Note: Descriptions are shown in the official language in which they were submitted.
CA 02159093 2003-06-25
60557-5084
1
INTUMESCENT COMPOSITION A,NU MET~iUD OF USE
This invention relates to flexible intumescent
compositions suitable fox use as firestops in
deterring the spread of fire, smoke, and fumes as may
happen during a fire in a building.
Fire, smoke, and fumes in confined spaces, such
as multi-floor buildings, can be extremely life
threatening. Frequently, if fire originates in the
space between a floor and ceiling of such a structure,
the fire, anal resultant smoke and fumes, will tend to
spread to other open spaces in the building,
especially to open spaces above the point of origin of
the fire.
The spaces between conduits, piping, and the
like, and the floors and ceilings through which they
pass are known as "through-penetrations." If not
protected by fire resistant materials, a through
penetration offers an area of low resistance to tire,
smoke and fumes, and in essence may serve as a chimney
for heat, flame and fumes. Through penetrations may
be filled with commercially available fire retardant
and intumescent putties, caulks, wraps, sheets, or
mats, known in the art as "firestops."
Representative firestop products are disclosed in in
assignee's U.S. Pat. Nos. 4,273,879, 4,364,210,
4,467,577, and 4,952,615. Other intumescent fire
retardant materials have been used, such as those
known under the trade designations "Palusol"
(commercially available from BASF] and "Expantrol"
(commercially available from 3M Co.), the latter being
an alkali metal silicate*
These firestop products and others have been
widely used for reducing or eliminating the chimney
effect for through-penetrations and pass the rigorous
American Society of Testing and Materials (ASTM) fire
WO 94/24227 PCT/LTS94/01747
2
endurance test (ASTM E°814) after intumescing and
charring wherein the material is not easily blown out
of penetrations when subjected to water hose pressure
such as may be present during fire fighting.
Therefore, essential characteristics of a firestop
material include the ability to expand and to char,
and for the charred material to have sufficient
strength to withstand the test requirements.
In spite of the above compositions, there exists
a need in the art for a firestop composition which
retains its expansion, low compression set, and
flexibility after long-term exposure to oxidative
environments.
The present invention relates to flexible,
intumescent (heat expanding), fire retardant
compositions having the capability of expanding many
times their original volume (preferably at least 8
times) when exposed to heat. The compositions may be
applied in sheet or molded form and remain in their
flexible unexpended state until such time as they are
subjected to temperatures on the order of 110°C or
higher, as, for example, upon exposure to fire in a
burning building. When heated, the inventive
compositions readily intumesce (expand) to seal gaps
in through-wall or through-floor penetrations, thus
providing a seal against smoke, vapors, flame, water,
and steam.
The compositions of the invention have excellent
compression set and expansion properties coupled with
excellent char strength of the charred material.
These terms are specifically defined herein.
The fire-retardant compositions of the present
invention are an advance over the art because they
exhibit superior aging properties (are more resistant
to degradation due to ozone and oxygen) and low
compression set in the unexpended state, and superior
char strength in the expanded state; further,
CA 02159093 2003-06-25
60557-5084
8
preferred compositions employ ingredients which are believed
to be less toxic than known r~~.,rn~,osita.c,~ns.
Thus, one aspect o.f tree pz:eseru. invention is a
flexible, fire-retardant, intumescent composition
characterized by:
a) an i.ntumescent rnateriai.;
b) an effective am<.~unt c~f a stabiml.izing agent; and
c) an elastomers.c ~~>rganuc f:~inder throughout which
the intumescent material and stabilizing agent are
dispersed, the elastumeric binder being at least partially
crosslinked, wherein the stabilizing agent consists
essentially of compounds sel~Yct~rci f~~orr~ the group consisting
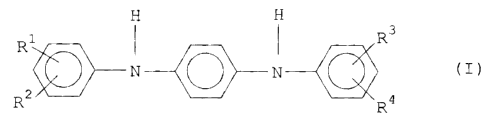
of compounds within general formula I
N
1 R3
R y~~~
'1 ~ _ ~ ',,'~ ~''% 4
R _.R
wherein R1-R'~ inclusive are independently selected from the
group consisting of hydrogen ("H'°) and alkyl groups having
from 1 to about 5 carbon atoms, with the provisos treat:
i) either both of R1 and R"'', or both R3 and R4 must
be alkyl groups having from 3. to abaut 5 ~,:~arban ator~as; and
ii) the stabil.izinc~ agent has a melting point
ranging from about 105 to about 125°C.
According to one aspect of the present invention,
there is provided a flexible, fire-retardant, intumescent
composition having a compression set less than 20s
CA 02159093 2003-06-25
60557-5084
3a
comprising: a) 15 to 8U weight percent hydrated alkali
metal silicate intumescent:. mat:eria:l; ~::>) a. stab:ilizirng agent;
and c) 15 to 40 weight percent. char-forming elastomeric
organic binder throughout wh:i.~:h ttze xr~turr~escent maters.al and
stabilizing agent: are dispersed, the elastomeric binder
being at least partially crosslinked, wherein the
stabilizing agent: consista esser.~tial~l.~° of a compound
selected from the group cansisting of compounds within
general formula I:
1o Ri ~, H ._ H __ Rs
r
;'
~',, ~........_... ~ .._....._. i' ,~ ~ ~,
,,
' i
>, 4
wherein R1-Rg inclusive are independently selected from the
group consisting of hydrogen arid alkyl groups having from
1 to about 5 carbon atoms, w:~.tra term px.favisos treat:: i)
either both of R1 and Rz, or both Rj and R~' are alkyl groups
having from 1 to about 5 carban at.arr~s; anc~ ii) the
stabilizing agent has a melting point ranging from about
105°C to about 125°C.
