Note: Descriptions are shown in the official language in which they were submitted.
-- WO 94/19837 21 5 6 4 8 9 PCT/US93/01714
AN ELECTRODE PLATE CONSTRUCTION
Feld of the In~ lion
This invention relates to the construction of a battery electrode plate that is
resi~ to cllPrnicql attack and corrosion, and provides an electrolyte-tight seal.
R~rl~. uund of the Invention
Even though there has been considerable study of qlt~rnq~ive electrochemical
systems, the lead-acid battery is still the battery of choice for general purposes such as
starting a vehicle, boat or airplane engine, emergency lighting, electric vehicle motive
power, energy buffer storage for solar-electric energy, and field hardware, bothindustrial and military. These batteries may be periodically charged from a generdtor
or other source of suitable DC power.
The conventional lead-acid battery is a multi-cell structure. Each cell generally
comprises a set of vertical inter~igit ~ed monopolar positive and negative plates formed
of lead or lead-alloy grids col,l~inillg layers of electroch~-mic~lly active pastes or active
materials. The paste on the positive electrode plate when charged comprises leaddioxide (PbO2), which is the positive active material, and the negative plate contains
a negative active material such as sponge lead. An acid electrolyte based on sulfuric
acid is interposed between the positive and negative plates.
Lead-acid batteries are inherently heavy due to the use of the heavy metal lead
inconstructingtheplates. Modern aUe~ toproducelight-weightlead-acidbatteries,
especially in the aircraft, electric car and vehicle fields, have placed their emphq~ on
producing thinner plates from lighter weight materials used in place of and in
co.llbi..alion with lead. The thinner plates allow for the use of more plates for a given
volume, thus increasing the power density of a conventional lead-acid battery.
However, the extent to which conventional battery pe-~.. a,.ce can be improved upon
is limited by its inherent construction.
21 .5 6 ~ 8 3 -2- PCT/US93/01714 ~
Bipolar batteries are not new and have been known for some time and offer the
potential for illlplove,llent over monopolar battery technology. Bipolar batteryconstruction comprises a series of electrode plates that each contain a negative active
material on one side and a positive active material on the other side, hence the terms
"bipolar" and "biplate". The biplates are serially arranged in such a fashion that the
positive side of one plate is directed toward the negative side of an opposing plate.
The bipolar battery is made up of separate electrolytic cells that are defined by biplates
of opposing polarities. The biplates must be impervious to electrolyte and be
electrically conductive to provide a serial connection between cells.
The bipolar battery is ch~dc~ ed by having improved current flow over that
of conventional monopolar batteries. The enhqn~e~ current flow is the result of
through-plate current transfer from one polarity of the biplate to the other. In a
conventional monopolar battery the current must travel from one electrode plate to
another of opposite polarity via a conductive path which commonly is circuitous and
of relatively considerable length. The signifi~qrltly shortened intercell current path
inherent in the bipolar battery reduces the battery's internal re~i~tq-n~e, making it more
effici~nt than the conventional monopolar battery in both discharging and charging
modes of operation. This reduced internal resi~tqn~ e permits the construction of a
bipolar battery that is both smaller and lighter than its equivalent monopolar battery,
making it a highly desirable alternative for use in the aircraft, military and electric
vehicle industry where considerations of size and weight are of major importance.
The bipolar battery, however, is not without its own difficulties and problems
which, hef~lorofe, have resisted efficient resolution and solution. A first suchdifficulty is related to the choice of materials for the conductive sheet used to make up
the biplate. The bipolar construction in its simplest form would use lead or lead alloy
for the conductive sheet and intercell partition. However, since the lead of theconductive sheet is corroded at the positive (anode) side by both the action of
overcharge (recharge) and by the current-producing interaction between the conductive
sheet and the active material itself (discharge), in time the lead sheet will be corroded
entirely through from the positive side. Once penetrated, the electrolyte from the cell
on the positive side has direct contact with the electrolyte on the negative side and a
short circuit is established between adjacent cells. In short order, the positive material
on one side of the now-penetrated conductive sheet becomes fully discharged against
the negative active material on the opposite side.
This short circuit condition will result not only in a loss of voltage from the
two cells, but will also introduce a very high resi~ ce in the series-connected bipolar
cells due to the near total conversion of both active materials to lead sulfate, which is
'~ Wo 94119837 2 1 5 6 4 8 9 ' ~/US93101714
3--
nol co~ lcting. The greatly increased r~;~ e will render the battery nearly useless
at all but very low disch~e rates and will entirely defeat the fiml1~rnPnt~lly low
resi.~ re inherent in bipolar construction.
Another problem in the construction of the bipolar battery is one inherent with
the construction of lead-acid batteries in general. This involves achieving an
electrolyte-tight seal about a conductive battery member passing from a position inside
the battery and in intimate contact with the battery electrolyte through the battery
housing to the battery's outside surface. In a convel-~ional monopolar lead-acid battery,
problems of u~-w~led electrolyte leakage occurs at the termim~l post seal where a
tellllillal post (conductive member) passes through the battery cell cover. The unique
electro-rhemi~try oc.;u.,h~g at such seal interface predicts that a true seal cannot be
obtained so long as an oxide film exists on the conductive member. Eventually the seal
will fail either by creep corrosion, resulting in the migration of the electrolyte to the
battery surface, or around the end of the conductive member to the adjacent cell, or
fail by crevice corrosion (also called nodular corrosion) resulting in the mech~nic~
separation or failure of the seal.
The problem related to achieving an electrolyte-tight seal about a conductive
battery member in intimate contact with the battery electrolyte as the conductive
member passes through the battery housing is well known and understood in the art and
was addressed in a paper entitled "Vulcanized Rubber Post Seal For Lead-Acid
Batteries A New Generic Type" presented before the 1988 INTELEC Il,l~lllalional
Teleco,."..ln-ic~ions Energy Conference. The paper idP,ntifiPd two dirr~renl types of
corrosion mPrh~ni~m~, creep and crevice or nodular corrosion, that was responsible
for electrolyte migration in electrolytic batteries.
In bipolar battery construction, a bipolar plate is the conductive member which
is in intimate contact with the battery electrolyte. The bipolar plate extends from its
position in contact with the electrolyte to a position at its edges where it is sealed to
a barrier between a~jacP-nt cells; it may extend to an exterior surface of the battery.
At the position defining the battery surface the biplate must interact with other battery
components to form an electrolyte-tight seal. The problem of achieving an electrolyte-
tight seal is exacerbated in bipolar construction because the electrolyte-tight seal must
occur about the full perimeter of each biplate, making the total area of seal much
greater than that encou-l~eled in conventional monopolar battery construction.
It is seen, therefore, that a need exist for a bipolar battery electrode plate
construction that will afford h.,pluved protection from rhPmic~l and electrolytic attack,
serving to both extend battery service life and improve the predictability of battery
pe.ro,l"ance. It is desirable that the electrode plate be constructed using methods
_ WO 94/19837 21~ 6 4 8 ~ PCT/US93/01714
_5_
Summary of the Invention
This invention addresses and fulfills the need id~Pntifie~ above. It does so by
providing an electrode plate structure for a bipolar battery which is of reduced weight
and has a long life ~;Ape.:l~,cy. The bipolar electrode and the overall battery are
m~nllfa~lrable by econol"ic and e-fficient procedures. The battery materials are well
- known and readily ~-.. P~ le to estr~li.chP~ rer~ ion and recycling process. The
electrode can be sealed to other battery col,.pol.enb in a manner which ~rl~ ively
resists leakage during the useful life of the battery.
Generally speaking, in terms of structure, this invention provides an e1ectrode
construction for an electrolytic bipolar battery, which construction includes a
structurally rigid and electrically conductive core member. At least one corrosion
resi~ layer, con~ isillg a material which is both corrosion le~i~,lanl and able to
participate in the electrochemical reaction, is in intimate interface with the surface of
the conductive core member. An interface layer, co"",lishlg a material that bothsubstrnti~lly participates in the electrochemical reaction and fuses or forms an intimate
interface with a positive-side active material, is in intimate interface with the surface
of the corrosion resi~,l layer. A positive-side active layer, co-"~ ing a material that
is capable of ~"~l",~ l participation in the electro~hP-mic~l reaction at the anodic or
positive side of the electrode plate, is in intimate inl~lr~ce with the surface of the
interface layer. A negative-side active layer, conl~,lisil,g a material that is capable of
substantial participation in the electro~hPmic~l reaction at the cathodic or negative side
of the electrode plate, is in intimate interface with the surface of the conductive core
member opposite the core member surface accollllllodating the corrosion I e~i~.l layer.
