Note: Descriptions are shown in the official language in which they were submitted.
CA 02159005 2002-04-18
1
"METHOD AND APPARATUS FOR ENCAPSULATION OF
BIOLOGICALLY-ACTIVE SUBSTANCES IN CELLS"
Technical Field
The present invention relates to methods and apparatuses for the encapsulation
of
biologically-active substances in various cell populations. More particularly,
the present
invention relates to a method and apparatus for the encapsulation of
allosteric effectors of
hemoglobin in erythrocytes by electroporation to achieve therapeutically
desirable changes
in the physical characteristics of the intracellular hemoglobin.
Background of the Invention
In the vascular system of an adult human being, blood has
a volume of about 5 to 6 liters. Approximately one half of this
volume is occupied by cells, including red blood cells (erythrocytes), white
blood
cells (leukocytes) and blood platelets. Red blood cells comprise the majority
of the
cellular components of blood. Plasma, the liquid portion of blood is
approximately
'0 94/21117 PCT/US94/03189
2159005
2
90 percent water and 10 percent various solutes. These solutes
include plasma proteins, organic metabolites and waste products,
and inorganic compounds.
The major function of red blood cells is to transport
oxygen from the lungs to the tissues of the body, and transport
carbon dioxide from the tissues to the lungs for removal. Very
little oxygen is transported by the blood plasma because oxygen is
only sparingly soluble in aqueous solutions. Most of the oxygen
carried by the blood is transported by the hemoglobin of the
erythrocytes. Erythrocytes in mammals do not contain nuclei,
mitochondria or any other intracellular organelles, and they do
not use oxygen in their own metabolism. Red blood cells contain
about 35 percent by weight hemoglobin, which is responsible for
binding and transporting oxygen.
Hemoglobin is a protein having a molecular weight of
approximately 64,500. It contains four polypeptide chains and
four heme prosthetic groups in which iron atoms are bound in the
ferrous state. Normal globin, the protein portion of the
hemoglobin molecule, consists of two a chains and two B chains.
Each of the four chains has a characteristic tertiary structure in
which the chain is folded. The four polypeptide chains fit
together in an approximately tetrahedral arrangement, to
constitute the characteristic quaternary structure of hemoglobin.
There is one heme group bound to each polypeptide chain which
can reversibly bind one molecule of molecular oxygen. When
hemoglobin combines with oxygen, oxyhemoglobin is formed.
When oxygen is released, the oxyhemoglobin is reduced to
deoxyhemoglobin.
Delivery of oxygen to tissues depends upon a number of
factors including, but not limited to, the volume of blood flow,
the number of red blood cells, the concentration of hemoglobin in
the red blood cells, the oxygen affinity of the hemoglobin and, in
certain species, on the molar ratio of intraerythrocytic
hemoglobins with high and low oxygen affinity. The oxygen
affmity of hemoglobin depends on four factors as well, namely:
(1) the partial pressure of oxygen; (2) the pH; (3) the
"0 94/21117 PCT/US94/03189
2159005
3
concentration of 2,3-diphosphoglycerate (DPG) in the
hemoglobin; and (4) the concentration of carbon dioxide. In the
lungs, at an oxygen partial pressure of 100 mm Hg,
approximately 98% of circulating hemoglobin is saturated with
oxygen. This represents the total oxygen transport capacity of the
blood. When fully oxygenated, 100 ml of whole mammalian
blood can carry about 21 ml of gaseous oxygen.
The effect of the partial pressure of oxygen and the pH on
the ability of hemoglobin to bind oxygen is best illustrated by
examination of the oxygen saturation curve of hemoglobin. An
oxygen saturation curve plots the percentage of total oxygen-
binding sites of a hemoglobin molecule that are occupied by
oxygen molecules when solutions of the hemoglobin molecule are
in equilibrium with different partial pressures of oxygen in the
gas phase.
The oxygen saturation curve for hemoglobin is sigmoid.
Thus, binding the first molecule of oxygen increases the affinity
of the remaining hemoglobin for binding additional oxygen
molecules. As the partial pressure of oxygen is increased, a
plateau is approached at which each of the hemoglobin molecules
is saturated and contains the upper limit of four molecules of
oxygen.
The reversible binding of oxygen by hemoglobin is
accompanied by the release of protons, according to the equation:
HHb+ + 02 Hbp2 + H+
Thus, an increase in the pH will pull the equilibrium to the right
and cause hemoglobin to bind more oxygen at a given partial
pressure. A decrease in the pH will decrease the amount of
oxygen bound.
In the lungs, the partial pressure of oxygen in the air spaces
is approximately 90 to 100 mm Hg and the pH is also high
relative to normal blood pH (up to 7.6). Therefore, hemoglobin
will tend to become almost maximally saturated with oxygen in
the lungs. At that pressure and pH, hemoglobin is approximately
98 percent saturated with oxygen. On the other hand, in the
'0 94/21117 PCT/US94/03189
2159005
4
capillaries in the interior of the peripheral tissues, the partial
pressure of oxygen is only about 25 to 40 mm Hg and the pH is
also relatively low (about 7.2 to 7.3). Because muscle cells use
oxygen at a high rate thereby lowering the local concentration of
oxygen, the release of some of the bound oxygen to the tissue is
favored. As the blood passes through the capillaries in the
muscles, oxygen will be released from the nearly saturated
hemoglobin in the red blood cells into the blood plasma and
thence into the muscle cells. Hemoglobin will release about a
third of its bound oxygen as it passes through the muscle
capillaries, so that when it leaves the muscle, it will be only about
64 percent saturated. In general, the hemoglobin in the venous
blood leaving the tissue cycles between about 65 and 97 percent
saturation with oxygen in its repeated circuits between the lungs
and the peripheral tissues. Thus, oxygen partial pressure and pH
function together to effect the release of oxygen by hemoglobin
A third important factor in regulating the degree of
oxygenation of hemoglobin is the allosteric effector
2,3-diphosphoglycerate (DPG). DPG is the normal physiological
effector of hemoglobin in mammalian erythrocytes. DPG
regulates the oxygen-binding affinity of hemoglobin in the red
blood cells in relationship to the oxygen partial pressure in the
lungs. The higher the concentration of DPG in the cell, the lower
the affulity of hemoglobin for oxygen.
When the delivery of oxygen to the tissues is chronically
reduced, the concentration of DPG in the erythrocytes is higher
than in normal individuals. For example, at high altitudes the
partial pressure of oxygen is significantly less. Correspondingly,
the partial pressure of oxygen in the tissues is less. Within a few
hours after a normal human subject moves to a higher altitude,
the DPG level in the red blood cells increases, causing more DPG
to be bound and the oxygen affinity of the hemoglobin to
decrease. Increases in the DPG level of red cells also occur in
patients suffering from hypoxia. This adjustment allows the
hemoglobin to release its bound oxygen more readily to the
tissues to compensate for the decreased oxygenation of
WO 94/21117 PCT/US94/03189
2159005
hemoglobin in the lungs. The reverse change occurs when people
acclimated to high altitudes and descend to lower altitudes.
As normally isolated from blood, hemoglobin contains a
considerable amount of DPG. When hemoglobin is "stripped" of
5 its DPG, it shows a much higher affinity for oxygen. When DPG
is increased, the oxygen binding affinity of hemoglobin decreases.
A physiologic allosteric effector such as DPG is therefore
essential for the normal release of oxygen from hemoglobin in
the tissues.
While DPG is the normal physiologic effector of
hemoglobin in mammalian red blood cells, phosphorylated
inositols are found to play the same role in the erythrocytes of
some birds and reptiles. Although IHP is unable to pass through
the mammalian erythrocyte membrane, it is capable of combining
with hemoglobin of mammalian red blood cells at the binding site
of DPG to modify the allosteric conformation of hemoglobin, the
effect of which is to reduce the affinity of hemoglobin for
oxygen. For example, DPG can be replaced by inositol
hexaphosphate (IHP), which is even more potent than DPG in
reducing the oxygen affmity of hemoglobin. IHP has a 1000-fold
higher affmity to hemoglobin than DPG (R.E. Benesch et al.,
Biochemistry, Vol. 16, pages 2594-2597 (1977)) and increases the
P50 of hemoglobin up to values of 96.4 mm Hg at pH 7.4 , and 37
degrees C(J. Biol. Chem., Vol. 250, pages 7093-7098 (1975)).
The oxygen release capacity of mammalian red blood cells
can be enhanced by introducing certain allosteric effectors of
hemoglobin into erythrocytes, thereby decreasing the affinity of
hemoglobin for oxygen and improving the oxygen economy of
the blood. This phenomenon suggests various medical
applications for treating individuals who are experiencing
lowered oxygenation of their tissues due to the inadequate
function of their lungs or circulatory system.
Because of the potential medical benefits to be achieved
from the use of these modified erythrocytes, various techniques
have been developed in the prior art to enable the encapsulation
of allosteric effectors of hemoglobin in erythrocytes.
'VO 94121117 PCT/US94/03189
2159OD5
6
Accordingly, numerous devices have been designed to assist or
simplify the encapsulation procedure. The encapsulation methods
known in the art include osmotic pulse (swelling) and
reconstitution of cells, controlled lysis and resealing,
incorporation of liposomes, and electroporation. Current
methods of electroporation make the procedure commercially
impractical on a scale suitable for commercial use.
