Note: Descriptions are shown in the official language in which they were submitted.
r t
.J
p 1 f
1 _
.,
' ~ A 1 1 1
-
~t~E, Pi~J i~J Ti;~ ~~.:~~d~C~. 21 5 8 7 5 7
TEXT 'FR~i~t3tAit9~1
-1-
COVERED STENT AND STENT DELIVERY DEVICE
Background of the Invention
The present invention relates to a st mt which
can be used within a vessel of the body of a living
animal or a living human. This invention also
relates to a device for delivering the stmt to the
treatment site. The stmt includes a flexible
tubular body which has a specific diameter at an
unloaded state but which can be contracted to a
smaller diameter by the application of force such as
by radially compressing the stmt or by pulling the
ends of the stent apart. This feature makes the
stent particularly useful for mechanical transluminal
implantation in biliary ducts, respiratory tracts,
the esophagus, blood vessels or the like. The stmt
delivery device includes a first tube having a
central lumen for accommodating a guidewire and a
flexible hose folded over itself and removably
surrounding the first tube. The stmt is placed
around the first tube and held in a radially
contracted state by the flexible hose. In this
manner, the stent can be delivered percutaneously and
transluminally to a treatment site in a body vessel.
The stmt is deployed by rolling the flexible hose
off of the stent to allow the stent to radially self-
expand. Once the stent is deployed, the stmt
delivery device can be withdrawn.
Prior radially self-expanding stents have an
open mesh construction. After positioning such a
stent in a body vessel, tissue may grow through the
spaces between the wires of the stent. In many
applications, such an occurrence is not detrimental
to the efficacy of the stent. Indeed in many cases
such tissue ingrowth is desirable because it helps to
I~M~N~ ~~
A
. .. .
2158757
-2-
keep the stent in place preventing migration of the
stmt .
However, in certain applications, tissue
ingrowth could be detrimental. For example, if the
stmt is to be placed in a body passage that has
tumor growth therein to maintain the patency of the
body passage, tumor ingrowth through the stmt would
limit the effectiveness of the stmt. Indeed tumor
ingrowth could completely block the body vessel. In
addition, such tumor ingrowth would permanently
"lock" the stmt in place. In certain applications
where the ability to remove the stmt is a
consideration, that is undesirable.
Prior delivery devices for radially self-
expanding stems generally perform in accordance with
their intended purposes. Typical prior delivery
devices have a moveable tubular member that
constrains the stmt in a contracted state on an
inner catheter. The tubular member is removed from
contact with the stmt to allow the stmt to be
deployed. In certain devices, the tubular member is
folded over itself to form a double-walled section.
However, such prior delivery devices may not be
totally effective in delivering a stmt to a
treatment site. For instance, friction between the
walls of the double-walled section of the moveable
tubular member as the walls move past each other can
make removal of the tubular member from the stmt
difficult. One means of minimizing this problem is
by the application of pressure between the walls of
the tubular member to move the walls of the tubular
member away from contact with each other. However,
this makes the delivery device difficult to operate.
The operator of the delivery device must continuously
I~ME.NDEt~ S!' F[T
.., - .,'
21 587 5 7
-3-
monitor the pressure to ensure that the pressure is
maintained in a certain range. If the pressure is
too low, friction forces will not be overcome. If
the pressure it too high, the delivery device could
rupture.