The electrode plate may comprise a single corrosion r~,is~,l layer or a
plurality of corrosion resi~,~,l layers. With respect to an electrode plate comprising
more than one corrosion resi,~lt layer, each corrosion resi~lanl layer can exhibit a
decreased degree of corrosion reCictrnce and an increased degree of participation in the
electrochemical reaction as a function of distance from the electrode core member. A
preferred corrosion lesi~ layer may comprise pure lead or a lead-alloy selected from
the group consisting of lead-tin, lead-indium, lead-calcium, lead-silver, lead-al.. ~.;.. -~
and lead-anli",olly or any co",bil-~ion thereof.
With respect to the interface layer, a prer~rred material may comprise either
pure lead or a lead-alloy selected from the group consislh~g of lead-tin, lead-indium,
lead-calcium, lead-silver, lead-al.. i.. ~.. , and lead-antimony or any colllbilldion
thereof. With respect to the positive-side active layer, a pr~Çe,led material is lead
dioxide. With respect to the negative-side active layer, a prere.led material is sponge
lead.
Wo 94/19837 2 1 5 ~ 4 g ~ ~/US93/01714
conu,lcrcially and economically feasible from materials readily and practically
available. It is also desirable that the electrode plate construction farilit~e the
rer~ tion and recovel~ of recyclable materials used in battery construction. Further,
it is highly desirable that the battery electrode plate be constructed in a manner
f~rilh~ing an electrolyte-tight seal when used in conju~ ion with other battery
members.
- 6 ~ 8 9
WO94/19837 PCT/US93/01714
The corrosion resistant layers and interface layer are applied
by metal ~l A~; ng or ci mi 1 ~r process which results in the formation
of metallurgical bonds between the layers. The positive-side and
negative-side active materials are applied to respective sides of
the electrode plate by conventional application methods well known
by those skilled in the art.
The electrode plate is a conductive battery me_ber which can
extend from a position within the battery in intimate contact with
the battery electrolyte, through a seal zone defined by another
battery component or the battery housing and to the outside battery
surface. The conductive battery member is prepared for use in
forming an electrolyte-tight seal by stripping the oxides away from
a portion of the surface of the conductive battery me_ber that lies
within the seal zone. In a non-oxi~i 7i ng atmosphere, the ~oYi~ized
conductive surface is coated with a protective layer that is oxygen
imp~ ~~hle, chemically and electrochemically stable, and capable of
joining with the battery housing or nonco~ ctive battery component
to form an electrolyte-tight seal. The battery is constructed, and
an electrolyte-tight seal is achieved, by pl~i ng the protected
portion of the conductive member within the seal zone and causing
the protected portion of its surface to intimately mate with and
connect to another battery ~_c ,~.~nt or the battery housing.
According to one aspect of the invention, there is provided an
electrode for an electrochemical battery in which the electrode is
to participate with an electrolyte in an electrochemical reaction,
the electrode comprising:
a conductive structural electrode core ~
a conductive interface material for contact by and corrosive
participation with a positive active material in the electrochemical
reaction in the presence of the electrolyte;
a conductive protective =aterial carried by the electrode core
member and carrying the interface material and which resists
corrosion to a greater extent than the interface material, which
participates in the electrochemical reaction less actively than the
interface material and which can form an intimate relation with the
positive active material.
45~.
- 6A -
According to a further aspect of the invention, there is
provided an electrode for an electrochemical battery in which the
electrode is to participate with an electrolyte in an
electrochemical reaction, the electrode comprising:
a structural electrode core member formed by a material which
is conductive and readily corrodible by the electrolyte:
a conductive interface material for contact by and corrosive
participation with a positive active material in the electrochemical
reaction in the presence of the electrolyte;
a conductive in~rr~ te body interposed between the
electrode core member and the interface material and which resists
corrosion to a greater extent than the interface material, which
participates in the electrochemical reaction less actively than the
interface material and which can form an intimate relation with the
positive active material.
According to yet a further aspect of the invention, there is
provided an electrode for an electrochemical battery in which the
electrode is to participate with an electrolyte in an
electrochemical reaction, the electrode comprising:
a conductive electrode core member;
a conductive corrosion resistant body in intimate contact on
one side thereof with a surface of the core member;
a conductive interface body in intimate contact with an
opposite side of the corrosion resistant body, the interface body
being comprised of a material which is both capable of substantial
participation with the electrolyte in the electrochemical reaction
and of forming an intimate relation with a positive-side active
material applied to it;
the corrosion resistant body being defined of a material which
can participate in the electrochemical reaction with the electrolyte
at a lower level of activity than the interface body; and
a conductive negative-side active material carrier in intimate
contact with a surface of the core m~her opposite the corrosion
resistant body.
- 6B -
8 g
According to still yet a further aspect of the invention,
there is provided an electrode plate for an electrical battery in
which the electrode is to participate with an electrolyte in an
electrochemical reaction, the electrode plate comprising;
an electrode core m~mher that is structurally rigid and
electrically conductive;
at least one corrosion resistant layer comprised of a material
that resists corrosion by the electrolyte and participates in the
electrochemical reaction in the presence of the electrolyte, the
corrosion resistant layer intimately engaging with a sur~ace of the
core m~mher;
a layer of interface material capable of substantial
participation in the electrochemical reaction in the presence of the
electrolyte, the interface layer comprising lead-antimony alloy and
intimately eng~gi ng with a surface of the corrosion resistant layer;
a positive-side active material capable of substantial
participation in the electrochemical reaction, the positive-side
active material being lead dioxide in intimate contact with the
interface layer; and
a negative side active material capable of substantial
participation in the electrochemical reaction, the negative-side
active material being sponge lead in intimate conductive relation to
the surface of the core me_ber opposite the positive-side active
material.
According to still yet a further aspect of the invention,
there is provided a method for making an electrode for an
electrolytic storage battery which uses a selected electrolyte to
produce an electrochemical reaction with the electrode, the method
comprising the step of conductively interposing between a conductive
structural core m~mher and a conductive body of material which
participates actively with the electrolyte in the reaction an
int~ ~dj~te quantity of a conductive material which participates
less actively with the electrolyte in the reaction.
According to still yet a further aspect of the invention,
there is provided a method for preparing, for leak-resistant 5~ling
to a selected component of a housing of an electrolytic storage
battery, a conductive member which is to extend from a position
within the battery in intimate contact with a battery electrolyte
into a seal zone with a n~nCon~l~ctive component of the battery, the
45 ~ method comprising the steps of;
g
~ 6C --
~DOYi ~i ~i ng at least a selected area of an external surface of
the conductive member which lies within the seal zone;
coating the ~oYi~ized area of the m~mher with a material
which adheres securely to the '- and which is impervious to the
passage of oxygen therethrough; and performing the ~oYi~izing and
coating steps in an environment free of oxygen in a form which can
produce an oxide layer at the surface of the conductive member.
.
According to still yet a further aspect of the invention,
there is provided a method for achieving an electrolyte-tight seal
in a lead-acid storage battery about conductive battery members in
intimate contact with the battery electrolyte that extend through
the body of the battery to its outside surface, the method
comprising the steps of;
-
deoxidizing a selected surface area of a conductive battery
member that lies within a seal zone by contacting the me~her by a
re~l~ing agent;
washing and drying the surface area of the ~oYi~ized
conductive battery m~mher;
applying a protective coating to the portion of the deoxidized
surface of the conductive member surface that lies within the seal
zone;
performing the deoxidizing, washing and applying steps in an
environment free of oxygen in a form which can produce an oxide
layer on the member; and
ass ling the battery in such a manner that the portion of
the conductive battery member carrying the protective coating is
pl~ce~ in direct contact with a non-oxidizable battery - ~on~nt
defining the seal zone.
According to still yet a further aspect of the invention,
there is provided an electrode unit for a bipolar electric storage
battery, the unit having opposite metallic surfaces, and
characterized in that the unit surfaces have peripheral margins
which are free of oxides and are coated with a material which is
impervious to the passage of oxygen therethrough.
