Note: Descriptions are shown in the official language in which they were submitted.
WO 94/19091 21 ~ ~ ~ 11 PCTISE94/00030
1
PROCESS AND APPARATUS FOR ABSORBING HYDROGEN SULPHIDE
This invention relates to a process and an apparatus
for absorbing hydrogen sulphide, more specifically a pro-
cess and an apparatus in black-liquor evaporation for the
r selective removal, by liquid absorption, of hydrogen sul-
phide from a gas containing hydrogen sulphide as well as
carbon dioxide.
Hydrogen sulphide is absorbed chiefly in the form of
hydrogen-sulphide ions (HS-) but also in the form of sul-
phide ions (S2-). In the following, the total content of
these ions will be collectively referred to as the "total
sulphide content" or, more briefly, as the "sulphide con-
tent".
It is well-known that hydrogen sulphide can be
removed from hydrogen-sulphide-containing gases by being
absorbed in an alkaline aqueous solution, such as sodium
hydroxide, or by using ethanolamine, such as monoethanol-
amine and diethanolamine. The absorption method may, for
instance, be used for producing hydrogen sulphide in pure
form, and optionally further processing to sulphur in a
Claus process. If the gas contains carbon dioxide in
addition to hydrogen sulphide, the carbon dioxide will
also be absorbed in the alkaline solution. Carbon dioxide
has approximately the same solubility in water as hydro-
gen sulphide, and the carbon dioxide will therefore com-
pete with the hydrogen sulphide for being absorbed in the
solution. Hydrogen sulphide and carbon dioxide are
absorbed in an alkaline aqueous solution of e.g. sodium
hydroxide in accordance with the formulae below.
H2S + OH' -~ HS- + H20 (1)
H2S + 20H- -~ S2- + 2H20 (2)
C02 + OH- -~ HCOg- (3)
C02 + 20H- ~ COg2- + H20 (4)
WO 94/19091 ~ ~ ~ PCT/SE94100030
2
The selectivity for hydrogen sulphide, i.e. the ratio of
mole of absorbed hydrogen sulphide to mole of absorbed
(hydrogen sulphide + carbon dioxide), is directly pro-
portional to the hydrogen sulphide and carbon dioxide
contents of the gas. Thus, the competition on the part of
carbon dioxide is especially pronounced when the gas con-
tains more carbon dioxide than hydrogen sulphide, as is
mostly the case in actual practice. If a gas contains,
say, 1$ by volume of hydrogen sulphide and 10$ by volume
of carbon dioxide and efforts are made to absorb the
hydrogen sulphide in a sodium hydroxide solution, the
selectivity for hydrogen sulphide is merely 10$, i.e. 90$
of the gas absorbed is made up of carbon dioxide, which
means that as much as 90$ of the sodium hydroxide is
spent in absorbing carbon dioxide.
In an effort to remedy the above inconvenience in
the absorption of hydrogen sulphide from gases containing
hydrogen sulphide as well as carbon dioxide, methods for
selective absorption of hydrogen sulphide have been deve-
loped. For instance, efforts have been made to selective-
ly absorb hydrogen sulphide in solutions of strong oxi-
dising agents, such as potassium permanganate, sodium
dichromate or ferric salts. In other selective methods,
use is made of alkaline solutions, such as sodium carbo-
nate or potassium carbonate solutions, the operational
conditions being carefully adjusted in the absorption.
More detailed information about this prior-art technique
is found in an article by C. Oloman, F.E. Murray and J.B.
Risk, entitled "The Selective Absorption of Hydrogen Sul-
phide from Stack Gas", Pulp and Paper Magazine of Canada,
5 December 1969, p. 69 ff, as well in an article by
E. Bendall, R.C. Aiken and F. Mandas, entitled "Selective
Absorption of H2S from Larger Quantities of C02 by
Absorption and Reaction in Fine Sprays", AICHE Journal
(Vol. 29, No. 1), January 1983, p. 66 ff.
An instance of the prior art is described in US-
3,554,859, which relates to a method for recovering sul-
WO 94/19091 PCT/SE94100030
3
phur from furnace gases generated in the combustion of
e.g. black liquor. The combustion gases, which contain
hydrogen sulphide and carbon dioxide, are contacted with
a gas containing molecular oxygen and with an aqueous
alkaline solution containing sodium ions, e.g. in the
form of sodium hydroxide and sodium carbonate or sodium
carbonate and sodium hydrogencarbonate. Thus, hydrogen
sulphide is absorbed from the gas and oxidised to thio-
sulphate. The absorption is rendered even more effective
by an addition of ferric oxide, the sulphide concentra-
tion of the solution being thus maintained at a very low
level, i.e. the sulphide content of the solution should
be minimised.