EP 0 481 365 relates to a device for widening a
stenosis in a body vessel, the device made of a
shape-memory alloy which radially expands at a
temperature above ambient temperature and below body
temperature. DE 4 022 956 relates to an intraluminal
rail, consisting of a wire enclosed by an
electrically insulating sheathing and shaped into a
basket which detachably encloses an inflatable
balloon, wherein the clearances stretching in the
direction of the circumference are kept free. The
clearances are formed in the manner of a mesh by a
regularly recurring approximation and bonding of the
sheathing of neighboring sections of the wire and
circumferentially expandable, whereby the sheathing
can be softened by heat at least once in order to
facilitate dilation by applying an electrical voltage
to the wire. DE 3 918 736 relates to metal grid
stem s for the permanent dilation of arterial
strictures which are coated with a thin layer of
polytetrafluoroethylene. 410 88/01924 relates to a
grasping means comprising an inner wall section and
an outer wall section each comprised of flexible
material with an annular region therebetween. GB 2
195 257 relates to a device for implantation by
insertion of a substantially tubular, radially
expandable prosthesis, comprising in combination such
prosthesis and concentric therewith a flexible probe
with means for maintaining said prosthesis in a
radially contracted state and for releasing same at
~M~.ND~'~ S~'trT
-4 - 21 58757
the desired location, said means for maintaining the prosthesis
comprising a hose concentrically surrounding said probe and
radially surrounding the prosthesis to form a compartment
therefor, characterized in that the probe has a central axial
channel enabling supply of a liquid flushing medium at its other
end and that the probe is provided with at least one radial
aperture opening into the prosthesis compartment to enable
flushing of the prosthesis compartment to remove gases therefrom
before implantation.
Therefore, it would be desirable to provide a stmt
that will maintain the patency of a body vessel and reduce the
tissue ingrowth through the stmt.
It would also be desirable to provide a stmt that is
removably placeable within a body vessel.
It would be further desirable to provide a stmt
delivery device that can deploy a stmt at a treatment site with
little difficulty.
Summary of the Invention
The invention provides a radially expandable stent,
comprising: at least one first thread element which extends in
a helix configuration along a center line of the stmt and
having a first direction of winding; at least one second thread
element which extends in a helix configuration along the center
line of the stmt and having a second direction of winding so as
to cross the at least one first thread element and form a lock
between the thread elements thereat and form a plurality of
interstices between each of the thread elements; the first thread
element and the second thread element defining a stmt inner
75394-5
-5- 2158757
surface and a stent outer surface; and a flexible matrix of
polymeric material surrounding and encircling each of the first
thread element and the second thread element and said lock
formed therebetween and occluding the interstices between each
of the first thread element and the second thread element, the
matrix being integral with and radially expandable with and
covering the stmt and preventing tissue ingrowth therethrough;
wherein the stmt is adapted to assume a radially contracted
state and a radially expanded state, and the first thread
element and the second thread element form an axially directed
angle greater than 60° when the stmt is in the radially
expanded state, the stmt exerting a radial force sufficient to
fixedly implant the stmt in a body vessel.
The flexible matrix is preferably applied to the
stmt by dip coating the stmt in a bath of silicone rubber and
an organic solvent. The thickness of the matrix film can be
controlled by the ratio of the silicone rubber and organic
solvent in the bath and by the number of dip coatings to which
the stmt is subjected.
The stmt delivery device includes an elongate and
flexible length of inner tubing with a central lumen for
accommodating a guidewire. The stmt is placed on this tubing
in a radially contracted state for transport to the treatment
site. A flexible hose surrounds the tubing and is folded over
itself to form a double-walled section. This double-walled
section surrounds and confines the stmt in a radially
75394-5
- 5a - ~ 1 5 87 5 7
contracted state on the tubing. To facilitate the movement of
the flexible hose away from the stmt, at least that portion
of the hose that contacts itself in the double-walled section
is lubricous. This lubricous characteristic can be achieved by
placing a lubricous coating on the surface of the hose that
contacts itself in the double-walled section of the hose, by
injecting a lubricous liquid into the space between the walls
of the double-walled section or by forming the flexible hose
from a naturally lubricous material. This makes the stmt
delivery device of the present invention
64680-837
I
21 587 5 7
-6-
easy to use and makes simple the deployment of a
stmt therefrom.
When it is desired to deploy the stmt at a
treatment site, the flexible hose is rolled back
proximally to first expose the distal end of the
stmt. This allows the operator of the stmt
delivery device first to align properly the distal
portion of the st mt in the body vessel. When proper
alignment is obtained, the operator can continue to
roll the flexible hose proximally to completely
uncover the stmt and allow it to radially self-
expand into engagement with the vessel wall.
