Note: Descriptions are shown in the official language in which they were submitted.
21S6618
-
Bifunctional chelating agents, their technetium and rhenium
complexes, methods for their production, and radiopharma-
ceuticals containing these compounds.
This invention relates to new technetium and rhenium
chelates, methods for their production, and radiopharmaceu-
ticals containing these compounds as well as to conjugates
of these compounds with substances that accumulate selec-
tively in diseased tissue, in particular, peptides and
proteins. This in~éntion further relates to the production
of agents containing these compounds and their application
in radiodiagnostics.
Radioactive metal ions have been in use in medical
diagnostics and for therapy for a longer period of time.
For example, gamma-ray emitters such as the Tc-99m isotope
lS were used for detecting tumours. ~-ray emitters such as the
Re-186, Re-188, and Re-189 isotopes were applied in tumour
therapy. Technetium-99m is the most frequently applied
radionuclide in nuclear medicine as it is particularly
suitable as an isotope for in-vivo diagnostics due to its
favourable physical properties (no corpuscular radiation,
physical half-life of 6 h, y-radiation 140 keV) and the low
exposure to radiation resulting from them. Technetium-99m
is easily gained from nuclide generators in the form of
pertechnetate. It can be immediately used in this form to
produce kits for clinical routine applications.
The efficiency of radionuclides in in-vivo diagnostics as
well as for therapeutical purposes is dependent on the
specificity and selectivity of the labelled chelates with
regard to the target cell. An improvement of these
properties can be achieved by coupling the chelates with
biomolecules according to the "drug targeting" principle.
Antibodies, their fragments, hormones, growth factors and
substrates of receptors and enzymes are the obvious
substances for use as biomolecules. For example, British
' i 21S6618
-
patent application GB 2,109,407 describes the use of
radiolabelled monoclonal antibodies against tumour-
associated antigens for in-vivo tumour diagnostics. Direct
labelling of protein using technetium-99m, either via donor
groups (amino, amide, thiol groups, etc.) of the protein
(Rhodes, B.A. et al., J. Nucl. Med. 1986, 27, 685-693) or
by introducing complexing agents (US Patent 4,479,930 and
Fritzberg, A.R. et al., J. Nucl. Med. 1986, 27, 957) were
described likewise. These experimental methods, however,
are not available for clinical application because their
selectivity is too low and their background activity is too
high to facilitate in-vivo imaging.
The first receptor-binding radiopharmaceuticals were
described by J. P. DiZio et al. (J. Nucl . Med. 1992, 33;
558-569 and Bioconjugate Chem. 1991, 2; 353-366). These
compounds were not found suitable for in-vivo imaging. The
fact that the compounds described have to be extracted from
the labelling medium has proved to be a great disadvantage.
These conditions are unsatisfactory as a user should be
exposed to as little radiation as possible when preparing
the kit and because of the fact that only few clinics have
suitable laboratories for carrying out such work.
Cyclic amines as described by Volkert et al. (Appl. Radiol.
Isot. 1982, 33; 891-896) and Troutner et al. (J. Nucl. Med.
1980, 21; 443-448) as well as N2S2 and N3S systems are per
se of little use as tissue-specific radiopharmaceuticals as
they lack reactive groups to combine with biomolecules or
proteins (M. Borel et al., Appl.Radiat.Isot. 1992, 43, 425-
436).
While pH values from 10 to 13 are required for labelling
the above mentioned cyclic amines as well as N2O2 systems
known from literature (Pillai, M.R.A. et al.; Inorg. Chem.
1990, 29, 1850-1856), N2S2 and N3S systems can already be
complexed, as a rule, at pH values from 9 to 11. But these
conditions are still too extreme for labelling unstable
.2i~
-
conjugates of biomolecules and macromolecules. This is all
the more true as some systems such as L,L-ethylene
dicysteine (L,L-EC) can only be sufficiently labelled at pH
values ~ 10 (Verbruggen, A.M. et al.; J. Nucl. Med. 1992,
33, 551-557). MAG3 as it is known from literature even
requires exposure for ten minutes to temperatures from 90
to 100 C (Bannister, K.M. et al.; J. Nucl. Med. 1990, 31,
1568-1573) to be ~roduced in a radiochemical purity that is
sufficient for clinical applications. These conditions are
not satisfactory ~or everyday clinical routine.
N2S2 and N3S systems as described by G. Bormans et al.
(Nucl. Med. Biol. 1990, 17, 499-506) and in European Patent
Specifications EP 0 173 424 and EP 0 250 013 meet the
requirement of sufficient s~ability of the respective
technetium-99m complexes but are discharged too rapidly and
without specific accumulation in the organism. Thus they
are only used clinically, though to a limited extent, in
renal function diagnostics. Their use is limited mainly
because the demand has increased for substances that accu-
mulate specifically in diseased tissues.
To solve the refined problems of nuclear medicine, there isan increasing need for stable bifunctional complexing
agents that do not show the disadvantages mentioned when
complexing and are capable of forming conjugates with bio-
molecules and macromolecules without considerably affectingthe specif-icity and selectivity of the latter. At the same
time, all requirements regarding radiation dose, stability,
and solubility have to be met to use these compounds in
human beings.
It is thus a problem of this invention to provide these
compounds and conjugates, to create a fairly simple method
for their production, and to provide a kit formulation for
clinical application of these compounds/conjugates
according to the invention. The present invention solves
this problem.
61~
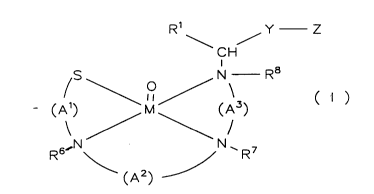
The compounds according to the invention are characterized
by the general formula (IJ
Rl\ /Y Z
CH
( ) ~M/(A3) ( I )
R6~N N~R7
.(A2)
wherein
(Al), (A2), and (A3) are same or different and represent
the general formula
O (CHR )I < (CHR9)m
(CR4R5)k
X
their free valences being linked arbitrarily with the
respective nitrogen or sulfur atoms,
and wherein
s 215661X
R3 and Rs are same or different and represent a hydrogen
atom, or a methyl or ethyl group, and
R4 is a hydrogen atom, a branched or unbranched alkyl group
containing 1 to 4 carbon atoms, the C atoms of which may
optionally carry additional amino groups, N(RaRb) groups
(with Ra and Rb being either same or different and repre-
senting branched or unbranched alkyl or acyl residues
containing 1 to 20 carbon atoms, the C atoms of which may
optionally carry an additional hydroxy, carboxy, or amino
group), hydroxy groups, thiol groups, halogens, carboxy
groups, alkoxy carbonyl groups containing 1 to 20 carbon
atoms, acyloxy groups containing 1 to 12 carbon atoms,
amino carbonyl grou~s, sulfonyl groups, amino sulfonyl
groups, or phosphorus residues,
R2 and R9 are same or different and represent the same as
R4,
k, 1, and m are same or different and stand for the numbers
0, 1, 2, 3, or 4, and
X is a hydrogen atom, a carboxy group, an alkoxy group
containing 1 to 20 carbon atoms, an alkoxy carbonyl group
containing 1 to 20 carbon atoms, an acyloxy group
containing 1 to 20 carbon atoms, an amino carbonyl group, a
sulfonyl group, an amino sulfonyl group, a phosphoric acid
residue, a carboxymethyl amino carbonyl group, a p-amino-
phenyl group, a p-hydroxyphenyl group, a halogen atom, a
hydroxy group, an amino group, an N(RaRb) group (with Ra
and Rb being either same or different and representing
branched or unbranched alkyl or acyl residues containing 1
to 20 carbon atoms, the C atoms of which may optionally
carry one additional hydroxy, carboxy, or amino group), a
hydrazine group, a hydrazide group, a cholesteryl
oxycarbonyl methyl amino carbonyl group, a cholesteryl
oxycarbonyl group, a cholesteryl oxycarbonyl methyl
6 21~661~
-
oxycarbonyl group, another steroid or a derivative of an
ethinyl or ethenyl steroid,
a substituent of the formula
zl_yl_ (CH2) i-Q-CO-
where
Q is an -NH- or -Q-,
Zl has the same meaning as Z,
yl has the same meaning as Y, and
i has the same meaning as m,
a substituent of the formula
v - u - Ql ~-
where
Q1 is an -NH-, -C0-, or -O-,
U is a bond, a group of the formula -(OCH2C0) h- with h=1-3,
or a suitable linker for coupling with bio- or macromole-
cules, and
V is a hydrogen atom, a hydroxy group, an N(RaRb) group
(with Ra and Rb being either same or different and repre-
senting branched or unbranched alkyl or acyl residues
containing 1 to 20 carbon atoms,the C atoms of which may
optionally carry one additional hydroxy, carboxy, or amino
group), a biomolecule, or a macromolecule, and
Y is an unsaturated unbranched or branched chain of up to
12 carbon atoms containing at least one double and/or
triple bond which may, optionally, carry additional one or
several and at any position in the chain, hydroxy, carboxy,
alkoxy, amino, or substituted amido groups containing 1 to
20 carbon atoms in the alkyl and/or aryl residue,
Z is a hydrogen atom, a halogen atom, a carboxy group, a
hydroxy group, an alkoxy carbonyl group containing 1 to 20
carbon atoms, an acyloxy group containing 1 to 20 carbon
21~6618
atoms, an alkoxy group containing 1 to 20 carbon atoms, a
cholesteryl oxycarbonyl methyl amino carbonyl group, a
cholesteryl oxycarbonyl group, a cholesteryl oxycarbonyl
methyl oxycarbonyl group, another steroid or a derivative
S of an ethinyl or ethenyl steroid,
a substituent of the formula
V - U - Ql - -
where
Q1 is an -NH-, -C0-, or -0-,
U is a bond, a group of the formula -(OCH2C=0) h- with h=l-
3, or a suitable linker for coupling with bio- or macro-
molecules, and `~-
V is a hydrogen atom, a hydroxy group, an N(RaRb) group
(with Ra and Rb being either same or different and repre-
senting branched or unbranched alkyl or acyl residuescontaining 1 to 20 carbon atoms, the C atoms of which may
optionally carry one additional hydroxy, carboxy, or amino
group), a biomolecule, or a macromolecule, and
M is an element having the atomic number 43 or 75,
Rl has the same meaning as R4,
R6, R7, and R8 are same or different and represent a hydro-
gen atom, an alkyl group containing 1 to 4 carbon atoms,
the C atoms of which may carry additional hydroxy, carboxy,
or amino groups, or a group of the formula -CH2-X in which
X has the above meaning
and their hydrosoluble salts.