By using a carbonate solution, such as a sodium car
bonate solution, instead of a hydroxide solution, such as
a sodium hydroxide solution, the selectivity for the
absorption of hydrogen sulphide can be augmented to about
30-50$. The reactions taking place during such absorption
can generally be rendered as follows.
H2S + C032- r-- HS- + HCOg- (5)
C02 + C032- + H20 ~ 2HCOg-
When the absorption solution is a carbonate solu-
tion, the hydrogen sulphide is absorbed almost instanta-
neously, whereas the carbon dioxide reacts only slowly
with the carbonate ions to form hydrogen carbonate ions.
Owing to the high content of hydrogen carbonate generated
when using a carbonate solution as absorption medium,
there is the additional advantage of a "counterpressure"
(equilibrium pressure) to the absorption of carbon
dioxide, as appears from the equilibrium formula (6)
above.
~A problem that arises when using a carbonate solu-
tion as absorption medium is that only a relatively low
sulphide content can be achieved in the solution; owing
to the reduction of the absorption capacity caused by
WO 94/19091 PCT/SE94/00030
. _.
4
the formation of hydrogen carbonate ions. Thus, it is
extremely difficult to attain sulphide contents exceeding
about 0.30 mole/1. As a result, prior-art methods for
selective absorption of hydrogen sulphide by means of an
absorption medium in the form of a carbonate solution
have not met with much success, despite the great demand
for such a method in the many fields where hydrogen-sul-
phide-containing and carbon-dioxide-containing gases are
generated. Examples of such fields of application are
petroleum refinement, coal-gas production and, in par-
ticular, the combustion of black liquor carried out in
the sulphate pulp industry.
When recovering chemicals in the sulphate industry
in accordance with the conventional Tomlinson process,
the black liquor is burnt in a soda recovery unit,
resulting in the generation of steam and the formation of
a melt chiefly consisting of sodium carbonate and sodium
sulphide. The melt is then dissolved in water and caus-
ticised, so that the sodium carbonate is converted to
sodium hydroxide and white liquor is obtained, which may
then again be used for digesting wood. For many reasons,
including the risk of an explosion when a tube in the
soda recovery unit bursts, efforts have in recent years
been made to develop new processes for the combustion of
black liquor, in which the black liquor is pyrolysed
under reducing conditions and in which no melt is formed.
Such processes can be collectively referred to as "black-
liquor evaporation", and one instance thereof is the so-
called SCA-Billerud process (E. Horntvedt and J. Gomy,
Paper Trade Journal 158 (1974):16, pp 32-34). In this
process, the black liquor is pyrolysed in a reactor under
such temperature conditions that dust, which chiefly con-
sists of sodium carbonate and carbon, and a combustible
gas, which inter alia contains sulphur compounds, are
formed. Another instance of black-liquor evaporation is
given in US-4,872,950, which relates to a method for
thermal decomposition of black liquor with concurrent
WO 94119091 ~ ~ ~ PCTISE94100030
supply of oxygen in an amount short of the stoichiometri-
cally required amount, at a pressure above 10 bar, and at
such a temperature that no melt is formed. The evapora-
tion results in the formation of a solid phase, which
5 chiefly consists of sodium carbonate, and a gaseous
phase, which chiefly consists of hydrogen sulphide, car-
bon monoxide, carbon dioxide, hydrogen, water vapour, and
methane.
EP 459,962 is concerned with the cleaning of process
gas in black-liquor evaporation. In the cleaning, sulphur
compounds and sodium compounds are removed from the gas
by the contact with alkaline solutions containing
hydrogen sulphide ions and hydroxide ions. The cleaning
involves at least two stages. In the first stage, the gas
is passed through a venturi nozzle along with an alkaline
solution, so that melted aerosol drops of black liquor in
the gas are absorbed by the solution. Then, the gas is
contacted with a solution containing hydroxide ions and
hydrogen sulphide ions in a molar ratio exceeding 4:1.
