Note: Descriptions are shown in the official language in which they were submitted.
WOg4/2~ PCT/GB94/00542
~8930
"
TOPICAL HYPERBARIC DEVICE
The present invention relates to topical hyperbaric
devices for treating wounds and lesions on a patient's
body by bringing a therapeutic gas into contact with the
area to be healed.
Hyperbaric apparatus for the treatment of open wounds and
lesions by application thereto of a therapeutic gas such
as oxygen, with or without pharmacologically acceptable
additives, is generally known, for example from US-A-
5154697 and US-A-4801291. Specifically it has been
discovered that the treatment of open lesions and wounds
in a hyperbaric chamber with oxygen promotes granulation,
raises capillary blood oxygen levels, and elevates the
Redox potential thereby suppressing bacterial growth.
In the above-identified prior art, a collapsible topical
hyperbaric apparatus is revealed comprising a shell
defining a substantially closed internal chamber
arrangeable between an expanded and a collapsed
configuration, said shell having an opening therethrough
communicating with said internal chamber, a ring within
said internal chamber secured to said shell about said
opening, said internal ring about said opening being
capable of conforming to a shaped surface of the patient's
body and adhering thereto, gas-introducing means for
introducing a therapeutic gas into the internal chamber
for expanding said shell from a collapsed to an expanded
configuration and pressure relief means to control flow
rate thereby to therapeutically treat a portion of the
patient's body.
~0 These arrangements were provided with strap means for
securing the hyperbaric apparatus to the body so that the
adhesive means retained a hermetic seal during the
treatment period. Generally the means for securing the
W094/2~ PCT/GB94/00542
21589~ 2
hyperbaric apparatus to the body is a strap or band, or
other means of compressing the device onto the body.
As stated in the prior art a good seal between the skin
adjacent the wound and the adhesivè layer is important
otherwise the oxygen or other therapeutic gas will tend to
leak from the device.
Previously, especially in the prior art, it was thought
necessary to provide for a through flow of therapeutic gas
such as oxygen in order to achieve good therapeutic
results. However, for reasons which are described below,
this arrangement is now not only superfluous but counter-
productive, since it tends to induce oxygen toxaemia
caused by excessive exposure to hyperbaric oxygen, where
oxygen is the therapeutic gas. Thus in the prior art
devices a certain level of leakage was in fact permissible
especially when the hyperbaric device was adhered to the
skin without a strap or band to hold the same down. In
practice in order to utilise the through flow device some
means of circulating the oxygen is required and this means
that the device is only utilisable in a hospital
environment.
Good adherence of the device to the skin is also a
function of the adhesive used. However, another important
factor lies in the design of the chambers. If reference is
made to Figure 3 of US-A-5154697 and Figure 4 of US-A-
4801291 it will be seen that once the hyperbaric device
has been inflated with therapeutic gas, it is no longer
possible to apply direct pressure to the sealing area.
Thus, once the device has been positioned and inflated,
further attempts to perfect sealing can only be effected
by applying overall pressure to the inflated device rather
than by specific point loading on a desired portion of the
adhesive band.
W094/2~ PCT/GB94/00542
21~8930
This situation becomes more important when lower volumes
of therapeutic gas are available i.e. when no significant
through flow of gas is envisaged after application, or
when only a limited quantity of therapeutic gas is
available.
One object of the present invention, therefore, is to
provide an arrangement allowing said adhesive layer to be
resealed during use without affecting the inflated sheet
material adversely.
Morphologically the most beneficial effects of hyperbaric
gas treatment, and particularly hyperbaric oxygen
treatment, is the stimulation of endothelial cell
proliferation and generation of new blood capillaries. New
blood capillaries can in fact be seen by with naked eye
during successful treatment as early as two days after the
commencement thereof. These new capillaries are stimulated
in mild hypoxia and in practice at pressures of up to 1.03
to 1.04 atmospheres for a predetermined period. Above and
below this range of values the wound is not beneficially
effected. ~xygen toxicity is a condition resultant from
excess oxygen exposure and results in distortion of the
Krebs cycle which directly or indirectly leads to
degeneration of the endothelial cells and destruction of
newly formed capillaries stimulated by the hyperbaric
therapy.
