Note: Descriptions are shown in the official language in which they were submitted.
~ 094/~770 2 1~ ~ 3 ~ ~ PCT/F~4/00136
A METHOD OF TREATING ENDO-OSTEAL MATERIALS
This invention relates to the use of bisphosphonates for
the treatment of endo-osteal materials including implants
to be used in surgery before their introduction into the
human body. The invention concerns particularly the
addition of a certain amount of a bisphosphonate to the
sterile preservation medium for the endo-osteal materials.
In recent years intensive studies have been made on
artificial endo-osteal materials, especially implants, to
be introduced in the human body such as artificial joints,
fixation plates in skeleton, hips and dental implants.
Substantial efforts have been made with respect to
materials which give high mechanical strength as well as
good biological affinity.
Surgical techniques involving the use of endo-osteal
prostheses including implants (screw implants, blade
implants, pin implants etc) into bone tissue are extensi-
vely used in orthopaedic and dental surgery as a result of
the progress made in somatological engineering.
Endo-osteal prostheses can roughly be divided into two
groups: those comprising a metallic substrate on the one
hand and ceramics and glass-ceramics (bioceramics) on the
other hand. Metallic prostheses possess excellent strength
properties but rather poor biocompatibility. Titan and its
alloys are the most frequently used metallic prostheses in
both orthopaedic and dental surgery. In order to enhance
the osseointegration and bone bonding process the metallic
substrate is normally plasma-spray coated e.g. with apatite
or hydroxyapatite to promote the bone bonding process. As
examples of endo-osteal orthopaedic prostheses can
mentioned Tricon-M and Allopro knee prostheses, Ortholoc
tibial prosthesis and Monk, DF-80 and Authopor hip
prostheses. Among the frequently used metal substrate
implants in the dental field can be mentioned pure titanium
2 ~ 6 5
W094/~770 PCT/FW/00136
implants (Nobelpharma, Swede-VentR, IMZ). Among coated
metallic substrate implant can be mentioned BonefitR, a
titanium substrate with titanium plasma coating; Steri-
OssR, a hydroxyapatite-coated metal alloy substrate and
Calcitec, a hydroxyapatite coated titanium substrate. The
ceramic implants are e.g. based on polycristalline
aluminium oxide, Al2O3 (Frialit). Glass-ceramics and
bioceramics include various compositions of glasses,
ceramics and glass-ceramics having ability to bond to bone.
Endo-osteal materials to be introduced in the human body
must be preserved strictly under sterile conditions before
use. The preservation can take place under dry conditions
or in a sterile solution. All the dental implants used are
packed in steril glass syringes. These syringes are either
empty or contain fysiologic saline solution. Dental
implants are normally packaged in small ampouls in some
milliliters of sterile sodium chloride solution. The hip
prostesis are packed in sterile containers, all parts in
their separate containers without any liquid.
The preservation of the prostheses and implants before
their use is not just a problem relating to sterility. It
is known that the biocompability of an implant is highly
associated with the surface property of the material. It is
therefore of great importance that the surface layer is
carefully controlled and specified at atom level. Two
implants initially manufactured from the same material can
aquire completely different bioactivity properties
depending on how the material is treated. Sterilization can
for example vary between two similar implants and thus
result in totally different biocompability for the two
similar implants. The problem relating to the risk of
cont~m;n~tion of the surface of the implant resulting in
inactivation of the implant surface has been realized, and
suggestions to overcome the problem have been made. US
patent 4,712,681 describes a method of packaging artifical
implants in sterile and cont~min~tion-free manner according
2160565
094/~770 PCT/F~4/00136
to which the implant is packaged in an inner capsule made
of the same material as the implant itself. US 4,763,788
suggests a rather similar solution of the cont~m;n~tion
problem; it represents a modification of the double capsule
system presented in US 4,712,681.
The sensitivity of the implant surface to particles in the
surrounding has thus been regarded as a difficulty to be
overcome.
This invention is based on the idea to take advantage of
the sensivity phenomenon and bring the surface of the endo-
osteal material in close contact with agents having a
positive influence on the biocompatibility of the endo-
osteal material. This is practically carried out by adding
a biocompatibility promoting agent to a solution in which
the endo-osteal material is going to be preserved before
its use.