The following references describe the incorporation of
polyphosphates into red blood cells by the interaction of
liposomes loaded with IHP: Gersonde, et al., "Modification of the
Oxygen Affinity of Intracellular Haemoglobin by Incorporation
of Polyphosphates into Intact Red Blood Cells and Enhanced 02
Release in the Capillary System", Biblthca. Haemat., No. 46, pp.
81-92 (1980); Gersonde, et al., "Enhancement of the 02 Release
Capacity and of the Bohr-Effect of Human Red Blood Cells after
Incorporation of Inositol Hexaphosphate by Fusion with Effector-
Containing Lipid Vesicles", Origins of Cooperative Binding of
Hemoglobin, (1982); and Weiner, "Right Shifting of Hb-02
Dissociation in Viable Red Cells by Liposomal Technique,"
Biology of the Cell, Vol. 47, (1983).
Additionally, U.S. Patent Nos. 4,192,869, 4,321,259, and
4,473,563 to Nicolau et al. describe a method whereby fluid-
charged lipid vesicles are fused with erythrocyte membranes,
depositing their contents into the red blood cells. In this manner
it is possible to transport allosteric effectors such as inositol
hexaphosphate into erythrocytes, where, due to its much higher
binding constant IHP replaces DPG at its binding site in
hemoglobin.
In accordance with the liposome technique, IIW is dissolved
in a phosphate buffer until the solution is saturated and a mixture
of lipid vesicles is suspended in the solution. The suspension is
then subjected to ultrasonic treatment or an injection process, and
then centrifuged. The upper suspension contains small lipid
vesicles containing IHP, which are then collected. Erythrocytes
are added to the collected suspension and incubated, during which
time the lipid vesicles containing IHP fuse with the cell
'YO 94%21117 PCT/US94/03189
215-9a05
7
membranes of the erythrocytes, thereby depositing their contents
into the interior of the erythrocyte. The modified erythrocytes
are then washed and added to plasma to complete the product.
The drawbacks associated with the liposomal technique
include poor reproducibility of the IHP concentrations
incorporated in the red blood cells and significant hemolysis of
the red blood cells following treatment. Additionally,
commercialization is not practical because the procedure is
tedious and complicated.
In an attempt to solve the drawbacks associated with the
liposomal technique, a method of lysing and the resealing red
blood cells was developed. This method is described in the
following publication: Nicolau, et al., "Incorporation of
Allosteric Effectors of Hemoglobin in Red Blood Cells.
Physiologic Effects," Biblthca. Haemat., No. 51, pp. 92-107,
(1985). Related U.S. Patent Nos. 4,752,586 and 4,652,449 to
Ropars et al. also describe a procedure of encapsulating
substances having biological activity in human or animal
erythrocytes by controlled lysis and resealing of the erythrocytes,
which avoids the RBC-liposome interactions.
The technique is best characterized as a continuous flow
dialysis system which functions in a manner similar to the osmotic
pulse technique. Specifically, the primary compartment of at
least one dialysis element is continuously supplied with an aqueous
suspension of erythrocytes while the secondary compartment of
the dialysis element contains an aqueous solution which is
hypotonic with respect to the erythrocyte suspension. The
hypotonic solution causes the erythrocytes to lyse. The
erythrocyte lysate is then contacted with the biologically active
substance to be incorporated into the erythrocyte. To reseal the
membranes of the erythrocytes, the osmotic and/or oncotic
pressure of the erythrocyte lysate is increased and the suspension
of resealed erythrocytes is recovered.
In related U.S. Patent Nos. 4,874,690 and 5,043,261 to
Goodrich et al. a related technique involving lyophilization and
reconstitution of red blood cells is disclosed. As part of the
WO 94/21117 PCT/US94/03189
2159005
8
process of reconstituting the red blood cells, the addition of
various polyanions, including inositol hexaphosphate, is
described. Treatment of the red blood cells according to the
process disclosed results in a cell with unaffected activity.
Presumably, the IHP is incorporated into the cell during the
reconstitution process, thereby maintaining the activity of the
hemoglobin.
In U.S. Patent Nos. 4,478,824 and 4,931,276 to Franco et
al. a second related method and apparatus is described for
introducing effectively non-ionic agents, including inositol
hexaphosphate, into mammalian red blood cells by effectively
lysing and resealing the cells. The procedure is described as the
"osmotic pulse technique." In practicing the osmotic pulse
technique, a supply of packed red blood cells is suspended and
incubated in a solution containing a compound which readily
diffuses into and out of the cells, the concentration of the
compound being sufficient to cause diffusion thereof into the cells
so that the contents of the cells become hypertonic. Next, a trans-
membrane ionic gradient is created by diluting the solution
containing the hypertonic cells with an essentially isotonic
aqueous medium in the presence of at least on desired agent to be
introduced, thereby causing diffusion of water into the cells with
a consequent swelling and an increases in permeability of the
outer membranes of the cells. This "osmotic pulse" causes the
diffusion of water into the cells and a resultant swelling of the
cells which increase the permeability of the outer cell membrane
to the desired agent. The increase in permeability of the
membrane is maintained for a period of time sufficient only to
permit transport of least one agent into the cells and diffusion of
the compound out of the cells.
Polyanions which may be used in practicing the osmotic
pulse technique include pyrophosphate, tripolyphosphate,
phosphorylated inositols, 2,3-diphosphoglycerate (DPG),
adenosine triphosphate, heparin, and polycarboxylic acids which
are water-soluble, and non-disruptive to the lipid outer bilayer
membranes of red blood cells.
'0 94/21117 2159005 PCT1US94/03189
9
The osmotic pulse technique has several shortcomings
including low yield of encapsulation, incomplete resealing, lose of
cell content and a corresponding decrease in the life span of the
cells. The technique is tedious, complicated and unsuited to
automation. For these reasons, the osmotic pulse technique has
had little commercial success.
Another method for encapsulating various biologically-
active substances in erythrocytes is electroporation.
Electroporation has been used for encapsulation of foreign
molecules in different cell types including IHP red blood cells as
described in Mouneimne, et al., "Stable rightward shifts of the
oxyhemoglobin dissociation curve induced by encapsulation of
inositol hexaphosphate in red blood cells using electroporation,"
FEBS, Vol. 275, No. 1, 2, pp. 117-120 (1990).
The process of electroporation involves the formation of
pores in the cell membranes, or in any vesicles, by the application
of electric field pulses across a liquid cell suspension containing
the cells or vesicles. During the poration process, cells are
suspended in a liquid media and then subjected to an electric field
pulse. The medium may be electrolyte, non-electrolyte, or a
mixture of electrolytes and non-electrolytes. The strength of the
electric field applied to the suspension and the length of the pulse
(the time that the electric field is applied to a cell suspension)
varies according to the cell type. To create a pore in a cell's
outer membrane, the electric field must be applied for such a
length of time and at such a voltage as to create a set potential
across the cell membrane for a period of time long enough to
create a pore.
Four phenomenon appear to play a role in the process of
electroporation. The first is the phenomenon of dielectric
breakdown. Dielectric breakdown refers to the ability of a high
electric field to create a small pore or hole in a cell membrane.
Once a pore is created, a cell can be loaded with a biologically-
active substances. The second phenomenon is the dielectric
bunching effect, which refers to the mutual self attraction
produced by the placement of vesicles in a uniform electric field.
VO 94/21117 2159005 PCT/US94103189
The third phenomenon is that of vesicle fusion. Vesicle fusion
refers to the tendency of membranes of biological vesicles, which
have had pores formed by dielectric breakdowns, to couple
together at their mutual dialectic breakdown sites when they are
5 in close proximity. The fourth phenomenon is the tendency of
cells to line up along one of their axis in the presence of high
frequency electric fields. Thus, electroporation relates to the use
in vesicle rotational prealignment, vesicle bunching and dielectric
constant or vesicles for the purpose of loading and unloading the
10 cell vesicle.
Electroporation has been used effectively to incorporate
allosteric effectors of hemoglobin in erythrocytes. In an article
by Mouneimne, Y et al., "Stable Rightward Shifts of
Oxyhemoglobin Disassociation Constant Induced by Encapsulation
of Inositol Hexaphosphate in Red Blood Cells Using
Electroporation", FEBS, Vol. 275, No. 1, 2, pages 11-120.
Mouneimne and his colleagues reported that right shifts of the
hemoglobin-oxygen dissociation in treated erythrocytes having
incorporated IHP can be achieved. Measurements at 24 and 48
hours after loading with IHP showed a stable P50 value indicating
that resealing of the erythrocytes was permanent. Furthermore,
it was shown that red blood cells loaded with inositol
hexaphosphate have a normal half life of eleven days. However,
the results obtained by Mouneimne and his colleagues indicate that
approximately 20% of the retransfused cells were lost within the
first 24 hours of transfusion.
The electroporation methods disclosed in the prior art are
not suitable for processing large volumes of sample, nor use of a
high or repetitive electric charge. Furthermore, the methods are
not suitable for use in a continuous or "flow" electroporation
chamber. Available electroporation chambers are designed for
static use only. Namely, processing of samples by batch.
