Note: Descriptions are shown in the official language in which they were submitted.
CR113/AA
219933
1
ABSORBENT ARTICLE
The present invention relates to an absorbent
article. Mere particularly the invention relates to an
elasticated absorbent article wherein the elastication is
provided by an elastomeric hot melt adhesive in the solid
state, which adhesive has an elasticity in the molten
state, as shown by its essentially Newtonian behaviour at
the processing (application) temperature, which is very
low and in rnany cases negligible.
In the manufacture of many different types of
article it is necessary to bond an elastic material to a
non-elastic substrate. One example is in the manufacture
of disposab7.e diapers where elastic strips are used to
provide leg and waist elastication. Typically, the
elastic strips consist of stretchable rubber which is
fixed to they body of the diaper by layers of adhesive,
for example hot melt adhesive, affixed to both surfaces.
This arrangement involves a number of difficulties.
For example, the elastic strip is often covered with an
anti- blocking agent such as talc during manufacture to
prevent the strip bonding to itself. However, this
anti-blockir,~g agent can, in turn, render bonding of the
elastic strip to the body of the article much more
difficult. Furthermore, since the hot melt adhesives
which are used do not themselves have real elastic
properties, application of adhesive tends to "kill" the
stretch of that portion of the elastic to which it is
2
applied. Finally, the use of the two materials, rubber
and adhesive, is costly.
A compc>sition having adhesive properties on
application from the molten state and preferably also
pressure ser.~sitive adhesive properties, and elasticity
comparable t.o that of rubber, whilst also possessing
suitable rhe~ological properties for melt processing, is
highly desirable since it would be capable of replacing
the rubber and the adhesive used at present and would
represent a considerable advance in the manufacture of
absorbent articles such as diapers.
Many types of composition are in use commercially as
hot melt adhesives and a much wider range of compositions
have been suggested for use in this field. A hot melt
adhesive can be defined as a composition which shows
adhesive properties when applied to a substrate in the
molten state. This does not preclude the composition
also showing adhesive properties at room temperature,
e.g. pressure sensitive adhesive properties. In general,
hot melt adhesives are formulated for properties such as
adhesion to a variety of surfaces, heat stability and
processing properties rather than for elasticity and any
attempt to increase elasticity has a detrimental effect
on adhesion and other desirable properties of the
composition. As yet, none of the commercial hot melt
adhesive com~~ositions combine good adhesion with elastic
properties c~~mparable to those of rubber.
3
Natural rubber is generally too viscous to be used
as a basis for hot melt adhesives and cured rubber cannot
be melted. Many hot melt adhesives are based on
thermoplast:LC block elastomers since these can be melted.
Many such e:Lastomers with a variety of properties are
available commercially. For use as an adhesive,
thermoplastic block elastomers generally have to be
blended with tackifying agents to improve adhesion.
Other additives such as plasticisers may also be
necessary depending on the application.
Certain prior art proposals have attempted to
provide hot melt adhesives which also have elastic
properties. For example US-A-4 418 123 attempts to
provide a self-adhering elastic having a combination of
elastic and adhesive properties. The composition is
defined in very broad terms as a combination of a block
copolymer comprising at least one substantially amorphous
rubbery polymeric mid block and at least two glassy
poly(vinylarene) end blocks, together with a midblock
associating resin and an endblock associating resin. All
of the specific examples (with the exception of a
comparative example with unsatisfactory properties) are
based on styrene-isoprene-styrene block copolymers.
Despite the claims made for them, as far as the present
applicants are aware, none of the compositions
exemplified in US-A-4 418 123 show true elastic
properties comparable to those of rubber combined with
2~ 59933
good adhesion and processability (see Comparative Example
A below) .
Similarly EP-A-0 424 295 (HB Fuller France SARL)
relates to a thermoplastic elastic intended particularly
for elastication of diapers which comprises:
a) at least one synthetic rubber which is of the
block copol~~ner type comprising at least one rubber
middle block: and at least two glassy end blocks;
b) 20 to 150 by weight based on the block
copolymer of at least one "tackifying" resin which
associates ~~ith the middle block of the copolymer;
c) 10 to 50$ by weight based on the block
copolymer of at least one "tackifying" resin which
associates with the terminal blocks of the copolymer; and
d) 5 to 35~ based on the weight of the composition
of a mineral oil.
The compositions exemplified in EP-A-0 424 295 are
generally based on SIS and an example of a composition
based on SBS shows unsatisfactory properties (see
Comparative Example B below).
It has now been found that, by careful selection of
the components, hot melt adhesives can be produced having
the desirable combination of properties referred to
above, i.e. good adhesion from the melt (and in many
embodiments also at room temperature), elastic properties
comparable to those of rubber; and good processibility
from the melt and that these compositions are
CA 02159933 1999-06-25
particularly suited to the elastication of absorbent
articles such as diapers.
SUMMARY OF THE INVENTION
In accordance with one embodiment of the invention, an
elasticated absorbent article wherein the elastication
is provided by an elastomeric hot melt adhesive
composition comprising at least one tackifying resin and
at least one thermoplastic elastomer selected from the
group consisting of styrene/butadiene/styrene (SBS)
block copolymers and blends of styrene/butadiene/styrene
with styrene/isoprene/styrene (SIS) block copolymer on
which SIS is present in an amount not more than 50% by
weight of the total block copolymer, and wherein the
composition
a) is capable of bonding, when applied from the
molten state, to materials selected from the group
consisting of plastic and cellulosic materials and
mixtures thereof°, with a 90° peel force of not lower than
0.5 N/cm;
b) has a tensile strength retention after 50
cycles of at least 40%; and
c) has a viscosity of not more than 120,000 cps
at 180°C and an applied shear of 80 sec-1.
In accordance with a further embodiment, an elasticated
absorbent article wherein elastication is provided by an
elastomeric hot melt adhesive composition comprising:
i) 10 to 80% by weight of a styrenic block copolymer
comprising at lE~ast two styrenic end blocks and at least
one rubbery mid. block per molecule and containing less
than 40% by weight of the total block copolymer of a
diblock copolymer containing one styrenic block and one
rubbery block pesr molecule;
ii) 20 to 90% of a tackifyng resin compatible
essentially only with the rubbery mid blocks;
iii) 0 to 40% of: at least one plasticizer; and
CA 02159933 1999-06-25
5a
iv) 0 to 20% of an aromatic resin; and wherein the
composition
a) is capable of bonding, when applied from the molten
state, to materials selected from the group consisting
of plastic and cellulosic materials and mixtures thereof
with a 90° peel. force of not lower than 0.5 N/cm;
b) has a tensile strength retention after 50 cycles of
at least 40%; .and
c) has a viscosity of not more than 120,000 cps at
180°C and an applied shear of 80 sec-1.
DEVILED DESCRIPTION OF THE INVENTION
The present invention provides an elasticated
absorbent article wherein the elastication is provided by
' an elastomeric hot melt adhesive composition comprising
at least one thermoplastic elastomer and at least one
tackifying resin, the thermoplastic elastomer(s) being a
styrene/butadiene/styrene (SBS) block copolymer or a
blend of styrene/butadiene/styrene with
styrene/isoprene/styrene (SIS) block copolymer on which
SIS is present in an amount equal to or less than 50$ by
weight of the total block copolymer the composition being
characterised in that:
a) it is capable of bonding, when applied from the
molten state, to plastic and/or cellulosic materials with
a 90° peel force of not lower than 0.5 N/cm (as herein
defined);
b) it has a tensile strength retention after 50
cycles (as herein defined) of at least 40$: and
CA 02159933 1999-06-25
Sb
c) it has a viscosity of 120,000 cps or less at
180°C and an applied shear of 80 sec-1.
Feature a) referred to above relates to the adhesive
properties oi: the composition and in all cases the
composition should have the properties of a hot melt
adhesive in that it is capable of bonding appropriate
substrates, typically plastic and/or cellulosic
materials, when applied from the molten state. In
particular, "capable of bonding" means that the
215993
6
composition is capable of showing adhesion on plastic
and/or cellulosic materials sufficient especially for
application in the construction of hygienic absorbent
articles. G~~hen applied from the molten state between two
substrates c>f plastics and/or cellulosic materials the
composition gives a bond strength, measured as 90° peel
of not lower than 0.5 N/cm. The composition at a weight
of 5 g/m2 is. applied in the molten state between the
substrates a.nd 48 hours after bond formation the 90° peel
strength is measured at 23°C and at a separating speed of
300 mm/min.
As described in more detail below, many compositions
as disclosed. herein also bond appropriate substrates at
room temperature and may show the properties of a
pressure sensitive adhesive.
Feature b) referred to above relates to the elastic
properties of the composition. The test which is used is
described in more detail below and involves measuring the
extent to which elastic properties are retained over 50
cycles of stretching and relaxation. The range over
which the composition is stretched is related to the
modulus of the composition and the likely degree of
stretching of the composition in use. The figure of 40~
for tensile strength retention indicates that the elastic
properties of the composition are comparable with those
of natural rubber and are preferably superior thereto.
Preferably the tensile strength retention is at least
50$, more preferably at least 60$.
~i59933
Feature c) above relates to the processability of
the composition and a viscosity of 120,000 cps or less at
180°C (applied shear 80 sec-1) indicates that the
composition can be applied using conventional apparatus
for use with hot melt adhesives. Preferably the
viscosity is 60,000 cps or less, more preferably 30,000
cps or less. It is also highly desirable that the
composition according to the invention show substantially
Newtonian rlzeological behaviour, in particular viscosity
does not vary significantly with applied shear. As
discussed in more detail below, many compositions as
disclosed hE~rein show Newtonian behaviour at intended
processing l.emperatures, e.g, around 180°C.
Compositions used to provide elastication in the
absorbent products according to the invention can be
formulated with any desired modulus depending on the
precise nature of the desired end use. However the
modulus has effects on the main properties of the
composition and it is convenient to divide the
composition:> as disclosed herein into low modulus and
high modulu:~ compositions.
Low modulus compositions are defined as compositions
having a moclulus of 0.5 MPa or less at 500 elongation
(six times the initial length of sample) measured at 23°C
under an elongation rate of 500 mm/minute. Generally low
modulus comF~ositions have a modulus in the range 0.05 to
0.5 MPa, preferably 0.05 to 0.3 MPa. Low modulus
compositions generally show good adhesive properties at
2~~9~
g
room temperature and may also be pressure sensitive
adhesives. These compositions are usually stretched
immediately after they are formed, for example after
extrusion from the melt as a strip or thread. Stretching
may take place immediately before or during application
to an artic:Le so that the compositions are effectively
applied in i:he stretched state. Low modulus compositions
are typical:Ly used under an elongation of 400$ to 1000.
Since ~_ow modulus compositions will usually be
stretched inunediately after extrusion it is desirable
that they should have a relatively high setting point so
that they solidify quickly on extrusion. Preferably the
setting point (measured by the Dynamic Mechanical
Analysis method described in more detail below) is at
least 80°C, more preferably at least 100°C.
