Note: Descriptions are shown in the official language in which they were submitted.
,. ,
"NITROGEN-SELECTIVE ZEOLITIC ADSORBENT
FOR USE IN AIR SEPARATION PROCESS"
The present invention relates in general to use of a
novel zeolitic adsorbent in air separation processes to
selectively adsorb nitrogen and, more particularly, to the
use in such processes of a nitrogen-selective adsorbent
containing zeolite X having a framework Si/Alz ratio of 2.0
to 2.4 and having a cation population at available cation
exchange sites comprising from 60 to 89 equivalent percent
Ca** ions, from 10 to 40 equivalent percent Na* ions and from
zero to 10 equivalent percent K* ions and wherein the total
cation equivalency contributed by Ca**, Na* and K* canons is
at least 90 percent.
BACKGROUND
The separation of air into its two principal constitu-
ents, nitrogen and oxygen, is an important commercial opera-
tion which produces several hundred billion cubic feet of
each material every year. Where large quantities of either
constituent are required, as, forexample, oxygen in the
manufacture of steel, the large capital costs of cryogenic
systems can be justified, and cryogenic procedures are
generally employed. For operationswith smaller require-
ments, oxygen and nitrogen can also be produced by pressure-
swing adsorption (PSA) processes. In PSA processes, com-
pressed air is pumped through a fixed bed of an adsorbent
exhibiting an adsorptive preference for one of the main
constituents whereby an effluent product stream enhanced in
the non-adsorbed (or lesser adsorbed) constituent is
obtained. Compared to the.cyrogenic processes, PSA air
separation processes require relatively simple equipment and
are relatively easy to maintain. PSA processes, however,
-1-
( ~ I
have lower product recovery and higher energy consumption
than the cryogenic processes. For these reasons,
improvements in the adsorption processes remain an important
goal_ One principal means of improvement is the discovery
and development of-better adsorbents.
The use of crystalline zeolitic--molecular sieves as
selective adsorbents for nitrogen, particularly from air, is
well known in the art. The general class of zeolites having
pore diameters of at least 4.6 Angstroms was proposed in US-
A-3140931 for this service. The use of the particular
zeolite species, zeolite X, containing as cations at least
one member of the group consisting of strontium, barium or -
nickel was proposed for this separation in US-A-3140932.
The relative merits of the various alkali metal cation forms
of zeolites, including zeolite X, were discussed in US-A-
3140933. In US-A-4557736, a binary ion-exchanged form of
zeolite X was taught as a preferred adsorbent for the
adsorption of nitrogen from air. In US-A-4481018, a set of
activation conditions which substantially avoids both frame
work and cation hydrolysis in polyvalent cation forms of
faujasite is disclosed.- These compositions, particularly in -
the Mg'*, Ca'*, Sr'*, and/or Ba'* cation forms, are alleged to
be superior adsorbents for the separation of nitrogen from
air. Based on selectivity values for N~/Oz obtained from
gas chromatographic studies, this '018 patent teaches that
the higher the degree of Ca'* exchange the greater-the
selectivity and capacity of the faujasite-type adsorbent for
the separation of nitrogen from air. The minimum calcium
content disclosed is 50% and the preferred adsorbents
contain greater than 80 equivalent percent calcium cations.
In sum, calcium, strontium and lithium cations are viewed by
the prior art as desirable constituents of zeolitic
adsorbents for use in nitrogen separation. Other cations
such as sodium are viewed as less desirable.
-2-
4 ~ I
v y
ERIEF DESCRIPTION OF THE DRAWINGS
Fig 1 plots nitrogen residual loadings of_CaNaX2.0
(i.e. X zeolite having a silica/alumina ratio of 2:1 and
Ca'' and Nal' cationa) after desorption to 10.7 kPa (1.55
psia) as a function of calcium exchange level at 40°C, 20°C
and 0°G, respectively.
Fig. 2 plots nitrogen delta loading of CaNaX2.0 between
14.7 and 1.55 psia as a function of calcium exchange level at
40'C, 20'C and 0'C, respectively.
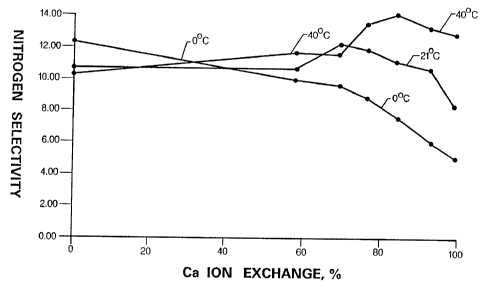
Fig. 3 plots nitrogen operational selectivity of
CaNaX2.0 as a function of calcium exchange level at 40'C,
20'C and 0'C, respectively.
Figs. 4, 5, and 6 plot nitrogen residual loading, delta
loading, and operational selectivity of CaNaX2.3 as a
function of calcium exchange level at 40'C, 20'C and 0'C,
respectively.
Figs. 7, 8, and 9 plot nitrogen residual loading, delta
loading, and operational selectivity of CaNaX2.5 as a
function of calcium exchange level at 40'C, 20'C and 0',
respectively.
Fig. 10 gives desorption rate of CaX2.0 (97% Ca) at
22'C.
Fig. il gives desorption rate of CaNaX2.0 (75% Ca) at
22'C.
Fig. 12 gives desorption rate of CaX2.3 (97% Ca) at
22'C.
-3-
,,
~~~7~~~
dig. 13 gives desorption rate of CaNaX2.3 (77% Ca) at
22'C.
~ig. 14 gives desorption rate of CaX2.5 (97% Ca) at
22'C.