According to one aspect of the invention an effective
amount of a bisfosphonate is added to the solution to be
used for the preservation of endo-osteal materials such as
artificial joints, hip prostheses, fixation plates, dental
and other implants. The use of endo-osteal prostheses
having been storaged in this manner optionally in
combination with a systemic bisphosphonate therapy is
strongly believed to result in a much lower failure rate
compared to the situation where no bisphosphonate is added
to the presevation solution for the endo-osteal prosthesis.
Bisphosphonates are synthetic organic compounds
structurally related to pyrophosphate in that the
pyrophosphate P-O-P-bond is replaced by a P-C-P-bond. In
contrast to pyrophosphate, bisphosphonates are resistent to
enzymatic hydrolysis in osseous tissue. The bisphosphonates
are potent inhibitors of bone resorption and they have been
successfully used in the treatment of hypercalcemia caused
by various reasons. A great number of bisphosphonates have
2160~5
W094/~770 PCT/F~4/00136 ~
been studied, but only clodronate, etidronate and
pamidronate have reached wider clinical use.
The main effect of the bisphosphonates is their ability to
inhibit bone resorption, but contrary to the effect on
mineralization, the mechanism involved is cellular (Fleisch
H, Drugs 1991; 42: 919-44). These different effects vary
greatly according to the structure of the individual
bisphosphonate compound. The half-life of circulating
bisphosphonates is very short, in the order of minutes to
hours. Of a given dose, 20 to 50 % is taken up by the
skeleton, the rest being excreted in the urine. The half-
life in bone is far longer and depends upon the turnover
rate of the skeleton itself.
A review (Mian M et al., Int J Clin Pharmacol Res. 1991;
11: 107-14) of 126 publications on clinical studies
concerning the use of clodronate in the therapy of bone
disease, involving 1930 patients, in order to evaluate the
tolerability and the effects following short- and
long-term administration of this drug, indicates that
clodronate therapy does not have any clinically significant
side-effects and confirm its tolerability and safety.
Of the many compounds belonging to the bisphosphonate
family, clodronate has been widely used in hypercalcemia
and osteolysis of malignancy (Bonjour J P and Rizzoli R,
Calcif Tissue Int 1990; 46 Suppl: 20-25). All published
reports indicate that clodronate can normalize plasma
calcium in the majority of hypercalcemic, rehydrated cancer
patients in whom increased bone resporption is the
prevailing disturbed calcium flux (Fleisch H, Drugs 1991;
42: 919-44).
Various phosphonate compounds are also reported in the
patent literature as being useful in the treatment of
anomalous mobilization and deposition of calcium phosphate
salts (bone mineral) in mammals. Reference is made to US
2 1 6 0 5 6 5 rCT/FV4/00136
patents 3,678,164; 3,662,066; 3,553,314; 3,553,315;
3,584,124; 3,584,125 and 3,641,246. US 3,683,080 discloses
the use of clodronate and various other phosphonates for
the treatment of anomalous calcification involving soft
tissues and arthritic conditions. US 4,234,645 discloses
clodronate as useful in the treatment of various collagen
diseases.
As discussed above, bisphosphonates are well documented
with respect to their ability to inhibit bone resorption in
connection with various diseases. The use of these
compounds to promote bone tissue formation subsequent to
surgical operations relating to endo-osteal prosthesis such
as hip prostheses, plates used in internal rigid fixation
and various kinds of implantations; osteomyelitis after
decorticalization of necrotics from the mandible or bone
transplantations has, however, never been suggested.
Particularly in dental implantation surgery, patients with
severe atrophy of the mandibular alveolar process are
difficult to treat by conventional implant techniques. At
the abutment connection operation mobile fixtures are found
frequently. About half of the number of recorded failures
occurred under the healing period (Adell R et al., Int J
Oral & Maxillofac Surg 1990, 5: 347-359). Autogenous bone
grafts used for severely resorbed ridge augmentation
usually resorb to a considerable extent (Baker R D et al.,
J Oral Surg 1970; 37: 486-89).
The effect of clodronate on hydroxyapatite has been
extensively studied. Although the effect of clodronate on
hydroxyapatite is well documentated, the use of clodronate
or other bisphosphonates to preserve hydroxyapatite coated
or otherwise coated endo-osteal prostheses including
implants has never been suggested. Neither has been
suggested the use of bisphosphonates to activate the
uncoated metal surface of such prostheses or implants.
