Note: Descriptions are shown in the official language in which they were submitted.
. '
.1
21~8~.~~.
THERMOPLASTIC MULTILAYER FILM FOR USE IN PACKAGING
WATER
FIELD OF THE INVENTION
This invention relates generally to thermoplastic films, and in particular to
gas-barrier, multilayer films suitable for the packaging of water.
BACKGROUND OF THE INVENTION
Water is currently typically packaged in rigid containers such as glass or
plastic bottles or other differently shaped plastic containers such as bricks
or
containers having a cylindrical, conic, bi-conic, or pyramidal form. In the
case of
rigid plastic containers, the most commonly used resins are polyvinylchloride,
and more preferably polyester e.g. polyethylene terephthalate (PETS.
As a matter of fact the mouldings obtained from these resins show
remarkable transparency and gloss and excellent mechanical strength which are
all properties needed for this specific use; some of them also possess good
gas-
barner properties.
Said resins, however, do alter the taste (odour and flavour) of the packaged
water, even if not dramatically, during the few month period of storage which
is
generally accepted by the current distribution practice (typically 8-12
months). In
other words the taint transfer from container to product during normal
storage,
using these types of resins; can be considered as barely acceptable, the term
"taint" being here defined, according to the terminology widely accepted in
this
field, as "unpleasant odour or flavour imparted to food through external
sources". Water is known in fact to be the most susceptible food product as
far as
taint is concerned since water contaminants can be perceived by the human
senses at extremely low concentrations, for example parts per million (106),
ppm,
or even parts per billion (109), ppb, and taint occurrence is actually the
most
limiting factor as far as the choice of packaging materials is concerned.
.. .--
2158121
,~ _
Rigid containers, furthermore, suffer from a main, general, drawback
which is related to the fact that they require huge volumes for the storage
and
distribution of filled containers and also huge volumes for the storage and
transport of discarded containers, such as empty water containers for
disposal.
This is a problem particularly felt in emergency situations where big amounts
of
packaged water must be transported and delivered in a very short period of
time. Flexible containers, such as flexible bags or pouches of thermoplastic
materials, would clearly obviate these problems as it would be possible to
nest
such bags or pouches without leaving almost any empty volume between the
packages. Furthermore, for the same amount of water to be packaged, flexible
containers would require a much lower amount of starting plastic material than
rigid containers and as a consequence thereof a much lower amount of plastic
waste should be disposed of.
A water package consisting of a flexible thermoplastic container, filled with
water and then sealed, was recently put on the market but its
commercialisation
was later discontinued. Its five-layer, symmetrical structure comprised a gas-
barrier layer of ethylene-vinyl alcohol copolymer, two tie layers of modified
polyolefin and as the abuse-resistant and the heat-sealable, contact, layers,
an
LDPE (a highly branched ethylene homopolymer having a density within the
range of from about 0.915 to about 0.925 g/ cc).
With this structure, just owing to the LDPE of the heat-sealable layer,
undesirable taint of the packaged water, as evidenced by a series of
analytical
sensory tests carried out in our premises by a panel of sensitive assessors,
occurred soon after packaging.
WO 93/ 02859 describes the use of VLDPEs (i.e. linear ethylene-a-olefin
copolymers with a density lower than 0.915 g/cc obtained by the conventional
Ziegler-Natta technology) with a heat seal initiation temperature of less than
about 100°C for the manufacture of pouches for packaging of flowable
materials,
. _. . 3 2158121
including liquids. The use of the specific VLDPEs is only aimed at improving
the
heat-sealing performances and thus avoid Ieakers while no reference at all is
made to the taint problem. -
Recently a new type of ethylene copolymers has been introduced which is
the result of a new catalyst technology. Examples of introductory journal
articles
include "Exxon cites 'Breackthrough' in olefin polymerisation" - Modern
Plastics,
July 1991, p.61; "Polyolefins gain higher performance from new catalyst
technologies" - Modern Plastics, Oct. 1991, p.29; and Plastics Technology,
Nov.
1991, p.15.
These new resins which are produced using so-called "metallocene
catalysts" essentially differ from the previously known linear polyethylenes,
obtained by using conventional Ziegler-Natta polymerisation catalysts, in a
much higher uniformity in chain Ierigth, average comonomer content, and
comonomer incorporation along the chain. In contrast to the Ziegler-Natta
polymers they are characterised as having a narrow molecular weight
distribution (MWD) and a narrow compositional distribution (CD).