Another aspect of the present invention is to be found in
the requirement that whereas the wound area in the skin is
exposed to therapeutic gas, unaffected areas are covered
~ preferably by the adhesive layer. This allows the
therapeutic gas to act directly on the wound area while
also allowing the adhesive to overlay a maximum area of
undamaged adjacent skin.
W094/2~ PCT/GB94/00542
21~89~ 4
Another object, therefore, is to provide a topical
hyperbaric device which can be readily adapted to locate
precisely over an irregularly shaped wound so that (a) the
adhesion of the device to the skin is not unnecessarily
impaired or reduced so that excess leakage of therapeutic
gas is avoided.
It has recently become apparent that for neo-
vascularization and wound healing, oxygen pressures of
about 1.03-1.04 atmospheres are required rather than the
2-3 atmospheres previously used. Also, because of the
problems associated with excess oxygen exposure, it is
usual to treat patients for comparatively short periods,
for example 20 minutes twice a day to about four hours
once a day for up to four days with a three or four day
rest period per week. Even so, some toxicity effects may
be observed after eight weeks of such a regimen.
It follows that hyperbaric oxygen therapy requires a
delicate balance between enough therapeutic gas to cause
revasculisation etc., and avoiding excess therapeutic gas
exposure. It is therefore both unnecessary and unwise to
provide for oxygen through-flow since this not only
unnecessarily complicates the device and support surfaces
associated with it, but also tends to increase oxygen
toxaemia.
For these reasons a single charge of therapeutic gas such
as oxygen is usually enough for therapeutic purposes so
long as the adhesive skin adhesion is leak-proof. I a
preferred arrangement means are provided to purge the
applied device of ambient air.
It is also an object of this invention to provide a
hyperbaric chamber which can be readily self-applied in
the home if appropriate. To this end it is, in practice,
necessary to provide an economic system which is readily
94/2l323
I~CI`~ 94/0~542
- ~15893~
applied by those who are essentially unskilled. This
requires both an economic, readily self-applied chamber
;and a source of pressurised therapeutic gas such as oxygen
in a readily hand-portable form. In practice, once a
topical hyperbaric device has been removed the adhesive
properties thereof are usually too poor for the device to
be reaffixed. Thus it is usual to replace it with a new
device.
~ u9, t~e .ir)velltiol~ furtllel- o~ject ;rl t~le ~r~vlsioll
lo of al) assemb.ly Or ~ topical llyp~rb~ric ~vi.c~ wit~ a llan~-
por-table source o~ t)lerapeutic ~as sucll a~ oxygell, w~lic
assem~Jy allow~ tlle topical llyperb~ric devi.ce to be
char~ed Wit~l ~ des.ired pressilre (e.g. 1.~ atmos) witllout
necessarily requ;ring the use of a pressure gauge. Of
course, a pressure gauge call be a~plied ~o a hal~-portable
gas cylillder and arrange~ suc~l tl~at 1.4 atmosplleres can be
readily read Or~ from a desired value.
_ _
US-A-4224941 provides a topical hyperbaric device comprising
a gas diffusion resistant flexible and/or resilient sheet
material including an adhesive layer adapted to affix to the
skin, said adhesive layer surrounding a treatment area, at
least one release sheet disposed over the adhesive layer and
a conduit for the supply of a therapeutic gas to the treatment
area, the sheet material being formed of a generally planar
sheet.
The present invention is characterized in that the adhesive
layer is disposed upon the intended skin contact portion of
the sheet, said release layer at least overlaying said
adhesive face, and in that the hyperbaric device is adapted
to retain a single charge of therapeutic gas over a treatment
period.
The sheet material may be formed with a blister to
~v
AMENDED SHE~T
IPEA/EP
Z15893~
-- 6
accommodate a predetermined volume of therapeutic gas without
stressing the interface in use between the skin and the
adhesive layer.
5 Said US-A-42Z4941 provides the features given above, and
additionally discloses that the sheet material is in the form
of a inflatable bag, and that the adhesive layer is disposed
about the peripheral underside portion at least of the portion
of the bag adjacent the treatment area.