The present invention relates to a method of treating endo-
W094/~770 2 1 6 0 5 6 5 PCT/F~4/00136
osteal materials to be used in surgery for sterile and
biocompatibility promoting storage characterized in the
embedding the endo-osteal material in an aqueous solution
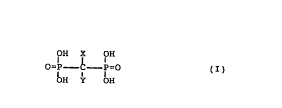
comprising an effective amount of a compound of formula (I)
OH X OH
o=P c b= ( I )
OH H
wherein X is H, OH, Cl, F or a methyl group and Y is Cl,
' ( CH2 ) 2 N ( CH3 ) - ( CH2 ) 4-CH3 ~ - ( CH2 ) n-CH3 or -(CH2)n-NH2, where
n is zero or an integer being l to 8, -NHZ, where Z is
pyridinyl or cycloheptyl, SZ', where Z' is pyridinyl or
chlorosubstituted phenyl or Y is a pyridinylsubstituted
lower alkyl chain; or a non-toxic, pharmaceutically
acceptable salt or ester thereof, after which the endo-
osteal material is either
- removed from the solution, dried and sterilized, or
- sealed in a vessel comprising a bisphosphonate solution
and sterilized.
The invention concerns also the packaged endo-osteal
material to be used in surgery for sterile and
biocompatibility promoting storage characterized in that
said endo-osteal material treated with a solution of a
bisphosphonate of the formula (I) or a non-toxic,
pharmaceutically acceptable salt or ester thereof dissolved
in an appropriate solvent.
The term "endo-osteal material" shall be understood to
include all kinds of endo-osteal prostheses and parts
thereof to be introduced in the human body, e.g. artificial
joints, hip prostheses, fixation plates for the skeleton
and implants, especially dental implants.
Particularly valuable members of formula (I) for the
purpose of this invention are clodronate, where X and Y
~16 0 5 6 ~ PCT/F~4/00136
both are Cl; pamidronate, where X is OH and Y is
-(CH2)2-NH2; alendronic acid, where X is OH and Y is
- ( CH2 ) 3-NH2; neridronic acid, where X is OH and Y is
- ( CH2 ) 5-NH2; risedronic acid, where X is OH and Y is
is 3-pyridinylmethyl; tiludronate, where X is H and Y is 4-
chlorophenylthio; YM-175, where X is H and Y is
cycloheptylamino; BM-210995, where X is OH and Y is
- ( CH2 ) 2-N ( CH3 ) - ( CH2 ) 4-CH3; and etidronate, where X is methyl
and Y is OH. The most preferable compound for the purpose
of the invention is clodronate or its pharmaceutically
acceptable salts or esters.
The pharmaceutically acceptable salts and esters useful in
the practice of this invention can be described by formula
(II)
IOM X ~M
O=P -C l=O (II)
OM Y OM
wherein X and Y are as defined above and M is hydrogen, a
pharmaceutically acceptable cation, preferably an alkali
metal cation such as sodium or potassium, or an alkyl or
aryl moiety, e.g. an alkyl of 1 to 4 carbon atoms or
phenyl.
The suitable amount of bisphosphonate to be added to the
treatment solution ranges from 0.5 to 100 mg/ml, preferably
1 to 10 mg/ml.
The treatment can be performed for example as follows:
After manufacturing the endo-osteal prostheses they are
embedded in a solution of 1 to 6 mg/ml of a bisphosphonate
for a period ranging from about 20 minutes to 7 days. After
that the endo-osteal prostheses can be dried and sterilized
by gamma-radiation and stored in dry condition before their
use. Alternatively the endo-osteal prostheses can be stored
in the same or a similar bisphosphonate solution as that in
21~
W094/23770 PCT/~94/00136
which they were embedded. In this case the bisphosphonate
solution is sterilized by gamma-radiation.
The treatment with bisphosphonates apply to endo-osteal
prostheses having a ceramic surface as well as those having
no ceramic surface.
The inventive idea has been verified by animal and clinical
tests. According to two separate studies with clodronate
disodium, the methods and results of which are presented in
detail below, the effect of clodronate on bone tissue
formation is demonstrated.
