Note: Descriptions are shown in the official language in which they were submitted.
2157187
Pyrrol- ~inic compounds and their ph~ -^e7ltically acceptable
salts, processes for their preparation and pharmaceutical
c~ tions cont~;nin~ them.
Field of the invention
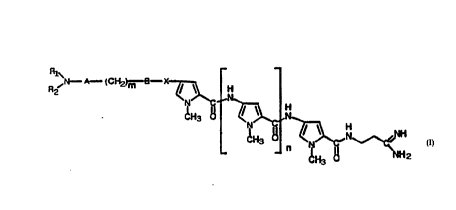
The present invention refers to pyrrol-amidinic compounds of
general formula (I)
r~~1CI~d m 3 K Q~ H
(I)
and their pharmaceutically acceptable salts wherein :
n is O or an integer ranging from 1 to 4
m is O or an integer ranging from 1 to 4
A is selected from the group consisting of cyclohexyl, p-
phen.-lene, l-methylpyrrole, thiophene, thiazole, imidazole, furan,
isoxazole, oxazole, triazole, pyridine. pyrrole;
B is selected from the group consisting of : a simple chemical
bonc. -CO-NH-CH(R3~ NH-CO-CH(R3)- wherein R3 is H or the side
15 ~ch~7~ of a natural alpha-aminocarboxylic acid;
X is selected from the group consisting of : -NHCO-, -CONH- and
A~ D SH~
~1~7I87
wherein :
i) Rl and R2 are equal and are selected from the group consisting
of : oxiranomethyl, l-aziridinomethyl, C2_4 alkyl optionally
substltuted in position 2 with an OH, C2_4 alkoxy, halogen
or -OSO2R4 group wherein R4 is selected from the group consisting
of Cl_4 alkyl or phenyl
or
ii) Rl = H and R2 is as above described
provided that :
when B = chemical bond, n is different from 1
when X = -CONH-, B = simple chemical bond, m is different from O
when B = -CO-NH-CH(R3)- X is different from -NHCO-
when B = -~H-CO-CH(R3)- X is different from -CONH-
Furthermore the invention relates to processes for the preparation
of the above mentioned compounds, to their pharmacologically active
salts and to pharmaceutical compositions contAin;ng them.
Prior art
.~ntibiotic dystamicine is a known compound of formula (II) :
HCONH
~;~rl H H
A~',EN~:3rD Sl~
21571B7
belcnging to the pyrrol-~mi~inic group and showing interesting
antiviral activity, for example against the herpetic viruses and
~oloney sarcoma virus, is characterized by the ability to interact
reversibly and selectlvely with DNA sequences rich in dA and dT
bases thereby interfering both in the replication and transcrlption
process [see Arcamone in B. Pull~an and J. Jorterez (eds)
"Molecular basis of specificity in nucleic acid - drug interaction"
369-383, lggo Kluwer Academic Publishers].
As is known, antiviral and antitumoral agents nowadays used in
therapy are characterized by serious side effects, limiting their
use in a large number of cases which on the contrary should take
advantage from the therapy; moreover therapeutical progresses are
necessary in the clinical treatment of important solid tumors, as
for example pl11 m~n~ry tumors and ovarian tumors, not responding
adequately to any treatment nowadays in use.
Pyrr~ mi~inic compounds having chemical structures similar to the
one described in the present application, and showing
antitumor and antiviral properties. are described in: EP - A - 388
948; ~P - A - 246 868; Anti-Cancer Drug Design (1986), 1. 235-244;
Bioc;-emical Pharmacology ( 1993), 45. 1536-39; J. Med. Chem. ( 1989) .
32, , ,4 - 778.
A r~uisite for the therapeutic progress in this particular field
is therefore the discovery of compounds having molecular moieties
alls-~ing them to increase the selectivity in inhibiting viral
AM~NcED ~ET
3a 2 1 5 7 1 8 7
proliferation and the proliferation of tumoral rather than healthy
ceIls.
Detailed description of the invention
The present invention has the aim to render available new
antitumoral and antiviral compounds and in particular compounds
analogous to dystamicine containing new chemical modifications at
~r
AM~NC~D ~ffEET
WO 94/20463 PCT~EP94/005~7
the N-terminal side chain level, and/or contnin;n~ a different
number of pyrrolic residues if compared to the natural product.