Brief Description of the Drawings
The above and other objects and advantages of
this invention will be apparent upon consideration of
the following detailed description, taken in
conjunction with the accompanying drawings, in which
like reference characters refer to like parts
throughout and in which:
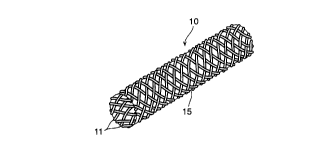
FIG. 1 is a perspective view of the covered
stent of this invention clearly showing the braided
configuration of the thread elements;
FIG. 2 is a perspective view of the covered
stmt of this invention in a radially contracted
state clearly showing the braided configuration of
the thread elements;
FIG. 3 is a cross-sectional view taken along
line 3-3 in FIG. 9.
FIG. 4 is a detailed view in perspective of a
portion of the stent of this invention without the
covering to show the braided configuration of the
thread elements;
FIG. 5 is a detailed sectional view of a portion
of the stmt of this invention without the covering
~,~G~}n~n
.. .... ...._. _ ~~...._. .~._w._ ... . ~...~...... . _ _ _. .. _ _.__ _._.._
._ _.__ _ . .. _____ . ..~.._
I
2158757
to show the braided configuration of the thread
elements;
FIG. 6 is a diagrammatic sectional view of a
portion of the covered stmt of this invention
showing the flexible polymeric matrix located along
the exterior of the stmt;
FIG. 7 is a view similar to the view of FIG. 6
with the flexible polymeric matrix located along the
interior of the stent;
FIG. 8 is a side view of the stmt delivery
device of this invention with a covered stmt loaded
therein;
FIG. 9 is an enlarged side view of the area
encircled at 9 in FIG. 8;
FIG. 10 is an enlarged side view of the area
encircled at 10 in FIG. 8; and
FIGS. 11-14 are side views of a distal portion
of the stmt delivery device and the covered stent
of the present invention in various stages of a stmt
deployment operation in a body vessel.
Detailed Description of the Invention
In FIG. 1 there is shown an example of the
covered st mt 10 of the present invention in an
unloaded condition. Covered stmt 10 is in the form
of a cylindrical tubular body. Stent 10 is formed by
a number of individual thread elements 11. Some of
these elements extend in helix configuration in one
direction axially displaced in relation to each~other
having the center longitudinal axis of st mt 10 as a
common axis. The other elements extend in helix
configuration in the opposite direction and are also
axially displaced in relation to each other having
the center longitudinal axis as a common axis. Thus
thread elements 11 extend in two directions and cross
ttJIFN(~~t7 ,~~~j~~
. ,
2~1 5 8 7 5 7
_8_
each other in a braided over and under configuration.
Thread elements 11 of stmt 10 are preferably
arranged symmetrically so that the same number of
thread elements are used in each direction of a
winding. The number of thread elements needed is a
function of the diameter of stmt 10 in an unloaded
condition. For a stent having a diameter of 10
millimeters preferably 24 thread elements are used.
Thread elements 11 are helically wound about a
cylindrical mandrel. One set of thread elements is
wound in one direction while the other set of thread
elements is wound in the opposite direction.
Thread elements 11 should be maintained in
tension. Insufficient tensile force may allow the
individual thread elements to depart from their
configuration causing the braided structure of stmt
l0 to unravel. When thread elements 11 are properly
tensioned, a slight impression is formed in the
overlying thread element at each intersection. See
FIGS. 4 and 5. Each thread element is thus deformed
such that it is bent over other thread elements and
partly circumscribes these other thread elements.
Generally, only the parts of the respective thread
elements lying on top of the crossing thread elements
as seen in the radial direction have been subject to
bending. These impressions, or saddles, tend to lock
the thread elements relative to one another at the
intersections. This maintains the stent
configuration without the need for welding or other
bonding of thread elements 11 at their intersections.