Preferred compounds according to the invention of the
general formula (I) are characterized in that the residue Z
is a hydrogen atom, a steroid, an ethinyl steroid or an
ethenyl steroid.
8 2156618
Furthermore, compounds according to the invention of
general formula (I) are preferred that are characterized in
that residue Z is a hydrogen atom, a steroid, an ethinyl
steroid or an ethenyl steroid, and that residue X is a
hydrogen atom, a halogen atom, a carboxy group, an amino
group, or an amido group containing 1 to 20 carbon atoms in
their alkyl and/or aryl residue.
In addition, compounds according to the invention of
general formula (I? are preferred that are characterized in
that residue Z is a hydrogen atom, a steroid, an ethinyl
steroid or an ethenyl steroid, and that residue X is a
hydrogen atom, a halogen atom, a carboxy group, an amino
group, or an amido ~roup containing 1 to 20 carbon atoms in
the alkyl and/or aryl residue, Y is an ethinylide group,
residues Rl, R3, R6, R7, and R3 represent hydrogen atoms,
and k, 1, and m each represent the number 0.
Another group of preferred compounds according to the
invention of general formula (I) is characterized in that
residue Z is a hydrogen atom, and residue X is a choles-
teryl oxycarbonyl methyl amino carbonyl group, a choles-
teryl oxycarbonyl group, a cholesteryl oxycarbonyl methyl
oxycarbonyl group, another steroid or a derivative of an
ethine or ethene steroid.
Furthermore, preferred compounds according to the invention
of general formula (I) are characterized in that residue Z
is a hydrogen atom, residue X is a cholesteryl oxycarbonyl
methyl amino carbonyl group, a cholesteryl oxycarbonyl
group, a cholesteryl oxycarbonyl methyl oxycarbonyl group,
another steroid or a derivative of an ethine or ethene
steroid, residue Y is an ethinylide group, and that
residues R1, R3, R6, R7, and R8 represent hydrogen atoms,
and k, 1, and m each represent the number 0.
A third group of preferred compounds according to the
invention of general formula (I) is characterized in that
9 21S6618
residue Z is a hydrogen atom, residue X is a hydrogen atom,
a carboxy group, or a substituent of the formula
V - U - Ql _
where
S Ql is an -NH-, -CO-, or -O-,
U is a bond, a group of the formula -(OCH2CO)h- with h=1-3,
or a suitable linker for coupling with bio- or macromole-
cules, and
V is a hydrogen atom, a hydroxy group, an N(RaRb) group
(with Ra and Rb being either same or different and
representing branched or unbranched alkyl or acyl residues
containing 1 to 20 carbon atoms, the C atoms of which may
optionally carry an additional hydroxy, carboxy, or amino
group), a biomolecule, or a macromolecule.
Other preferred compounds according to the invention of
general formula (I) are characterized in that residue Z is
a hydrogen atom, residue X is a hydrogen atom, a carboxy
group, or a substituent of the formula
V - U - Ql _
where
Q1 is an -NH-, -CO-, or -O-,
U is a bond, a group of the formula -(OCH2CO)h- with h=1-3,
or a suitable linker for coupling with bio- or macromole-
cules, and-
V is a hydrogen atom, a hydroxy group, an N(RaRb) group(with Ra and Rb being either same or different and repre-
senting branched or unbranched alkyl or acyl residues
containing 1 to 20 carbon atoms, the C atoms of which may
optionally carry an additional hydroxy, carboxy, or amino
group), a biomolecule, or a macromolecule, that residue Y
is an ethinylide group, and that residues Rl, R3, R6, R7,
and R8 represent hydrogen atoms, and k, l, and m each
represent the number 0.
- lo 21S6618
A fourth group of preferred compounds according to the
invention of general formula (I) is characterized in that
residue Z is a hydrogen atom, and residue X is a hydrogen
atom, a carboxy group, an alkoxy group containing 1 to 20
carbon atoms, an alkoxy carbonyl group containing 1 to 20
carbon atoms, an acyloxy group containing 1 to 20 carbon
atoms, an amino carbonyl group, a sulfonyl group, an amino
sulfonyl group, a-phosphoric acid residue, a carboxymethyl
amino carbonyl group, a p-aminophenyl group, a p-hydroxy-
phenyl group, a ha~ogen atom, a hydroxy group, an amino
group, an N(RaRb) group (with Ra and Rb being either same
or different and representing branched or unbranched alkyl
or acyl residues containing 1 to 20 carbon atoms, the C
atoms of which may optionally carry an additional hydroxy,
carboxy, or amino group), a hydrazine group, or a hydrazidegroup.
In addition, compounds according to the invention of
general formula (I) are characterized in that residue Z is
a hydrogen atom, and residue X is a hydrogen atom, a
carboxy group, an alkoxy group containing 1 to 20 carbon
atoms, an alkoxy carbonyl group containing 1 to 20 carbon
atoms, an acyloxy group containing 1 to 20 carbon atoms, an
amino carbonyl group, a sulfonyl group, an amino sulfonyl
group, a phosphoric acid residue, a carboxymethyl amino
carbonyl group, a p-aminophenyl group, a p-hydroxyphenyl
group, a halogen atom, a hydroxy group, an amino group, an
N(RaRb) group (with Ra and Rb being either same or
different and representing branched or unbranched alkyl or
acyl residues containing 1 to 20 carbon atoms, the C atoms
of which may optionally carry an additional hydroxy,
carboxy, or amino group), a hydrazine group, or a hydrazide
group, that residue Y is an ethinylide group, and that
residues R1, R3, R6, R7, and R8 represent hydrogen atoms,
and k, l, and m each represent the number 0.
- 11 2156618
The present invention further relates to compounds of the
general formula (II)
Rl\ /Y Z
T CH
~S N R8
(A~) (A3) ( I I )
R6~N N~R7
(A2)
'~.
wherein Al, A2, A3, R1, R6, R7, R8, Y and Z have the meaning
specified above and wherein T is a hydrogen atom, an
acetate group, a benzoate group, a p-methoxybenzyl group,
an acetamidomethyl group, a benzamidomethyl group, a
trimethyl acetamidomethyl group, a hydroxy acetyl group, or
another suitable sulfur protective group.
Another object of this invention are conjugates of com-
pounds according to the invention of general formulae (I)
and (II) with substances that accumulate selectively in
tissues. The fragments are bonded amidically, imidically,
or ester-like, depending on the kind of substances to be
conjugated. If the substances that accumulate in the
tissues are peptides, proteins, antibodies, or fragments
thereof, they are bonded amidically through their amino
groups; if the substances that accumulate in the tissues
contain hydroxy groups, they are bonded ester-likei and if
the substances that accumulate in the tissues contain
aldehyde groups, they are bonded imidically.
~ 12 ~ 1~ 6618
Such substances accumulating in tissues are preferred that
accumulate in diseased tissues, above all in tissues that
have atherosclerotic plaques.
s
Furthermore, conjugates according to the invention are
preferred that are characterized in that the substances
that accumulate in diseased tissue are peptides such as
endothelines, partial endotheline sequences, endotheline
analogues, endothe~ine derivatives, or endotheline
antagonists.
Particularly preferred conjugates according to the
invention are characterized in that the peptides comprise
the following sequences or parts thereof:
cys-ser-cys-ser-ser-leu-met-asp-lys-glu-cys-val-tyr-
phe-cys-his-leu-asp-ile-ile-trp,
cys-ser-cys-ser-ser-trp-leu-asp-lys-glu-cys-val-tyr-
phe-cys-his-leu-asp-ile-ile-trp,
cys-thr-cys-phe-thr-tyr-lys-asp-lys-glu-cys-val-tyr-
tyr-cys-his-leu-asp-ile-ile-trp,
cys-ser-ala-ser-ser-leu-met-asp-lys-glu-ala-val-tyr-
I
phe-cys-his-leu-asp-ile-ile-trp,
~ 13 21~66i8
cys-ser-cys-asn-ser-trp-leu-asp-lys-glu-cys-val-tyr-
phe-cys-his-leu-asp-ile-ile-trp,
cys-ser-cys-lys-asp-met-thr-asp-lys-glu-cys-leu-asn-
phe-cys-his-gln-asp-val-ile-trp,
~
ala-ser-cys-ser-ser-leu-met-asp-lys-glu-cys-val-tyr-
phe-ala-his-leu-asp-ile-ile-trp,
~ .
ala-ser-ala-ser-ser-leu-met-asp-lys-glu-ala-val-tyr-
phe-ala-his-leu-asp-ile-ile-trp,
cys-ser-cys-ser-ser-trp-leu-asp-lys-glu-ala-val-tyr-
phe-ala-his-leu-asp-ile-ile-trp,
cys-val-tyr-phe-cys-his-leu-asp-ile-ile-trp,
N-acetyl-leu-met-asp-lys-glu-ala-val-tyr-phe-ala-his-leu-
asp-ile-ile-trp,
or the partial sequence
his-leu-asp-ile-ile-trp
or the cyclic amino acid sequences
Cyclo-(Dtrp-Dasp-pro-Dval-leu),
Cyclo-(Dglu-ala-alloDile-leu-Dtrp).