According to another aspect of the present
invention, there is provided a flexible, fare-retardant,
intumescent composition having a compression set less than
20% comprising: a) 1.5 t:o 80 weight percent hydrated alkali
metal silicate intumescent material; b) a stabilizing agent;
and c) 15 to 40 weight percent non-cha:r_-f.:orming elas>tomeric
organic binder and a char-forming additive throughout which
the intumescent material and st:a,xailizing agent are
dispersed, the elastomez:°ic b_~.nder being at: least partially
crosslinked, wherein the stabilizing agent consists
essentially of a compound sea.ectecl. from thm group cc>nsisting
of compounds within general formula I
CA 02159093 2003-06-25
60557-5084
3b
N H
l.r-1:,~ ,..-'~:~ R 3
v
~i~
R ,~ ~ ~,. R4
wherein Rl-R'~ inclusive are i.ndependent:ly selected from the
group consisting of hydrogen and alkyl groups having from
1 to about 5 carbon acorns, w:.i.t.h tk~e px~:>visos that:. ; i)
either both of Rl and R', or both R3 and R~ are alkyl groups
having from 1 to about 5 carborn. atoms; and i:i) the
stabilizing agent has a melting point ranging from about
105°C to about 125°C.
The stabilizing agexlt: :i.s either an antioxidant
and/or an antiozonant, defined as a compound or mixture of
compounds capable of reducing or preventing degradation of
the organic binder due to exposure of the inventive
compositions to oxygen and/o:rv ozone>. An "effective amount"
of stabilizing agent means t'ue stabilizing agent is present
in an amount sufficient
WO 94/24227 PCT/US94/01747
4
to render the expansion and compression set properties
of the composition after prolonged exposure to
oxidative conditions substantially unchanged. The
term "consists essentially of,'° when referring to the
stabilizing agent, means the stabilizing agent may
contain impurities which do not render compounds
within general formula I ineffective in prolonging the
expansion and compression set properties.
Surprisingly, stabilizing agents useful in the
present invention unexpectedly provide the intumescent
compositions of the invention with a good compression
set property without substantially hindering the
intumescent (expansion) property. The expansion
direction may be controlled by laminating the
composite of the invention to a metal foil, such as
aluminum foil or stainless steel foil.
As used herein the term "fire-retardant°' means
the compositions of the invention retard the spread of
flame, smoke, and/or fumes by charring under exposure
to heat, and thus not degrading under the heat of fire
and exposure to a water hose stream. The term
"intumescent" refers to swelling or expanding under
conditions of exposure to fire or heat, typically by
the expulsion of water vapor. The term '°chlorinated
elastomeric binder" refers to a substantially
crosslinked polymeric binder having a plurality of
chlorine substituent groups, allowing the composition
to retard fire spread. The term "char strength'° is a
measure of the strength of the expanded carbonaceous
residue ("char") formed from the composition of the
invention after exposure to temperatures above about
110°C.
The term °'compression set" is defined by the
tf
equation (1 - ) 100 where tf/t; is the ratio of
t;
WO 94/24227 PCT/US94/01747
composition thickness after compression and release to
original composition thickness (i.e. before
compression). Compression set is an indication of the
degree of cure of binder in a composition of the
5 invention. If the organic binder is not fully cured
(i.e., crosslinked), compositions within the invention
will deform inelastically after an applied force is
released. A composition within the invention is
acceptable if it has a compression set less than 20~,
more preferably less than 10%, most preferably 0%,
since the composition will substantially return to its
original thickness after compression and release.
The term "expansion ratio" when used herein in
reference to the inventive intumescent compositions is
the ratio of the expanded volume (caused by heating or
exposure to heat) to initial volume of a sample of the
composition of the invention under standard heating
conditions.
It was found that the degree of cure of the
organic binder affected the expansion ratio of
compositions within the invention. Compositions which
were undercured apparently provided no resistance to
the expansion gases from the intumescent component and
allowed the expansion gases to escape. On the other
hand, binders which were overcured apparently provided
excessive resistance to the expansion gases and did
not allow the expansion to take place. Control of
this process is critical to the development of a
composition that will perform as installed and
continue to perform after it has aged.
Stabilization agents useful in the compositions
r of the invention consist essentially of compounds
selected from the group consisting of diaryl
paraphenylenediamines within general formula I, the
diaryl paraphenylenediamines having melting point
ranging from about 105 to about 125C. An especially
preferred stabilizing agent is commercially available
CA 02159093 2003-06-25
60557-5084
from Goodyear Chemical Co., Akron, O~, under the trade
designation "Wingstay" 100 AZ. This is a proprietary
mixture of diaryl pare-phenylenediamines within
general formula I having a melting point ranging from
108-114°C. The starting materials far synthesizing
this particular stabilizing agent are believed to be
orthotoluidine, mixed xylidenes (ortho, mete, and
pare), and hydroquinane. Thus, reaction products
would typically comprise the di~ mixed (ortho, mete,
and/or pare) xylidene-, diorthatalyl-, and orthotolyl-
mixed (ortho, mete, or pare) xylidene
paraphenylenediamines. Although net wishing to be
bound to any particular theory, the methyl groups (and
longer chain alky3 groups, ~.f present) on the xylidene
appear to decrease the basicity of n~.trogens in the
reaction product, and thus apparently decreasing
premature curing of the compositions within the
invention.
