec:ul_ar we iglats fall
within this range. however , itr view o(.' the stability
and availabilii~y, of alkylbenzenes i.t; is preferable to
select al..kyl.ben.zenes (b) having .l Lo 4 alkyl groups,
each groul;~ <:onLaritrirrg 1 to 40 cur'hoir ari.orins acrd the
total amount of carbon atoms in t,ho <rlkyl. group being
ZO to 40, and more preferably Lo se.lec~. alky:Lbenzenes
having 1 to 4 alkyl groups, each group c:ontaining 1 to
30 carbon atoms and the total. amount caf carbon atoms in
the alkyl group being 20 to 30.
>Jxarnp:les of al.ky.l. groups conl;ai.n:inrg 1 to 40
carbon atoms arc' tnethyl_, et-hyl, propyl. (i.ncluding all_
isomers) , buty:l (it~cl_ud:itrg al:l i.somors) , pentyl
(:inc_ludingr al.l_ isotner:~) , hexy:L ( i m:.l.uc.l:i.ng a.ll_ isomers) ,
heptyl ( i.nclud.Lng all. isomers ) , oc:ty.t ( including all
isomers) , nonyl (inc:luding all isomers) , decyl
( including al l isomers) , undec:yl. ( i.nc:luding all
isomers) , dodec:yi ( incvl_uding al.l isomers) , tridecyl_
(including a:l_1. isomers) , tetradecyl. (including all
isomers ) , perrtadecyl ( :i nclud ing <i L:1 i somers ) , hexadecyl
( i.nc:luding a:ll i somers ) , Ire~p t~adec.;y:l ( i rtcludi.ng all
isomers) , octadecyl. (incl.udirug~ al..l isomers) , nonadecyl
( including all. i sourer s ) , is:osylg~rouys ( i.nc:luding all
isomers), henicosyl. groups (including all isomers),
CA 02155166 2004-07-15
docosyl groups (including all isomers), tricosyl groups
(inc:Luding all isomers), tetracosyl groups (including
all isomers), heptacosyl groups (including a:Ll
isomers), hexacosyl groups (inc:luding all. isomers),
heptacosyl groups ( i.ncluding all i comers ) , oct<~cosyl_
groups (including all isomers), nonacosyl groups
(including all isomers), triacontyl. groups (including
all isomers), hentriacontyl groups (including all
isomers), dotriacontyl groups (including all i.somers),
tritriacontyl groups (including all isomers),
tetratriacontyl groups (including all isomers),
pentatriacontyl groups (including all isomers),
hexatriacontyl groups (including all isomers),
heptatriacontyl groups (including all isomers),
1J oc:tatriacontyl groups (including all isomers),
nonatriacontyl groups (including all isomers) and
tetracontyl groups (including all isomers).
These alkyl groups may be straight-chain or
branched-chain ones. However, in view of the stability
and viscosity of the alkylbenzene, branched-chain alkyl
groups are preferable; and branched-chain alkyl groups
that are derived from an oligomer of an olefin such as
propylene, butene or isobutylene are preferable in
view of their availability.
The number of alkyl groups in the alkylbenzenes
defined in the above (b) is confined to 1 to 4.
However, in view of the stability and availability of
CA 02155166 2004-07-15
- 12 -
the alkylbenzenes, it is the most preferable to select
alkylbenzenes having one or two alkyl groups, i.e., <~
monoalkylbenzene, a dialkylbenzene and a mixture
thereof.
It is also possible to employ not only the
alkylbenzenes defined in the above (b) which have the
same molecular structure, but also those having
different molecular structures as long as there are
satisfied the conditions that they contain 1 to 4 alkyl
1.0 group, each group containing 1 to 40 carbon atoms and
the total amount of carbon atoms in the alkyl group
being 20 to 40.
Although there is no specific restriction
imposed on the viscosity of the alkylbenzenes
1.5 constituting the component (A) of the refrigerator oil
composition of this invention, it is preferable to
select alkylbenzenes having a kinematic viscosity of 3
to 50mm2/s, preferably 4 to 40mm2/s, and
preferably 5 to 35mm2/s at a temperature of 40°C.
20 There is no restriction placed on the
manufacturing method of the alkylbenzene oil
constituting the component (A) of the refrigerator oil
composition of this invention, and the alkylbenzene oil
can be manufactured according to the following
25 synthesizing methods.
Aromatic compounds which may be used as a raw
material include benzene, toluene, xylene,
CA 02155166 1995-09-25
- 13 -
ethyl.benzene, methyl.ethylbenzene, dic:~tlryltoenzene and a
mixture thc,reof. f11ky1ating agents, which may be used
herein include a 1_ower mono-olefin srtch as ethy:l_ene,
propylene, butene or isobutylene: prcefcera.bly an ole-Cin
oI' a straight-c:train or branched-ctrai.r~ type having 6 to
40 carbon atoms that is obtained h,y the polyrneri.zation
of propylene; an olefin of a straighi.-chain or
branched-chain type having f to 40 carbon atoms that is
obtained by the thermal decornpos i ti.orr cof wax, heavy
oil, a petroleum fraction, polyethylene, polypropylene
or the like; an olefin of a straight-chain type having
6 to 40 carbon atoms i;hat is obtai.rred toy separating n-
paraffin from a petro:Learn fraction such as kerosi.ne or
gas oil and then catal.yt.ical.l.y transforming t;he n-
paraffin into an olefin; and a rnixture of these
o:Lefins.
An alkylating catalyst for use in tare alkylati_on
includes a conventional c:atal.yst exempla Pied by a
Friedel-Crafts cater:Lyst such as a l.umi rmrn ~;:h:Loride or
zinc chloride; or an acidic c:atal.yst such as sui_furic:
acid, phosphoric acid, s:i:iico-t=ungstic acid,
hydrofluoric acid or activated clay.
The al_kylbenzene o.i.l.. c:orrsti.tutir~g the component
(A) of the refrigerator <.~il composi.tion of i~his
invention may be obtained by mixing separately prepared
aa_kylbenzenes having a mol.ecul..ar weight ranging from
200 to 350 wi th alk3~lbenzenes havi ng <i mo7_ec:ular weight
CA 02155166 2004-07-15
- 1~
of less than 200 or more than 350 in a ratio as defined
by this invention. However, it is advisable in
practice to obtain a distillate containing at least 60~
by weight of alkylbenzenes having a molecular weight;
ranging from 200 to 350 through distillation or
chromatography from a mixture of alkylbenzenes which is
manufactured according to the~method explained above or
is available in the market.
Meanwhile, the component (B) of the
refrigerator oil composition of this invention is a
synthetic oil containing oxygen. Preferable examples
of this component (B) are an ester, polyglycol, ketone,
polyphenyl ether, silicone, polysiloxane and
perfluOrO ether. Among them , ( c ) ester , ( d ) polyglycol ,
(~') ketone and a mixture of them are especially
preferable.
Examples of (c) esters are a dibasic ester, a
polyol ester, a complex ester, a polyol carbonate and a
mixture of them.
Examples of dibasic esters are those that can be
obtained by reacting a dibasic acid having 5 to 1.0
carbon atoms such as glutamic acid, adipic acid,
pimelic acid, suberic acid, azelaic acid or sebaci_c
acid with a monohydric alcohol having 1 to 15 carbon
atoms and an alkyl group of a straight-chain or
branched-chain type, such as methanol, ethanol,
propanol, butanol, pentanol, hexanol, heptanol,
CA 02155166 2004-07-15
- 15 -
octanol , nonanol , decanol , undecanol , dodecanol ,
trie~e~~anol , tetradecanol or pentadecanol . These esters
may also be used as a mixture. More particularly,
these esters include ditridecyl glutarate, di 2-
ethylhexyl adipate, diisodecyl adipate, ditridecyl
adipate, di 2-ethylhexyl sebacate and a mixture of
them.
Examples of polyol esters are esters between a
diol or a polyol having 3 to 20 hydroxyl groups and a
1.0 fatty acid having 6 to 20 carbon atoms. More
particularly, such diols include ethylene glycol, 1,3-
propanediol, propylene glycol, 1,4-butanediol, 1,2-
butanediol, 2-methyl-1, 3-propanediol, 1, 5-pentane
di of , necopentyl glycol , 1 , 6- hexanediol, 2-ethyl--2-
methyl-1, s- propanediol, 1, 7- heptanediol, 2-methyl-2-
propyl-1 > 3- propanediol, 2 , 2-diethyl-1, 3- propanediol,
l , g- octanediol, l , 9- nonanediol, ~ , lo- decanediol,
l ,11- undecandiol and 1,12- dodecandiol. specifi c'
examples of polyol are polyalcohols such as trimet.hylol-
. ethane , trimethylolpropane, trimethylolbutane, di-
(trimethylolpropane), tri-(trimethylolpropane),
pentacrythritol, di-(pentaerythrito_L), tri-
(pentaerythritol), glycerin, polyglycerin (glycerin
dimer to icosamer), 1,3,5-peritanetr101, sorbitol,
sorbitan, sorbitol-glycerin condensate, adonitol,
arabitol, xylitol and mannitol; saccharides such as
xylose, arabinose, ribose, rhamnose, glucose, fructose,
CA 02155166 2004-07-15
- 16 -
galactose, mannose, sorbose, cellobiose, maltose,
isomaltose, trehalose, sucrose, raffinose, gentianose
and melezitose, the partially etherified products of
these polyalcohols and saccharides; and
methylglucoside. More particularly, such fatty acids
include pentanoic acid, hexanoic acid, heptanoic acid,
octanoic acid, nonanoic acid, decanoic acid, undecanoic
acid, dodecanoic acid, tridecanoic acid, tetradecanoic
acid, pentadecanoic acid, hexadecanoic acid,
heptadecanoic acid, octadecanoic acid, nonadecanoic
acid, icosanoic acid, oleic acid (these fatty acids may
be of a straight-chain or branched-chain type), or a
neo acid where a carbon atom thereof is quaternary.
More specifically, valeric acid, isopentanoic acid,
caproic acid, enanthic acid, 2-methylhexanoic acid, 2-
ethylpentanoic acid, caprylic acid, 2-ethylhexanoic
acid, pelargonic acid, 3,5,5-trimethylhexanoic acid may
be preferably empoyed as a fatty acid. Polyolester may
contain a free hydroxyl group. Preferable examples of
the pOly01 ester are esters of a hindered al.coho~_ such
as neopentyl glycol , trlmethylOletharie, trimethylol-
propane , trimethylolbutane, di-(trimethylolpropane),
tri-(trimethylolpropane), pentaerythritol , di-
(pentaerythritol) and tri-(pentaerythritol). Specific
examples are neopentylglycol 2-ethyl.hexanoate,
trimethylolpropane caprate, trimethylolpropane
CA 02155166 1995-09-25
~~ ~~~ ~~s
- L~ -
pelargonat;e, pentaerythritol 2-et;hyllrexanoa:rte,
pentaerythritol pelargonate or a mixt=ure of them.