In the first test, the effect of clodronate on bone
regeneration was tested in rabbit tibia. An experimental
model involving free bone transplantation to the tibia was
developed. The tests revealed that clodronate had a
positive effect on bone regeneration in the donor cavity
and in the free bone grafts transplanted using a titanium
screw. Clodronate-treated tibias were more quickly and more
extensively vascularized than the control tibias.
The results of human studies, where the patients had an
extra implant that was removed after a certain period of
time, demonstrated that clodronate-medicated patients
exhibited a more rapid bone formation than the unmedicated
control group.
Hydroxyapatite and bisphosphonbate molecules form a surface
and not a chemical bond. In the surface there is a place
for calcium atoms. Thus the calcium concentration is high.
The composition of hydroxyapatite and bisphosphonate
forming a layer on the surface of the endo-osteal
prosthesis or other implant is helpful in bone regeneration
after surgery.
The effect of bisphosphonates for the treatment of implants
has also been studied in tests reported below.
2I6056~
094/~770 PCT/F~4/00136
Because of the close structural and pharmacological
relationship between clodronate and its analogues as
represented by formula (I) above it is justified to believe
that the remaining members of formula tI) also are
effective to promote the biocompatibility of endo-osteal
prostheses including implants during their storage.
EXPERIMENTS
I. Effect of clodronate on bone reqeneration in rabbits
The aim of the study was to determine whether clodronate
had a positive effect on vascularization and bone formation
in the tibia of a rabbit in which bone was transplanted
with the aid of a titanium screw.
Materials and methods
Sixteen skeletally mature (3.5 - 3.9 kg) New Zealand white
male rabbits were used. The animals were divided into two
groups. Each group consisted of eight animals (16 tibiae).
One group received clodronate disodium (BonefosR, Leiras Oy,
Finland) 25 mg/kg i.m. twice a week. The other group
(control) was untreated.
The rabbits were anaesthetized with an i.m. injection of
2.8 mg of KetalarR (Parke-Davis, Spain) and 2.0 ml of
RompunR (Bayer, Germany).
The proximal ends of both tibiae were exposed and the
periosteum removed from the operative area. A piece of
cortical bone 4 mm across was removed using a trepan bur. A
0.6 mm titanium implant screw (Filpin, Filpol Dental,
Ireland) was screwed through the piece. The piece,
perforated with the implant, was screwed into place 3 mm
above the donor cavity. Reference is made to Figure l
representing the rabbit tibia, where A means the implant, T
W094/23770 2 ~ 6 0 ~ ~ 5 PCT ~ 4/00136
the transplant and F the donor cavity. The upper drawing of
the Figure represents the cross section and the lower
drawing the tibia as seen from above.
The animals were divided into two groups: microangiography
was performed on eight animals and histological staining
specimens was carried out from the other eigth animals.
Roentgenological ex~min~tions with two steel wires with
knots twisted around the tibiae to determine the exact
positions of implant and donor cavity were performed.
Reference is made to Figure 2, which discloses a lateral
roentgen picture of tibia in the operation area. The
letters A, T and F have the same meaning as in Figure 1.
Histological evaluation
Eight animals were killed for histological evaluations at
various times after implantation: after 14 days (2
rabbits), 21 days (4 rabbits) and 35 days (2 rabbits). The
number of control and clodronate-treated animals was the
same each time.
Tibiae were fixed with 5 % phosphate-buffered formalin and
toluine blue st~ining and hematoxylin eosin (HE) were
carried out. Specimens were inspected under a light
microscope and adverse effects or signs of inflammation
were recorded.
Microangiography
Eight animals (4 controls, 4 treated) were killed after 21
days by means of an i.v. dose of pentobarbital.
Before death the abdominal artery and vein were exposed and
an 18-gauge angiocath was inserted and tied in place. A 20
ml syringe cont~;ning heparinized saline was used to infuse
the abdominal artery. Infusion continued until a clear
venous effluent emerged from the transacted abdominal
~094/~770 ~1 6 ~ PCT/F~4/00136
veins. A 100 ml syringe filled with an orange-colored
silicone rubber compound (Micro-FilR, Canton Biomedical,
Boulder CO, USA) was then injected until orange effluent
emerged from the abdominal veins. After the compound had
set for 4 hours, the tibiae were separated. The specimens
were then sequentially dehydrated according to the cleaning
technique of the manufacturer.