21~6618
_ 14
The compounds according to the invention of the general
formula (I)
CH
S ~ M / (A3)
6,N N ~ R7
~(A2)
are produced according to a method known to a person
skilled in the art, for example, by reacting, in the
presence of a reductant (such,as Sn(II) chloride or
dithionide) and, optionally, an auxiliary ligand (such as
sodium citrate or sodium tartrate), technetium-99m in the
form of pertechnetate which is easily eluted from molyb-
denum-technetium generators, with a compound of the general
formula (II)
Rl ~Y Z
T CH
S N R8
(A') ( ~3)
R6~N ~N ~ R7
(A2)--'
2156618
wherein Al, A2, A3, Rl, R6, R7, R8, Y, Z, and T have the
meaning specified above.
The reaction is preferably carried out in an aqueous medium
at room temperature. The SH protective group is split off
in situ or according to the methods known to a person
skilled in the art from the relevant literature, e.g. basic
hydrolysis, reductive decomposition, etc. (see, for
example, "Protective Groups in Organic Synthesis", T.W.
Greene, John Wiley and Sons 1981).
Another object of the present invention are methods for the
production of compo*nds according to the invention of the
general formula (II)
T CH
S N R8
(A1) (/3) ( ll )
R6,N ~ N~R7
(A2)
characterized in that, in a generally known way,
a) a compound of the general formula (III)
16 2156618
R'\ /Y Z
CH
Hal N--R8
(A~ 3) ( I I I )
6,N N ~ R7
(A2)
<
wherein
Al A2, A3, Rl, R6, R7, R8, Y and Z have the meaning
specified above and~ Hal represents a halogen,
is reacted with a compound of the general formula
T - S~ M+
wherein M+ is an alkaline metal cation and T has the
meaning specified above,
or,
b) a compound of the general formula
HN(R6)-A2-N(R7)-A3-N(R8)-Y-Z
wherein A2, A3, R6, R7, and R8 have the meaning specified
above, are reacted with
a compound of the general formula
T - S - Al - W
wherein Al and T have the meaning given in Claim 11, and W
represents a leaving group which enables A1 to react with
free amino acids.
The N-haloacetyl amides required as parent substances are
produced according to methods known from the relevant
literature by acylating an amino function using haloacetyl
`_ 1 21S6618
halides (JACS, 1969, 90, 4508; Chem. Pharm. Bull. 1981,
29(1), 128).
The required amines of the general formula
HN(R6) -A2-N(R7) -A3-N(R8) -Y-Z
are synthesized according to methods of peptide synthesis
known from the relevant literature (see, for example, "The
Practice of Peptide Synthesis", M. Bodanszky and A.
Bodanszky; Spring~r Verlag, 1984, and Fieser, Reagents for
Organic Synthesis 10, 142). N-protected a- and ~-amino
acids are coupled, according to methods known to a person
skilled in the art such as a (primary or secondary) amine
addition/ eliminati~on reaction, with N-unprotected organic
molecules using a carbonyl compound (such as acid chloride,
mixed anhydride, activated ester). After separating the
amino protecting group according to methods known from
literature (such as basic hydrolysis, hydrogenolysis,
reductive decomposition using alkaline metals in liquid
ammonium), the remaining amino acid is re-derivated in a
second reaction using an N-protected a- and ~-amino acid
according to methods known from the relevant literature
(e.g. the carbodiimide method). The amino protecting group
is separated according to the above-mentioned methods
described in the literature.
The compounds of the general formula
T - S - Al - W
that are also required are synthesized by converting the
carboxy group(s) of respective carboxylic acids according
to methods known to a person skilled in the art, for
example, according to the diimide method (Fieser, Reagents
for Organic Synthesis 10, 142), via a mixed or cyclic
anhydride (Org. Prep. Proc. Int. 1975, 7, 215), or using an
activated ester (Adv. Org. Chem. Part B, 472).
18 215 6618
The compounds according to the invention show the desired
properties to a great extent. The radionuclides required
for their application are stably bound in the complex.
The coupling options of the chelating agents according to
the invention are of particular interest; these agents can
be viewed as being trifunctional as they facilitate
coupling via a multiple bond as well as via a carboxy or
amino group besides metal complexing in Al, A2, or A3.
It is of particular relevance to the invention that
labelling methods be provided which permit rapid and
quantitative reacti~on of the compounds of the invention
under more gentle conditions than those of known methods,
and immediately before use of the radiopharmaceutical.
It is of great advantage to link the chelating agent with
steroids via the multiple bond as this allows both coupling
to a -CH2 group of the steroid or bonding to the 17-ethinyl
or 17-ethenyl group of steroids.
Some technetium-99m labelled steroid derivatives have a
surprisingly strong affinity for their respective steroid
hormone receptor. Thus, the technetium complex of 3,17~-
dihydroxy-17a-(5-[2-benzoylthioacetyl-glycyl-glycyl]-
amino-pent-1-(E)-en-3-in)-1,3,5-estratriene (Example 13b)
showed a similar receptor affinity as 17~-estradiol in an
in-vitro cytosol test (Carlson, K.E. et al. 1989, 32, 345-
355). This makes these compounds excellently suited for re-
ceptor imaging using technetium-99m and for the diagnosis
and therapy of steroid-dependent tumours.
Another advantage of the present invention is the fact
that, due to the option of linking either via the carboxy
group or via the multiple bond, it allows to control the
solubility and pharmacokinetics of the complexes by chemi-
cal substitution at the level of complexing agents.
19 21~661~
Biodegradable groups or linkers in X are of particular
significance here.
Compounds containing lipophilic substituents in X or Z show
surprising properties, such as the compound of Example 9,
as they accumulate in atherosclerotic plaques and thus
provide a way to visualize atherosclerotic modifications on
vessel walls.
Selection of appropriate bio- and macromolecules (e.g.
monoclonal antibod~es, steroids, ...) in X or Z yields
complexes according to the invention that show a surpris-
ingly great extent of tissue and organ specificity and may
therefore contribute excellently to solving a number of
diagnostic and therapeutical problems.
The complexing ligands obtained in this way of the general
formula (II) in which Z is a hydrogen atom and/or ligands
of the general formula (II) containing an ethinyl function
may be coupled with iodovinyl steroids according to methods
known to a person skilled in the art (e.g. R. Rossi et al.,
Tetrahedron 1983, 39, 287). The iodovinyl steroids required
are synthesized according to methods known from the
relevant literature (e.g. H. Hofmeister et al., Tetrahedron
1986, 42, 357S).
The carboxy groups of compounds of the general formula (II)
may be converted, for example, according to the diimide
method (Fieser, Reagents for Organic Synthesis 10, 142),
via a mixed or cyclic anhydride (Org. Prep. Proc. Int.
1975, 7, 215), or using an activated ester (Adv. Org. Chem.
Part B, 472).
The chelating agents are coupled to bio- or macromolecules
according to methods known to a person skilled in the art
by converting the carboxy group(s) at the chelating agent,
for example, according to the diimide method (Fieser,
Reagents for Organic Synthesis 10, 142), via a mixed or
-- 20 21S6618
cyclic anhydride (Org. Prep. Proc. Int. 1975, 7, 215), or
using an activated ester (Adv. Org. Chem. Part B, 472), and
subsequent formation of a covalent bond by reaction with a
nucleophilic group of the bio- or macromolecule.
The chelating agents obtained in this way may also be
linked with bio- or macromolecules that are known to accu-
mulate to a particularly great extent in the organ, organic
part, or tissue to be examined. Among such molecules are,
for example, enzymes, hormones, polysaccharides such as
dextrane or starches, porphyrins, bleomycins, insulin,
prostaglandins, steroid hormones, amino sugar, amino acids,
peptides such as polylysine, proteins (such as immoglobu-
lins, monoclonal antibodies, lectins), lipides (including
liposomes), and nucleotides of the DNA and RNA types.
Conjugates with albumins such as human serum albumin, with
antibodies such as monoclonal antibodies, antibodies spe-
cific to tumour-associated antigens, or antimyosin are of
particular significance here. Suitable synthetic polymers
such as polyethylene imines, polyamides, etc. may be linked
instead of biological macromolecules.
The pharmaceuticals produced from these conjugates are
suited for application in tumour diagnostics and tumour
therapy, diagnosis of atherosclerosis, or receptor imaging.
Bonding affinity and specificity of the produced pharmaceu-
tical to the target organ, target organ part, or targettissue should be impaired only slightly, or not at all, by
forming the conjugate.
The radiopharmaceuticals of the invention are also produced
in a generally known way by dissolving or suspending the
complexing agents according to the invention and their
conjugates in an aqueous medium, optionally by adding the
adjuvants common in galenics, and subsequent optional
lyophilization of the solution or suspension. Among the
suitable additives are physiologically tolerable buffers
(such as tromethamine), auxiliary ligands (such as sodium
21 ~156618
,
citrate or sodium tartrate), reductants (such as tin (II)
chloride) or, if required, electrolytes such as sodium
chloride, or, if required, (one of) the adjuvants common in
galenics (such as lactose, mannite) and/or surfactant(s)
(such as lecithine, Tween~, Myrj~). The composition of
additives used should facilitate the production of the
compounds according to the invention.
Another object of the present invention is a kit for pro-
ducing radiopharmaceuticals consisting of a compound of the
general formula (I~), a reductant and, optionally, an
auxiliary ligand, said substances being either dry or in
solution, instructions for use including instructions for
reacting the compounds described with technetium-99m or
rhenium in the form of a pertechnetate or perrhenate
solution.