45 ~
-- WO 94/19837 21 5 6 ~ 8 9 PCT/US93/01714
-7 -
Dc~ Jtion of the D~
The above-m~ntiQned and other features of this invention are set forth in the
following detailed description of the pr~sG~ y pr~relred and other embodi"~ of the
invention, which description is presenled with re~rellce to the acco",~h"yi"g dla~ lgs
S wherein:
FIG.lis a perspective view of a bipolar battery C~ y-iSil~ the electrode plate
provided in the practice of this invention;
FIG.2is a perspective view of an electrode plate as provided in the practice
of this invention;
FIG.3is a cross-sectional view of one embodiment of the electrode plate;
FIG 4.is a cross-sectional view of one another embodiment of the electrode
plate;
FIG.5is an exploded perspective view illu~lld~hlg the interrelationship of an
electrode plate with other battery components in the construction of a bipolar battery;
FIGs. 6(a) through 6(e) are a series of enlarged cross-section~l views of an
electrode plate illustrating successive stages in the corrosion of the electrode plate
during its useful life;
FIG. 7.is a graphic depiction of the variation in composition of a metallic
layer interposed between the electrode core member and the positive-side active
material in the practice of this invention showing the variation of the materialcomposition through the layer;
FIGs. 8(a) through 8(e) are enlarged cross-sectional views illustrating
progressive steps in a method practice of this invention for providing an electrolyte-
tight seal about the peripheral portion of an electrode plate;
FIG. 9 is a cross-section~l view of another embodiment of the method for
providing an electrolyte-tight seal; and
FIG. 10 is a fra~ .y cross-sectional elevation view illu~,~i"g another
method for providing an electrolyte-tight seal at a ",a,~i"al edge of an electrode plate
for a bipolar battery.
Wo 94/19837 ~ PCTIUS93/01714
215~489 -8-
Detailed D~ lion
The construction of an electrode plate for use in a battery is generally not a
large concern when the battery is a conventional monopolar lead-acid type battery. In
the con~e,llional lead-acid battery, each positive and negative electrode plate is typically
made from lead. Each electrode is of only one polarity on each of its sides, hence the
name monopolar. Lead and alloys of lead are chosen as the prere.led electrode
material for two reasons. First, it has an ability to participate in the electrochçmic~l
reaction that creates the flow of electrons or current flow of the battery. Second, it has
an ability to fuse with and form an intimate contact with the active material at the
surface of the electrode plate. The positive and negative electrodes in a conventional
battery are arranged in a repeating pattern of negative and positive plates separated by
a nonconductive member. The plates are immersed in an acid electrolyte which
fa~ilir.~es the ion transfer and electrochemical reaction between plates of opposing
polarities. A cell in a co"~,entional monopolar battery comprises a group of electrode
plates, a volume of electrolyte, and a cell partition usually made from a
nonconductive/chemically inert polymeric material.
The construction of a bipolar battery involves the placP.ment of an electricallycondllctive sheet between adj~(ent cells of the battery such that separation of the
electrolyte in the respective cells is ~ d by the conductive sheet. Positive active
material is applied to one side of the sheet, while negative active material is applied to
the opposite side. In this way, the positive active material is exposed to the electrolyte
of one cell and the negative active material is exposed to the electrolyte of the adjacent
cell. The conductive sheet thereby forms a single structure electrically connecting two
adjacent half cells, one positive and one negative. By repeating this arrangement of
conductive sheets s~li"g successive cells, a battery of any desired voltage can be
constructed simply by building up the required number of cells into a stack.
In its simplest form, the conductive sheet between the cells to which the activematerials are applied can be metallic lead or lead alloy, as is true with conventional
monopolar lead-acid batteries. The only re4uh~,llenl which must be observed is that
the sheet be truly conLil,uoL~s (without holes) and be sealed at its perimeter to preclude
contact between the electrolyte of adjacçnt cells along paths around the edges of the
electrode. The conductive sheet in a bipolar battery serves multiple functions in the
completed cell: that of intercell partition, and that of current collector for active
materials in each of the adi~cçnt cells. Because the electrically conductive sheet holds
or is in contact with active materials of both positive and ne~alive polarity, it is said
to be bipolar (or two poles). When this construction me~od is utilized, the conductive
~ WO 94/19837 ~ ~ 5 6 4 ~ 9 - . PCT/US93/01714
,~_ g
sheet with both active materials ~t~hed is called a biplate, and the stacked series of
biplates is called a bipolar battery.
An advantage of bipolar batteries over conventional monopolar batteries lies
in the reduction of internal rc~ nt~e which is directly related to its construction. In
a co"vc-,lional monopolar battery the electron transfer g~.~w~ted by the electro~hP-mi~1
reactions oc.;u,li..g at the active surfaces of each electrode must progress through the
active material of each electrode, through a conductor to a plate of opposite polarity
in an adjacent battery cell, and then through the active material of that plate in merely
moving from one cell to the next within the battery; that path is then repeated, on
average, as an electron moves from cell to cell in a monopolar battery before reaching
the battery negative terminal where it is available for use as electric current. The total
electron path in a monopolar battery can be quite long. This path of current flow is
characterized by an inherent resist~n~e (lead is not highly conductive) which causes a
reduction in the current output of the battery.
Because of the bipolar nature of each electrode plate in a bipolar battery, the
electron transfer occurs through the thic~nP,cs of the biplate. The biplate thickness is
many times smaller than the intercell electron path length in a monopolar battery,
decreasing the internal rP~ict~nce of the bipolar battery. This reduced internalre~ict~nce permits the construction of a bipolar battery that is both smaller and lighter
than its equivalent monopolar battery, making it a highly desirable alternative for use
in the aircraft, military and electric vehicle industry where considerations of size and
weight are of major importance.
The biplate of a bipolar battery is required to serve many dirr~cu~ functions.
The biplate must: (1) participate in the electrochemical reaction causing the evolution
of free electrons, (2) serve as a cell partition, plcven~ing the migration of electrolyte
betwveen ~jacent cells, (3) i~ni~ y and effectively support the relevant active
materials of a battery cell, (4) conduct electricity as effiri~ntly as possible, and (5) due
to the inherent construction of the bipolar battery, cooperate with other battery
components to form an electrolyte-tight seal about the biplate edges which may be on
the battery's outside surface. Therefore, the material chosen for the biplate is an
hlll)ol~a"~ co~cidp~ration in the construction of an effective, long lasting bipolar battery.
There are many factors which influence the m~eri~hc suitable for use as battery
electrode plate. For example, the electrode plate must be both structurally rigid to
support the active surface during the life of the battery, and it must be chPmi~ y and
electroch~mi~'ly stable as well. Lead and certain alloys of lead are not well suited for
use as biplates in a lead acid bipolar battery, even though they have superior ability to
fuse with and support positive and negative active m~Pri~lc for such batteries. Lead
WO 94/19837 ~1~6 ~8 9 PCT/US93/01714
at the positive side of the biplate is corroded both by the action of ovelcha~ ~ and by
the very interaction with the active material, lead dioxide (PbO2) which is PCcenti-ql to
the current creation process. Thus, in time, a lead biplate would be corroded entirely
through from the positive side. Once the biplate is ptn~d~d, the electrolyte from the
positive side of the biplate will come in direct contact with electrolyte at the negative
side and a short circuit will be established between q~dj-~cPnt cells, thereby causing the
positive active material on one side of the biplate to be rapidly fully discharged against
the negative active material on the opposi~e side. That short circuit will greatly
increase the internal ~ ce of the battery.
To preclude the failure of an entire stack of bipolar cells due to corrosion
pe"~dlion of one conductive intercell partition, it is necp~scqry that the partition
include a conductive material which is sufficiently chPmirqlly l~i~ to the action of
strong acids and electroch~PmicLqlly resistant to the action of oxidation so that through-
corrosion of the partition cannot occur within the life of other components of the
battery. Only relatively recently have materials which met the criteria of beingconductive but completely chemically inert become available in the form of conductive
polymers. However, such polymers are uncuited for use as a conductive material in
the construction of a bipolar battery because they are unable to wilhs~and the
electrochemical oxidation action that occurs at the positive-side active material and are
unable form a bond with and intimq~ely interface with the battery active material.
In the conventional monopolar lead-acid battery construction, the establichm~nt
of a sqticfq~tory boundary layer interface between the active material and the current
collector relies upon a complex chemistry of intermediate oxides and corrosion
products produced from the metallic current collector itself. Corrosion of the current
collector, then, is a neces~y prerequisite for making and ~ contact between
the conductive sheet of the biplate and the active material, particularly at the positive
side. If non-corroding Illdle. ials are used to fabricate the conductive intercell partition
of a bipolar battery, then the corrosion necessary for s~icfactory i-"el~ce between the
conductive partition and the active material would not be formed and, conceq ~ently,
electrical pe~"ll~ ce would not be realized.