A second aspect of the invention is characterised in that said
skin contact portion of the adhesive layer further, supports
an external pressure ring about the treatment area adjacent
the adhesive layer and independent of the sheet material,
which ring allows pressure to be exerted towards the interface
between the skin and the adhesive layer in use, and in that
the hyperbaric device is adapted to retain a single charge of
therapeutical gas over a treatment period.
In a third aspect of the invention there is provided an
assembly of a topical hyperbaric device comprising a gas
diffusion resistant flexible and/or resilient sheet material
including an adhesive face adapted to affix the sheet material
to the s~in, said adhesive face surrounding the treatment
area, at least one release layer disposed over said adhesive
face, a conduit for the supply of therapeutic gas to the
treatment area, and a readily hand-portable canister of
therapeutic gas for operative inter-connection with said
conduit.
A final aspect of the present invention is characterised in
that the release layer extends over a major proportion of the
treatment area prior to use, and wherein the wound shape is
sized- from the release layer or a gauging layer attached
thereto prior to use, whereby the device adheres to the skin
adjacent the wound so that the device adheres over as large
an area of skin as possible.
AMENDED SHEET
IPEA/~P
2~8931~
- 6 ~
The sheet material may optionally be formed into a bag with
an opening defined by the adhesive means in the form of a ring
S for disposition about the treatment area. A moist dressing
material may be disposed on a portion of
AMENDED SHEE~T
IP~ P
W094/2~ ~ 16 8 9 3 ~ PCT/GB94/00542
the internal face of the bag which is opposed to the
opening so that the dressing overlays the treatment area
only in the substantial absence of hyperbaric pressure.
The adhesive means may be formed of a flexible/resilient
sheet coated on both surfaces with an adhesive, for
example a thin hydrocolloid having a thickness of 0.2 mm
to 0.65 mm and preferably about 0.45 mm. The release sheet
may be a silicone coated release paper for removal prior
to adhesion to the skin.
In a preferred form of the invention the adhesive is
double-sided and has the release sheet secured to one
surface thereof. To the other surface is secured the inner
edges of the flexible/resilient bag so as to secure a firm
seal between the flexible/resilient material and the
adhesive layer. Most preferably the bag is secured to the
adhesive means adjacent the intended aperture to the
treatment area, but is not secured over the whole outer
area of the adhesive means. The adhesive means preferably
extends therefore outwardly of its point of adherence with
the bag, said adhesive means over this portion being
covered by a second annulus so that the area around the
aperture can be secured to the skin without direct contact
therewith.
The present invention also seeks to improve upon prior art
devices and to provide an economic alternative to the
foregoing, by providing a topical hyperbaric device formed
from a single operative sheet of flexible and/or resilient
plastics material provided with adhesive to at least a
- portion of one face thereof. Accordingly in this aspect,
the hyperbaric device in accordance with the present
invention has the general conformation of a traditional
bandage or wound dressing but is provided with means for
the supply of a therapeutic gas to a treatment area.
W094/2~ PCT/GB94/00542
215~93~ 8
The devices of the present invention are economical to
manufacture and because they utilise comparatively small
volumes of therapeutic gas are particularly adapted for
out-patient treatment which has hitherto not been
generally available with the devices of the prior art.
The gas diffusion resistant flexible and/or resilient
material is preferably a transparent plastics material
such as polyalkylene or PVC sheet with an adhesive face
over part or all thereof. Other materials utilised in the
art for adhering bandages for wound dressings etc. are
generally suitable for this. For example, they may
comprise a thin hydrocolloid adhesive having a thickness
of 0.20-0.60 mm (preferably 0.45 mm) backed by a 50
polyalkylene film, such as polyethylene.
The adhesive may extend over all of the operative face of
the sheet material, in which case a dressing material may
be adhered to the portion of the adhesive face which forms
the intended treatment area. The dressing may be a
traditional multi-layer woven material or a
pharmacologically acceptable foam material for example.
Either may be impregnated with a pharmacologically active
material, for example an antiseptic, an antibiotic, or an
anti-inflammatory preparation, and/or may be absorbent.
The dressing may be moistened with sterile water or saline
solution. Alternatively the dressing may be a medicated
alginate dressing as known in the art. Very often the
dressing is not necessary.