The radiopharmaceuticals according to the invention are
applied at doses from 0.1 mCi to 50 mCi, preferably at a
dose between 0.5 mCi and 30 mCi, per 70 kg of a patient's
body weight. Details of appli~ation and dosage are de-
scribed, for example, in "Radiotracers for Medical Applica-
tions" CRC Press, Boca Raton, Florida. The radiopharmaceu-
ticals are designed for intravenous administration. For
radiodiagnostic and radiotherapeutic purposes, the complex
compounds/conjugates are used in the form of complexes with
radionuclides of elements having the atomic numbers 43 or
75.
The radiopharmaceuticals according to the invention meet
the various requirements to be appropriate for use as
radiopharmaceuticals in radiodiagnostics and radiotherapy.
They are excellently suited for accumulating in target
tissues after i.v. administration, thus permitting a non-
invasive diagnosis of the respective tissues. Solubility
in water of the radiopharmaceuticals according to the
invention is ensured, if required, by adjuvants common in
galenics as described above. In addition, the radiopharma-
22 215 6618
ceuticals according to the invention do not only have greatin-vitro stability but also a surprisingly great in-vivo
stability; therefore, there is no, or at least no clini-
cally relevant, release or exchange of the radionuclide
bound in the complex.
The preferred radiopharmaceuticals according to the inven-
tion are furthermore characterized in that they contain the
compounds according to the invention of the general formu-
lae (I) or (II) in the form of liposomes, and that the
compound according to the invention of general formula (I)
is optionally prepared in a kit using technetium or rhenium
in the form of their permetallates.
The radiopharmaceut`icals according to the invention may
also be used in radioimmuno- or radiation therapy. These
differ from radiodiagnostics only in the type and quantity
of radionuclide used. Its objective is to destroy tumour
cells using short-wave rays of a fairly short range. Among
the suitable ~-ray emitting isotopes are rhenium-186 and
rhenium-188.
The radiopharmaceuticals according to the invention are
suited for non-invasive in-vivo imaging of tissues
containing steroid receptors, for example, steroid hormone
dependent tumours.
The radiopharmaceuticals according to the invention are
suited for non-invasive in-vivo imaging of atherosclerotic
vessel changes, for example, of plaques.
All in all, new complexing agents and metal complexes have
been successfully synthesized that open up new ways for
diagnostic and therapeutic medicine. This is desirable
mainly with a view to the development of novel imaging
procedures for nuclear diagnostics.
2156618
_ 23
The invention shall now be explained in more detail by the
following examples.
21~6618
24
_
Example 1
a) S-benzoyl thioacetyl glycyl glycyl propargyl amide [la]
A solution of 215.1 mg (1.22 mmol) of potassium thiobenzoate
in anhydrous DMF is added by dropping, and in a nitrogen
atmosphere, to a solution of 150 mg (0.61 mmol) of chloro-
acetyl glycyl glycyl propargyl amide in anhydrous DMF. Then,
catalytic quantities of sodium iodide are added, and the
batch is heated for 2 hours to 110 C. After cooling down to
room temperature, ~he reaction mixture is stirred into 1 N
HCl and extracted with acetic ester until the product is no
longer contained in the aquèous phase. The organic phases
are evaporated to dryness under reduced pressure, again
taken up in acetic ~ster, washed with water, and dried above
Mg2SO4. After drawing off the solvent under reduced pres-
sure, the product is recrystallized from methanol or, if re-
quired, purified by column chromatography using CH2Cl2/MeOH
(9:1).
Yield: 76~ of the title compound [la]
Cl6Hl7N304S (MW: 347.39
lH NMR (d6-DMS0):
= 3.11 (t; lH, -C-CH)
3.69 (d; 2H, -NH(CH2)CO-)
3.78 (d; 2H, -NH(CH2)C0-)
3.84 - 3.88 (m; 2H, -NH(CH2)-C-CH)
3.90 (s; 2H, -SCH2CO-)
7.55 - 7.61 (m; 2H, ArH)
7.69 - 7.74 (m; lH, ArH)
7.93 - 7.97 (m; 2H, ArH)
8.20 -(t; lH, -NH-)
8.27 (t; lH, -NH-)
8.50 (t; lH, -NH-)
2156618
b) Technetium-99m complex tlb] of S-benzoyl thioacetyl
glycyl glycyl propargyl amide
A solution of 0.5 mg (1.44 ~mol) of S-benzoyl thioacetyl
glycyl glycyl propargyl amide [la] in 50 ~l of 1 N soda lye
is diluted with 250 ~l of phosphate buffer (Na2HPO4,
0.5 mol/l, pH 8.5). Afterwards, 50 ~l of 0.15 molar
trisodium citrate dihydrate solution and 2.5 ~l of 0.2
molar tin(II) chloride dihydrate solution are added. The
reaction mixture i,s mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 mol;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
Example 2
a) 2-acetyl thiosuccinyl glycyl glycyl propargyl amide
~2a]
5.89 g of (37.5 mmol) of glycyl glycyl propargyl amide are
dissolved in a nitrogen atmosphere in 60 ml of anhydrous
DMF, mixed with 8.50 g (48.7 mmol) of acetyl mercaptosuc-
cinic anhydride and kept agitated overnight at room
temperature. The reaction mixture is then evaporated to
dryness under reduced pressure. The residue is suspended in
methanol, filtered off by suction, and recrystallized from
water.
Yield: 61~ of the title compound [2a]
Cl3H17N3O6S (MW: 343.36)
26 2156618
H NMR ( d6 - DMSO ) :
= 2.35 (s; 3H, -COCH3)
2.66; 2.83 (2x dd; 2H, -(CH2)COOH)
2.99 (t; lH, -C--CH)
3.70 - 3.77 (m; 4H, 2x -NH(CH2)-CO)
3.88 (dd; 2H, -NH(CH2)-C-CH)
4.35 (t; lH, S-CH(CH2COOH)-CH2-)
8.07 (t; lH, -~NH-)
8.12 (t; lH, -NH-)
8.26 (t; lH, -NH-~
b) Technetium-99m complex [lb] of 2-acetyl thiosuccinyl
glycyl glycyl p~opargyl amide
A solution of 0.5 mg (1.45 ~mol) of 2-acetyl thiosuccinyl
glycyl glycyl propargyl amide [2a] in 50 ~l of 0.1 N soda
lye is diluted with 250 ~l of phosphate buffer (Na2HPO4,
0.5 mol/l, pH 8.5). Afterwards, 50 ~l of 0.15 molar
trisodium citrate dihydrate sPlution and 2.5 ~l of 0.2
molar tin(II) chloride dihydrate solution are added. The
reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than 95~.
27 21~ 6618
Example 3
a) S-acetyl thioacetyl glutaminyl glycyl propargyl amide
[3a]
A solution/suspension of 2.0 g (8.3 mmol) of glutaminyl
glycyl propargyl amide in 80 ml of anhydrous DMF is slowly
mixed with a solution of 5.3 g (8.4 mmol) of S-acetyl
mercaptoacetic N-hydroxysuccinimide ester in anhydrous THF.
The mixture is hydrolyzed after 4 hours of stirring at room
temperature. Then~the solvent is removed under reduced
pressure, and the residue recrystallized from
methanol/water.
Yield: 45~ of the title compound [3a]
Cl4Hl9N306S (~W: 357.36)
lH NMR (d6-DMSO):
~ = 1.64 - 1.92 (m; 2H, -CHCH2CH2COOH)
2.31 (s; 3H, -COCH3)
2.36 (t; 2H, -CHCH2CH2COOH)
3.02 (t; lH, -C--CH)
3.70 (s; 2H, -NH(CH2)-CO)-
3.73 - 3.80 (m; lH, -CHCH2CH2COOH)
3.89 (dd; 2H, -NH(CH2)-C--CH)
8.09 (m; lH, -NH-)
8.19 (m; lH, -NH-)
8.26 (m; lH, -NH-)
b) Technetium-99m complex [3b] of S-acetyl thioacetyl
glutaminyl glycyl propargyl amide
A solution of 0.5 mg (1.40 ~mol) of S-acetyl thioacetyl
glutaminyl glycyl propargyl amide [3a] in 50 ~1 of 0.1 N
soda lye is diluted with 250 ~l of phosphate buffer
(Na2HPO4, 0.5 mol/l, pH 8.5). Afterwards, 50 ~1 of 0.15
molar trisodium citrate dihydrate solution and 2.5 ~1 of
0.2 molar tin(II) chloride dihydrate solution are added.
The reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
2156618
28
-
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V.); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than 95~.
,
Example 4
a) S-acetyl thioac~tyl glycyl glutaminyl propargyl amide
~4a]
A solution/suspension of 2.0 g (8.3 mmol) of glycyl
glutaminyl propargyl amide in 80 ml of anhydrous DMF is
slowly mixed with a solution of 5.3 g (8.4 mmol) of S-
acetyl mercaptoacetic N-hydroxysuccinimide ester in
anhydrous TH~. The mixture is hydrolyzed after 4 hours of
stirring at room temperature. Then the solvent is removed
under reduced pressure, and the residue recrystallized from
methanol/water.