While the Ziegler-Natta catalysts are typically composed of metal halides
activated by an organometallic co-catalyst, e.g. titanium or.magnesium
chlorides
complexed with trialkyl aluminum, the metallocene catalysts are organometallic
compounds containing one or more cyclopentadienyl ligands attached to
metals, such as hafnium, titanium, vanadium, or zirconium, typically in the
presence of a co-catalyst such as for instance an oligomeric methyl alumoxane.
The uniqueness of these catalysts resides in the steric and electronic
equivalence
of each catalyst position which results in a singular activity and selectivity
of the
catalyst system. For this reason, metallocene catalyst systems are often
referred
to as "single site" owing to the homogeneous nature of them, and polymers and
copolymers produced from them are often referred to as single site resins by
their suppliers.
. . _ i i
- 4 2158121
In recent years several resin suppliers have been researching metallocene
catalyst technology. The following brief discussion should be viewed as
representative rather than exhaustive of this active area of the patent
literature.
EP-A-416,815 discloses the preparation of single-site ethylene-a-olefin
- 5 copolymers using monocyclopentadienylsilane complexed to a transition
metal.
US-A-4,306,041 describes the use of metallocene catalysts to produce
ethylene copolymers which have narrow molecular weight distributions.
US-A-5,088,228 relates to the production of ethylene copolymers of 1-
propene,1-butene,1-hexene, and 1-octene using metallocene catalysts.
US-A-4,935,397 discloses the production of single-site ethylene copolymers
using metallocene catalysts.
US-A-5,084,534 discloses the use of bis(n-butylcyclopentadienyl)zircoruum
dichloride to produce high molecular weight polyethylene with specific
polydispersity and density.
US-A-5,055,438 and US-A-5,057,475 disclose the use of
monocyclopentadienyl catalysts having a unique silicon bridge.
jP 63/175004 employed bis(ciclopentadienyl)ethoxy-zirconium chloride for
the preparation of homogeneous ethylene copolymers.
JP-B-1,101,315 discloses the use of bis(cyclopentadienyl)zirconium
dichloride for the preparation of ethylene-butene copolymers.
WO 93/03093 discloses the use of single site interpolymers with a
composition distribution breadth index of at least 50% in heat sealed articles
and
films.
WO 93/12151 describes high molecular weight linear copolymers of
ethylene and a linear a-olefin having at least 10 carbon atoms obtained by
this
new technology.
2158121
i: ~
WO 93/08221, US-A-5,272,236, and US-A-5,278,272 describe long-chain
branched single-site ethylene-a-olefin co-polymers-characterised by a high
shear
sensitivity and therefore a better processability.
Single-site ethylene-a-olefin copolymers are actually marketed by Dow (the
5 Affinity brand) and by Exxon (under the trade-names ExactTM).
Among the improved physical properties of the single-site ethylene
copolymers there may be cited better optics, improved impact-resistance, and
controllable melt characteristics ("New plastic resins search for their niche"
-
Packaging, March 1994, p.25-26). It is also known that in the single-site
copolymers the low molecular weight and the high molecular weight "tails" are
greatly reduced. While the absence of high molecular weight, ethylene-rich,
portions results in the improvement of the optics, reduction of the low
molecular
weight fractions reduce the "extractables" i.e. the shorter polymer chains
that are
soluble in apolar solvents such as pentane or hexane.
It is the object of the present invention to provide a convenient, effective
way of storing water in flexible containers, e.g. bags or pouches, made from a
thermoplastic, multilayer film so as to reduce the room required for storage
and
the amount of plastic material to be disposed of while maintaining the taste
of
the packaged water unaltered for Iong periods of time.
Accordingly thereto, the present invention provides a gas-barrier,
multilayer, film which is particularly suited for the manufacture of bags or
pouches for effectively and efficiently packaging water without taint
occurrence.
SUMMARY OF THE INVENTION
In one aspect of the present invention, a thermoplastic, multilayer film
suitable for water packaging comprises at Ieast a gas-barrier layer, and an
innermost surface, heat-sealable, layer which will be the layer in contact
with the
packaged water and is characterised in that said innermost surface heat-
sealable
2158121
~. _
layer essentially consists of a homogeneous single-site catalysed copolymer of
ethylene and an a-olefin having from four to eighteen carbon atoms.
In another aspect of the present invention, a method for packaging water in
a thermoplastic, flexible container is characterised by the fact that the
flexible
container is made from a multilayer film which comprises at least a gas-
barrier
layer and an innermost surface, heat-sealable, layer which will be the layer
in
contact with the packaged water wherein said innermost surface heat-sealable
layer essentially consists of a homogeneous single-site catalysed copolymer of
ethylene and an a-olefin having from four to eighteen carbon atoms.