These compounds, show a marked antitumoral and antiviral activity,
as well selectivity in the inhibition of tumoral cells and viruses
with respect to the healthy cells.
The compounds according to the present invention are those of
general formula (I)
R2~N--~(Cl~m ~ b N
~H3 n ~ ~~ 2
(I)
wherein:
n, m, A, B, X, R1, R2 are as previously described, and their
pharmaceutically acceptable salts.
Besides, the invention, refers to pharmaceutical compositions
containing the above mentioned compounds, or pharmaceutically
acceptable salts thereof formed with inorganic acids such as
hydrochloric, hydrobromic, sulfuric, nitric and the like or with
organic acids such as acetic, propionic, succinic, malonic, citric,
~artaric, methansulfonic, p-toluensulfonic and the like.
~ 94/20 ~ PCT~EP94/00557
- 5 2 1 5 7 1 8 7
According to the present invention, preferred are the ,- -ln~ of
formula (I) wherein:
n is as above defined
m is zero or an integer comprised between 1 and 3.
A = cyclohexyl, p-phenylene, l-methylpyrrole, th;ophpne~ thiazole,
~ ole, furan, isoxazole, oxazole, triazole, pyridine, pyrrole.
B is a simple bond, or when it is a -C0-NH-CH(R3)- group or a -NH-
C0-CH(R3)- group, R3 is preferably methyl, isobutyl, sec-butyl,
hydroxymethyl, mercaptomethyl, carbamoylmethyl, benzyl, 4-
hydroxybenzyl, 5-imidazolylmethyl, 2-carbamoylethyl, 2-
methylthioethyl, l-hydroxyethyl, 3-~l~ni~inopropyl~ 4-aminobutyl Rl
and R2 represent preferably an ethyl group, 2-hydroxyethyl, 2-
chloroethyl, methansulfonylethyl.
The following compounds are particl-l~rly preferred:
3-[1-Methyl-4-tl-methyl-4-t4-tN,N-bis(2-chloroethyl)amino~bPn~PnP-
aminocarbonyl]pyrrol-2-caL-bo~y- ;do]pyrrol-2-
carbo~y~ido]propionr idine hydrochlorate;
3-tl-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[4-[N,N-bis(2-
chloroethyl) amino]benzeneaminocarbonyl]-pyrrol-2-carbo~ -ido]
pyrrol-2-carboxyamido]pyrrol-2-carboxyamido]pyrrol-2-carbox~ ido]
propion~mi~ine hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[4-
[N,N-bis(2-chloroethyl)amino]benzPne~minocarbonyl]pyrrol-2-
carboxyamido]pyrrol-2-carboxyamido]pyrrol-2-carboxyamido]pyrrol-2-
carboxyamido] pyrrol-2-carboxyamido]propionamidine hydrochlorate;
WO 94/20463 PCT~EPg4/00557
3-tl-Methyl-4-[l-methyl-4-[4-[N~N-bis(2-chloroethyl)amino]
butPnf idQ]pyrrol-2-carboxyamido]pyrrol-2-csrboxyamido]propiona-
midine hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-{4-[N,N-bis(2-
chloroethyl)-amino]benzenbut~n~mi~n]pyrrol-2-c&-bo~y&~ido]pyrrol-2-
csrboxy 'do]pyrrol-2-ca.l,o~ys~ido]pyrrol-2-
caLLu~y~ido]propionnmi~i n~ hyd.-ochlorate;
3-[1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-~1-methyl-4-[N,N-
bis(2-chloroethyl)amino]ben7~nebùt~n~ ~o]pyrrol-2-carboxyamido]
pyrrol-2-carboxyamido]pyrrol-2-ca.boxy ido] pyrrol-2-ca~bGxy do]
pyrrol-2-carboxyamido]propinn~ ~ine hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[4[N,N-bis(2-chloroethyl)amino]benzyl-
amino carbonyl]pyrrol-2-carbony ~o]pyrrol-2-
caL-bo~y~ ;~o]propion~mi~in~ hydrochlorate;
15 3-[1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[4-[N,N-bis(2
chloroethylamino)benzylaminocarbonyl]pyrrol-2-carboxyamido]pyrrol-2-
carboxyamido]pyrrol-2-carboxyamido]pyrrol-2-ca.-bo~y ~ido]propiona-
midine hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[2-[4-[N,N-bis(2-chloroethyl)amino)
phenyl] et h ~n ~ inocarbonyl]pyrrol-2-carboxyamido]pyrrol-2-carboxa
mido] propionamidine hydrochlorate;
3-~1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[2-[4-[N,N-bis
(2-chloroethyl) amino]phenyl]eth~n~minocarbonyl]pyrrol-2-carboxa-
