Note: Descriptions are shown in the official language in which they were submitted.
21S9588
~O g4/23777 PCT/E:94/00020
Description
INTRADERMAL INJECTION DEVICE
Technical Field
This invention relates to a device for ~dministering intradermally
5 a medicament in liquid form.
The use of the term "intradermal" herein is intended to embrace
subcutaneous.
Background Art
Many drugs, active ingredients or medications, hereinafter
10 referred to collectively as medic~rnents, require ~dministration by
injection. This mode of ~iministration tends to be inconvenient and
also painful. Patients may be required to self-~-lmini~ter a medicament,
such as insulin in the case of diabetics, and various alle~ ts have been
made to make this mode of ~dministration more convenient, thereby,
15 increasing patient compliance. Thus, "pen" type injectors have been
proposed.
There is a need for a device which can be used to automatically
inject a metered dose of a medicament intradermally with the minimum
of trauma. Such a device should ideally include a needle or needles
20 which enter the skin to a predetermined depth and allow precise
penetration of the skin by the patient.
Disclosure of the Invention
The invention provides a device for ~dmini~tering intradermally
a metered dose of a medicament in liquid form, said device comprising
25 means for creating a vacuum so as to secure the device to the skin
during medicament delivery, means for penetrating one or more skin
layers and delivering said metered dose and means for releasing said
WO 94123777 PCT/IE94/00020
~ 59S88 2
vacuum for removal of Ihc device following delivery of the metered
dose.
The device according to the invention can be used with a
minimum of trauma by pa~ients as well as by medical and nursing staff,
5 to administer a range of medicaments, including peptides and
polypeptides, by the intradermal route as hereinbefore defined.
Preferably, the means for creating the vacuum comprises a
piston arrangemen~ and an associated system of air channels.
Further, preferably, the means for penetrating the or each skin
10 layer comprises a plurality of hollow needles in commllnication with a
reservoir for the medicament.
The creation of the vacuum can cause the skin to be drawn
towards, and penetrated by, said penetration means.
ln one embodiment, the vacuum is created circumferentially of
15 the penetrating means. For example, the vacuum can be generated
circumferentially of a housing for the device.
In the case when the penetrating means comprises a plurality of
hollow needles, a vacuum can be created circumferentially of each
needle defining said penetrating means.
The piston arrangement, when such is present, is preferably
actuated by a plunger mechanism integral with the piston. Further,
preferably, the piston impinges on a displaceable membrane defining a
boundary wall of the reservoir so as to expel the contents of the
reservoir through the penetrating means when the piston is depressed.
Further, preferably, ~he plunger has an internal air duct which is open
to the atmosphere.
.ro 94/23777 ~ PCT/IE94/00020
~1~9588
When it is desired to release the vacuum and, thereby, the device
from the skin of a patient following administration of the medicament,
the piston is retracted.
The penetrating means can be movable within the device, such
5 that when the vacuum is generated, said penetrating means is
simultaneously or sequentially displaced towards the skin for
penetration thereof.
According to a preferred embodiment, actuation of the device
simultaneously creates the vacuum and causes movement of the skin
10 penetrating means for penetration of the skin.
For example, following application of the device to the skin and
actuation thereof, the vacuum generated can draw the skin against a
plate structure in the device disposed on a skin-contacting surface
thereof. The plate structure then maintains the skin in position while a
15 set of needles is caused to descend and pass through a plurality of
apertures in said plate structure and enter the skin to a predetern~ined
depth.
Alternatively, a set of needles is fixed in position on such a plate
structure and the act of drawing the skin against the plate causes the
20 needles to penetrate the skin. The medicament is then delivered as a
single dose through the set of needles.
The wlthdrawal of the needles from the skin can release the
vacuum or vlce versa.
The device according to the invention is preferably in the form
25 of a disposable pre-filled product for once-off use, although the device
can be readily adapted for continued use by the provision of filling
means for replenishing the reservoir. ln the case of a refillable device,
the device is suitably provided with means for indicating when a
quantity, corresponding to one or more metered doses, has been
30 inserted therein.
WO 94/23777 PCT/IE94/00020
2~,S9S~ 4
The medicament can have almost any viscosity, provided that it
can be dispensed through the penetrating means at a rate which is
pharmaceutically effective and acceptable.
The penetrating means will suitably be manufactured from
5 medical-grade stainless steel. However, the penetrating means can also
be composed of a suitable inert plastics material.
Other parts of the device may also be manufactured from
suitable plastics materials.
The device according to the invention is suitable for the
10 administration, including self-administration, of a wide range of
medicaments, including peptides and polypeptides. The device
according to the invention is especially suitable for the ~clministration
of insulin.
The device according to the invention is also suitable for the
15 administration of vaccines in methods of vaccination.
The device according to the invention can be used to inject a
medicament intradermally or subcutaneously automatically as desired
by suitable adjustment of the length of the needles or the extent to
which the needles or other penetrating means are movable relative to
20 or within the device.
The device according to the invention allows for precise
penetration of the skin which is an advantage over currently available
automatic injection devices.
The device according to the invention can also be used to inject
25 controlled release formulations, suitably in a lipophilic base, allowing
for convenient subcutaneous injection of depot formulations.
The device may be enclosed in a sterilised package until required
for use. Furthermore, the needles or other penetrating means will
094/~3777 21S9S~8 ~/IE94/00020
normally be either covered by a peel-off strip or stuck in an
impermeable pad to prevent the medicament from leaking out of the
device while in storage.
The invention will be further illustrated by the following
5 description of embodiments thereof given by way of example only with
reference to the accompanying Drawings in which like parts are
indicated by like reference numerals.