Yield: 61~ of the title compound [4a]
Cl4H1gN3O6S (MW: 357.36)
1H NMR (d6-DMSO):
~ = 1.60 - 1.88 (m; 2H, -CHCH2CH2COOH)
2.30 (s; 3H, -COCH3)
2.28 (t; 2H, -CHCH2CH2COOH)
3.04 (t; lH, -C3CH)
3.68 (s; 2H, -NH(CH2)-CO)
3.70 - 3.77 (m; lH, -CHCH2CH2COOH)
3.88 (dd; 2H, -NH(CH2)-C3CH)
4.19 (s; 2H, -SCH2CO-)
8.11 (m; lH, -NH-)
8.23 (m; lH, -NH-)
_ 29 2156618
8.35 (m; lH, -NH-)
b) Technetium-99m complex [4b] of S-acetyl thioacetyl
glycyl glutaminyl propargyl amide
A solution of 0.5 mg (1.40 ~mol) of S-acetyl thioacetyl
glycyl glutaminyl propargyl amide [4a] in 50 ~l of 0.1 N
soda lye is diluted with 250 ~l of phosphate buffer
(Na2HPO4, 0.5 mol/1, pH 7.5). Afterwards, 50 ~l of 0.15
molar trisodium citrate dihydrate solution and 2.5 ~l of
0.2 molar tin(II) chloride dihydrate solution are added.
The reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HP04; 0.01 M;
pH 2.0) 50:50 (V/V); flow ratê: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95%.
Example 5
a) S-(p-methoxybenzyl) cysteinyl glycyl glycyl propargyl
amide [Sa]
9.8 g (0.02 mol) of N- tert. -butoxycarbonyl-S-(p-methoxyben-
zyl) cysteinyl glycyl glycyl propargyl amide are dissolved
at 0C in trifluoroacetic acid and stirred in an ice bath
for 4 hours. Then the solution is evaporated to dryness
under reduced pressure, the residue is mixed with ethanol
and evaporated once again. The residue is purified by
column chromatography above silica gel [CH2Cl2/CH3OH
(5:1)]. The product crystallizes after evaporating.
Yield: 93~ of the title compound [5a]
_ 30 2156618
C18H24N4O4S (MW: 392.48)
H NMR (CD30D):
= 2.54 (t; lH, -C-CH)
2.63 - 2.86 (m; 2H, -CHCH2S-)
3.48 - 3.s2 (m; lH, -CHCH2S-)
3.71 (s; 2H, -NH(CH2)CO-)
3.77 (s; 3H, -OCH3)
3.85 (s; 2H, --NH(CH2)CO-)
3.92 (s; 2H, -CH2Ar)
3.97 (dd; 2H, .~NH(CH2)-C-CH)
6.86; 7.25 (AA'BB'; 4H, ArH)
b) Technetium-99m complex of cysteinyl glycyl glycyl
propargyl amide~[Sb]
A solution of 0.5 mg (1.27 ~mol) of S-(p-methoxybenzyl)
cysteinyl glycyl glycyl propargyl amide [5a] in liquid HF
is stirred for 15 minutes at 0C. After evaporating the
solvent at room temperature, the residue is taken up in
50 ~l of 1 N soda lye and diluted with 250 ~l of phosphate
buffer (Na2HPO4, 0.5 mol/l, pH 7.5). Afterwards, 50 ~l of
0.15 molar trisodium citrate dihydrate solution and 2.5 ~l
of 0.2 molar tin(II) chloride dihydrate solution are added.
The reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, S ~m;
gradient from 100% A to 100% B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0;01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
~ 31 2156618
Example 6
a) N-[2-acetylthio-3-cholesteryl oxycarbonyl propionyl]-
glycyl glycyl propargyl amide t6a]
A solution of 5.0 g (8.9 mmol) of 3-acetylthio cholesterol
succinate in anhydrous THF is cooled in an ice bath and
mixed in excess with dry triethyl amine. Afterwards, 1.9 g
(17.8 mmol) of ethyl chloroformate are added by dropping.
The batch is allowed to warm up to 0C, then mixed with
3.0 g (18.0 mmol) of glycyl glycyl propargyl amide and
stirred at room temperature for 3 hours. The reaction
mixture is hydrolyzed with water and extracted with acetic
ester until the product is no longer contained in the
aqueous phase. The organic phases are evaporated to dryness
under reduced pressure, again taken up in acetic ester,
washed with water, and dried above Mg2SO4. After drawing off
the solvent under reduced pressure, the product is
recrystallized from methanol or, if required, purified by
column chromatography using CH2Cl2/MeOH (9:1).
Yield: 67~ of the title compoûnd [6a]
C40H61N306S (MW: 712.01)
H NMR (CDC13):
= 0.68 - 2.08 (m; 41H, steroidal)
2.30 (d; 2H, -C=CHCH2- steroidal)
2.32 (s; 3H, -COCH3)
2.76 - 3.00 (m; 2H, -(CH2)COO-)
3.10 (t-; lH, -C-CH)
3.66 - 3.73 (m; 4H, 2x -NH(CH2)CO-)
3.84 (d; 2H, -NH(CH2)-C--CH)
4.39 (t; lH, -S-CH(CH2COOH)-CH2-)
4.52 - 4.63 (m; lH, -(CH)O steroidal)
5.48 (d; lH, -C=CH- steroidal)
8.05 (t; lH, -NH-)
8.11 (t; lH, -NH-)
8.23 (t; lH, -NH-)
2~6618
_ 32
b) Technetium-99m complex [6b] of N-t2-acetylthio-3-
cholesteryl oxycarbonyl propionyl]-glycyl glycyl
propargyl amide
A solution of 0.5 mg (0.7 ~mol) of N-[2-acetylthio-3-
cholesteryl oxycarbonyl propionyl]-glycyl glycyl propargyl
amide [6a] in 300 ~1 of DMSO is mixed with 30 ~l of 1 N
soda lye. Afterwards, 50 ~l of 0.15 molar trisodium citrate
dihydrate solution and 0.4 to 0.9 mCi of pertechnetate
solution from a Mo_99/Tc-99m generator are added. The
reaction mixture is mixed in portions with 10 ~l of 0.2
molar tin(II) chloride dihydrate solution, incubated at
room temperature for 10 minutes, and filtered (0.2 ~m
filter). ~
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 30 min;
eluent A: acetonitrile/water 50:50 (V/V);
eluent B: acetonitrile; flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~-
Example 7
a) Cholesterol-~N-(2-acetylthio succinyl glycyl glycyl
propargyl amide-4-yl)glycine] ester ~7a]
A solution of 2.0 mg (5.8 mmol) of 2-acetylthio succinyl
glycyl glycyl propargyl amide [2a] in anhydrous THF is
cooled in an ice bath and mixed in excess with dry triethyl
amine. Afterwards, 1.3 g (11.6 mmol) of ethyl chloroformate
are added by dropping. The batch is allowed to warm up to
0C, then mixed with 5.0 g (12.0 mmol) of glycine
cholesterol ester and stirred at room temperature for 3
hours. The reaction mixture is hydrolyzed with water and
extracted with acetic ester until the product is no longer
_ 33 21S6618
contained in the aqueous phase. The organic phases are
evaporated to dryness under reduced pressure, again taken up
in acetic ester, washed with water, and dried above Mg2SO4.
After drawing off the solvent under reduced pressure, the
product is recrystallized from methanol or, if required,
purified by column chromatography using CH2Cl2/MeOH (9:1).
Yield: 41% of the title compound [7a]
C42H64N306S (MW: 769.06)
lH NMR (CD30D):
~ = 0.68 - 2.10 (m;'41H, steroidal)
2.32 (d; 2H, -C=CHCH2- steroidal)
2.33 (s; 3H, -COCH3)
2.81 - 3.10 (m; 2H, -CH(CH2)COO-)
3.08 (t; lH, -C--~H)
3.41 (d; 2H, -NH(CH2)CO-)
3.64 - 3.71 (m; 4H, 2x -NH(CH2)CO-)
3.83 (d; 2H, -NH(CH2)-C-CH)
4.35 (t; lH, -S-CH(CH2COOH)-CH2-)
4.61 - 4.72 (m; lH, -(CH)O- steroidal)
5.38 (d; lH, -C=CH- steroidal)
8.05 (t; lH, -NH-)
8.09 (t; lH, -NH-)
8.13 (t; lH, -NH-)
8.25 (t; lH, -NH-)
b) Technetium-99m complex [7b] of cholesterol-tN-(2-
acetylthio-succinyl glycyl glycyl propargyl a~; de-4-
yl)glycine] ester
A solution of 0.5 mg (0.65 ~mol) of cholesterol-N-[2-
acetylthio-succinyl glycyl glycyl propargyl amide-4-
yl)glycine] ester [7a] in 300 ~1 of DMSO is mixed with
30 ~1 of 0.1 N soda lye. Afterwards, 50 ~1 of 0.15 molar
trisodium citrate dihydrate solution and 0.4 to 0.9 mCi of
pertechnetate solution from a Mo-99/Tc-99m generator are
added. The reaction mixture is mixed in portions with 10 ~l
~ 34 21~661~
of 0.2 molar tin(II) chloride dihydrate solution, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100% B within 30 min;
eluent A: acetonitrile/water 50:50 (V/V);
eluent B: acetonitrile; flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95%.
Example 8
a) N-dodecanoyl-S-(p-methoxybenzyl) cysteinyl glycyl
glycyl propargyl amide [8a]
2.0 g (5.1 mmol) of S-(p-methoxybenzyl) cysteinyl glycyl
glycyl propargyl amide [5a] are dissolved in CH2Cl2 and
mixed with a small amount of NEt3. Afterwards, 1.2 g (5.5
mmol) of dodecanoic acid chloride in CH2Cl2 are added by
dropping and stirred at room temperature for 2 hours. The
mixture is hydrolyzed with water and extracted with CH2Cl2.
After drying, the product is evaporated under reduced
pressure and the residue chromatographed using CH2Cl2/MeOH
(9:1) above silica gel.