A complex ester is an ester obtained by t°cacting
a fatty acid and a dibasic acid with a monolrydric
alcohol and a polyol.. Lxatnpi.es of l'szt l:y uc i.ds , dibasi <.:
acids, monohydric alcohols and po.lyols useful in this
case may be the same rrs exemplif'ie<j above with
reference to dibasic: esters and p<alyoi. esters.
A po:lyo:l carbom:rte ester i.s an aster obtained by
reacting carbonic acid wit! a monohydr ic: alcohol and a
polyol . Examples of mortohydr i c rxl.c:oho Ls and polyols
useful in this case tray be not. only the same as
exemplified above, but a:Lso polyglycol obtained through
homopolymerizat:i_on or copolymerizatiotr of a dio 1. , and
products which are obtainable by the addition reaction
o.f po7_yglycol wi th the po7_yol rnen t:ioned above .
Lxanrples of polyg.Lyc:ol. (cl) suitable in th:LS case
are polya:lkylene glyc:o.ls, Ethers i,hereof and modified
compounds thereof.
2p Examples of polyal.kylene glyco:Ls a:re
homopolymers or copol_yrners of an alky 1_ene oxide such as
ethylene oxide, propylene: oxide or t:~ut~ylene oxide. When
a polyalkylene glycol. i.s formed of a <:opolymer of
alkylene oxides different; in structure from eacYr other,
there is no restrioi;i.on placed on the polymeriza.t:i.on
form of oxyalkylene groups, i.e., the form may be
random copolymer.ization or block copo.l.ymerization.
CA 02155166 2004-07-15
- l~ -
An ether of the polyalkylene glycol is a
compound wherein the hydroxyl group of the polyalkyl_ene
glycol i.s etherified. Examples of ethers of the
polyalkylene glycol are monomethyl ether, monoethyl
ether, monopropyl ether, monobutyl ether, monopent-yl.
ether, monohexyl ether, monoheptyl ether, monooctyl
ether, monononyl ether, monodecyl ether, dimethyl
ether, diethyl. ether, dipropyl ether, dibutyl ether,
dipentyl ether, dihexyl ether, diheptyl ether, dioctyl
ether, dinonyl ether and didecyl. ether.
Examples of the modified compounds of 1 polyglycol
are an adduct of polyol with an alkylene oxide or an
etherified product of polyol. Examples of polyol
useful in this case may be the same as those
exemplified i.n reference to polyol esters.
Examples of ketones (e) mentioned above are ones
which contain in the molecule at least one group
represented by the following general formula:
O
-C-C-C-
(More particularly, the ketones (e) include
ketone compounds represented by the following formulas
(1), (2) and (3), and a mixture of these ketone
compounds.
(R'-C)m-X-(R2)n ___-_-(1)
CA 02155166 1995-09-25
,.~ .~ ,~ i ~~
y 4~ ,..
- l S3 -
wherein X is au m+n valent. aromatic ring or an
al.ky.1-substi tuted <r.r<>mat:i c r i rtg W rv.i.rrg 6 to 5U carbon
atoms, preferably E; t-o 20 carbon at;c.~ms; 1~t1 and R~ may
be i;lre same or dif:(°er:w:nt: and are eac:lr tr hydrocarbon
group having 1to 50 carbon atoms, lrr~:L'c~rably 1 to 3U
carbon atoms, peel'erak~l_e examples thc~reoi' being an
alkyl , phenyl or alky:ipltetryl group ; m and n rnay be tyre
same or di.i'ferent integers and arse emc:lr 1. to 20,
t~reCerably 1. to 10.
Preferable examples oI' the atwomatic r:Lng
representi.trg X axre a k~enzene t~ing, a naplrthal_ene ring',
an anthracene r:Lng, <r phenanthrene ring acrd an alkyl-
substituted arornat.ic ring wherein ai. least one hydrogen
atom on said aromati_<:: rings is subst:i tuted by an alkyl
L5 group having J to 20 carbon atoms .
Preferable examples caf R1 and R~ are rnethyl,
ethyl_ , propy:l ( Lnclud.i rrg al_:l i sonrers ) , butyl ( including
all i.sorners) , peutyl ( inclucli_rrg a:Ll isc:nnor~s) , hexyl
(including all. isomers), troptyl (i_nc:luding al__L
isomers), octyl (including all isomers), nonyl
( including all isomers ) , dec:yl ( includ i.ng all isomers ) ,
undecyl. ( .incl.ud.i.ng ~jl..l isomers ) , dodecyl ( including all
isomers) , tridecyl. ( i.rrc:lud:ing all. isomers) , tetradecyl
( :inc:luding al:l i comers ) , p~ni~adec.:yl ( including a_l.l
Z5 isomers ) , hexadecy:L ( irrc.Ludi.ng a1.1 isomers ) , heptadecyl
( including all isomers ) . oc:tadecy_t( iuc:ludirrg~ all
isomers ) , nonadecy_i ( i.ncl_uding al l isomers ) , icosyl
CA 02155166 1995-09-25
~a,
. rJ ~i ~. ~) '~:~
p _
(including al.l isomers) , henic:osyl ( including al_1
isomers), doc:osyl (including al.l isomers), tricosyl
( including all i somers ) , tetrac:osyl ( irtc:luding rzl.l_
isomers ) , hep tac:osy:l ( including al_1 i sortrers ) , hexac:osy l_
( including a.ll isomers ) , hep t<zcosyl ( includitrg all
isomers) , ocl;acosy:l ( ino.luding al 1. isomers) , rtonac:osyl
( including a L.l_ son~ers ) , tar iac~ont:yl ( i.nc:a.udi.ng a:L:L
isomers ) , phony 1. , tol_5~1_ ( itrcl.uding a) 1 i som~rs ) , xyl.yl
(including al..l isomers) , ethylptrerty:i_ ( inc,l ud.i.ng all.
isomers) , prc>pylphony.l (incl.uding all i.scuners) ,
ethylmethylphenyl (i_ncluding al.l isomers), buty:l.phenyl
( including all. isomers) , d.iethy:Lphenyl ( inclu.ding all
isomers), pentylphenyl. (inc_Luding all isomers),
hexy:Lphenyl ( :i.n<:luding al l i comers ) , hop t.ylphenyl
( including a.11 isomers ) , octylphenyl( .includ:ing all
isomers ) , nonylphenyl ( including nllisomers ) ,
decylphenyl (inc:luding all isomers) , undecylphenyl
( including al_1 isomer s ) , dodecyl_~~henyl ( irrcludi_ng all_
isomers) , tridec:ylphenyl ( inc:l.uding al.l lSOIrrE'.rS) ,
tetradecylpherryL (inc:luding al_1 isomers) ,
pentadecylphenyl (inca.uding al. 1. isornc~rs) ,
hexadecyl.phenyl ( including trl1. i.sconer s ) ,
heptadecyl.phenyl ( i nclud ing al:L isomers ) ,
octadecyl.phenyl_ ( inc;ludiug al.l isomers ) ,
nonadec:ylphenyl. ( inc:lrrdi.ng al:L isomers ) , icosylphenyl
( inc:l.udl.ng al.l isomers ) , hc~n:ic:osy:Ll~henyl ( i.nc:Lulling all
isomers ) , doc:osy lphenyl. ( including al l i somers ) ,
CA 02155166 1995-09-25
f~
l _
tricosylphenyl (inc:ludirrg all isomers) and
tetracosylphenyl_ ( inc:Luding a1.:1_ i sonretvs ) .
0 0
IZ'3_(~-[_.1Z4_L~_lL,-R' __.__-_(2)
wherein 1Z3 and R' may be i~he same or different
and are individual_l.y a hrydroc:arbon group having 1 to 50
carbon acorns , prefe~rrrt~ly 1 to ,30 tarpon acorns ,
preferab:l y an zt.l_ky.l , a lv>henyl or ao ,:rlkylpltenyl. group ;
R4 is an al.kyl..ene group having 1 t;o 19 carbon atoms,
preferably 1 to 1_0 carbon atoms; L, is an integer of 1.
to 5, preferably 1 to 3.
1'referabl.e examples of R'~ anti R' are alkyl,
phenyl and alkyl.pheny:l. groups by which are exemplified
R1 and R~ in the compounds represeni:ed by the general
formula ( 1_ ) .
r'urther, R4 is preferably <zrr al.kylene group
which includes tnethylene , ethylene ( i nc:.l.uding a7 1
isomers) , propyl..ene (inc:luding a1 L isomers) , butylene
( inc:luding al.l isomers ) , pentylene ( i ncl_ucling al l
isomers) , hexylene (i.ncluding all isomers) , heptylene
( including all isomers ) , oc:tyl.ene ( inc.Luding all
isomers ) , nonyl.ene ( including all. isomers ) or decylerre
group ( incl.uding~ a1.1 i.sorners ) .
(
Ei 7 (~ 5 9
A-[-()-(R. 0)p-l~ -(,-(R 0)c.l-R ~r __._(;1)
CA 02155166 1995-09-25
wherein A i s a monolydric t=o i cosah3ydri.c alcohol
residue ; Cti' , 1z7 an<:l R3 may be the sarnc or different arid
are indi.vidua:lly ar:c a:Lkylerue group laving 1. to 4 carbon
atoms ; R1j is a hydrocarbon group , prel'erabl.y an alkyl ,
phenyl or a1_ky.Lplretty l group each having 1 t;o 50 carbon
atoms, pret'erab7_y 1. t;o 30 c:arbors acorns; F~ and q rnay be'
the same or different integers and are individually 0
to 30, prei'erably 0 to 20; and r is an integer of 1 to
20.