In addition, this allows a suitable length of the
tubular braid to be cut in order to make a st mt of
the desired length. The cut length of the tubular
braid essentially maintains its cylindrical shape at
h!~.~~.i~(i~~1 a't~''
21 587 5 7
-9-
the end sections.
In order to further improve the radial stability
of stmt 10, the axially directed angle between
crossing thread elements should be at least 600,
preferably greater than about 90' and even more
preferably greater than about 100' when stent 10 is
in an unloaded condition. The greater the angle, the
higher the stability of stmt 10 under external
pressure.
Thread elements 11 forming stmt 10 can be made
from a biocompatible and flexible yet rigid material
such as various polymers, e.g. Kevlar~, and metal
such as stainless steel. Other materials include
alloys substantially based on cobalt, chromium,
nickel and molybdenum, the alloying residue being
iron. In addition, thread elements 11 cam be formed
from a core and a tubular case surrounding the core.
This configuration can enhance the radiopacity of
stmt 10. For example, the core can be constructed
of tantalum for radiopacity while the case can be
constructed of a cobalt-based alloy such as an alloy
available under the brand name Elgiloy°, "Phynox" and
"MP35N".
The diameter of stent 10 can be changed by
radially compressing stent 10 or by axially
displacing the ends of stent 10 relative to each
other. In FIG. 2 there is illustrated how stent 10
according to FIG. 1 has been given reduced diameter
by moving the ends away from each other in the
direction of the arrows. Since stmt 10 must engage
against the wall of the body vessel in which stmt 10
is to be placed with certain pressure in order to
64680-837
' . , ~ '~1
2158'57
-10-
remain fixed, the diameter of stmt 10 in the
radially contracted state must be smaller than the
diameter of stent 10 at free expansion.
Stent 10 is covered by a matrix 15 of a
flexible, polymeric material such as silicone rubber,
polyurethane or Teflon . Other flexible and
biocompatible polymers could also be used.
Preferably silicone rubber is used. Matrix 15 can
take the form of a film or a braided or woven
covering.
Matrix 15 is preferably applied to stmt 10 by
dip coating. Liquid silicone rubber is mixed with an
organic solvent, preferably xylene, to make the
silicone rubber flowable. For a stent having a
diameter of 10 mm, 24 thread elements and a braid
angle of 1100 and where the stmt is supported in the
silicone rubber and xylene bath only at the ends, a
ratio of 27% silicone rubber to xylene is preferably
used. Using this arrangement only one dip coating is
needed to completely cover stmt 10. For a stmt
having a diameter of 20 mm, 36 thread elements and a
braid angle of 1100 and where the stent is supported
in the silicone rubber and xylene bath by an internal
mandrel, a ratio of 18% silicone rubber to xylene is
preferably used. Using this arrangement 3 to 5 dip
coatings is needed to completely cover stmt 10.
Additional coats could be applied to stmt 10
beyond what is described above. However, if too many
coats are used, the flexibility of the resulting
stmt will be compromised making it difficult to load
the resulting stmt on a delivery device on to deploy
the resulting stmt at a treatment site. It has been
found that a coating about 0.010 cm (.004 inchesl
thick is preferable. Alternatively, if a matrix 15
~i ~c ~D~D ~~
9
_. , - ~ ,
2158757
-11-
with more flexibility is desired, matrix 15 can take
the form of a braided or woven covering.
Stent 10 can be dip coated by supporting the
ends of stmt 10, by using an external mandrel or by
using an internal mandrel to support stmt 10 when it
is dipped in the bath of silicone rubber and xylene.
When stmt 10 is dip coated by supporting the ends of
stmt 10, the silicone rubber surrounds thread
elements 11 so that the silicone rubber only extends
in the interstices between thread elements 11 and
there is little excess silicone rubber on the outside
or inside of stmt 10. See FIG. 3 and FIG. 14. When
stmt 10 is supported by an external mandrel the
silicone rubber coating tends to extend toward the
outside of thread elements 11 forming stmt 10. See
FIG. 6. When stmt 10 is supported by an internal
mandrel the silicone rubber tends to extend toward
the inside of thread elements 11 forming stmt 10.