Yield: 80% of the title compound [8a]
C30H46N4OsS (MW: 574.79)
H NMR (d6-DMSO):
= 0.85 (t; 3H, -(CH2)8CH3)
1.19 - 1.30 (m; 16H, -(CH2)8CH3)
1.46 - 1.54 (m; 2H, -CH2-CH2CONH)
2.08 - 2.20 (m; 2H, -CH2-CH2CONH)
2.51 - 2.79 (m; 2H, -CHCH2S)
3.08 (t; lH, -C-CH)
3.74 (s; 3H, OCH3)
3.67 - 3.78 (m; 4H, 2x -NH(CH2)CO-)
~ 35 2156618
3.76 (d; 2H, -CH2Ar)
3.85 - 3.88 (m; 2H, -NH(CH2)-C_CH)
4.49 - 4.55 (m; lH, -CHCH2S-)
6.85; 7.23 (AA'BB'; 4H, ArH)
8.04 - 8.09 (m; 2H, 2x -NH-)
8.22 (m; lH, -NH-)
8.33 (m; lH, -NH-)
b) Technetium-99m complex [8b] of N-dodecanoyl cysteinyl
glycyl glycyl ~ropargyl amide
A solution of 0.5 mg (0.9 ~mol) of N-dodecanoyl cysteinyl
glycyl glycyl propargyl amide [8a] in liquid HF is stirred
for 15 minutes at 0~C. After evaporating the solvent at
room temperature, the residue is dissolved in 300 ~l of
DMSO and mixed with 30 ~l of 1 N soda lye. Afterwards, 50
~l of 0.15 molar trisodium citrate dihydrate solution and
0.4 to 0.9 mCi of pertechnetate solution from a Mo-99/Tc-
99m generator are added. The reaction mixture is mixed in
portions with 10 ~l of 0.2 molar tin(II) chloride dihydrate
solution, incubated at room temperature for 10 minutes, and
filtered (0.2 ~m filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
21~6618
36
Example 9
a) N-(3-hexadecyl aminocarbonyl-2-acetylthio-propionyl)
glycyl glycyl propargyl amide [9a]
A solution of 2.0 g (5.8 mmol) of 2-acetylthio succinyl
glycyl glycyl propargyl amide [2a] in anhydrous THF is
cooled in an ice bath and mixed with 1.7 g (7.0 mmol) of
hexadecylamine. Afterwards, 1.47 g (7.0 mmol) of dicyclo-
hexyl carbodiimidefin anhydrous THF mixed with 2 ml of
triethyl amine are added by dropping at 0C. The batch is
allowed to warm up to room temperature and stirred for 3
hours at this temperature. The reaction mixture is mixed
with a few drops of~acetic acid, hydrolyzed with water and
extracted with acetic ester until the product is no longer
contained in the aqueous phase. The organic phases are
evaporated to dryness under reduced pressure, again taken up
in acetic ester, washed with water, and dried above Mg2SO4.
After drawing off the solvent under reduced pressure, the
product is recrystallized from methanol or, if required,
purified by column chromatography using CH2Cl2/MeOH (9:1).
Yield: 69~ of the title compound ~9a]
C29H50N4OsS (MW: 566.81)
H NMR (d6-DMSO):
= 0.87 (t; 3H, -CH2CH3)
1 16 - 1.35 (m; 28H, -(CH2)l4CH3)
2.33 (s; 3H, -COCH3)
2.73 - 3.10 (m; 4H, -(CH2)-)
3.07 (t; lH, -C--CH)
3.67 - 3.76 (m; 4H, 2x -NH(CH2)CO-)
3.85 - 3.89 (m; 2H, -NH(CH2)-C-=CH)
4.29 - 4.35 (m; lH, -S-CH(CH2COOH)-CH2-)
7.99 (t; lH, -NH-)
8.07 (t; lH, -NH-)
8.15 - 8.23 (m; 2H, 2x -NH-)
_ 37 21S6618
b) Technetium-99m complex [9b] of N-(3-hexadecyl
aminocarbonyl-2-acetylthio-propionyl) glycyl glycyl
propargyl amide
A solution of 0.5 mg (0.9 ~mol) of N-(3-hexadecyl
aminocarbonyl-2-acetylthio-propionyl) glycyl glycyl
propargyl amide[9a] in 300 ~l of DMSO is mixed with 30 ~l
of 1 N soda lye. Afterwards, 50 ~l of 0.15 molar trisodium
citrate dihydrate solution and 0.4 to 0.9 mCi of pertechne-
tate solution from~a Mo-99/Tc-99m generator are added. The
reaction mixture is mixed in portions with 10 ~l of 0.2
molar tin(II) chloride dihydrate solution, incubated at
room temperature for 10 minutes, and filtered (0.2 ~m
filter). ~
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
Example 10
a) N-dodecanoyl homocysteinyl glycyl glycyl propargyl
amide ~lOa]
A solution of 2.0 g (6.7 mmol) of N-dodecanoyl
homocysteinyl thiolactone in anhydrous THF is mixed with a
solution of 1.2 g (7.0 mmol) glycyl glycyl propargyl amide
in DMF. After adding 1 ml of triethyl amine, the batch is
refluxed for 3 hours. It is then evaporated under reduced
pressure, the residue is taken up in CH2Cl2 and washed with
diluted HCl. The organic phases are washed neutrally with
water and dried above Mg2SO4. After evaporating the solvent
under reduced pressure, the product is recrystallized from
38 21~ 66 18
methanol and, optionally, purified by column chromatography
using CH2Cl2/MeOH (9:1).
Yield: 58~ of the title compound [lOa]
C23H40N404S (MW: 468.66)
lH NMR (d6-DMSO):
= 0.86 (t; 3H, -(CH2)8CH3)
1.20 - 1.33 (m; 16H, -(CH2)8CH3)
1.48 - 1.57 (m; 2H, -CH2-CH2CONH-)
2.10 - 2.19 (m;j 2H, -CH2-CH2CONH-)
2.56 - 2.73 (m, 2H, -CHCH2CH2SH)
3.10 (t; lH, -C-CH)
3.38 - 3.44 (m; 2H, -CHCH2CH2SH)
3.52 (s; lH, -SH)
3.65 - 3.73 (m; 4H, 2x -NH(CH2)CO-)
3.86 - 3.90 (m; 2H, -NH(CH2)-C--CH)
4.48 - 4.54 (m; lH, -CHCH2CH2SH)
8.05 - 8.10 (m; 2H, 2x -NH-)
8.24 (t; lH, -NH-)
8.35 (t; lH, -NH-)
b) Technetium-99m complex tlOb] of N-dodecanoyl
homocysteinyl glycyl glycyl propargyl amide
A solution of 0.5 mg (1.06 ~mol) of N-dodecanoyl homocys-
teinyl glycyl glycyl propargyl amide [lOa] in 300 ~l of
DMSO is mixed with 30 ~l of 1 N soda lye. Afterwards, 50 ~l
of 0.15 molar trisodium citrate dihydrate solution and 0.4
to 0.9 mCi of pertechnetate solution from a Mo-99/Tc-99m
generator are added. The reaction mixture is mixed in
portions with 10 ~l of 0.2 molar tin(II) chloride dihydrate
solution, incubated at room temperature for 10 minutes, and
filtered (0.2 ~m filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4i 0.01 M; pH 2.0);
~ 39 2156618
eluent B: acetonitrile/phosphate buffer (Na2HP04; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
Ex~mple 11
a) N-decanoyl homocysteinyl glycyl glycyl propargyl amide
[lla]
A solution of 2.0 g (7.4 mmol) of N-decanoyl homocysteinyl
thiolactone in anhydrous THF is mixed with a solution of
1.35 g (8.0 mmol) ~f glycyl glycyl propargyl amide in DMF.
After adding 1 ml of triethyl amine, the batch is refluxed
for 3 hours. It is then evaporated under reduced pressure,
the residue is taken up in CH2Cl2 and washed with diluted
HCl. The organic phases are washed neutrally with water and
dried above Mg2SO4. After evaporating the solvent under
reduced pressure, the product is recrystallized from
methanol and, optionally, purified by column chromatography
using CH2Cl2/MeOH (9:1).
Yield: 67~ of the title compound [lla]
C21H36N404S (MW: 440.61)
H NMR (d6-DMSO):
= 0.85 (t; 3H, -(CH2)6CH3)
1.18 - 1.30 (m; 12H, -(CH2)6CH3)
1.45 - 1.53 (m; 2H, -CH2-CH2CONH-)
2.11 - 2.22 (m; 2H, -CH2-CH2CONH-)
2.55 - 2.70 (m; 2H, -CHCH2CH2SH)
3.09 (t; lH, -CaCH)
3.37 - 3.42 (m; 2H, -CHCH2CH2SH)
3.50 (s; lH, -SH)
- 3.65 - 3.75 (m; 4H, 2x -NH(CH2)CO-)
3.87 - 3.92 (m; 2H, -NH(CH2)-C_CH)
4.48 - 4.53 (m; lH, -CHCH2CH2SH)
8.08 - 8.12 (m; 2H, 2x -NH-)
~ 40 2156618
8.22 (t; lH, -NH-)
8.32 (t; lH, -NH-)
b) Technetium-99m complex [llb] of N-decanoyl
homocysteinyl glycyl glycyl propargyl amide
A solution of 0.5 mg (1.1 ~mol) of N-decanoyl homocysteinyl
glycyl glycyl pro~argyl amide [lla] in 300 ~1 of DMSO is
mixed with 30 ~l of 1 N soda lye. Afterwards, 50 ~1 of 0.15
molar trisodium citrate dihydrate solution and 0.4 to 0.9
mCi of pertechnetate solution from a Mo-99/Tc-99m generator
are added. The reaction mixture is mixed in portions with
10 ~l of 0.2 molar tin(II) chloride dihydrate solution,
incubated at room t~e~mperature for 10 minutes, and filtered
(0.2 ~m filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95% .