A.lcohol_s for the alcohol residue A, include
aliphatic monohydric alcohols suc:lr as methanol,
ethanol , propanol. ( including all isomers ) , butarrol
( including all. isomers ) , pen t;ano.l. ( i rrc: l.uding all
isomers) , hexano.l (i.rm:l.uding all. i.scmers) , heptano:l
(including al.i isomers), oct;anol (inc:Luding a:11
i_sotners) , nonanol (inc:luding all isomers) , decanol
(including a17. isomers), undecanol. (incl.ud.ing all
isomers) , dodecanol ( i.nc:ludirrg a11. isomors) , tridecanol
( including al.l _i.somers) , tei:r~adecvatrol ( including al.l
isomers ) , pentadecanol ( i nc:luding sill isomers ) ,
hexadecanol (including all isomers), heptadecanol
( incl.uding all. isomers ) , octadecano.l ( i.ncludi.ng all
isomers) , nonadecanol. (inc:l.ud:ing a.l.:L isomers) ,
icosanol (including all isomers), tuenicosanol
(including all isomers), docosanol_ (inc:luding all
isomers), tricosano:L (including al.l isomers) and
tetrac:osanol ( .irrc:ludi.rrg all. i comers ) ; cl iols such as
CA 02155166 2004-07-15
- 23 -
ethylene glycol, 1,3-prOparied101, propylene glycol,
1, 4- butanediol, 1, 2- butanediol, 2-methyl-l , 3-propane
d.iol, 1,5- peritaned101, neopentyl glycol, l,s-hexane_.
diol, 2-ethyl-2-methyl-1,3- propanediol, 1,7-heptane _
diol, 2-methyl-2-propyl-1,3- propanediol, 2,2-diethyl-
l , 3- propanediol, i , g- octanediol, l , s- nonanediol,
l , lo- decanediol, 1,11-undecandiol, and 1,12-dodecan -
diol; polyols including polyhydric alcohols such as
trimethylolethane, trimethylolpropane, trimethylol-
butane, di-(trimethylolpropane), tri-(trimethylol-
propane), pentaerythritol, di-(pentaerythritol), t=ri-
(pentaerythritol), glycerin, polyglycerin (glycerin
dimer to icosamer consisting of 2 to 2Q glycerin
monomers), 1,3,5- pentanetriol, sorbitol, sorbitan,
sorbitol-glycerin condensate, adonitol, arabitol,
xylitol and mannitol, and saccharides such as xylose,
arabinose, ribose, rhamnose,.glucose, fructose,
galactose, mannose, sorbose, cellobiose, maltose,
isomaltose, trehalose, sucrose, raffinose, gentianose
and melezitose, the partially etherified products of
these polyalcohols and saccharides; and
methylglucoside.
Preferable examples of alkylene groups
representing R5, R7 and Rg are methylene, ethylene
(including all isomers), propylene (including all
isomers) and butyl.ene (including all isomers).
CA 02155166 1995-09-25
a
Z~.~~'~~ ~~f~
(referable examples o1' R'~ _are alkyl, phenyl and
alkylphenyl groups by which are exernl.~l i.f'ied ftl and R~
i.n the c:ornpouncls reps esented by i=he>, yerrera.7 formulct
(1).
'fhe lower .l irni i, o f' tire con t;en t. ca P t;he c;ornponen t
(A) i.n a reI'ri.gerator oit. (a rnixecl base oi.l) of this
invention comprising the components (A) and (B) is at
least 70i by weight, 1:>re('erabl.y azt le<zst 75a by weight,
more preferably at Least 80% by weight, bused on the
total weight of the components (A) and (l3) , wtii.l_e the
upper 1_imit of the content o1' the component (13) is not
more than 30~ by weight, preferably nc~l: more Lhan 25%
by weight, more preferab:.l.y not more t;han ;~0°~ by weight,
based on the total weight.: of Lhe componE.n~ts (A) and
(g),
On the other Lrand , the upper' 1 irni t of thc.
content of the component (A) in a refr-igerator oil (a
mixed base of l) of t:h.is i anent ion cornlari sing the
components (A) and (13) is not more tlrun 99% by weight,
preferably not rnore than 95% by weight;, more preferably
not more flan 90 ., by weight , based on t;tre total weight:
of the components (A) and (13), while tire lower limit of
the content of the component (13) is ai: least. 1% by
weight, preferably at least 5~ by wei.g~ht, more
preferably at least 10% by weight, based crn the total
weight of the components (A) and (B).
CA 02155166 2004-07-15
If the lower limit of the content of the
component (A) is Less than 70% by weight based on the
total weight of the components (A) and (B), a long germ
reliability of the refrigerator oil composition will
undesirably deteriorate. On the other hand, if the
upper limit of the content of the component (A) is more
than 99% by weight based on the total weight of the
components (A) and (B), the return of the refrigerator
oil composition in a refrigerating system will
to undesirably deteriorate.
The refrigerator oil of this invention comprises
(A) an alkylbenzene oil (alkylbenzenes) and (B) a
synthetic oil containing oxygen as defined above, which
can be suitably used as a refrigerator oil for an HFC
refrigerant containing HFC-134a and/or HFC-125 without
accompaniment of an additive. However, it is also
possible to use in the form of a refrigerator oil
composition containing therein any of various additives
as required.
In particular, it is preferable to blend a
phosphorus oompound (C) in view of improving the
refrigerating apparatus in wear resistance and load
resistance by the use of the refrigerator oil
c~.omposition. The phosphorus compound (C) means in
this case at least one kind of a phosphorus compound
selected from the group consisting of phosphoric
esters, acidic phosphoric esters, amine salt of acidic
CA 02155166 2004-07-15
- 26 -
phosphoric esters, chlorinated phosphoric esters and
phosphorous esters.
These phosphorus compounds are esters obtained
by a reaction between phosphoric acid or phosphorous
acid and an alkanol or polyether type alcohol, and are
also derivatives o'f said esters.
Examples of phosphoric esters are tributyl
phosphate, tripentyl phosphate, trihexyl. phosphate,
triheptyl phosphate, trioctyl phosphate, trinonyl
1.0 phosphate, tridecyl phosphate, triundecyl phosphate,
tridodecyl phosphate, tritridecyl phosphate,
tritetradecyl phosphate, tripentadecyl phosphate,
trihexadecyl phosphate, triheptadecyl phosphate,
tri.octadecyl phosphate, trioleyl phosphate, triphenyl
phosphate, tricresyl phosphate, trixylenyl phosphate,
cresyldiphenyl phosphate and xyleyldiphenyl phosphate.
Examples of acidic phosphoric esters are
monobutyl acid phosphate, monopentyl acid phosphate,
monohexyl acid phosphate, monoheptyl acid phosphate,
monooctyl acid phosphate, monononyl acid phosphate,
monodecyl acid phosphate, monoundecyl acid phosphate,
monododecyl acid phosphate, monotridecyl acid
phosphate, monotetradecyl acid phosphate,
monopentadecyl acid phosphate, monohexadecyl acid
phosphate, monoheptadec:yl acid phosphate, monooctadecyl
acid phosphate, monool.eyl acid phosphate, dibutyl acid
phosphate, dipentyl acid phosphate, dihexyl acid
CA 02155166 1995-09-25
,, a A
~~.'~~,~~.~~
7 _
phosphate, d3.ttc~pt;y:L acid phosphate, dioctyl acid
phosphate , d inorry 1 ac: i d phosphate , d i.decyl. ac.i cl
phosphate, diundecyl. ac:Ld phosphate, didodec:yl acid
ptrosphat.e, ditri_dec:yl acvi.d phosphate, ditetradecyl acid
phosphate , d ipent=aclec:yl acid phosphate , dioctadecyl.
acid phosphate anti d i « L eyl ac i_d phosphate .
Examples oi' am i ne srzl is oi' acidic: phosphoric
esters are methyl. au.rnirre, ethyl arsine, propyl amine,
butyl amine , pertty:l. um.i.ne , hrxyl arsine , heptyl amine ,
oc:tyl. amine, dimet;hyl amine, diethyl_ arsine, dipropyl.
amine, dibutyl arninE , dipentyl arninE;, dihexy:L arsine,
diheptyl amine, dioc~t;,yl amine, trirnet:hyl. amine,
triethyl amine, tri.propyl. arn.ine, trik~utyl_ amine,
tripentyl ami_tie, trihexyl amine, triheptyl amine and
trioctyl_ amine of the acidic phosphoric ester.
Examples of chl.or.i.nat;ed phosphoric est;ers are
tris-dic:hloropropyl. phosphate, Iris-ch:L.oroethyl
phosphate , tr is---chloroplrenyl phosphate and
polyoxyalkylene bis I chi ( ~'ltloroalky.l. ) J phosphate .
Examples of phosphorous est;ers arc' dibutyl. phosphite,
dipentyl pltosphite, diluexyl pltosphite, ditreptyl_
phosphite, dioctyl phosphite, dinonyl phosphite,
didecyl_ pltosphi.te, di.undecyl. phoshhite, didodecyl_
phosphite, diol.eyl phosphite, diphenyl phosphite,
di.cresyl phosphate, tributyl phosphate, tr ipentyl
phosphi to , tri.ttexyl phosl:>hi t;e , tr iheptyl phosphi to ,
trioctyl phosphi.te, t;ri.nonyl phosphate, tridec;yl
CA 02155166 2004-07-15
2g _
phosphite, triundecyl phosphite, tridodecyl phosphit:e,
tr.ioleyl phosphite, triphenyl phosphite and tricresyl
phosphite. It.is also possible to use a mixture of
these compounds.
These phosphorus compounds may be incorporated
into a refrigerator oil composition in any desired
mixing ratio. However, it is generally preferable to
add these phOSphOruS compounds in the ratio of 0.005
to 5.0 parts by weight, more preferably 0.01 to 3.0
L0 parts by weight, based on 100 parts by weight of the
total amount of the alkylbenzene oil (A) and the
synthetic oil containing oxygen (B).
If the amount of the phosphorus compound added
is less than 0.005 part by weight based on 100 parts by
weight of the total amount of the components (A) <rnd
(B), any substantial effect on the improvement of
wear resistance and loading resistance would not be
attained by the addition of said compound. On the
other hand, if the amount of the phosphorus compound
added exceeds 5.0% by weight based on 100 parts by
weight of the total amount of the components (A) and
(B), it may give rise to undesirable corrosion in a
refrigerating system during its use for a long period
of time.
The improvement in wear resistance and loading
resistance to be attained by the addition of the
phOSphOruS compound is one of the features of the
CA 02155166 1995-09-25
'~,~~~a~~~
refrigerator oil cc~tnpositioo ofi' this invention. l_t is
eertalnJ_y possible tc'o achieve more or less act
improvement; in wear r°esistancc anc.I l.c>adin.g resisi;artee,
even wi th i:he use of PAG ( po_l.yal.kyletre g:Lyc:ol.. ) or an
ester which is eactr known as use('u_I. I'or a reC'ri.gerator
oil used with FI1~(~. However, t;lre ei'I'oci: thazt can be
attained by the use of these conventional compounds i.s
far less than the el'1'ect i;c~ lae ac:ttieved by the use of
the refriger<-zt;or oil composition c~f' t,lti.s ittvc:~nt.ion.