See FIG. 7. Preferably, stmt l0 should be supported
at the ends or by an external mandrel during the dip
coating process. The matrix resulting from this
process tends to be stronger and more tear resistant
so that it is better able to prevent tissue ingrowth
through the interstices between thread elements 11
forming stmt 10.
Although the dip coating process described above
is the preferred process for covering the stmt of
this invention, matrix 15 can also be applied by
other methods such as by injection molding or spray
coating stmt 10 with the polymeric material.
Stent 10 is placed on a stmt delivery device 20
in.a radially contracted state for delivery to the
treatment site in a body vessel. Stent 10 is carried
by the distal portion of delivery device 20. The
r,~~~rio~o s~~
2158757
-12-
proximal portion of delivery device 20 generally
remains outside of the body for manipulation by the
operator.
Delivery device ~0 comprises an elongated, inner
tube 30, preferably having an axially extending lumen
35 therethrough. A distal portion of inner tube 30
is flexible and can be made from nylon or any other
suitably flexible biocompatible polymeric material.
At its distal end, inner tube 30 is provided with a
head 31, through which lumen 35 continues. Head 31
serves to facilitate the insertion of delivery device
through a narrow opening in a body vessel. The
proximal portion of inner tube 30 is preferably
formed from stainless steel or some other suitably
15 rigid metal alloy. The proximal end of the distal
portion of inner tube 30 is bonded to the distal end
of the proximal portion of inner tube 30 in any
conventional manner such as by using a standard
adhesive.
20 A proximal tube 50 surrounds the proximal
portion of inner tube 30 in coaxial fashion.
Preferably proximal tube 50 is formed from
polyurethane. The proximal end of proximal tube 50
is connected to a valve body 40 having a side port
41. An extension tube 45 extends from side port 41
to an opening 42. This arrangement allows fluid to
be injected through extension tube 45 and between
proximal tube 50 and inner tube 30. -
A moveable hose 55 surrounds the distal portion
of inner tube 30. Hose 55 is rolled over itself to
form a double-walled section. The proximal end of
the inner wall 56 of the double-walled section is
connected directly to inner tube 30. The proximal
end of the outer wall 57 of the double-walled section
AME~~DED S''t r~
._
i
21 587 5 7
-13-
is connected to the outer surface of the distal
portion of proximal tube 50. These connections can
be achieved by any conventional means such as by a
standard adhesive. This arrangement allows hose 55
to be rolled off stmt 10 placed on the distal
portion of inner tube 30. By moving valve body 40 in
the proximal direction, outer wall 57 of hose 55
slides proximally over inner wall 56. This causes
inner wall 56 to "roll back" off of stmt 10. To
facilitate movement of hose 55 off of stmt 10, at
least that portion of inner wall 56 that contacts
outer wall 57 in the area where hose 55 is folded
over to form the double-walled section should be
lubricous.
The lubricous characteristic can be achieved by
adding a lubricous substance to this surface of hose
55; injecting a lubricous liquid between inner wall
56 and outer wall 57 or forming hose 55 from a
naturally slippery material such as Teflon .
In the preferred embodiment, at least the
surfaces of inner wall 56 and outer wall 57 that face
each other in the double-walled section are coated
with a lubricous hydrophilic coating. Preferably a
hydrophilic coating manufactured and sold by The
Hydromer Company under the designation 2018-M is
used. Other materials include polyethylene oxide and
hyaluronic acid. When wet the hydrophilic coating
becomes lubricous and thus reduces friction between
inner wall 56 and outer wall 57 of the double-walled
section of hose 55 as outer wall 57 moves past inner
wall 56. This facilitates the removal of the double-
walled section of hose 55 from stent 10..
Preferably, hydrophilic material is added to
hose 55 during the assembly of delivery device 20.