Continuos use of a "static" chamber results in over heating of the
chamber and increased cell lysis. Furthermore, the existing
technology is unable to incorporate a sufficient quantity of IHP in
a sufficient percentage of the cells being processed to dramatically
'0 94/21117 PCT/US94/03189
2159005
11
change the oxygen carrying capacity of the blood. In addition,
the prior art methods require elaborate equipment and are not
suited for loading red blood cells of a patient on site. Thus, the
procedure is time consuming and not suitable for use on a
commercial scale. What is needed is a simple, efficient and rapid
method for encapsulating biologically-active substances in
erythrocytes while preserving the integrity and biologic function
of the cells. The potential therapeutic applications of biologically
altered blood cells suggests the need for simpler, and more
effective and complete methods of encapsulation of biologically-
active substances, including allosteric effectors of hemoglobin in
intact erythrocytes.
There are numerous clinical conditions that would benefit
from treatments that would increase tissue delivery of oxygen
bound to hemoglobin. For example, the leading cause of death in
the United States today is cardiovascular disease. The acute
symptoms and pathology of many cardiovascular diseases,
including congestive heart failure, myocardial infarction, stroke,
intermittent claudication, and sickle cell anemia, result from an
insufficient supply of oxygen in fluids that bathe the tissues.
Likewise, the acute loss of blood following hemorrhage,
traumatic injury, or surgery results in decreased oxygen supply to
vital organs. Without oxygen, tissues at sites distal to the heart,
and even the heart itself, cannot produce enough energy to sustain
their normal functions. The result of oxygen deprivation is tissue
death and organ failure.
Although the attention of the American public has long
been focused on the preventive measures required to alleviate
heart disease, such as exercise, appropriate dietary habits, and
moderation in alcohol consumption, deaths continue to occur at an
alarming rate. Since death results from oxygen deprivation,
which in turn results in tissue destruction and/or organ
dysfunction, one approach to alleviate the life-threatening
consequences of cardiovascular disease is to increase oxygenation
of tissues during acute stress. The same approach is also
-VO 94/21117 PCT/US94/03189
2159005
12
appropriate for persons suffering from blood loss or chronic
hypoxic disorders, such as congestive heart failure.
Another condition which could benefit from an increase in
the delivery of oxygen to the tissues is anemia. A significant
portion of hospital patients experience anemia or a low "crit"
caused by an insufficient quantity of red blood cells or
hemoglobin in their blood. This leads to inadequate oxygenation
of their tissues and subsequent complications. Typically, a
physician can temporarily correct this condition by transfusing
the patient with units of packed red blood cells.
Enhanced blood oxygenation may also reduce the number
of heterologous transfusions and allow use of autologous
transfusions in more case. The current method for treatment of
anemia or replacement of blood loss is transfusion of whole
human blood. It is estimated that three to four million patients
receive transfusions in the U.S. each year for surgical or medical
needs. In situations where there is more time it is advantageous
to completely avoid the use of donor or heterologous blood and
instead use autologous blood.
Often the amount of blood which can be drawn and stored
prior to surgery limits the use of autologous blood. Typically, a
surgical patient does not have enough time to donate a sufficient
quantity of blood prior to surgery. A surgeon would like to have
several units of blood available. As each unit requires a period of
several weeks between donations and can not be done less than
two weeks prior to surgery, it is often impossible to sequester an
adequate supply of blood. By processing autologous blood with
IHP, less blood is required and it becomes possible to completely
avoid the transfusion of heterologous blood.
As IHP-treated red cells transport 2-3 times as much
oxygen as untreated red cells, in many cases, a physician will need
to transfuse fewer units of IHP-treaded red cells. This exposes
the patient to less heterologous blood, decreases the extent of
exposure to viral diseases from blood donors and minimizes
immune function disturbances secondary to transfusions. The
ability to infuse more efficient red blood cells is also
VO 94/21117 2159005 PCT/US94/03189
13
advantageous when the patients blood volume is excessive. In
other more severe cases, where oxygen transport is failing, the
ability to rapidly improve a patient's tissue oxygenation is life
saving.
Although it is evident that methods of enhancing oxygen
delivery to tissues have potential medical applications, currently
there are no methods clinically available for increasing tissue
delivery of oxygen bound to hemoglobin. Transient, 6 to 12 hour
elevations of oxygen deposition have been described in
experimental animals using either DPG or molecules that are
precursors of DPG. The natural regulation of DPG synthesis in
vivo and its relatively short biological half-life, however, limit
the DPG concentration and the duration of increased tissue P02,
and thus limit its therapeutic usefulness.
Additionally, as reported in Genetic Engineering News,
Vol. 12, No. 6, April 15, 1992, several groups are attempting to
engineer free oxygen-carrying hemoglobin as a replacement for
human blood. Recombinant, genetically modified human
hemoglobin that does not break down in the body and that can
readily release up to 30% of its bound oxygen is currently being
tested by Somatogen, Inc., of Boulder Colorado. While this
product could be useful as a replacement for blood lost in
traumatic injury or surgery, it would not be effective to increase
Po2 levels in ischemic tissue, since its oxygen release capacity is
equivalent to that of natural hemoglobin (27-30%). As are all
recombinant products, this synthetic hemoglobin is also likely to
be a costly therapeutic.
Synthetic human hemoglobin has also been produced in
neonatal pigs by injection of human genes that control
hemoglobin production. This may be a less expensive product
than the Somatogen synthetic hemoglobin, but problems with
oxygen affmity and breakdown of hemoglobin in the body are not
solved by the method.
What is needed is a simple, efficient and rapid method for
encapsulating biologically-active substances, such as IHP, in
erythrocytes without damaging the erythrocytes.
'VO 94/21117 PCTIUS94/03189
2159005
14
Summary of the Invention
The present invention relates to a method and apparatus for
the encapsulation of biologically-active substances in various cell
populations. More specifically, the present invention provides an
automated, self-contained, flow apparatus for encapsulating
allosteric effectors, such as inositol hexaphosphate, in red blood
cells, thereby reducing the affinity of the hemoglobin for oxygen
and enhancing the delivery of oxygen by red blood cells to
tissues. Encapsulation is preferrably achieved by electroporation;
however, it is contemplated that other methods of encapsulation
may be used in practicing the present invention. The method and
apparatus of the present invention is equally suited to the
encapsulation of a variety of biologically-active substances in
various cell populations.
The apparatus and method of the present invention is suited
to the incorporation of a variety of biologically-active substances
in cells and lipid vesicles. The method and apparatus of the
present invention may be used for introducing a compound or
biologically-active substance into a vesicle whether that vesicle is
engineered or naturally occurring. For example, the apparatus
and method of the present invention may be used to introduce IHP
into erythrocytes.
The encapsulation of inositol hexaphosphate in red blood
cells by electroporation according to the present invention results
in a significant decrease in the hemoglobin affinity for oxygen
without affecting the life span, ATP levels, K+ levels, or normal
rheological competence of the cells. In addition, the Bohr effect
is not altered except to shift the 02 binding curve to the right.
Lowering the oxygen affmity of the erythrocytes increases the
capacity of erythrocytes to dissociate the bound oxygen and
thereby improves the oxygen supply to the tissues. Enhancement
of the oxygen-release capacity of erythrocytes brings about
significant physiological effects such as a reduction in cardiac
VO 94/21117 PCT/US94/03189
21JR005
output, an increase in the arteriovenous differences, and
improved tissue oxygenation.
The modified erythrocytes prepared in accordance with the
present invention, having improved oxygen release capacities,
5 may find their use in situations such as those illustrated below:
1. Under conditions of low oxygen-partial pressure,
such as at high altitudes;
2. When the oxygen exchange surface of the lung is
reduced, such as occurs in emphysema;
10 3. When there is an increased resistance to oxygen
diffusion in the lung, such as occurs in pneumonia or
asthma;
4. When there is a decrease in the oxygen-transport
capacity of erythrocytes, such as occurs with
15 erythropenia or anemia, or when an arteriovenous
shunt is used;
5. To treat blood circulation disturbances, such as
arteriosclerosis, thromboembolic processes, organ
infarct or ischemia;
6. To treat conditions of high oxygen affinity of
hemoglobin, such as hemoglobin mutations, chemical
modifications of N-terminal amino acids in the
hemoglobin-chains, or enzyme defects in
erythrocytes;
7. To accelerate detoxification processes by improving
oxygen supply;
8. To decrease the oxygen affmity of conserved blood;
or
9. To improve the efficacy of various cancer
treatments.
According to the method and apparatus of the present
invention, it is possible to produce modified erythrocytes which
contribute to an improved oxygen economy of the blood. These
modified erythrocytes are obtained by incorporation of allosteric
effectors, such as IHP, by electroporation of the erythrocyte
membranes.
CA 02159005 2006-05-12
16
The incorporation of the biologically-active substances into the cells in
accordance with the method of the present invention, including the
encapsulation
of allosteric effectors of hemoglobin into erythrocytes, is conducted
extracorporally via an automated, flow electroporation apparatus. Briefly, a
cell
suspension is introduced into the separation and wash bowl chamber of the flow
encapsulation apparatus. The cells are separated from the suspension, washed
and
resuspended in a solution of the biologically-active substance to be
introduced into
the cell. This suspension is introduced into the electroporation chamber and
then
incubated. Following electroporation and incubation, the cells are washed and
separated. A contamination check is optionally conducted to confirm that all
unencapsulated biologically-active substance has been removed. Then, the cells
are prepared for storage or reintroduction into a patient.