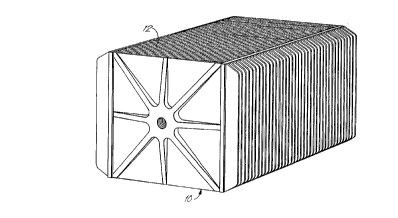
An electrode plate 12 (FIG.2) constructed according to the present invention
is used in the construction of a bipolar battery 10 shown in FIG. 1. In that bipolar
battery, the electrode plates (biplates) are stacked in serial fashion, progressing from
the front to the rear of the battery, defining both the length and the cross-sectional
shape of the battery. FIG. 1 illustrates an embodiment of the bipolar battery having
bipolar plates shaped in the configuration of a square or rectangle, but other plate
configurations, such as a circle, oval, hexagon and the like, can be used if desired.
wo 94,lg837 21 5 6 4 8 9
- 1 1 -
FIGs.2 and 3 illustrate a preferred embodiment of electrode plate 12 accordi-lg
to the present invention. The electrode plate Co~ iscs a positive-side active material
14 at the surface of its positive side. A proleclive coating 16 .,u~ u~ds the edge and
periphery of both the negative and positive sides of the electrode plate. As shown in
FIG. 3, biplate 12 co",~,ises a core member 18 that serves as a structurally rigid
conductive ".t",ber for the electrode plate. With respect to the positive (anodic) side
of the electrode plate to the right of dashed line 15, a C(JIlu~ion IC~ li~l layer 20is in
intimate contact with the core ~"t"~b~l 18. The corrosion re~ layer 20 is
interposed between the core member and an interface layer 22 capable of both
sub~ iql participation in the desired electrochemical reaction and of fusing andforming an intimate interface with the positive-side active material 14. The interface
layer 22 is interposed between the corrosion ,esi~-l layer 20 and the positive-side
active material 14. The positive-side active layer occupies the positive-side surface of
the electrode plate and is fused to or intimq~ely carried by the interface layer 22. With
respect to the negative (cathodic) side of the electrode to the left of dashed line 15, a
negative-side active material 26 is carried in intimate relation by an adjacent face of a
cathode liner 27 which covers the c~hodic side of core member 18.
With respect to electrode core member 18, it is desired that it be structurally
rigid, electrically conductive and farilh~e the reclq-m~ion and recuve,y of recyclable
materials used to construct the battery. It is not necescq~y that the core member be
chemically or electrochemically stable as previously required in bipolar batteryconstruction. The reason for this is that the corrosion resi~ll layer 20 protects the
core ~l-t",bel from corrosion by chemic-q-l and electrochemical processes during the
useful life of the battery 10.
Suitable materials for electrode core member 18 may include known metals and
metal alloys such as carbon steel, copper, alu~ .., iron, stainless steel and the like.
However, other materials such as graphite prepreg, ceramics, and carbon based
materials as well as conductive polymers such as metal epoxies, metal polyolefins,
metal thermoplastics, metal thermosets, and metal polyca.l,orlales, polymer composites
with conductive additives of SnO2, Ti40" and graphite/carbon particulate or fiber and
the like may also be used. These and other suitable core materials may contain the
- conductive metal material in the form of either grains or fibers. In general, any type
of material that possesses the re luisile structural strength and the ability to conduct
electricity can be used to make the core member of the present invention. Materials
having high ratios of strength to weight are plc;r~lled.
Pl~f~,Ied core ll~hllbc. materials include carbon steel, iron and alu...;..-~.... A
particularly prcrell~d material is carbon steel because it is readily rechqim~l le and
WO 94/19837 PCT/US93/01714
- 2156489 12
recyclable. Although the use of alu,~ l would be advantageous because of its
relatively light weight, its use would adversely affect the ability to reclaim and recover
the recyclable the llld~el-'A otherwise used in constructing the battery 10. As
illusLldLed in FIG. 2, the core sheet can be configured as a r~l~;ular flat of a given
thir~nPc~. However, the particular size of the core sheet is ultim~ p~ly d~enda lL on
its particular application. For instance, a core sheet may be configured to
acco"ll"odate its use in an electric wrist watch, calculator, or camera. At the other
extreme, a core sheet may be configured to accomm~ d--~e its use in a sub",~i"e or
commercial airplane. As an example, a core sheet used to power an electric vehicle
can be configured in the shape of a rectangle having a~pro~i,lldLe dimensions of 25 by
20 centimeters and a thickness in the range of from 0.2 to 0.8 millimetPn~. A
particularly preferred electrode core member may have a thir-l~nPcs of applo~illldlely
0.4 millimeters. Although the preferred shape of the core member is rectangular, other
configurations such as square, round, oval, hexagonal and the like may also be used.
In addition, the core member may be configured other than at a flat sheet; for example,
the core member may be corrugated for strength and rigidity or for otber reasons.
As to the layer 20 of corrosion resis~a lL mqt~riql~ it is desired that this material
be chosen from a class of materials which is corrosion resistant to some extent and
which participates in the battery's positive electrochPmiç-q-l reaction to some extent. It
is desired that the corrosion resi~ Iayer exhibit sim~lltq-neous properties of corrosion
resi~tq-nre and electrochemical participation in an inversely related manner. For
example, a corrosion resi~L~,L material having high corrosion resistant properties should
display a low degree of electrochPmicql participation.
These simultaneous cha acL~isLics and their inverse relationship is particularlydesirable to assure a gradual increase in the corrosion penetration resistance of the
electrode plate, moving from the active material to the core member, to prevent the
sudden colro~ive penetration of the core member and the resulting sudden decrease in
battery pel~",lance. The corrosion resi~L~L layer is interposed between core member
18 and the sllhstqntiqlly less corrosion ~esisLan~ layer 22 which actively participates self-
destructively in the desired electrochemical processes with active material 14 in the
presence of an electrolyte from which core member 18 is protected by layer 20. Layer
22 is a corrodible layer. When the corrodible layer is penetrated by the batteryelectrolyte, as it nP,cçscq~ily will be in due course as a con~equPnre of use of the
battery, the electrolyte will interact with the less active corrosion resi~L layer,
providing a height~n~Pd degree of col-osion pen~L~àLion re~ re to the core member.
With respect to lead-acid batteries and the related electroch~-mir,,ql reaction, a
material Colll~ illg lead provides the degree of electrochemical participation desired.
~ W O 94/19837 215 6 4 ~ 9 PCTAUS93/01714
1 The concu,le"lly desired corrosion resi.,L~IL chdla~leli~7lic may be acuuired by using
a lead alloy colll~ ing a material that is both chemically comp3~ihle with lead and
corrosion resi~l when alloyed with lead. Plere~led materials for constructing the
corrosion resi~ l layer may co",~,ise pure lead or a lead alloy selected from the
group con~ goflead-indium, lead-calcium, lead-tin, lead-silver, lead-alu~.. ;n~.. and
lead-al,limon~ or any co~bi"dion thereof. Other alloying agents such as polonium,
copper, arsenic, sulfur, ca l...i-...., bismuth, and zinc may be added to the chosen lead
alloy in order to change the physical properties of the corrosion resisl~ult layer. A
plèrellèd corrosion lesis~ll layer may comprise up to a~lo~h~ ~Ply 0.1 percent by
weight of these additional alloying agents.
A particularly prere~red material for constructing corrosion resistant layer 20
is one comprising lead-tin (PbSn) alloy. Lead-tin alloy is particularly prerelled because
of its low relative cost and its ready availability. A lead-silver alloy would provide the
best corrosion ~eci~ e7 but it has relatively high material cost. A prere,.ed lead-tin
alloy comprising lead-tin comprises in the range of from 0.01 to 5 percent by weight
tin and has a thickness in the range of from 0.04 to 0.2 millim~ters. A lead-tin alloy
col"~"ising less than 0.01 percent by weight tin will not exhibit the degree of corrosion
r~ e desired to protect the core member from chPmir~l and electrorll~-mic~l
attack. A lead-tin alloy co,.",~ g greater than 5 percent by weight tin will notparticipate in the electrochemical reaction to the degree desired to ,.~h.~i" optimal
battery p/,.ro",-ance upon exposure with the battery electrolyte. A particularlypreferred corrosion lesisl~ll layer comprises d~pro~h~tely 2 percent by weight tin and
has a thickness of a~Jpro~h---~ely 0.08 millimetrrs. In order to achieve enh~nre~l
corrosion protection a small amount of silver (Ag) in the range of from 0.001 to 1
percent by weight may be added to the lead alloy (i.e., lead-tin) or to any combination
of alloys chosen for the corrosion ,~si~,~,l layer. A particularly prere"èd corrosion
LeSiSI~ll layer comprises ~pro~il"~ely 0.3 percent by weight silver.
The corrosion lesisl~l~ layer may be joined to the surface of the core member
by using known terllniqu~s that causes two materials to intim~Ply interface with and
bond to each other including the use of cl~lAing, conductive adhesives, co",p,ession
welding, hot dip coating, fluid film deposition, and plating or the like. A cl~ ing
process is p~erè~red because such a process creates an intimate metallurgical bond
between the two di~imil~r materials. An intimate bond between the layers of
di~imil~r materials is desired in order to ,..~ i...i,e the conth~ctivity between the layers.