,~~t~i0~0 SH~'FT
'"' \ . .
i
2158757
-14-
In order for the hydrophilic material to adequately
bond to hose 55, the material used to manufacture
hose 55 must be matched to the hydrophilic material
used. It has been found that polyurethane works well
as the material to form hose 55. In particular, a
blend of 65D and 75D polyurethane provides sufficient
flexibility to allow hose 55 to roll over itself yet
still be soft enough and compatible with the
hydrophilic material so it can be properly coated.
Preferably the blend is composed of 50% 65D
polyurethane and 50% 75D polyurethane.
During the assembly of delivery device 20, one
side of hose 55 is coated with the hydrophilic
material after hose 55 (outer wall 57) has been
connected to proximal tube 50. Isopropyl alcohol is
first applied to one side of hose 55 to clean the
surface and remove the waxy film resulting from the
plasticizers in the polyurethane. Next that same
side of hose 55 is coated with the hydrophilic
material. The surface of hose 55 should be flushed
with alcohol for about 30 seconds. Similarly, that
surface of hose 55 should be flushed with the
hydrophilic coating for about 30 seconds. It has
been found that this technique deposits sufficient
hydrophilic material on inner wall 56 and outer wall
57 to allow hose 55 to be rolled back with minimal
friction when the hydrophilic material is wet.
Once delivery device 20 has been assembled and
is ready for use, the hydrophilic coating is wetted
with physiological saline solution by injecting the
solution through extension tube 45, past proximal
tube 50 and into the space between inner wall 56 and
outer wall 57 of the double-walled section of hose
55. Excess fluid exits from the hole 59 formed
A~PFPzO~il ',,'~'r~..,.
' . ~ ._
21 587 5 7
-15-
toward the distal end of the double-walled section of
hose 55. In this same manner, a lubricous fluid such
as polyethylene glycol can be injected into the space
between inner wall 56 and outer wall 57 of the
double-walled section to provide the lubricous
characteristic of hose 55 in place of adding a
lubricous hydrophilic material to hose 55 as
described above.
To deliver stmt 10 to a treatment site in a
body vessel, stent 10 is placed in a radially
compressed state in a surrounding relationship to the
outer distal end of inner tube 30. Stmt 10 is
constrained on inner tube 30 by the double-walled
section of hose 55. It is important that stmt 10
not be confined too tightly on inner tube 30. Hose
55 should apply just enough force to stmt 10 to hold
stmt 10 in place. The double-walled section of hose
55 can be removed from surrounding relation to stmt
10 by pulling valve body 40 and proximal tube 50 in a
proximal direction. The double-walled section
"rolls" off of stmt 10. No sliding movement takes
place between stent 10 and inner wall 56 which
contacts stmt 10. Along with the movement of the
double-walled section in a proximal direction, the
distal end of stmt 10 will be exposed in a radial
direction to engagement against the wall of the body
vessel. See FIG. 13. As the double-walled section
of hose 55 continues moving proximally, more of stent
10 expands in a radial direction until the entire
length of stmt 10 is exposed and engages the wall of
a body vessel. See FIG. 14.
Lumen 35 is used to allow stmt delivery device
20 to follow a guidewire (not shown) previously
inserted percutaneously into the body vessel. Lumen
_.,.
. ~ n~ F jl, i~~~ ,~r~ .:
f.'~.
ri
;i
2158757
-16-
35 of inner tube 30 can also be used to introduce a
contrast fluid to the area around the distal end of
delivery device 20 so that the position of delivery
device 20 may be easily detected for example by using
X-ray technique.
Thus it is seen that a covered stent is provided
that maintains the patency of a body vessel and
reduces tissue ingrowth through the stmt. In
addition, a stmt delivery device is provided that
minimizes friction between moving parts and that can
deploy a covered stmt at a treatment site with
little difficulty. One skilled in the art will
appreciate that the described embodiments are
presented for purposes of illustration and not of
limitation and that the present invention is only
limited by the claims which follow.
r,ntw~f'.F'~ :~~"