The release layer is an essentially non-adhesive layer
which forms a weak bond with the adhesive disposed on the
sheet material. The release layer may be formed in a two
or three layer partially overlapped configuration whereby
the removal of one such layer allows a portion of the
adhesive face to be exposed for contact With the 5kin
about the wound. This arrangement allows the device to be
WO94/213~ 2 15 8 9~ 0 PCT/GB94/00542
correctly orientated relative to the wound and secured in
this orientation while the other release layer or layers
are removed so that the device is correctly orientated on
the skin without folds or rucks.
The flexible and/or resilient sheet material is preferably
transparent and may be planar, or may be formed by non-
elastic stretching or thermo-forming with a pocket over
the treatment area to retain a dressing therein, or merely
to provide a greater area for the containment of
therapeutic gas. In such an arrangement where the dressing
is not present, the adhesive does not extend over the
treatment area.
The conduit for the supply of therapeutic gas is connected
between the treatment area of the device and a canister of
the therapeutic gas. It is sealed into the treatment area
in a gas tight fashion, optionally by the use of an
adhesive filler such as a clear silicone filler.
In a preferred form of the invention the conduit is
comprised of a length of a flexible transparent
pharmacologically acceptable plastics tubing, for example
Trygon (Registered Trade Mark), R-3603. In such an
arrangement an aperture is formed in the sheet material in
the treatment area, the end of the conduit is passed
through said aperture and the conduit is secured to the
exterior face of the sheet material by means of a
secondary layer of an adhesive sheet material disposed
over the conduit and adjacent portions of the exterior
face of the sheet material to secure the conduit. In order
to ensure a fluid-tight seal it may be necessary to coat
the exterior of the conduit with a secondary adhesive
layer such as a silicone adhesive.
One of the reasons that hyperbaric therapeutic gas devices
have been restricted in use is that the cylinders of
WO 94/2L~23 PCT/GB94/005n
21~89~ 10
therapeutic gas tend to be heavy and require man-handling
to the bedside. Alternatively sophisticated conduiting is
re~uired in a hospital to deliver the therapeutic gas to
the bedside. This has necessitated the utilisation of a
hospital bed for hyperbaric treatment.
The devices of the present invention are suitable for use
in out-patient departments because the applicants also
provide a readily hand-portable therapeutic gas cylinder
for utilisation with the hyperbaric device of the present
invention.
The hyperbaric gas is usually oxygen, but may be other
gases, for example an inert gas such as nitrogen, and may
comprise other additives if therapeutically indicated.
The hand-portable canisters in accordance with the present
invention may be a one-shot canister with an outlet
adapted for interconnection with the conduit and means
being provided to release pressurized therapeutic gas in
the one-shot canister at least to the conduit. There is
sufficient gas in the one-shot canistér to fill the
treatment area once. The conduit may then be tied to
prevent gas seepage, or the device may be provided with a
one way valve or clamp to the same end.
Further, the canister or other device may be provided with
means to supply the therapeutic gas to a treatment area at
a predetermined pressure value, for example 1.03- 1.04
atmospheres. This may be effected by including a pressure
responsive layer in the device which changes colour with
changes in pressure.
In a preferred form of the invention the sheet material is
provided with a closeable vent, the vent may comprise at
least one aperture and preferably a plurality of small
apertures formed, for example, by laser drilling, and
WO94/213~ 21~ ~ 9 3 ~ PCT/GB94/00542
_
11
overlaid to the desired extend with a gas diffusion vent
layer, whereby temporarily pealing back the vent layer
allows gas to escape from the device.
This arrangement allows ambient air from the affixed
device to be vented to the exterior prior to use and for
limited purging of the device with the therapeutic gas
before the gas pressure is increased to operational
values. With the correct selection of adhesives for the
vent layer it can be arranged that the vent layer will
separate from the sheet material in use if the interior
pressure of therapeutic gas exceeds a preset value. This
provides a means for protection against inadvertent over-
pressurisation and hence the formation of leakage path
between the skin and the overlaying adhesive layer as will
be formed by excessive internal pressures, may be avoided.