The high molar ratio of hydroxide ions to sulphide ions
results in the absorption solution having a low sulphide
concentration. The alkaline solutions used in the absorp-
tion, such as white liquor or wash liquor, have high pH
values of about 13-14, resulting in poor selectivity for
the absorption of hydrogen sulphide. Furthermore, the
absorption solution employed has a low carbonate content,
and it is specifically indicated that green liquor, which
has a high carbonate content, cannot be used as washing
solution.
In order to be able to recover the chemicals used in
black-liquor evaporation, i.e. the evaporation of black
liquor by combustion in a reducing atmosphere, and pro-
duce from these chemicals white liquor to be used in the
manufacture of pulp, it is necessary that the hydrogen
sulphide can be removed from the generated gas. Since the
gas also contains carbon dioxide, the latter will compete
with the hydrogen sulphide in the liquid absorption, and
WO 94/19091 ,~ PCTlSE94/00030
6
since the gas has a low content of hydrogen sulphide
(about 0.5-2$) while the carbon dioxide content is some
20 times higher (about 10-20~), conventional liquid
absorption results in unsatisfactory recovery of hydrogen
sulphide.
Thus, there is a demand for a method of separating,
from a gas generated in black-liquor evaporation and con-
taining hydrogen sulphide as well as carbon dioxide, the
hydrogen sulphide at a high degree of separation and a
high degree of selectivity, as well as absorbing the
hydrogen sulphide in a liquid such that a high sulphide
content is obtained therein.
According to the invention, it has been found that
countercurrent and multistage absorption, which uses a
carbonate-containing alkaline solution as absorption
medium and during which the pH of the solution is adjust-
ed to about 9-12 by the addition of a hydroxide and not
by the addition of fresh carbonate, involves a high
degree of separation of hydrogen sulphide as well as a
high selectivity for hydrogen sulphide. Thus, the inven-
tion enables a selectivity for the absorption of hydro-
gen sulphide of 60-70$, as well as a degree of separa-
tion of hydrogen sulphide of about 90-99~. According to
the invention, the total sulphide content of the outgo-
ing absorption solution is high, i.e. above about
0.30 mole/1, preferably above about 0.47 mole/1, and
usually is in the range of about 0.30-1.30 mole/1, pre-
ferably in the range of about 0.47-1.1 mole/1, and most
preferred in the range of about 0.65-1.0 mole/1. This
solution can be utilised for producing white liquor in
the manufacture of sulphate pulp.
To be more specific, the invention provides a pro-
cess which is of the type mentioned by way of introduc-
tion and in which the gas is countercurrently brought ,
into multistage contact with circulating carbonate-con-
taining alkaline solutions, the pH of which is adjusted
during the absorption to about 9-12 by the addition of
WO 94/19091 PCT/SE94100030
.:
a hydroxide, such that the hydrogen sulphide is absorbed
to a total sulphide content exceeding about 0.30 mole/1
in the outgoing solution in contact with the incoming
gas.
The invention further provides an apparatus which is
of the type mentioned by way of introduction and which is
characterised in that the apparatus comprises a container
having a gas inlet and a gas outlet, that the container
contains a packing arranged in a number of successive
stages, that the apparatus has means for supplying a
carbonate-containing solution to the last stage, as seen
in the feed direction of the gas, that each stage has
means for supplying the carbonate-containing solution
through the stage countercurrently to the gas and for
recycling the solution across the stage, that the appara-
tus has conduits arranged between the stages for supply-
ing a partial flow of the solution from one stage to a
preceding stage, as seen in the feed direction of the
gas, and that the apparatus has means for supplying a
hydroxide to the carbonate-containing solution in at
least one of the stages in order to adjust the pH of the
solution to about 9-12, as well as an outlet conduit from
the first stage, as seen in the feed direction of the
gas, for discharging liquid having a total sulphide con-
tent exceeding 0.30 mole/1.
Further distinctive features of the invention will
appear from the following description and the appended
claims.
By the expression "carbonate-containing alkaline
solution", as used herein, is meant an aqueous solution
containing carbonate ions (C032-). Preferably, this solu-
z tion is an alkali metal carbonate solution, such as a
solution of sodium carbonate, potassium carbonate or
lithium carbonate. Sodium carbonate is especially pre-
ferred, being as it is easily available as well as fairly
inexpensive. The carbonate concentration of the solution
is not critical, but suitably is about 0.1-3 M with
WO 94/19091 ~ PCT/SE94/00030
8
respect to carbonate, preferably about 1-2.5 M, and most
preferred about 2 M.