In still another aspect of the present invention a flexible container, e.g. a
bag or a pouch, to be used for water packaging is made from the above defined
multilayer film.
DEFINITIONS
For the purposes of the present application the term "film" is intended to
refer to any flexible and flat or tubular flattened sheet of thermoplastic
material
having a thickness up to 250 ~, typically ranging from about 50 to about 150
~,,
and preferably from about 75 to about 130 ~,. For the purposes of this
application
this term also includes those structures which are conventionally indicated as
"laminates".
The terms "core" or "core layer" as used herein refer to an interior Iayer of
a
multilayer film having an odd number of layers. In the case of a symmetrical,
palindromic film, the same number of layers is present at either side of the
core
layer.
The terms "surface" or "surface layers" as used herein mean a layer of a
multilayer film which comprises a surface thereof.
The terms "polymer" or "polymer resin" generally include but are not
limited to, homopolymers, copolymers, such as for instance, block, graft,
' 218121
.
random, and alternating copolymers, etc. as well as blends and modifications
thereof.
The term "copolymer" as used herein is intended to denote polymers of two
or more comonomers. Therefore, altough the present specification generally
discusses ethylene-a-olefin copolymers, such term is intended to encompass
copolymers of ethylene with one or more a-olefins or of ethylene with an a-
olefin and another comonomer.
The term "polyolefin", as used herein, generally refers to thermoplastic
polymers obtained by polymerisation or copolymerisation of relatively simple
(C2-Cl~olefins which may contain other comonomers wherein the olefin units
are however present in higher amounts with respect to the other comonomers;
including, but not limited to, homopolymers, copolymers, terpolymers, blends
and modifications of such relatively simple polyolefins. Are specifically
included
therein homopolymers such as polyethylene and polypropylene, propylene
copolymers, ethylene-a-olefin copolymers, ethylene-vinyl acetate copolymers,
and ethylene-acrylate or ethylene-methacrylate copolymers.
The term "polyethylene" as used herein refers to a family of resins obtained
by polymerising ethylene molecules. By varying the catalysts and the methods
of
polymerisation, properties such as density, melt index, crystallinity, degree
of
branching and molecular weight distribution can be regulated over wide ranges.
v Polyethylenes having densities below about 0.925 g/ cc are called low
density
polyethylenes (LDPE), those having densities ranging from about 0.926 to about
0.940 g/cc are called medium density polyethylenes (MDPE) and those having
densities ranging from about 0.941 to about 0.965 g/cc and over are called
high
density polyethylenes (HDPE).
The term "polypropylene" refers to a thermoplastic resin obtained by
homopolymerising propylene units according to known processes.
215812.
The term "propylene copolymer" refers to a propylene copolymer with
ethylene and/or 1-butene wherein the propylene units are present in a higher
amounts than the ethylene and/. or butene units.
The term "ethylene-a-olefin copolymer" designates a copolymer of ethylene
with one or more (C4-C1g)-a-olefin preferably selected from the group
consisting of copolymers or terpolymers of ethylene with 1-butene, 4-methyl-1-
pentene, 1-hexene, and 1-octene. Ethylene-a-olefin copolymers can be prepared
using Ziegler-Natta or metallocene single site (constrained geometry)
catalyst.
The heterogeneous ethylene-a-olefin copolymers prepared using Ziegler-Natta
catalysts are generally classified as linear low density polyethylene (LLDPE),
having a density usually in the range of from about 0.915 g/ cc to about 0.925
g/ cc, linear medium density polyethylene (LMDPE), having a density usually in
the range of from about 0.926 to about 0.941 g/cc, and very low density
polyethylene (VLDPE), having a density lower than 0.915 g/cc.
The term "ethylene-vinyl acetate copolymer" (EVA) as used herein refers to
a copolymer formed from ethylene and vinyl acetate monomers wherein the
ethylene derived units in the copolymer are present in major amounts and the
vinyl acetate derived units in the copolymer are present in minor amounts.
As used herein the term "ethylene acrylate" or "ethylene-methacrylate
copolymer" refers to the product obtained by copolymerisation of ethylene with
acrylate or methacrylate monomers of formula CH2=C(R)-CO-OX wherein R is
hydrogen or methyl and X is hydrogen, (C1-C~alkyl, or a metal cation
preferably selected from Na+, Zn++, wherein the ethylene units are present in
a
higher amount than the acrylate or methacrylate units.
The term "polyamide" means a high molecular weight polymer having
amide linkages, and refers more specifically to synthetic polyamides, either
aliphatic or aromatic, either in crystalline or amorphous form. Exemplary of
r
synthetic polyamides are various nylons.