Example 12
a) N-hexanoyl homocysteinyl glycyl glycyl propargyl amide
[12a]
A solution of 2.0 g (9.3 mmol) of N-hexanoyl homocysteinyl
thiolactone in anhydrous THF is mixed with a solution of
1.7 g (10.0 mmol) of glycyl glycyl propargyl amide in DMF.
After adding 1 ml of triethyl amine, the batch is refluxed
for 3 hours. It is then evaporated under reduced pressure,
the residue is taken up in CH2Cl2 and washed with diluted
HCl. The organic phases are washed neutrally with water and
dried above Mg2SO4. After evaporating the solvent under
- 21~6618
41
reduced pressure, the product is recrystallized from
methanol and, optionally, purified by column chromatography
using CH2Cl2/MeOH (9:1).
Yield: 60~ of the title compound [12a]
5C17H2sN44S (MW: 384.50)
H NMR (d6-DMSO):
= 0.86 (t; 3H, -(CH2)2CH3)
1.20 - 1.31 (m; 12H, -(CH2)2CH3)
1.47 - 1.54 (m; 2H, -CH2-CH2CONH-)
2.10 - 2.19 (m;' 2H, -CH2-CH2CONH-)
2.56 - 2.68 (m; 2H, -CHCH2CH2SH)
3.11 (t; lH, -C--CH)
3.36 - 3.40 (m; 2H, -CHCH2CH2SH)
3.51 (s; lH, -SH)
3.67 - 3.76 (m; 4H, 2x -NH(CH2)CO-)
3.85 - 3.89 (m; 2H, -NH(CH2)-C--CH)
4.47 - 4.54 (m; lH, -CHCH2CH2SH)
8.03 - 8.10 (m; 2H, 2x -NH-)
8.20 (t; lH, -NH-)
8.29 (t; lH, -NH-)
b) Technetium-99m complex tl2b] of N-h~yAn~yl
homocysteinyl glycyl glycyl propargyl amide
A solution of 0.5 mg (1.3 ~mol) of N-decanoyl homocysteinyl
glycyl glycyl propargyl amide [lla] in 300 ~l of DMSO is
mixed with 30 ~l of 1 N soda lye. Afterwards, 50 ~1 of 0.15
molar trisodium citrate dihydrate solution and 0.4 to 0.9
mCi of pertechnetate solution from a Mo-99/Tc-99m generator
are added. The reaction mixture is mixed in portions with
10 ~l of 0.2 molar tin(II) chloride dihydrate solution,
incubated at room temperature for 10 minutes, and filtered
(0.2 ~m filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
2156618
42
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
Example 13
a) 3,17~-dihydroxy-17a-[5-(2-benzoyl thioacetyl glycyl
glycyl) amino pent-l-(E)-en-3-in]-1,3,5-estratriene
[13a]
A suspension of 120.0 mg (0.24 mmol) of 17~-hydroxy-17a-
iodovinyl-1,3,5-est~atriene-3-tetrahydropyranyl ether,
0.3 g (0.85 mmol) of S-benzoyl thioacetyl glycyl glycyl
propargyl amide [la], 10.0 mg (0.044 mmol) of benzyl
triethyl ammonium chloride, 10.6 mg (9.2 ~mol) of
tetrakis(triphenyl phosphine)palladium(0), and 8.15 mg
(0.043 mmol) of copper(I) iodide in 4 ml of toluene is
stirred at room temperature for 72 hours. Then the mixture
is mixed with water, extracted twice with toluene, and
dried above Mg2SO4. After evaporating the solvent under
reduced pressure, the residue is taken up in 5 ml of THF,
mixed with 150 mg (0.6 mmol) of pyridine toluene-p-sulfonic
acid in 5 ml of ethanol, and refluxed for 3 hours. Then the
solvent is evaporated under reduced pressure, and the
residue is purified by column chromatography using
CH2Cl2/MeOH (8:1).
Yield: 41~ of the title compound [13a]
C36H4lN3O6S (MW: 643.80)
H NMR (d6-DMSO):
~ = 0.86 (t; 3H, -CH3 steroidal)
1.15 - 2.30 (m; 13H, steroidal)
2.65 - 2.74 (m; 2H, -CH2- steroidal)
3.72 (d; 2H, -NH(CH2)CO-)
3.81 (d; 2H, -NH(CH2)CO-)
3.90 (s; 2H, -SCH2CO-)
215661~
43
4.02 (d; 2H, -NH(CH2)-C-C-)
5.65; 6.35 (AB; 2H, -CH=CH-)
6.43 (d; lH, steroidal)
6.50 (dd; lH, steroidal)
7.00 (d; lH, steroidal)
7.44 - 7.49 (m; 2H, ArH)
7.51 - 7.56 (m; 2H, ArH)
7.90 - 7.96 (m; 2H, ArH)
8.23 (t; lH, -NH-)
8.27 (t; lH, -NH-)
8.80 (t; lH, -NH-)
b) Technetium-99m complex ~13b] of 3,17~-dihydroxy-17a-[5-
(2-benzoyl thioà-~etyl glycyl glycyl) amino pent-1-(E)-
en-3-in]-1,3,5-estratriene
A solution of 0.5 mg (0.8 ~mol) of 3,17~-dihydroxy-17a-[5-
(2-benzoyl thioacetyl glycyl glycyl) amino pent-1-(E)-en-3-
in]-1,3,5-estratriene [13a] in 250 ~l of ethanol is mixed
with 50 ~l of 1 N soda lye and incubated at room
temperature for 15 minutes. Afterwards, 50 ~l of 0.15 molar
trisodium citrate dihydrate solution and 2.5 ~l of 0.2
molar tin(II) chloride dihydrate solution are added. The
reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
HAMILTON PRP-1 column, 125 x 4.6 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: acetonitrile;
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.001
mol; pH 7.4) 50:50 (V/V); flow rate: 2.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
21S6618
44
Example 14
a) 17~-hydroxy-17a-[5-(2-benzoyl thioacetyl glycyl glycyl)
amino pent-1-(E,Z)-en-3-in]-1,3,5-estrene-3-on [14a]
A suspension of 120.0 mg (0.28 mmol) of 17~-hydroxy-17a-
iodovinyl-4-estrene-3-on, 0.3 g (0.85 mmol) of S-benzoyl
thioacetyl glycyl glycyl propargyl amide [la], 10.0 mg
(0.044 mmol) of benzyl triethyl ammonium chloride, 10.6 mg
(9.2 ~mol) of tetr~kis(triphenyl phosphine)palladium(0),
and 8.15 mg (0.043 mmol) of copper(I) iodide in 4 ml of
toluene is stirred at room temperature for 72 hours. Then
the mixture is mixed with water, extracted twice with
toluene, and dried above Mg2SO4. After evaporating the
solvent under reduced pressure, the residue is taken up in
5 ml of THF, mixed with 150 mg (0.6 mmol) of pyridine
toluene-p-sulfonic acid in 5 ml of ethanol, and refluxed
for 3 hours. Then the solvent is evaporated under reduced
pressure, and the residue is purified by column
chromatography using CH2Cl2/MeOH (8:1).
Yield: 39~ of the title compound [14a]
C36H43N3O6S (MW: 645.80)
H NMR (d6-DMSO):
= 0.95 (t; 3H, -CH3 steroidal)
0.82 - 2.50 (m; 20H, steroidal)
3.68 (d; 2H, -NH(CH2)CO-)
3.80 (d; 2H, -NH(CH2)CO-)
3.87 (s; 2H, -SCH2CO-)
3.94 - 3.98 (m; 2H, -NH(CH2)-C--C-)
5.60; 5.95 (AB; 2H, -CH=CH-)
5.82 (s; lH, steroidal)
7.41 - 7.45 (m; 2H, ArH)
7.49 - 7.54 (m; 2H, ArH)
7.85 - 7.90 (m; 2H, ArH)
8.24 (t; lH, -NH-)
8.29 (t; lH, -NH-)
8.78 (t; lH, -NH-)
~ 45 21~6618
b) Technetium-99m complex [14b] of 17~-hydroxy-17a-[5-(2-
benzoyl thioacetyl glycyl glycyl) amino pent-1-(E,Z)-
en-3-in]-4-estrene-3-on
A solution of 0.5 mg (0.8 ~mol) of 17~-hydroxy-17a-[5-(2-
benzoyl thioacetyl glycyl glycyl) amino pent-1-(E,Z)-en-3-
in]-4-estrene-3-on [14a] in 250 ~l of ethanol is mixed with
50 ~l of iN soda lye and incubated at room temperature for
15 minutes. Afterwards, 50 ~l of 0.15 molar trisodium
citrate dihydrate solution and 2.5 ~l of 0.2 molar tin(II)
chloride dihydrate solution are added. The reaction mixture
is mixed with a pertechnetate solution (0.4 to 0.9 mCi)
from a Mo-99/Tc-99m~generator, incubated at room
temperature for 10 minutes, and filtered (0.2 ~m filter).
Labelling is analyzed using HPLC:
HAMILTON PRP-1 column, 125 x 4.6 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: acetonitrile;
eluent B: acetonitrile/phosph~te buffer (Na2HPO4; 0.001
mol; pH 7.4) 50:50 (V/V); flow rate: 2.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~ .
Example 15
a) S-acetyl thioacetyl sarcosyl glycyl propargyl amide
[lSa]
A solution of 1.54 g (8.4 mmol) of sarcosyl glycyl
propargyl amide in 30 ml of anhydrous THF is slowly mixed
with a solution of 5.3 g (8.4 mmol) of S-acetyl
mercaptoacetic acid N-hydroxy succinimide ester in
anhydrous THF. The mixture is stirred at room temperature
for 4 hours and then heated for 30 minutes to 40C. After
cooling, the solvent is evaporated under reduced pressure,
and the residue is purified by column chromatography above
~ 46 2 1~ 6618
silica gel [CH2Cl2/CH3OH (9:1)]. The product crystallizes
from ethanol.