According to the invention, it is important that the
carbonate-containing alkaline solution has a pH of at
least about 9. pH values below about 9 result in unsatis-
factory absorption of hydrogen sulphide, and there is
even a risk that hydrogen sulphide already absorbed be
released from the solution. However, the pH of the solu-
tion should not be too high, since this would have an
unfavourable effect on the absorption of hydrogen sul-
phide as compared with the absorption of carbon dioxide.
Thus, the pH of the solution should not exceed about 12
in order that the absorption of carbon dioxide should not
be too considerable. Preferably, the pH of the solution
is in the range of about 10.0-11.5, especially in the
range of about 10.0-11.0, and most preferred in the range
of about 10.2-10.8. If the pH of the solution is adjusted
within this last narrow range, optimum separation of
hydrogen sulphide is obtained.
As appears from the equilibrium reactions (5) and
(6), hydrogen carbonate ions (HCOg-) are formed in the
absorption of hydrogen sulphide and carbon dioxide. This
means that the pH of the absorption solution decreases as
the absorption of hydrogen sulphide and carbon dioxide
proceeds. When the pH of the solution goes below about 9,
the absorption of hydrogen sulphide becomes unsatisfac-
tory, as indicated in the foregoing, and there is instead
a risk that hydrogen sulphide already absorbed will be
released from the solution. If this is to be avoided, the
solution has to be regenerated, i.e. its pH be increased
to above the lower permissible limit for a state of equi-
librium between gaseous H2S and sulphide content of the
liquid at the temperature and pH value at issue. However,
the pH value must not be increased to above about 12,
in which case the absorption of carbon dioxide would
become predominant. As a result of the increase of the
pH of the solution brought about by the addition of a
WO 94/19091 PCT/SE94/00030
g
hydroxide, such as an alkali metal hydroxide, e.g. NaOH,
in accordance with the invention, the hydrogen carbonate
ions formed are reconverted to carbonate ions according
to the following equilibrium reaction.
OH- + HC03- ~ COg2- + H20 (7)
Being thus regenerated, the carbonate solution can
absorb more hydrogen sulphide according to the reaction
(5) above. Since the pH of the solution is adjusted by
the addition of a hydroxide and maintained within the
given range of about 9-12, preferably about 10.0-11.5,
especially about 10.0-11.0, and most preferred about
10.2-10.8, the absorption of carbon dioxide is kept on
such a low level as to be negligible.
As mentioned above, the carbonate-containing alka-
line solution is regenerated by the addition of a
hydroxide. Basically, use can be made of any hydroxide
that does not have an adverse effect on the absorption of
hydrogen sulphide and is capable of increasing the pH of
the solution from the given lower limit of about 9 to the
desired value, such as a value not exceeding about 12.0,
preferably not exceeding about 11.5, and most preferred
not exceeding about 10.8. According to the invention, use
is preferably made of hydroxides of alkali metals or
alkaline-earth metals, such as sodium hydroxide (NaOH),
potassium hydroxide (KOH), lithium hydroxide (LiOH),
calcium hydroxide (Ca(OH)2) and magnesium hydroxide
(Mg(OH)2). Sodium hydroxide is the most preferred, for
reasons of availability and cost.
The temperature of the liquid used a.n the absorption
according to the invention is not particularly critical,
and may vary within a wide range but preferably should be
below about 80°C, since there is a risk that the absorp-
tion of hydrogen sulphide decreases at temperatures of
about 80°C and above. It is preferred that the tempera-
ture is in the range of from room temperature, i.e. about
WO 94/19091 PCT/SE94/00030
20°C, to about 80°C, more preferably in the range of
about 40-70°C, especially about 50-70°C, and most
preferred about 60-70°C.
According to the invention, it has been found that
5 the selectivity for hydrogen sulphide in the liquid
absorption is optimised by carrying out the absorption in
such a manner that the flow of gas and the flow of the
absorbing liquid are countercurrent, and by having a tur-
bulent flow of gas and a laminar flow of liquid. Further-
10 more, the separation of hydrogen sulphide is promoted if
the volume of absorbing liquid is large compared to the
volume of gas from which hydrogen sulphide is absorbed.