9 2158121
The term "polystyrene" generally .refers to those polymers which are
obtainable by polymerisation of styrene or styrene derivatives, e.g.
divinylbenzene, vinyltoluene, and a-methylstyrene, or copolymerisation of the
above monomers with other vinyl comortomers, e.g. butadiene, acrylonitrile,
methyl acrylate, malefic anhydride, and the like comonomers, as well as to the
rubber modified polystyrenes (impact-resistant polystyrenes) and to their
blends.
The term "polyester", as used herein, refers to bi-oriented, heat-set, poly-
ethylene terephthalate.
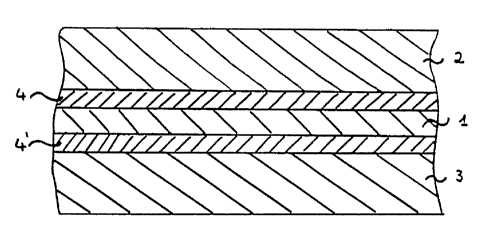
BRIEF DESCRIPTION OF THE DRAWING
The invention may be further understood with reference to the sole
drawing, Figure 1, showing a cross-section of a film according to a preferred
embodiment of the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is directed to a thermoplastic, multilayer film
suitable for use in water packaging comprising at least a gas-barrier layer,
and
an innermost surface, heat-sealable, layer, said multilayer film being
characterised in that said heat-sealable layer essentially consists of a
. homogeneous single-site catalysed copolymer of ethylene and an a-olefin
having
from four to eighteen carbon atoms.
'y'~ With reference to Figure 1, in a preferred embodiment the multilayer film
includes a gas-barrier layer 1, and an innermost surface, heat-sealable, layer
2; an
outermost surface, abuse-resistant, layer 3, and two tie layers 4 and 4'
interposed
. between the gas-barrier layer and the surface layers are preferably present.
. 25 Additional layers may also be present if desired.
The gas-barrier layer 1 is preferably comprised of a non-chlorine containing
organic polymer which is substantially impermeable to oxygen gas; i.e. an
organic polymeric material which is essentially free from chlorine and has an
w '2158121
-
oxygen transmission rate (tested as a 25 ~. thick film) of less than about 150
cc/m2.day.bar at 25 °C and 0% relative humidity (R.H.) (ASTM D-3985j:
Preferably it exhibits a permeability to oxygen gas of less than 100
cc/m2.day.bar and more preferably less than 30 cc/m2.day.bar, where the
5 permeability measurements are taken at 25°C and 0% R.H. In addition
to
substantial impermeability to oxygen gas, it will further be appreciated that
the
gas-barrier layer of the film according to the present invention also exhibits
barrier properties to carbon dioxide, nitrogen and hydrogen sulfide gases, as
well as to other gases and odors. Scope of this oxygen barrier layer is in
fact to
10 avoid transmission of odors and aromas from the outside environment,
through
the packaging material, to the packaged water.
Non limiting examples of non-chlorine containing organic polymers in
accordance with the present invention include vinyl alcohol containing
polymers, such as ethylene-vinyl alcohol copolymer (EVOH) and polyvinyl
alcohol (PVOH), polyacrylonitrile, nylon, bi-oriented PET and the like
polymers
either alone or blended with each other or another polymer. Preferably, the
non-
chlorine containing organic polymer is ethylene-vinyl alcohol copolymer and
the
gas barrier layer is preferably comprised of EVOH either alone or optionally
blended with a polyamide.
The gas-barrier layer 1 may also be comprised of a chlorine containing
polymer substantially impermeable to oxygen. Examples of such a polymer are
the vinylidene chloride copolymers (PVDC) wherein a major amount of the
polymer comprises vinylidene chloride and a minor amount comprises one or
more unsaturated monomers copolymerisable therewith, such as vinyl chloride,
acrylonitrile, and alkyl acrylate comonomers.
The innermost surface, heat-sealable, layer 2 essentially consists of a
homogeneous, single-site catalysed, copolymer of ethylene and an a-olefin
having from four to eighteen carbon atoms and a density of from about 0.88 to
CA 02158121 2005-07-05
50229-1
11
about 0.935 g/cc, preferably of from about 0.90 to about 0.920 g/cc, or of a
blend
of such single-site polymers.
Preferably the a-olefin will. contain from four to eight carbon atoms and
even more preferably it will be selected from 1-butene, 1-hexene, 4-methyl-1
pentene, and 1-octene.