Alternatively the therapeutic gas canister may be of the
multi-shot type, in which case a pressure actuated valve
on said canister may be utilised to control the
therapeutic gas supply and preferably its pressure via the
conduit to the treatment area. Alternatively the vent
arrangement alluded to above may be used. Again, means
may be provided to prevent seepage cf the gas via the
conduit in use when the canister is removed. This may, as
previously stated, be effected by means of clamps, tying
or by means of the use of one way valves within the
device.
The devices according to the invention may be provided
with a moisture indicator within the device; such a
moisture indicator being secured within the device or
attached to the dressing so that it changes colour as it
dries. This enables the dressing, where used, to be
replaced or re-moistened as necessary. The devices of this
invention are useful in the treatment of conditions such
as leg ulcers, wounds, post-operative
WOg4/2~ PCT/GB94/00542
215~93 12
lesions/wounds,haematomas, burns, skin grafts, sports
injuries and frost bite. Specific conditions successfully
treatable by the devices in accordance with the present
invention include osteomyelitis, burns and scalds,
necrotizingfaciitis, pyoderma gangreneosum, refractory
ulcers and diabetic foot ulcers.
Of these pyodermic gangreneosum responds dramatically to
hyperbaric therapy.
The devices according to the invention may be configured
in any convenient size, for example from about 1 cm in
diameter or diagonal, to body sized dependent on the
intended treatment. The device may be designed to cover a
square, rectangular, ovoid or circular or irregularly
shaped treatment area as desired.
One aspect of the present invention is also directed to
devices in accord with the present invention incorporating
a secondary gas cushion. Such a gas cushion is adapted to
protect the device when the same has been applied to an
area
such as an elbow, knee or heel against inadvertent knocks.
According, therefore, to a further feature of the
invention there is provided a secondary gas diffusion
resistant layer overlaying the primary gas diffusion
resistant layer, said secondary layer being formed of a
flexible and\or resilient sheet material secured about
said primary layer by an adhesive facing, said secondary
layer being provided with a secondary conduit for supply
of a gas, optionally a therapeutic gas, after or
simultaneously with, the supply of the therapeutic gas to
the treatment area.
The device may comprise means, for example an LCD chip or
a pressure-activated strip material which changes colour
~V094/21323 2 1 5 8 ~ 3 0 I'~I(,r`94/~0~42
-
1~
or form on an illcrease in pressure; tl~ereby to iJl~icate a
~aximum fill con~ition, or to indicate t~at furtller
therapeutic gas is re~uired.
The invention wil] now be describe~, by way of
illustration only, witll reference to tlle accompanying
drawings whereill:
Fiqure 1 shows a vertical cross-section througll a device
of the invention from a first direction,
Fiqure 2 sllows a vertical cross-section tllrougl~ a device
of tlle illventior~ Lrom a second directioll,
Fiqure 3 sllows a vertical cross-sectioll tllrough a device
according to ~igure 1 i.n situ over ~ lesion,
Fiqure 4 shows a vertical cross-section tllrougll a device
in accordance wittl ~igures 1 and 2, b~lt incorporating a
one-way valve,
Fiqure 5 shows a diagrammatic side view from above oE a
rectallgular device according to ~igure 1.
Fiqure 5~ shows a cross-section tl~rougt~ the vent yortior
of Figure 5,
Fiqure 6 shows a vertical cross-section througll anotller
device according to the invention;
Fiqure 7 shows a vertical cross-section througl~ the
devices o~ Figure 6 inflated over a lesion, and
Fiqure ~ sl~ows a plan view from below o~ tlle device as
shown in Figure 6 prior to removal of tlle release slleet.
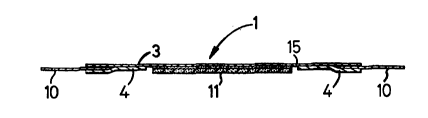
With reference to Figures 1 to 5 of t~le accompanyillg
drawings a topical hyperbaric device (1) is provide~ Wittl
a flexible plastics transparent sheet material in tlle
general form of a rectangle or square having a dimensio
3~ of 100 mm. 'I'lle slleet material (3) is formed over tl~e
entirety of its lower surface wittl an ad~lesive layer (3A),
whereas the upper layer is essentially non-ad~lesive.