mido]pyrrol-2-carboxyamido]pyrrol-2-carboxyamido]pyrrol-2-
~ 94/20463 PCT~EP94/00557
';~
carboxyamido]prop;onr ~ine hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[3-[4-[N,N-bis(2-chloroethyl)amino]
phenyl]propylaminocarbonyl]pyrrol-2-chl-bo~y do]pyrrol-2-carboxa-
mido] propisn~ ~ine hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[1-methyl-4-tl-methyl-4-[3-[4-tN,N-bis(2-
chloroethyl)amino]phenyl]propylsminocarbonyl]pyrrol-2-
carboxy do]pyrrol-2-caLbo~y6~ido]pyrrol-2-carboxyamido]pyrrol-2-
carboxy- do] prop;on r ~; n~ hydrochlorate;
3-[1-methyl-4-[1-methyl-4-[1-methyl-4-tl-methyl-4-tl-methyl-4-t3-
t4-tN~N-bis(2-chloroethyl)amino]phenyl]propylr ;noc~G-lyl]pyrrol-
2-carbG~y do]pyrrol-2-caL-bo~y do]pyrrol-2-caLbu~y ~o]pyrrol-2-
carbo~y- ido]pyrrol-2-caLboxy do]propionr '~;ne hydrochlorate;
3-tl-Methyl-4-[1-methyl-4-[4-[N,N-bis(2-chloroethyl)amino]benzyl
carbo~yr ~o]pyrrol-2-carboxyamido]pyrrol-2-
carboxy- do]propion~ ;~;ne hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[4-tN,N-bis(2-
chloroethyl)amino]benzylcarbo~y do]pyrrol-2-carbo~y ~o]pyrrol-
2-carboxyamido]pyrrol-2-carboxy ;~o]pyrrol-2-carboxyr i~o]
propionr i~i ne hydrochlorate;
3-[1-Methyl-4-tl-methyl-4-[2-[4-[N,N-bis(2-chloroethyl)amino]
phenyl]ethylcarboxyamido3pyrrol-2-carboxyamido]pyrrol-2-carboxy-
amido]propionamidine hydrochlorate;
3-[1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[2-[4-[N,N-bis
- (2-chloroethyl)amino]phenyl]ethylcarboxyamido]pyrrol-2-
carboxyamido]pyrrol-2-carboxyamido]pyrrol-2-carboxyamido]pyrrol-2-
WO 94/20463 PCTAEP94/00557
~ ~ ~ 8
ca ~o~y~ido]propionamidine hydrochlorate;
3-[1-Methyl-4-[1-me~hyl-4-[1-methyl-4-[N,g-[4-EN,N-bis(2-chloro-
ethyl)amino]benzoyl]glycylamino]pyrrol-2-carbG~y ~o]pyrrol-2-
c&~-bo~y do]pyrrol-2-carbo~y do]propionr i~inP hydrochlorate;
3-~1-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[N,a-[4-[N,N-
bis(2-chloroethyl)amino]benzoyl]glycylamino]-pyrrol-2-
ca~o~y do]pyrrol-2-carbo~y ~o]pyrrol-2-ca~-~o~y ~o]pyrrol-2-
calbo~y ;do]propi on~ ine hydrochlorate;
Compounds of general formula (I) can be prepared according to the
following processes:
a) reacting the ~ nd of fo. 1~ (III)
Rl~
N-A-(CH2)m-B-COOH
R2
(III)
wherein B is a chemical bond or the -CONHCH(R3)- group wherein R3
is as above defined, and m, A, Rl and R2 are as above defined, or a
reactive derivative thereof, with a compound of formula (IV)
H2N
_ CH3 `~ --P H2
(IV)
94/204K3 ~ PCTAEP94/00557
. 9 ~ ~
wherein p is an integer comprised between 2 and 6, thereby
obt~ining the compounds of formula (I) wherein X = -CONH-, B is a
~'~ c~1 bond or the -CO-NH-CH(R3)- group and m, n, A, B, Rl, R2
and R3 are as above defined;
a') reacting the compound of formula (V)
Rl
/ N A (CH2)m B NH2
R2
(V)
wherein B is a chemical bond or the -NHCOCH(R3)- group, wherein R3
is as above defined, and m, A, R1 and R2 are as above defined, with
a c ,_~tn~ of formula (VI)
HOOC~
~N ~NH
_ CH3 1 --PH2
(VI)
wherein p is an integer comprised between 2 and 6, thereby
obt~ining the compounds of formula (I) wherein X = -NHCO-, B is a
chemical bond or the -~'H-CO-CH(R3)- group and m, n, A, B, R1, R2
and R3 are as above defined;
WO 94/20463 PCT~EP94/00557
b) reacting the compound of formula (VII)
A (CH2~X
N~--COOH
~H (VII)
wherein m, A, X, R1 and R2 are as above defined, with a compound of
formula (IV)
HOOC~
~N ~N~
CH3 --P H2
(IV)
wherein p is an integer comprised between 1 and 5, thereby
obt~inin~ the compounds of formula (I) wherein B is a chemic~l bond
and m, n, A, X, R1 and R2 are as above defined.