It is also possibie i'or the purpose oI' i_tnprovi_ng
stability to incorporate in t:ite refrigerator oil
composition of t;his invention at l east one kind of an
epoxy compound selected from the group consisting of:
(1) Phenylglycidyl_ ether type epoxy compounds,
(~) Alkylglyc:idyl ether type epoxy cc.~utloourzds,
(3) Glycidyl ester type epoxy compounds,
(4) Aryl oxirane compounds,
(5) Alkyl oxirane compounds,
(6) Alicyclic epoxy compounds,
(7) Epoxidized fatty monoesters,
(8) Cpoxid:i~ed vegei:able oils.
Examples of phenyl.glyc:idyl. et;tier type epoxy
compounds ( 1 ) are ph.enylg lycidyl ether and
alkylphenylglycidyl ether. 'Itze alkylpitertylglycidyl
ether used herein may be one Having 1 to ;.. alkyl groups
each containing L to 13 carbon atoms, preferably one
having one alkyl group containing 4 to 1U carbon acorns .
CA 02155166 1995-09-25
y, n
~~.~~~~:~.~
- ,30 -
Examples o.t' such prei'erabl.e alkylptrc~nylglyc:idyl ethers
are n-buty.l_phenylglycidy7. ettrer, i.-buty:l.l:~henylglycidyl.
ether , sec-bra tyl pheny.Lglyc i.dyI ether , tert;-
butyl.pheny..l,g.Lycidyl ei;her, pentylphenylglycidyl ether,
trexylphenylg~:l_yc.~idylether, hepty.lphenyl.glycidyl ether,
octylphenylglycidyl ether, nonylplrenylglycidyl ether
and decyl.phenylglycidyl ether.
Exampl.c>s of alky.Lglyci.dyl_ ether type epoxy
compounds (2) are decyl.gLycidyl ether, undecylgLycidyl
ether, dodecy.l.glyc:idy~.1 ether-, Lri<lecy.L~,rl_ycidyl ether,
tetradecylglyc:idyl etLrer, z-ethyltrexy:Lg:l.yci.dyl ether,
neopentylglycoldigl:yc:idyl ether, trinrethylolpropane
triglycidyl ether, pentac:rythritol tetraglycidyl ether,
1,6-hexadiol diglyc:Ldy:1 tether, sorbitol polygl.ycidyl
1.5 ether , po l_yai_kyl eneg7 ycol. monog.lyc.L.dy L etl:rer and
polyalkyleneglycol. d_iglyoidyl ether.
l3xampl.es of gl.ycidyl ester Lype epoxy compounds
(3) are phenylglyc:i<kyl ester, alkylglyc:idyl ester and
al.kenylglyc:idyl ester. L'rei'erable examples thereof' are
gl.yci_dyl 2,2-dimethyloctanoate, glycickyl benzoate,
glycidyl acryl.ate and glycidyl methacryl.ai;e.
Ex<~mples of a.ryl_ oxirane compounds (4) are 1,2-
epoxystyrene acrd alk.yl.-:1. , z-epoxystyrerze .
Examples of alkyl oxirane compounds (5) are 1,2-
epoxybutane , L , 2--epoxypentane , 1 , 2-el>oxyhexane , 7_ , 2-
epoxyheptane , 1 , 2-epoxyoctane , 1 , 2-epcaxyno~nane . J_ . 2-
CA 02155166 1995-09-25
~~..:~r~~~~
- 31 -
epoxydecane , 1 , 2-epoxyundec:ane , 1. , 2-cpoxydodecane , 1. , 2-
epoxytridecane , 1 , c -epoxyte t:radec:ane , L , 2-
epoxypentardecane , 1 , 2-epoxylrexadecar~c: , 1 , 2-
epoxyheptadecanc~ , 1 , 2-epoxyoctadec:anc , 1 , 2-
epoxynonadecane and 1- , 2-epoxyei c:osmrrc~ .
Examples of a 1 i cyc 1 i c epoxy ccaml:~ounds ( 6 ) are
1 , 2-epOXyC',yclohexato 1. , 2-el~oxycyc:lc.~l>entu.ne , 3 , 4-
epoxycyclohexyl_tuethyl.._~3 ~l-c,pc>xycyc.Lolrexane c:arboxylate,
bis (;:3, 4-epoxycyc: l.ohexy:Lmetlryl ) ad ipnte , exo-a? , 3-
epoxynorbornane , bi s ( 3 , 4-epoxy-6--
methylcyclohexylmeth~j.l.) adipate, 2-(7-
oxabicyclo[4.1.OJhep(:-3-yl)-spiro(l_,3-clioxane-5,3'-
[ 7 ]oxabicyclo [ 4 . 1 . 0 ) ) heptane , 4-- ( 1_' -rnethy.Lepoxyethyl ) -
1 , 2-epoxy-2-methylc;yc.v:l_ohexmue and 4-epoxyethy 1.-1 , 2-
epoxyc:yclohexane .
Examples of c~poxiciized fatty monoesters (7) are
an ester formed by reach ng an epoxidized fatty acid
having 1.2 to 20 carbon at:orna wi t:lr au n:l.cohol. having 1.
to 8 carbon atoms, phenol or an a_lkyllpheno7, In
particular, epoxystearates such ars L~ut:yl., hoxyl,
benzyl , cyclohexyl , methoxyethyl , phenyl_ and
butylphenyl esters of epoxyst:earic acid are prei'"erred.
Examples of epoxidized veget:abl_e oils (8) are
epoxy compounds oI' a veget=able o:i.l suc:lo as soybeain oil,
linseed oil or cottc.~nseed oil.
Among these epoxy compounds, the more preferred
ones are plrenylgLycidyl_ ether type epoxy <:ornpounds,
CA 02155166 2004-07-15
- 32 -
glyc:idyl ester type epoxy compounds, alicyclic epoxy
compounds and epoxiciized fatty monoester are preferred.
Among them, pheny.Lglycidyl. ether type epoxy compounds
and glycidyl ester type epoxy compounds with
phenylglycidyl ether, butylphenylglycidyl ether and
alkylglycidyl esters being the most preferred.
These epoxy compounds may be incorporated into a
refrigerator oil in any desired mixing ratio. However,
i.t is generally preferable to incorporated these epoxy
compounds in the ratio of 0.1 to 5.0% by weight, more
preferably 0.2 to 2.0% by weight, based on 100 parts by
weight of the total amount of the alkylbenzene oil (A)
and the synthetic oil containing oxygen (B).
1t is of course possible to employ these
phosphorus compounds and epoxy compounds jointly.
It is also possible, if required, to use singly
or jointly suitable conventional additives in the
refrigerator oil for the purpose of improving the
properties of the oil composition of this invention.
The suitable additives include, anti-oxidants of a
phenol. type such as ci.i-tert-butyl-p-cresol and
bisphenol A or of an amine type such as phenyl-a-
naphthylamine and N,N-di(2-naphthyl)-p-phenylene
diamine; anti.-wear additives such as zinc
dithioph osphate; extreme pressure agents such as
chlorinated paraffin and sulfur compounds; an oiliness
improvers such as a fatty acid; antifoaming agents such
CA 02155166 1995-09-25
1 ~::) ~~ ~. ~7
- 33 -
as si7...i_cone-tyL~e ones; metal inac;t.ivators such as
ben2otriazole; visc:osi.t;y index improvers; pour point
depressants; and detergent-dispersant:s. 'These
additives may be used singly or in combination. 'These
additives can be generally added in <r r°atio c>f not more
than 10% by weight, more preferably not more than 5~ by
weight, based on 100 parts by weight oi' the total
amount of ttte al_ky_iberrzene oi.l (A) and Lhe synthetic
oil containing oxygen (B) .
'I he refrigerants used, togettrer with t:he
refrigerator oil composition of this invention, in a
refrigerator include an alkane fluorid<~ having 1_ to ,3
carbon atoms, preferably 1 to 2 carbon atoms and
containing at least 40% by weight of 1,1,1,2-
tetrafluoroethane (fIFC-134x) and/or an alltane fluoride
having 1 to 3 carbon atoms, preferabay J 1:0 2 carbon
atoms and containing at least 20% by weight, preferably
at least 30% by weight, more preferably at 1_east 40% by
weight of pentafluoroethane (IIFG-125) .
'There is no rest~ric:tion placed con the kinc.I of
HFC (hydrofluorocarbon) to be mixed with HFC-134a
and/or HFC-125. The IiL~C includes tri.('luoromethane
(HFC-23), difluoromethane (HFG-32), 1,1,2,2-
tetrafluoroethane (IIFC-134) , 1, 1, 1-trif iuoroethane
(HFC-143a) or 1,:1-difl.uoroethane (IiI~C;-l_52a) .
Examples or the HFC refrigerant: containing
1,1,1,2-tetrafluoroethane (I-IF(:-134x) and/or
CA 02155166 1995-09-25
. ;~ :~ ~ ~i ~
- 34 -
pentafluoroethane (HF(>-125) that at:~e uset'u.l. in this
invention are I~1FC-134a alone ; HFC-125 alone ; a mixture
of IIFC-1.34a/l~F(~-32 irn a rat;i.o of 60-H0~ by wc~ightl40-
20% by weight, a mixture of tII~'C-7.~34a/HI~C-32/iIFC-1.25 in
a ratio of 40-70% by weight/:15-35'% by weight/5-40 o by
weight, a rnixi:ure of III~(~-:125/IiI~C--,32 in a ratio of 30-
601 by weight/70-40 a by we:igltt, a mixture of IiFC-
125/HFC-143a in a ratio of 40-60~ by wc~.igtrt./Ei0-40% by
weight and a rni.xtur~~ c>(' IIhC-7.25/IItO:;-1.34a/EiF(:-143a in a
ratio of 35-55% by wei ght/l.-7 5°~ by wc~ ight/40-60°o by
weight.
More part;icu_iarly, the III~C refrigerant mixtures
are R404A (I-IFC-125/IIFC-143a/IIfC-134a in a ratio of 44%
by weight/52~ by weight/4 o by weight) , t1407C (HfC-
32/IihC-125/lil.~C;-.134a in a ratio oI' 2,3'% by weig~Lrt/25% by
weight/52'% by weight;) , R410l1 (ILFC-32/IIFC-125 in <r ratio
of S0 o by weight/50~~ by weight ) , t1410L3 ( IIFC-32/HFC-l25
in a ratio of 45~ by weight/55% by weight) and 8507
(HFC-125/IIFC-143a in a ratio of 50°~ by weigtzt/50~ by
weight.