In accordance with the present invention and with reference to the
preferred embodiment, blood is drawn from a patient, the erythrocytes are
separated from the drawn blood, the erythrocytes are modified by the
incorporation of allosteric effectors and the modified erythrocytes and blood
plasma is reconstituted. In this manner, it is possible to prepare and store
blood
containing IHP-modified erythrocytes.
The apparatus of the present invention provides an improved method for
the encapsulation of biologically-active substances in cells including an
apparatus
which is self-contained and therefore sterile, an apparatus which can process
large
volumes of cells within a shortened time period, an apparatus having improved
contamination detection, cooling and incubation elements, an apparatus is
entirely
automated and which does not require the supervision of a technician once a
sample is introduced into the apparatus.
Thus, the present invention seeks to provide an automated, continuous
flow encapsulation apparatus.
CA 02159005 2006-05-12
17
A further aspect of the present invention seeks to provide an automated,
continuous flow electroporation apparatus.
A further object of the present invention seeks to provide a continuous
flow encapsulation apparatus which produces a homogenous population of loaded
cells or vesicles.
Another aspect of the present invention seeks to provide a continuous flow
electroporation device which produces a homogenous population of loaded cells
or vesicles.
Another aspect of the present invention seek to provide a sterile and
nonpyrogenic method of encapsulating biologically-active substances in cells.
Another aspect of the present invention seeks to provide a method and
apparatus which results in stable resealing of cells or vesicles following
electroporation to inimize lysis of the modified cells or vesicles after
electroporation.
Another aspect of the present invention seeks to provide a flow
encapsulation apparatus which produces a modified cell population from which
all exogenous non-encapsulated biologically-active substances have been
removed.
Another aspect of the present invention seeks to provide an electroporation
apparatus which produces a modified cell population from which all exogenous,
non-encapsulated biologically-active substances have been removed.
Another aspect of the present invention seeks to provide a method and
apparatus that allows continuous encapsulation of biologically-active
substances
in a population of cells or vesicles.
A further aspect of the present invention seeks to provide a method and
apparatus that achieves the above-defmed aspects, features, and advantages in
a
single cycle.
Another aspect of the present invention seeks to provide a continuous flow
electroporation chamber.
CA 02159005 2006-05-12
18
Another aspect of the present invention seeks to provide an improved and
more efficient method of encapsulating biologically active substances in cells
than
those methods currently available.
A further aspect of the present invention seeks to provide a population of
artificial cells suitable for medical use.
It is a further aspect of the present invention to provide a composition
suitable for use in the treatment of conditions and/or disease states
resulting from
a lack of or decrease in oxygenation.
Other aspects, features, and advantages of the present invention will
become apparent upon reading the following detailed description of the
preferred
embodiment of the invention when taken in conjunction with the drawing and the
appended claims.
Brief Description of the Drawings
Fig. 1 is a schematic diagram of a first embodiment of a continuous flow
encapsulation apparatus.
Fig. 2 is a schematic diagram of a second embodiment of a continuous
flow encapsulation apparatus.
Fig. 3 is a top view of a first embodiment of the flow electroporation
chamber with electrodes.
Fig. 4 is a top view of a first embodiment of the flow electroporation
chamber without electrodes.
Fig. 5 is a side view of a first embodiment of the flow electroporation
chamber.
Fig. 6 is an end view of a first embodiment of the flow electroporation
chamber.
Fig. 7 is a side view of an electrode for use with the first embodiment of
the flow electroporation chamber.
CA 02159005 2006-05-12
18a
Fig. 8 is a front view of the electrode of Fig. 7
Fig. 9 is an exploded perspective view of a second embodiment of the
flow electroporation chamber.
Fig. 10 is a perspective view of the flow electroporation chamber of Fig.
9 with the chamber being assembled.
Fig. 11 is a graph comparing the effect of various fieldstrengths, under
static or flow conditions, on the % oxygenation of IHP-encapsulated red blood
cells.
Fig. 12 is a table comparing the effects of various fieldstrengths, under
static or flow conditions, on the Pm value of IHP-encapsulated red blood
cells.
: "O 94/21117 2153005 PCT/US94103189
19
Fig. 13 is a table comparing the survival rates of red blood
cells subjected to electroporation under static and flow conditions
at various fieldstrengths.
Detailed Description of the Invention
The present invention provides an automated, self-
contained, flow apparatus for encapsulating allosteric effectors,
such as inositol hexaphosphate, in red blood cells. The apparatus
of the present invention combines the features of a plasmaphoresis
apparatus with those of a flow electroporation apparatus to form
an automated, self-contained flow electroporation device. The
present invention further comprises a new flow electroporation
chamber that allows use of the chamber under flow rather than
static conditions. It is contemplated that the method and apparatus
of the present invention may be used to encapsulate a variety of
biologically-active substances in diverse cell populations.
Additionally, the present invention provides a population of
modified cells having physical characteristics that make the cells
particularly useful for treating conditions which demand or
benefit from an increase in the delivery of oxygen to the tissues.
In accordance with the method of the present invention, a
homogenous population of IHP loaded red blood cells can be
obtained with reduced contamination and a reduced propensity to
lyse following encapsulation. The treated red blood cells exhibit
normal life spans in circulation. Using the present invention, red
blood cells of a patient in need of the treatment can be quickly
loaded and retumed to the patient's circulation.
The method of operation of the apparatus of the present
invention is described below with reference to the preferred use
of the apparatus, i.e., the encapsulation of allosteric effectors of
hemoglobin in red blood cells. Inositol hexaphosphate is the
preferred allosteric effector to be used with the present invention.
Other sugar phosphates, such as inositol pentaphosphate, inositol
tetraphosphate, inositol triphosphate, inositol diphosphate and
diphosphatidyl inositol diphosphate, can also be used. Other
.r0 94/21117 2159005 PCT/US94/03189
suitable allosteric effectors include polyphosphates such as
nucleotide triphosphates, nucleotide diphosphates, nucleotide
monophosphates, and alcohol phosphate esters. In case of certain
mutations of hemoglobin, e.g. "Zurich" hemoglobin, organic
5 anions such as polycarboxylic acids can be used as allosteric
effectors. Finally, it is possible to use inorganic anions such as
hexacyano ferrate, phosphate or chloride as allosteric effectors.
Red blood cells that have been loaded with inositol
hexaphosphate according to the present invention can be used to
10 treat a wide variety of diseases and disease states. The IHP loaded
red blood cells made according to the present invention can be
administered to a patient undergoing a heart attack thereby
increasing the oxygen delivery to the ischemic heart tissue and, at
the same time, reducing the cardiac output. The IHP-loaded red
15 blood cells made according to the present invention also can be
used to treat any ischemic condition including, but not limited to,
stroke, diabetes, sickle cell disease, burns, intermittent
claudication, emphysema, hypothermia, peripheral vascular
disease, congestive heart failure, angina, transient ischemic
20 disease, disseminated intravascular coagulation, adult respiratory
distress syndrome (ARDS) and cystic fibrosis. A detailed
description of the medical applications of compositions prepared
in accordance with the method of the present invention is also
provided below.
Continuous Flow Encapsulation Apparatus
The method of operation of the apparatus of the present
invention is described below with reference to the preferred use
of the apparatus, i.e., the encapsulation of allosteric effectors of
hemoglobin in red blood cells by electroporation. It is to be
understood that the apparatus may be adapted to accommodate
other cell populations or vesicles, and other biologically active
substances. Additionally, the apparatus maybe adapted to utilize
methods of encapsulation other than electroporation.
Briefly, in accordance with the present invention, a sample
of blood is introduced into the continuos flow encapsulation
NO 94/21117 PCT/US94/03189
2159005
21
apparatus. If red blood cells are being collected, the blood can
either be drawn directly from a patient or can be previously
drawn blood. The blood is initially separated into red blood cells,
plasma and white blood cells, and waste products. The waste
products include the diluent and various blood solutes remaining
in the supematant after centrifugation. They are stored in a waste
reservoir within the apparatus. The blood plasma and white
blood cells are also retained in a reservoir within the system
while the red blood cells are admixed with the substance to be
encapsulated. The suspension of red blood cells is then subjected
to electroporation. Following electroporation, the red blood cells
are incubated under conditions which allow the cells to reseal.
They are then processed and washed to eliminate exogenous, non-
encapsulated biologically-active substances. When the cells have
been processed, the red blood cells containing the encapsulated
substances can be optionally reconstituted with the blood plasma
and white blood cells. The reconstituted blood may then be
retumed directly to the patient or can be stored for later use.
Although described as discrete steps, the process is essentially
continuos.
A first embodiment of the present invention is described
with reference to Fig. 1, which schematically illustrates the
structure of the continuous flow encapsulation apparatus of the
present invention.
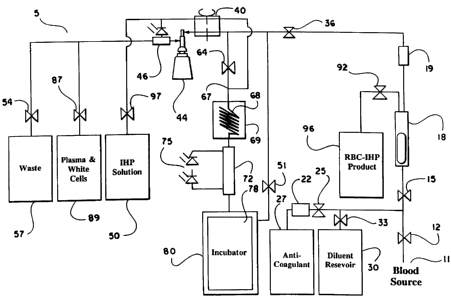
In accordance with the present invention, a volume of
whole blood is admitted into the electroporation system 5 at input
11. The blood sample may optionally be drawn directly from a
patient into the electroporation system 5, or the blood may be
drawn at an earlier time and stored prior to introduction into the
system 5. Valve 12 is opened to admit the sample into the system
5. Simultaneously, valve 25 is opened and pump 22 is engaged
to admit an anti-coagulant from the anti-coagulant reservoir 27.