Such a high ratio of liquid to gas is obtained by recycl-
ing the absorbing liquid which is contacted with the
hydrogen-sulphide-containing gas.
Moreover, the contact between the hydrogen-sulphide-
containing gas and the absorbing liquid (the carbonate-
containing solution) involves several stages, preferably
two or three stages, and most preferred three stages.
Such multistage contact has the advantage of shortening
the length of the individual stages, so that the pH of
the carbonate-containing solution does not have time to
fall below about 9 in the individual stages, while at the
same time the sulphide content can be kept low in the
uppermost stage. Preferably, each stage has such an
extent or length that the pH of the solution at the end
of the stage has fallen to about 10.0-10.2, the liquid
being then drawn off to be regenerated by means of a
hydroxide and then be recycled to the stage at issue.
For clarifying purposes, the invention will now be
described with reference to the accompanying drawing,
which shows a preferred embodiment of the apparatus
according to the invention.
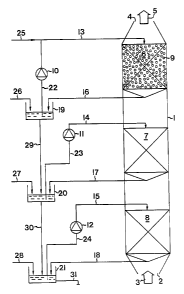
The inventive apparatus comprises a tower or con-
tainer 1 having an inlet 2 for a gas 3 generated in
black-liquor evaporation and containing hydrogen sulphide
as well as carbon dioxide. At the opposite end of the
WO 94/19091 ~ ~ ~ ~ PCTISE94I00030
11
apparatus, there is provided an outlet 4 for gas 5 from
which hydrogen sulphide has been removed by liquid
absorption. The contact between the hydrogen-sulphide-
containing gas and the carbonate-containing solution
involves three stages 6, 7 and 8. Each stage contains a
packing 9, as hinted at in stage 6 in the drawing. To
optimise the selectivity for hydrogen sulphide in the
absorption, the packing 9 has such a shape as to generate
a laminar flow of liquid through the stages 6, 7 and 8.
According to the invention, it has been found that a
packing in the form of corrugated plates is especially
suitable for this purpose. The packing may, for instance,
be made of plastic or metal.
The contact between the hydrogen-sulphide-absorbing
carbonate-containing solution and the hydrogen-sulphide-
containing gas is carried out in countercurrent fashion.
To this end, each stage has means for supplying the car-
bonate-containing solution through the stage countercur-
rently to the gas, as well as means for recycling the
solution across the stage. As shown in the drawing, these
means are made up of pumps 10, 11 and 12 which, via con-
duits 13, 14 and 15, feed the carbonate-containing alka-
line solution to the respective stages 6, 7 and 8, as
well as conduits 16, 17 and 18 conducting the solution
from the respective stages to collecting vessels 19, 20
and 21. From these collecting vessels, the solution is
recycled across the stages through conduits 22, 23 and 24
which are connected to the pumps 10, 11 and 12, respec-
tively. Fresh carbonate solution, preferably sodium car-
bonate solution, is fed to the last stage 6, as seen in
the feed direction, through a conduit 25 from a supply
(not shown) of sodium carbonate solution.
Instead of supplying fresh carbonate solution to the
last stage, the carbonate solution can be generated in
the last stage by supplying sodium hydroxide solution to
this stage and allowing the hydroxide solution to absorb
carbon dioxide from the gas, such that a carbonate-con-
WO 94119091 PCT/SE94100030
2~.a~~~~
12
taining solution is obtained according to the reactions
(3)-(4) above.
In order to adjust (increase) the pH of the absorp-
tion solution, a hydroxide, preferably a sodium hydroxide
solution, may be supplied to the collecting vessels 19,
20 and 21 through conduits 26, 27 and 28, respectively,
conducting alkali from a supply (not shown), which pre-
ferably is common all the conduits. The supply of sodium-
hydroxide solution for adjusting the pH of the absorption
solution is regulated on the basis of the measured pH
values of the solutions in the collecting vessels 19, 20
and 21 (not shown).
As appears from the drawing, the different stages
are furthermore interconnected by conduits 29 and 30 for
feeding a partial flow of absorption solution from one
stage to a preceding stage, i.e. from the stage 6 to the
stage 7 as well as from the stage 7 to the stage 8.
Finally, an outlet conduit 31 is arranged for dis-
charging sulphide-containing liquid from the collecting
vessel 21 and the stage 8.
The invention will now be further elucidated with
the aid of a non-restricting Example.