High modulus compositions are defined as
composition; having a modulus of greater than 0.5 MPa at
500 elongation (six times the initial length of sample)
measured at 23°C under an elongation rate of 500
mm/minute. Preferably the high modulus compositions have
a modulus of from 1 MPa to 10 MPa. Since pressure
sensitive adhesive character is generally inversely
proportional to modulus, compositions with high modulus
are often applied from the molten state although some may
retain sufficient pressure sensitive adhesive character
to be applied at room temperature. Application from the
melt implies that the composition is applied without
stretching with stretching generally taking place in use
2j X9933
9
and this applies particularly to compositions with a
modulus of 1 MPa or higher. For this reason high
solidification temperature is less critical for high
modulus compositions but for convenience these are also
preferably formulated to have a setting point of at least
80°C, more :preferably at least 100°C. High modulus
compositions are generally used at a lower degree of
stretching. They are capable of giving sufficient
elastic for~~e at low deformation (typically no higher
than 50~). However, in cases where the compositions
retain sufficient pressure sensitive adhesive character
so that the~~ can be applied at room temperature in a
stretched slate, they can be used at an elongation of up
to 400.
The essential components of the composition used to
provide elastication in the absorbent products according
to the invention are a thermoplastic elastomer and a
tackifying resin and these will now be discussed in
general ternns .
Thermoplastic elastomers are a very interesting
chemical and technological class of polymers which are
distinguished by their characteristic behaviour. At room
temperature they behave as cured rubbers showing high
elasticity but in contrast to cured rubbers they can be
melted and reprocessed in the same way as normal
thermoplastics.
This behaviour results from a particular chemical
structure. Most thermoplastic elastomers are block
2j 5993
copolymers, i.e. their molecules are formed by blocks of
different natures linked together. Different blocks can
alternate along the chain as relatively short blocks
(multiblock structure of the form A-B-A-B-A etc); or the
molecules can have a three block structure of the form
A-B-A where A are terminal blocks and B is a central
block of a different nature (linear three block
copolymers); or the molecules can have a "radial" or
"star" structure represented as (AB)x where all midblocks
B are chemically linked together at a central point and
terminal blocks A are radially disposed each at the end
of block B. Structures formed by only two blocks
(diblocks) ~~f the form AB are ineffective as
thermoplastic elastomers in terms of their elastic
behaviour.
The chemical nature of the different blocks can be
varied and 'the resulting copolymers can be classified for
example as ~~olyurethanes, polyesters, polyethers,
polyether- ester amides, etc. However, a common
characterisi_ic is the following: different blocks are
physically incompatible so that they are mutually
insoluble. The material can thus be considered an
heterogeneous system in which different blocks, even if
chemically :Linked in the same molecule, exist as separate
entities. Blocks A of different molecules tend to
associate together in microscopic regions or "domains"
with the same happening for blocks B. The material so
formed has an heterogeneous structure of domains A and B,
2~5~~3
each well s~=_parated, with the one present at the lower
level being dispersed microscopically in the other one
which constitutes a continuous phase. This continuous
phase is generally formed by "soft" or rubbery blocks B
which give i_o the material its elastic properties, while
the dispersE~d phase A is formed by "hard" non-elastomeric
blocks. Be:Low the glass transition temperature or
softening point of the hard blocks each molecule of the
copolymer has its A blocks fixed in at least two points,
i.e. they are "confined" in the hard domains.
Accordingly, the rubbery part of the molecule can undergo
stretching but without flowing relative to other
molecules and when the external stretching force is
relaxed it returns to its initial position for reasons of
entropy.
Thus in thermoplastic elastomers, the hard blocks
work as a physical vulcanization and the advantages of
this proces~;ing are clear. The chemical linkages that
form the vulcanized structure of a standard rubber cannot
be removed by heating and at sufficiently high
temperature the rubber simply begins to decompose. On
the other hand, in thermoplastic elastomers heat can
effectively melt the hard domains the material can thus
be melted anal processed, but hard domains giving back the
pseudo-vulcanization, are formed again simply by cooling
the material. It is apparent from the above explanation
that diblock.s, which contain only one hard and one soft
block, cannot contribute to elastic properties.
2~ 5993
12
Diblocks can improve processibility but their
content in the material must be confined within certain
limits so that they do not reduce elasticity to an
unacceptable extent. In addition, the total amount of
hard blocks is important; too low a content will give
poor elastic properties (similar to an insufficiently
cured rubbe:r), whereas too high a content will make the
material belZave as a very hard, super- cured rubber,
again with ~~ery poor elasticity.
Amongst= thermoplastic elastomeric block copolymers,
the so called Styrenic Block Copolymers (SBC) are well
known and widely used in many applications on account of
their very good properties. Styrene block copolymers as
a class are described for example in Thermoplastic
Elastomers: A Comprehensive Review, Legge, Holder &
Schroeder (Eds), Hauser Publishers (1987), Chapters 3, 4
and 12(1). They can have the structures already
mentioned above as:
-. multib.'Lock A-B-A-B-A-B- ..etc.
- linear triblock A-B-A
- radial or "star" polymers (AB)x where x > 2.
A represents a "hard" block of a vinyl-arene polymerized
monomer, generally styrene or alpha-methyl-styrene; and B
represents ~~ "soft midblock" generally formed by a
rubbery monomer such as poly(butadiene), (isoprene),
(ethylene- butylene) or (ethylene-propylene) rubbers.
The content of diblock molecules A-B in such
products can be as high as 80~ by weight and in special
2~ 5~~3~
13
commercial :products can even form the totality of the
polymer. These products are used for particular
applications because, for the reasons discussed above
they have nc~ or very poor elastic properties. Diblocks
can help processing and improve adhesive properties but
in order to retain good elastic characteristics their
content in 1=he thermoplastic elastomeric block copolymer
should be kE~pt lower than 40$ by weight.
SBC's are widely used as substitutes for vulcanized
rubbers, their hardness, modulus and general mechanical
and elastic properties being strongly related to the
content of hard blocks, formed especially by polystyrene.
They have also found use as base polymers for hot melt
adhesives because of their generally good mechanical
characteristics, easy tackification of their rubbery
midblocks and good thermal stability which make them
superior to traditional bases for hot melts such as
ethylene-vinylacetate copolymers. However the main
object of standard compositions has been to optimize
adhesive properties with retention of at least some of
the elastic properties, typical of the base polymer, not
being taken into account.
Thermoplastic elastomeric block copolymers known as
SBC's, typically have the following characteristics:
- They are formed by two kinds of monomers each
polymerized in blocks of the same monomer units, the
blocks being distinct even if chemically linked inside
2~ ~~~ 33
14
the copolymer molecule. Moreover the two kinds of blocks
must be mutually incompatible.
- The structure according to which the two kinds of
present blocks are linked in the molecule can be:
- a:Lternating multiblock as .... A-B-A-B-A-B....
- t:riblock linear as A-B-A
- radial or star structure as (A-B)x where x > 2.
- "A" represents blocks of a polymer derived from a
vinyl- arenE~ monomer, typically styrene or
alpha-methy:L-styrene. They are called hard blocks
because at room temperature these polymeric species are
hard, glass5r and fragile materials being under their
glass transition temperature (Tg).
Typically u:~eful constituents for hard blocks have Tg
well above zoom temperature and preferably higher than
90°C.
- "B" re~~resents blocks of a rubbery polymer having a
Tg < 0°C and preferably < -40°C.
Typically these "soft" blocks are formed by rubbers such
as polybutacliene, polyisoprene, poly-ethylene-butylene
and poly- ethylene-propylene.
In the common technological lexicon the resulting
thermoplastic elastomeric block copolymers are often
referred to by the abbreviations SBS, SIS, SEBS and SEPS
respectively.
As already discussed in terms of the mechanism of the
generation of elastic properties in these type of
polymers, and particularly the function of hard blocks in
~1 ~~~3~
15
giving a physical vulcanization to the polymer, useful
SBC's contain at least two hard blocks "A" per molecule
and at least one soft block "B". Molecules formed by one
block of A and one block of B (the so called diblocks)
should, for use in the present invention, be kept lower
than 40$ by weight in the base polymer.
It is widely recognised in the literature that
differences exist between SIS and SBS copolymers which
are relevant to the formulation of hot melt adhesives.
SBS copolymers generally cost less than comparable SIS
copolymers and SBS copolymers can be synthesized to
exhibit beta=er elasticity than comparable SIS copolymers.
However it has not hitherto been possible to take
advantage o:E these potentially advantageous properties of
SBS copolymers as a result of the fact that SBS
copolymers have not generally shown adequate adhesive
properties and SIS copolymers are much easier to tackify.
For this reason hot melt adhesives have generally been
formulated rising SIS copolymers as the predominant SBC.
Both US-A-4118123 and EP-A-0424295, which are discussed
above, clearly prefer SIS as the SBC on which the
compositions are based and neither document discloses a
composition based on SBS which has satisfactory
properties.
It has been found according to the present invention
that compositions in which SBS copolymers are the main
styrene block copolymers) can be produced with
satisfactor5r adhesive properties. At the same time these
2~ X9933
16
compositions retain the advantages of SBS copolymers with
respect to elasticity which have been mentioned above.
Thus, , compared to compositions based on other SBC's,
better elasi=is properties, quicker elastic return, more
flat stress,~strain diagrams even at elongations > 1000
and give compositions of better processability (more
Newtonian rheological behaviour). However, it should be
noted that direct comparison between SIS and SBS based
compositions is very difficult since the compositions
need to be ~=ormulated in different ways. Accordingly, it
would not gE:nerally be possible to substitute an SBS
copolymer for an SIS copolymer in a hot melt composition
and obtain :>atisfactory properties and other adjustments
need to be made to the formulation depending on the
nature of the SBC in order to obtain optimum results.
The way in which compositions according to the invention
should be formulated to obtain the desired properties is
discussed in more detail hereinafter.
Thus, t:he compositions used to provide elastication
according to the present invention are based on SBS
copolymers or a blend of SBS/SIS in which SIS is present
at levels equal or less than 50~ by weight of the total
block copol5~ner.
All thermoplastic elastomers can be processed in the
molten state using various technologies and in various
apparatus, in all cases showing in the solid state
properties :similar to those of a cured rubber.
Potentially all thermoplastic elastomers can be made
17
21 X9933
adhesive. Some adhere well enough in the molten
conditions to different substrates. However it is
clearly highly desirable to obtain thermoplastic
elastomers which are capable of adhering at room
temperature or at only moderately elevated temperature to
various substrates.
Thus, whilst pure thermoplastic elastomers have some
adhesivity at high temperature, this adhesivity can be
conveniently enhanced both in terms of the strength of
the bonds formed with different substrates and in terms
of the rangE~ of temperatures at which strong bonds are
formed.
This enhancement is obtained by the use of at least
one suitable: tackifying resin. More particularly, much
better adhesive properties and even self adhering
properties at room temperature (pressure sensitive
behaviour) c:an be obtained by blending thermoplastic
elastomers with the materials known as tackifying resins
which, as a class, are well known in the literature.