The intumescent material is preferably a granular
hydrated alkali metal silicate such as described in
U.S. Pat. No. 4,273,8?9, fibs average particle size of
the intumescent material may range from about 0.2 mm
to about 2.0 mm, with about 95 weight percent of the
particles being greater than 0.2 mm. The preferred
alkali metal silicates described in the '879 patent
are granulated sodium silicates having a moisture
content of about 5 to about 30 weight percent, a
silicon dioxide (Sio2) to sodium oxide (Na20) ratio
ranging from about 2.0:1 to about 3.?5:1, and particle
sizes ranging from about 0.2 mm to 2.0 mm (i.e. about
95% of the particles being greater than 0.2 mm).
In preferred compositions of the invention, the
uncombined sulfur content is preferably less than 5
weight percent, more preferably less than 2.5 weight
percent, even mare preferably less than 1 weight
percent. It has been found by the inventor that when
the stabilizing agent known under the trade
CA 02159093 2003-06-25
60557-5084
7
designation "Wingstay" 100 A~ is included in the
compasition of the invention, not only dues the
composition exhibit acceptable compression set when
uncombined sulfur is zero or a de minimis amount, but
the expansion of the composition is also maximized.
It has also been found that use of curing
accelerators captaining sulfur linkages, such as
dimethylthiuram manosulfide (available under the trade
designation "Unads" from Akzo Chemicals, Akron, C~H)
and diorthotolylguanidine (~rOTG), provide surprisingly
improved long-term retention of char expansion of
material aged at elevated temperature, especially when
uncombined sulfur is ami.tted or held to a de minimis
amount.
In the rubber chemistry art, relative weights of
ingredients are frequently expressed as "parts per
hundred weight of elastomer'", ar "'~"HT~." Examples of
compositions within the invention having amounts of
ingredients expressed in this manner are given in the
Examples which follow. Cn a weight percentage basis,
the flexible, intumescent, fire-retaz°dant compositions
of the invention preferably campra.se from about 15 to
about 80 weight percent of hydrated alkali metal
silicate, from about 15 to about 40 weight percent of
an organic binder, at most ~0 weight percent of an
organic char-forming component, and from about 0.1 to
about 1.5 weight percent of diaryl
paraphenylenediamines within general formula I.
The organic binder is preferably an elastomer,
more preferably a chlorinated elastomer such as the
polychloroprene elastomers kpown under the trade
designation "Neoprene" and the like. Elastomeric
binders are preferably selected from a class of
organic char-forming elastamers such as natural rubber
and synthetic rubbers such as polyisaprene and
polychloroprene rubbers. An especially preferred
elastamer is polychloroprene because it has excellent
CA 02159093 2003-06-25
60557-5084
8
aging properties, good weatherability and by itself is
a char former when e~cposed to fire or heat.
As polychloroprene elastomers and like elastomers
are char-forming by nature, it is not necessary to use
a char-forming binder additive. Both °'Neoprene G and
"NeopreneMW" type chloroprene rubbers are useful in
the invention as organic binders. '°Neoprene G" rubber
differs from "NeopreneMW" Types in that the G types
are interpolymerized with sulfur and contain a thiuram
disulfide stabilizer. The W types are homopoly~n~ers of
chloroprene or copolymers of chloroprene and 2,3-
dichloro-1,3-butadiene, and contain no sulfur, thiuram
disulfide, or other additives that are capable of
decomposing to yield either free sulfur or a
TM
vulcanizer accelerator. Neoprene G polychloroprene
elastomers suitable for use in the invention have
number average molecular weights ranging from about
20,000 to about 950,000. Neoprene W polychloroprene
elastomers suitable for use in the invention have
number average molecular weights ranging from about
180,000 to about 200,000. The weight percentage of
chlorine in polychloroprene elastomers known under the
trade designation "Neoprene°' types W and G ranges .from
about 35 to about 37 weight percent, other useful
polychloroprene elastomers include those known under
the trade designations "Baypret~" (~iobay Corp.j and
"Butachlor~' (A. Schulman Co.~.
If the organic binder is non-char-forming, a
char-forming additive must be added to the
compositions of the :invention. Suitable non-char-
forming polymers include chlorinated polyethylenes
such as those known under the trade designations
"Parachlor" (from Uniroyal Chemicals and "Tyrin" (from
Dow Chemical); chlorosulfanated polyethylenes such as
those known under the trade designation "Hypalon" (du
Pont); polybutene; and polysulfide polymers.
CA 02159093 2003-06-25
60557-50&4
Char-forming resins useful in the compositions of
the invention include phenolic resins, palycarboimide
resins, urea-aldehyde resins, and melamine-aldehyde
resins. The general term "phenalic" includes
phenol-formaldehyde resins as well as resins
comprising other phenol--derived compounds and
aldehydes.
It is frequently desirable, although not a
requirement, to add a nitrogen-phosphorous compound
such as those known under the trade designation "NH
1197", from Great Lakes Chemical Corp., West Lafayette
TM TM TM
IN; "Amgard ND", "Amgard EDAF"', "Amgard NIA", from
Albright and Wilson, Richmond, VA; and "Exolet IFR-
10", Hoechst Celanese Corp. Somerset, NJ. When heated
in combination with the other components of the
composition, these materials contribute to the
formation of a highly refractory char.