F'ig. 15 gives desorption rate of CaNaX2.5 (77% Ca) at
22'C.
SUMMARY -
It has now surprisingly been found that zeolite X,
particularly those forms having a Si/A12 molar ratio of from
2.0 to 2.4, which contains a charge-balancing cation
population comprising from 60 to 89, preferably 60 to 80,
equivalent percent Ca" cations, from 10 to 40 equivalent
percent Na' cations and from zero to 10 equivalent percent R'
cations, and wherein the total cation equivalency contributed
by Ca", Na' and K' is at least 9o percent, is significantly
more effective as an adsorbent for the separation of air in a
pressure-swing adsorption process in the temperature range of
50oto -20oC at pressures in the range of 5 to 506.5 kPa
(0.05 to 5.0 atmospheres) than is the same zeolite X that has
been calcium exchanged to a greater or a lesser extent.
Accordingly, the present invention resides in a cyclic
process for separating nitrogen from a mixture thereof with
oxygen which comprises (a) providing an adsorption bed
containing as an adsorbent a zeolite having the crystal
structure of faujasite, a framework Sioz/A1z03 molar ratio of
from 2.0 to 2.4 and containing from 60 to 89 equivalent
- 4 -
. ,. ,
., 2$~74~I
percent Ca" rations, from 10 to 40 equivalent percent Na'
rations and from zero to 10 equivalent percent K' rations,
the total ration equivalency contributed by Ca", Na' and K'
being at least 9o percent; (b) passing said mixture of
nitrogen and oxygen into said adsorption bed at a temperature
of from -20'C to 50'C until an internal bed pressure rises to
the range of 13.8 to 506.8 kEa (2 to 73.5 psia) and nitrogen
is selectively adsorbed on said zeolite adsorbent; (c) dis-
charging unadsorbed oxygen as a product from the adsorption
bed at the adsorption pressu~ and (d) decreasing the bed
pressure to a final desorption pressure within the range of
101.4 to 0.7 kPa (I4.7 to 0.1 Asia) to desorb the adsorbed
nitrogen and discharge desorbed nitrogen from the bed.
Fn a particularly preferred embodiment of the present
invention, the zeolite X adsorbent has a SiOZ/A1z03 molar
ratio of from 2.0 to 2.35 and its ration population
comprising from 65 to 80~ calcium and 20 to 35~ sodium
rations and is substantially free of potassium rations.
DETAILED DESCRIPTION
It is well known that nitrogen has a quadrupole moment
of 0.31 A3 and for that reason interacts more strongly with
zeolitic rations than does oxygen which has a quadrupole
moment of only about 0.10 A3. Being more strongly adsorbed,
nitrogen is selectively adsorbed on zeolites from mixtures of
nitrogen and oxygen in accordance with thermodynamic
principles. This selectivity for nitrogen adsorption is the
basis for the numerous air separation processes utilizing
fixed-bed pressure-swing adsorption-desorption cycles. The
- 5 -
,,
~~~~4~I
properties of the zeolite cations are the most important
factors which bear on the selectivity and capacity for
nitrogen adsorption. Lithium and calcium as zeolitic
cations have been shown to exhibit particularly strong
affinities for nitrogen.
In the case of calcium-exchanged forms of zeolite X,
the effect of increasing calcium content has been reported
in US-A-4481018. Therein it is alleged that better
selectivity and capacity is achieved When the calcium canon
content exceeds about 80 equivalent percent. The
improvement in nitrogen adsorption efficacy is ascribed to
the fact that the calcium and other polyvalent metal cations
of the zeolite X employed are predominantly in the
dehydrated/dehydroxylated state. In view of these
teachings, it is the general understanding by those skilled
in the art that where a particular metal cation form of
zeolite exhibits good adsorptive properties with respect to
nitrogen, the higher the content of that particular metal
cation the better the performance in air separation.
- 6 -
, -~ ,
.. ~ ,
It has now unexpectedly been found that in the case of
calcium-exchanged forms of zeolite X, the peak performance in
air separation under certain temperature and pressure
conditions is not obtained when the calcium content is at a
maximum, but rather when the degree of calcium exchange is in
the range of 60 to 89 equivalent percent, especially in the
range of 65 to 80 equivalent percent. It is even more
surprising to find that the improvement caused by the reduced
calcium exchange level results in an effective adsorbent only
if the SiOZ/Alzo; ratio of X zeolite is in the range of 2.0 to
2.4.
While not wanting to be bound by any particular theory
or theories, it is believed that too much reliance has
heretofore been placed on the charge density of the calcium
cation without due regard for other factors. At least with
respect to calcium-exchanged forms of zeolite X, hereinafter
designated CaNaX, the nitrogen selectivity and capacity are
complex functions of cation composition and zeolite structure
as well as the temperature and pressure conditions under
which the adsorbent and the nitrogen containing gas are
brought into contact.
The nitrogen selectivities of CaNaX compositions
reported in the literature are often measured with
chromatographic techniques, with the nitrogen pressure over
the adsorbent being quite low. Since the nitrogen
selectivity of CaNaX is sensitive to pressure, the high
selectivity values reported are not applicable to higher
nitrogen pressures. The present invention is based at least
in part upon the discovery that, depending upon the level of
calcium exchange, the nitrogen selectivity of CaNaX can drop
,. ,
. "
significantly as pressure increases. Furthermore, pressure
used in the adsorption step of a practical commercial scale
air separation process is substantially higher than the
nitrogen pressure in a chromatographic measurement.
Therefore, the selectivity as determined by chromatographic
method has very little relevancy to the adsorption step in a
commercial pressure swing adsorption process.