When thermoplastic elastomers are assembled at room
temperature (e. g. because it is desired to pre-stretch
them in the solid state and bond under tension) it is
necessary that they exhibit the typical behaviour of true
pressure sensitive adhesives and this generally requires
a blend of a. thermoplastic block elastomer and tackifying
resin. It should be noted that in order to enhance the
adhesive properties of the thermoplastic elastomer (both
at high temperature and at room temperature), only the
2~ 59933
18
soft (rubbe:ry) blocks of its molecule should be modified
by the tack:ifying resin. Thus, only interactions between
the soft (rubbery) blocks and a resin, substantially
compatible with them, causes the generation of tack;
while the eventual modification of hard blocks with a
resin never leads to the development of adhesive
behaviour.
Not only do the hard blocks not exhibit any adhesive
activation but their eventual modification by a
tackifying resin could "soften" their mechanical
strength. This risks impairing their ability to function
as "centers of physical vulcanization" for the elastomer,
consequentl~~ destroying elastic behaviour.
So, for the various thermoplastic block elastomers,
depending on the chemical nature of their soft and hard
blocks, suitable tackifying resins can be identified
which must ~~e compatible (i.e. soluble and capable of
creating the appropriate physical modification of the
system) only with the soft or rubbery blocks, whilst
compatibility with the hard blocks is as low as possible
or even zero, in order to retain as much as possible of
primary elastic properties of the polymer. However, the
amount of tackifying resin must be controlled since the
addition of quantities of tackifying resin(s), which are
too large, even if the tackifying resin is compatible
only with the midblocks (soft blocks) and fully
incompatible with the hard blocks, could still impair the
elastic properties of the resulting formulation. In any
2159933
19
case, the addition of the resin constitutes a dilution of
the concentration of the hard block domains, weakening
their ability to function as centers of "physical
crosslinking" for the elastomer. Thus both the content
of hard domains in the base thermoplastic block elastomer.
and the content of the elastomer in the final formulation
must be sucih to ensure a sufficient final concentration
of hard domains in the formulation to retain appropriate
levels of "~~hysical vulcanization" and thus of elastic
properties.
Therefore, on the one hand, it is important to
control the final concentration of hard blocks in the
composition. On the other hand, the addition of resins)
which are compatible only with the hard blocks and their
domains, is completely ineffective in the development
and/or improvement of adhesive properties. Resins
compatible with the hard blocks will, by swelling the
hard domains, stiffen the composition, increase modulus
and (comparE~d to similar levels of tackifying resin
compatible with the midblock) will tend to increase
viscosity. In a system already containing a tackifying
resin compatible with the soft domains, the addition of
resins compatible with the hard domains will also
decrease the adhesivity. Accordingly, in general terms,
only limited quantities of resins compatible with the
"hard blocks" can be used without too great an impairment
of the overall properties of the adhesive elastic hot
melt. Generally they will only be used in special cases,
2~ ~993r~
20
for example, if an additional increase in modulus is
required fo:r some applications; or (using high softening
point hard block compatible resins) if a higher setting
temperature or a better temperature resistance is
desired.
According to the present invention the compositions
provide elastication to absorbent products in which they
are applied without the use of any glue, these being for
example structures where elastication has been obtained
conventiona7_ly by elastic formed of vulcanized rubbers
bonded to tree structure by means of a glue. Thus, one
material (the adhesive elastic hot melt) can substitute
for the use of two materials (the rubber and the glue to
fix it) with a substantial saving in costs. Normally
rubber elastics are covered with talc to prevent sticking
of the elastics in the packaging. Talc can give rise to
problems at the stage of adhesion with glues.
Moreover the thermoplastic, adhesive elastic hot
melt can be directly extruded in varied geometrical forms
directly during the construction of product which are to
be elasticat.ed. It can be extruded as strands or yarns,
as bands, as films, etc. Structures such as bands or
films can be also foamed before the extrusion, obtaining
elasticated structure which are particularly soft.
Elastication can be also applied according to non-linear
(curved) geometries which makes the anatomical fitting,
of the product comprising the elastication, to the
wearer's body particularly good. This is very difficult
21
to obtain with standard rubber yarns of ribbons. Under
different geometrical forms the adhesive elastic hot
melts can be applied both in an already stretched or an
unstretched state. In the first case the extruded melt
is cooled immediately after the extrusion die and
stretched at the desired elongation. In this case it is
advisable that it possesses the following properties:
- a relatively high setting point so that it
solidifies immediately after the extrusion and can be
elastically stretched. An elastic stretching can be
given only to a solid material, because any force applied
to a molten or semisolid material will cause only a
plastic len~~thening along the direction of force without
any elastic tension.
- good pressure sensitive properties of adhesion
because the adhesive elastic hot melt will contact the
substrate(s1 when already cold, e.g. at room temperature.
When the material is applied without any prior
elastic stretching and directly contacted to the
substrate(s;l to which it has to adhere at the outlet of
the extrusion die, pressure sensitive behaviour is less
important bE~cause bonding is made when the material is
still above room temperature. In this case besides the
geometrical forms mentioned above, the material can be
applied to 1=he substrates) e.g. by spraying or
fiberization or by similar processes obtaining a network
of interconnected short fibers or a network of fibers
having indeoinite length which can have both a random or
2I 599 ~
22
a geometrically regular network structure (e. g. each
fiber can form a spiral).
All these features are particularly suitable for the
elastication of absorbent articles, in particular
hygienic absorbent articles such as diapers and
catamenials. The use of materials applied in an already
stretched form can substitute for all of the presently
used rubber elastics in baby and adult diapers, in
catamenials etc., when it is desired to have parts of the
product already under elastic tension offering, as a
result of their extreme versatility, the possibility of
new elasticated structure of practically infinite
variety. In this case, another advantage of elastic,
adhesive hot melts over rubber elastics is worthy of
note. As will be shown in more detail below, the
preferred elastic hot melt compositions have a
stress/stra:in diagram that is much flatter than a rubber
elastic, i.e. even if already under tension a further
stretching (e.g. due to the movements of the wearer of
the absorbent article) causes a very low increase in
modulus and in the tensile strength that is perceived by
the wearer. This is especially true for low modulus
composition,.
Compositions used to provide elastication in
absorbent articles according to the invention that are
more conveniently applied in the unstretched state are
typically uaed to give elastic return to
structures/products only when the whole final
CA 02159933 1999-06-25
23
structure/product is subject to some deformation during
use. Normally in this case the typical deformations that
are given :in use to an absorbent article are very
limited, e..g. of the order of 5-50$. Accordingly, it is
necessary i=hat the adhesive, elastic hot melt contained
in these si:ructures is able to respond with a sufficient
elastic rei:urn force to external stresses even at these
low elongat_ions. For these applications, it will be
generally snore convenient to use formulations at higher
modulus.
In summary the use of thermoplastic block
elastomers, in different physical forms and with
different application processes, for the elastication of
structures and particularly of hygienic articles, is very
advantageous.
The formulations based on SBC's which are used to
provide elastication according to the present invention
show optimum properties, typical of hot melts, ranging
. . 2159933
24
from compositions that can more conveniently be strongly
bonded to substrates at high temperature from the melt to
about 50°C, to compositions that retain a permanent
strong adhesivity on most substrates even at room
temperature being true pressure sensitive adhesives.
Moreover th~~ compositions are characterized by retention
of distinct elastic properties from the base
thermoplastic block copolymer, showing all of the typical
behaviours 'that define an elastomeric material in the
technological sense.
Thus when stretched in the solid state and when the
stress is relaxed they will return quickly to their
initial len~~th with only minor permanent (plastic)
deformation. The formulations preferably having a
distinct pressure sensitive character, can be applied
even at room temperature, both in the unstretched or
preferably in the stretched state for the elastication,
in different geometrical forms, in absorbent products,
in particular hygienic products such as baby and adult
diapers or adult incontinence products different from
diapers, or feminine catamenials. Compositions having
lower pressure sensitive character will be more
convenientl~~ be applied at temperature over 50°C, in the
stretched or preferably in the unstretched state for the
elastication of the same structures and products. In
particular when applied in the unstretched state they
will work ai: limited extension, e.g. up to 50~ (i.e.
final stret<:hed dimension = 1.5 times initial dimension).
215933
25
In order to show even at these low extensions a
distinct elastic return force, these formulations will
generally have a higher modulus than the previous ones,
the two kinds of materials being, in fact, the extremes
of one field of formulations all of which are both
excellent hot melt adhesives and retain excellent elastic
properties, the passage from one to another being
gradual.
As already indicated, compositions used according to
the invention can be divided into "low modulus" and "high
modulus" formulations, this distinction being based on
their modulus value and on their behaviour as pressure
sensitive a~~hesives.
Thus t:he present invention makes use of a family of
compositions based on at least one thermoplastic elastic
block copolymer in which SBS copolymers) are the main
copolymers) and at least one tackifying resin
essentially compatible with the soft (rubbery) blocks of
the aforementioned copolymer, the tackifying resin being
used mainly to improve adhesivity, both at high and at
room temperature of the aforementioned copolymer which
compositions provide elastication to the absorbent
articles. 'the compositions are extrudable and in the
solid state retain a distinct elastic behaviour typical
of elastome~rs from which they are derived. As typical
examples and without any limitation, these compositions
can be extruded and applied in the form threads, yarns,
bands, continuous films, networks of fibers both
2159933
26
continuous or having a finite length and in which fibers
have both a random orientation or a geometrically regular
conformation etc. As examples of applications in the
field of absorbent articles, they can be used for the leg
elastication of diapers, as elastic waistband in the
same, for the elastication of catamenials and of adult
incontinent products other than diapers, for the internal
elastication of the structures of all the aforementioned
products, for reinforcement under mechanical stress of
their absorbent cores and for giving them stretchability
and resiliency etc.
A lower modulus generally means that adhesive
materials have a more aggressive adhesivity, so that the
low modulus formulation generally have higher tack
typical of pressure sensitive adhesives. They are able
to form very strong bonds with many substrates on simple
contact even at room temperature or in any case lower
than 50°C.
Ratios between hard and soft blocks in the base
thermoplastic elastomeric copolymer are very important in
determining the elastic behaviour and the mechanical and
adhesive properties.
Generally the higher the content of hard blocks
(that conventionally will be referred to as "styrene
content") the higher the modulus, the more evident are
elastic properties, the quicker is elastic return after
relaxation of stretching but the lower is adhesivity and
especially pressure sensitive behaviour. All this is
2I X9933
27
true provided that the level of hard blocks does not
become so high that it forms the continuous phase and the
material becomes a hard and no longer elastic material.
Useful SBC's can contain from 10 to 50$ of styrene
by weight. However, when modified with the tackifying
resin, the :behaviour of the resulting composition will be
clearly governed, in terms of all of the aforementioned
properties, by the resulting content of styrene or of
hard blocks in the compositions so that it is determined
both by the level of styrene in the base copolymers) and
by the content of copolymers) in the final composition.