The refrigerator oil composition according to
this invention is generally present in a refrigerator
in the form of a fluid composi tiorr in which the
refrigerator ail composition is mixed with the al.kane
fluoride as mentioned a:rbove. The mixing ratio of the
refrigerator oil composition to the refrigerant (alkane
fluoride) in i;lris fluid composition may be optionally
CA 02155166 1995-09-25
~, ~~
- 35 -
determined, but is trenerall.y a rat:i.o of l_ t:o 500 parts
by weight, of t;lre re('ri.gerc-zCor o.i.l c:onrposit-ion
preferably 2 to 400 par is by weight, ofi' t:he
refr igerator o i. L cornpc>s i t ion per 100 parts by weight; of
the re rr i ger<>_nt .
5i.ttc;e t:lte refrigerator oi.l <:orrrposit.ion according
to this i.nverttion is excell.enl: irr electric: properties
and low i.n hygroscopicat,y, i t; is pari,ic;ularl.y suited
1'or use i n mn a i r cond i t i.ortcr or a re l'ri gerat:or
provided wi ttt a se<zled compressor of a reciprocating
type or rotary t:ype. This rel'rigerat..or oil composition
is also suited for case in an air conditioner or
dehumidifier for vehicles, a freezer, a refrigerating
chamber , an autornat.tc: vending machine , a show-case or a
1.5 cooling system 1'or a chemic<zl p:l.ant. 'I'tri.s refrigerator
oi.l composition is also applicable to a c:otnpressor of a
centrifugal type.
Brief' Descrit~tion of the f)raw::rn~
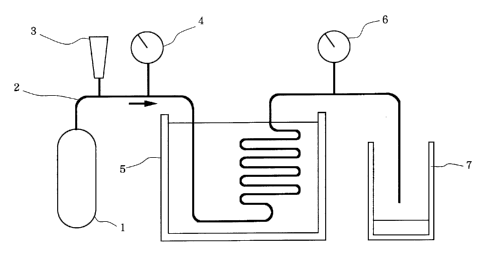
l~ig. 1 i.s a schematic view o(' l.he t:esting
apparatus used in the tvaJ_uation 'Pest Nos. 4 and 8,
wherein the reference trurneraL 1. represent; a
refrigerating tank; 2, a copper conduit; 3, a flow
meter; 4 and 6, manometers respectively; 5, a
thermostatic tatrk; aancl 7, an oil pair.
Descrit~tion ol' t;he Preferred Etnbodirnents
This invention will be 1'urttrer explained with
referenc;e to the Collow.i.ng examples acrd comparative
CA 02155166 1995-09-25
., .a ~~ r
t a.t y
- 3E~ -
examples. I-Iowever, it should be noted t=hat: Ltteso
examples are not; intended to restrict; in arty manner tire
scope of this invention.
Examples 1 to 2F and Comparative Lxarnples 1 to 13
The properti es oi' the Case oi.l s used in t=hese
Lxamples and E;onrparat ive Lxampl.es are represented in
'fable 1, and the additives used therein are shown in
Table 2. 'ftte distribution of mol ec:ui ar weights of
alky~_henzenes i.n mixture was measured by means caf mass
sped=rometry.
20
S
CA 02155166 1995-09-25
__ 37 -
1't3b l o
~._.._. __I<1 __.._ .._....-_- _-.__
nen ._...
it
i~~:
__._....
B ase oil i viscosity nloleculurwt. stribution
di
I 2 ,,,
(mrri ~_~ .-.._~.__wt. -_
_. /.> -_ ___ ~,
__- _. _~~_.
':._
40~:' 1.00"(;<200 200-300 3U1--350>350
A Alkyl benzene 8 . 2 _ :> :)3 2 0
3 10
(branched- '
chain t ~e
i
B Alkyl benzene I 5 2 . 4 (>8 1.4 14
. 3 S34
(branched-
chain t a '
i Alkyl.. benzene l 6. 3.:15 20 ''0 1~~ 41
C 9
(branched-
chain t a ~_ _-__ ~ _-___~.._________.._...____.__
.__
D Alkyl benzene l_2.6 2.62 0 83 15 2
~
(branched-
chain t a
K Alkyl benzene 29.0 4.30 2 4:) 2~4 25
(branched-
chain t a
F Alkyl benzene ;35.2 4.52 2 38 35 25
(branched-
chain t a
. - _.. _ _. _ __~ ._....__ _-_
_..
r
G Alkyl benzene 60.8 5.91 3 32 30 35
(branched-
c h a i n t;
a
~
__~_._... _...._.
_.-. ___~_ ___.- ___.-____.._...-__-- _-
_____
'
H Alkyl benzene 72.6 6.40 3 22 26 49
I
(branched-
chain t a ~_-_,_ __-~ _ _
i Alky1 t>anzana 1 5.4 3. 18 0 ~~(>1 i 30 9
l
(straight- '
~, ~ ~' __ ___-.._.._.._ _...- _ .__ _-_
i _ __ _ __
_. _,__
__._,_
J'Alkyl benzene ''S 4 . 1 45 43 11
. 6 33
(straight- i,
i
c: h a i n t:
y p a )
_'_ _- ~ ... _... _..- ___.._
._ ...- _-___
..._.
'
K Refined
j naphthenic i 32.5 4.71 _.-_
m ineral oil
__ ___ v
CA 02155166 1995-09-25
21~~~~.
- 38 -
'I'abLe 1 (continued)
1(lnematl~ -._-.._ ___. __._._.
Base oil vi.scosit:y Mole>cu:lar w't. clist;ribut;ion
( mrn ~ !~s ) __~ _ ~_. ( w t. . -o
40°(, 100°(; <z00__.._.__2..UU_:..~jUU -_301-3 50 >350
L 'I'etraester
(neopentylglyc:ol/ 7.4 2.05 w--
2-ethylhexanoic
a c i d - _._ _____-_._..__~_- _-_-.__ --
M Tetraester
(pentaerythritol/ 66.;j 8.18 ---
ethylhexanoic
acid & 3,5,5-
trimethylhexanoic
ac i d __ .._u.. _._.. _.____...._-__~_____-_
N Di 2- 11.3 3.19 -----
ethylhexyl f
sebacate
0 Complex ester 59.'i 9.71 ---
P polyethylene
pol_yglycol Lf>1 . 3 ~ 32 . 2 5 Number--average Mol .14't . 2500
d i m a t h 1 a t h a r _ -~ ~ -______...-. ~_«__-__
Q Polypropylene
glycol 32.5 6.71 Number-average h1o1.1Nt.690
monobut lether _ ' ._ _ .____...----_-
R Tetrapropyl-
etherified 41..4 7.18 Number--average Mol .11't.950
product of i
propyleneox:i.de
addition ' i
product of ~- [ i
m a t h y 1 ~;1 c o s i d a __~_-.~_.r..-____..__. ____. _ _ __._ __... .. __
._ _-_.-~____-~-___
_.
S Carbonate oU'
trimethylol- 42..1 ~i 5.59 ~ _ __
propana/batanol__~_ _-_: _.__.__...-_ __~___~._~-___-_._
T Acetylized 54.,:~ 4.77.
a1k lbanzc.na I __. _~ ____
_ - ~_ __ _
U Alkyl benzene
(branched- 15.2 2.90 1. 72 L7 10
chain type ) _.~_.__-.--_. _.._._._____.___. _.. __- -_-__. - -_
CA 02155166 2004-07-15
- 39 -
[Note]
A, C, D, E, F, H and U: 'these oils were each
produced by distilling a rnJ.xt=ure of monoalkylbenzenes
and d.ialkylbenzenes which hack hec~n prepared from, as
raw materials, benzene and branched-chain olefins
consisting of propylene dinners to octamers and having 6
to 24 carbon atoms, by reacting them in the presence of
hydrofluoric acid as an alkylating catalyst.
B: A mixture of A and E (50% by weight:50% by
weight).
G: A product obatained by the re-distillation of
H.
I and J: These oils were produced by distilling
a mixture of monoalkylbenzenes and dialkylbenzenes
l-5 which had been prepared from, as raw materials, benzene
and n-paraffin having 9 to 18 carbon atoms and
separated from a kerosene fraction, by reacting them in
the presence of hydrofluoric acid as an alkylating
catalyst.
p: An ester obtained by reacting adipic acid,
neopentyl glycol and 3,5,5-trimethylhexanol together.
T: A product obtained by reacting the
alkylbenzenes of A with acetyl chloride in the presence
of aluminum chloride.
CA 02155166 2004-07-15
- 40 -
Table 2
Additive Name of Compound
A Tricresyl phosphate
B Dioleylhydrogen phosphate
C Di(2-ethylhexyl) acid phosphate
D Para-tertiarybutylphenylglycidy_1 ether
E Neodecanoic glycidyl ester
F 2,6-ditertiarybutyl-p-cresol
Various kinds of refrigerator oil compositions
of this invention have the compositions shown in
'fables 3 and 4, respectively. The refrigerator oil
compositions thus obtained were each subjected to an
evaluation test for their long-term operability as
illustrated in 'fables 3 and 4 (Examples 1-26).
[Evaluation 'test 1]
A household room air conditioner having a
refrigerating capacity of 2.5kw was filled with 3508 of
a test oil and 10008 of a mixed refrigerant consisting
of HF(:-134a/HFC-32 in a ratio by weight of 70o to 30%
was placed in a thermostatic room kept at an
atmospheric temperature of 43°(: and then subjected to a
continuous operation of 500 hours while setting the air
conditioner to maintain the room at 25°C, in order to
evalutate the test oil for its operability.
[Evaluation Test 2]
CA 02155166 1995-09-25
~~ ~ J ~ ~~ ~~
- 41 -
A luc~useh.ol.d three-door type r~~I'riger-ntor having
an effective inner volume of :300 liters was Pi.l.ted with
180g of a refr:igera.nt consisting oi' lil~C-1.34a and 1508
of a test oil , housed in a thermostatic: r°oorn kept at: an
atmospheric terni~erature of 4;:3c'G and then subjected to m
continuous operation of 500 hours wh.iie setting the
temperatures of the freezing chamber and the cooling
chamber to -18°(~' and 3°C respectively, in order to
evaluate the test oil for its oiler°<:rb.i 1 i t;y (or
Performance).