In a PSA air-separation process, desorption is a
required process step. For an efficient adsorbent, a maximum
decrease of nitrogen loading over the shortest desorption
time interval must be achieved, so that the adsorbent can be
regenerated with minimal use of a vacuum pump or the oxygen
product. In other words, in order to have an efficient
desorption step in a PSA air separation process it is
necessary for the adsorbent to have a low nitrogen affinity
at the desorption or purge pressure. Since the nitrogen
pressure in a chromatographic measurement is well within the
desorption pressure of a PSA process, a high nitrogen
selectivity observed chromatographically suggests that such
an adsorbent will have poor desorption characteristics in a
PSA process.
Ideally, for PSA air separation, an adsorbent
should exhibit a low nitrogen affinity at low pressures
and a high nitrogen affinity at high pressures. In theory,
and confirmed by actual experience, it is not possible to
have such a nitrogen adsorbent. The next best thing
is an adsorbent whose nitrogen selectivity does not
decline steeply as the adsorbate pressure increases.
_ g _
~ 7
,i
2~~746~
In accordance with the present invention it has been
found that for CaNaX adsorbents, the decrease in nitrogen
affinity with increasing nitrogen pressure is, to a
significant degree, dependent upon the level of calcium ion
exchange. For highly calcium X, i.e., 90 equivalent percent
or greater, the decrease in nitrogen affinity is steep. For
CaNaX having a 60 to 89t Ca" level, the affinity for nitro-
gen at very low pressures is not very high and it does not
decrease rapidly when pressure increases. As a result, the
operational nitrogen selectivity of moderately calcium-
exchanged CaNaX can be higher than that of high CaX. More
importantly, at the desorption pressure the nitrogen is
easily desorbed from the moderately exchanged CaNaX, thus
significantly facilitating the regeneration of the adsorbent.
As a result the overall performance of the particular CaNaX
compositions employed in this invention is significantly
better than that of the more highly exchanged CaX.
To evaluate the potential of a material as a PSA air
separation adsorbent, the following three criteria should be
considered: 1. Residual nitrogen loading which is the nitro-
gen loading at the desorption pressure. A good PSA air
adsorbent should have low residual nitrogen loading. 2. The
nitrogen delta loading which is the difference between the
loading at adsorption pressure and the loading at the desorp-
tion pressure. A good PSA air adsorbent should have a high
nitrogen delta loading. 3. The operational selectivity which
is defined as the nitrogen delta loading divided by the
oxygen delta loading. A good air separation adsorbent should
have a high operational selectivity for nitrogen.
_ g _
i ~ 7
The temperature at which the process is to be carried
out is also an important factor in the performance of
adsorbents in air separations. Industrial PSA air
separation vessels are usually large and process cycle times
are short relative to the time needed to heat up or cool
down the massive amount of adsorbent involved. As a
consequence, the process cycle is carried out under
adiabatic or near adiabatic conditions. The heat
of adsorption of nitrogen on CaNaX zeolites is
significant. The heat released by adsorption is carried
forward by the feed and product gas and accumulates to form a
heat front. A portion of the heat front may leave the column
with the product gas. The remaining portion of the front is
pushed back into the column during the desorption stage of
the process cycle. During the desorption, kinetic energy is
consumed to free nitrogen from the adsorbent and thus reduces
the temperature of nitrogen and the adsorbent. The heat of
desorption creates a cold front in the bed, a portion of
which leaves the bed along with the waste gases. The remain-
ing portion of the cold front is pushed backward through the
column by the feed gas,during the next adsorption step.
After many adsorption-desorption cycles a steady state
will be reached. At the steady state, some portion of the
bed can be significantly cooler than ambient. It is, there-
fore, imperative to utilize an adsorbent whose adsorptive
properties are not unduly impaired by such temperature chang-
es. The present invention is based in part upon the determi-
nation that the adsorptive properties of highly calcium
exchanged forms of zeolite X are easily impaired by a. drop in
- 10 -
~~
~~~'~4 ~~
temperature but in the case of the moderately exchanged CaNaX
are not so impaired. Even a moderate decrease of adsorption
temperature can greatly increase the residual nitrogen load-
ing and decrease the nitrogen operational selectivity of a
highly calcium exchanged X zeolite. Our investigations also
establish that adiabatic desorption will cause a greater
temperature drop on high CaX than on CaNaX.
In a PSA air separation process, the productivity of
adsorbent is determined not only by the nitrogen delta load-
ing and the nitrogen operational selectivity but also by
length of cycle time. An adsorbent which can function in a
shorter cycle time will have a higher production rate. The
cycle time is determined by how rapidly the adsorbent can be
regenerated and made ready for the next adsorption step. It
has been found that nitrogen desorbs much faster from a
moderately exchanged CaNaX than from a highly exchanged CaX.
In sum, after taking PSA air separation conditions into
consideration, it has been found that moderately calcium
exchanged CaNaX is a superior adsorbent to highly exchanged
CaX.
The present invention is illustrated and exemplified by
the data shown in tabular form in TABLES I through V and in
the graphs of Figures 1 through 15 of the drawings. The
following adsorbent compositions, the methods for their
preparation and the testing procedures were utilized in
obtaining the aforesaid data:
- 11 -
-~ ,
.. _ ~.
2~~'~~~I
Preparation of the Starting Zeolite X Compositions:
Samples of Zeolite X having Si/AlZ molar ratios of 2.0,
2.3 and 2.5, respectively, were prepared. The sample having
a Si/Ah ratio of 2.0 was synthesized hydrothermally at 70'C
in the mixed Na' - K' cation form, i.e., NaKX2.0, in
accordance with the technique disclosed in British Patent
1,580,928, using sodium hydroxide, potassium hydroxide,
sodium silicate, aluminum hydroxide and water as the re-
agents. As initially crystallized, the NaIQC2.0 contained
about 25 equivalent percent K' cations with the remainder
being Na' cations. The K' cations were substantially all
removed and replaced by Na' cations by a thorough ion ex-
change using an aqueous NaCl solution.