Too low a l~svel of final block styrene will give poor
elastic properties. Too high a level will increase the
modulus and decrease the adhesivity to an unacceptable
extent. Increasing final styrene level in the
composition by increasing the content of copolymers)
will increase excessively the viscosity and decrease
processability. So both the level of copolymers) in the
composition and their content of styrene should be chosen
to optimize the final styrene content and thus all the
above mentioned properties. Optimum ranges will be
indicated below.
If desired the rubbery part of the SBC can itself be
cured (in a similar manner to the curing of natural or
synthetic robbers) by using suitable chemical or physical
means, in particular curing systems known for synthetic
rubber which are not activated by heat. This will have
~1 ~993~
28
the effect of increasing the modulus of the overall
composition.
The tac:kifying resin is added mainly to improve
adhesive properties of the base copolymers) even to the
extent of arriving at the typical behaviour of a pressure
sensitive adhesive. Moreover it improves the
processabil~.ty of the thermoplastic elastomer both by
giving to treat composition lower absolute values of
viscosity (<~s compared to the pure block copolymer) and a
rheological behaviour that, at the indicated levels of
resin, is practically Newtonian, i.e. the viscosity is
dependent only on temperature and does not change with
applied stress, a property which is very advantageous for
easy process>ing. It is known that SBC's which have many
very intere:~ting characteristics, may be difficult to
process as a result of non-Newtonian behaviour in the
molten state' as pure materials. This means that not only
do they show very high viscosity but also, under the
influence only of temperature, they do not appear to melt
even at very high temperatures near 200°C. They can even
begin to thermally decompose before showing a distinct
fluid state. In order to make them flow and so to be
able to process them, it is necessary to apply
temperature and high mechanical stress. In any case
processing of pure SBC's is difficult, viscosities are
high and highly dependent on the combination of
temperature and applied stress.
21 ~~93~
29
The basic composition of SBC and tackifying resin is
capable of ~~iving materials which, whilst retaining very
good elastic and adhesive properties, are also easily
processable because of both relatively low viscosities
and of pracl:.ically Newtonian (or acceptable
Newtonian- hike) rheological behaviour. This latter
property was measured as variation at constant
temperature (180°C) of the viscosity under two levels of
applied shear rate, 20 and 80 sec-1. The ratio of these
two viscosii:ies is hereinafter called the "Newtonian
Index" (N. I ,. ) .
An ideal Newtonian fluid should have N.I. - 1 while
a pure SBC could have, in the same conditions an N.I.
even > 6; i,.e. the viscosity variation, only due to the
variation oi= applied shear rate from 20 to 80 sec-1 is
more than 6 times which can cause severe problems for
regular and easy processing. For easy processability it
is necessar5r that the compositions have only limited
variation oi: viscosity at constant temperature with
variation oi: applied shear rate.
More particularly, it is preferred that compositions
show a Newtonian or almost Newtonian rheological
behaviour based on the molten material at 180°C, by
comparing tree viscosities under a shear rate of 20 and 80
sec-1. Preferred compositions do not show a variation in
viscosity > 50~ i.e. a ratio between viscosities (N. I.)
not higher than 1.5.
21~9~3~
30
It has been found that the most preferred
composition; based on SBS's have an almost ideally
Newtonian bE~haviour, with an N.I. not higher than 1.05.
In ordESr to retain sufficiently the elastic
properties of the base polymer it is necessary that the
tackifying resin is compatible mainly with and preferably
essentially only with, the soft, rubbery blocks of the
block copol5rnner and does not interfere to a significant
extent with hard blocks. This is governed both by the
chemical nature of the resin and by its molecular weight.
The compatibility of the resin with the rubbery blocks
and its incompatibility with the hard blocks can be
measured, for example, by determining the variation of Tg
of soft and hard blocks deriving from the addition of
resin. In F>articular, incompatibility with the hard
blocks is considered satisfactory if their Tg (originally
at 100°C if they are formed from polystyrene) is not
changed more than 15°C by the addition of 100 parts of
resin to 10C1 parts of copolymer. Measurements of the two
Tg's requires appropriate equipment. Accordingly both
the experience of formulators and the technical
literature of resin suppliers can be taken into account
to determine which tackifying resins are chemically
compatible with the soft blocks and incompatible with the
hard blocks of SBC's. As used herein, the term
"compatible essentially only with the soft blocks" means
that a tacki.fying resin is compatible with the soft
blocks of the copolymer and is incompatible with the hard
2~ ~993~
31
blocks to tile extent that Tg of the hard blocks is not
significant:Ly changed and more preferably decreased by no
more than 1.'~°C on admixture of 100 parts of tackifying
resin to 10c) parts of copolymer. Preferably the Tg of
hard blocks is not decreased at all.
More specifically a suitable main tackifying resin
will be chosen from the following chemical groups which
have high compatibility with soft blocks of SBC's and low
or no compatibility with their hard blocks;
- hydrocarbon resins
- aliphat:ic resins
- polytel:pene resins
- terpene: phenolics
- synthet:ic C5 resins
- synthet:ic C5/C9 resins
- rosins and rosin esters
as well as their totally or partially hydrogenated
derivatives.. They can be used as the pure resin or also
in blends.
When more than one resin is used, the main
tackifying resin system, defined as the essential
resin/blend of resins present at least at a level of 50~
of the total. amount of resin, are characterised by having
a softening point between 85 and 150°C and more
preferably between 100 and 140°C (all softening points
being measured by the well known Ring & Ball (R & B)
method) .
21 ~9~3~
32
Tackifying resins having softening point < 85°C are
considered to have a prevailing plasticizing effect which
may in any case be important for the development of good
adhesive and. elastic properties but is to be
distinguished from the tackifying effect. This is due to
the fact that in the processing of the present elastic
hot melt compositions quick setting of the material after
extrusion is desirable, especially for compositions which
are to be stretched before application on the substrate,
which is clearly possible only with solid materials. For
this reason it is desirable that the setting point of
these compositions is not less than 80°C and more
preferably greater than or equal to 100°C.
Setting point is most accurately determined by using
the technique known as Dynamic Mechanical Analysis under
sinusoidal stress, which is well known in the science and
technology of polymers and adhesives. According to this
technique three main rheological parameters of the
material are determined as a function of temperature:
- elastic or storage modulus G'
- the viscous or loss modulus G "
- the angle ° (delta) and its tangent, being the phase
shift between G' and G " .
G' is higher than G " when the material is solid.
When the material is fluid G " becomes higher than G'.
Naturally, G' is higher at low temperature and the
reverse at high temperatures. The crossing temperature
33
between G' and G " is taken as the true Theological
solidification (or melting) point of the material.
For us~~ according to the present invention, it is
preferable that the crossover temperature for the
composition is greater than or equal to 80°C and more
preferably greater than or equal to 100°C.
The po:>ition of the crossover point is dependent on
many physical parameters of the hot melt. However, it
has been found that the main influence is the softening
point of thE: main tackifying resin and a secondary
influence i:~ the content and molecular weight of the
copolymer. So the tackifying resin should preferably
have a softening point from 85 to 150°C and more
preferably from 100 to 140°C provided that in any case
the overall composition has a true Theological
solidification temperature at least of 80°C and more
preferably at least of 100°C.
Beside:. the thermoplastic elastomeric block
copolymer and the main tackifying resin, compositions can
contain additional components which improve specific
properties. A more detailed description of the
composition~~ and of their principal properties is given
below.
For practical reasons of clarity of description,
further description will relate specifically to "low
modulus" anf, "high modulus" compositions.
The low modulus compositions are elastic,
extrudable, adhesive compositions based on at least one
21 ~~933
34
thermoplastic elastomeric block copolymer, suitably
modified by the proper addition of at least one
tackifying ~=esin essentially compatible with its soft
blocks. ThE~ polymer, or at least the polymer present at
the highest level, is a polystyrene/ polybutadiene block
copolymer. In this embodiment the compositions have a
modulus of 0.5 MPa or less, essentially from 0.05 MPa to
0.5 MPa and preferably less than or equal to 0.3 MPa; the
modulus being measured at 23°C at 500$ elongation (six
times the initial length of sample) under an elongation
rate of 500 mm/minute. Moreover the compositions have
viscosities at 180°C and with an applied shear rate of 80
sec-1 of 120000 centipoise (cps) or less and preferably
60000 cps or less and more preferably 30000 cps or less.
The low modulus compositions will typically contain
from 10 to 80$ by weight, and more preferably from 15 to
50~ by weight, of SBC or of a blend of SBCs having the
following characteristics:
- a molecular structure that can be multiblock, linear or
radial (star) provided that it contains per molecule, at
least two hard-blocks formed by a vinyl-arene polymer and
preferably polystyrene or poly-alpha-methyl-styrene, and
at least one' soft or rubbery block, the soft block of the
SBC, or of t:he SBC present at the highest level, being
polybutadiene. The diblock content in the SBC(s) should
be kept lower than 40~ by weight.
- the aromatic content (conventionally referred to
hereinafter as "block styrene content") of the SBC(s) can
35
vary from 10 to 50~ by weight and preferably from 20 to
50~ by weight.
However in order to retain significant elastic
properties, both the SBC(s) level in the final
composition and the styrene content thereof should be
chosen so t~o have a final block styrene content in the
composition from 3 to 17~ by weight and preferably from 6
to 15~ by weight.
The composition also contains tackifying resin or a
blend of the same essentially compatible with the soft
blocks of SBC.
The preferred resins belong to the chemical groups known
as:
- hydrocarbon resins
- aliphai~ic resins
- polyterpene resins
- terpenE~ phenolic resins
- synthel:ic C5 resins
- _ synthei:ic C5/C9 resins
- rosin <ind rosin esters
as well as i:heir totally or partially hydrogenated
derivatives thereof.
The tackifying resin/blend of resin has/have a R&B
softening point from 85 to 150°C and preferably from 100
to 140°C. '.Che level of such resin/blend of resin in the
composition can be from 20 to 90~ by weight. However, in
a preferred embodiment the content of resin/blend of
resin descr:Lbed above is from 30 to 55$ by weight the
2159933
- - 36
remainder being formed by the additional components
described below which enhance elastic and/or adhesive
properties.
In any case both the level and the softening points
of the tack.ifying resin/blend of resins as well as those
of additional components described below will be chosen
so that the final composition has a true Theological
setting temperature (measured as crossing temperature of
G' and G ") not below 80°C and preferably not below
100°C.
It has also been found that adhesive and/or elastic
and/or mechanical properties of the binary blends
SBC/tackifying resin can be improved by using additional
components.