A suitable anticoagulant is heparin, although other anticoagulants
can be used. The preferred anticoagulant is ACD. Valves 15 and
36 are also opened and pump 40 is engaged. The admixture of
anticoagulant and whole blood passes through a filter 18 and a
WO 94/21117 PCT/US94103189
2159005
22
pressure evaluation system 19 that monitors the flow through the
apparatus, and is collected in a blood separation and wash bowl
44 which is activated when pump 40 is engaged. A sensor
indicates when the blood separation and wash bowl 44 has been
filled with red blood cells. When it has been filled, they blood
supply is stopped. The steps involving separation of the blood
components can be accomplished by a plasmaphoresis apparatus,
such as the plasmaphoresis apparatus manufactured by
Haemonetics Corporation (Haemonetics Corporation, Braintree,
MA).
As explained above, when pump 40 is engaged in a
clockwise direction, the blood separation and wash bowl 44 is
engaged and the anti-coagulant and whole blood suspension is
centrifuged to separate the plasma, white blood cells, red blood
cells, and waste. Valve 87 is opened to admit the plasma and
white blood cells into the plasma reservoir 89.
Optionally and dependent on the cell population being
processed by the apparatus, the cells retained in the blood
separation and was bowl 44 are then washed. Valves 33, 15,
and 36 are opened to admit saline buffer from the diluent
reservoir 30 into the blood separation and wash bowl 44 which
contains the red blood cells. Pump 40 is still engaged. The red
blood cells are then washed and centrifuged. The preferred saline
buffer is a .9% sodium chloride solution, although other
physiologically isotonic buffers can be used to dilute and wash the
red blood cells. Valve 54 is opened to admit the waste into the
waste reservoir 57 during the washing process. Again, the waste
is stored in the waste reservoir 57 and the red blood cells are
retained in the blood separation and wash bowl 44. The wash
process is repeated if necessary.
Following separation of the red blood cells, pump 40 is
reversed, pump 22 is turned off, valves 12, 15, 33, 36, 25, 87,
and 54 are closed, and valves 97 and 64 are opened. The IHP
solution is pumped out of the IHP reservoir 50 while,
simultaneously, red blood cells are pumped out of the blood
separation and wash bowl 44 towards the cooling coil 68. The
WO 94/21117 2159005 PCT/US94/03189
23
red blood cells and IHP solution are admixed in the tubing of the
apparatus at junction 67 and then pumped through the cooling
coil 68. In a preferred embodiment of the present invention, and
as explained in detail below, the IHP solution and red blood cells
may be admixed in the separation and wash bowl 44 before being
admitted into the cooling coil 68.
The preferred concentration of IHP in the solution is
between approximately 10 mMol and 100 mMol with a more
preferred concentration of approximately 23 to 35 mMol, and a
most preferred concentration of 35 mMol. The preferred IHP
solution comprises the following compounds, in the following
concentrations:
35 mMol IHP salt neutralized with 35 mMol IHP acid
toapHof7.3
33 mMol K2HPO4
7.0 mMol NaH2
30.6 mMol KCL
6.4 mMol NaCI
7.3 mMol Sucrose
5.0 mMol ATP
A second IHP solution for use with the present invention
comprises the following compounds, in the following
concentrations:
23 mMol IHP salt neutralized with HCI to a pH of 7.3
40 mMol K2HPO4
7 mMol NaH2
The IHP may be obtained from Sigma Chemical Company of St.
Louis, Missouri.
The hematocrit of the suspension is preferably between
approximately 30 and 80 with the most preferred hematocrit of
approximately 50. Pump 40 is designed to pump both red blood
WO 94/21117 PCTIUS94/03189
24 2159005
cells and IHP solution and can be adjusted so that the final
hematocrit entering the cooling coil 68 can be predetermined.
After mixing, the red blood cell-IHP suspension is then
pumped through a cooling coil 68. Cooling can be achieved with
a water bath or with a thermo-electric based cooling system. For
example, cooling coil 68 is immersed in a cooling bath in the
cooling reservoir 69. When the red blood cell-IHP suspension
passes through the cooling coil 68, the suspension is cooled to a
temperature of between approximately 1 C and 12 C, preferably
approximately 4 C. Cooling the red blood cells ensures the
survival of the pore created in the cell membrane during the
electroporation process. The use of a cooling coil aids in the
speed of cooling by increasing the surface area of the sample in
contact with the cooling element. Optionally, the cooling coil can
be surrounded by a thermo-electric heat pump.
Certain applications may require heating of the cell
suspension prior to electroporation. In such a case, a heating coil
may replace the cooling coil 68. The maximum temperature
tolerated by red blood cells is approximately 370C.
A thermoelectric heat pump works by extracting thermal
energy from a particular region, thereby reducing its
temperature, and then rejecting the thermal energy into a "heat
sink" region of higher temperature. At the cold junction, energy
is absorbed by electrons as they pass from a low energy level in
the p-type semiconductor element, to a higher energy level in the
n-type semiconductor element. The power supply provides the
energy to move the electrons through the system. At the hot
junction, energy is expelled into a heat sink as electrons move
from a high energy level element (n-type) to a lower energy level
element (p-type).
Thermoelectric elements are totally solid state and do not
have moving mechanical parts or require a working fluid, as do
vapor-cycle devices. However, thermoelectric heat pumps
perform the same cooling functions as freon-based vapor
compression or absorption refrigerators. Thermoelectric heat
pumps are highly reliable, small in size and capacity, low cost,
vrO 94/21117 215DO05 PCTIUS94/03189
low weight, intrinsically safer than many other cooling devices,
and are capable of precise temperature control.
The preferred thermoelectric heat pumps for use in the
present invention are manufactured by MELCOR Materials
5 Electronic Products Corp. of Trenton, New Jersey. The
thermocouples are made of high performance crystalline
semiconductor material. The semiconductor material is bismuth
telluride, a quatemary alloy of bismuth, tellurium, selenium, and
antimony, doped and processed to yield oriented polycrystalline
10 semiconductors with properties. The couples, connected in series
electrically and in parallel thermally, are integrated into modules.
The modules are packaged between metallized ceramic plates to
afford optimum electrical insulation and thermal conduction with
high mechanical strength in compression. Modules can be
15 mounted in parallel to increase the heat transfer effect or can be
stacked in mullet-stage cascades to achieve high differential
temperatures. Passing a current through the heat pump generates
a temperature differential across the thermocouples, with
maximum ratings of 700 C and higher.
20 After cooling, the red blood cell-IHP suspension enters the
electroporation chamber 72 where an electric pulse is
administered from a pulse generator 75 to the red blood cell-IHP
suspension, causing openings to form within the cell membranes
of the red blood cells. Optionally, an automatic detection system
25 will turn the pulse generator 75 on when the chamber 72 is filled
with red blood cell-IHP suspension. An electrical pule is applied
to the suspension every time the chamber 72 is filled with
unencapsulated cells. A conventional electroporation chamber
may be used when the operation of the apparatus is static, namely,
when single discrete batches of cells are processed. In a
preferred embodiment of the present invention a flow
electroporation chamber is used. In one embodiment, a flow
electroporation chamber 72 is constructed of clear polyvinyl
chloride, and contains two opposing electrodes spaced a distance
of 7 mm apart. The distance between the electrodes will vary
depending on the flow volume and fieldstrength. Preferably, the
94/21117 PCTIUS94/03189
26 2159005
flow electroporation chamber 72 is disposable. The
electroporation chamber may also be constructed of polysolfone,
which is preferably for use with certain sterilization procedures,
such as autoclaving. A detailed description of the structure and
5 construction of the flow electroporation chamber is provided
below.
The red blood cell-IHP suspension passes between the two
electrodes of the electroporation chamber 72. When a suspension
of non-treated cells enter the chamber 72, an electrical field of I
10 to 3 KV/cm is created and maintained for a period of I to 4
milliseconds, preferably for a period of 2 milliseconds with a 1.8
ml flow chamber. Preferably, the IHP-red blood cell suspension
is subjected to three high voltage pulses per volume at a
fieldstrength of approximately 2600 to 3200 V/cm per pulse. The
pulse of current across the cell membranes causes an electrical
breakdown of the cell membranes, which creates pores in the
membranes. IHP then diffuses into the cell through these pores.
Following electroporation, the red blood cell-IHP
suspension enters an incubation chamber 78 where the suspension
is incubated at room temperature for an incubation time of
between approximately 15 minutes and 120 minutes with the
preferred incubation time of 30 to 60 minutes. Optionally, the
red blood cell-IHP suspension is incubated for approximately 5
minutes at a temperature of approximately 370 C, and at least 15
minutes at room temperature. The incubation chamber 78 may
optionally be surrounded by a heating means.80. For example,
the heating means 80 can be a water bath or can be a
thermoelectric heat pump.
Optionally, the incubator 78 contains a resealing buffer
which aids in resealing and reconstitution of the red blood cells.