Using a scalpel, cross-sections were cut through the mid-
portions of the grafts for viewing and slide photography
under a dissecting microscope. The absolute number of
vessels penetrating the transplant host junction was
counted by means of color transparencies (Eppley B et al.,
J Oral Maxillofac Surg 1988; 46: 391-98).
The vessel count was performed in the specimen where the
most vessels were observed. Vessels were counted on two
separate occasions by the same observers and the results
were averaged. If the variation between two values was
greater than 10 %, a third count was undertaken and the
three counts were averaged. Vessel counts in both groups
were compared using a paired t-test; P values less than
0.05 were considered significant.
Results
The clinical observations revealed that all wounds healed
uneventfully.
Evaluation of angiogenesis
When counting the vessels, most of them were clearly
visible. It was, however, difficult to count the small
vessels in the bone-transplant and bone-donor cavity
junctions. Because of the variation in the two values by
the same observer the third count was undertaken in five
specimens.
W094/23770 216 0 ~ 6 ~ PCT/F~4/00136 ~
Donor cavities
The number of vessels penetrating into the donor cavities
was greater in rabbits treated with clodronate than for the
control. The results are given in Table I below and the
difference is statistically significant (P < 0.05).
Table I
Number (x) of vessels penetrating donor cavity
x S.D. Number of tibiae
________________________________________________________
Control 12.3 4.6 8
Clodronate treated 26.3 4.0 8
The difference in the amount of vessels can also be
observed from the photographs of Figures 3 and 4. Figure 3
discloses a 21-day specimen from a rabbit treated with
clodronate. Implant and transplant are located in the
centre of the picture. The donor cavity is seen to the
right of the transplant. It can be seen that many vessels
penetrate the donor cavity and transplant. Figure 4 shows a
21-day specimen from an untreated rabbit. Only a small
number of vessels penetrated the transplant.
Transplants
The transplants in the tibiae from the clodronate-treated
animals became vascularized sooner and more extensively
than in the tibiae from the control. The difference was
statistically significant (P < 0.05). The results are
presented in Table II.
~ W094/23770 216 0 5 6 5 PCT/~94/00136
13
Table II
Number (x) of vessels penetrating transplant
x S.D. Number of tibiae
________________________________________________________
Control 4.75 1.7 8
Clodronate treated 13.0 4.0 8
The vessels penetrated closer to the centre of the cavity
in the medicated rabbits than in the control group. In the
medicated rabbits the number of vessels from one side of
the specimen was greater than from the opposite side.
Histological findings
No signs of adverse tissue reactions or inflammation were
observed when the specimens were studied under the light
microscope.
Donor cavity
The 14-day control specimens exhibited slight collagen
formation and were partly devoid of histologically visible
elements in the middle part of the cavity. The clodronate-
treated specimens exhibited more collagen formation than
the control specimens. No empty spaces were seen. At three
weeks, the control specimens exhibited only slight bone
formation at the outer edges of the cavity. The inner part
of the cavity was mainly filled with collagen and a sharp
line between the cavity and bone was clearly seen. The
clodronate-treated donor cavities were almost completely
filled with new bone. Collagen was still found between new
bone in the three-week specimens.
The five-week control cavities were partly filled with new
bone, and the line between drilled cavity and bone was
W094/~770 ~ PCT/F~4/00136
14
still seen in most parts of the cavity. The clodronate-
treated cavities were completely filled with new bone and
the drilling line was visible but the resolution between
the donor cavity and old bone had started. Figure 5
illustrates a five-week control cavity. Bone regeneration
is seen in middle of cavity and in drilling lines. The line
between drilled cavity and bone is still seen in most parts
of cavity. New bone formation with osteoblasts occurs
occasionally in drilling line and also in centre of
cavity. Figure 6 illustrates a five-week clodronate-treated
cavity. Cavity is completely filled with new bone and
drilling line is still visible but there is a fusion
between donor cavity and cortical bone. Figures 7 and 8
represent greater magnifications of Figure 6. In Figure 7
solid new bone and osteoblasts can be observed. Figure 8
shows that cortical and new bone are almost completely
fused.