Adhesive properties can be enhanced by adding
limited quantities of high molecular weight rubbers such
as polyisoprene, polybutadiene, polyisobutylene, natural
rubber, but~~l rubber, styrene/butadiene rubber (SBR) or
styrene/isoF~rene rubber (SIR) and blends thereof. These
polymers have high viscosities and, in the uncured state,
have poor elastic properties. However, adding them in
quantities up to 15$ by weight of the formulation and
using polymers with Mooney viscosities ML (1+4) at 100°C
from 30 to 70, the resulting compositions show improved
pressure sensitive adhesive properties whilst still
retaining final viscosities within a useful range and
without any detrimental effect on final elastic
properties. A particularly suitable SBR is the product
CA 02159933 1999-06-25
37
TM
sold by Enichem under the trade name EUROPRENE SOL 1205
TM
and by Fina under the trade name FINAPRENE 1205. This
product is described as an SHR in which styrene is
partially distributed in blocks. Of the total styrene
content of 25% by weight, from 15 to 18% has a block
structure .and the remainder is randomly co-polymerised
with the butadiene.
Plast:icization of the composition can have very good
effects nolt only on the adhesive properties and on the
viscosity but can also even improve elastic behaviour by
reducing the internal (molecular) frictions that
dissipate elastic energy during stretching and subsequent
relaxation" In general, the composition may contain up
to 40% by weight of plasticizer(s).
In a preferred embodiment, the compositions contain
at least one of the following plasticizers:
- up to 40% by weight of a tackifying resin with a
softening point from 50 to 85°C,
- up to 20% by weight, and preferably up to 15% by
weight, of a liquid hydrocarbon resin, rosin ester or
polyterpene~ resin with a softening point not higher than
30°C,
- from 3 to 30% by weight and preferably from 5 to 15%
by weight of a paraffinic or naphthenic mineral oil
having an aromatic content of less than 10% by weight in
order not to interfere with the styrenic domains,
- up to 15% by weight of a liquid polyisoprene or
depolymerized natural rubber or polyisobutylene,
CA 02159933 1999-06-25
_ . 38
polybutenE~ or polypropylene oils and the liquid
copolymers thereof, for example PARAPOL (Exxon), or LIR
( from KUR~IRAY) .
The amount of plasticizer should be such that the
setting temperature is not lowered beyond the limit
referred t:o above. In a preferred embodiment the total
plasticizes content in a low modulus formulation is not
less than 10% by weight and not higher than 40% by
weight.
In low modulus compositions, the use of aromatic
resins, which have no effect on adhesive properties,
which interfere with the hard blocks of SBC's and which
stiffen the composition and tend to increase viscosity,
is generally not desirable and the preferred level of
aromatic resins is zero. However, limited quantities of
an aromatic resin or a blend of aromatic resins, for
example 20% by weight or less, more preferably 10% by
weight or less can be used as a reinforcement for
compositions which have low total styrene content (say up
to 6%) or which include significant amounts of SIS, for
example 30'~ by weight or more of SIS based on the total
SBC(s). In fact SIS copolymers, especially the ones
which have a styrene content < 30% by weight when diluted
2into the composition by resin and other additives, can
show an inadequate (too low) modulus and poor
characteristics of elastic return as a result both of the
intrinsic :lower modulus of SIS and the low concentration
of styrene,. acting as a physical vulcanizing agent. In
2.~~993~
39
this case the aromatic resin can both increase modulus to
a useful level and increase the density of hard domains,
that are swollen by the resin. Useful aromatic resins
have a softening point from 115 to 160°C and are
chemically identified as derivatives of styrene,
alpha-methyl-styrene, vinyl-toluene, coumarone- indene
and copolymers thereof; alkyl-aryl resins etc.
Apart from the components discussed above the
compositions can contain the usual additives such as
antioxidants, U.V. inhibitors, pigments and colouring
materials, :mineral fillers etc. Generally in a total
amount up to 20~ by weight.
Without limitation as to their most suitable
processing .and use, the low modulus formulations are
typically used in the stretched state at typical
extension levels of 400-1000. They are characterized by
very high elongation at break (over 1100 and often over
1400$) and very good adhesive, often pressure sensitive
adhesive properties.
In order to simulate the application of the
composition into an absorbent article, pressure sensitive
adhesive properties were measured as loop tack (or "Quick
Stick Tack";i and 90° peel according to the standard
methods FIN~~T Test MEthod No 9 for the loop tack and
FINAT test r4ethod No 2 for the 90° peel, modified as
defined herE~in.
- For both tests the compositions were applied on a
polyester f~Llm at a weight of 80 g/m2.
21~~~3~
40
- Adhesive properties, both as loop tack and 90° peel,
were measured on a polyethylene film fixed on the
standard test plate.
- Loop tack values were expressed as peak values,
ignoring tree initial peak.
- The 90° peel was evaluated after compression by a
400 g roll passed back and forth, i.e. two passes, one in
each direction, and measurements were made 20 minutes
after contact of adhesive and polyethylene.
The compositions used according to the invention
generally have a loop tack > 5 N/cm and 90° peel > 7 N/cm
(separating speed = 300 mm/min). Materials which can
usefully be bonded and assembled at room temperature into
a hygienic product are considered to be those which show
on polyethylene loop tack > 2.5 N/cm and 90° peel
strength > 3 N/cm.
High modulus formulations are elastic, adhesive,
extrudable compositions similar to those described
previously and having the following characteristics:
1) They have a modulus higher than 0.5 MPa and more
preferably not lower than 1 MPa up to 10 MPa.
2) At 180°C and with an applied shear rate of 80 sec-1,
they have viscosities of 80000 cps or less preferably
50000 cps or less and more preferably 35000 cps or less.
3) They are based on the same types of SBC's referred
to previously, but which have a final block styrene
CA 02159933 1999-06-25
- - 41
content of from 15 to 30% by weight and preferably from
15 to 25% by weight.
4) The SBC or blend of SBC's which is used has a
diblock content not exceeding 25% and preferably not
exceeding :LO%. The most preferred polymers are those
with no content of diblocks such as those marketed by
DEXCO-Co wider the trade name VECTOR.
5) The preferred SHC(s) contain from 20 to 50% by
weight of styrene and the preferred level of SBC or blend
of SBC's in the composition is from 35 to 75% by weight,
provided that both the styrene content of SHC's and their
level in the composition are such as to match the
requirement of point 3) above about final styrene
content.
6) The tackifying resin/blend of tackifying resins
has/have the same chemical and physical characteristics
as already discussed above. However, the preferred
content is from 20 to 40% by weight.
71 The content of high molecular weight rubbers such as
polyisoprene, polybutadiene, polyisobutylene, natural
rubber, SIR and SBR should not exceed 10% by weight and
preferably is less than 5% by weight based on the total
composition.
8) The total content of plasticizers, as previously
described ahould not exceed 25% by weight.
9) Aromatic resins or blend of the same are still
preferably avoided for their detrimental effect on
adhesive and stress/strain properties (steeper
259933
42
stress/stra.in diagrams; generation of a yield point and
consequently of an unrecoverable plastic deformation).
However, as in the previous case, these materials can be
present at levels up to 20~ by weight with acceptable
properties in the composition provided that the
non-adhesive/non elastomeric part of the composition
does not exceed 50$ by weight of the total composition.
This non-adhesive/non-elastomeric part is formed by the
sum of the total styrene content in the composition plus
the content of aromatic resin/resins.
Other requirements and other possible further
components and additives remain the same. In particular
it is still required that the true rheological setting
temperature (measured as the crossing point between G'
and G ") is not lower that 80°C and preferably not lower
that 100°C.
Again with no limitations on their processing and
use, these high modulus compositions are often applied in
the unstretched state, especially the ones having moduli
> 1 MPa. This preferred use is due to the fact that they
are capable of giving sufficient elastic return forces
even at low deformations (typically not higher than 50~)
which are often met during use of stretchable hygienic
articles which can conveniently be made elastic and
resilient in this way. This is also due in part to the
fact that assembling with the composition in the
stretched state implies the need to apply the composition
at about room temperature and so requires that it adhere
2~~993~
43
strongly to substrates even in these conditions (pressure
sensitive adhesive properties). The pressure sensitive
character of adhesives tends to be inversely proportional
to their elastic modulus.
However, some of the high modulus compositions
according to the invention still retain distinct and
useful pressure sensitive behaviour (loop tack on PE >
2.5 N/cm~ 90° peel on PE > 3 N/cm) and can be applied
also at room temperature and in the stretched state at
typical elongations up to 400$ with the elongations at
break of these compositions typically over 900.
Adhesive properties are measured under the same
conditions as for low modulus compositions.
The unexpectedly good level of elasticity of the
compositions according to the invention can be measured
as retention of tensile strength after cyclic
deformation. This is a test that simulates conditions in
use on a hy~~ienic article where the movements of the
wearer can ~~ause further and subsequent elongations of
the elastic;ated parts which, for optimum behaviour, must
regain their initial length with only minor losses of
tensile strength. All the compositions are tested
starting from an already stretched state and are given a
further elongation of about 15$ of the initial stretched
length, in order to simulate movements of the wearer.
The composii=ions were cyclicly stretched and released
fifty times from the initial elongation to the further
stretched elongation.
219933
44
The ~ retention in tensile strength at the initial
elongation after 50 cycles of stretching at a speed 500
mm/minute, compared to the initial tensile strength, was
taken as a measure of the elasticity of the materials.
Tests were performed at room temperature on bands 2.54 cm
wide.
- Low modulus compositions were stretched at an
initial elongation typical of intended applications of
800 and then cyclicly further stretched and released 50
times between 800 and 920$ (ie from 9 to 10.2 times the
initial length of the sample).
- High modulus compositions were tested in the same
manner but at an initial elongation typical of intended
applications of 300 and under 50 cycles of further
elongation and relaxation between 300 and 345$.
The term "tensile strength retention after 50
cycles" as used herein refers to the test described
above. A natural rubber vulcanized elastic produced by
the company JPS Elastomerics which is used in the leg
elastication of diapers and applied at an initial
stretched deformation of 220 was taken as a reference
and was cyclicly deformed 50 times between 220 and 255
It was found that under these conditions the natural
rubber vulcanized elastic, after 50 cycles, had an
average retention of tensile strength equal to 47$ of the
initial tensile strength at 220. This level of
retention of tensile strength was considered indicative
of good elastic behaviour.
219933
- - 45
More generally it was observed that materials that
do not lose more that 60~ of their initial tensile
strength in. these test conditions, show good elastic
behaviour. Retention of tensile strength less than 40~
represents unsatisfactory elasticity as indicated by slow
return to the initial length after release of stretching,
high permanent plastic deformations etc.
As mentioned above the present invention provides an
elasticated absorbent article wherein the elastication is
provided by an elastic adhesive composition as disclosed
herein.
The absorbent article may be a baby diaper, a diaper
for incontinent adults, an incontinence garment, a
sanitary napkin, a pantiliner, etc. The adhesive
composition may provide elastication to the article in,
for example, the waist or leg area of a diaper or
generally i:n any area where elastication is required.
The present invention will now be illustrated with
reference t~o a diaper, although this should not be
considered as in any way limiting on the overall scope of
the inventi~~n.
In the accompanying drawings:
Figure 1 is a perspective view of a diaper.