Vulcanizing agents and activators are
crosslinking agents are added to help the elastomer
cure, and accelerators help control the rate of the
crosslinking reaction» Preferred crosslinking agents
are those which do not contain uncombined sulfur, such
as zinc oxide and magnesium o~cide. Dne particularly
useful vulcanization agent is ma~gr~esium oxide having a
high surface area to volume ratio, known under the
TM
trade designation "Maglite D" from C.P. Hall, Chicago,
IL. This magnesium oxide actually acts as a
wlcanization retarder when used in conjunction with
zinc oxide, since the two chemicals compete for
available chlorine or other halogen in the preferred
chloraprene elastomer binders. ~~.nc oxide is a Lewis
acid which serves as an activator far vulcanization.
The flexible, fire-retardant int:umescent
composites of the present invention can range from
soft putty-like compositions to a hard rubber. This
range of hardness is achieved by selectively varying
CA 02159093 2003-06-25
60557-5084
amounts of the individual components of the
composites.
For example, an effective amount of a plasticizes
is preferably added to the inventive compositions to
5 help make them flexible, preferably extrudable. The
amount of plasticizes added ranges from about 2.5 to
about 7.5 weight percent based on weight of
composition. Preferred plasticizers include
naphthenic oil (cycloparaffin) phosphates, such as
10 isodecyl Biphenyl phosphate, other phosphate esters,
and the like. one preferred plasticizes is that known
under the trade designation "'Calsol" ~24t7,
commercially available from Calumet Lubricants,
Chicago, IL. This particular plasticizes is a Beverly
hydrogenated naphthenic oil phosphate.
The compositions of the invention may include
fillers to adjust hardness and reduce cost. Fillers
which may be added to the compositions of the
invention include quartz sand (silica), colorants,
clay, fly ash, blowing agents, perlite, vermiculite,
inorganic fibers such as glass fibers and mineral
wool, and organic fibers. Fillers may make the
composition stiffer ("harder"). A preferred filler is
alumina trihydrate, commercially available under the
trade designation "Solem" SB332, from J.M» Huber,
Solem Division, Norcross, GA» Colorants are useful
for product identification; preferred colorants are
the various forms of iron oxide (FezO, Fe30,~, or Fe2U3) .
Conventional antioxidants and ant.iazonants 'may be
used in the inventive compositions, provided they do
not interfere with the beneficial effects of the
stabilization agent. One useful conventional
antioxidant is that known under the trade designation
T111
"Agerite Stalite S" commercially available from R.T.
Vanderbilt, Norwalk, CT, which comprises a mixture of
octylated diphenylamines. A useful conventional
antiozanant is a wax antiozonant commercially
CA 02159093 2003-06-25
60557-5084
11
available from Uniroyal Chemical, Akron, oIi under the
trade designation '"Sunproof ~~~ " This particular.
antiozonant .is a blend of aliphatic hydrocarbons. The
hydrocarbons tend to rise to the outer surface of the
expanding composition and provide a barrier to ozone.
The ingredients which comprise a precursor mill
base of the cured intumescent co~npositi.ons
of the invention are typically and preferably mixed
using a conventional rubber mill or 5anbury processing
to equipment. After compounding, the precursor mill base
is calendered into 0.635 cm thick sheets and laminated
with alurninurn foil. In another procedure, the
precursor mill base is formed into 0.635 cm thick
sheets on a rubber mill and then laminated in a press
on one side with aluminum foil and hexagonal wire and
on the other side with fifteen mil (0.038 cmj steel
foil. The laminated sheets are then vulcanized in an
oven at about 90°C until the campression set of the
intumescent composition reaches an acceptable level,
which generally takes 5 or 6 days.
In a preferred article embodiment of the present
invention, the expansion direction of the intumescent
composition of the invention is effectively controlled
by laminating a restraining layer thereto. Use of a
restraining layer can provide assurance that the char
formed during the intumescent reaction to fire and
heat is generated so that the penetration cavity is
optimally filled. Upon exposure to temperatures
greater than about 110°C, the restrained intumescent
sheet expands in a direction substantially
perpendicular to the restraining layer, i.e., into the
penetration, so as to optimally fill it rather than
expanding isotropically as would be the case with an
unrestrained intumescent sheet. L7seful restraint
layers are disclosed in commonly assigned U.S. Pat.
No. 4,467,577 .and include metal foils, sheets, and
screens made from aluminum, copper, steel, and lead;
WO 94/24227 PCT/US94/01747
12
heavy paper and cardboard such as a Kraft-type paper;
high temperature rubber and plastic sheets such as are
made from silicones and epoxies, screen and cloth made
from inorganic fibers
such as glass fibers and high temperature organic
fibers such as polyaramid.
Preferably an intumescent precursor composition
of the invention is either laminated to aluminum foil
on one side or to aluminum foil and hexagonal wire on
one side and steel sheeting on the other. The
intumescent precursor composition is cured by
vulcanizing by exposure to heating means, such as a
hot air oven. The vulcanization temperature must be
controlled to avoid pre-expansion of the intumescent
but sufficiently high to allow the elastomer to cross-
link to the proper compression set. As cure
increases, compression set decreases. A five or six
day vulcanization at 90°C (194°F) is preferred in
order to achieve compression sets less than 20%.