If required, the thi-~nec~ of the composite foil may be readily controlled by
collvelllional rolling ter~ ni~U~s.
WO 94/19837PCT/US93/01714 ~
i 21S6489
-14-
More than one corrosion lesi~lt layer or thi~1rness may be used when it is
desired that the increase in corrosion penetration res;~ e occurs in a more gradual
manner after penetration of layer 22 by the electro1yte; see FIG. 4. Where an
additional layer of corrosion re~ material is used as with corrosion resis~ Iayer
20 it is desired that this material be chosen from a class of materials that is both
corrosion resi~,l to a lesser extent than the initial (i.e., a~liarçnt to core 18) collusion
rcsi~ l layer 20 and participates in the electrochem~ reaction to a greater extent
than the initial corrosion resi~l layer. In the context of lead-acid batteries and the
related electroch~-miçi~l reaction, a material colll,ulishlg pure lead provides the degree
of electrochemical participation desired. The sim~llt~n~us corrosion r~
characteristic may be ac.lui,cd by using a lead alloy co",l"isil-g a material that is both
chçmi~i~lly compatible with lead and corrosion resis~l~. Prere~red materials forconstructing an additional corrosion resistant layer may comprise pure lead or a lead
alloy selected from the group consisli"g of lead-indium, lead-calcium, lead-tin, lead-
silver, lead- alu~ .. ? and lead-antimony or any co",bil,dion thereof. As with the
initial corrosion l~is~,l layer 20, the same alloying agents may be added to thechosen lead alloy in the same proportion in order to achieve a particular physical
property.
A particularly preferred material for constructing an additional corrosion
resi~ l layer 28 is lead-tin (PbSn) alloy. A prere~ed lead-tin alloy comprises in the
range of from 0.01 to 3 percent by weight tin and has a thir~nP~s in the range of from
0.04 to 0.2 millimpt~rs. A particularly plc~relled additional corrosion lesisl~,l layer
28 comprises alJIJroAillld~ely 1 percent by weight tin and has a thickness of
approximately 0.08 millimPtf?rs. In order to achieve improved participation in the
electro~hemic~l reaction an amount of ~l~h~onr (Sb) in the range of from 0.2 to 15
percent by weight may be added to the lead alloy (i.e., lead-tin) or any combination
of alloys chosen for the additional corrosion lesisl~l layer. A particularly prerelled
additional corrosion resis~ll layer comprises approxi,l,dlely 1 percent by weight
alllh"ony.
If plural corrosion re~ l prute~live layers for the electrode structural core
are to be made from similar lead alloys, the property of decledsing corrosion resist~n~e
can be achieved by gradually decreasing the proportion of alloying agent in the material
with each additional layer proceeding away from the core toward the side of the
electrode where corrosion occurs when the battery is operated in its discharge mode.
This explains why, in a pr~r~lled embodiment, the initial corrosion resi~ layer
pluAh"~le the core member will comprise a greater proportion of alloying agent than
that contained in each subsequçnt corrosion rcsi~l layer.
~ WO 94/19837 2 1 5 6 4 ~ 9 ~ PCT/US93/01714
-15-
Each successive corrosion r~ layer may be joined to the surface of the
pre-;u,~ol or previous corrosion ~ t layer by using the same process as that used
to affix layer 20 to core 18.
An electrode plate of the present invention may comprise as many corrosion
lesi~l layers as desired to simlllt~nP~Qusly achieve a more gradual increase in
corrosion pcl,ch~lion r~ re and decrease in electrochPmic~1 rea.;livi~ as one
proceeds towards core member 18 from the layer of positive-side active material 14.
This may be desirable where the electrode plate is to be used in a battery application
requiring a high degree of reliability, such as with airplanes, s~Pllit~s or space vehicles
and the like. Each subsequPnt corrosion resis~l layer can contain a proglessively
smaller proportion of the same alloying agent, such as tin in presently the practice of
the invention, preferred embodiment, or dirrele.~t layers can contain dirr~.e.~l alloying
agents in desired concelllla~ions.
~or example, an electrode plate may comprise a core member, a first corrosion
resisLiml layer, a second corrosion resi~l layer, a third corrosion resis~ll layer and
a forth corrosion resi~ layer in succç-~sion proceeding from the electrode structural
core member towards the positive active material. In such event, ~ ..i..g that lead-
tin is used as the basic alloy, each corrosion re~is~l layer will have a lesser
proportion of alloying agent, i.e., tin, than that of the pl~ or or prior corrosion
resi~,l layer.
With respect to the layer 22 of interface material capable of substantial
participation in the electrorhemic~l reaction with the positive-side active material 14,
a material collll,lishlg lead provides the degree of electrorhPmir~l participation desired
for use in a lead-acid battery. An additional requirement, however, is that the interface
layer be capable of fusing with and forming an intimate interface with the positive
active material used in a lead battery, namely lead dioxide (PbO2). The ability of the
hll ~race layer to fuse with and form an intimate interface with the active material is
hllpo~ l because the pt~ru~l-,allce of a battery is largely dependant on the ability of
the active material surface to both participate with the battery electrolyte in the
electrorhe-mic~l reaction and transfer electrons to the electrode core member. Optimal
electron transfer (electrical con~nctivity) from the active material to the core member
is realized when the active material is in intimate i..l~,. r.~ ~e with the interface layer.
Optimal participation of the active material in the electrochemical reaction is realized
when the active material is in intimate interf~e with the interface layer, and thus
capable of w;~ g the chçmir~l and electrochemical ell~ilo~ cnt of a battery
without falling âway from the electrode plate.
WO 94/19837 ~ PCT/US93/01714 ~
2l~6~89
-16-
Therefore, lead-alloys Cc~ lising a material capable of fusing and forming an
intimate interface with lead dioxide are pl~ruled ~ the material for electrode layer 14.
P~relred materials for constructing the h-~urace layer may comprise pure lead or a
lead alloy selected from the group cofici.cli..g of lead-indium, lead-~calcium, lead-tin,
lead-silver, lead-alu~.. ;n-,.. and lead-antimony or any combination thereof. As with
the corrosion r~i~L~.l layer 20, the same alloying agents may be added in the same
proportion to the lead alloy chosen to construct interface layer 22 in order to achieve
a particular physical property.
A particularly prer~..ed interface material is lead-~u-Li",u--y (PbSb) alloy.
A-~ -o"y is the plerelled alloying agent due to its ability to corrode while .. ~;.. 1; ;.. ;ng
an intimate interface with the lead dioxide positive-side active material. A preferred
lead-antimony alloy Colll~li5CS in the range of from 0.2 to 15 percent by weight~llhllolly and has a thirlrnec~ in the range of from 0.06 to 0.3 m~ m~t~rs. An
interface layer colll~ illg less than 0.2 percent by weight antimony will not exhibit the
degree of fusion with the active material desired in the construction of an electrode
plate. An i"lel race layer CO"-~ lg more that 15 percent by weight antimony will not
exhibit the desired degree of participation in the electrochemic~l reaction. A
particularly preferred interface layer co,--L,-i~es ~proxi...dely 3 percent by weight
a lti---ony and has a thickness of ~p~.o~i,.,ately 0. l millim~ters. In order to achieve
penetration corrosion resi~t~nre an amount of tin (Sn) in the range of from 0.0l to 5
percent by weight may be added to the lead alloy (i,e,. lead-an~ orly) or any
combination of alloy chosen for the interface layer. A particularly p~eruled interface
layer comprises dl)pruxilll~lely 0.8 percent by weight tin.
The layer 22 of the interface material may be applied to the surface of the
adjacent corrosion resi~ult layer by a cl~ing process which is pr~ru.ed for the
reasons already stated.
The positive-side active material 14 is chosen to participate sul,~ tially in the
electrochemical reaction desired ûn the positive or anodic side of the battery electrode
plate. For use in a lead-acid battery system, the p.~ru,ed positive-side active material
co,--~ es lead dioxide (PbO2) paste. The positive-side active material may be applied
by conve--lional methods well known to those skilled in the art to the surface of the
interface layer 22.
With respect to the selection of the negative-side active material it is desiredthat the material chosen participate SU1JS~ IIY in the electroc~ernir~l reaction taking
place on the negative or cathodic side of the battery electrode plate. For use in a lead-
acid battery system the pr~u-ed negative-side active material comprises sponge lead.