Transplants
The soft tissue and periosteum above the transplants
contained more collagen in the clodronate-treated group
than in the control animals at all stages. Fourteen-day
control specimens exhibited necrotic bone with invading
collagen. Treated transplants were beginning to be resorbed
at their outer edges. Figure 9 represents a side-view of
four-week clodronate-treated rabbit's tibia. New bone
covers transplant. Periosteum is intact but thinner than
that above non-operated area. Implant and transplant are in
the middle of this specimen. Donor cavity is to the right
from transplant and is the reason for new bone formation in
normally empty rabbit's spongious bone.
Twenty-one-day transplants were partly resorbed. New bone
in the resorbed areas was seen in the treated tibiae. No
bone formation was seen in control transplants. Bone
formation around the implant in the cortical bone area was
solid in the clodronate-treated group. Figure lO represents
~ W094/~770 21 ~ ~ 65~ PCT/F~4/00136
a clodronate-treated 21-day specimen. Transplant is partly
resorbed and replaced with new bone. The letters A and T
represent implant and transplant, respectively, as in
Figure 1, and E represents new bone adjacent to transplant
and cortical tibia.
In 35-day specimens there was new bone formation almost
throughout the transplants in the treated tibiae. Only
solid bone was seen in the control transplants.
Regeneration of transplants occurs through microvascu-
larization of the transplant. In a rat embryo study, Ray
(Ray R D, Clin Orthop 1977; 87: 43-48) showed that
vascularization of a rat embryo takes 3 to 4 weeks. In a
review article, Burchardt (Burchardt H, Clin Orthop 1983;
174: 28-42) states that cancellous bone differs from
lS cortical grafts as far as rates of revascularization are
concerned. He suggested that revascularization of
cancellous grafts can occur within hours as a result of
end-to-end anastomoses from host vessels. Revascularization
may be completed within two weeks (Ray R D; reference as
above). A cortical graft is not penetrated by blood vessels
until the sixth day (Ray R D; reference as above). Twenty-
one days was selected on the basis of the results of a
report by Eppley and co-workers (Eppley B et al., J Oral
Maxifollfac Surg 1988; 46: 391-98) as bone regeneration
time after implantation. They found that the
vascularization of bone grafts in rabbits reached a m~xi mum
after 21 days.
The results of the present study confirm the results of
earlier reports (Bonjour J P; Ray R D; both references
given above) as far as the control group is concerned. In
the medicated rabbits vascularization occurred more quickly
than in the control group. The histological findings show
clearly that clodronate-treatment makes better bone. The
results of the study suggests that bisphosphonates,
particularly clodronate, are useful in implant and bone
5 ~ ~
W094/~770 PCT/F~4/00136
transplant patients where there is a high risk of failure
of bone regeneration.
II. Human tests
Material and methods
The material of this study were 20 edentulous patients.
They all came to the Institute of Dentistry, University of
Turku, for an implantation procedure. The Institutional
Review Board of the Faculty of Medicine at the University
of Turku received the project in order to determine whether
human subjects are placed at risk. The unanimous decision
made by the Institutional Review Board was that the human
subjects concerned in this activity would not be placed at
any risk. Patients gave permission for an explantation of
an extra implant. lO patients got a daily dose of 1600 mg
clodronate disodium until the extra implant was removed
(the medicated group) and lO patients got placebo. The
medication and placebo administration, respectively,
started one week before the surgery and continued for three
weeks after the surgery.
Surgical technique
Routine method with five Astra implants was used. Fig. ll
is a front view human mandible with four Astra implants,
where A means implants, E explanted implant with bone and N
is the mandibular nerve. To avoid disturbances in neural
function implants are usually placed between the ends of
mandibular nerve. At the operation an extra 4 mm screw was
installed in the midline of the m~n~ ible.
Bone remodelling
At a separate operation the 4 mm extra implant was removed
with a trephan bore after 4 (lO patients, equally from both
groups) and 12 weeks (lO patients, equally from both
21~056~ -
094/~770 PCT/F~4/00136
groups). The specimens were imbedded in acrylic blocks and
divided in midline in two pieces. To the one piece a
histological ~XAminAtion was performed. The other one was
taken to a SEM-electromicroscopic exAmin~tion.