[Evaluation Pest 3]
An evaluation test was conducted using tyre same
test oils as those which were rec:ogn:i.zed as LSE-~,ing
excellent in the above Lval.uation ~l'est~s 1. and 2 by the
I5 use of a rolling piston type compressor in which 50g of
a refrigerant consisting of 1-lT'C-1.34a <rnd 70g or a test
oil were filled. 'Then, t:hc~ compressor so fi.ll.ed was
subjected to a continuous operation c>f' 1_000 hours under
the conditions ol' a delivery pressure oi' 161cgI'/cmZG, an
inlet pressure of Okgf/cm~G, a revolvi_ug :peed o('
300rpm and a test temperature of 160«(',. After 1..000
hours of the test, t=he surface roughness of sliding
surface portion of t-he compressor vanes was measured.
For the purpose of comparison, Che same,
evaluation tests as those conducted above were also
performed on various rc:fr:ige.rator oil compositions as
indicated in Tabl_~ 5 , i. . a . , a composi t.i on comprising
CA 02155166 1995-09-25
~ ~, ~. ~ ~a
.><. '~~ ~31 t~
- 4z -
only an a.llcy.ibenzene «i.i c:otrtuirti.ttg Less Lltatt 60'i by
weight (based ott the total we.ighL oi.' the component (A) )
of alkylbenzenes having a molecular weight of 200 Lo
350 in the component (A) (Cornparat:ivEe l3xarnpl_es 1 to 4) ;
a eompositi.on comprising a re1'ine~cl rtaphthene--based
tn:inera7_ oii as the component: (A) ((;otnp<,trat.iVE', C:xample
5 ) ; a c:ompos i ti.on con i;ai.n:ittg~ on ly t:tte c:oml:~c~nont ( B )
(Comparati.ve Lxarnples 6 to ;3) ; <rrtd a.t composition
containing tyre component (f3) in a mixing ratio -i'alling
outside the range defined by this invettLion
(Comparat.ive Examp:Les lU to 13). The results of these
tests are also shown in 'table 5.
20
CA 02155166 1995-09-25
- 43 -
r, r,
p ~
~_
I I
L~ Ca O O.. O i cn GO
00 N i
a a
r-~ r-,
O O r-i n t0
' ~ M
t0 Ll O ~ O I W . Gt.. C~ VJ
.n
I O p p
a a a a
n n
0 0
I I N
tt7 CO O O O i i '.~ V7
'
t~ M ~
a a
n n
0 o r-, c_
I
v
~r c0 ~ ~ I cz,. v~ v~
00 .-aI o d
O a a a
_r
a
r,
w o r--i
.er
p I I
ra ca ~n a I
. I W n
oa
u,I I Q
a a
r-~ r--~
O
O p
I I .-i
N ~ c~ Te O I I va V'i
of N I I ~
a a t
n
o r-,
o I I
C ~ ..7 1 I v~ t/~
a m n I I p
a a
N
E,
n O
COU .c7
v v
O E[
v
" ' N H
.--tN M O
O .i..r
ai ~.r Y 1.~ U
O N fn N td
b Q: W O 4r
~f b f- t--. H v~
Ca ~
+~
C
~ V II
_ G 'T7
.' ~C 47 va
' _ p +~
4t fd
O O ~ N
Q r 4m --n a..n
a
U
G. N
CA 02155166 1995-09-25
xr
_.
_
0 0 '
N ,
, -.
Gs, ~'i I I N ~ .
o O
rn .-i I i o
a a
n n
C O
I I .-r
.-w w o pa I I cn vi
o
o~ c~ I I o
'S~O a a
O ~
Q
.~ ~
r-'~C n
W D n
O O o I I
V ..-IW u'a .~ I I tn C/~
v Oe m7 1 I O
a a
m
0 0
I I
a~ Ga E I i v~ va
c c
I I a
a a
n n
O O n n v~
I O M
e0 Ca V7 I Fa . Gc. V7 t/~
tn u'a
N I ~-1 O O
a a a a
I
.. .. ,. a
6 CO U
O 6
~' V F-,
N M O
Y
+n y.~ay -. V
N ,~' tn fn N f0
N 'b Q7 N V 4-,
'C! H f- E- ~n
f=q d'
__.-....
r
O
O
O V II
C
TJ
CO N
N
H
V~ H
3f ~V
O O N
~
a r
a a~ H o
cu
U ,v "~
0..
N
CA 02155166 1995-09-25
~~~~~~~a~
_ 4p _
-, .-,
O G r-~ r,
Ca . ~ ,~-~ ,n
CO ',~. (.S~ CY fn I%]
~7 LC:n
a0 .-~ O p
a '~ a a
n n
O o n ~r
C
i
C o ~ o ~ W in
00 N e-1 I O
a am a
n
O o
I I .-r
-i "'~ CP' f I !n VJ
O o
oa .-~ 1 I p
a
n n
O O
I I
- m- ~ I I tn fn
O
a a I I
a
r,
w o o r-., ,--, ..r
W a ~n
1 - o a o I ra , u. vJ rro
oe .-, I o o a
a a a a
n n
O O N
~" I I
U' O.. I I Cn (n
O O
00 N I I O
a a
n n
O o tG
I I
-~ cz, m o I I rn vi
o
CO N I I C7
a a
rn
4.
C W U .C
v ~ r
O B
~ N H
-~ 7 .-1 C~7M O
O
W H U
N VJ W V1 f0
t~ b ~ ~ ~ 4.
tC T7 f-- f-~f- N
CO C -,
r
N
N H
C Q7
O U II
N ~
ZI'
N H
.H a
n Y
O ~ f~
O O
4 ~-' 4r .H.n
..-.
6 i L O
td
O ~ N
>
U
CA 02155166 1995-09-25
~~~~., ~~,.~
cr ..., va
~fi -
n
O o n
n
O .-1 I
N O O O
O "~ ~, Cn
C~
op N ,-i
a a a .Q~ O
n n
O O
N 'a .~ I
O O
I f/J G7
I I o
n n
O o r-,
I O
N 1 O CD' U I In V
O .
7
Oa .--~ p I 0
a a a
'b
n n
O p ~
u'~
G M '.,~ I
O
N .". ~ O CY1 1 t~ V7
p
a a I
~ O " O
O .-,
U p,
id
n n
W O O r-i
M
N O I
O
N F''"o mN ~ I cn W
~ ~ .
a a ,~ (
O
n n
O O I--i M
. . .-1 I O
N W O Cb U I t/3 C/7
O
00 N O I
a a a
n n
O ~
O r-i r-, r-~ ~o
O . . O O M O
N A W v7 ~I O
W '
, . Cv (~ VJ
t- N . r-1 O O
M
a a a a a
N
H
_ _ n U
C C7 U .C
v
O p
a.,
> ~ N M O
O
*' au ~ +, U
QJ
!~ fn f~ Id
'b O O N 4~
't7 H F- F- v~
C
nt
N
N
O U I
I
G
'b
td
N
6
~
N ~ N
~
O . O dj
~
i-~ O
(Si
O O ?..
>
U ~y
O
CA 02155166 1995-09-25
~~.-::~ ~l.~i
__
~
p ~
I O I N
I ~ o Kt c~ vi
r
I o r Q
a
O
1
O I T
c o I I v~ rn
I O I I p
a
~ ~ .d
O O
I I o N u~a I
' N
AC O . O I I N .r N
Z ..H
00 o I I ~ N <r I
a N
N a
~ ~
'O .d
k O
O ~ s7 .t7
N N
O I O N C7 I
CJ
Wit'CC O O~ O C . I co ~c7
..., .
.w
O N ~ .
I C~ ~ I
> a a a N N
r
4r
R1 ~ ~
O O ,Ly .b
y ~
~ I O N . I
o
N
O cn I~ p m o I ~t~
.-~
N i
U ao N I I M O n I
a ~
N
a
r~
~ ~ ' ~
L~ CI
o O ~ ~ .G .c7
N N
~n O I
N
N U W ,Z"e upC7 . Cx., . N u'7 I
.-r ...,
.
O O N N N I
N
a a a a
~ ~ .b .d
O O ~ .C7 .C
ev N
I ~ O N era 1
' N
r-i U ~ .~ lf7 I [y ...1 M I
..r
I O N N N
N
a a a
4.
n N
<C CO U ,n
v...
O p
N N
.....
..-n N M O
. , +~
r... w y~ ~ V
N ~ t~ m rn ~V
N 'fl N N N 4r
'b E-. ~-- H H
Ca ~ ..H
N
o v II
...r
e~ a~ v~
v~ ae
O O ~ N
~
a w .-.
N > x
0., N
CA 02155166 1995-09-25
~.~~=s ~.~~
__
r ri
O O n p
O I t0
w o ~r ~ I v~ v,
o
I o
a a a
n r~
0 0
I I co
.-~ rs, O' I I w v)
o o
~ mn t 1 c
a a
.b
C r-~ r~
O O n
+' ~ O I
C .W C.aa ~ O C 1 f!) f/'~
O
O u'a ~n ~ I p
U a a a
O
6 O O ap
a!O I I sr
se.-i CG ~ O I 1 V~ C/~
o
W ~ ~ I I O
a a
O
tb n
t-n O r- w 7
1 , O I r-
CLoa I O O cC, I t~) C~
9 I O .-i I O
O .-1 a
U a
r~
O c0
I I I r-
0o I O O I I V) (n
1 O 1 I O
a
r.. ~. ~ N
6 f~ U .C
O
,-.~ N it
.r 7 ~ N . O
O .,r
au .w~ 1-n au V
N V) fn N ~C
b O N ~ H-r
td b F-" f- (- m
~C C
N
C O
O v I
I
..r C
'C
a~ ~ N
d
~
a~
v) 1-~
if tC
O O d
~
A, 4~ a,
Y .-i
9 i~ 1-, O
cc!
O ~ G7
>
U C1.
N
CA 02155166 2004-07-15
- 49 -
Examples 27 to 30 and Comparative Examples 14 to .17
There were prepared various kinds of the
refrigerator oil compositions of this invention having
their respective compositions shown in Table 6
(Examples 27 to 30). The refrigerator oil compositions
thus prepared were subjected to the following Oil--
return Property Test 1 as indicated below. 'L'he results
obtained are shown in Table 6.
[Oil-return Property Test 1]
An experimental apparatus shown in Fig. 1 was
employed, and 5.Og of an oil was filled into the
portion of the thermostatic tank in which the copper
conduit of 1.5m in length and 0.0036m in inner diameter
was dipped. The temperature of the thermostatic tank
was set to -220°C, and HFC-134a was allowed to flow at
a flow rate of O.OOlm3. 30 minutes later, the amount of
oil collected in the oil pan was measured, and, based
on this measurement, an oil-return ratio was calculated
according to the following equation.