As with the positive-side active m~teri~l, the negative-side active material may be
~_ WO 94/19837 215 6 ~ 8 ~ PCT/US93/01714
-17-
applied by col,venlional methods to a prote.,live layer 27 on the n~dlive side of the
electrode core, the plol~live layer serving to protect the core material from attack by
the electrolyte by rhPmicql and electrochemirql processes. The negative side p-oleclive
layer can be pure lead or any desired lead alloy. Layer 27 prcreldbly is affixed to the
core by a metal c~ iing process. Layer 27 can be relatively thin as it is not attq-r~PA
or corroded to any si~ifirqrlt degree as the battery undergoes dis~ h~g and charging
processes.
CO~ lelll with the rur~oing, FIG. 4 illusllalcs a prefc"ed embodiment of an
electrode plate 29. Electrode 29 comprises a core member 18 that serves as a
structurally rigid conductive member for the electrode plate. With respect to the
positive (anodic) side of the electrode plate to the right of the dashed line 15, a first
corrosion ~esi~l layer 20 (e.g.~ Iead-tin alloy COIII~liSillg a~plO,~ P~y 3 percent by
weight tin) is in intimate contact with the core member 18. A second corrosion
rèsis~ll layer 28 (e.g., lead-tin alloy CO~ IiSillg a~lo~-illlàlely 1 percent by weight
tin) of lessor corrosion lCSi~ and more electrochP-mic~l active properties than
corrosion resi~ull layer 20 is in intimate surface contact with layer 20. An hlle,Lce
material layer 22 (e.g., a lead~ ill,on~ alloy) having the capacity to both subst~nti~lly
participate in the electrochemir~l reaction and fuse with or form an intimate interface
a the positive-side active material 14 is in intimate surface contact with the surface of
layer 28. A positive-side active material 14 (e.g., lead dioxide) forms the surface of
the positive side of the electrode plate and is in intimate interface with the surface of
material layer 22. At the negative (cathodic) side of the electrode plate to the left of
the dashed line, a negative-side active material layer 26 is in intimate surface contact
with the adjacelll surface of a core protecting layer 27 which is in intimate surface
contact with structural core 18 over the whole of the negative side of the core.Although corrosion resi~l layers and an interface layer capable of both
s.~bs~ electrochemical participation and fusing with or forming an immPAi ~e
interface with the positive-side active material have been described above as separate
and distinct thiC~nPssp~ of a multi-layered electrode construction, a single thickness of
material can be used to achieve the purposes and functions described above. For
example, instead of providing separate corrosion le~i,~l~ and interface layers, an
electrode plate may be constructed in which a single layer of dir~el~ ially alloyed lead
is present between a structural core sensitive to the battery electrolyte and the positive
active material.
FIG. 7 pertains to an example of a single layer 60 of material having at
di~elelll locations through its thir~ness~ dir~lenl degrees of the properties of corrosion
r~ .re, on the one hand, and particip~il)n in the electrorh~mir~l reaction and
Wo 94/19837 - PCT/US93/01714 ~
~ ~B489 -18- ~
intimate asso~ation and- cooperation with the positive-side active material. The single
layer 60 colllplis~ a lead-alloy having a decreasing pro?ollion of alloying agent
~sis~l~ to peneLIa~ion corrosion (tin in this example) and an ineredsillg proportion of
alloying agent (~ullhl~ony in this example) capable for forming an intimate interface
with the positive-side active material proceeding through the layer from a surface 61
engaged with a supl/o~ g surface of core member 18 at the left side of the layer to the
positive-side active material 14 at the right side of the layer. Thus, in FIG. 7 curve
62 is read with a % Sn scale 63 at the left side of FIG. 7 and curve 64 is read with a
9~ Sb scale 65 at the right side of the same Figure. This variation in alloy composition
yie1ds the desirable sim~lltqnP~ous ellaldclelislics for the single thic~mP~ of decreasing
corrosion resi.~t~nre, and of increasing participation in the electrocl emicql reaction and
h~creasillg capability to fuse and form an intimate interface with the positive-side active
material the further a unit volume of the layer lies from structural core 18 to the
positive-side active material 14. Although layer 60 comprises varying IlliAlules of
lead-tin and lead-a.llhllolly, other materials in addition to or in subslilulion for those
alloying metals may be used to define a dirrelelllially alloyed layer having the desired
properties.
A dirrelell~ially alloyed electrode layer, such as layer 60, can be defined by
applying the dopant (i,e,. alloying metal) by diffusion processes with different dopant
materials to the opposite surfaces of a host metal (e.g., lead) layer of desired thickness.
The dirr~ ially alloyed layer then can be affixed to an electrode structural core
member to fully cover the core member surface by use of any suitable procedure or
process, a metal clqA~ ing process pelr~,lllled in a reducing environment being
preferred.
Workers skilled in the art and science of battery design and mqnllf,q,ctllre will
readily appreciate that an electrode construction according to the foregoing description
of electrode constructions 12 and 29, or of an electrode in supporting the differentially
alloyed electrode layer 60 represented in FIG. 7, has particular utility in a bipolar
battery. The electrode has exterior surfaces defined of materials which cooperate
effi~iPntly and effectively with suitable positive and negative active materials so that
the desired galvanic processes can occur at the opposite sides of the electrode. The
core of the electrode can be defined by any suitable constructive material, p~er~lably
a c~llul-on metal, which is strong yet relatively light and which has other desired
properties such as compatibility with the processes used to reclaim and recycle the
3~ materials used to mqnllf~ctllre the electrode in the first instance. The material of the
core Ille~llbe~ c. n be selected and defined principally in terms of the functions the core
member is to serve in the electrode without regard to how that material may react with
~ WO 94/19837 215 6 ~ 8 9 PCT/US93/01714
-19-
j .
or be att~rlrrd by the electrolyte to be used with the finished elè-ctrode. The destructive
and corrosive galvanic (electroc~mir~l) processes of the battery occur at the positive
side of the electrode, and the electrode can be defined to cause those processes to occur
effi~ P.ntly.
Further, workers skilled in that same art and science will understand the role
played during the useful life of the electrode by the electrode layer i,ltel",ediate the
core member and the positive face of the electrode. That role in illuslld~ed in the
series of views of FIGs. 6(a) through 6(e) in the acco"~ g dla~hlg~. It is well
known that the corrosive galvanic processes productive of electron release do not occur
truly uniformly across the surface between, for example, lead and lead dioxide in the
presence of an electrolyte such as, for example, sulfuric acid; on a macroscopic level
the corrosion process may be perceived to be uniform, but at a local or microscopic
level that is not the case. The reason is that the metal or other conductive material
which carries the battery positive active material is not truly homogeneous and
amorphous. It has local differences due to impurities, crystal structural, grainorientation and other factors, most of which are known and understood. As a
con~equen~e, the Co1105iVC positive galvanic process occurs more actively and
vigorously at some places along that electrode surface than at other places. The results
is that pits develop at local sites on the active material surface and propdgale through
the material defining that surface more rapidly than at other places in that material.
Those local pitting phenomena are treated statistically, i.e., on average basis, in the
design of a battery of given kind intPnded for a specific purpose or for general purpose
use. That is, no storage battery relying on galvanic processes is pe~ f~~l or life-
limitless; all such batteries have a design life expectancy and, like people, some live
longer than others, but on average they have a defined life. Therefore, workers skilled
in the art and science to which this invention pertains will ~pleci~le that this invention
exists within the context of that reality. That reality has more dire implications and
consequPnres in a bipolar battery than in a monopolar battery. In a bipolar battery,
the biplates are serially arranged, and the colrosive penetration of a biplate by the
battery electrolyte results in rapid failure of the pair of cells separated by that biplate.
On the other hand, in a monopolar battery in which separate positive and negative
electrodes are inter~igit~Pd within a given cell (and plural cells are serially conncc~ed
to achieve a desired voltage), the collosive pc"et~dion of a single positive electrode
does not cause the cell to fail ca~Llophically but merely to suffer a gradual reduction
in pelrolllldl ce, and such reduction in p~"rulll~ance within a cell of a monopolar battery
does not signifi~~ntly burden other cells in the battery. In a bipolar battery, however,
the cd~l,ophic failure of a pair of cells by reason of electrolytic penc~,dlion of the
W O 94/19837 215 6 4 8 9 PCTrUS93/01714 _~
-20-
biplate between them causes the internal res;~l~nf~e of the battery to rise. That increase
in internal re~i~t~nre s~ iently burdens other cells in the same battery that their
mortality rate rises. Thus, once one biplate in a bipolar battery fails, the degenerative
illness to which all galvanic battery are inherently subject shifts, in effect, to a higher
gear and the entire battery soon fails in a relatively short time. That overall failure,
if it occurs suddenly, could place the user of the battery at risk or at a significant
disadvall~dge. On the other hand, if a bipolar battery fails more gradually and
predictably, the user has time, more or less at his convenience, to replace the battery
and avoid that risk or inco..~ellience. This invention, for the reasons set forth alone,
assures that the failure of a bipolar battery, usually toward the end of its design life,
will occur gradually and predictably rather than suddenly.