The preferred composition of the resealing buffer is provided
below:
WO 94/21117 PCT/US94/03189
27
RESEALING BUFFER
1. Combine
Sodium chloride 150 mMol
Potassium chloride 8 mMol
Sodium phosphate 6 mMol
Magnesium sulfate 2 mMol
Glucose 10 mMol
Adenine 1 mMol
Inosine 1 mMol
Penicillin G 500 units/ml
Chloram henicol 0.1 m ml
H. Add
BSA 3.5%
Calcium chloride 2 mMol
In the preferred embodiment of the present invention, no
resealing buffer is used.
Following incubation, valve 51 is opened and pump 40 is
engaged and the red blood cell-IHP suspension is returned to the
blood separation and wash bowl 44 from the incubation chamber
78. The excess IHP solution is removed from the red blood cell
suspension by centrifugation. The waste IHP solution is directed
to waste reservoir 57. Valves 33, 15 and 36 are then opened to
admit a volume of diluent into the blood separation and wash
bowl 44. The red blood cell-IHP suspension is then centrifuged
and the supematant is discarded in the waste reservoir 57 through
valve 54 leaving the red blood cells in the blood separation and
wash bowl 44. A saline buffer is added to the modified red blood
cells from the diluent reservoir 30. The cells are washed and the
supernatant is discarded following centrifugation. The wash
process is repeated if needed.
Optionally, as the waste is removed from the separation and
wash bowl 44 it passes through a contamination detector 46 to
detect any free IHP in the waste solution thereby confirming that
WO 94/21117 PCT/US94/03189
21590 95
28
exogenous non-encapsulated IHP has been removed from the
modified red blood cells. The contamination detection system
relies on optical changes in the washing buffer. After the
modified red blood cells have been washed and centrifuged, the
supematant passes through the contamination detector 64 before
it is deposited in the waste reservoir 57. If exogenous, non-
encapsulated IHP remains in the washing buffer, The discarded
solution will be turbid. The turbidity is due to the reaction of
IHP with calcium, which is a component of the wash buffer. The
contamination detector 46 uses an optical detection system.
Preferably, the light source is an LED and the detector is a
photodiode. The voltage difference of the photodiode will
indicate the amount of IHP in the wash solution. The
contamination detector 46 is optional.
Following washing, the IHP-red blood cell product is
optionally reconstituted with the plasma and white blood cells
which had been retained in reservoir 89. The treated red blood
cells may be collected in a reinjection bag, either in a
preservation media or in the autologous plasma of the patient.
The IHP-loaded red blood cells obtained can be
administered directly back into the patient or the cells can be
stored for later use. The IHP in the red blood cells is not released
during the normal storage time.
A preferred embodiment of the present invention is
described with reference to Fig. 2, which schematically illustrates
the structure of the continuous flow encapsulation apparatus of
the present invention. Again, the method of operation of the
apparatus is described with reference to the preferred use of the
apparatus, i.e., the encapsulation of allosteric effectors of
hemoglobin in red blood cells by electroporation. It is to be
understood that the apparatus may be adapted to accommodate
other cell populations or vesicles, and other biologically active
substances. Additionally, the apparatus maybe adapted to include
other methods of encapsulation.
In accordance with the present invention, a sample of whole
blood is admitted into the electroporation system 10 at input 11.
rVO 94/21117 PCT/US94/03189
21590 0 5
29
Valve 12 is opened to admit the sample into the system 10.
Simultaneously, valve 25 is opened and pump 22 is engaged to
admit an anti-coagulant from the anti-coagulant reservoir 27.
Valves 15 and 36 are also opened and pump 40 is engaged.
The admixture of anticoagulant and whole blood passes
through a filter 18 and a pressure evaluation system 19, and is
collected in a blood separation and wash bowl 44 which is
activated when pump 40 is engaged. A sensor indicates when the
blood separation and wash bowl 44 has been filled with red blood
cells.
When pump 40 is engaged in a clockwise direction, the
blood separation and wash bowl 44 is engaged and the anti-
coagulant and whole blood suspension is centrifuged to separate
the plasma, white blood cells, red blood cells, and waste. Valve
87 is opened to admit the plasma and white blood cells into the
plasma reservoir 89.
Optionally, the cells retained in the separation and wash
bowl 44 are then washed and centrifuged. Valves 33, 35, 15,
and 36 are opened to admit saline buffer from the diluent
reservoir 30 into the blood separation and wash bowl 44 which
contains the red blood cells. Valve 12 is closed and pump 40
remains engaged.
During washing, valve 54 is opened to admit the waste
into the waste reservoir 57 during the washing process. Again,
the waste is stored in the waste reservoir 57 and the red blood
cells are retained in the blood separation and wash bowl 44. The
wash process is repeated if necessary. A contamination detection
system may optionally be installed between the separation and
wash bowl 44 and the waste reservoir 57 to control the wash
process.
Following separation of the red blood cells, pump 40 is
reversed, pump 22 is turned off, valves 12, 15, 33, 35, 36, 25,
87, and 54 are closed, and valve 97 is opened. If the cells were
washed, pump 22 was previously turned off and valves 12 and
25 had been closed. The IHP solution is pumped out of the IHP
reservoir 50 and into the separation and wash bowl 44 containing
. 0 94/21117 PCT/US94/03189
~~~~~0a'
the red blood cells. There, the red blood cells and IHP are
admixed to form a suspension.
The preferred concentration of IHP in the solution is
between approximately 10 mMol and 100 mMol with a more
5 preferred concentration of approximately 23 to 35 mMol, and
with a most preferred concentration of 35 mMol. The preferred
IHP solution comprises the following compounds, in the
following concentrations:
10 35 mMol IHP salt neutralized with 35 mMol IHP acid
to a pH of 7.3
33 mMol K2HPO4
7 mMol NaH2
30.6 mMol KCL
15 6.4 mMol NaCI
7.3 mMol Sucrose
5.0 mMol ATP
The IHP may be obtained from Sigma Chemical Company of St.
20 Louis, Missouri.
The hematocrit of the suspension is preferably between
approximately 30 and 60 with the most preferred hematocrit of
approximately 50. Pump 40 is designed to pump both red blood
cells and IHP solution and can be adjusted so that the final
25 hematocrit entering the cooling coi168 can be predetermined.
The steps of collecting the specimen, separating the cells
from the specimen, washing the cells, and combining the cells
with IHP can be done with an apheresis-blood washing machine,
such as that manufactured by Haemonetics corporation. The
30 apheresis-blood washing machine is coupled to the flow
electrophoresis apparatus described herein to form a continuos
flow electroporation apparatus.
After combining the red blood cells with the IHP solution,
pump 40 is again reversed, valve 97 is closed and valve 64 is
opened. The red blood cell-IHP suspension is then pumped
through a thermoelectric cooling coil 68. A blood bag from a
WO 94/21117 PCT/US94/03189
2159005
31
blood warming set, such as the blood bag provided in the
Fenwal Blood Warming Set manufactured by Baxter Healthcare
Corporation can be used as the cooling coil 68. When the red
blood cell-IHP suspension passes through the cooling coil 68 in
the cooling reservoir 69, the suspension is cooled to a
temperature of between approximately 1 C and 12 C, preferably
approximately 4 C. Optionally, a pump may be added to the
apparatus between the cooling coil 68 and cooling reservoir 69,
and the electroporation chamber 72, to ensure a constant flow
rate and compensate for fluctuation in volume that occurs when
the cooling coil 68 is filled.
Optionally, the pre-cooling step may be eliminated and the
red blood cell-IHP suspension may be directed to the
electroporation chamber 72 immediately after admixing. In such
an instance, the cooling coil 68 and cooling reservoir 69 would
be eliminated from the continuos flow encapsulation apparatus
10. Cooling prior to electroporation may not be required if the
temperature of the electroporation chamber is sufficiently cool to
maintain the cells suspension at 40C.
After cooling, the red blood cell-IHP suspension enters the
electroporation chamber 72. The chamber 72 is maintained at a
temperature of approximately 40C. As the red blood cell-IHP
suspension passes through the flow electroporation chamber 72,
an electric pulse is administered from a pulse generator 75 to the
suspension causing openings to form within the cell membranes of
the red blood cells.
The red blood cell-IHP suspension passes between two
electrodes of the electroporation chamber 72. Figs 3 to 10
describe the electroporation chamber. In a preferred
embodiment of the present invention, when a suspension of non-
treated cells enters the chamber 72, the IHP-red blood cell
suspension is subjected to approximately three high voltage pulses
per volume at a fieldstrength of approximately 2600 to 3200
V/cm per pulse. The charge created across the cell membranes
causes an electrical breakdown of the cell membrane, which
vrO 94/21117 PCT/US94/03189
21a9'005
32
creates pores in the membrane. IHP then diffuses into the cell
through these pores.