TEST METHODS
To determine compression set of an intumescent
composition of the invention, 1 inch (2.54 cm)
diameter discs were die cut from a sheet of a
precursor composition. A MTS (Material Testing
System) equipment was used to compress the samples.
The initial thickness (t;) of the sample was measured.
The sample was compressed to 1/2 of its original
thickness and held in compression for 30 seconds. The
force was released and the sample was removed. After
no less than 5 minutes and no more than 10 minutes,
the final thickness (tf) was measured.
The percent compression set was determined by the
following equation:
COMPRESSION SET = (1 - tf/t;) X 100
A high number for compression set indicates poor
recovery of the sample°s original thickness; whereas,
a low number indicates good recovery. Compression set
CA 02159093 2003-06-25
60557-5084
13
is indicative of the degree of cure. A poorly cross-
linked polymer (cure is minimal will exhibit poor
recovery and high compression set. Dn the other hand,
a well cross-linked polymer (cure is advanced) will
exhibit good recovery and low compression set. The
preferred compression set values are less than 20%.
To determine the expansion ratio, the die cut
disc was waxed, cooled and the initial volume measured
from its loss in weight in water. The disc was then
placed into a preheated furnace at 350°C for 15
minutes. The sample was removed and the volume of the
expanded material (final volumes was determined by
using Archimedes principle of water displacement,. The
expansion is represented bye
X = Final Valume/lnitial Volume
High volume expansions are the most desirable.
Therefore, an expansion ratio of at least 8 is
preferred for this invention.
Materials bescriptioa
"Neoprene W" is the trade designation of
polychloroprene, a 35-3~% chlorinated elastamer,
available from E.I. DuPont de Nemaurs, Wilmington, DE.
"Wingstay" 100 AZ is the trade designation of an
antiozonant and antioxidant commercially available
from Goodyear Chemical c:o., Akron, OH, and is a
mixture of diaryl pare-phenylenediamines having a
melting point ranging from about 108 to 114°C;
1M
"Wingstay" 2~ is the trade designation of an
antiazonant commercially available from Goodyear
Chemical Co., Akron, DH, and is a styrenated
diphenylamine liquid adsorbed on fumed silica so that
the fumed silica is 30% by weight
CA 02159093 2003-06-25
60557-5084
14
rM
~~BritSil HS-240" is the trade designation of a
hydrated sodium silicate, commercially available from
Philadelphia Quartz Co., Valley Forge, PA;
TM
"Solem" 58332 is the trade designation of alumina
trihydrate, commercially available from J.M. Huber,
Solem Divisian, Norcross, GA;
TM
"Agerite Stalite S" is the trade designation for an
antioxidant commercially available from R.T.
Vanderbilt, Norwalk, CT, which compr~.ses a mixture of
octylated diphenylamines;
"Sunproof Jr~." is the trade designation for an
antiozonant comprising a waxy blerad of aliphatic
hydrocarbons, cammercially available from Uniroyal
Chemical, Akron, OH;
TM
"Maglite D" is the trade designation for a
vulcanization agent comprised of magnesium oxide
having a high surface area to volume ratio,
commercially available fram C.P. ~~all, Chicago, IL;
and
"Mag Beads" is the trade designation of a vulcanizing
agent comprised of a dispersion of magnesium oxide in
a hydrogenated cyclaparaffin oil, commercially
available from Elastochem, Chardon, OH.
"Iron Oxide PF-~5" is the trade designation for iron
oxide commercially available from Bailey Engineers,
Fairfield, AL.
TM
"Calsol" 8240 is the trade designation for a
plasticizer commercially available from Calumet
Lubricants, Chicago, IL, and is a severly hydrogenated
cycloparaffinic oil;
CA 02159093 2003-06-25
60557-5084
5
"Elastozinc" is the trade designation for a
vulcanizing agent, comprised of a dispersion of zinc
oxide in a hydrogenated cycloparaffinic oil,
commercially available from Elastochem, Chardan, OH.
"DOTG" is the trade designation of an accelerator,
diorthotolylguanidine, commercially available from
American Cyanamid, Charlotte, NC.
r~
l0 "Unads" is the trade designation of a dimethylthiuram
monosulfide accelerator, commercially available from
Akzo Chemicals, Akron, OH.
n~
"PB-RM-S-80" is a trade designation for a sulfur
15 dispersion in an ethylene copolymer binder, from
Elastochem, Chardon, OH.
Microfine wettable sulfur is a product of George Gulf
Sulfur, tlaldosta, GA.
In the following Examples, all parts and
percentages are by weight unless otherwise specified.
Euampxes
Examples i-13 and Comparati~re Examples A-E
The expansion and compression set properties of
compositions of Examples 1-13 (inventive) were
compared with the compositions of Comparative Examples
A-E (the compositions are presented in Tables 1-3j.
The compositions were compounded in a laboratory
utilizing a miniature mixer of 350 mL capacity with
cam mixing blades. The mixer was powered by a torque
rheometer known as "Plasticorder"° Model DR-2071,
commercially available from C.~. Erabender
Instruments, Inc., South Hackensack, NJ. It is
referred to as the "5rabender" mixer.
CA 02159093 2003-06-25
60557-5084
16
The mixing and sample preparation procedure were
as follows:
1. The elastomer was added to the mixer and
mixed for five minutes.
2. With the exception of hydrated sodium
silicate, the remaining ingredients were added and
mixed within the next 15 minutes.
3. The hydrated sodium silicate was added and
the composition mixed for 5 minutes.