Brief Description of the Drawings
In the accompanying Drawings:
Fig.l is a front elevation in section of a first embodiment of
the device according to the invention;
Fig. 2 is a plan view of the device of Fig. 1 taken on the line
11-11;
Fig. 3 is a front elevation in section of a second embodiment
of the device according to the invention; and
Fig. 4 is a plan view of the device of Fig. 3 in the direction of
the arrow.
Modes for Carrying Out the lnvention
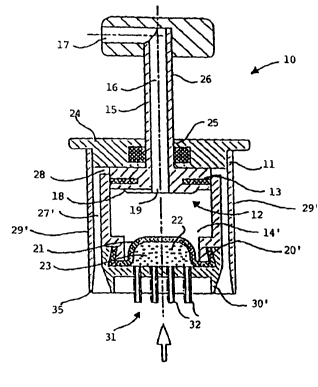
Referring to Figs. 1 and 2 of the Drawings, there is indicated
20 generally at 10, a device for administering intradermally to a patient a
metered dose of a medicament in liquid form. The device 10
comprises a housing 11 and a piston arrangement, indicated generally
at 12, comprising ~ piston 13 movable in sealing engagement within a
hollow chamber 14 disposed internally of the housing 11. The piston
25 13 is provided with an integral plunger mechanism 15 for actuation by
the person administering the medicament. The plunger 15 has an
internal airway 16 which is substantially L-shaped and which
WO 94/23777 ~CT/IE94/00020
~ 59~ 6
communicates with the atmosphere via an outlet 17. End 18 of the
piston 13, which travels within the chamber 14, serves to close the end
of the chamber 14 distal from the skin, in use, apart from a second
outlet 19 of the airway 16. The end of the chamber 14 proximal to the
5 skin, in use, is defined by an elastomeric membrane 20 defining a
boundary wall 21 of a reservoir 22 for the medicament 23.
The piston arrangement 12 is similar to that used in a
conventional hypodermic syringe and thus end 18 of piston 13 which
travels within chamber 14 has a circumferentially extending flexible
10 grooved sealing means.
The housing 11 has a cap 24 with an aperture 25 through which
shaft 26 of the plunger mechanism 15 passes in sealing engagement
therewith.
An air chamber 27 which communicates via extension 28 with
15 the space above end 18 of the piston 13 when the piston is depressed is
disposed intermediate walls 29 of the housing 11 and the walls of the
chamber 14 and is continuous within a skin-contacting part 30 of the
housing 11 containing penetrating means 31 comprised of a plurality of
needles 32. The air chamber 27 is also in communication with an
20 aperture 33 surrounding each needle 32.
The air chamber 27 can be replaced by a plurality of narrow
bore air chambers, typically four, spaced equidistantly about the
circumference of the device 10 and being continuous with the space
above end 18 of the piston 13 when the piston is depressed and with the
25 skin-contacting part 30.
The device 10 is provided with a circumferentially extending
elastomeric sealing ring 34 for engagement with the skin in use.
ln use, when it is desired to administer a liquid medicament such
as insulin, the person administering the medicament, who may be the
30 patient, selects the site on the skin at which the medicament is to be
~O 94/23777 21 59 588 PCT/IE94/00020
adrninistered. The device lO is applied to the skin, the plunger
mechanism 15 is depressed in the direction of the skin, causing the
piston 13 to move towards the membrane 21.
The hollow chamber 14 is open to the atmosphere via outlet 17.
5 Depression of the piston 13 causes a reduction in pressure in space 28
and causes air to be sucked into said space via the air chamber 27,
resulting in the creation of a vacuum at the site of application of the
device 10. The creation of ~he vacuum causes the skin to be drawn
towards and penetrated by the needles 32. As the plunger is fully
10 depressed, the medicament 23 is delivered through the needles 32 in
response to the action of the piston 13 depressing the membrane 20
which forces the medicament 23 out of the reservoir 22 and into the
needles 32 followed by intradermal delivery. To release the device 10
from the skin (not shown) following medicament delivery, the piston
15 13 is retracted releasing the vacuum and thereby releasing the device
10.
Best Mode for Carrying Out the Invention
Referring to Figs. 3 and 4 of the Drawings there is illustrated a
modification of the device lO depicted in Fig. 1 and wherein like parts
20 are accorded like reference numerals.
The hollow chamber 14' and the skin-contacting part 30' each
has a slightly different shape relative to the hollow chamber 14 and the
skin-contacting part 30, respectively, of the embodiment depicted in
Fig. 1. Likewise, the membrane 20' in sit~ has a shape dictated by the
25 interengaging parts l 4' and 30'.
In addition to skin-contacting part 30', there is also provided a
peripherally-extending flange 35 which, in use, rests on the skin (not
shown). An air chamber 27' is formed intermediate walls 29' of the
housing l l ~nd externally of the hollow chamber 14' and the skin-
30 contacting part 30'.
WO 94/23777 PCT/E:94/00020
~ S9~ 8
In the embodiment of Fig. 3, the needles 32 are not eachprovided with an aperture in contact with the air chamber 27'. Rather,
when the flange 35 engages the skin, in use, and the piston 13 is
depressed, air is sucked from the air chamber 27' into space 28 above
5 piston 13, so as to create a vacuum circumferentially and externally of
the part of the device 10 housing the needles 32. The creation of the
vacuum causes the device 10 to be held firmly in contact with the skin
during delivery of the medicament. To release the device 10, the
piston 13 is retracted in the same manner as for the embodiment
10 depicted in Fig. 1.