TM
Examples of suitable single-site ethylene copolymers are Exxon's Exact 3006
(an ethylene-butene-hexene terpolymer with a density of 0.910 g/ cc and M.F.I.
of
1.2 g/10'), Exact 3016 (an ethylene-butene-hexene terpolymer having a density
of
0.910 g/ cc and a M.F.I. of 4.5 g/ 10'), Exact 3033 (an ethylene-butene-hexene
terpolymer having a density of 0.900 g/cc and a M.F.I. of 1.2 g/10'), Exact
3034
(an ethylene-butene-hexene' terpolymer having a density of 0.900 g/cc and a
M.F.I. of 3.5 g/10'), Exact 3028 (an ethylene butene copolymer having a
density
of 0.900 g/cc and a M.F.I. of 1.2 g%10'), Exact 3026 (an ethylene butene
copolymer having a density of 0.905 g/ cc and a M.F.I. of 2.25 g/ 10'), Exact
3024
(an ethylene butene copolymer having a density of 0.905 g/ cc and a M.F.I. of
4.5
g/ 10'), Exact 3001 (an ethylene butene copolymer having a density of 0:910 g/
cc
and a M.F.I. of 4.5 g/10'), Exact 3025 (an ethylene butene copolymer having a
density of 0.910 g/cc and a M.F.I. of 1.2 g/10'), Exact 3027 (an ethylene
butene
copolymer having a density of 0.900 g/ cc and a M.F.I. of 3.5 g/ 10'), Exact
401'1
(an ethylene butene copolymer having a density of 0.888 g/cc and a M.F.I. of
2.2
g/10'), Exact 2010 (an ethylene hexene copolymer having a density of 0.920
g/cc
and a M.F.I. of 1.5 g/10'), Exact 2009 (an ethylene hexene copolymer having a
TM
density of 0.922 g/cc and a M.F.I. of 3.0 g/10'),Dow Affinity PL1840 (an
ethylene
octene copolymer having a density of 0.908 g/cc and a NLF.I. of 1.0 g/10'),
Dow
Affinity PL1880 (an ethylene octene copolymer with a density of 0.902 g/ cc
and
a M.F.I. of 1.0 g/10'), Dow Affinity FW1650 (an ethylene octene copolymer with
a density of 0.902 g/cc and a M.F.I. of 3.0 g/10'), Dow Affinity FM1570 (an
ethylene octene copolymer with a density of 0.915 g/cc and a M.F.I. of 1.0
g/10'),
I2 21~812~
Dow Affinity SM1250 (an ethylene octene copolymer with density of 0.885 g/cc
and a M.F.I. of 3.0 g/10'), Dow Affinity PL1845 (an ethylene octene copolymer
with a density of 0.910 g/cc anc~ a M.F.I. of 3.5 g/10'), Dow Affinity PF1140
(an
ethylene octene copolymer having a density of 0.895 g/cc and a M.F.I. of 1.6
g/10'), Dow Affinity HF1030 (an ethylene octene copolymer having a density of
0.935 g/cc and a M.F.I. of 2.5 g/10'). All of these products are actually
commercially available with the brand name indicated above.
Additional layers can be present in the multilayer film according to the
present invention. As an example, an outermost surface, abuse-resistant, layer
3
is generally present. In particular, when the gas-barrier layer comprises a
vinyl
alcohol containing polymer, said outermost surface, abuse-resistant, layer
must
be present. The outermost surface, abuse-resistant, layer 3, when present, is
preferably composed of a polyolefin, such as polypropylene, polyethylenes,
linear polyethylenes, single-site linear polyethylenes, ethylene-vinyl acetate
copolymers, etc., either alone or blended with each other or with other
polymers.
Alternatively, it may also be comprised of a polyamide, such as various types
of
nylons, or of a polystyrene.
Also, tie or adhesive layers 4 and 4' can be present, interposed between the
surface layers and the gas-barrier layer. The tie or adhesive layers serve to
adhere the gas-barrier layer and the surface layers together, when the
selected
materials comprising those layers are not naturally compatible and therefore
not
able to adhere to one another after coextrusion. When tie layers are present
in
the multilayer film according to the invention they will be comprised of
materials which provide structural integrity to the multilayered barrier
structure
without substantially affecting the barrier properties of the gas-barrier
layer or
the mechanical and physical properties of the surface layers. Non limiting
examples of tie layers include functionalized polyolefins such as anhydride
or/ and acid modified polyolefins.
2158121
Other different layers may be present to further improve the mechanical
and physical properties of the overall structure. Said additional layers may
generally comprise polyolefins (typically ethylene-vinyl acetate copolymers,
and
polyethylenes) and polyamides (either aliphatic or aromatic polyamides).