Example 1
A test was performed for the selective removal of
hydrogen sulphide from a gas generated in black-liquor
evaporation. Use was made of an apparatus of the type
described above and shown in the accompanying drawing.
Absorption took place at atmospheric pressure, and
the incoming gas had a temperature of about 60°C and con-
tained 1.13 mole ~ of hydrogen sulphide and 16.9 mole
of carbon dioxide. The gas was saturated with water
vapour at the temperature at issue, which corresponded
to about 18.7 mole ~ of water. The incoming gas flow was
38,280 Nm3/h, giving the gas a velocity of about 3.1 m/s
in the absorption tower. The absorption tower had a
height of 6.25 m, and the two first stages each had a
height of 1.5 m, whereas the last stage, as seen in the
WO 94119091 ~ PCT/SE94100030
''
13
feed direction of the gas, had a height of 1 m. Each
stage was provided with a packing of the type Mellapack
500 from Sulzer. The diameter of the tower was 2.3 m.
Fresh absorption solution, consisting of 8.8 m3/h
of 2 M sodium carbonate solution having a temperature of
about 60C, was supplied to the last stage in the tower
along with recycled absorption solution, such that a
total of about 50 m3/h of absorption solution was sup-
plied to the last stage in the tower. The pH of the
absorption solution supplied was about 11.0, which was
reduced to about 10.2 during the passage of the solution
through the stage owing to the absorption of hydrogen
sulphide. After passing through the stage, the solution
was supplied to a 1.5-m3 collecting vessel where the
solution was regenerated by the addition of a 2.5 M
sodium hydroxide solution having a temperature of about
60C, such that the pH of the solution was again raised
to about 11Ø Then, the regenerated solution was recycl-
ed by means of a pump to the last stage in the absorption
tower for renewed absorption of hydrogen sulphide.
About 11 m3/h of the absorption solution was drawn
off from the collecting vessel of the last stage to the
collecting vessel of the intermediate stage, whence about
50 m3/h of the absorption solution having a pH of about
11.0 was pumped, as in the previous stage, to the inter-
mediate stage, whence the absorption solution was drawn
off at a pH of about 10.2 to be recycled to the collect-
ing vessel. In the collecting vessel, the solution was
regenerated by the addition of a 2.5 M sodium hydroxide
solution having a temperature of about 60C, as in the
previous stage.
From the collecting vessel of the intermediate
stage, about 13.5 m3/h of the absorption solution was
drawn off to the collecting vessel of the first (lower-
most) stage, whence 50 m3/h of the absorption solution
having a pH of about 11.0 was pumped to the first stage,
as seen in the feed direction of the gas. After passing
WO 94/19091 PCTISE94/00030
14
thi$ stage and there absorbing hydrogen sulphide, the
solution, now having a pH of about 10.2, was drawn off
to the collecting vessel. In the collecting vessel, the
solution was regenerated as in the previous stages by the
addition of a 2.5 M sodium hydroxide solution having a
temperature of about 60°C, such that the pH of the
regenerated solution was about 11Ø All in all, about
8.6 m3/h of the 2.5 M sodium hydroxide solution was
supplied to the collecting vessels of the three stages.
From the collecting vessel of the first (lowermost)
stage, about 17.4 m3/h of the solution having a sulphide
concentration of 1 mole/1 was drawn off. The gas leaving
the absorption tower contained 0.113 mole ~ of hydrogen
sulphide and 16.4 mole ~ of carbon dioxide. In this test,
the degree of separation of hydrogen sulphide was about
90$, and the selectivity for hydrogen sulphide in the
separation was about 67$.
By having a high sulphide content as well as a high
content of alkali metal carbonate, preferably sodium car-
bonate, the solution having undergone absorption accord-
ing to the invention is exceptionally well suited for the
production of white liquor to be used in the manufacture
of sulphate pulp. According to the invention, the solu-
tion leaving the absorption tower after the absorption of
hydrogen sulphide has, as indicated in the foregoing, a
sulphide content exceeding about 0.30 mole/1, preferably
exceeding about 0.47 mole/1. Usually, the sulphide con-
tent is in the range of about 0.30-1.30 mole/1, prefer-
ably in the range of about 0.47-1.1 mole/1, and most pre-
ferred in the range of about 0.65-1.0 mole/1. As stated
above, the carbonate content suitably is about 0.1-3 M,
preferably about 1-2.5 M, and most preferred about 2 M.