4. The material was removed from the mixer and
pressed into 0.25 inch (0.635 cm) thick sheet with
0.002 inch (0.127 mm) thick aluminum foil laminated on
one side. A Wabash press, Model 75~1~1~-2TM, at 60°C
(140°F), was used to press and laminate the material.
5. The aluminum foil laminated sheet was then
placed far 35 days in an oven heated to 90°C (1~4°Fj.
6. Samples were taken periodically during the 35
days by die cutting 1 inch (2.54 cm) diameter discs
from the sheet.
7. Compression set and expansion ratio were
determined on the compositions of Examples 1-1.3 and
Comparative Examples A-E. The data presented in
Tables 4-6 provided an assessment of the advancement
of the crosslinking of the el.astomer and of the
accelerated aging characteristics of the compositions
(compression Set and expansion ratio). It can be seen
by comparing the inventive compositions (those
containing the stabilizing agent known under the trade
designation "Wingstay" 100 AZ) with the comparative
compositions (those not containing "Wingstay~ 100 ,AZ)
that excellent expansion and compression sets were
obtained.
CA 02159093 2003-06-25
60557-5084
17
TABLE 1 COMPOSITIONS
VARIODB CONCENTRATIONS OF 8~1L~"UR IN INTUMESCENT
FIRESTOp COMF08ITIONB
INGREDIENTS
SAMPLE
DES~:GNATION
EX.
1
EX.
2
EX,
9
EX,
4
EX.
5
PHR* PHR PHR PHR PHR,
TM 100 100 100 100 300
Neo rene W
TM
Win sta 29 1.00 100 1.00 1.00 1.00
Win sta TM 100AZ 3 . 00 3 00 3. 3. 00 3. 00
t30
Maglite D 4.00 4.00 4.00 4.00 4.00
Alumina Trihydrate32.1 32.1 32.1 32.1 32.1
Iron Oxide PF-95 5.00 5.00 5.070 5.00 5.00
TM
Calaol 8240 _21,? 21.7 21."' 21.7 21.7
~~ -
ElastozincM 5.68 5.68 5.~a8 5.68 5,68
Sulfur 2.00 4.00 1.00 0.50
TM
Brit Sil HS-240 230 230 230 230 230
*pHR = parts per hundred weir.,~ht ~f el.sstomer
CA 02159093 2003-06-25
60557-5084
1$
TABLE 2
INTUMESCENT FIRESTOP COMP08ITIONB
INGREDIENTS SAMPLE
DESIGNATION
EX.
b EX.
7 EX.
8 EX.
9 A
B C
PHR PHR PHR I'HR PHR PHR PHR
Neoprene W 100 100 100 i00 I00 100 100
Agerite S.S. ---. ___. ___ _~ 2,0( 2.06 2.00
Wingstay 29 ~ '~ 1.00 1.00 1.00 ---- --- --
Wingstay 100AZ3,.00 --3 3.0(1 3.00 ___ ~.. ~_
Maglite deb __~ 4 ~ _.__. 4.00 4.00 4.00
D~ -. .
Mag BeadsTM(Mg0)____ b.l5r _--~_ b.15~ ---- ---~ ----
Alumina
Trihydrate 32.1 32.1 32.1 32..1 32.1 32.1 32.1
Iron Oxide 5.00 5.00 ~ 5.005.00 5.00 5.00 5.00
PF-95
Calsol 8240TM21.7 19.b= 21.7 19.6= 2I.7 21.7 21.7
Elastozinc'M 5.68 5.68 S.bB 5.68 5.68 5,68 5.68
2 S a l fur . 25 ---- -.-.- .~__- l , ---. ---
0 tap
TM
PB (RM-S)-80 _--_ 1.25 ---- 1.25 -- ---- -
DOTG TM 1.50 --~- 1.50 1.50 ~---- ---~ ---
Unads TM 1.00 ---- 1.00 1.010 ---- ---~ --
Sunproof Jr.TM---- ---- ----.-_.~ ---- --- 3.00
-
2 Brit Sil HS-240TM230 230 230 230 230 230 230
5
dispersion of Mg0 in hydrogenated oil e.~uivalent to 4.00 PHR M,gO
compensated for the amount of oil in Mag Beads
3 dispersion of sulfur in an ethylene copolymer binder e~urvalent to 1.00 PHR
sulfur
CA 02159093 2003-06-25
60557-5034
19
TAHLE 3
=NTUMESCENT F"TRESTOB coMPOSTTZON8
INGREDIENTS SAMPLE PI
DESIGNATIO
EX. EX" 11 D E EX. EX. 13
10 12
PNR PHR PHR PHR PHR PHR
Neoprene W 100 100 100 100 100 100
Agerite S.S. 2.00 2.00 2.00 2.00 2.00 Z.00
Wingstay 29 -- _..-_ _~ ..__
Wingsta3100AZ2.00 2.00 --- --- 2.00 2.00
Maglite DT"" 4.00 4.00 4.00 4.00 4.00 4.00
Mag Beads'"(Mg0)_.~ ~... _.._- .._.....