During electroporation, an electrical field of 1 to 3 KV/cm
is created and maintained for a period of 1 to 4 milliseconds. The
preferred pulse length is 3 to 4 milliseconds, with a most
preferred pulse length of 2 milliseconds. Pulse length is defined
as 1/e. At a flow rate of approximately 10.6 ml/minute, the
preferred number of pulses is 3, at the preferred pulse rate of
0.29 Hz. The fieldstrength is defined as the voltage over the
distance between the electrodes. The distance between electrodes
is measured in centimeters. The preferred electrical parameters
are as follows:
Exponential Pulse: pulse length = 1.5 to 2.5 ms
field strength = 2.7 to 3 KV/cm
Following electroporation, the red blood cell-IHP
suspension enters an incubation chamber 78 where the suspension
is incubated at room temperature for an incubation time of
between approximately 10 minutes and 120 minutes with a
preferred incubation time of 30 minutes. Optionally, the red
blood cell-IHP suspension is incubated for approximately 5
minutes at a temperature of approximately 370C, and at least 15
minutes at room temperature. The incubation chamber 78 may be
surrounded by a heating means 80. Any heating means 80 can be
used in practicing the present invention. The preferred heating
means 80 are a water bath or a thermoelectric heat pump.
Optionally, the incubator 78 contains a resealing buffer
which aids in resealing and reconstitution of the red blood cells.
In the preferred embodiment of the present invention, no
resealing buffer is used.
Following incubation, the red blood cell-IHP suspension is
returned to the blood separation and wash bowl 44 when valve
51 is opened and pump 40 is engaged. The excess IHP solution is
removed from the red blood cell suspension by centrifugation.
The waste IHP solution is directed to waste reservoir 57. Valves
WO 94/21117 PCT/US94/03189
2159005
33
33, 37, 15 and 36 are then opened to admit a volume of post
wash solution from reservoir 31 into the blood separation and
wash bowl 44. In a preferred embodiment of the present
invention, the post wash solution comprises a .9% NaC12 solution,
including 2.0 mM CaC12 and 2.0 mM MgC12. Any physiological
saline may be used.
After addition of the post wash solution, the red blood cell-
IHP suspension is then centrifuged and the supernatant is
discarded in the waste reservoir 57 through valve 54 leaving the
red blood cells in the blood separation and wash bowl 44. The
wash process is repeated until all unencapsulated IHP has been
removed.
Optionally, as the waste is removed from the separation and
wash bowl 44 it passes through a contamination detector 46 to
detect any free IHP in the waste solution thereby confirming that
exogenous non-encapsulated IHP has been removed from the
modified red blood cells. The contamination detector 46 is
optional.
Following washing, the red blood cells containing W may
be reconstituted with the plasma and white blood cells retained in
reservoir 89. Pump 40 is engaged and valves 87, 36, and 92
are opened. The modified red blood cells and plasma and white
blood cells are pumped to reservoir 96. A filter may be installed
between reservoir 96 and valve 92 to remove any aggregates or
other impurities from the reconstituted modified blood.
The IHP-loaded red blood cells obtained in accordance with
the method of the present invention can be administered directly
back into the patient or the cells can be stored for later use. The
IHP in the red blood cells is not released during the normal
storage time.
It is contemplated that continuos flow encapsulation
apparatus of the present invention may be modified to utilize
other encapsulation methods.
Furthermore, it is contemplated that the continuous flow
encapsulation apparatus may be adapted to process various diverse
WO 94/21117 2 ~ ~ 9005 PCTIUS94103189
34
cell populations. Furthermore, the apparatus may be used to
encapsulate biologically active substances in artificial vesicles.
It is also contemplated that the continuos flow encapsulation
apparatus of the present invention may be use to encapsulate a
broad range of biologically active substances.
The flow electroporation apparatus of the present invention
may be separated from the plasmaphoresis apparatus of the
present invention. The blood cooling system, peristaltic pump,
electroporation chamber, pulse generator, and electronics
comprising the flow electroporation apparatus may be linked to a
plasmaphoresis apparatus and interface with the controls of that
machine.
While this invention has been described in specific detail
with reference to the disclosed embodiments, it will be
understood that many variations and modifications may be
effected within the spirit and scope of the invention as described
in the appended claims.
Flow Electroporation Chamber
During electroporation, the insertion rate of IHP is linearly
dependent on the voltage administered to the cells. Generally, the
higher the voltage, the more IHP is encapsulated; however, cell
lysis is also increased and cell survival is decreased. The
efficiency of an electroporation system may be judged by cell
survival after electroporation. Poor cell survival indicates very
low efficiency. The amplitude and duration of the electrical pulse
is responsible for the electric breakdown of the cell membrane
and creates pores in the pole caps parallel to the electric field.
Thus, the factors to be considered in designing an electroporation
system include the field strength, the pulse length and the number
of pulses.
A perfect electroporation target is shaped like a sphere, so
its orientation does not effect the efficiency of the applied field.
When the target is spherical, a single pulse with an field strength
above the threshold can electroplate 100 % of the target. Red
blood cells are disk shaped. Because of their shape and
NO 94/21117 PCTIUS94/03189
2159005
orientation in the electroporation chamber, only approximately
% of the cells are electroplated during a single pulse. To also
electroporate the other 60 %, the fieldstrength can be increased.
This increases the stress on the red blood cells in proper
5 orientation to the electric field and leads to lower survival rates
of the cells.
To achieve more efficient encapsulation while reducing the
incidence of cell lysis and death, a flow electroporation chamber
utilizing short duration multiple pulses was developed. With the
10 flow-through rate steady and a steady field voltage, it was
determined that plurality of pulses would insert maximal
quantities of IHP with minimal 2 to 24 hour lysis. A multiple-
pulse system allows an increase in the cell survival rate without
increasing the field strength. When a multiple-pulse system is
15 used, orientation of the cells is not as critical as it is when a
system is a single pulse system is used. The lower field strength
is much more gentle to the red blood cells. It is much easier to
electroporate every single cell in the multiple pulse system,
because the timing between the flow rate of the red blood cells
20 through the chamber and the electroporation pulses, and the
orientation of the cells is not as crucial as in a single pulse system.
The flow multiple-pulse electroporation system also increases
both the short term and the long term survival of red blood cells
when compared to the single pulse method.
25 Figs. 11 to 13 illustrate the effects of various field
strengths, under static or flow conditions, on the % oxygenation
of IHP-encapsulated red blood cells over a range oxygen
pressures; on the P50 value of IHP-encapsulated red blood cells
(two concentrations of IHP solutions were compared); and, on the
30 survival rates of red blood cells subjected to electroporation. All
readings were taken 24 hours after electroporation. The results
indicated that multiple pulses at comparatively low fieldstrengths
produce optimal encapsulation results.
A cooled electroporation chamber is preferred to keep the
35 red blood cells at a constant temperature during the
electroporation process, thereby enhancing their survival rates.
WO 94/21117 2159n 0a- PCT/US94103189
36
This is accomplished by removing the excess heat created by the
electrical pulse during the electroporation process. The excess
heat may be removed either by cooling the electrodes or cooling
the entire flow electroporation chamber. In accordance with the
preferred embodiment of the present invention, the electrodes
themselves are cooled.
During the electroporation process, blood is pumped
through an inlet in the electroporation chamber and the red blood
cells are subject to a series of electrical pulses as they travel
through the chamber. They exit out the other end of the
chamber. The chamber can be made of any type of insulating
material, including but not limited to ceramic, teflon, plexiglass,
glass, plastic, silicon, rubber or other synthetic materials.
Preferably, the chamber is comprised of glass or polysulfone.
Whatever the composition of the chamber, the intemal surface of
the chamber should be smooth to reduce turbulents in the fluid
passing through it. The housing of the chamber should be non-
conductive and biologically inert. In commercial use, it is
anticipated that the chamber will be disposable.
In a preferred embodiment of the present invention, the
electrodes that comprise part of the electroporation apparatus can
be constructed from any type of electrically or thermally
conductive hollow stock material, including but not limited to
brass, stainless steel, gold plated stainless steel, gold plated glass,
gold plated plastic, or metal containing plastic. Preferably, the
surface of the electrode is gold plated. Gold plating serves to
eliminate oxidation and reduces the collection of hemoglobin and
other cell particles at the electrodes. The surface of the
electrodes should be smooth.
The electrodes can be hollow, to allow cooling by liquid or
gas, or the electrodes can be solid, to allow for thermoelectric or
any other type of conductive cooling. Cooling could also be
accomplished by cooling the electroporation chamber itself, apart
from cooling the electrodes.
WO 94/21117 2159005 PCT/US94/03189
37
Preferably, the flow electroporation chamber is disposable.
A detailed description of two embodiments of the electroporation
chamber of the present invention is provided below.
In one embodiment, the flow electroporation chamber is
constructed of clear polyvinyl chloride, and contains two
opposing electrodes spaced a distance of approximately 7 mm
apart. The electroporation chamber is a modification of a
chamber obtained from BTX Electronic Company of San Diego,
California. However, when the electroporation chamber is used
continuously, it overheats and the survival rate of the cells
processed by the apparatus decreases over time. To correct the
overheating problem that occurred when the apparatus was used
in a continuos flow manner, a continuous flow electroporation
chamber was designed. A detailed description of the structure of
the continuous flow electroporation chamber is provided below.
Figs. 3 through 8 show one embodiment of the flow
electroporation chamber 72 of the present invention. As can be
seen in Fig. 3, the flow electroporation chamber 72 includes a
housing 100 having two electrodes 102 inset on opposing sides of
the housing 100 of the electroporation chamber 72. The housing
100 includes an inlet channel 104 at one end and an outlet
channel 106 at the other. The inlet 104 and outlet 106 channels
include connectors 108 and 109 respectively, preferably of the
male Luer variety. The connectors 108 and 109 are hollow and
form the inlet 104 and outlet 106 channels into the interior of
the electroporation chamber 72.