Furthermore, and totally optionally even if not preferably, the outer surface
layer, the gas-barrier layer, the optional tie layers or any other additional
optional layer may also contain additives such as antistatic materials,
pigments,
plasticisers, ultraviolet absorbers, and the like.
The heat-sealable, innermost surface layer, on the other hand, should not be
compounded but employed in the extrusion process essentially as provided by
the supplier.
In the multilayer film of the present invention the thickness of the gas-
barrier layer will generally depend on its gas-barrier properties and will be
determined on the basis of said properties so as to provide the overall
multilayer
film with an oxygen permeability, measured at 25°C and 0% R.H. of less
than 100
cc/m2.day.bar, preferably less than 50 cc/m2.day.bar, and even more preferably
less than 25 cc/m2.day.bar.
Generally, however, gas-barrier layers of from 3 to 30 ~,, preferably of from
4 to 16 ~, and even more preferably of from 5 to 12 ~, are employed.
- 20 The innermost surface, heat-sealable, layer has a thickness which is
generally higher than 5 ~, preferably higher than 10 ~., and even more
preferably
higher than 15 ~..
The thickness of the outermost surface, abuse-resistant, layer, if present, is
not critical and layers as thin as few microns can be suitable. It is anyway
necessary, in case a moisture susceptible polymer, such as a vinyl alcohol
containing polymer, is used as the gas-barrier layer, that on each side of
said gas-
barner layer there is a sufficient thickness of one or more non-hydrophilic
polymers which provides for a suitable moisture barrier. In the preferred five-
14 ~1~81~1
layer structure showed in Figure 1, the tie layers are only a few microns
thick
(e.g. 4 to 8 ~, thick) and the surface layers are each from about 25 to about
45 ~.
thick.
The multi-layer structures according to the present invention may be
prepared by methods well known in the art. As an example they may be
coextruded through a coextrusion feedblock and die assembly (either flat or
annular) to yield a film of the desired thickness wherein the several layers
adhere together already at the molten stage as the film exits the die. The
film
exiting the die can be rapidly quenched or in case of an annular die it can be
"hot-blown" in order to reduce the film thickness, and then rapidly cooled
down.
Alternatively said structures may be formed by extrusion coating whereby
a substrate of one or more layers obtained by extrusion or coextrusion is
contacted with one or more hot molten polymers as said polymers exit the die.
A further alternative method of manufacturing the films according to the
invention comprises combining two or more mono- or multi-layer films
prepared as described above, by the process generally referred to as
"lamination", either by means of an adhesive or by the application of heat and
pressure.
The multilayer films according to the present invention may optionally be
subjected to an energetic radiation treatment, including but not limited to
corona
discharge, plasma, flame, ultraviolet, and high energy electron treatment.
Irradiation with high energy electrons is typically carried out to create
cross-
linking in the polyolefin containing layers. Suitable radiation dosages, if
applied,
would be up to 20 MRad and typically within the range of from 2 to l2MRad.
Conversion of the multilayer film into bags or pouches is conventional in
the art and may be performed by submitting the film, either in the form of a
single or double wound flat film or in the form of a tubular film, to a series
of
cutting and heat-sealing steps wherein the heat-sealable layer of the film is
heat-
CA 02158121 2005-07-05
. 50229-1
sealed to itself. Preferably,.however, for the manufacture of flexible
containers
for water packaging, the -film according to the invention is converted into
pouches directly in a FFS (Form-Fill-Seal) machine. Vertical and horizontal
FFS
machines which could suitably be employed are currently manufactured by
5 Onpack, Prepac, Waterline, and other equipment producers. Typically, before
the "form" step, the flat film is sterilised by passing it into a hydrogen
peroxide
solution or under a LTV lamp. Then it is dried, if necessary by a sterile air
jet,
formed into a pouch, typically a pillow pouch or a self-standing, gusset,
pouch,
by several sealing steps, either overlapping or inside-to-inside seals, filled
with
10 water and finally sealed. For hygienic reasons the overall process is
carried out
under sterile conditions and in a sterile environment. The flexible containers
which can be manufactured with the film according to the present invention
generally have a volume up to 2 I, preferably up to 11.
As anticipated, the thermoplastic, multilayer film according to the present
15 invention is particularly suited for water packaging.