Figure 2 is a partially cut away plan view of the
disposable diaper of figure 1 opened out into planar
configuration.
CA 02159933 1999-06-25
46
Disposable diapers having many different basic
designs are known to the art and reference can be made,
for example, to U.S. Patent Re 26152, U.S. Patent
3860003, U,S. Patent 4,808,178, U.S.
Patent 4324245, U.S. Patent, 4337771, U.S. Patent 4352355
and U.S. Patent 4253461.
The diaper illustrated in Figure 1 is based on the
disposable diaper design disclosed in US Patent 3860003.
The diaper shown in Figure 1 is merely for illustration
and it will be appreciated that the present invention can
be applied to any other design of diaper.
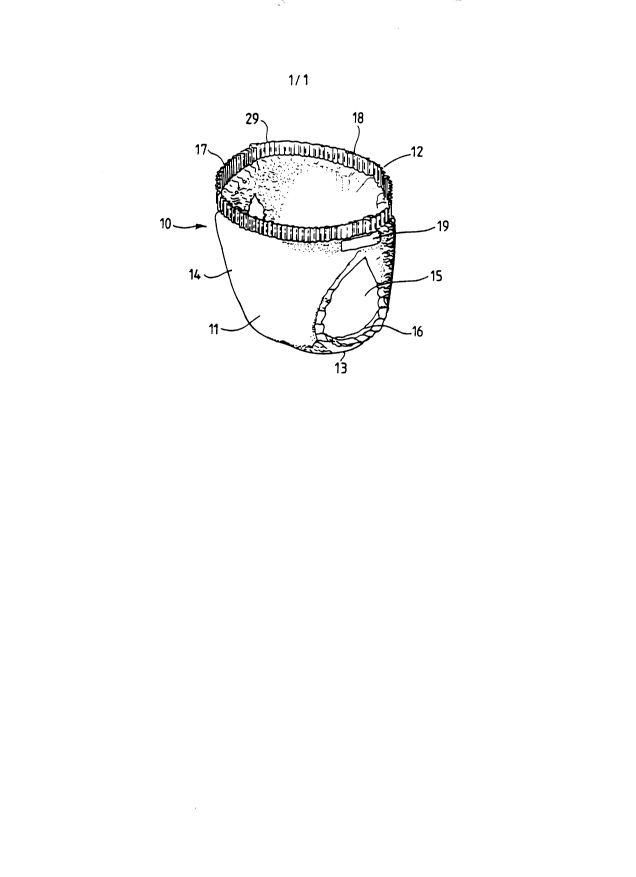
Figure 1 depicts a disposable diaper indicated
generally as 10 which is shown in
perspective in a configuration as if it were applied
about an infant. Disposable diaper 10 comprises a front
portion 11 and a rear portion 12 with a crotch portion 13
interposed therebetween. In use, crotch portion 13 is
placed between the legs of the infant and front portion
11 and rear portion 12 are placed, respectively, along
the front a.nd rear lower portions of the wearer's trunk.
Topsheet 15 forms the inner surface of disposable diaper
10 while ba.cksheet 14 forms its outer surface. Side
flaps (or leg cuffs) 16 fit about the wearer's thighs.
In use, front waistband 17 and rear waistband 18 are
placed adjacent the wearer's waist regions on,
respectively, the front and rear portions of the wearer's
trunk. Disposable diaper 10 is held in position about
the wearer by fastening tape 19. Outer margin of
2.~~~~3~
47
waistband 29 is shown in Figure 1 as the upper edge of
disposable ~~iaper 10. Figure 2 is a partially cut away
plan view of disposable diaper 10 opened out into a
planar configuration. Topsheet 15 is, in this
illustration, the upper surface of the diaper while
backsheet 1~4 is the lower surface. Absorbent element 21
is interpos~sd between topsheet 15 and backsheet 14.
As illustrated, disposable diaper 10 is generally
symmetrical about longitudinal center line 25 and lateral
center line 26. While this is a preferred configuration,
it is not nE~cessary that disposable diaper 10 be
symmetrical,. An asymmetric orientation about lateral
center line 26, as when crotch portion 13 is transposed
toward front: waistband 17, is quite useful.
Disposable diaper 10 is provided with elastic
members 22 in the side margins thereof running generally
parallel to longitudinal center line 25. In the
embodiment illustrated, two elastic members 22 are placed
on either side of disposable diaper 10; single or
multiple elastic members can be used.
Fastening tapes 19 are secured to disposable diaper
10 adjacent rear waistband 18.
Front Hraist elastic element 23 and rear waist
elastic element 24 are positioned, respectively, in front
waistband li' and rear waistband 18 and adjacent outer
margin of waistband 27. In the embodiment illustrated in
Figures 1 and 2, disposable diaper 10 comprises elastic
waist elements in both the front and the rear waistbands.
2j~9933
48
The elastication could however be only in the front or
rear waistband.
The elastication 23 and 24 as shown in Figures 1 and
2 extends across essentially the entire lateral width of
disposable diaper 10. The elastication 23, 24 may only
extend across a portion of the lateral width of the
diaper. Preferably they extend across a major portion of
the lateral width of the disposable diaper. The
elasticatio;n 23, 24 is provided by the elastomeric hot
melt adhesive as described herein.
One major function of backsheet 14 is to prevent
body fluids from escaping from disposable diaper 10 and
soiling the wearer's outer garments and other surfaces in
contact with the disposable diaper. Any compliant,
non-irritating planar material which is impermeable to
body fluids can be used as backsheet 14. Conventional
materials may be used as a back sheet, for example those
as described in the aforementioned patents and
application concerning diapers. A preferred backsheet is
formed from polyethylene film having a thickness of from
about 0.012 to about 0.051 millimetre (mm).
Breathable backsheets (i.e. backsheets that permit
the passage of vapor and air while preventing the passage
of liquid) useful in the present invention are described
in, for example, US 3156242, US 3881489, US 3989867, US
4341216.
The size of backsheet 14 is dictated by the exact
diaper desi<~n selected and the size of the infant
CA 02159933 1999-06-25
49
intended to be the wearer; it can be readily ascertained
by those s);illed in the art.
Topshe~et 15 can be any compliant, soft feeling, non-
irritating (to the wearer's skin) planar material. It
functions t:o contact the wearer's skin, to receive fluid
discharges, to allow the discharges to pass readily
therethrough into the absorbent element, and to isolate
the wearer's skin from the fluids in the absorbent
element. fo aid in effective perfonaance of the last
function, the topsheet is preferably hydrophobic.
Topsheet 15 can be a permeable layer made from
natural or synthetic fibers or mixtures thereof,
non-woven fabric made from natural or synthetic fibers or
mixtures thereof, apertured plastic film, porous foam, or
the like. ;Examples of suitable topsheets are described
in the aforementioned patents and patent application.
A preferred topsheet is spun bonded non-woven
polyester fabric made from fibers of from 2.2 to 2.5
denier, having a basis weight of 17 grams (g) per square
meter (M2). Another preferred topsheet material has a
basis weighs: of 22 g per M2 and comprises 65$ (by weight)
staple lengi:h, 1.5 denier polyester fibers (such as Kodel
type 411 po7lyester fibers as sold by Tennessee Eastman
Corporation, Kingsport, Tennessee) 15$ crimped, staple
length, 1.5 denier rayon fibers: and 20$ acrylic
co of
p ymer binder (such as Celanese CPE 8335 as sold by
Celanese Co=-poration of Charlotte, North Carolina).
2159~3~
50
"Staple length" refers to fibers having a length of at
least 15 mn~.
Still another preferred topsheet is constructed from
polypropylene fibers which have been carded and thermally
bonded in a spaced-apart pattern. Fibers 3.8 centimetres
(cm) long and of from 1.5 to 3.0 denier are suitable. A
preferred topsheet of this type has a basis weight of 24
g per M2.
Suitable topsheets can also be constructed from
apertured plastic films such as those described in U.S.
Patent 4342314, U.S. Patent 4341217 and U.S. Patent
3929135.
As with the case of backsheet 14, the size of
topsheet 15 is dictated by the exact diaper design
selected.
Absorbent element 21 can be any means which is
generally compressible, conformable, non-irritating to
the wearer's skin, and which is capable of absorbing and
retaining fluids.
Absorbent element 21 can be constructed from any of
a variety of materials commonly used in disposable
absorbent articles and which are described in the
hereinbefore incorporated patents. Examples of suitable
absorbent materials include creped cellulose wadding,
absorbent foams, absorbent sponges, super absorbent
polymers, and, preferably, comminuted and airlaid wood
pulp fibers commonly referred to as absorbent fluff. An
21599~~
- - 51
absorbent fluff having a density of from 0.05 to 0.175 g
per cm3 is generally acceptable.
As in the case of backsheet 14 and topsheet 15, the
size of ab:>orbent element 21 is dictated by the exact
diaper design selected.
Optionally, absorbent element 21 can have associated
with either or both planar faces envelope tissues (not
illustrated in the drawings) comprising any permeable
material well known to those skilled in the art, such as
wet strength tissue paper. When used, envelope tissues
are generally coextensive with absorbent element 21 and
either coterminous therewith or folded up and about the
laterally extending margins thereof. Envelope tissues
can optionally be secured to absorbent core 21 by any
means well known to those skilled in the art.
Absort~ent element 21 is interposed between backsheet
14 and topsheet 15. The diaper design selected
determines whether or not the three elements are
coterminous although, in general, either backsheet 14 or
topsheet 15 or both extend beyond the margins of
absorbent element 21.
Optionally, backsheet 14 can be secured to absorbent
element 21 by any convenient means (not illustrated in
the drawings) well known to those skilled in the art.
Examples of suitable means are parallel beads of adhesive
(such as hot melt adhesive) and double sided adhesive
tape; each extend essentially the entire longitudinal
length of absorbent element 21. The backsheet 14 and
2~ ~9 3~
52
absorbent element may be secured together using the
elastomeric: hot melt adhesive as disclosed herein.
Elastic members 22 serve to contract or gather the
cuffs
(longitudinally extending margins) of disposable diaper
and maintain them in contact with the legs of the
wearer thereby providing improved fit and reducing fluid
leakage from the diaper. The elastic members are
provided by the elastomeric hot melt adhesive as
disclosed herein.
The length of elastic elements 22 is dictated by the
precise diaper design chosen. In the design illustrated
in Figures 1 and 2, elastic elements 22 extend a major
portion of the longitudinal length of disposable diaper
10, but terminate outside the waist regions of disposable
diaper 10.
Elastic members 22 are affixed to the disposable
diaper 10 in a stretched or unstretched state, during or
after production of the diaper. For example, disposable
diaper 10 can be contracted in crotch portion 13, for
example by pleating) and elastic members 22 can be
affixed to the contracted disposable diaper 10 while the
elastic members are in their relaxed or unstretched
orientation.
Front waist elastomeric element 23 and rear waist
elastomeric element 24 are each formed of elastomeric hot
melt adhesive material as disclosed herein.