As seen in Figs 4 and 5, an intemal chamber 110 extends
most of the length of the housing 100 and is sized to receive the
two electrodes 102. The internal chamber 110 includes beveled
surfaces 111 for receiving the internal edges of the electrodes
102. The internal chamber 110 is thus formed by the internal
surfaces of the electrodes 102 and the internal surfaces of the
housing 100. The internal chamber 110 is connected to the inlet
104 and outlet 106 channels.
As can be seen in Figs. 7 and 8, the electrodes 102 of the
electroporation chamber 72 of Figs. 3 to 6 are comprised of flat,
: O 94/21117 PCr/US94/03189
2159005
38
elongated, hollow shells. The electrodes 102 include cooling
inlets 112 and cooling outlets 114 at their ends. As described
above, the rear surfaces of the electrodes 102, or the surface to
the left in Fig. 7, fits flush against the beveled surface 111 of the
housing 100.
The electroporation chamber 72 is designed such that the
cell suspension to be subjected to electroporation enters the
electroporation chamber 72 through the inlet 104 and expands to
fill the internal chamber 110. As the red blood cell suspension
flows through the internal chamber 110 a pulse or charge is
administered across the width of the intemal chamber 110.
To maintain a relatively constant temperature during the
electroporation process, cooling fluid or cooling gas is pumped in
the cooling inlet 112 and out the cooling outlet 114 so that the
electrodes 102 are maintained at approximately 40 C.
Figs. 9 and 10 display a second embodiment of the flow
electroporation chamber 172. As can be seen in Figs. 9 and 10,
the flow electroporation chamber 172 includes a hollow housing
200 substantially rectangular in shape. Two electrodes 202 are
inserted into the interior of the housing 200 directly opposite one
another, flush against the housing 200 walls. The flow
electroporation chamber 172 further comprises an inlet channel
204 at one end and an outlet channel 206 at the other end of the
housing 200. The inlet 204 and outlet 206 channels include
connectors 208 and 209 which are attached by tubing 216 to a
cell suspension supply that supplies the cell suspension, i.e. the
IHP-red blood cell suspension, to the electroporation chamber
172. The connectors 208 and 209 and inlet 204 and outlet 206
channels serve to direct the cell suspension into and out of the
housing 200.
As can be seen in Fig. 10, one end of the inlet channel 204
and one end of the outlet channel 206 extends into the interior of
the housing 200 forming an internal chamber 210. The internal
chamber 210 is thus formed by the internal surfaces of the
electrodes 202, the intelnal surfaces of the housing 200 and the
intemal surfaces of the of the inlet 204 and outlet 206 channels.
VO 94/21117 PCT/US94/03189
2159005
39
As can be seen in Figs. 9 and 10, the electrodes 202 of the
flow electroporation chamber 172 comprise flat, elongated,
hollow shells. The electrodes 202 include cooling inlets 212 and
cooling outlets 214 at their ends, through which a gas or fluid
may be pumped through the electrodes 202 to maintain a constant
temperature during electroporation.. The electrodes 202 are
connected to a pulse generator by cables 220.
As with the chamber described above, the electroporation
chamber 172 of Figs. 9 and 10 is designed such that the
suspension to be subjected to electroporation enters the
electroporation chamber 172 through the fluid inlet 204 and
expands to fill the intemal chamber 210. As the red blood cells
suspension flows through the internal chamber 210, a pulse or
charge is administered across the width of the internal chamber
210 between the electrodes 202. To maintain a relatively
constant temperature during the electroporation process, cooling
fluid or cooling gas is pumped in the cooling inlet 212 and out
the cooling outlet 214 of the electrodes 202 through the
connectors 208 and 209 so that the electrodes 202 are
maintained at approximately 40C. It is also possible that the inlet
channel 204, outlet channel 206 and connectors 208 and 209 can
be made as a solidly integrated glass part, rather than separate
components.
It is contemplated that the flow electroporation chamber
172 maybe constructed from drawn glass or any other highly
polished material. It is preferable that the interior surface of the
electroporation chamber 172 be as smooth as possible to reduce
the generation of surface turbulence. Drawn glass components
are highly consistent with perfect surface finishes. Furthermore,
they are stable and inert to blood components. They are also
relatively inexpensive, which is desirable for a disposable
electroporation chamber.
The electrodes may also be comprised of drawn glass,
electroplated with colloidal gold. Again, the surfaces of the
electrodes should be highly finished, highly conductive, yet
biologically inert. Gold electroplate is durable and inexpensive.
.10 94/21117 PCT/US94/03189
2159005
Fluidic connection can be accomplished using commonly available
parts.
The flow electroporation chamber may be constructed
either as a part of the entire flow encapsulation apparatus, or as
5 an individual apparatus. The flow electroporation apparatus may
then be connected to a commercially available plasmaphoresis
machine for encapsulation of particular cell populations. For
example, the flow electroporation chamber maybe connected to
commercially available plasmaphoresis equipment by electronic
10 or translational hardware or software. Optionally, a pinch-valve
array and controller driven by a PC program can also be used to
control the flow electroporation apparatus. Similarly, current
power supplies are capable of establishing the power levels
needed to run the flow electroporation chamber or flow
15 encapsulation apparatus.
While this invention has been described in specific detail
with reference to the disclosed embodiments, it will be
understood that many variations and modifications may be
effected within the spirit and scope of the invention as described
20 in the appended claims.
Application of IHP treated red blood cells
The present invention provides a novel method for
increasing the oxygen-carrying capacity of erythrocytes. In
25 accordance with the method of the present invention, the IHP
combines with hemoglobin in a stable way, and shifts its oxygen
releasing capacity. Erythrocytes with IHP-hemoglobin can
release more oxygen per molecule than hemoglobin alone, and
thus more oxygen is available to diffuse into tissues for each unit
30 of blood that circulates. Under ordinary circumstances, IHP is
toxic and cannot be tolerated as an ordinary drug. Attachment of
IHP to hemoglobin in this novel procedure, however, neutralizes
its toxicity. In the absence of severe chronic blood loss, treatment
with a composition prepared in accordance with the present
35 method could result in beneficial effects that persist for
approximately ninety days.
vVO 94/21117 PCT/US94/03189
2159{305
41
Another advantage of IHP-treated red blood cells is that
they do not lose the Bohr effect when stored. Normal red blood
cells that have been stored by conventional means do not regain
their maximum oxygen carrying capacity for approximately 24
hours. This is because the DGP in normal red blood cells diffuses
away from the hemoglobin molecule during storage and must be
replaced by the body after transfusion. In contrast, red blood
cells treated according to the present invention are retain their
maximum oxygen carrying capacity during storage and therefore
can deliver maximum oxygen to the tissues immediately after
transfusion into a human or animal.
The uses of IHP-treated RBC's is quite extensive including
the treatment of numerous acute and chronic conditions including,
but not limited to, hospitalized patients, cardiovascular
operations, chronic anemia, anemia following major surgery,
coronary infarction and associated problems, chronic pulmonary
disease, cardiovascular patients, autologous transfusions, as an
enhancement to packed red blood cells transfusion (hemorrhage,
traumatic injury, or surgery). congestive heart failure,
myocardial infarction (heart attack), stroke, peripheral vascular
disease, intermittent claudication, circulatory shock, hemorrhagic
shock, anemia and chronic hypoxmia, respiratory alkalemia,
metabolic alkalosis, sickle cell anemia, reduced lung capacity
caused by pneumonia, surgery, pneumonia, trauma, chest
puncture, gangrene, anaerobic infections, blood vessel diseases
such as diabetes, substitute or complement to treatment with
hyperbaric pressure chambers, intra-operative red cell salvage,
cardiac inadequacy, anoxia - secondary to chronic indication,
organ transplant, carbon monoxide, nitric oxide, and cyanide
poisoning.
Treating a human or animal for any one or more of the
above disease states is done by transfusing into the human or
animal between approximately 0.5 and 6 units (1 unit = 500 ml)
of IHP-treated blood that has been prepared according to the
present invention. In certain cases, there may be a substantially
complete replacement of all the normal blood in a patient with
NO 94/21117 PCT/US94/03189
2159005
42
IHP-treated blood. The volume of IHP-treated red blood cells
that is administered to the human or animal will depend upon the
indication being treated. In addition, the volume of IHP-treated
red blood cells will also depend upon concentration of IHP-
treated red blood cells in the red blood cell suspension. It is to be
understood that the quantity of IHP red blood cells that is
administered to the patient is not critical and can vary widely and
still be effective.
IHP-treated packed RBC's are similar to normal red blood
cells in every category except that the IHP-treated packed red
blood cells can deliver 2 to 3 times as much oxygen to tissue per
unit. A physician would therefore chose to administer a single
unit of IHP-treated packed red blood cells rather than 2 units of
the normal red blood cells. IHP-treated packed red blood cells
could be prepared in blood processing centers analogously to the
present blood processing methods, except for the inclusion of a
processing step where the IHP is encapsulated in the cells.
While this invention has been described in specific detail
with reference to the disclosed embodiments, it will be
understood that many variations and modifications may be
effected within the spirit and scope of the invention as described
in the appended claims.