Certain of the test procedures reported hereafter were
performed on pure, i.e., unbonded, zeolite samples, and in
others bonded agglomerates were utilized. In the latter
case, the binding agent was an attapulgite-type clay avail-
able from the Floridin Company under the trademark lYiinugel.
The agglomerates, in the form of beads and extruded pellets,
were prepared by conventional methods well known in the art.
To prepare partially calcium exchanged adsorbents, a
batch exchange technique was used. The zeolite, either in
powder or aggregate form, was immersed in a calcium chloride
solution at 80 to 95'C with constant agitation. Typically
this operation lasted 1 to 2 hours. The calcium exchange
level was controlled by the quantity of zeolite and calcium
chloride used. To prepare high calcium exchanged zeolite X
powder, a technique of repeated batch exchange with great
excess of calcium chloride was used. To prepare high calcium
- 12 -
2~~~~~~
exchanged aggregates, a column exchange technique was used.
Zeolite aggregates were placed in a heated column and pre-
heated calcium chloride solution was passed through the
column to displace and flush away the exchanged sodium or
potassium ions. Further details of the ion-exchange proce-
dures appear in the numbered Examples, below.
Example 1.
In this Example are reported the preparation, composi-
tion and adsorption properties of those CaNaX compositions in
which the Si/A1z framework ratio is 2Ø These compositions
are denominated CaNaX2Ø The CaNaX2.0 samples were prepared
by batch ion-exchanging NaX2.0 clay-bonded agglomerates in
the form of 8x12 beads. In general, 50 grams of starting
NaX2.0 beads (dry weight) were added to 1 to 2 liters of
aqueous 0.15M to 1M CaCl2 solution adjusted to a pH of 9.0
using Ca(OH)Z. In each instance the exact quantity and
concentration of the CaClz solution were selected in view of
the targeted degree of Ca" ion-exchange to be achieved.
Where necessary, multiple batch exchange procedures were used
to obtain a product of the desired degree of ion-exchange.
In each batch exchange the zeolite-containing beads were
stirred in the CaCly solution while the solution was heated
from ambient room temperature up to 90'C and thereafter the
stirring continued for one hour. The exchanged beads were
recovered by filtration in a Buchner funnel and rinsed with
500 ml of hot water at a pH of 9.0 adjusted by the addition
of Ca(OH)z. Thereafter the beads were stirred in 1 liter of
water at 90'C and a Ca(OH)i adjusted pH of 9.0 for 30 min-
utes, recovered by filtration and dried in air. In all,
seven CaNaX2.0 samples, denominated hereinafter as la, through
- 13 -
v I ~ 1
lg, respectively, were prepared. The particulars of the
preparations and the chemical compositions of the products
are set forth in Table I and Table II, respectively, below.
All of the agglomerate beads contained 12 wt. t clay binder.
Example 2.
Six powder and two beaded samples of calcium-exchanged
NaX having a Si/AlZ molar ratio of 2.3 were prepared by the
same general procedures described in Example 1. The princi-
pal difference in the procedures was the temperature of the
ion-exchange medium which was 95'C in these preparations
instead of the 90'C temperature used in Example 1. The
details of the ion-exchange procedures and the chemical
composition of the ion-exchanged products, denominated as
samples 2a through 2h, are set forth below in Table I and
Table II, respectively.
Example 3.
Using an as-synthesized sodium zeolite X having a Si/A1z
molar ratio of 2.5, clay-bonded 8 x 12 beads were ion-
exchanged with Ca'' ions. Four samples, denominated 3a
through 3d, respectively, were prepared. Samples 3a and 3b
were ion exchanged using the batch technique of Examples 1
and 2, and samples 3c and 3d were ion-exchanged using the
column technique wherein an aqueous CaCl2 solution is passed
through a heated column containing the zeolite-containing
beads. Details of the ion-exchange procedures and the
chemical compositions of the exchanged products are set forth
in Table I and Table II, respectively.
- 14 -
2I~~~~1
Example 4.
The nitrogen and oxygen isotherms of activated samples
la through 1g and the NaX2.0 starting material of Example 1
were determined at 40'C, 20'C, 0'C and -20'C using a
sartorius balance. The samples were activated in a glass
tube in a system equipped with an oil diffusion pump and a
liquid nitrogen trap by heating from ambient room temperature
up to 510'C over the period of 10 hours and maintaining the
final temperature for 6 hours. A pressure of 1.33 x 10-6
kPa (1 x 10-S torr) was achieved at the end of the
activation procedure. In the measurement of the nitrogen
and oxygen isotherms the temperature of each test sample was
controlled by placing the sample chamber in a constant
temperature bath or a tube furnace, as appropriate. Between
0 to 506.7 kPa (zero psia to 73.5 psia), 13 data points were
taken. The nitrogen loadings at exactly 10.7 to 101.4 Kpa
(1.55 and 14.7 psia) were determined by intrapolation. The
difference of the two values is the nitrogen delta loading.
The oxygen loadings at 25.5 kPa (3.7 psia) were also
determined by intrapolation. The nitrogen delta loading
divided by the oxygen delta loading between 25.5 kPa (3.7
psia) and 0 kPa (zero psia) gives what is termed the
operational selectivity. Values of residual loading, delta
loading and operational selectivity are listed in Table III
bElow, and plotted in Figures 1, 2 and 3 of the drawings.