Alumina 32.1 32.1 32. 32.1 32.1 32.1
I
Trihydrate _
Iron Oxide 5.00 5.00 5.00 5.00 5.00 5.00
PF-95
Ca1so18240'""21.7 21.7 21.7 21.7 21.7 21.7
Elastozinc"" 5.6$ 5.68 5.6$ .5.68 5.6$ 5.68
Sulfur .25 --- ---~- .ZS .ZS ----
2 PB (RM-S)-80 --- ~__ ~ __,.
0
DOTG ~" 1.50 I .50 I .50 I .50 1,50 I ,50
UnadS f"" I .00 1.00 I .00 1.00 I .00 I .00
Sunproof Ir:""~ -~ ~ 3.00 3.00
Brit Sil HS-240'"'230
230
230
230
I 230
230
TABLE 4
EXPANS10N RATIOS (?~ AND COIvIPRESS10N SETS (CS) OF
SAM1"LES AGED AT 90'C
DAYS SAMPLE
DESIGNATION,
X/CS
AGES
AT EX. 1 EX. 2 EX. 1 EX. 4 EX. 5
90 c
3 1 12.8/30.914.7/17.9 15.5/17.112.9 27.3 13.6/31.0
5
2 18.2/16.010.9 7.91 9.62/5.195.28 11.1 11.9 16.4
_..... ., w..,
3 16.8 15.97.62,4.40 6.5279 1Ø5 7.7914.6 9.84
5 11.3/6.465.96/4.15 5.62/4.969.22/4.55 9.55/5.15
7 9.45/4.315.04/3.53 4.99/4.526.27 2.49 '7.94 3.89
4 14 6.54/5.394.12/8.97 4.46/15.72S34/4.25 6.21/2.89
0
21 6.12/6.084.59 2.86 3.99/6.375.35 1.26 5.77 3.29
5.77/5.134,51/3.93 3.88/4.965.41/1.79 5.84/3.33
WO 94/24227 ~ ~ ~ ~ ' PCT/US94/01747
TABLE 5
5 E%PANSION RATIOS (%) AND COMPRESSION SETS (CS) OF
SAMPLES AGED AT 90°C
DAYS SAMPLE
AGED DESIGNATION,
X/CS
_
10 AT EX.6 EX.7 EX.8 EX.9 A B C
90'
C
1 11.8/26.812.7/22.012.8/32.513.3/9.654.49/42.36.98/32.65.64/21.8
2 17.2/14.111.519.5114.7/20.75.11/10.68.88/28.87.54/38.97.61/21.0
3 10.5/11.78.22/6.5313.2/16.44.99/5.3311.3/28.38.12/37.810.4/22.5
1 5 8.22/10.26.07/4.5510.8/11.34.86/5.7113.7/27.69.17/29.411.6/25.4
5
7 9.33/8.535.61/5.469.81/8.74.95/3.9313.9/20.69.70/31.913.0/23.1
1
14 9.38/8.125.00/3.689.04/7.695.09/3.5712.9/9.3313.0/23.914.3/17.2
21 8.59/7.134.93/1.328.31/6.545.38/4.7810.7/9.4013.2/22.713.5/15.0
35 8.46/4.914.91/1.477.90/4.765.40/3.229.45/8.7512.7/17.212.4/10.1
TABLE 6
E%PANSION RATIO (%) AND COMPRESSION SETS (CS) OF
SAMPLES AGED AT 90°C
DAYS SAMPLE
DESIGNATION,
X/CS
AGED
3 AT 90' EX. 10 EX. 11 D E EX. 12 EX. 13
0 C
1 8.67/33.66.91/35.35.82/39.76.86/35.111.3/31.48.42/33.4
2 13.2/28.014.8/31.87.40/37.610.7/29.618.0/22.514.3/24.8
3 13.8/17.415.7/24.410.2/34.011.5/22.613.4/18.616.2/20.9
5 13.4/14.816.2/16.415.3/29.213.5/18.913.3/12.615.3/12.6
3 7 12.7/16.814.4/14.316.2/27.213.1/19.214.1/9.9113.4/9.54
5
14 12.9/10.813.8/9.6714.3/16.113.8/17.112.6/7.8311.8/7.48
21 13.2/9.2112.3/10.613.9/15.814.2/15.111.7/7.4211.1/7.14
11.3/7.6011.1/7.0511.6/9.9511.9/12.810.2/3.719.90/4.71
The composition of Example 13 was also compared
with a composition of similar nature except using
previously known and conventional ingredients, as
CA 02159093 2003-06-25
60557-5084
21
detailed in 'table "3. The expansion ratio and
compression set data are given in Table 8.
TABLE 7
COMPARI80N OF F8-195 (PREBE~TT PRODOCT~ AND
Example 13 COMPOS1TION8
Composition Function FS-195 Ex.