The several views of FIGs. 6(a) through 6(e) depict a localized area of a biplate
according to this invention at s-~bst~nti~lly regular intervals over the life of the biplate,
illu~LI~lh~g the difference in corrosion potential between the interface layer 22 and the
corrosion resistant layer 20.
FIG. 6(a) shows a cross-sectional view of the biplate COIIIpli,illg a core
member 18, a corrosion lesi~ layer 20, an interface layer 22, and a positive-side
active material 14. FIG. 6(a) shows the biplate at a point in time when the interface
layer 22 has just begun to corrode and form lead dioxide (PbO2) which combines with
the positive-side active material 14.
FIG. 6(b) depicts the same area of the electrode plate at a point in time when
the interface layer has been subs~ ly corroded. FIG. 6(c) depicts the same area of
the electrode plate after an equivalent interval of time, illu~Ll~lingthe through corrosion
of the interface layer such that positive-side active material 14 is P~tPnf~ing to the
surface of the corrosion resi~L~IL layer 22. FIG. 6(d) depicts the same area of the
electrode plate after an equivalent interval of time, illustrating the initial corrosion of
the corrosion lesi~lànt layer. Like the hlte.race layer, the corrosion product of the
corrosion r~isL~Il layer is lead dioxide which combines with the positive-side active
material layer 14.
FIG. 6(d) shows the difference in corrosion potential between the corrosion
resisLanl layer and the h,l~lrace layer. Specifically, the superior corrosion resi~t~n~e
of the corrosion re~i~ku,l layer is illustrated by it re~i~Le~ g only a Illal~ hlal degree of
corrosion loss when colllpared to the signifi~nt corrosion loss r~i~red by the
interface layer the interface layer as ll.ea~ured from the time of FIG. 6(c).
FIG. 6(e) depicts the electrode plate after an equivalent interval of time, and
clearly illu~LIat~,s the large dirrel~lce in corrosion potential between the interface layer
22 and the corrosion resi~l layer 20. Similar to FIG. 6(d), the superior corrosion
~ wO 94/19837 215 6 ~ 8 3 PCT/US93/01714
-21-
re~b~ll properties of the corrosion resi~ layer is shown by its marginal cullûsion
loss when co",y~red to the signific~nt decrease in area r~i~lerPd by the interface layer
as l"ca~.lred from the time of FIG. 6(d).
As noted above, another problem long known to exist with storage batteries is
that of effectively sealing a cQn~ln~tive battery component to a battery housing at a
connPction of the cu~ onelll to the housing so that electrolyte cannot leak through the
connection. That problem becomes acute in bipolar balle.ies where each biplate must
be sealed along its pc,illletcr to other battery components to define a chamber on each
side of the biplate from which electrolyte cannot leak around the biplate to an a~ ent
cha,nbel or to the exterior of the battery. This invention addresses that problem in the
ways shown in FIGS. 8, 9 and 10.
An electrode plate 12, e.g., according to the present invention comprises a
plo~ec~ e coating along its pélhllel~, edge and on the whole of a peripheral portion of
each OyyOSi~è face of the plate as shown in FIG. 2. That coating covers deoxidized
areas on the surfaces and edges of the electrode plate. If the protective coating is to
be used to form an electrolyte seal about the peripheral portion of the electrode plate
the coating must be oxygen i"")el",eable, chemically and electrochemically inert, and
be capable of hl~ela~ g with a nonconductive battery member to form an electrolyte-
tight seal. The plo~ ive coating is applied to the edges and the peripheral margin of
both the positive and negative sides of the electrode plate p~cre~d,ly before the positive
and negative active materials are applied to the electrode plate, for the purpose of
elimin~ting electrolyte migration that can occur along a surface of a conductive member
of a battery, where the surface is corroded by the battery electrolyte, as the conductive
member extends through or to the battery's outside surface.
While not wishing to be bound to any particular theory, it is believed that the
migration of battery electrolyte across the surface of a conductive member is caused
two dirrèlell~ corrosion mPc~ , creep corrosion and crevice or nodular corrosion.
These corrosion mech~ni~ms are well known in the art and were the topic of a paper
entitled "Vulcanized Rubber Post Seal For Lead-Acid Batteries A New Generic Type"
presented at the 1988 Intelec Inlel"~ional Teleco.. ~.-ic~tions Energy Conrelence.
Both types of corrosion are believed to occur at the surface of the conductive member
which is connected to a nonconductive battery member or the housing of the battery.
This corrosion is believed to occur in the presence of an oxide film at the surface of
the conductive member, which film enables the development of porous microscopic
irregularities on the surface of the conductive member. It is believed that the presence
of these irregularities f~Cilit~t~s the migration of electrolyte along that surface, as by
capillary action through the oxide film itself and in other ways, and pre~"~ the
WO 94/19837 215 6 4 8 9 PCT/~'S93/01714
-22-
formation of an electrolyte-tight seal by known methods other than by intimate
interaction of the conductive battery member and the nonconductive battery member
by extreme pre~,~,ure.
This invention provides an electrolyte-tight seal without reliance upon the
S application of extreme pr~C,.,.l~es to form a connection of biplate accol.li"g to this
invention to an adjacent nol,conductive culllpol.~,n~ of a bipolar battery. It does so by
removing the oxide film from the surfaces of the biplate via which connection to other
battery components are to be made, and then coating the dP~xidi7Pd surface with an
oxygen hlll,tlllll able protective coating. One method of pIe~.uing a biplate 12 for
effective leak proof connection to another battery CO~ Olle~ , such as incl~h~ing frame
member 30 (see FIG. 5) is illustrated in FIGS. 8(a) through 8(e). FIGS. 8(a) through
8(e) are cross-sectional views of electrode plate 12 at an q~ ent one edge of the plate.
FIG. 8(a) illustrates the electrode plate as mqnl~factllred before treatment to apply a
protective coating 42.
All metals oxidize when exposed to oxygen or to air which contains oxygen.
Depending on the metal, the oxide forms more or less rapidly and to varying thickness.
As a concequPn~e, biplate electrode 12 nPcPssqrily will have a film of oxide on all
exposed surfaces when its mqmlfactllre, as described above, has been completed. That
oxide covering is depicted in FIG. 8 as oxide film 36. Some portions of that film are
desirable, notably those portions which cover the areas of the biplate which are to be
contacted by the battery active materials, notably the positive active material which,
in the c~e of biplate 12, is lead dioxide itself. However, in marginal areas 34 of the
plate surface, where the biplate is to be connected between a pair of incu~ ng frames
30, that oxide film is very undesirable.
As shown in FIG. 8(b), the surfaces of the biplate where the presence of oxide
film 36 is not undesirable are coated with a mqc7ring layer, but areas 34 and the edges
of the biplate are not masked. The mqclring layer is formed of a material which is
inert to a reducing environment. Then, as depicted in FlG. 8(c), the masked biplate
is placed in a suitable reducing envhunlllt;--~ which is free of any and all forms of
oxygen which can react with the materials of the biplate to form oxides of thosematerials. The reducing e.lviro~ ent removes oxide film 36 from all areas of thebiplate which are not covered by mqcking material 38, thus to provide oxide free areas
40 at the plate margins and edges.
Next, while the biplate continues to be in a reducing or oxygen-free
em~irollllltl,l, a layer of an oxygen impermeable material, that is both chemis~lly and
electrochemir~lly stable and capable of joining with the battery housing or
nonconductive battery member to form an electrolyte-tight seal, is applied to areas 40
~ wo 94~19837 2 I ~ 6 ~ 8 ~ PCT/US93/01714
-23 -
and to the biplate edges to form on those areas and edges a coating 16 which protects
those deoxidized surfaces from contact by oxygen or by co--,younds co~ .;n~ oxygen
in a form capable of reacting with the biplate ll~à~ ials to form oxides of them.
Th~eaner, as shown in FIG. 8(e), mqCl~ing material 38 is removed from the surfaces
of the biplate inside now-prole.,led areas 40 to expose those surfaces.
The method step of l.,.llovillg oxide film 36 can be achieved by using well
known co"-,ll~cial processes such as immersing the electrode plate in a chemicalreducing agent. The coating step (FIG. 8(d)) prcre.ably is performed in a non-
oxidizing ~mosphere such as in a vacuum or in a nitrogen ~ o~yhere in order to
prevent the lc;ru--llalion of an oxide film on the unmq~d electrode plate surfaces.