Oil-return ratio (wt.%)
=(Amount (g) of oil collected)/5.0(g) X 700
For the purpose of comparison, the same
evaluation test as mentioned above was conducted using
a refrigerating oil composition containing only the
component (A) as the base oil as indicated in Table 6.
The results obtained are shown in Table 6.
CA 02155166 1995-09-25
~'~~ a x ~e
~. ~ eJ ,~. ~ ~~
- JO -
o .-, r-,
N I ~ ~ r_
.-~ .._,p I GC ~,
o I o p
.-w a a
a
O
Q
5 n
O
X an 1 I I c0
W -~ ~~ O I I I
O I 1 1
N
> a
.r,
n
CC O n
C~.~n I o I r-
9 -~ C O I C 1 N
O O I r, 1
U ~--i a
a
n
O
sr' I I I
--~ <C O / I - I N
O I / I
a
n n
O O n n
O .-1 ~n,-r
M ~-.V'7~z If7W L. M
00 ~ O O
a a a a
n n
O o
1 I o
N '- u~ z v I I c-~
O .-. I I
O a a
C1
?! n n
W O O n
. . O I rn
C' W ..a 1f'~C / M
7
O ~ ~ 1
a a a
n n
O o
I I a~
N ~ .n -W n I I M
O ~ I I
a a
b.
n N
W V S7
~
~ ,.u
O
~-r 41 O
>
O .- . a,
a ~
O
~V "fl O9
CO C C
L,
~
C ~ 3f
0 Y
Q7
Y
i.~ ~.~ 4.
..~ ?Q I v
.N
U
CA 02155166 2004-07-15
- 51 -
Examples 31 to 56 and Comparative Examples 1_8 to 30
There were prepared various kinds of the
refrigerator oil c.~.ompositions of this invention having
their respective compositions shown in Tables 7 and 8
(Examples 31 to 56). The refrigerator oilcompositions
thus prepared were subjected to an evaluation test for
their long-term operability as indicated below. 'The
results obtained are shown in Tables 7 and 8.
[Evaluation Test 4]
A household room air conditioner ~lavirig a
refrigerating capacity of 2.5kw was filled with 3508 o.f
a test oil and 10008 of a mixed refrigerant consisting
of HFC-125/HFC-32/HFC-134a in a ratio of 25~ by
weight/52% by weight/23% by weight, was placed :in a
1.5 thermostatic room kept at an atmospheric temperature of
43°C, and then subjected to a continuous operation of
500 hours while setting the air conditioner to maintain
the room at 25°C, in order to evaluate the test oilfor
its operability (or performance).
[Eval.uation Test 5]
A household three-door type refrigerator having
an effective inner volume of 300L was filled with 1508
of a test oil, and 1808 of a mixed refrigerant
consisting of HIFC-125/HFC-134a/HFC-143a in a ratio of
44% by weight/4% by weight/52% by weight, placed in a
thermostatic room whose atmospheric temperature was
kept at 43°C and then subjected to a continuous
CA 02155166 1995-09-25
l ' .! j(~ [/[;;"
~.~~C~.J:.~~
- 52
operation of 50t) hours while sett:.ing tile temperatures
of the rreez.i.ng chamber <~nd the cc.)ol..i_ng~ charrlber to -
18°C and 3°C respectively, i_rl order to evaluate the
test oil for operat;i.:l.i ty (per-.Pc)rmancc~) .
[L:valuation 'Pest 6 ~
Arl evaluation lest was eonciuc:t.ed tazsing the same
test oils as those which were rec:og-n_i zed as being
excellent in the above L;valuation Pests 4 and 5 by the
use of a roll i rl~r piston type compressor , i n whictl 70g
of a test oil and 5Ug of a mixed refrigerant consisting
of HFC-125/HFC-32 in rz ratio of 50 ~ by weighl;/50% by
weight were filled. 'Then, the compressor so filled was
subjected to a continuous operation of 1000 hours under
the conditions of a delivery pressure ol' l6kgi'/crn~(', an
inlet pressure of Oikgl.'/cm~G, a revo_Lving speed of
3000rpm and a test temperature of 16U°t;. After 1_000
hours of the test, the surface roughness of sliding
surface portion of the compressor vanes wus measured.
For the purpose o:l' <:ompari son , t;he same
evaluation tests as conducted above were also performed
on various refrigerator oil. coml:)os:itions as i.ndi.cated
in 'fable 9, i.e. , a c:ornpc)si.tion comprising only an
al.kylbenzene oil al_kylberlzenes c:orltai.ni.ng less than 60~
by weight (based on t;he total weight of the component
(A)) of the alkylbenzenes having a molecular weight of
200 to 350 i.n the component (A) (Comparative Cxamples
18 t0 ~~. ) ; ~l C:OCTIpOSl tlOrl CorrlpC Lslrlg a ref l_IlE:d
CA 02155166 1995-09-25
~' v ~~ f
- ~;3 -
napLrthene-~asec'1 mir'iera:L c'~il as ttie ~:eonpottent: (A)
(Comparat.ive ~xampl.e ~2); a c~.otnpos.ition ooritrzi.nirtg only
the componen t ( i3 ) ( Comparative hxamp I es 2,3 to 2Ep ) ; and
a composition c: ontarining ttae componc:rrt (Li) in a mixing
ratio fall_i.ng outsi.cle the range de('it~ed by ti:ri.s
invention ( Comparat:ive Exanrp l es z7 to 30 ) . 'I'h.e resin t=s
of these i:ests are also shown in Takr L a 9 .
15
25
CA 02155166 1995-09-25
.>
~. ~ ,"~ ~ ~ ~ ~-~
- 54 -
o
i
I
'~ ca " ~
~ v~ v~
M N
C7
a a
n n
O O
I l(7 M
I
w . cs. rr~ vi
' ~ I 0
a a o
a a
n n
~> I C7
i C.7
I
I vi v~
G~ M I I
C>
a a
n n
O O
I
m '-i
M ~ ~ ~ I
~
w VJ U,7
I o
O a a a
C1
6
v
w O r-,
M I I 'r'
~-i
M co a I
v
. I v~ vv .
v~ ~n I I
a a
n n
O O
M
N I I
.-1
M ~ O .~ I I
O
V7 (/J
o~ N I I
C~
a a
n
o r~
r"i O I
M ~ ,n ..a e-1
.
I I !/J tn
a a
O
H
_ _ n N
21 U .c7
v v ~ rJ
O q
p
H
~," tn to O
O
y ~ r V
N vW A N f~
b N N d ~,
T7 H (-~ E~ v~
c4
.
y
f~
VJ f,/j
U I
I
"'' ~ C/~
~ N
..,
~q
H
iC!
N
C1 y.~ ,~,
Ae ,...,
H p
~C
O O
~
U ~y
y
CA 02155166 1995-09-25
,~., ;..,
_ ,, _
O o ,a,
N
'~1'LL ~ G I I !/) fn
O
c7 .-~ I I o
a a
n n
O o
I I
W CSa i i !n f/J
O O
op N
'C ~ a a O
N
O Q
C E9
_. ___-
C W O ;-t M
O O . O
v e~ W ~ I I c~ r~
tr
..,i a mn I I o
a a
n
t~
' 0 0 I I
'-,
M O I~ I I V7 !/)
O 'J
a>' r-t I I Q
tJ 1J
n r-,
O O r~ ,-t p
I O M
M L7 In I G1 . Gz. t/a (~
~7 Lf'~
t~ I
~ O
U ~J U
N
i1 /~
n
O
v
H
> ~C' u-~ t0 O
iJ
i-! ,p.Jy,pV
N ...~ fn N M td
'O C) N 47 4.
'b H E- E-~t~
Pa c~
N
C O
O V I
I
..w ~
.b
N N
N
m
H
t0
O O O
~
C4 y..,
a~ .-,
4 O
cC
o a~ z
'-' >
U p
CA 02155166 1995-09-25
.f
. ~ ~l ~ ~i '.;~
- ~s -
0 0 ~ ~ M
~r co ~ c~ a. v~ v~
wi ~
00 .-~ 0 0 0
a a a a
n n
O O n .q,
O I p
tN d' .~ c,~ I (/~ U.7
O O .
0o N e-, I O
a a a
n n
0 0
I I
C -~ O' i v7 t/~
o o
a~ .-a i
a a
n n
O O
I f ~t
O ~ I I v~ W .
o
o? a I I O
O ,
,
4
k n n
W O o r-~ ,---, "..,
I u~ tra .-r
O ..a I G1 . fs..VJ (~
o .
I o o p
a a a a
n
O O .qi
I I
~" C7 f1, I I f/J V7
O O
N I I O
a a
n
C O
C~
~' fs-, O' I I VJ (/)
O O
o N I I O
a a
H
n n N
C 0.7 U .G
r
O E3
~. N F,
> sr ~a .c O
o .,~
,.. ,..,~..,v
N cn N N R!
b N N O 4~
.b ~--.E--.f--.v~
m c~
C O
O v il
G
'C1
Y f~
r-1 ~
~ i.I
N 22 L.,
~V
O O v
~
0. 4w au
y r.
f3 H O
~ ~c7
O ~ Q7 :r
>
U I1,
N
CA 02155166 1995-09-25
~~.~~~.E~?
__
O o ,--, '-, m
' O
0
o ~ ~.
Oa N '-1 O O
a a a a
n n
O O
4
u7 O O ~ O I I t/JG7
a a I I O
n n
O O r-n
'd" . r-I I O
~-~ ~ o U I m vi
o .
v~ .-r O I p
a a a
n
.b
r. N n n
.,.r.--n O O n
N O.M . . ~-1 I O
C q Is~ -- Te na I v~ v~
o o
O iC1 00 N O I O
CJ ~C a a a
~ W
n
O O r-r .i,
N . . O I O
Gx.. m O cue' I f~ VJ
O
00 N .-r I O
a a a
n n
O O n
_ I O
~
wa W O Pa U I (n V)
O
00 N O I O
a a a
n
r~ I
O o n ,-~ ,-, ,n
O . . O O M p
~a CW (n ~ A . Gz. t~ VJ
n u~
t N r7 .-~ O O
a a a a a
N
F~
n n O
C !b U .b
v ~ ~ a.. n
O
> .y ra ea O
O .
a~ ~ ~ ~ v
~ N N N
T7 N Q> O 4.