Example 5.
Following the procedure described in Example 4, supra,
the nitrogen residual loading, nitrogen delta loading and the
nitrogen operation selectivity of NaX2.3 and CaNaX2.3 samples
of 2a through 2h, respectively, of Example 2 were determined.
The results are tabulated in Table IV. All of these samples
- 15 -
.
are unbonded, i.e., they do not contain clay, zeolite powder.
Figs. 4, 5 and 6 of the drawings disclose residual loadings
and operational selectivity data as functions of calcium
exchange levels and adsorption temperatures.
Example 6.
Following the procedure described in Example 4, supra,
the nitrogen residual loading, nitrogen delta loading and the
nitrogen operation selectivity of NaX2.5 and CaNaX2.5 samples
3a, 3b and 3c of Example 3 were determined. The results are
tabulated in Table V. Figures 7, 8 and 9 of the drawings
disclose residual loadings and operational selectivity data
as a function of calcium exchange levels and adsorption
temperatures.
Example 7.
To evaluate the desorption time needed for an adsorbent
in a PSA cycle, the nitrogen desorption rate was measured
with a Sartorius balance. The activated adsorbent was first
brought into contact with nitrogen at a pressure of 101.3 kPa
one atmosphere). After adsorption equilibrium was reached, the gas
was rapidly evacuated by a vacuum pump. The pump down step was
terminated when pressure reached 1.4 kPa (0.2 psia). It
required about 10 seconds to reach that pressure. The
adsorbent was then allowed to reach its equilibrium and the
weight loss of the adsorbent was continuously monitored. The
final pressure in the Sastorius balance is a function of
adsorption capacities of the adsorbent. For CaNaX of various
silica to alumina ratios and different calcium exchange
- 16 -
v ~ f
~ ~ ~ ~I5'~46~
levels, the final pressure is in the range of 1.72 to 2.62 kPa
(0.25 to 0.38 psia). Since nitrogen has large heat of desorption, a rapid
desorption under adiabatic conditions causes a drop in
adsorbent temperature. Vacuum desorption in a Sartorius
balance closely approximates an adiabatic process. It is,
however, very difficult to measure the sample temperature in
a Sartorius baiance directly, and accordingly the temperature
after desorption was estimated by a comparison of the
residual loading with the nitrogen isotherms at 20', 0' and
-20'C, respectively.
(a) The nitrogen desorption curve for sample lg of
Example 1, a highly calcium exchanged (97~) zeolite X having
a Si/A12 molar ratio of 2.0, is shown in Fig. 10 of the drawings.
To obtain the data, the vacuum-activated sample was first equilibrated -
in the Sartorius balance with nitrogen at 112.4 kPa (16.3 psia).
The balance was then rapidly evacuated to 1.38 kPa (0.2 psia) and
the sanq~le allowed to reach equilibrium with a consequent rise in
pressure to 2.62 kPa (0.38) psia). Also shown in Fig. 10 are the
expected equilibrium nitrogen loadings at 2.62 kPa (0.38 psia)
for the temperatures 20°C,,O°C and -20°C as determined by
extrapola-
tion from 10.3 to 2.62 kPa (1.5 to 0.38 psia). It appears from
these data that the desorption in the Sartorius balance
likely chilled the zeolite sample temperature to about 0'C.
(b) Using essentially the same procedure as in part
(a) above, the nitrogen desorption curve for Sample ld of
Example 1 containing 75 equivalent percent calcium cations
was determined. These data are set forth in Fig. 11. The
final nitrogen pressure in the balance was 1.72 kPa (0.25 psia) and
the residual loading about 0.09 mmol/gram. From inspection of the expected
equilibrium nitrogen loadings at 1.72 kPa (0.25 psia)
- 17 -
,. ,
2~~'~462
(by extrapolation fran 10.3 kPa (1.5 psia)) and 20°C, 0°C and -
20°C
also shown in Fig. 11, the sample appears to have been
chilled during desorption to a temperature of about 10'C.
(c) Using the sample procedure as in part (a) above,
the nitrogen desorption curves for Sample 2h of Example 2
containing 97 equivalent percent calcium cations was deter-
mined. These data are set forth in Fig. 12. The final
nitrogen pressure in the balance was 2.55 kPa (0.37 psia) and the resid-
ual loading was about 0.38 mmol/qram. The equilibrium
nitrogen loadings at 2.55 kPa (0.37 psia) at 20°C, 0°C and -
20°C,
respectively, also shown in Fig. 12, were determined by
extrapolation fran 10.34 to 2.55 kPa (1.5 psia to 0.37 psia). The sample
appears to have been chilled during desorption to a temperature
of about 3°C.
(d) Using the same procedure as in part (a) above, the
nitrogen desorption curve for sample 2g of Example 2 contain-
ing 77 equivalent percent calcium cations was determined.
These data are set forth in Fig. 13. The final nitrogen pres-
sure in the balance was 1.93 kPa (0.28 psia) and the residual load-
ing was about 0.07 mmol/gram. The expected equilibrium
nitrogen loadings at 1.93 kPa (0.28 psia) at 20°C, 0°C and -
20°C,
respectively, determined by extrapolation from10.34 to 1.93 kPa
{1.5 psia to 0.28 psia), are also shoran in Fig. 13. Fran these data the
sample appears to have been chilled during desorption to a
temperature of about 5'C.