Yield: 88~ of the title compound [15a]
Cl2Hl7N3O4S (MW: 299.35
lH NMR (d6-DMSO):
= 2.34 (s; 3H, -COCH3)
2.81 (s; 3H, -N-CH3)
3.07 (t; lH, -C--CH)
3.70 (d; 2H, -NH(CH2)CO-)
3.82 (s; 2H, -~H(CH2)CO-)
3.86 - 3.90 (m; 2H, -NH(CH2)-C-CH)
3.93 (s; 2H, SCH2CO-)
8.13 (t; lH, -NH-)
8.22 (t; lH, -NH-)
b) Technetium-99m complex tl5b] of S-acetyl thioacetyl
sarcosyl glycyl propargyl amide
A solution of 0.5 mg (1.67 ~mol) of S-acetyl thioacetyl
sarcosyl glycyl propargyl amide [15a] in 50 ~1 of 0.1 N
soda lye is diluted with 250 ~l of phosphate buffer
(Na2HPO4, 0.5 mol/l, pH 8.5). Afterwards, 50 ~l of 0.15
molar trisodium citrate dihydrate solution and 2.5 ~l of
0.2 molar tin(II) chloride dihydrate solution are added.
The reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~.
47 2156618
Example 16
a) 2-(S-acetylthio)succinyl sarcosyl glycyl propargyl
amide [16a]
8.89 g (48.5 mmol) of sarcosyl glycyl propargyl amide are
dissolved in a nitrogen atmosphere in 60 ml of anhydrous
DMF, mixed with 8.5 g (48.7 mmol) of acetyl mercaptosuccin-
anhydride and stirred for 1 hour at 40C. The product is
precipitated with ether after cooling and centrifuged off.
The crystalline product is recrystallized from THF.
Yield: 90~ of the title compound [16a]
Cl4HlgN3O6S (MW: 357.39)
H NMR (d6-DMSO):
= 2.34 (s; 3H, -CO~H3)
2.78 (s; 3H, -N-CH3)
2.70 - 3.04 (m; 2H, -(CH2)COOH)
3.07 (t; lH, -C--CH)
3.68 - 3.73 (m; 4H, 2x -NH(CH2)CO-)
3.85 - 3.89 (m; 2H, -NH(CH2)-C-CH)
4.33 - 4.38 (m; lH, -S-CH~CH2COOH)-CH2-)
8.12 (t; lH, -NH-)
8.23 (t; lH, -NH-)
12.68 (broad; lH, -COOH)
b) Technetium-99m complex [16b] o~ 2-(S-acetylthio)
succinyl sarcosyl glycyl propargyl amide
A solution of 0.5 mg (1.4 ~mol) of 2-(S-acetylthio)
succinyl sarcosyl glycyl propargyl amide [16a] in 50 ~1 of
0.1 N soda lye is diluted with 250 ~1 of phosphate buffer
(Na2HPO4, 0.5 mol/l, pH 8.5). Afterwards, 50 ~1 of 0.15
molar trisodium citrate dihydrate solution and 2.5 ~1 of
0.2 molar tin(II) chloride dihydrate solution are added.
The reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
21~6618
_ 48
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100~ A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
95~ .
Example 17
a) (2-(S-acetylthio)succinyl sarcosyl glycyl propargyl
amide-4-yl)-cys-ser-cys-ser-ser-leu-met-asp-lys-glu-
cys-val-tyr-phe-cys-his-leu-asp-ile-ile-trp tl7a]
A solution of 50 mg of 2-(S-acetylthio)succinyl sarcosyl
glycyl propargyl amide [16a] and 15 mg of N-hydroxy suc-
cinimide in anhydrous, freshly distilled DMF is cooled down
to -15C and mixed with 28 mg-of dicyclohexyl carbodiimide
in anhydrous DMF. The reaction mixture is stirred for 2
hours at -5C, then 2 hours at room temperature, and then
cooled down to -15C. Precipitated N,N'-dicyclohexyl urea
is filtered off. The filtrate is mixed with a solution of 1
mg of cys-ser-cys-ser-ser-leu-met-asp-lys-glu-cys-val-tyr-
phe-cys-his-leu-asp-ile-ile-trp (endotheline 1) in
anhydrous DMF and stirred for 20 hours at room temperature.
The reaction mixture is evaporated under reduced pressure
at room temperature. A flocculent precipitate emerges after
adding diethyl ether by dropping. The precipitate is cen-
trifuged and purified by preparative HPLC (gradient: aceto-
nitrile/phosphate buffer). When the buffered eluate is neu-
tralized, the organic solvent portion is blown off usingnitrogen, and the residue is lyophilized. The crystalline
product is kept in a protective gas atmosphere (Ar).
MW: calc.: 2831.3
det.: 2831.6 (F~3-MS)
49 21~6618
b) Technetium-99m complex tl7b] of (2-(S-acetylthio)
succinyl sarcosyl glycyl propargyl amide-4-yl)-cys-ser-
cys-ser-ser-leu-met-asp-lys-glu-cys-val-tyr-phe-cys-
his-leu-asp-ile-ile-trp
A solution of 0.5 mg of 2-(S-acetylthio) succinyl sarcosyl
glycyl propargyl amide-4-yl)-cys-ser-cys-ser-ser-leu-met-
asp-lys-glu-cys-val-tyr-phe-cys-his-leu-asp-ile-ile-trp
[17a] in 50 ~l of 0.1 N soda lye is diluted with 250 ~l of
phosphate buffer (~a2HPO4, 0.5 mol/l, pH 8.5). Afterwards,
50 ~1 of 0.15 molar trisodium citrate dihydrate solution
and 2.5 ~1 of 0.2 molar tin(II) chloride dihydrate solution
are added. The reaction mixture is mixed with a
pertechnetate solut~on (0.4 to 0.9 mCi) from a Mo-99/Tc-99m
generator, incubated at room temperature for lO minutes,
and filtered (0.2 ~m filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x ~ mm, 5 ~m;
gradient from 100% A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
80~.
- Example 18
a) Cyclo(trp-leu-val-pro-asp)-cys-gly-gly-propargyl amide
tl8a]
A solution of 133.0 mg of cyclo(trp-leu-val-pro-asp) and
41.3 mg of hydroxy benzotriazole in 6 ml of anhydrous,
freshly distilled DMF is cooled down to -15C and mixed
with a solution of 51.7 mg of 1-ethyl-3-(3-dimethyl
aminopropyl) carbodiimide hydrochloride in 8 ml of DMF. The
reaction mixture is stirred for 2 hours at -5C and another
-- so 21~6618
2 hours at room temperature. Then, a solution of 134.0 mg
of S-(p-methoxybenzyl) cysteinyl glycyl glycyl propargyl
amide [5a] in anhydrous DMF is slowly added by dropping.
After 7 more hours of stirring, the solution is evaporated
under reduced pressure, and the peptide precipitated with
ether. The protecting groups present are split off by
stirring in liquid HF. After evaporating the HF, the
residue is purified by preparative HPLC (gradient:
acetonitrile/phosph~ate buffer). When the buffered eluate is
neutralized, the organic solvent portion is blown off using
nitrogen, and the residue is lyophilized. The crystalline
product is kept in a protective gas atmosphere (Ar).
MW: calc.: 865.0
det.: 865.2 (FAB-MS)
b) Technetium-99m complex [18b] of cyclo(trp-leu-val-pro-
asp)-cys-gly-gly-propargyl amide
A solution of 0.5 mg of cyclo(trp-leu-val-pro-asp)-cys-gly-
gly-propargyl amide[18a] in 300 ~l of phosphate buffer
(Na2HPO4, 0.5 mol/l, pH 8.5) is mixed with 50 ~l of 0.15
molar trisodium citrate dihydrate solution and 2.5 ~l of
0.2 molar tin(II) chloride dihydrate solution are added.
The reaction mixture is mixed with a pertechnetate solution
(0.4 to 0.9 mCi) from a Mo-99/Tc-99m generator, incubated
at room temperature for 10 minutes, and filtered (0.2 ~m
filter).
Labelling is analyzed using HPLC:
MERCK nucleosile column, 125 x 4 mm, 5 ~m;
gradient from 100% A to 100~ B within 7.5 min;
eluent A: phosphate buffer (Na2HPO4; 0.01 M; pH 2.0);
eluent B: acetonitrile/phosphate buffer (Na2HPO4; 0.01 M;
pH 2.0) 50:50 (V/V); flow rate: 1.0 ml/min.
Radiochemical purity of the Tc-99m complex is more than
90~ .
~~ 51 21~661~
Example 19
Accumulation of N-(3-hexadecyl aminocarbonyl-2-acetyl
thiopropionyl) glycyl glycyl propargyl amide, technetium-
99m complex, in atherosclerotic vascular lesions of WHHLrabbits
N-(3-hexadecyl ami~ocarbonyl-2-acetyl thiopropionyl) glycyl
glycyl propargyl amide (produced according to Example 9a)
is labelled as described in Example 9b.
99.9 GBq (2.7 mCi) of the substance labelled according to
Example 9b were dilù'ted to l ml with phosphor-buffered
saline and administered via the ear vein to a narcotized
WHHL rabbit, Rompun/Ketavet (1:2). The rabbit was killed 5
hours after the application, and an autoradiogram as well
as a Sudan(III) staining were carried out to visualize the
atherosclerotic plaques (Figure 1). The accumulation factor
between normal and atherosclerotic walls was between 3 and
8 depending on the thickness of the plaques (Sudan(III)
staining).