'b t- E- f~ m
C~ C
td
O rJ I
I
N V)
N
6
it
H
~
O O N
~
G1 4.n
a~ -r
Fr O
ct1
O
>
U p,
O
CA 02155166 1995-09-25
~~ ~- ~. ~
~.1
e..k z.~ ~4.~ ~,~
_ i~
o '-, Q.,
tr I o I
N I .~~ O 6 . I V7 (/J
I O .-, I c
N
a
n
O pp
N I Te O I I v~ W
I O I I O
a
n n 'fl T1
O o t7 G
N 6>
N , , _
'
N .14O ".L O I I ~ ~ 01 I
.-1
ao N I I c N -c~ I
4>
a a
_.
6
~C1 n n 'b 1ry
ac o o .-, ,Lt .C
N a;~
W ~ O I ~n o I
N N
N !SIO O~ O C I O .r .er I
..-i
rv ~ N .~ I 'E~ '~ I
Q7 G:~
> a a a
Y
N
r1 n b b
L1 O O ,fa ,CI
U7 d
o I I o N ~n 1
N
O N rs7O C,~ O I I .--.~ .-,
... .
r,
U oo N I I ~r ~ I
N N
a a
n n b b
O O r-n n Ly p
fU O
~ ~ o N O 1
N
.-~ U u'7y n CJ Cz. m n ..-nco I
..~
o~ .--1 O O N N N I
W
a a a a
O O n t! .Ly
N N
co . . I ~n o N o I
N
.-1 U W "y n I Cx.. o .r N I
i .r
aO ~~ I O N N N I
N
a a a
N
H
n r~v n N
C, Ca U .G
Y
O 19
N H
> .C. wa eo O
O
au Y Y JJ V
H ~ N
N 'p O N N 4-~
fd 'fl E~ E~' E-~(~
.H
id
tn
V
O V I
I
...a
au cd (n
N
~
i~
N ~Q L~
lC
O O d
~
0. ~ 4r ...-. +,
6 a 4w ~V O
V
>
U 0., N
CA 02155166 1995-09-25
r~~~ 3.
- 59 -
0 0
O . . O I
w
a a a O
n n
O O O
O7 . .
N (s, O'
O O
a a
O
'C7
N
n n
0 0
N
I i~
G N GG .."E C., I C/~ fn
O O
O u n .-, I o
V a a a
O
a ~ ,-,
F3 v O ,
f,
~r
~CN CC '~E I I C/7 (/;~
O O
a a
I I O
N
.....
-
n
o n
I '
N ~ . O I W n
m C
o
~I O a a I O
-
U r
-
a
n
O
I . I
N I cue' I I va (/)
O
1 O I I o
a
4.
n ~ N
C C7 U .c7
v ~ ~ t
a
. n
.
O
v
~
_ F~
> ~ ua en O
O .- y ,,
r' ~ ~ y V
N
V7 H V7 cd
b d O G7 4r
TJ H I--~ f--~VJ
~1 ~
Y
O V If
.,., ~ .a
a, nt N
N
..r E3
n a~
N ~ ..
O . O ~ N
f3 La
i fd
N >
o.
N
CA 02155166 1995-09-25
a
v
S.'tr
- E)0 -
Examples 57 to 60 and Comparative L:xarr~~les 30 Lo ;34
'i'here wera prepared various k i.nds oi-' the
refrigerator oi:l. cornposi dons of LhiS invention leaving
i:hei.r respective connposiLions shown irr '1'abl.e 1.0
(Examples 57 to 60) . 'T'hc>, roi'rigcraztcor oi..lc:omposiLions
thus prepared wore sut,jected to i.lre t'ol:l.c~win,~,r O:i:L-
return Property Test ~ as indi.cat.crd below. The results
obtained are shown irz Table 10.
[Oi.:l_-ret;urn Property 'Pest 21
An experimental. apparatus shown itz Fig. 1. was
employed, and 5.0g of an o.i...L was I'.il i.ed into the
portion of the i;hermostatic tank i.n whic:h the copper
conduit, 1..5m in length and 0.003(im in inner diameter,
was dipped. The temperature of tire Lhermostatic tank
was set to -220°C, and a mixed refrigerant consisting
of IIFC-125/HI~'(:-32/HFC-134a ( ~5 v~t . ~/z3 wt . o/5z wt . % )
was allowed to i'low at az f Low rate o i' U . OOl.m'~; . ,30
minutes later , l;he atnounL o1' tire: o:il co_Ilectc:d in the
oil pan was measured, and , based orr t lr:i s ;measurement ,
an oil-return ratio was o.alcul.ated according t;o the
following equation.
Oil-return r<~ti.o (wt . a, )
=(Amount (g) of oi:l c:ollecLed)/ >.0(g) x~ 7_00
For tire pt,trpose of comparison, Ltze same
evaluation test; ms mentioned above; was conducted using
a refrigerating oil composition containing only the
CA 02155166 1995-09-25
... "~ ~,~ ~ x
- Ei l
component (A) as the base oil as ind~sated in 'fable J0.
The results ol.~tained azre rilso shown i n 'fa.bl.e 1Ø
to
20
CA 02155166 1995-09-25
iw. a ~~ ~>
~~3.~.~~~
- Ep2 -
o r-~r-,
I - ma w
M -- O I C7 a. r.
O i O O
a a
a
N
6 n
R7 O
M I I I eo
W M - O I I I
O I 1 I
N .--r
7 a
N
N n
O r~
d N I O I ~C
M d' O I 4' I N
O O 1 .-rI
U .-~ a
a
n
O
I I I e~
M C O I I I N
O I I I
ri
a
n n
O O n n
O ~ t!7 .-i
tD n...1t7?-., ~t7CO GY M
t0 ~ O O
a a a a
r1 O O
O 1 I
z ~ I I M
I I
O a a
C1
~C n n
W O O n
, o I o0
eC tf ..-a LCD<C I M
J
00 r-i ~i 1
a a a
n n
O O
1 I
.n sC vra.-a wa I I M
I I
a a
t,
n n n O
C GC U .c7
L ~ a..
O
- -~ a7 O
O
+ y p
N c,
b
td 'd 00
CO ~C C
C
Nn
~ if
O
N ~
+' n 4~
i
I ~r
N
CL ~ O
B v
O
U
CA 02155166 1995-09-25
- Ei:3 -
As appaarettt ('rom the resin is oi' tire performance
eval.uati.<m t;est=s slrc~wrt itt 'i'ables ;3 anti 4 tts we:l.l as
shown in 'fables 7 wand 8, the ret'rig~erator o:i_1
compositions of th:i.s invention dial riot cause the
seizure of rel.'ri.geratiny compressor and were ex.cell.ent
i.n lubricity, thus making it possible to maintain a
high reliabil.i.ty for az L<>ng~ period ol' time.
In partict.tlar, the refrigerator oil c:ompositic>ns
o f' t~xazyo l es l ~i t.o ~4 , ~6 , 48 to >4 and 56 , each
containing a phosuphorus compound ( (; ) , indic:;~ted a
remarkable improvement in the surfao.<~ rouglanc~ss of
sliding surface portion of the compressor vanes over
the refrigerator oi.l composii;:ions of Examples 1 to 17,
25 , 31 to 47 and 55 , t:hus c;learl_y detnoustrating the
retnarkable ef f'e<: t oI' the phosuphorus compound ort the
improvement i_n wear resistance.
By cont,rzist , when there were used t:he
refrigerator oil compositions of (;omparative l3xamples 1
to 4 and 18 to zl shown respectively i.n Fi.gs. 5 and 9,
each comprising, as t;lne cotnpottent (A) , an alkylbenzene
oil (alkylbenzenes) containing less than 60% by weight
(based on the total weight of the component (A)) of the
a:lkylbenzenes having a molecular weight ranging frotn
200 to 350, the seizure oi' a refrigerating compressor
used was recognized, thus i.ndicat;i.ng that they cannot
be reliably used for a. 7.cong period of time. It was
also recognized that t:he gertc:ration of the sei.zure of
CA 02155166 1995-09-25
a.. ... ~ f~
~~~~.~~~>
- Ei4 -
the refrigerating compressor could not; bc: ;:zvo:i.ded even
of a phosuphorus cornpoutrd was tzdcaeci Lc~ tlae:~e
refrigerator oil compositions of the (.;omparative
hxamples. 'This tendency wszs also rec:ogn:ized in the
cases of the Comparative Examples 5 and '~2 usitrg a
naphthene-based mineral oil.. as the component (A).
On the other hand, when tire refril;erator oil
compositions ovf' Cornparat;ive F_xamp.les Ei arrd 23, each
c;ornlorising only peni;azerythri t<al ester as the component
(B) , and of Comp~arat:ive Cxamples 8 and z;i, each
comprising only polypropylene glycoi_rnonoalkyl ether as
the component (t3), were used, they indicated far poor
wear resistance as compared with the refrigerator oil.
composition of this i.nventi.on, even t;hou~;h ttrey did not;
cause the seizure a~i' the refrigerat::i.ng compressor.
Furt;her, the refrigerator oi.l compositions of
Comparative Examples 7 , 9 , z4 and 2E3 , which were
prepared by adding a phosuphorus compound (C) Lo the
same refrigerator oils as Lhose of Comparative Fxamp7es
g, g, 23 and 25, respectively, did not; exhibit any
substantial improvc~rnerrt in wear resi stance . 'This
clearly cienronstrates a synerg~.isti.c ei'i-'ect of the base
oil (t;he components (I1) and (l~) ) and a phosuphorus
compound (C) in the refrigerator o.il composition of
this invention.
On the other hand, as apparent; from the results
of oil-return property test shown in Digs. 6 and 10,
CA 02155166 2004-07-15
- 65 -
the refrigerator oil composition of this invention is
far excellent in oil-return property of t:he
refrigerator oil as compared with those of Comparative
Examples 14 to 17 and 31 to 34, each containing only
the component (A) as a base oil.
As explained above, the refrigerator oil
composition of this invention is suited for use in an
HFC$refrigerant containing HFC-134a and/or HFC-125,
beCauSe it enables the generation of seizure of
the refrigerating compressor to be avoided and 1S
excellent in lubricity, thus making it possible to
maintain a high reliability for a long period of time.
'therefore, the refrigerator oil composition of this
invention is highly useful as a refrigerator oil
composition to be utilized together with an HFC
refrigerant containing HFC-134a and/or HFC-125. As
explained above, it has been made possible to provide a
refrigerator oil composition suited for use with an HFC
refrigerant containing HFC-134a and/or HFC-125, and to
provide a refrigerator fluid composition comprising
such a refrigerator oil composition as mentioned above
and an HFC refrigerant containing HFC-134a and/or HfC-
125.