The amidation reactions of the compound of formula (III) with a
compound of formula (IV) wherein p is an integer comprised between
2 and 6, of the compound of formula (V), wherein Rl, R2, A, m and B
are as previously defined, with the compound of formula (VI),
wherein p is comprised between 2 and 6, and of the compound of
formula (VIIj, wherein R1, R2, A, m, X are as above defined, with a
compound of formula (IV) wherein p is an integer comprised between
1 and 5, can be carried out in the presence of co~Pncing agents as
~ 94/20463 ~ ~ ~ PCT~EPg4/00557
8~
11 -
DCC (dicyclnhpyylcarbo~ de) or EDC [l-dimethyl r~i n~propyl)-3-
~ ethylcarbo~ de hydrochlorate] and poss~hly in the presence of
hydroxybenzotriazole or BOP (benzotriazol-1-
iloxy(dimethylaminophncphonit '~ fluoride ph~sph~te) or by using a
reactive derivative of the acids (TTT) and (IV) as for example an
acylchloride, an acyl~ ole, an acyl~7i~e or an active ester,
such as 2,4,5 trichlororh~l~o~yester or N-oxysllcc;ni ~n~cter, or an
anhy~.-ide thereof.
Preferably the above defined ~ t~ on reactions are carried out
using molar ratio of from 1:1 to 1:3 in an inert organic solvent
for example dimethyl sulfoxide. hexamethyl phosphotriamide,
dimethylacetamide, or preferably ~ l fo. ~e in the pres~nce
of a con~nsing agent as above described and of N-
hy~-u~ybenzotriazole or BOP and in the presence of an organic base
as triethylamine, diisopropylethylamine and 1,8-bis(dimethylamino)-
naphth~l~ne.
The reaction temperature may be comprised between -lO C and 5O C
and the time required for the reaction ranges from 2 to 48 hours.
The reaction of the compound of foL .l~ (III) or the compound of
formula (VII) with the compound of fo. l~ (IV) may be csrried out
using a reactive derivative of the compound of formula (III) or of
the compound of formula (VII) of the above mentioned type, and
therefore accompliching the reaction in a biphasic system water-
organic solvent as Schotten-B~I ~nn amidation or in an organic
WO 94/20463 ~ PCT~EP94/00557
12
solvent as for example a hydro~yde, a carbonate or a bicarbonate of
an ~lkaline metal, preferably sodium, potassium, barium or an
organic ba e as triethylamine, diisopropyl~ ne, pyridine or N,N
dimethylaminopyridine.
The reaction is usually conducted at room temperature and the time
required for the reaction varies from 2 to 24 hours.
In the process (a), the compounds of formula (III) wherein B is a
chemical bond and m, A, Rl and R2 are as above defined, namely the
compounds of formula (VIII)
Rl\
N-A-(CH2)m~CH
R2
(VIII)
either are already commercially av~ hle or they are prepared by
conventional processes of the organic chemistry, starting from
known compounds as reported for example in J. Med. Chem. 32, 774
(1989) or J. Org. Chem. 26, 4996 or in J. Med. Chem. 33, 1177
( 1990) .
In the process (a) a compound of formula (III) wherein B is the
group -CO-NH-CH(R3)- and m, A, Rl, R2 and R3 are as above defined,
can be prepared by the hydrolysis of the compounds of formula (IX)
Rl~ .