(e) Using the same procedure as in part (a) above, the
nitrogen desorption curve for Sample 3d of Example 3 (con-
taining 98 equivalent percent calcium cations) was deter-
mined. These data are set forth in Fig. 14. The final
- 18 -
nitrogen pressure in the balance was 2.62 kPa (0.38 psia) and the
residual loading was about 0.18 mmol/gram. The expected
equilibrium nitrogen loadings at 2.41 kPa (0.35 psia) at 22°C,
30°C and
-20°C, determined by extrapolation from 10.34 to 2.62 kPa (1.5 to 0.38
psia), are also shown in Fig. 14. The sample appears to have
been chilled during desorption to a temperature of about
-13'C.
(f) Using the same procedure as in part (a) above, the
nitrogen desorption curve for Sample 3b of Example 3 contain-
ing 77 equivalent percent calcium rations was determined.
These data are set forth in Fig. 15. The final nitrogen pres-
sure in the balance was 56.88 kPa(8.25 psia) and the residual load-
ing was about 0.06 mmol/gram. From inspection of the expect-
ed equilibrium nitrogen loadings at 2.41 kPa (0.35 psia) at 22°C and
0'C, also shown in Fig. i5, the sample appears to have been
chilled during desorption to a temperature of about 3'C. The
expected equilibrium loadings were determined by extrapola-
tion fran 10.34 to 1.72 kPa (1.5 psia to 0.25 psia).
The effectiveness of an adsor-
bent for air separation is measured by three parameters,
i.e., nitrogen delta loading, nitrogen operational selectivi-
ty and residual nitrogen loading at the desorption pressure.
Taking these parameters into account, the data of the Tables
of the specification and the Figures of the drawings, includ-
ing the following specific observations, clearly establish
the superiority of the present invention.
The adsorption data of clay-bonded X2.0 samples are
given in Table III. The nitrogen residual loadings are
- 19 -
r ~ ~ 7
r 215746
plotted against calcium ion-exchange levels in Fig. 1. This
establishes that as the calcium cation level of the zeolite
adsorbent increases, the desorption of nitrogen progressively
becomes more difficult, therefore the residual loading in-
creases. At calcium levels of 90% or more, the residual
nitrogen loading becomes so high, product contamination
becomes a serious problem. The residual nitrogen level is
also sensitive to temperature. A combination of a 90% calci-
um level along with an operating temperature of 0'C brings
the residual nitrogen to an unacceptably high level. Since
cooling always occurs in PSA processes, these data demon-
strate that 90% or more calcium exchange can impair efficien-
cy of X2.0 in more than one way.
Fig. 2 gives the nitrogen delta loading between 101.3 to
10.69 kPa (14.7 and 1.55 psia) of CaNaX2.0 as a function of cal-
cium level and adsorption temperature. Initially as the calcium
content increases the delta loading of nitrogen also increases.
The delta loading reaches a broad maximum and starts to descend.
These data demonstrate that calcium exchange levels higher
than 90~ or lower than 608 are desirable.
Fig. 3 gives operational selectivity of X2.0 as a func-
tion of the calcium exchange level and temperature. Here is
a clear example how temperature can override the influence of
cation. At 40'C, the nitrogen operational selectivity in-
creases as the calcium content increases. Zt peaks around 85%
Ca" exchange and then begins to decline. At 20'C, the peak
operational selectivity is in the region of 60 to 80% calcium
content area. At temperatures of 0'C or lower, NaX2.0 has
higher operational selectivity than does CaNaX2Ø
- 20 -
7
At -20oC the nitrogen selectivity of CaNaX2.0 is
substantially lower than that of NaX2Ø
The adsorption data of X2.3 samples are given in Table
IV. Figs. 4, 5 and 6 give residual loadings, delta loadings
and operational selectivity data of X2.3 powder as a function
of calcium exchange levels and adsorption temperatures.
Since these samples do not contain clay, their loadings are
higher than corresponding bonded samples. Results of X2.3
samples are parallel to those of X2Ø The use of an X2.3
with 90~ or more of its cations in Ca" form and a process
temperature of 0'C is clearly not desirable. For an ambient
process cycle with significant temperature excursion, a
CaNaX2.3 with a calcium level of at least 60~, and less than
got, is preferred. Generally, the operational selectivity of
CaNaX2.3 is lower than that of the CaNaX2.0 adsorbent.
The adsorption data of bonded X2.5 beads are given in
Table V. These data along with the residual loadings, delta
loadings and operational selectivities plotted against calci-
um exchange levels in Figs. 7, 8 and 9, provide an outstand-
ing example that the performance of a PSA adsorbent is not
determined by single structure parameters. Silica-to-alumina
ratio is equally as important as calcium level. The nitrogen
delta loadings of X2.5 increase and the nitrogen operational
selectivities decrease as the calcium levels increase. This
is basically the same pattern observed in the case of X2.0
and X2.3, but with an important difference in detail. The
nitrogen delta loadings of CaNaX2.5 in the 60t to 90~ range
are very much lower than their X2.3 and X2.0 counterparts. It
is largely for this reason that CaNaX2.5 is not employed in
the practice of the present invention.
- 21 -
I ,
2~~?46I
The data of Fig. 1 shag that the residual loading or nitrogen
loading at 10.7 kPa (2.55 psia) increases rapidly as the calcium
content in the zeolite increases. The rate of increase is
inversely proportional to the adsorption temperature. At
0'C, the residual loading of 97% calcium exchanged X2.0 is
0.79 mmol/gm which is higher than its delta loading (0.74
mmol/gm). Accordingly, it would be much more difficult to
produce pure oxygen at 0'C using a CaX2.o adsorbent. At
20'C, its residual nitrogen loading is 0,39 mmol/gm, which is
high, but this adsorbent can function well using a proper
process cycle. On the other hand, with a calcium level at 76
equivalent percent the nitrogen residual loadings are 0.46
mmol/gm at 0'C, which is about same as the CaX at 20'C. The
residual loading of CaNaX2.0 at 20'C is 0.25 mmol/gm.