Electromicroscopic ex~min~tion in bone-implant interspace
and bone in three points with SEM/EDXA (energy dispersive
X-ray analysis) was made. At the four different places, two
in the upper cortical bone, one in the middle of the
implant and one in the bottom of that the following values
are calculated: sodium, calcium, phosphor, magnesium and
titan. Calciumtphosphor and calcium/magnesium ratio were
calculated in 12 points.
Results
Clinical treatment
All the wounds healed well. Two patients had problems with
their lower denture under the healing period. They were
treated by taking away a part denture. No side-effects were
recorded. One patient had pain in his hip orthopedic
prosthesis. Those disappeared after clodronate medication.
Histological examinations
One month-specimens
Because all the mandibles were considerably resorbed and
when the length of explanted implant was 4 mm biopsied bone
was cortical in all specimens. The histological results are
shown in Figures 12 and 13, which both disclose the bone-
implant specimen marked with E in Figure 11. Fig. 13
represents a greater magnification of Fig. 12. No spongious
bone was seen. Soft gingival tissue covering the implants
was healthy.
Histological eXAmin~tion revealed no more new bone in
medicated than control-mAn~ihles. There were no signs of
W094/~770 1~ 0~ PCT/F~4/00136
18
inflammation. The space between implant and bone was mainly
filled with collagen. In same points the contact between
bone and implant was close. This is natural, because
screwed Astra implants were used.
SEM-results
Table III shows the SEM results in human mandibles 4 weeks
after implantation of an extra Astra implant.The l0 000 x
SEM figure is the same as that Figures ll and 12. The exact
points where mineral concentrations are measured are shown
with small numbers in Figure 14. The mean values of those
standard points are given in Table III.
Table III
CaO P2O5 CaO/P2O5 Mg Na
______________________________________________________
control 56 30 l.8 l.2 8.2
medicated 72 40 l.8 0.9 2.8
The values are given in weight percent.
Histological and SEM-pictures were similar in both groups.
No differences under light and SEM-cross-over pictures were
seen. In one month specimens P2O5 and CaO are both
significantly greater in the medicated than in control
mandibles. This means that rapid bone formation had begun,
osteoclasts have resorbed bone. Osteogenesis is more
intensive in medicated than in control patients.
III. Immersion studies
The effect of bisphosphonates for the treatment of implant
surfaces before their use was verified in the following
study. Twenty skeletally mature rabbits were used for the
implantation of hydroxyapatite coated 6 mm IMZ-implants.
Ten rabbits got 25 mg/kg i.m. clodronate in two injections
2160i~5
094/~770 PCT/F~4/00136
one week before the surgery. Postoperative injections twice
a week were continued for l8 days. The remaining ten
rabbits did not get any systemic clodronate treatment
before or after the surgery. In the operation an IMZ-
implant was implanted into both femurs of each rabbit. Theimplants operated into the right femurs of the rabbits had
been immersed for five seconds in a physiological solution
containing 6 mg/ml clodronate before the implantation. The
implants operated into the left femurs of the rabbits were
not treated with clodronate before the implantation. The
specimens were analysed according to methods described
earlier. SEM ~x~mi nAtions were carried out and the total
amount of new bone (NB) as well as the amount of bone in
contact with the implant (IB, i.e. osseointegration) was
lS determined.
All the wounds of the twenty rabbits healed without
infections. Great variations in the NB and IB amounts were
found. Preli~inAry results show that systemic clodronate
treatment of the rabbits as well as immersion of the
implant in a clodronate solution before the implantation
highly enhance the NB and IB formation. The highest NB and
IB amounts were received where systemic clodronate
treatment of the rabbits were combined with the immersion
of the implant in a clodronate solution. Hydroxyapatite
coated IMZ implants immersed in a clodronate solution
before the implantation into systemically medicated rabbits
resulted in a more rapid bone remodelling than implantation
of untreated IMZ implants to similarly systemically
medicated rabbits.
Figure l5 shows an untreated implant operated into a
nonmedicated rabbit. Figure l6 shows an immersed implant
operated into a systemically medicated rabbit. The progress
of the bone formation is clearly observed in Figure 16.