N-A-(CH2)m-CO-NH-CH(R3)-COORs
R2 (IX)
~ 94l20463 - PCT~EP94100557
13 S71~7
wherein A, m. Rl, R2, R3 are as above defined and R5 is a
protecting group characteristic of the carboxylic group of
n9~ c as methyl, ethyl, t-butyl, benzyl, trimethylsilyl, the
hydrolysis of the compound of formula (IX) can be carried out
following the ~nown methods and processes of the organic chemistry
as for example reported in T. W. Greene Protective groups in
Organic Synthesis Wiley Interscience Pu~li cnti on 1981.
A compound of formula (IX) wherein A, m, Rl, R2 R3 and R5 are ~c
above defined, can be prepared by rescting a ~ d of foL 1~
(VIII) wherein A, m, Rl and R2 are as above defined or its reactive
derivative with a compound of fo. 1l ~ (X)
H2N-CH(R3)-COORs
(X)
wherein R3 and R5 have the above defined -~nin~,
A reactive compound of an acid of formula (VIII) can be the same
already reported in the present application for the compound of
formula (III) or for the compound of formula (VII) and the reaction
can be accomplished under similar conditions to those reported for
the amidation reaction of a compound of formula (III) or a compound
of formula (VII) with a compound of formula (IV).
The compounds of formula (X) either are commercially available or
can be prepared by the conventional processes starting from the
- corresponding aminoacids as described for example in E. Gross, J.
Meienhofer, The Peptides V. 3, p. 102-132, 1981. .~c~ ;c Press.
W O 94/20463 ~ PCT~EP94/00557
; 14
In the process (a') a compound of formula (V) wherein B is a
chemical bond snd m, A, R1 snd R2 are as above defined, nsmely
compounds of formula (XI)
Rl~
N-A-(CH2)m-NH2
R2
(XI)
either are commercially available or are prepared by the
conventional processes of the organic chemistry as ,-e~o,-ted for
example in J. Med. Chem. 33, 112 (199O).
In the process (a') a compound of fo. -l~ (V) wherein B is the
group -NHCOCH(R3) wherein m, A, R1, R2 and R3 are as above defined,
can be prepared by hydrolysis of the c ,_ ~ of formula (XII)
N-A-(cH2)m-NH-co-cH(R3)-NHR6
R2
(XII)
wherein A, m, R1, R2, R3 are as above defined and R6 is a
protecting group characteristic of the smino group of ~mino~ c as
trifluoroacetyl, benzyloxycsrbonyl. tertbutyloxycarbonyl, 9-
fluorenylmethyloxycarbonyl and trityl, the hydrolysis of the
compound of formula (XII) can be carried out following the methods
and processes known in the organic chemistry as for example
reported in T. W. Greene Protective group in Organic Synthesis
94/20463 ~ PCT~EP94100557
-,
Wiley Interscience Publication 1981.
A compound having formula (XII) wherein A, m, R1, R2, R3 and R6 are
as above defined can be prepared by reacting a compound of fG. 1
(XI) wherein A, m, R1 and R2 are as above defined, with a c _
of formula (XIII)
Hooc-cH(R3)-NHR6
(XIII)
wherein R3 and R6 are as above defined, or with its reactive
derivative.
A reactive derivative of an acid of formula (XIII) can be the same
as reported in this application for the compound having fo~
(III) or for the compound having formula (VII) and the reaction can
be carried out under similar conditions to those reported for the
amidation reaction of a compound of fo. 1~ (III) or a c .- ~ of
formula (VII) with a compound of fo, l~ (IV).
The compounds of formula (XIII) either are commercially avAil~hle
or are prepared by conventional procedures, startin~ from
aminoacids as described for example in E. Gross, J. ~ei~nhofer, The
Peptides V. 3, p. 102-132, 1981, Ac~ ic Press.
In the process (b), a compound of formula (VII) wherein m, A, X, R1
and R2 are as above defined, can be prepared as described in the
ZO International Patent applicstion No. WO 93/13739 published on 22nd
July 1993 in the name of the same applicant and herein reported by
reference.
WO 94/20463 ~ PCT~EP94/00557
~ ~ 16
In the processes (a) and (b) a compound of formula (IV) either is a
commercially av~ h]e compound or can be prepared by the known
methods tGazzetta Chimica It~ n~ 99, 632 (1969)].