CaNaX2.0 (76%) will be able to function even at 0'C and is,
therefore, much favored.
With reference to Fig. 2, at 0'C the nitrogen delta
loading iF at a maximum at a calcium content of 75%. At 20'C
and 40'C, it peaks at a calcium content of about 90%. ,Based
on the nitrogen delta loading, the 75% exchanged CaNaX2.0
would have a clear advantage over a 97% exchanged CaX2Ø
In Fig. 3 it is shown that a 76% exchanged CaNaX2.0 has
a higher operational selectivity than a 97% exchanged CaX2.0
at 20'C and 0'C. These data establish that the use of a
CaNaX2.0 provides greater flexibility in choosing process
conditions than is the case with CaX2Ø
With reference to the desorption curves of Fig. 10 and
Fig. 11, it can be seen that one minute into the desorption
- 2a -
,.
2IJ'~46_I.
process, the residual loading on sample lg (CaX2.0 (97% Ca)]
is six times that of sample ld (CaNaX2.0's (75% Ca)]. These
data suggest that the highly exchanged zeolite will require a
longer time to regenerate than the less highly exchanged
composition. Another important implication of these data is
that after desorption the highly exchanged material may have
a significantly Lower temperature than the more moderately
exchanged zeolite. The drop in adsorbent temperature com-
pounds the problems experienced with very highly Ca" ex-
changed forms of zeolite X because they are very inefficient
at low temperatures.
The nitrogen desorption curves of CaX2.3 (97% Ca) and the
expected equilibrium adsorption capacities at 2.55 kPa (0.37 psia)
are given in Fig. 12. The desorption curve of CaNaX2.3 (778
Ca) and its expected equilibrium adsorption capacities at 1.72
kPa (0.25 psia) are given in Fig. 13. The residual loading of
CaX2.3 is about 0.17 mmol/gm, and its estimated temperature
after desorption is about 3'C. The residual loading of
CaNaX2.3 is about 0.05 mmol/gm, and the estimated temperature
after desorption is about 9'C. Again, the 77% calcium-
exchanged X2.3 is a more efficient adsorbent than is the 97%
exchanged counterpart. ,
The nitrogen desorption curve of CaX2.5 (97% Ca) and its
expected equilibrium adsorption capacities at 262 kPa (38 psia)
are given in Fig. 13. The desorption curve of CaNaX2.5 (77~ Ca)
and its expected adsorption capacities at 1.72 kPa (0.25 psia) are given
in Fig. 14. The residual loading of CaX2.5 is about 0.17
mmol/gm, and its temperature after desorption is estimated to
be about -13'C. The equilibrium loading of CaNaX2.3 is about
- 23 -
,.
' ' 2~~~4~I
0.07 mmol/gm, and its temperature after desorption is
estimated to be about -3'C. It is surprising to see that
CaNaX2.5 has a residual loading equivalent to -3'C. However,
it is consistent with the fact that CaNaX2.5 is a poor PSA
adsorbent relative to CaNaX2.0 and CaNaX2.3.
- 24 -
' ' 2~ i'~4 ~I
X
a
0
Y
L L
N_ N_ N N N_ N N_ _N N N V N N ' N
v r
a
L If1 Y
L L i _ i V
YI i ~ V V
a a $ ~a a v a
a
$ & ~ s
a
s
S''3uYa ~ ~~~=r~~ a '~u, ~'''u~,,
= a = A = ~ ~ o o a o v
n 1n .o a i L a a L L ,'~u, ,'~u, N
0 0 o s L
i _Y h Y i
p _~ _ = Z 7 i .E n n n n n n n ~ H
i i i i ~ i N N Z
0 0 0 0 ~ ° ~ o o a o 'o ~ 'o
if N
i i ~ _ _~ ~ s i s
Y 4 4 4 V ~ Y i 4
~
Y d ~ 4 x ~ i i t i i ~ ~ i ~ tut
1 % ~ ~ ~ ~ 11 = a a a a a i a a r
___so ~aBS"aBaE~ HBESS
=srerru'~ ~'cy'u~'x t3 ~u ~ a
~ g a ~ B a E ~ ~ g' a a a a a ~ a lj ~ ~ o c o
.. r r .. a N a a a
a W o 1n o .. a o o rv ~ o .. o
111 .~ .. . o ~ . . ~ ~
D N v ~ N r J ~ N H O r N 1I1 ~ .0 U v N ~e ~e
4
1
W
r ! d a D a '~ 0 H ~ H N N N N ~ In M h a
V
L
L
a
0 0 0 o a o o n n n n n n 1n 1n ~
Y1 O N N N N N N N n N N N N N n N n Y1 N N Y1 111
N ~ a ~ ~ a C ~ N ~ a 1 ~ ~ N d N N d ~ N N _
1~ a ~ i i ~ ~ ~ i a s ~ a a i i i a
a a a a a a a s a a a a a a a a s a a a a
- 25 -
V
V
V
i S A $ w IC YoR a S o n ' ~ o ~ ~ S ~ I: ~ $
S . . r a
v ~
0 0 0 0 0 0 0 0 0 0 0 o a o 0 0 0 0 0 0 o a
.