Comparative sensory evaluations of water packaged in pouches made
from different films, either according to the present invention or according
to the
closest available prior art, have been made by a panel of highly sensitive
assessors. More particularly the samples which have been evaluated were five-
layer structures comprising an ethylene-vinyl alcohol copolymer as the gas-
barrier layer, two tie layers of modified polyolefins and two surface layers
consisting of either (a) a single-site ethylene-a-olefin copolymer for the
innermost surface layer and an LLDPE for the outermost surface layer (a film
according to the present invention), or (b) an LDPE for both innermost and
outermost surface layers (the commercialised structure) or again (c) an LLDPE
for both innermost and outermost surface layers (a comparative film). The
results obtained in these tests showed that while almost no taint could be
perceived with the containers obtained from the multilayer film according to
the
21581~2~
present invention even after a prolonged storage ~ or after storage in abuse
conditions (e.g. raised temperature), the taste cf water packaged in the
commercialised flexible container or in the comparative film was judged
unacceptable even after a short storage. These results were surprising and
could
not be expected on the basis of the properties known for the single-site co-
polymers including their low content of "extractables", as water, unlike most
foods including milk and other liquid foods, clearly has no lipophilic
character
and is not expected to dissolve and thus "extract" low molecular weight
polyethylene chains.
The good performances of the films according to the present invention, on
the other hand, might tentatively be related to the different catalyst systems
employed in the preparation of the different polymers and therefore to the
different trace contaminants present in the polymer resin.
A further object of the present invention is therefore a flexible,
thermoplastic, container for use in water packaging made from a multilayer
film
comprising at least a gas-barrier layer, and an innermost surface, heat-
sealable,
layer, wherein said heat-sealable Iayer essentially consists of a homogeneous
single-site catalysed copolymer of ethylene and an a-olefin having from four
to
eighteen carbon atoms.
The present invention is hereinbelow detailedly described by examples to
which the invention is not limited. In the examples, unless otherwise
specified,
"%" is on a weight basis.
Examples 1 to 5
The following five-layer structures are prepared either by the hot blown or
the tubular cast method by coextruding the innermost, heat-sealable, layers
made of the polymer resins indicated in Table 1 along with the following
standard structure
Tie layer of modified LLDPE (Bynel E409 by Dupont) - 5 ~.
1~ X158121
Gas-Barrier layer of EVOH (EVAL EPE105A by Kuraray) -10 ~,
Tie layer of modified LLDPE (Bynel E409 by Dupont) - 5 ~
Outermost surface, abuse-resistant, layer of LDPE (Dow 300R) - 40 ~.
Table 1
Example no. Innermost surface, heat-sealable, layer
1 Homogeneous single-site catalysed ethylene-butene-hexene
terpolymer with a density of 0.910 g/cc and M.F.L 1.2 g/10'
-. - (Exact 3006 by Exxon) - 40 ~,
- 2 Homogeneous single-site catalysed ethylene octene
copolymer with a density of 0.908 g/cc and M.F.L 1.0 g/10'
(Affinity PL 1840 by Dow) - 40 ~
3 Homogeneous single-site catalysed ethylene octene
copolymer with a density of 0.902 g/cc and M.F.L 1.0 g/10'
(Affinity PL 1880 by Dow) - 40 ~
4 Homogeneous single-site catalysed ethylene octene
copolymer with a density of 0.902 g/cc and M.F.I. 3.0 g/10'
(Affinity FW 1650 by Dow) - 40 ~.
5 Homogeneous single-site catalysed ethylene butene
copolymer with a density of 0.910 g/cc and a M.F.I. of 1.2
g/10' (Exact 3025) - 40 ~,
Comparative Examples 6 and 7
The following five-layer structures are prepared by the hot blown method
by coextruding the innermost, heat-selable, layers made of the polymer resins
indicated in Table 2 below along with the standard structure listed in the
foregoing Examples : Table 2
Comparative Example no. Innermost surface, heat-sealable, layer
6 Heterogeneous Ziegler-Natty catalysed ethylene
octene copolymer with a density of 0.914 g/cc
18 2158121
and M.F.I. 4.00 g/10'
. (Stamylex 09-046 F by DSM) - 40 ~,
7 Heterogeneous Ziegler-Natta catalysed
ethylene octene copolymer with a density of
. 0.919 g/ cc and a M.F.I. of 4.0 g/ 10'
(Stamylex 1046F by DSM) - 40 ~.
Examples 8 to 12
The following structures are obtained by flat cast coextrusion using the
Exact 3006 single-site terpolymer (35 u) as the innermost surface, heat-
sealable,
layer and coextruding it along with the following resins
Ex. 8 Tie layer of modified LLDPE (Bynel E409 by Dupont) - 5 ~
Gas-Barrier layer of EVOH (EVAL EPE105A by Kuraray) -10 ~.