In the process (a') a compound of fo. -lA (VI) can be prepared
according to the methods described in the International Patent
application No. W0 93/13739 p-~hl i che~ on 22nd July 1993 in the name
of the same applicant and herein reported by reference.
The compounds of the present invention have antitumoral and
antiviral activity, in particular they show high cytotoxicity
levels against the tumoral cel 1~ r lines.
Moreover the present invention relates to pharmaceutical
c~,~sitions comprising as active principle a compound of general
for .l~ (I) or a pharmaceutically acceptable salt thereof with a
pha.- --eutically acceptable vehicle or ~il u~n t .
A therapeutically effective amount of the compound of formula (I)
according to the invention is combined with an inert and
pharmaceutically acceptable vehicle. Conventional vehic~es can be
used and the ~ .itions can be prepared using the conventional
techniques. The compounds according to the present invention are
useful for human and animal therapeutical treatment.
In particular, the compounds according to the present invention are
useful as antitumoral and / or antiviral agents when ~i ni ctered
to patients in a therapeutically effective amount, for example a
suitable dosage for the a~in; ctation to adult patients can vary
25 from about O.1 and 100 mg per unitary dose from one to 4 times a
94l204C3 , PCTAEP94/00557
17
day.
BAMPLE 1
3-[1-Methyl-4-tl-methyl-4-[1-methyl-4-[1-methyl-4-t4-[N,N-bis(2-
chloroethyl)amino]benzenbut~n~ ;~n]pyrrol-2-c & ~o~y do]pyrrol-2-
cal~o~y i~o]pyrrol-2-carbo~n~ido]pyrrol-2-ca~ y do]propiona-
midine hydrochlorate
(I, X ~ -CONH-, A = p-phenylene, B z O m = 3, n = 2, Rl = R2 = 2-
chloroethyl).
4-[Bis(2-chloroethyl)amino~b~ 7~Pbutanoylchloride (801 mg, 2.49
mmoles), (obt~ined by the correspon~ing ca~Lo~ylic acid VIII, (A =
p-phenylene, m = 3, R1 = R2 = 2-chloroethyl) (75O mg, 2.49 mmoles)
by treatment with SOCl2 (1.2 ml) in tetrahydrofuran (25 ml) under
reflux), are ~iscolved in 25 ml anhydrous tetrahydrofuran and added
to a solution of 3-[1-Methyl-4-[1-methyl-4-~1-methyl-4-(1-methyl-4-
aminopyrrole-2-carboxyamido)pyrrol-2-carboxyamido]pyrrol-2-
carboxyamido]pyrrol-2-carboxyamido]propionamidine hydrochlorate
tIV, p = 4) (327 mg, 0.51 mmoles), [Gazzetta Chimica It~ n~, 99,
632 (1969)] and sodium bicarbonate (90 mg, 1.11 mmoles) in 40 ml
water.
~fter one hour stirring st room temperature, the reaction mixture
is evaporated to dryness and the residue is separated by
chromatography on silica gel (eluent CHC13/MeOH 7/3) thus obt~in;ne
276 mg I (X = -CONH-, A = p-phenylene, B = O, m = 3, n = 2, R1 = R2
= 2-chloroethyl) (yield 60 %).
-
W O 94/20463 PCT~EW4/00557
18
H-NMR (DMS0-d6), d : 1.82 (m, 2H), 2.25 (t, 2H), 2.45
(t, 2H), 2.64 (t, 2H), 3.50 (m, 2H), 3.70 (s, 8H), 3.82
(s, 3H), 3.85 (s, 3H), 3.86 (s, 3H), 3.87 (s, 3H), 6.69
(d, 2H), 6.90 (d, lH), 6.97 (d, lH), 7.04 (d, 2H), 7.08
(bs, 2H), 7.15 (d, lH), 7.18 (d, lH), 7.22 (bs, 2H). 8.19
(t, lH), 8.57 (bs,2H), 8.95 (bs, 2H), 9.76 (s,lH), 9.90
(m,3H)-
The following compound of formula (I) is also obtained by an
analogous proce~s:
3-~l-Methyl-4-tl-methyl-4-[4-~N,N-bis(2-chloroethyl)amino]be
but~nFImido]pyrrol-2-ca.bu~.y do]pyrrol-2-caLLo~y r~n] propiona-
midine hydrochlorate (I, X - -CONH-, A = p-phenylene, B = 0, m ~ 3,
n ~ O, Rl ~ R2 = 2-chloroethyl).