Cenrally disposed Otl l;lle Utlder6~de C)~ tlle 611eet mateL ial
(3) is an optional dressing (11) formed of a foamed
material moistened by sterile water and\or Witll a
AMEND D SHEE~T
IPEA/EP
~94t2132~ 2 1 5 8 9 3 JC~ 00542
p~)armacologica]ly acceptable alginate.
Io t)~e ullderside of t11e edges of t1~e 6~1eet materi.al (3) is
applied ~ primary relea~e ~a~er (~) wl~ic1 is secure~ to
the adhesive undersidé (3A) of the sheet material (3) and
generally conform~ to t~le exterior ~erip~lery t~1ereo~.
11owever in tlle arra1lyeme1lt as s11ow1~ ln Figure I tlle edge
portions of tlie primary release layer (~) do 1-ot adllere to
t~le edge portiorls o~ t~le ulldersi~e Or tt1e .slleet material
(3) but are left free. ~ secol~clary relea~e ]aye~ (l(l) is
secure~l ~o eacl) ~ara]l.el edge of ~he ~l1eet ma~erial (3).
With reEerel)ce par~;cula~ly to ~igl1re ~ it wil.L ~e noted
t~at t~1e dres~ir~g (l~) is pierce-l t~y ~l1e en~1 oE a col1duit
(5) Wil;.C~I extellds ~etweel~ the valve (6) Or calli.ster (2) to
the treatment area (16). Tile conduit (5) i.s ~ecured to tl1e
exterior face o~ tlle slleet material (3) by mealls of an
overla~ (7) preEerably formed of t~)e same material as tlle
s~eet material (3) with an ad~lesive un~erside t~1ereon.
r~le overlay (7) secures tl1e condui.t (5) to tlle exterior
face Or t~le sl1eet material (3) i11 a fluid tigllt ~as~1ion.
In order to reinEorce the fluid tigtlt efFect an ad~lesive
silicone filler may be applied to tlle exterior of ~le
conduit prior to adllesion of t11e overlay (7) to ensure
tl)at tlle col)duit is truly secured an(l tilat no gas seepage
can occur.
In an alternative form of the invention tl1e arrangeme1lt of
Figure 2 is repeated but tlle dressing (ll) wl1eJl present
is cut away over a portion so tl1at a one way valve (14)
may be inserted to prevent seepage of tile oxygen in the
reverse sense witllir) the conduit (5). The one way valve
~l4) may be a flexible sheet secured to tile under adllesive
face oE the slleet material (3) but provi~ecl Witll an
opening w11ich is securely closed by means of gaseous back
pressure.
AMENDED SHEET
IP~ P
WO94/213~ 21~ ~ 9 3 U PCT/GB94/00542
._ .
A general arrangement of the device is shown in Figure 5
which shows the device connected diagrammatically to a
hand-portable therapeutic gas cylinder wherein the valve
(6) has yet to be operated such that the hyperbaric device
(1) lies flat upon a flat surface.
With reference particularly to Figure 3 the hyperbaric
device in accordance with the present invention is shown
in diagrammatic form in situ upon the skin. Initially hair
is removed from about the wound site so that the adhesive
face of the sheet material (3) can firmly adhere to the
skin in a fluid-tight fashion. The device, as shown in
Figure 1, is then presented to the wound (13) in the skin
(12) and positioned generally thereover. It will be
appreciated that, as shown in Figure 1, the transparent
sheet (3) and the release layers (4, 10) result in an
arrangement where it is not possible to be sure that the
device is correctly orientated. For this reason a gap (15)
is left in the device between the peripheral edge of the
dressing (11) and the inner edge of the release layer (4).
Since this gap (15) is transparent it is possible to
ensure that the device is correctly orientated. With the
device held in position from one end, one of the release
layers (10) may be removed and the edge of the release
layer (4) folded back so that an adhesive edge of the
sheet material (3) may then be correctly orientated upon
the skin. When this has been pressed into firm adherence,
the release layer (4) and the remaining release layer (10)
are stripped away such that the sheet material (3)
adhesively overlays undamaged skin without rucks, creases
or gas releasing discontinuities.