~1 ~99~3
53
The elastication on the diaper may be provided by
the elastom.eric hot melt adhesive as disclosed herein in
the form of threads, yarns, bands, strips, continuous
films, networks of fibers both continuous or having a
finite length and in which the fibers have both a random
orientation or a geometrically regular orientation. The
threads or strips may be in linear or non-linear (curved)
geometry. The adhesive may also be in foamed state.
As will be shown in the following examples,
compositions which provide elastication in absorbent
articles according to the invention, both low and high
modulus, show good elastic behaviour, at the same level
or better than the natural rubber vulcanized elastic.
More specifically high modulus compositions retained
up to 67.5 of their initial tensile force and low
modulus compositions up to 59.8.
Elastic properties were also judged by the following
method: The compositions in the form of bands 2,54 cm
wide, were tested at 23°C and at a stretching speed of
1000 mm/minute, with 3 cycles of elastic hysteresis
between zer~~ and a typical possible elongation in
application i.e. 800 for low modulus and 300 for high
modulus compositions. The elastic energy of each cycle
evaluated ass the area of the cycle was recorded and the
ratio between the elastic energy of the third and the
first cycles was determined as retention of elastic
energy after 3 hysteresis cycles. For good elastic
behaviour it is believed that under these test
CA 02159933 1999-06-25
54
conditions,, a retention of elastic energy not lower than
30~ is required.
The invention is further illustrated by the
following examples which should not be considered as in
any way limiting on the invention. In the case of
proprietary products, details of their nature and
composition is that provided by the manufacturer.
The compositions of all of Examples 1 to 5 are
suitable for the elastication of absorbent articles in
accordance with the invention. The compositions of all
of Examples 1 to 5, when applied from the molten state
between plastics and/or cellulosic materials at a weight
of 5 g/m2 showed a bond strength well in excess of 0.5
N/cm measured as 90° peel.
EXAMPLE 1
An SBC polymeric system based on SBS
(styrene-butadiene- styrene block copolymers) was
formulated as follows:
CARIFLEX TR-4113 S 36$ by weight
EUROPRENE SOL 1205 g$
DERCOLYTE A 115 q5,g$
TM
FORAL 85-E 6$
TM
HERCOLYN D-E 4$
TM
IRC,ANOX 1010 0 . 2 $
259933
55
where:
- CARIFL1~X TR-4113 S is an oil-extended SBS copolymer
available from SHELL Co said to contain:
68.5 by weight of a linear triblock SBS having a styrene
content of 35~ by weight and with a diblock content < 20~
by weight
31.5 by weight of a naphthenic mineral oil, acting as a
plasticizer,, and containing less than 5~ of aromatics.
- EUROPRENE SOL 1205 is styrene/butadiene rubber (SBR)
available from ENICHEM. (The product FINAPRENE 1205
available from FINA is similar and could equally be
used.) It Ls described as a solution polymerized SBR
having a Mooney viscosity ML (1 + 4) at 100°C equal to 47
and a total styrene content of 25~ by weight. This
styrene is partially (typically from 15 to 18~)
distributed in blocks with the remainder being randomly
copolymerized with butadiene. The randomly copolymerized
styrene give's the rubbery part of the molecule the
chemical structure of an amorphous SBR, which contributes
to the development of particularly good pressure
sensitive adhesivity.
- DERCOLS'TE A 115 (the main tackifying resin)is
available from DRT. It is a polyterpene resin derived
from alpha- pinene having a softening point of 115°C.
- FORA1, 85-E is a tackifying resin composed of a
hydrogenated glycerol ester of rosin available from
HERCULES Co. It has a softening point of 85°C.
~~.~5~93
_ 56
- HERCOI~YN D-E is a liquid methyl ester of rosin
available i:rom HERCULES.
- IRGANC)X 1010 is a phenolic antioxidant available
from CIBA-C~EIGY.
The cc>mposition was found to have the following
properties:
- total styrene content = 10.6 by weight of which
10.1 is in blocks
- visco~;ity at 180°C at 80 sec-1 = 20520 cps
- modulu~,s at 500 elongation - 0.182 MPa (low modulus)
- elonga.tion at break > 1400 (1400 was the maximum
elongation achievable on the machine used for the
determination)
- rheological setting temperature (crossover point of
G' and G" ) - 125°C
- loop tack on PE = 8.5 N/cm
- 90° peel on PE = 16.3 N/cin
- tensile strength retention after 50 cycles between
800 and 920 = 59.8$
- elastic energy retention after 3 hysteresis cycles
between zero and 800 = 57.7
- Newtonian Index (N. I.) - 1.05.
The composition showed extremely good elastic and
adhesive properties and was considered completely
suitable for elastication of structures, particularly
hygienic absorbent articles. It was easily processable
i.e. extrudable, having quick setting (stretchable on
line) and having good pressure sensitive characteristics
2~ ~993~
allowing the formation of strong bonds on simple contact
with many substrates even at room temperature.
The formulation was:
CARIFLEX TR-4113S 38.8$ by weight
FINAPRENE 1205 8.9$
DERCOLYTE A115 26$
FORAh 85-E 26$
IRGANOX 1010 0.3$
The following properties were measured:
- total atyrene content = 11.5$ by weight of which
10.9$ is in blocks
- viscos:ity at 180°C at 80 sec-1 = 28180 cps
- modulus at 500$ elongation = 0.188 MPa (low modulus)
- elongation at break > 1400$
- rheoloc~ical setting temperature = 120°C
- loop tack on PE = 8.6 N/cm
- 90°C pE:el on PE = 10.4 N/cm
- tensilE~ strength retention after 50 cycles between
800 and 920$ = 59.1 $
- elastic: energy retention after 3 hysteresis cycles
between zero and 800$ = 48.3 $
- Newtonian Index (N. I.) - 1.01.
CA 02159933 1999-06-25
The composition was similar to that of Example 1,
the main variation being the fact that about 50% of the
high softer,~ing point tackifying resin was substituted by
a lower softening point resin and the only plasticizes
was the oil contained in CARIFLEX TR-4113 S (12.2% by
weight of the composition). Nevertheless it was found
that the composition still retained a very high
solidification temperature, quick setting as well as
optimum elastic and pressure sensitive'adhesive
properties and excellent processability, so that it was
easily possible to extrude and immediately stretch it
even to 800% in the form of very thin threads (diameter =
0.4 mm) that: could be applied for the side elastication
of an absorbent product.
EXAI~LE 3
A different system based on radial SHS was tested,
more precisely a blend of one SHS with relatively low
hard block content and one SHS with relatively high hard
block contenvt .
The formulation was as follows:
FINAPRENE 415 17.7% by weight
FINAPRENE 407. 7.0%
FINAPRENE 12C15 8.0%
TM
ZONATAC 115 KITE 45.8%
FORAL 85-E 6.3%
CA 02159933 1999-06-25
59
HERCOLYN D-:E 4.0~
TM
SHELLFLEX 4510 FC 11.0
IRGANOX 1010 0.2$
where:
- FINAPR1ENE 415 and FINAPRENE 401 are radial SBS
copolymers available from FINA. Both are supposed to
contain less than 20$ diblock and to be formed by a
"star" structure of four blocks of SB chemically linked
in a central point through the butadiene blocks.
FINAPRENE 415 contains 40~ by weight of block styrene and
. FINAPRENE 401 contains 22$ of block styrene.
- ZONATA(: 115 LITE is a hydrocarbon modified terpene
tackifying resin with a softening point of 115°C
available from Arizona Co. It is supposed to be based on
limonene modified with styrene.
- SHELLFhEX 4510 FC is a naphthenic mineral oil,
available from SHELL, which is supposed to have an
aromatic content < 5~.
The following properties were measured:
- total ~styrene content = 10.6$ by weight of which
10.1$ is in blocks
- viscosi.ty at 180°C at 80 sec-1 = 12810 cps.
- modulus~ at 500 elongation = 0.223 MPa (low modulus)
- elongat:ion at break > 1300$
- rheological setting temperature = 107°C
- loop tack on PE = 6.4 N/cm
- 90° peel on PE = 14 N/cm
60 ~~~~933
- tensil.e strength retention after 50 cycles between
800 and 920$ = 50$
- elasti.c energy retention after 3 hysteresis cycles
between zero and 800$ = 44.9$
- Newtonian index (N. I.) - 1.04.
The composition showed properties typical of a very good
and easily processable elastic, extrudable adhesive
material.
EXAMPLE 4
The following high modulus composition was made with the
formulation:
VECTOR 4461-D 44.8$ by weight
ZONAREZ7115 LITE 37$
FORAL 5-E 5$
8
ZONAREZALPHA 25 3$
PRIMOL 352 10$
IRGANOX1010 0.2$
where:
- VECTOR 4461-D is a linear SBS copolymer having 43$
by weight of styrene and non diblock content available
from DEXCO Co.
- ZONARE;Z 7115 LITE is a polyterpene tackifying resin,
having a softening point of 115°C, derived from limonene,
available from ARIZONA Co.
21 ~9 9 3
61
- ZONAREZ ALPHA 25 is a liquid tackifying resin (S. P.
- 25°C) derived from alpha-pinene having a very good
plasticizing effect. It is available from ARIZONA Co.
- PRIMOL 352 is a plasticizing, paraffinic mineral oil
available from EXXON, which is said to contain non
aromatics.
The following properties were measured:
- total block styrene content = 19.3 by weight
- viscosity at 180°C at 80 sec-1 = 16810 cps.
- modulus at 500$ elongation = 1.07 MPa (high modulus)
- elongation at break = 987
- rheological setting temperature = 111°C
- loop tack on PE = 4.3 N/cm
- 90° peel on PE = 6.5 N/cm
- tensile strength retention after 50 cycles between
300 and 345 = 67.5
- elastic energy retention after 3 hysteresis cycles
between zero and 300 = 63.3
Newtonian Index (N. I.) - 1.05.
The composition was a good elastic material useful
especially at low elongations. It showed acceptable
semi-pressure sensitive characteristics so that is can be
bonded to materials also at room temperature in the
stretched state.
1'1 V1 1ITT T G
The formulation was:
259933
62
VECTOR 4461-D 54.8$ by weight
ECR 368 35$
PRIMOL 352 10$
IRGANOX 1010 0.2$
where:
- ECR 36B is a hydrogenated hydrocarbon tackifying
resin, available from EXXON and having a softening point
of 100°C.
The following properties were measured:
- total block styrene content = 23.56$ by weight
- viscos:ity at 180°C at 80 sec-1 = 34000 cps.
- modulu;s at 500$ elongation = 1.61 MPa (high modulus)
- elongation at break = 947$
- rheological setting temperature = 114°C
- loop tack on PE = 1.3 N/cm
- 90° pec=1 on PE = 2.6 N/cm
-_, tensile strength retention after 50 cycles between
300 and 345' = 62.3$
- elastics energy retention after 3 hysteresis cycles
between zero and 300$ = 38.3$
- Newton:ian Index = 1.05.