13
Product
PHR PHR
% %
Neo reneMW* binder 100 100
23,0 24.5
A erite S.S.* antioxidant 2,06 2.00
0,474 0.490
Win eta TM100AZ**antiazonant -__-~- 2.00
---- 0.490
Sunproof Jr.**TM Antiozonant -~-~ 3.00
----- 0.735
Maglite D**"'" vulcanixer ---- 4.00
---- 0.980
Alumina filler -~--- 32.:1
---- 7.87
Trih drate**
Iron Oxide PF- colorant -M~-~- 5.00
----- 1.23
95**
Min-U-Sil-10 filler 32.0 ----
7.37 -___
2 Micron***
0
BTL 29-407 char former 23.8 5.48 ---- ----
(Phenolic
resin ***
Calsol 8240**'"" lasticizer ---- ---- 21.~ 5.32
DOP (dioctyl plaeticizer 3S.b 8.20 ----~ ----
hthalate)***
Elaetozinc**TM vulcanizes --_-- ---- 5.68 1.39
DOTG**TM accelerator ---- ---- 1.50 0.368
Sulfur*** vulcanizes 0456 0.105 ~----~----
3 Red Lead*** vulcanizes 5.1"7 1.19 ----~ --_-
0
UNADS'M TMTM * accelerator 5.1I 1.19 1.00 0.245
BRIT Sil HS-240*TMintumes~~;~nt 230 53.0 230 56.4
*used in both the present product l~nown under the
trade designation "F~-~~5", from 3M, and a
composition within the invention (Example 13)
**used only in the Example 13
***used only in the "FS--1'~5" product
CA 02159093 2003-06-25
60557-5084
22
TABLE 8
EXPANSION RATI08 (8) AND COMPRE88ION 8ET8 (C8)
OF BAMPLEB AGED AT 90°C ~'OR F8~-19.5 (PRESENT PRODOCT)
AND E7CAMPLE lit
SA~~~SPLE DES1GNATTC1N,
X ~S
..~.._,~,..._...._._..._,~
DAYS AGED AT 90C PRESENT FS-195 EX. 13
~'Rta~7~JCT
0 (not cured) _ 3.20/46.0
1 -~-~-~ 8.42/3.4
... 14.3/4.8
3 --- 16.2/20.9
5 - -~ ~ 15 . 3 12 . fi
7 X4,2 25.8 13.4 9,54
8 ~ 4 . 4 1"7. 3 ___.~
9 :~3.7~'~14 ___..
10 . ~4.5/2~.3
14 ~1.~_af l'~.9 ~ 11$/x.48.
~.
21 :~0,~/~1?4 11.1/rr.l4
35 a,2S/14.0 k 9.9a/4.~1
.._._w~._.. ~..._..._.~. _~
Examples i~-16: Wrap/8trips and Composite Sheets
For preparation of the fire retardant
compositions of these examples, a water cooled F-80
Banbury mixer was used. All ingredients except the
elastomer, plasticizes, and ~.ntumescent were
prebatched by dry blending. The elastomer,
plasticizes and intumescent were charged into the
Banbury mixer at low speed. The ram was lowered and a
ram pressure of 3.4 x 1.0~ N/xnz was x~.aintained. When the
temperature reached 82°C (180°F), the batch was
dropped, extruded, and calendered into 0.535 cm (0.25
inch) sheets which were laminated on one side with
aluminum fail for Examples 14 and 15. The sheets were
cured in an oven at ~0°C for 5 to 5 days.
For producing the laminates of Example 16, the
extruded material from the Banbury mixer was placed on
a 2 roll rubber mill, milled to X7.535 em (0.25 inch)
thickness, cut to size, and laminated on one side with
WO 94/24227
PCT/US94/01747
23
0.015 inch (0.38 mm) thick galvanized stainless
hexagonal wire screen and on the other side with
0.0025 inch (0.00635 cm) aluminum foil.
The laminate of Example 14 employed the flexible
fire-retardant intumescent composition as indicated in
Table 7 as Example 13 laminated to an aluminum foil.
This laminate was evaluated in restricting collars and
plastic pipe devices according to ASTM Test Method E
814. These laminates were installed around 10.2 cm
diameter polyvinyl chloride pipes. In comparison, a
laminate comprising compositions similar to the
composition of Table 7, denoted as 'Present FS-195
Product," was laminated to aluminum and tested in the
restricting collars and plastic pipe devices according
to the ASTM Test Method. Compared to the comparative
product, the inventive laminate surpassed flame-
through time by about one hour. That is, the mean
flame-through time for the laminate of Example 14
containing the composition within the invention was
approximately three and one half hours, whereas the
mean flame-through time for the comparative product
assembly was approximately two and one half hours.
For Example 15, the composition of Example 13 was
applied to an aluminum foil backing as described above
for Example 14. The laminate of Example 15 was tested
for two hour flame-through, one hour temperature, and
one hour backup hose stream tests according to the
above mentioned ASTM Test Method E 814. The laminate
of Example 15 was wrapped around a 20 inch (50.8 cm)
diameter steel pipe placed in a 29 inch (73.7 cm)
diameter opening of a 2.5 inch (6.35 cm) concrete
slab. Four wraps of the laminate were used on steel
pipe which was insulated with 3 inches (7.62 cm) of
glass fiber insulation. The two hour flame-through,
one hour temperature, and one hour backup hose stream
tests were passed by the laminate within the
invention.
.~
WO 94/24227 '~ PCT/US94/01747
24
In Example 16, the flexible intumescent fire
retardant composition of Example 13 was laminated
between aluminum foil and galvanized hexagonal
stainless steel wire screen as above described, and
was installed on one half of a 32 inch (81.3 cm)
y
square opening in a 4.5 inch (11.4 cm) concrete slab.
A comparative product laminate using the composition
of Table 7 indicated as °°FS-195°° laminated
between
aluminum foil and galvanized hexagonal stainless steel
wire screen was installed on the other half of the
square opening. In each half a 4 inch
(11.4 cm) diameter copper pipe was installed. The
installation followed the requirements and is
equivalent to Underwriters Laboratories' System 93 for
the bottom application. The laminate within the
invention passed the one hour flame-through test and
the hose stream test.
Various modifications and alterations of the
invention will become apparent to those skilled in the
art. The above disclosure and examples are not
intended to limit the scope of the intended claims but
are merely illustrative thereof.