With the device tl) located over the lesion (13) on the
skin (12) the conduit (5) is connected to the valve (6) of
the canister (2). The valve (6) is actuated to allow
sterile oxygen to pass into the treatment space (16)
whereby the dressing is raised from the lesion (13). With
WO94/21323 2 i ~ ~ 9 3 ~ 94/00542
lG
a or1e-s11ot canis~er (2) t~lere is alw~ys t1~e pos~ibility
t~lat ~)e oxyge~ providecl will exceed tlle volume of tlle
treatment ~pace (16) w.ithout raisi1lg this are~ t~ too 11i.gl
a pressure. For tll;.s reasorl ar1 operational vel1t: (P) may be
ir1corporated as s11ow11 in Fi~ures 5 al1d 5~. Ver1t (8)
comprises a plurality of laser drilled apert~1re~ (8~)
throug11 the slleet material (3) a1ld an aclhes.ive resea].able
ver1t layer (aB) w11ic11 can be ma1-ually li.fted ar~d replaced.
l`his allows tlle ~evice to be ~uryed of ambie1lt air after
lo fi~.illq alld l~l-iol- to fi11~ r~ g ~ith ~xygell
W11ere a o1-e-s1~ot cal1i~ter (2) ;~ uti.lised it m~r com~rise
a valve w11ic11 is operated by ma11ual. pre~sure ~ 1 llellce it
i.s possible to i11f1.ate tlle treatme11t ~pace (~ ) to tlle
required degree al1d to top up as required for a sirlgle ~se
o~ tlle device. ~ mult.i-s~1ot cal1i.ster car1 also br~ used for
more t~1an devi.ce and is similarly c~esigned
It is sometimes desirable that the dressing slloul~ be
moist and s~1ould be provided Wit~1 suitable ~herapeutic
adjuncts ~or t11e treatment of tlle lesion concerlled.
ZO ~lternatively, a desired level o~ moisture may be
incorporated in tlle therapeutic gas.
Tl~e pressure ring is provided wit11 a generally central
aperture and a plurality of concentric marki ngs to assist
wound prori]ing. ln use tlle wound sllape is cut from tl1e
çombined pressure ring and release layer, tl1e orientation
o~ tlle device cllecked on the patient, al~d t~lell t~le release
layer is removed and the adhesive race o~ tlle pressure
ring adheres to the s~in as would tl1e adhesive layer in
AMFNDE~ SHE~T
IP~ ~P
, g4/2132~ 2 1 ~ 8 ~ 3 0 I~{~ 94~00s42
-
1.7
the earlier descri~ed embodiment.
'I'urnillg now to Figures 6 to 8 o~ t~le accompanyillg
drawings, tilere is provided a secoll~ form of llyperbaric
device provided optionally wit~ an illterllal dressillg. ~litl
particular referellce to t~-e topical ~lyperbaric device (2~)
there is provided a flexible P~C slleet l~avir~g a qenerally
rectilinear col-~iguration as SI~OWIl i-l~ Figul-e ~. 'Ille ~'C
sheet is essentially ~lexible alt~ou~h it ~la~ a degree of
resil;el~ce an~l ;r~ tllis case i~ a ~00 grade caclmium an~
lead-free di.-decyl pllt~lalate plasticized calelldered PVC of
~ type generally u~ed for uri~le bags. 'llle ~vC 6lleet (21)
enclose~ a ilyper~ric ~pace (30) but is, in it~ pre-u~e
conditiotl, qerler~lly flat a~d e-)c.lo~e~ witllill a sterile
package.
Tl-e PVC sheet materi.al (21) may optionally be formed
internally witll a generally square dressing (~7) whicll is
of the alginate type and retains a desired amoullt of
sterile water. The central lower portion of the sheet material
(21) is formed with an aperture (31) which is opened on
removal of the release layer (23). The lower ~aces of the PVC
sheet material are pressed into contact with a double-sided
adhe~ive layer (22) to which they are firmly secured. The
outboard edqes of the double-sided adhesive layer (22) are
also covered with a release layer (23) so that the outer
portions of the double-sided adhesive layer (22) do not adhere
to the outer lower edges of the sheet material (21).
release sheet (23) overlays t~le double-sided adhesive
layer, said release s1~eet being a silicone-coated release
paper in accordance witl~ the knowll prior art. 'I`lle double-
sided adhesive layer (22) has an adllesive of a tllickness
of about 0.6 mm overlaying a central polyalkylene or PVC
film.