The elimin~ ~ion of oxides from the sealing areas of the electrode plate is important to
prevent electrolyte migration across the plate surface caused by the phenomenon of
nodular crevice ~lrosioll, and thus provide an electrolyte-tight seal.
~rote.:live coating 16 can be formed of any suitable material that forms an
oxygen barrier, is ch~mi~'ly and electrochemically stable, is electrically nonconductive
and capable of joining with the battery housing or nonconductive battery member to
form an electrolyte-tight seal. Pl~relled prole~;liv~ coating materials may include
lacquers, polymers, rubbers, plastics, silicones, adhesives, rubber cements and the like,
provided they are co--lyatible with the further processes to which the electrode plate is
subjected in the further .~ c of battery 10. The coating material can be a
m~teriql~ such as a heat a-;Livd~ble adhesive, which participates directly in the
definition of the seal or connection llltimqtely desired.
Although the method of accomplishing an electrolyte-tight seal between a
conductive battery member and another battery member has been described in reference
to an electrode plate used to construct a bipolar battery, this method applies to
conventional monopolar batteries as well. For example, the method of this invention
may be applied to accomplish a electrolyte-tight seal between a battery te~ ~" ~ and a
battery housing of a co"venlional monopolar lead acid battery. FIG. 9 illustrates a
battery terminal 44 and its inl~connection to a battery housing 46 as it passes from a
position within the battery, where it is in intimate contact with the battery electrolyte,
to a position outside the battery. At least a portion of the outer surface of terminal 44
which lies within a desired seal zone between itself and the battery housing is
ci~;u~l~rtlenlially deoxidized and prolecli~rely coated, as at 45, in the manner described
above. After assembly of the battery housing cover to the battery housing, an annular
space between the terminql and an aperture 47 in the housing cover can be filled with
â suitable sealant 48 to create a seal which is secure against leakage of the electrolyte.
W O 94/19837 ~. ~ TrUS93/01714 __
2156489 -24~
It has been found that certain alloys of lead, for example, develop oxide
coatings which are dif~lcult to remove by chemical reduction, reducing atmospheres or
abrasion in oxygen-free ~ ssphrres. One such alloy is lead-allti-.lo,ly alloy. In that
in~tanre, it is the tightnr$s of the bond of the surface oxide film to the underlying alloy
which makes such an alloy useful on the positive surface of a battery electrode; t_at
oxide layer fqcilit~rs the desired intimate contact and cooperation with lead dioxide as
a battery positive active material. If the instance of lead-~ulthl.ony alloys, and also
other alloys which develop tightly bound oxide films, the oxide film can be ~lloved
by the use of a flux. Therefore, with IGrerellce to FIG. 10, a presently pler~lled
procedure for p-èp~illg edge margins of an electrode biplate 62 having a surface layer
63 of lead-~"i.llolly alloy is to apply a layer 64 of lead-alloy solder (i.e., 50/50 lead-
tin) to the margin of that surface in the presence of a flux, pl~;r~lably â rosin-type flux.
The solder can be applied in air. As the solder is applied, to flux removes the topical
oxide layer from the lead-antimony and the solder immeAi~ely wets and covers theoxide free surface. As the solder solidifies on the biplate margin, it develops its own
oxide coating, but that oxide layer is readily removed in the ways described above,
e.g., by application of a reducing vq~mosphPre to enable coating 16 to be applied to bare
metal at the biplate margin and edges.
In certain in~tqnre~s it may be desirable to form a temporary oxygen
impermeable coating on the surface of a conductive metal used in battery construction.
Such a temporary oxygen barrier may be formed by applying a rosin flux to the surface
area of the conductive member to be protected. The flux acts to remove the topical
oxide layer and forms an oxide impermeable coating over the newly de~ri(li7~d
surface. This prol~live coating is temporary in nature because the flux is neither
rhemiçqlly nor electrically stable. Accoldill~ ly, before the con~luctive member is used
in the construction of a battery the telll~olaly coating is removed by known methods
and suhsthuted with a pel",alle"l coating that is oxygen impermeable, chrmir,qlly and
electrochemirqlly stable, and capable of h~leld~;ling with the battery housing or
nonconductive battery member to form an electrolyte-tight seal.
The method of obtaining an electrolyte-tight seal accordi-,g to this invention is
not limited to the construction of bipolar, monopolar, or even lead-acid batteries. It
is useful to eliminqte the migration of battery electrolyte that may occur along or
around a conductive battery member that extends to or through the outside surface of
the battery or which lies wholly within the battery and is to be sealed to an adjacent
insulative battery component.
FIG. 5 illustrates the relationship and coopt dion of an electrode plate
according to this invention with other battery components used in the construction of
~-- WO 94/19837 215 6 ~ 8 9 ~ PCT/US93/01714
-25-
bipolar battery 10. Three electrode plates 12 are arranged in serial fashion. A
noncollductive frame-like in~ ing member 30 ca~l,ying a st;yd~dling member 32 isinterposed between each a~j~c~pnt pair of electrode plates. In constructing the battery,
the electrode plates and the inc~ ting members are sandwiched together in intimate
contact with each other at their margins where they can be bonded together by a
suitable adhesive. The adhesive is applied to the deoxidized and prole~ ely covered
marginal surface of the electrode plate.
When the battery components are so sandwiched together, the outside edges of
the electrode plates and the inc~ ing members cooperate to define the outside surfaces
of bipolar battery 10. Accordingly, since each bipolar cell contains electrolyte and
each electrode plate is in intimate contact with the electrolyte, it is necPsC~ry that the
joint between each electrode plate and the ~dj~cent incul~ting members be electrolyte
tight. To accomplish this, the electrode plates 12 each comprise a protective coating
16 that covers the edge and periphery of the electrode plate in a manner that overlaps
and mates with the adjoining surface area of each incul~in~ member 30.
Separating members 32 are used in the construction of bipolar battery 10 to
ensure that each biplate is physically separated from and does not contact an adjoining
biplate. As illustrated in FIG. 5, the se~ ing member may be configured to fit
within the inside perimeter of each inclll~ting member 30. The main requirement for
a separating member is that it be nonconductive to electricity and permit ion transfer
to occur through it between adjoining biplates.
EXAMPLE OF THE PREFERRED EMBODIMENT
An electrode plate was constructed according to the practice of this invention
having a core ",el"ber made from iron. The core member was configured in the shape
of a flat re~ gular sheet having a thickness of a~;)ro~ a~ely 0.4 millimetPrs. A first
corrosion resistant layer having a thickness of applo~illlàlely 0.8 millimPtPrs and
co~ hlg a~proxillldlely 96.7 percent by weight lead, 3 percent by weight tin, and
0.3 percent by weight silver was joined to one surface of the core member by a
commercial cl~lding process. A second corrosion resi~l~,l layer having the same
thir~nP-cc of the first corrosion resi~lanl layer and colll~lisillg appro~i",alely 98 percent
by weight lead, 1 percent by weight tin, and 1 percent by weight a~lh"ony was applied
to the surface of the first corrosion r~is~l member by the same cl~kiing process used
to apply the first corrosion resistant layer.
A layer of interface material having a thickness of approximately 0.13
millimeters and colll~,lishlg d~,proxi",~ely 96.2 percent by weight lead, 3 percent by
weight ~llhllolly, and 0.8 percent by weight tin was applied to the surface of tbe
WO 94tl9837 2 156 ~ 8 9 PCTtUS93/01714 __
-26-
second corrosion resis~.L layer by the same cl~ing process used to apply the
corrosion resistant layers. A layer of positive-side active material C~lllpri~illg lead
dioxide was applied using conventional methods known in the art to the surface of the
int~rf~ce material.
S A thin pure lead foil having a thi-~n~ss of app-~ ely 0.1 millimeters was
applied to the surface of the core member opposite the positive-side active material by
co,l~ ,lcial cl~dding process. A layer of negative-side active material COlll~liSillg
sponge lead was applied using conventional means known in the art.
The f~legoil,g description of presently pl~r~lled and other aspects of this
invention has been presented by way of illuslldion and example. It does not present,
nor is it int~nded to present, an eYh~ tive catalog of all structural and procedural
forms by which the invention can be embodied. Variations upon and alterations of the
described structure and procedure can be pursued without departing from the fairsubstance and scope of the invention consi~l~--l with the foregoing descriptions and the
following claims which are to be read and h~ le~ed liberally in the context of the
state of the art from which this invention has advanced.