u
r
sv
a
p J ~ S 1N11~ ~ N ~ ~ A P J O ~ ~ S J S V N
O N V n H ~C p N n ~I d P N A O ~ n H A
U9
H
V
I
1
INU H N ' n A H ~ V ~DO N n O
~ ~ ~
~On P ~ d V ~ N N ~ V n V V
p O .0 V H n N O ' D V .0V N O V O A d V O O
r
H
I
H
%
N N h ~ ~ ~ h S ~ ~ ~ S h N
H H H H A 111H H H ~ A A ~ A ~ P O O
V V V V V V V V n V V V V V H
s
a
,
J ~ ~ ~ O r ~ ~ ~ N ~ J ~ h ~ ~ YV10
r
L ~ Nnr n n n ~ n ~ ~ ~ .~~ oxn n n n
% , l 1 1 l
J s
~ S H ~ O ~ H h S ~ Y N ~ N
Y
~ f
O n J V H V ~t V A .O p A p O H .OV H H ~O
J N N N N N N N N N N N N N N N N N N N N 1V
3
a
V
w
a u a .w a N ~ N N N N n n y
N ,. l
l l
a _~,
0 0 0 0 0 o a n n n n n n n H 1n H
0 n n H H
N N N N N N N N N N N N N N N N N
% % 1 a d % ~ % N J
' u
a ~ ~f i ~ ~ ~ ~ i ~l~ i a u a ~ i i i ~
V V V V V V V V V V V V V V V V V ~O
H J
t
- 26 -
~
~~~J~~~~
H
~ h A 0 ~ ~ ~ A ~ A O h ~ h ~ ~ ~ p H d H
, P
~
N/
i ~ N M N N O O N ~ O O 0 N O Y; Q ,0y~ r
t
_.
gg~
'a'~ox x '6g g o lS'b'~o 8 8 S g o 8
$r.~
w r o a o a a o a o a o , 0 0 0 o a o 0 0 0 0
"; 0
~
/
a -
~
a~ _
,n ~ V H ~ ~ ~ ~ n a ~ ~ A h A ~ 0 ~ ~ A
V
; N
~
J O O O O O O O O O O O O O O O O O 0 0 O O
~
H 1~
A
0
H
~
~ O ~ ~ r ~ N N ~ r ~ ~ ~ V h 0 N h
W
~
1t J 0 O 0 a O O 0 0 O O O a 0 0 O a 0 0 O O 0
~
< N
C
=V~
_Y
V V J V Y V N R O O O 0 O O O
V
O ~ J N N ~ N N
1
i
a D U U r 1 d U 11r A d D U 4 w~ P
a
H
H
~ ~ A P O n A P O r 0 A O Pl
i O 10P .0 0 P . P
0
V
/
lil
a
0 0 0 o a o o a o o a o 0 0 0 0 0 0
N N N N N N O N ~ N N % ~ ~ ~ % Nft
< / X i i i ftft i aN~tae t~~ x ~ e a
~ ~ ~ ~
i i ~ / ~ ~ '~ ~ '~ $ i ~ ~ ~ ~ o
~
a ~ ~ ~ ~ a x a a a a a a a a a a a a
n
- 27 -
.
2.~~'~4~1
~
gr
w~A
M
~yp p y~
~~ N ~QfIA ~ r ~ ~ ~ ~ ~ ~ ~ N ~ h r P. h
i N 1
i 1, ' n N N n O O r ~ N l
y ' A P O d .p V
V1
V
r
i
0
s
o x g ~ ~s~ o g ~s g a s $ ~ ~ s s s
A
V J O O O 0 0 0 O 0 O O 0 O 0 O 0 0 O 0 0 0
h 0
ga
~p p y1 ~ /I~ p1~
$Y~t~N J H 1~fl~ ~ A .nflPll~ A ~ A ~ ~ ~ O
O O O 0 O O O
O 0 O O O O O O O O O O 0
r ~ O
C
V
h
O ~ ~ r ~ N ~ ~ ~ ~ N h J V ~ N J V H
P W
..~ 0 0 O 0 O 0 0 0 0 0 O O 0 O O 0 0 0 O 0
~ 0
N
C
i
V
~.
1
W
a
0 O V V J J V V ~ ~ 0 0 O 0 0 0
~ O
~ J N ~ n N
N
H
1
b
a a a a ... ~ a a a .~- ~ a a N
N N N N N N N N N N N N N o
N
N
b
N
V%
.pA A .pO A .0 1~A .p0 A d A 1' .p A
O 0
~ ~ ~ ~ D P ~ ~ ~ ~ O P ~ ~ ~ ~ p
O
V~
ti
W
n n n n n n n n n n n n n n n n n n n n
n
N N N N N N N N N N N N N N N N
L 6 ~ N
~
i ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ E
~
a a a a a a V V V V ii v a a v V
'u
N
z0
,. ,
I
Y g P n V h YO V H N .Dh d
~ l
O ~ P O P O .p P h d P1
Yy N ~~
YJ
J
g r
_ 0 0 ~ o o ~ ~ ~ ~ a o
A
a
J~j 0 0 0 0 0 0 0 0 0 0 0 0
O
gH II
_
'~ ~~ ~ N PS ~ n v ~ ~ .novA,~
0 0 . 0 O O O . 0 0 0 0
0 O '
<V
i~
H
n o ' .p ' n in a r
V1 O N ~ N V ~ N V
J ~ O O O 0 O O O O O O O 0
_
Z Y
<
~u
_ A ~
0 0 0 o N o 0 o a'
V V V V N N
~ ~
I
i
~
r
n a a n o ~ n a a
~'~
n n n n n n n M n
b
N I
H
O ',g~ a O
1 1u
V
H 1f1Y1 If1IN 1f1 411P Y1
H Y
N N N i N N N ~ N N N
s ~ 3 ~ ~
~ 3 ~ s a a
H
- 29 -