Wit~l particular reference to Figure 8, t~le release slleet
AMENDED SHEE~T
IPEA/EP
-~94121323 Z1~ g9~ ~ rc r /c~ns4/nos~2
ln
(23) w~iC~1 over.1.ay~ t~1e double-sided adl1esive 1ayer (22)
is also provide~ Witl1 a target gri.d (33) printed
t~lereuE~on .
Similar to t~1e arrar~gement as StlOW~ .igures ~ ~rd 4
Figures 5 and 6 are provided witl~ a conduit (~5) to admit
hyperbaric oxygen. 6aid conduit is secured to tllo outer
upper face o tl1e material (21) ~y means Or a ColldU.it
overlay (26) careful]y secured as previously described
before.
In use tl~e release layer (~3) wlli~ sllou]d be tr~n~arent
and/or at 1.~a~t tt-~r~luce1lt is tem~?orarily wit1draw1~ from
t~le devi~e (Z()) a11d p1ace~ over t11~ wo~11lc1 or l l~e le~ioll.
lhe release layer (23) is provi~e~ as S~10W11 i11 ~iyure 8
with a tar~et grid and from t1~is grid it ca-l bo readily
seen w~lere tlle lesion is relative to the aperture (31).
parame~ic can ti~en draw an outli11e of t1~e wou~d 011 the
release s~leet relating its centre to tlle centre of t~le
grid (33) w~lereupo1l t~1e release s~1eet (23) is ot~ce more
laid upon the double-sided release layer (22). ~i.t11 tl~is
2~ ligl1tly adllered t1~eret:o the paramedic may t1~en cut round
the wound site so tl1at tl~e aperture formed in t~le device
(20) corresponds to tl)e edges of tlle wound or lesion to
w11ic11 it is to be applied. 111e remaining portions of t1~e
release slleet are t11e1l removed and the device located over
tl~e wound or lesion. 1`his is assisted because t~1e PVC
material (21) is transparent so tllat tlle wound can be
accur~ately ~ligned wit}1 the aperture formed ror it.
External pressure portions (24) are then pressed firmly into
contact with the skin (28) about the lesion so that the formed
aperture (32) is retained about the wound.
11yperbaric oxygen is the1l introduced via conduit (25) from
a-hand por~able oxygen cylinder ~2) 60 t~lat tll~ ~evice
formed tlle shape somewhat as s1~own ill Figurè 6. Care must
be taken not to overinflate tl1e device since tl1i6 may put
AMENDED SHEE~T
IPEA/EP
~094/21323 2 1 ~ 8 9 ~ 9 ~ 94/00542
an undue strain 011 tl1e adl)esive bol-d between tlle ski~ 8)
and tl1e device (2()). I{owever witll tlle l-al-d portable
device it is possible to top up tlle hyperbari 5 ~ressure as
necessary to a value usual~y Or about 1.03 to l.O4
atmosp1~eres.
Devices in accorclance wit11 tl~e present invel-ltioll may be
applied sucl1 as to recluce hypoxia but`avoid oxygen
toxaemia. Iypical regimens or 20 minutes twice a day, to ~
to 6 )lour treatments for ~ days o~ a week allow wounds and
other lesions to cure quickly.
It will be ap~)rec;~ted tl~at ~ecall~e tlle tllera~eutic gas
canisters are l~and-portable and because tlle devices in
accorclance wit1~ tl~e present inverltioll are essentially
disposable tlle assemblies in accordance witll tle present
inventiorl may be utilised 011 an out-patient basis and even
applied and utilised by motivated patients on some
occasions wit1~out professiol~al medical intervention.
Accordingly tlle arrangements of tlle present invelltioll are
cl1eap enougll and easy enougl- to use to bring l~yperbaric
treatment more widely illtO use.
The invention provides, tllerefore a topical llyperbaric
device and an assembly of a topica1 1~yyerbarlc clevice wit~
a readily 1~and-portable canister of tl~erapeutic gas suc1
as oxygen .
AMEND~D SHEET
IP!E~A/EP