The composition was made so to maximize modulus
whilst stil:L retaining acceptable elasticity and good
adhesivity at temperatures higher than room conditions.
Such a mate~_ial is more conveniently applied in the
unstretched state and bonded directly at temperature >
CA 02159933 1999-06-25
- - 63
50°C. It gives good elastic return forces even at very
low extensions, e.g, modulus at 20% elongation = 0.236
MPa. However, it also works well in the stretched state,
e.g. 300% elongation, and shows adhesive properties on PE
which are not negligible even at room temperature.
COI~ARATIV~ EXAI~LE A
The formulation was:
KRATON D1107 40.2% by weight
WINGTACK 95 32.9%
IC-145 26.5%
WESTO618 0.2%
- IRGANOX 1010 0.2%
where:
- KRATON D1107 is a linear SIS block copolymer
available from SHELL containing 14% by weight of styrene.
_ WINGTACK 95 is a synthetic polyterpene resin, having
a softening point of 95°C available from Goodyear Co.
- IC-145 is a coumarone-indene aromatic resin, having
a softening point of 145°C and available from the German
Company VFT.
- WESTON 618 is a phosphite based antioxidant
available from Borg Warner Co .
- IRGANO:~C 1010 is as described in Example 1.
This formulation was made in accordance with the
teaching of US-A-4 418 123 (Example IV). According to
CA 02159933 1999-06-25
64
the US patent the composition is said to have completely
satisfactory elastic, adhesive and processing properties.
The formulation of Comparative Example A has the
following differences from Example IV of US-A-4 418 123:
TM
1) The coumarone-indene resin CUMAR LX-509 is not
widely available in Europe and the equivalent resin
IC-145 was used. The resins are chemically similar but
IC-145 has a softening point of 145°C as compared to the
figure of 7.55°C reported for CUMAR LX-509 in the US
patent:
2) For practical reasons, the pigment (1.5$ titanium
dioxide) was omitted. The pigment was presumably present
in the orictinal formulation to mask the light brown
colour and would be expected to have a negligible effect
on adhesives and elastic properties.
The main properties can be summarised as follows:
- total block styrene content = 5.6~ by weight
- visco:~ity at 180°C at 80 sec-1 = 192000 cps
_ modulus at 500$ elongation = 0.365 MPa (low modulus)
- elongation at break > 1400 (1400$ was the maximum
elongation achievable on the machine used for this
determination)
- rheological setting temperature (crossover point of
G' and G" ) - 195°C
- loop tack on PE = 5.3 N/cm
- 90° peel on PE = 15.7 N/cm
- tensil.e strength retention after 50 cycles between
800 and 920$ = 34.3
.~. 21 ~~~~~
- elastic energy retention after 3 hysteresis cycles
between zero and 800 = 19.8
- Newtonian Index (N. I.) - 2.03.
It was found that the above formulation had good
adhesive, pressure sensitive characteristics as indicated
by the US patent in that as measured on PE under the
above described conditions, the loop tack was 5.3 N/cm
and the 90° peel was 15.7 N/cm. However it was also
found that both elastic and processing properties for an
extrudable material were unsatisfactory. In particular:
- The processability, especially the application under
thin strips or threads, was very difficult owing to the
extremely high viscosity and the highly non Newtonian
rheological behaviour.
- The mo~~ulus at 500 elongation was found to be 0.365
MPa and the elongation at break to be > 1400. However
elastic pro~~erties were unsatisfactory. In particular
under the cyclic deformation test between 800 and 920
the formulation was found to lose 65.7 of its initial
tensile strength after 50 cycles and to lose 80.2 of its
elastic energy after 3 hysteresis cycles between 0 and
800 making it unsuitable for the elastication of
products such as hygienic absorbent articles that are
stressed in use many times by subsequent stretchings due
to movements of wearer. It is worthy of note that 45$~of
the loss of tensile strength occurred between the first
and the second cycle, confirming an easy and
unrecoverab:Le plastic deformation.
CA 02159933 1999-06-25
66
C(~ARATIVE EXAI~LE H
The formulation was:
TM
TUFPRENE A 30.0$ by weight
ESCOREZ CR 368 55.0$
(:ATENEX P941 10.0$
TM
KRISTALEX 1.~'100 5.0$
with the addition of 0.2 parts per 100 parts by weight of
the above composition of the antioxidant IRGANOX 1010,
where:
- TUFPRF'sNE A is a linear SBS available from Asahi
Chemical C0. and containing 40$ by weight of styrene.
Diblock content is not specified by the manufacturer.
- ESCORF~Z CR 368 is a hydrogenated modified
hydrocarbon resin available from Exxon having a softening
point of 100°C.
_ CATENEX P941 is a paraffinic mineral oil available
from Shell which is supposed to have an aromatic content
< 5$ by we:i ght .
- FQtISTALEX F 100 is an aromatic resin based on
°-methyl si.yrene and styrene available from Hercules and
having a softening point of 100°C.
This :Formulation was made in accordance with the
teaching o:E EP-A-0424295 (Example V). The formulation of
comparative example B has the following difference from
Example V of EP-A-0424295:
7 2159933
0.2 parts per 100 parts by weight of the antioxidant
IRGANOX 1010 was added to the composition as set out in
Example V of EP-A-0424295. As would be apparent to any
person skilled in the art, attempting to compound and
process the composition exactly as disclosed in
EP-A-0424295, i.e. without an antioxidant would
undoubtedly have led to thermal degradation of the
system. In order to follow the teaching of EP-A-0424295
as closely as possible the antioxidant used in the
comparative example A of that document was used.
However, the antioxidant was added at the usual level of
0.2~ (this :being the level also used in the preceding
examples according to the invention) rather than the
unusually (and also unnecessary) high level used in
Comparative Example A of EP-A-0424295.
The main properties can be summarised as follows:
- total block styrene = 12~ by weight
- viscos:ity at 180°C at 80 sec-1 = 5620 cps
-_ modulua at 500 elongation = 0.204 MPa (low modulus)
- elongaition at break > 1300$
- rheoloc~ical setting temperature (crossover point of
G' and G" ) - 106°C
- loop tack on PE = 0.7 N/cm
- 90° peE~l on PE = 0.8 N/cm
- tensilE~ strength retention after 50 cycles between
800 and 920i~ = 21.3$
- elastic: energy retention after 3 hysteresis cycles
between zero and 800 = 25.0$
68
- Newtonian Index (N. I.) - 1.16
It was found that processability, as shown by
viscosity a:nd NI was acceptable although NI was high for
an SBS base~3 composition. However, the formulation had
poor pressure sensitive characteristics and values of 0.7
N/cm for loop tack and 0.8 N/cm for 90° peel show it to
be practica.Lly unusable as a low modulus elastic
adhesive, intended to be applied in the stretched state,
in the assembly of hygienic absorbent articles. Elastic
properties were unsatisfactory as indicated by the loss
of about 80'~ of the tensile strength of the composition
after 50 cycles of subsequent stretching and of 75~ of
its elastic energy after 3 hysteresis cycles.
The unsatisfactory properties of the composition may
be related i.o the diblock content of TUFPRENE A. This is
not stated by the manufacturer and direct measurement is
difficult. However the value provided by the
manufactures- for Tensile Set at break measured according
to ASTM method D412 is 47~. This compares to much lower
values of around 10~ or lower quoted for SBS copolymers
with a diblock content < 20~ by weight. On the other
hand SHELL technical literature gives the following
figures for Tensile Set at break for copolymers with a
high diblocl~: content
- KRATON D-1112 (SIS containing 40~ by weight diblock)
- 20~
- KRATON D-1118X (SBS containing 80$ by weight
diblock) -4Cn .
69
From this, it can be inferred that TUFPRENE A probably
has a diblock content well in excess of 40~.
It should be noted that the above results seem to be
inconsistent with the results reported in EP-A-0424295 in
at least some respects. Thus the 90° peel of 0.8 N/cm
reported above compares to 3.9 N/cm for 180° peel
derivable from EP- A-0424295 . This may be explained at
least in part by both the different peel angle, and the
compression used in the test (400 g as compared to 2 kg)
since the composition is stiff and bonding is very much
influenced by compression. It should also be noted that
the weight of adhesive per square meter is not specified
in EP-A-0424295 and this is extremely important in
determining bond strength. The tests used according to
the present invention provide a realistic measure of the
suitability of the compositions for use in the proposed
applications. The poor properties of the composition of
EP-A-0424295 may also be related to the combination of a
hard SBS (40~ styrene) with an aromatic resin at low
plasticises levels (10~).
EXAMPLE 6
This e:~ample relates to the stress/strain diagrams
of the compositions of Examples l to 5 and Comparative
Examples A and B. The elastic hot melt compositions
according to the invention should desirably have a
stress/stra:in diagram that is much flatter than a rubber
215993?
elastic, i.~. even if already under tension further
stretching (e.g. due to the movements of the wearer of
the absorbe:zt article) cause only a very low increase in
modulus and in the tensile strength that is perceived by
the wearer. This is especially true for low modulus
compositions and can be seen by measuring the average
increase in modulus for a given extension. Low modulus
compositions, which are typically applied in the already
stretched svtate, were judged as the mean increase in
modulus per 100 increase in elongation, by dividing by 8
the total increase in modulus between 0 and 800
elongation (nine times the initial length).
Referring to the low modulus compositions mentioned
in the abovEs Examples the results were as follows:
EXAMPLE N0. MEAN INCREASE IN MODULUS PER
100$
STRETCHING
1_ 0.044 MPa/100$ stretching
2 0.045 MPa/100~ stretching
3 0.063 MPa/100~ stretching
Comparative Example A 0.099 MPa/100~ stretching
Comparative Example B 0.079 MPa/100$ stretching
The high modulus compositions of Examples 4 and 5, which
are generally used in the unstretched state or in any
case at lowE~r elongations, were judged as mean increase
in modulus per 100 increase in elongation between zero
and 300$ final elongation:
71
EXAMPLE N0. MEAN INCREASE IN MODULUS PER
100$
STRETCHING
4 0.169 MPa/100$ stretching
5 0.284 MPa/100$ stretching
As a comparison, a natural rubber vulcanized elastic,
used for the leg elastication of diapers, even if applied
at much lower extension (typically 2200 showed, between
zero and 220$, an average increase in modulus of 0.89 MPa
per 100$ el~~ngation.
It is ;aossible to compare the behaviour of a natural
rubber elastic and of a low modulus composition according
to the invention.
In the case of rubber elastic, even limited
movements of the wearer that cause for instance a further
stretching ~~f the elasticated parts as low as say 10$
elongation, will cause a mean increase in modulus of
about 0.09 IKPa. By using a low modulus composition the
increase in modulus will be about 20 times lower and even
with the strongest high modulus compositions several
times lower so that the movements of the wearer of an
absorbent article elasticated by the compositions
disclosed in the present invention are much more free.
Accordingly, in these applications low modulus and low
variation of modulus with strain are a clear advantage.