lH-NMR (DMS0-d6), d : 1.80 (m, 2H), 2.24 (t, 2H), 2.50
(t, 2H), 2.62 (t, 2H), 3.50 (m, 2H), 3.70 (s, 8H), 3.81
(s, 3H), 3.83 (s, 3H), 6.68 (d, 2H), 6.87 (d, lH), 6.92
(d, lH), 7.04 (d, 2H), 7.13 (d, lH), 7.16 (d, lH), 8.18
(t, lH), 8.65 (bs, 2H), 8.94 (bs, 2H), 9.75 (s, lH), 9.83
(s, lH).
EXAMPLE 2
3-rl-Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[4-[N,N-bis(2-
chloroethyl~amino3benzen~ i~ocarbonyl]pyrrol-2-caL-bo~y -ido]pyrrol-
2-carboxyamido]pyrrol-Z-carboxyamido]pyrrol-2-carbo~y ido] propio-
namidine hydrochlorate
(I, X = -NHC0-, A = p-phenylene, B z 0, m = 0, n = 2, Rl = R2 = 2-
~ 0 94/20463 , I 8 7 PCT~EPg4/00557
19
chloroethyl).
(337 mg, o.84 mmoles) of 1-Methyl-4-~4-[N,N-bis(2-chloroethyl)-
amino]b~n~nPminocarbonyl] pyrrol-2-c~,~ohylic acid chloride (VII,
A = p-phenylene, X = -NHCO-, m - O, Rl = R2 = 2-chloroethyl),
(obtained by treatment of the carboxylic acid VII (A ~ p-phenylene,
X = -NHCO-, m = O, Rl = R2 = 2-chloroethyl) (322 mg, o.84 l~)
with SOC12 (4.2 mmoles) ~i~solved in CH2C12 and in the pr~nre of
dimethylfor de), are dissolved in 10 ml tetrahydrofursn and
added to a mixture of N-deformyldystamicine (221 mg, 0.42 mmoles)
10 and diisopropylethylamine (0.3 ml, 2.1 mmoles) in anhydrous EtOH (5
ml) .
The mixture is maintained 30 minutes under stirring at room
t~ ture, then ethylacetate is added up to the precipitation of
the raw product, which after separation by HPLC (H20/CH3CN/CF3COOH
15 56/44/0.1) gives 162 mg of I (X = -NHCO-, A = p-phenylene, B = O, m
= O, n = 2, Rl = R2 = 2-chloroethyl), (yield 45 %).
H-NMR (DMSO-d6), d : 2.60 (t, 2H), 3.50 (m, 2H), 3.70
(s. 8H), 3.81 (s, 3H), 3.86 (s, 3H), 3.88 (s, 3H), 3.92
(s, 3H), 6.74 (d, 2H), 6.96 (d, lH), 7.08 (s, 2H), 7.18
20 (d, 2H), 7.25 (d, lH), 7.28 (d, lH), 7.42 (d, lH), 7.54
(d, 2H), 7.69 (d, lH), 8.17 (t, lH), 8.50 (bs, 2H), 8.59
(bs, 2H), 9.49 (s, lH), 9.88 (s, lH), 9.94 (s, lH), 10.09
(s, lH)-
The following compound of formula (I) is also obtained by an
WO 94/20463 PCT~EP9S/00557
~ 20
analogous process:
3~ Methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[1-methyl-4-[4-
[N,N-bis(2-chloroethyl)amino]b~n7Pnr 'noc~rbonyl]pyrrol-2-
c~ y do]pyrrol-2-ca~bo~y ~o]pyrrol-2-carbo~y~ido]pyrrol-2-
5 C&~o~y ~o]pyrrol-2-car~y~ido] propionr ~ine hydrochlorate
(I, X = -NHC0-, A = p-phenylene, B = O, m = O, n = 3, Rl = R2 = 2-
chloroethyl).