Intermediate layer of polyainide (Ultramid KR-4407F by BASF)
- 12 ~,
Tie Iayer of modified LLDPE (Bynel E409 by Dupont) - 5 ~.
Outermost surface, abuse-resistant, layer of LDPE (Dow 300R) - 40 ~.
Ex. 9 Tie layer of modified LLDPE (Bynel E409 by Dupont) - 5 ~,
Intermediate layer of polyamide (Ultramid KR-4407F by BASF)
.. - 12 ~,
Gas-Barner layer of EVOH (EVAL EPE105A by Kuraray) -10 ~.
Intermediate layer of polyamide (Ultramid KR-4407F by BASF)
- 12 ~,
Tie layer of modified LLI7PE (Bynel E409 by Dupont)
- 5 ~,
Outermost surface, abuse-resistant, layer of LDPE (Dow 300R) - 40 ~.
Ex.10 Tie layer of modified LLDPE (Bynel E409 by Dupont) - 5 ~
Gas-Barrier layer of EVOH (EVAL EPE105A by Kuraray) -10 ~,
Intermediate layer of polyamide (Ultramid KR-4407F by BASF)
21~81~1
- 12~
Tie layer of modified LLDPE (Bynel E409 by Dupont) - 5 ~.
Intermediate layer Qf LDPE (Dow 300R) -15 ~,
Tie layer of modified LLDPE (Bynel E409 by Dupont)
-5 ~,
Outermost surface, abuse-resistant, layer of polyamide
(Lntramid KR-4407F by BASF) - 10 ~
Ex.11 Tie Iayer of modified LLDPE (Bynel E409 by Dupont) - 8 ~,
Gas-Barrier layer of EVOH (EVAL EPE105A by Kuraray) -10 ~
Tie layer of modified LLDPE (Bynel E409 by Dupont) - 8 ~.
Outermost surface, abuse-resistant, layer of ethylene-propylene
copolymer with a high ethylene content (Hifax 7029 by Himont)
~.
Ex.l2 Tie layer of modified LLDPE (Bynel E409 by Dupont)
15 -12 ~,
Gas-Barrier layer of copolyamide (Grilon CR9 by EMS) - 30 ~.
Comparative Example 13
Analysis of the film used in the commercialised pouches for water
packaging showed that the multilayer structure there employed was the
- 20 following one
Innermost surface, heat-sealable, layer of LDPE - approximately
40 ~.
Tie Iayer of modified LDPE - approximately 5 ~
Gas-barrier layer of EVOH - approximately 10 ~
Tie layer of modified LDPE - approximately 5 ~.
Outermost surface, abuse-resistant, layer of LDPE - approximately
40 ~.
2° ~1~8121
Sensory evaluations
The films according to the present invention have been submitted to
difference tests to measure specific effects by simple discrimination. More
particularly, multiple sample difference tests have been employed which are
only aimed at indicating whether samples are similar or different to a known
standard and provide reliable and statistically analyzable results.
For these tests small groups of pre-selected and trained judges are
employed. Each panellist is served a number of samples generally up~ to 6 or 7
as
a row of identical glasses, one of which is identified as a known standard.
The
unknowns may or may not include a hidden control identical to the known
standard. The panellist compares each coded sample with the standard and
assignes it a different score depending on the degree of difference between
the
known standard and the unknown test sample. In this difference intensity scale
"10" indicates no difference, "8" a very slight difference, "6" a slight
difference, "4"
a clear difference, "2" a large difference, and "0" an extremely large
difference.
The average score of all panellists for each sample is then calculated.
Results
from panellists who rate the hidden standard as different are however
discontinued.
In a group of tests the samples were kept at 38°C for 28 days before
being
tested, while in another group of tests they were kept at room temperature for
up to 8 months. For use in these tests the films were converted into 400-ml
pouches, filled with water of exactly the same source, and sealed.
In these tests the films according to the present invention received very
high scores.
In representative tests, water stored at 38°C in flexible containers
made
from the film of Example 1 received ~. mean score of 9.4 after 28 day storage,
while the water stored in containers made from the films of Comparative
- , . : .. . . ~ . . ~,. : :,
. _ . y 21 ~1~8121
. .
Examples 6 and 7 and 13 was assigned a mean score of 7.2 and 8.2 respectively
after 28 day storage.
The commercialized structure was tested after 7 and 42 day storage at
38°C
and it was assigned a score of 1.2 and 3.4 respectively.
Very good results are also obtained with the filins according to the present
invention after storage at room temperature for 3.5 and for 8 months
15
25