H-NMR (DMS0-d6), d : 2.60 (t, 2H), 3.50 (m, 2H), 3.70
(s. 8H), 3-81 (s, 3H), 3.85 (s, 3H). 3.87 (s, 6H), 3-92
10 (s, 3H), 6.72 (d, 2H), 6.95 (d, lH), 7.07 (m, 3H), 7.16
(d, lH), 7.21 (d, lH), 7.23 (d, lH), 7.26 (d, lH), 7.41(d,
lH), 7.52 (d, 2H), 7.68 (d, lH), 8.18 (t, lH), 8.47 (bs,
2H), 8.89 (bs, 2H), 9.48 (s, lH), 9.88 (s, lH) 9.90
(s, lH), 9.93 (s, lH), 10.09 (s, lH)
EXAMPLE 3
N-[4-~N,N-bis(2-chloroethyl)amino]benzoyl]glycine (III, A = p-
phenylene, m = 0, Rl = R2 = 2-chloroethyl, B = -CONHCH(R3)-, R3 =
H)
A mixture composed by glycine (600 mg, 8 mmoles),
20 bis(trimethylsilyl)acetamide (3.25 g, 16 mmoles), and
trimethylsilylchloride (0.2 ml, 1.6 mmoles) in CH2Cl2 (20 ml) is
kept under reflux for 2 hours.
After cooling to room temperature, (3 g, 10.8 mmoles) of 4-[N,N-
bis(2-chloroethyl)amino]benzoylchloride are added to the reaction
~0 94/20463 1S7187 PCT/EP94/00557
21
mixture, which is maintained under stirring at 40 C.
After 2 hours, the mixture is acidified with lN HCl and extracted
with CH2C12; the organic extracts are coll ected and extracted on
their turn with an aqueous solution of NaHC03, and the basic phase,
5 after acidification with lN HCl i~ extracted with CH2Cl2 thus
obt~;nine after evaporation of the organic phase, 1.32 g of III (A
= p-phenylene, m = O, R1 = R2 = 2-chloroethyl. B = -CONHCH(R3)-, R3
= H), (yield 52 ,X)
lH-NMR (DMSO-d6), d: 3.78 (m, 8H), 3.88 (d. 2H), 6.78
10 (d, 2H). 7.72 (d. 2H), 8.46 (t. lH).
BAI~LE 4
3-[1-Methyl-4-~1-methyl-4-[1-methyl-4-[N,a-[4-[N,N-bis(2-
chloroethyl)amino]benzoyl]glycylamino]pyrrol-2-ca~l~o~y 'do]pyrrol-
2-carl~o~y ~o]pyrrol-2-cE~ G~y do]propion~ 13in~ hydrochlorate
15 (I, X = -CONH-, A ~ p-phenylene, B z -CO~;n(R3)-, R3 = H, m = O, n
1, R1 = R2 = 2-chloroethyl).
308 mg (0.58 mmoles) N-deformyldystamicine, 136 mg (1 mmole) N-
hydroxybenzotriazole (HOBT), 182 mg (0.85) mmoles 1,8-bis-
(dimethylamino)-naphthalene and 191 mg (0.11 mmoles) [1-(3-
20 dimethylaminopropyl)-3-ethylcarbodiimide ( EDC ) are added to a
solution of 270 mg, (0.85 mmoles) III (A = p-phenylene, m = O, R1 =
R2 = 2-chloroethyl, B = -CONHCH(R3) -, R3 = H ), prepared as
described in example 3 and dissolved in anhydrous dimethylformamide
(45 ml).
25 The reaction mixture is maintained under stirring at room
WO 94/20463 PCT~EP94/00557
22
temperature for one hour, then ethylacetate is added up to
precipitation o~ the raw product, which after chromatography on
silica gel (eluent CH2Cl2/anhydrous EtOH/H2O 65/35/2) gives 275 mg
of I (X - -CONH-, A = p-phenylene, B ~ -CONHCH(R3)-, R3 ~ H, m - O.
n = 1. Rl = R2 = 2-chloroethyl) (yleld 60 %)
H-NMR (DMSO-d6), d : 2.64 (t, 2H), 3.50 (m, 2H), 3.78
(s, 8H), 3.82 (s, 3H), 3.84 (s, 6H), 3.97 (d, 2H), 6.78
(d, 2H), 6.91 (s. 2H), 7.O4 (d. lH), 7.14 (d, lH), 7.18
(d. lH), 7.21 (d, lH), 7.78 (d, 2H), 8.21 (t, lH), 8.52
(t, lH), 8.78 (bs, 2H), 9.O4 (bs, 2H), 9.89 (s, 2H), 9.95
(8, lH).